1. Introduction
The world’s population is aging quickly. In 2015, there were more than 900 million people over the age of 60 in the world, and by 2050, that number is projected to rise to 2 billion, or 22% of all people. By 2050, there will likely be 434 million people over the age of 80 [
1]. A common health issue for older adults is hearing loss. Ninety percent of those in their 80s and 50% of those in their 60s have at least slight hearing loss [
2].
In addition to reducing quality of life, hearing loss has been linked to social isolation, depression, cognitive decline, and communication problems. Consequently, there is a lot of interest in the rehabilitation of hearing loss in the elderly population [
3].
Auditory rehabilitation (AR) is most frequently accomplished with hearing aids (HA). Some HA users have complained of aural feedback, occlusion effects, and pain from the device filling up their ears. As a response [
3]. As a result, alternatives have been created over the last two decades. In addition to cochlear implants, auditory brainstem implants, and bone conduction (BC) devices, the active middle ear implant (AMEI) is one of the most often utilized devices [
4].
Growing technology advancements and surgical expertise in otological implantation have resulted in the field’s rapid growth and expansion. Particularly AMEIs have developed into a well-recognized rehabilitation technique to treat not only sensorineural hearing loss but also conductive hearing loss (CHL) or mixed hearing loss (MHL) in recent years by using different coupling options [
4].
Ugo Fisch performed the first AMEI implantation in 1996, where the floating mass transducer (FMT) was connected to the incus, providing mechanical waves that directly stimulated the inner ear. Since then, multiple attachment/coupling solutions have been developed to implant the AMEI in various sites, allowing for varied difficult middle ear problems, the short and long processes of the incus, the stapes head, the stapes footplate, [
5], and the round window (RW) [
6] are examples of structures.
The Active middle ear implants are an electromagnetic, implantable hearing implant that has been shown in numerous international tests to be effective and safe [
7,
8]. It is a good alternative to traditional hearing aids when they are unsatisfactory to patients, particularly when they do not provide enough hearing gain in noise or at high frequencies, or when they are contraindicated in patients due to anatomical causes or infection in the external ear canal [
9].
The AMEI consists of an external component, the audio processor (AP), and an implanted component, the vibrating ossicular prosthesis (VORP), which incorporates a receiver/stimulator, a conductor link, and a floating mass transducer (FMT). The AP sends information to the VORP, which causes the FMT to vibrate the mobile structure of the middle ear (i.e., incus, stapes superstructure, or the stapes footplate) or the inner ear (i.e., the round window membrane), stimulating the cochlear fluids [
10].
Despite this AMEI has been used with patients for many years, there is still a lack of scientific literature within the Middle East area about the efficacy and performance of this device in implanted patients. Therefore, the objective of this study is to evaluate audiological outcomes, quality of life, and complications in patients implanted with AMEI. The secondary objective is to investigate the required duration after implantation to reach satisfactory outcomes.
2. Materials and Methods
This retrospective study included all patients who underwent active middle ear implantation with a Vibrant SoundbridgeTM device (VSB) (MED-EL, Innsbruck, Austria) at a single referral Centre from August 2017 to October 2021. According to the manufacturer’s indication, the coupling modality was selected based on the patient’s middle ear anatomy and kind of hearing loss. This study was approved by the Institutional Review Board and done in accordance with the Declaration of Helsinki.
Participants
The study’s population was not limited by gender or laterality. The study included all patients who underwent middle ear implantation according to the criteria, Patients with conductive (including those with aural atresia, microtia, and external canal stenosis) and mixed hearing loss, as well as those with moderate to severe sensorineural hearing loss that has been stable for at least two years and unaided speech discrimination scores (SDS) of 50% or better. Patients with incomplete medical records and those who did not respond to postoperative follow-up were excluded.
Outcome Measures
All subjects underwent thorough audiological evaluations preoperatively and at 3 months, 6 months, and 12 months postoperatively. Pure-tone audiometry was conducted, including ear-specific air conduction (AC) and bone conduction (BC). Pure-tone average (PTA4) values were calculated as the mean thresholds at 0.5, 1.0, 2.0, and 4.0 kHz.
Speech testing was conducted and included the speech reception threshold (SRT) and speech discrimination scores (SDS) in quiet and in noise at 65 dB. All speech tests were performed using spondee Arabic words and phonetically balanced Arabic words. The contralateral ear was masked during all hearing tests.
Questionnaire
The Speech, Spatial, and Qualities of Hearing Scale (SSQ12) was administered to the patients. SSQ12 is a hearing-specific questionnaire consisting of 12 questions about the ability to cope with different listening situations before and after an intervention [
11]. The ‘‘benefit’’ version of this questionnaire (SSQ12) was specifically designed for retrospective assessment in cases in which no questionnaire was administered before intervention. The domains assessed by the SSQ12 include speech hearing (five questions), spatial hearing (three questions), and quality and ease of hearing (four questions). Each item of the SSQ12 can be rated on a 10-point scale ranging from -5 to +5. The midpoint (zero) corresponds to no change due to the intervention. The SSQ12 results in an overall score and scores in the three subscales (speech, spatial, quality).
3. Results
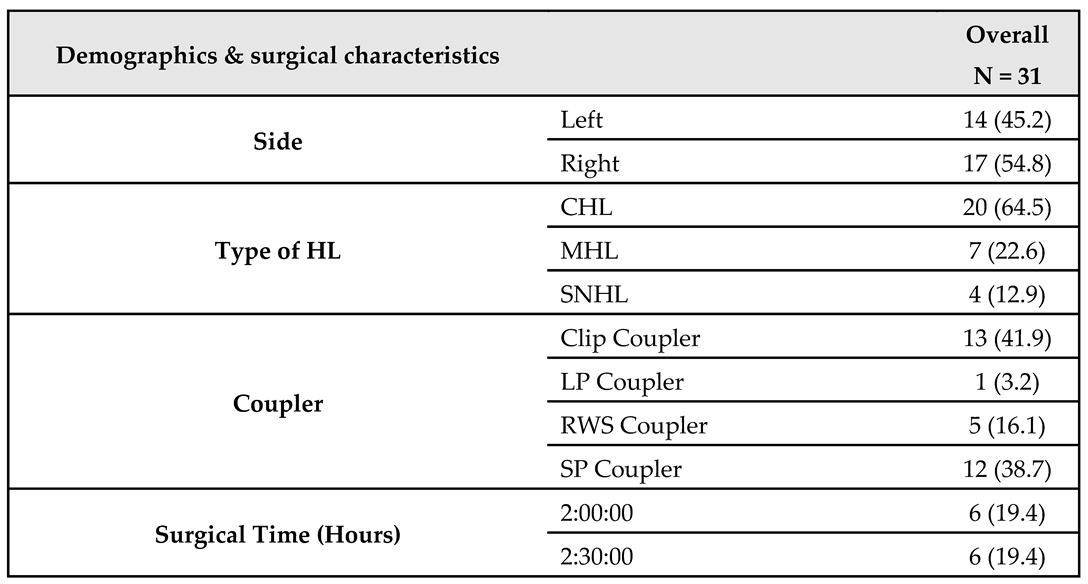
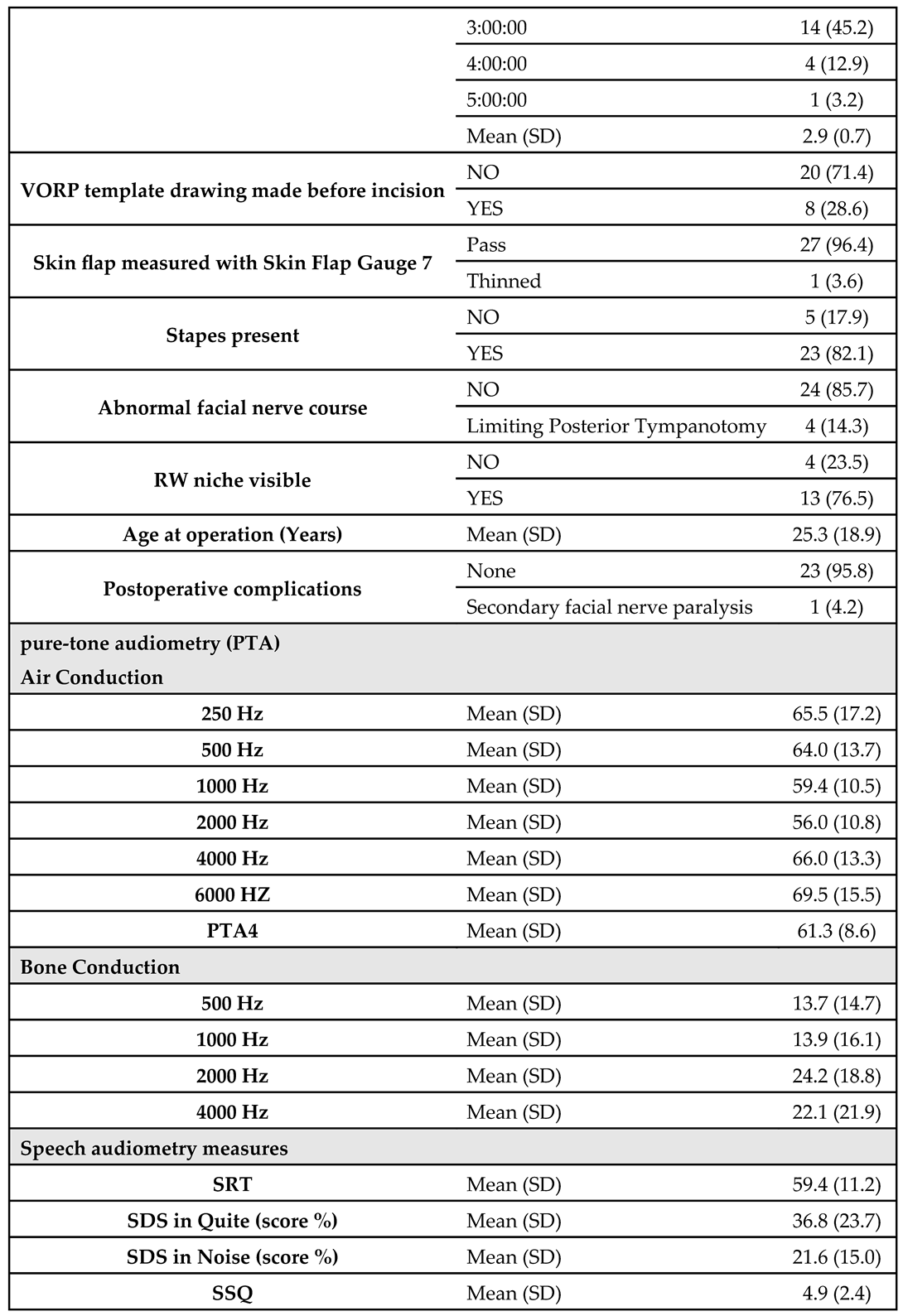
The study was carried out on 31 patients who used the AMEI system. About 54.8% of them were right-sided and the remaining 45.2% were left. Looking at the types of hearing loss, we found that 64.5% of patients had conductive, 22.6% had mixed, and 12.9% had sensorineural. Furthermore, Clip Couplers were used in 41.9% of patients, SP Couplers in 38.7%, RWS Couplers in 16.1%, and the remaining 3.2% used LP Couplers. Regarding the duration of surgery, about 45.2% of patients spent 3 hours, about 19.4% spent 2 and 2.5 hours for both, about 12.9% spent 4 hours, and the remaining 3.2% spent 5 hours. The overall mean surgical duration was 2.9 ± 0.7 hours.
The VORP template drawing has been made in 28.6% before the incision. 96.4% of patients passed the skin flap Gauge test (7 mm), while the remaining 3.6% were thinned. About 82.1% of patients had stapes bone and about 14.3% of them had limiting posterior tympanotomy, while the remaining 85.7% did not have any abnormal facial nerve course. About 76.5% of patients had visible round window niche with a mean age at operation of 25.3 ± 18.9 years. Furthermore, only one patient showed postoperative secondary facial nerve paralysis.
The measured pre-operative Air Conduction pure-tone audiometry (PTA) was 65.5 ± 17.2 at 250 HZ; 64.0 ± 13.7 at 0.5 KHZ; 59.4 ± 10.5 at 1 KHZ; 56.0 ± 10.8 at 2 KHZ; 66.0 ± 13.3 at 4 KHZ; and 69.5 ± 15.5 at 6 KHZ, while the mean PTA 4 was 61.3 ± 8.6. The pre-operative Bone Conduction pure-tone audiometry (PTA) was 13.7 ± 14.7 at 0.5 KHZ; 13.9 ± 16.1 at 1 KHZ; 24.2 ± 18.8 at 2 KHZ; 22.1 ± 21.9 at 4 KHZ. The mean pre-operative Speech Reception Threshold was 59.4 ± 11.2, the mean pre-operative speech discrimination score was 36.8 ± 23.7% in quiet and 21.6 ± 15.0% in noise. The mean pre-operative Speech, Spatial, and Qualities of Hearing Scale was 4.9 ± 2.4 (
Table 1).
Outcome measures:
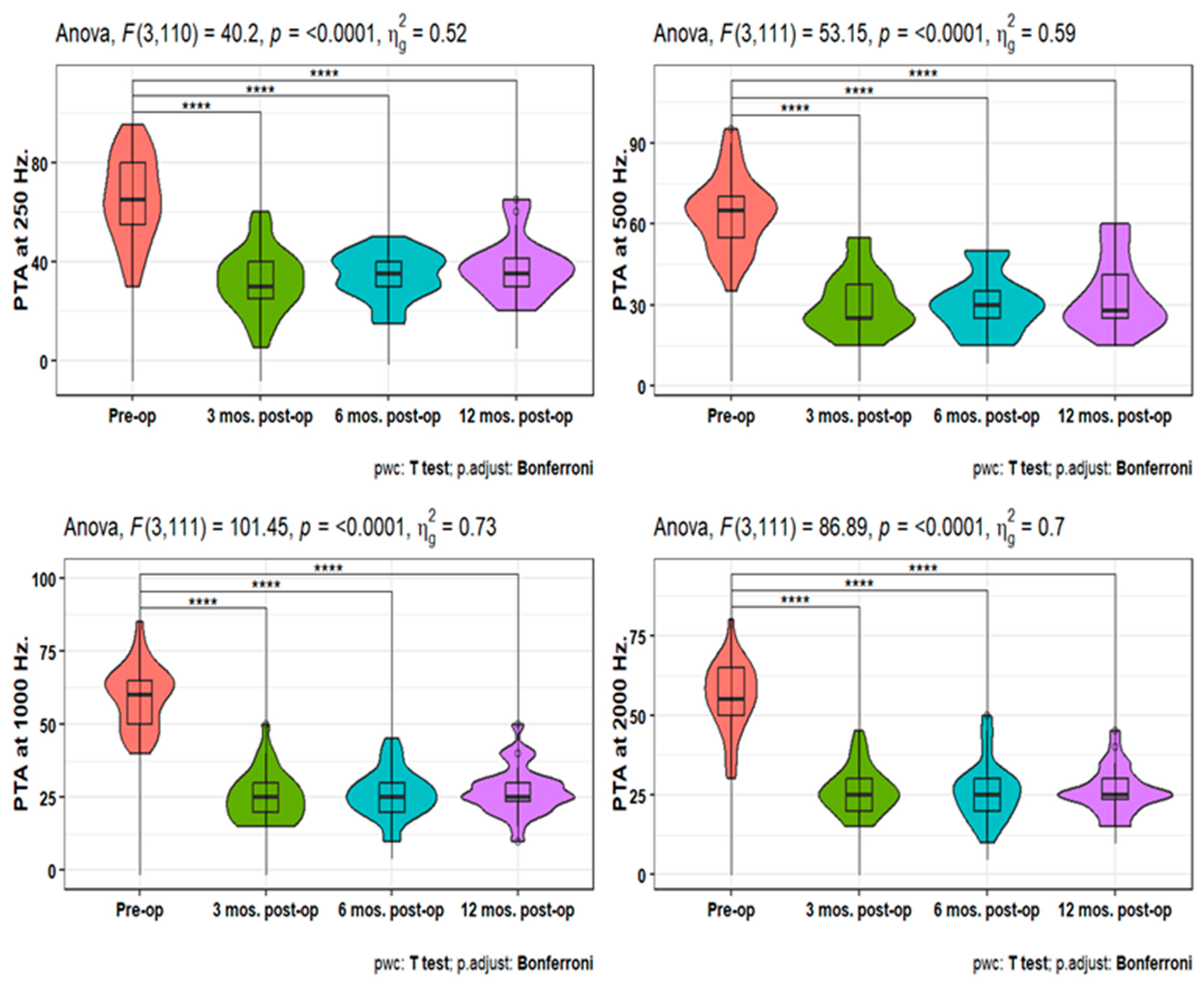
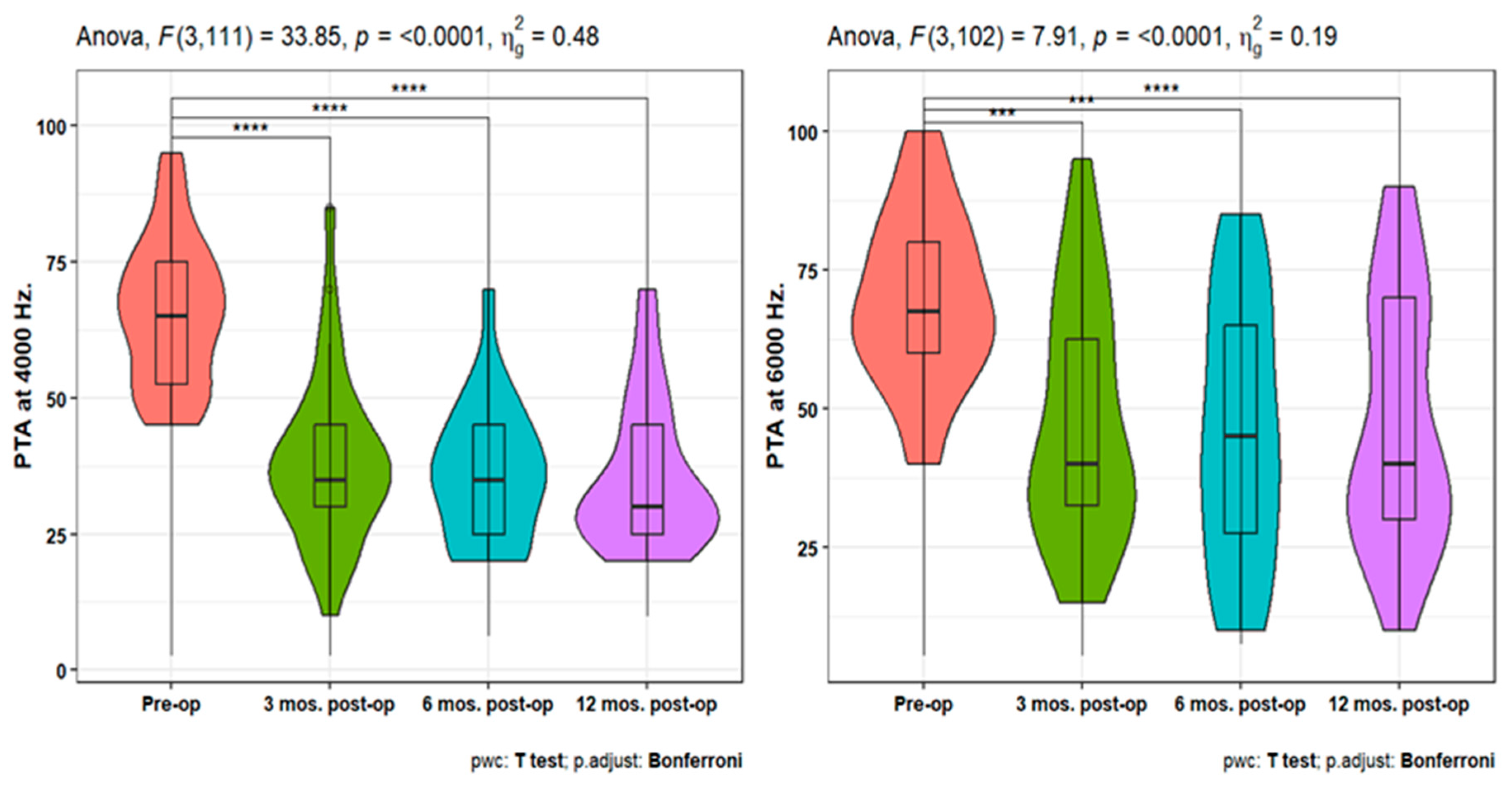
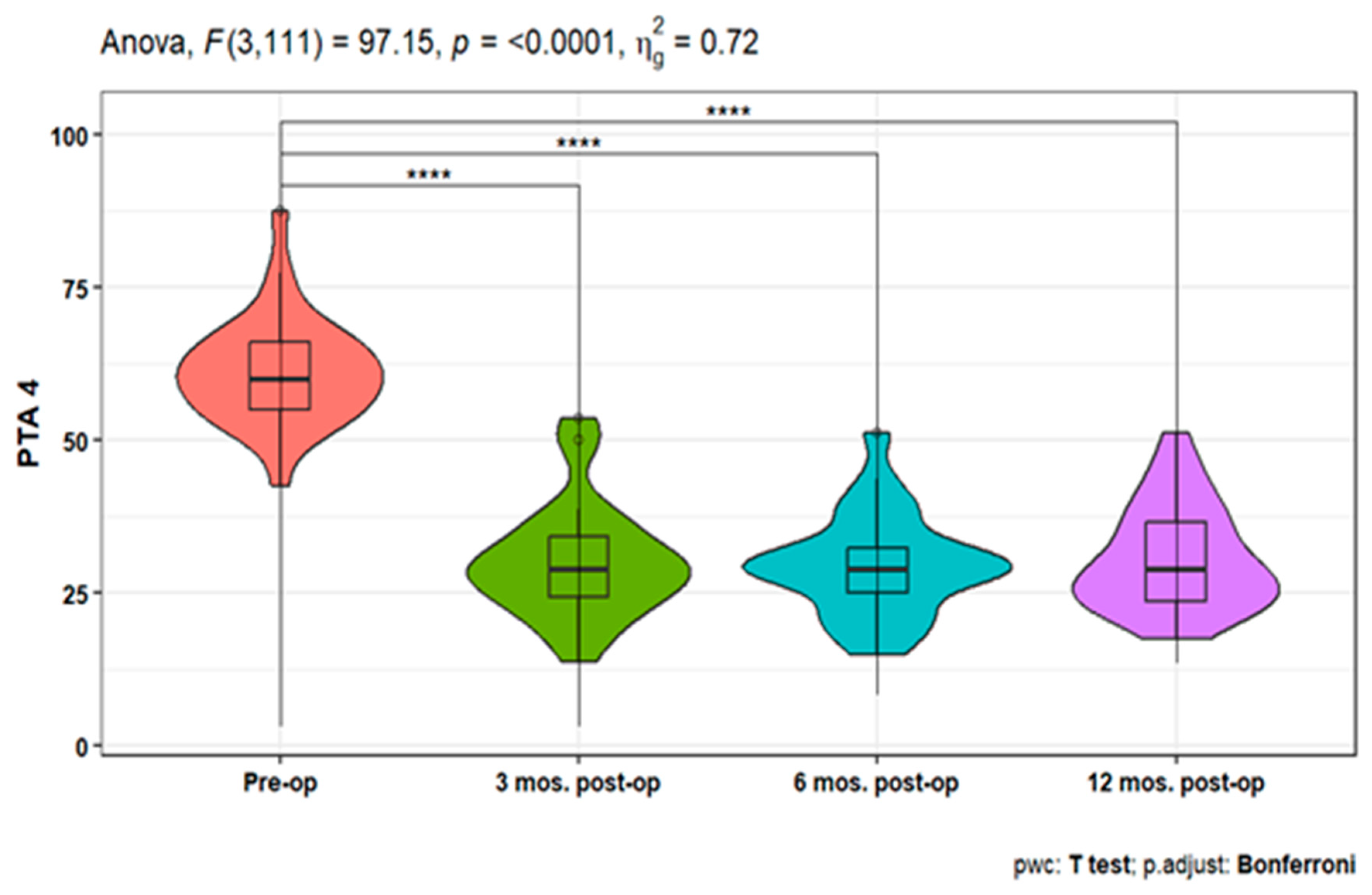
Pure-tone audiometry (PTA) measures showed significant improvement (p<0.001) at 3-, 6-, and 12-months post-operative compared to the pre-operative measures at 250, 500, 1000, 2000, 4000, and 6000 HZ. Also, PTA4 showed a significant improvement after 3-, 6- & 12-months post-operative compared to the pre-operative one. However, the pairwise comparisons of post-operative PTA4 at the tested time points did not show significant differences (
Figure 1,
Figure 2 and
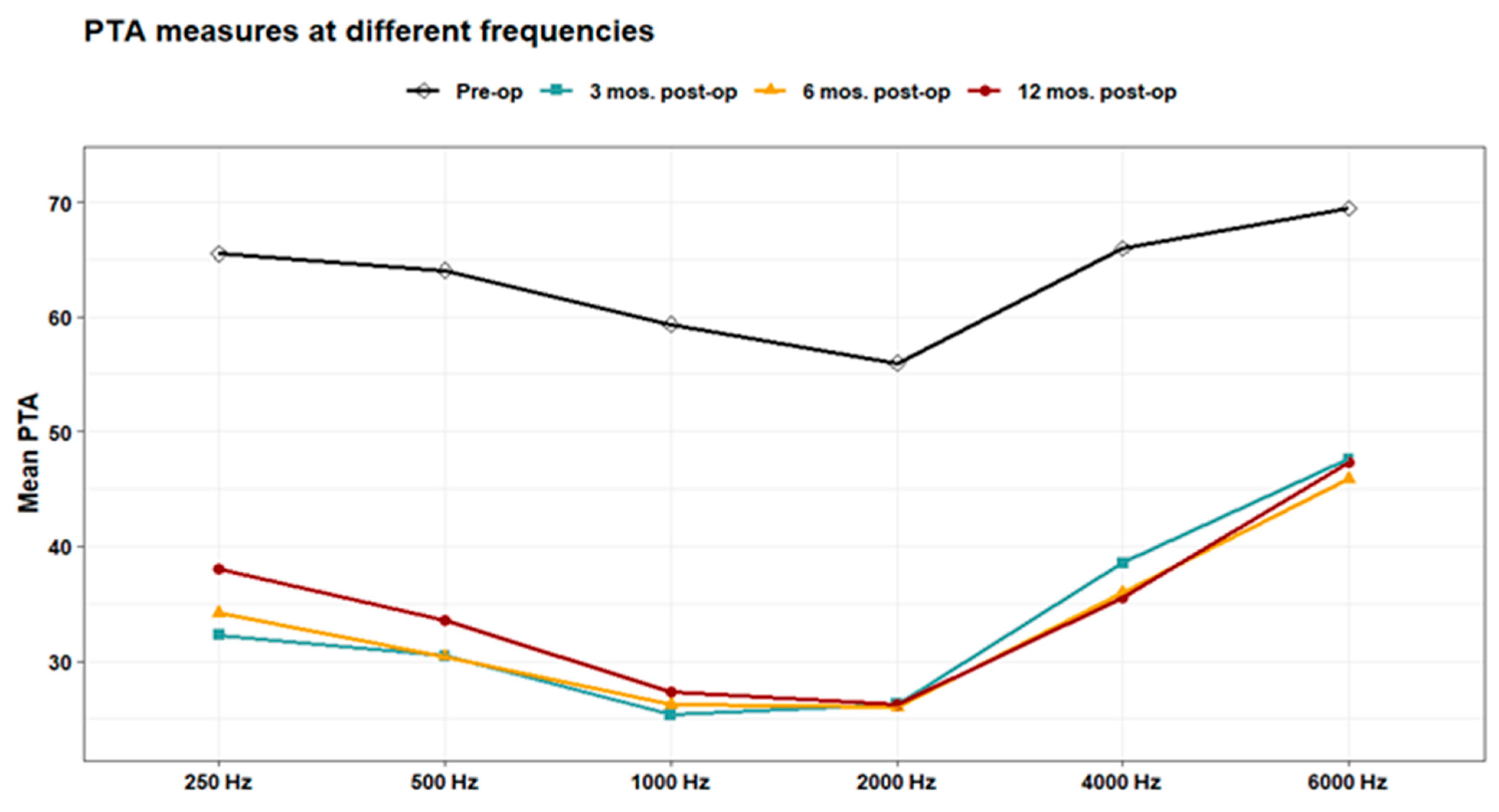
Figure 3). The line chart in (
Figure 4) also showed the non-significant differences between 3, 6 & 12 months post-operative PTA measures at different frequencies in contrast to the significant difference between PTA at every post-op time point compared to the pre-operative measures.
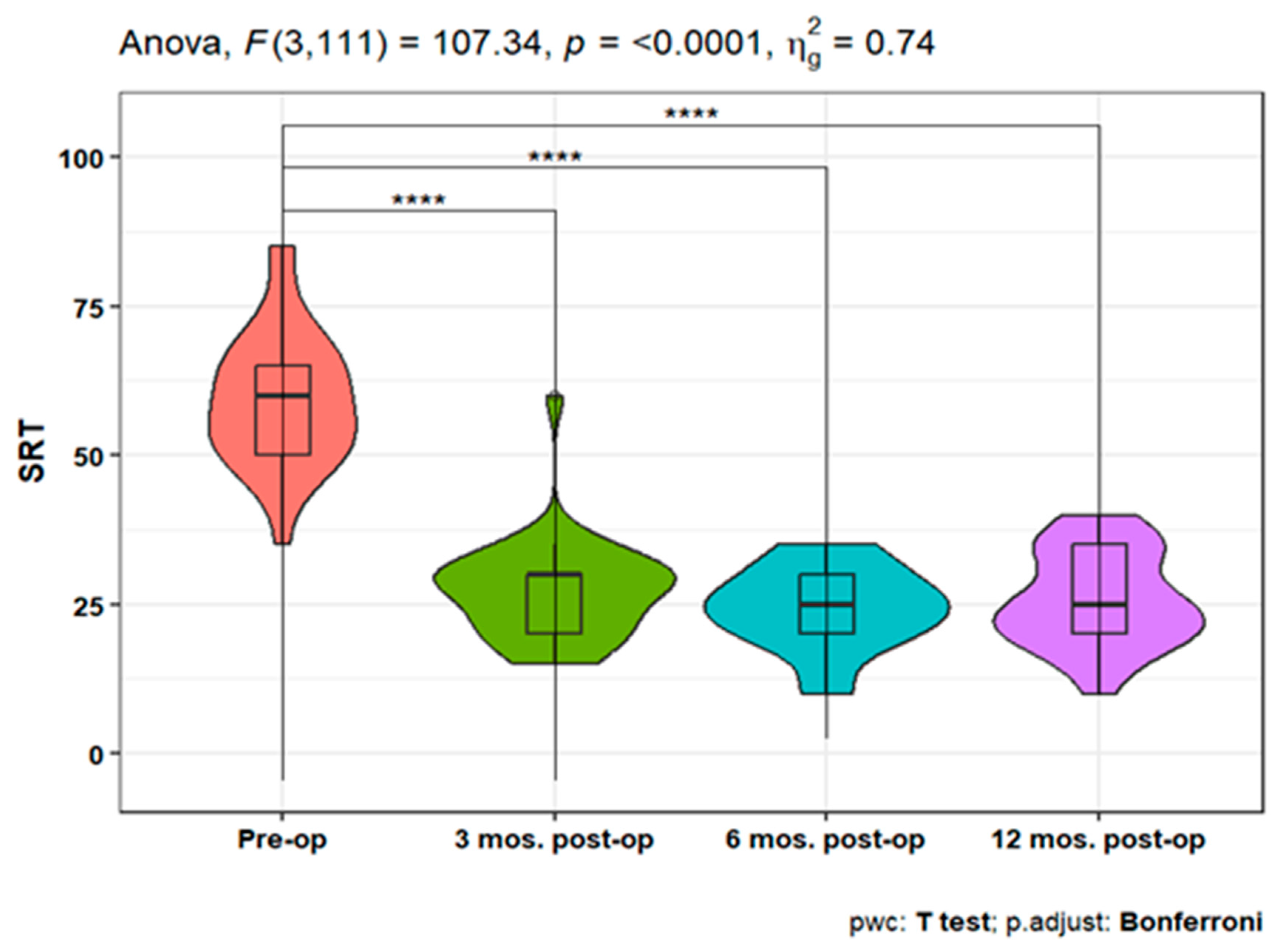
The speech reception threshold (SRT) showed a statistically significant improvement at 3, 6, and 12 months post-operative measures compared to pre-operative SRT (p<0.001). Moreover, non-significant differences were detected between the three postoperative time points (
Figure 5).
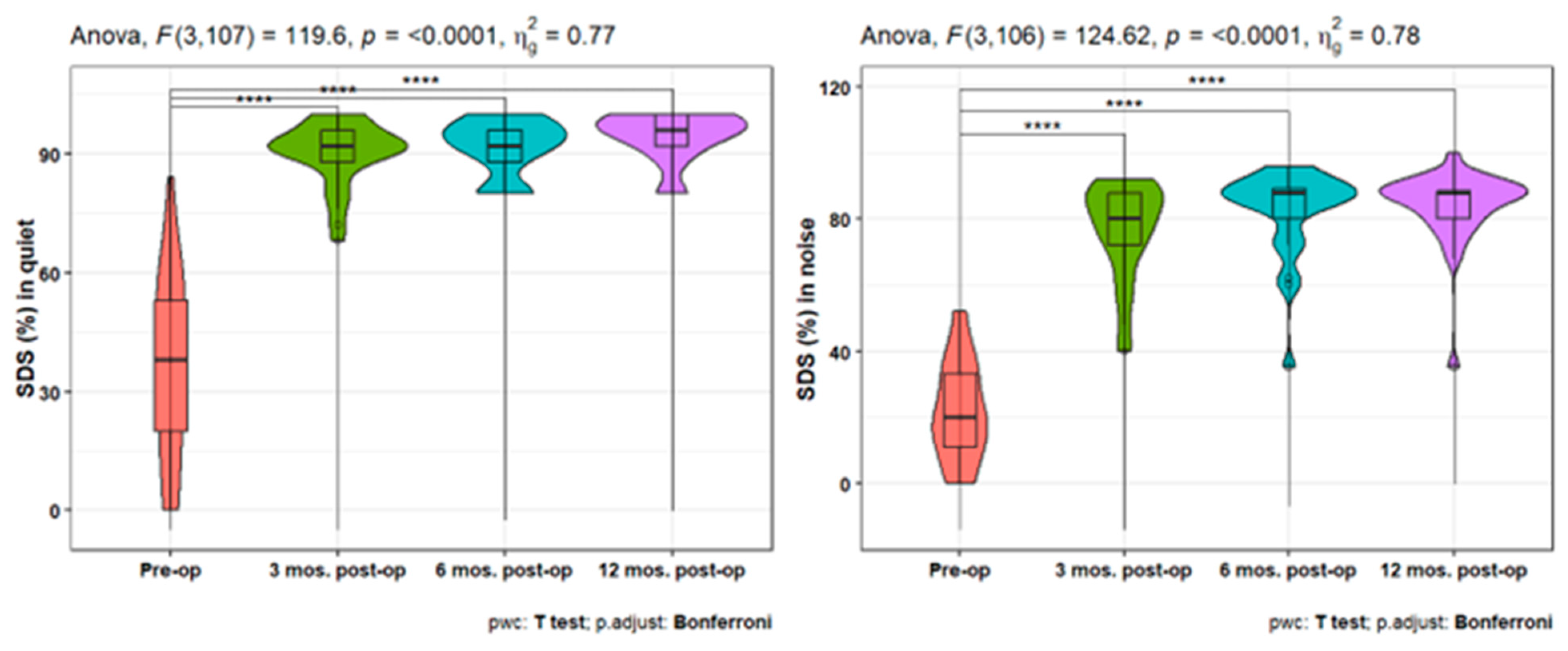
Furthermore, the speech discrimination scores (SDS) showed a statistically significant increase at 3-, 6-, and 12-months post-operative either in quiet or in noise measures compared to the pre-operative ones (p<0.001). In addition, there is a statistically significant increase in SDS at quiet after 12 months compared to the 3 months post-operative scores (p = 0.021) (
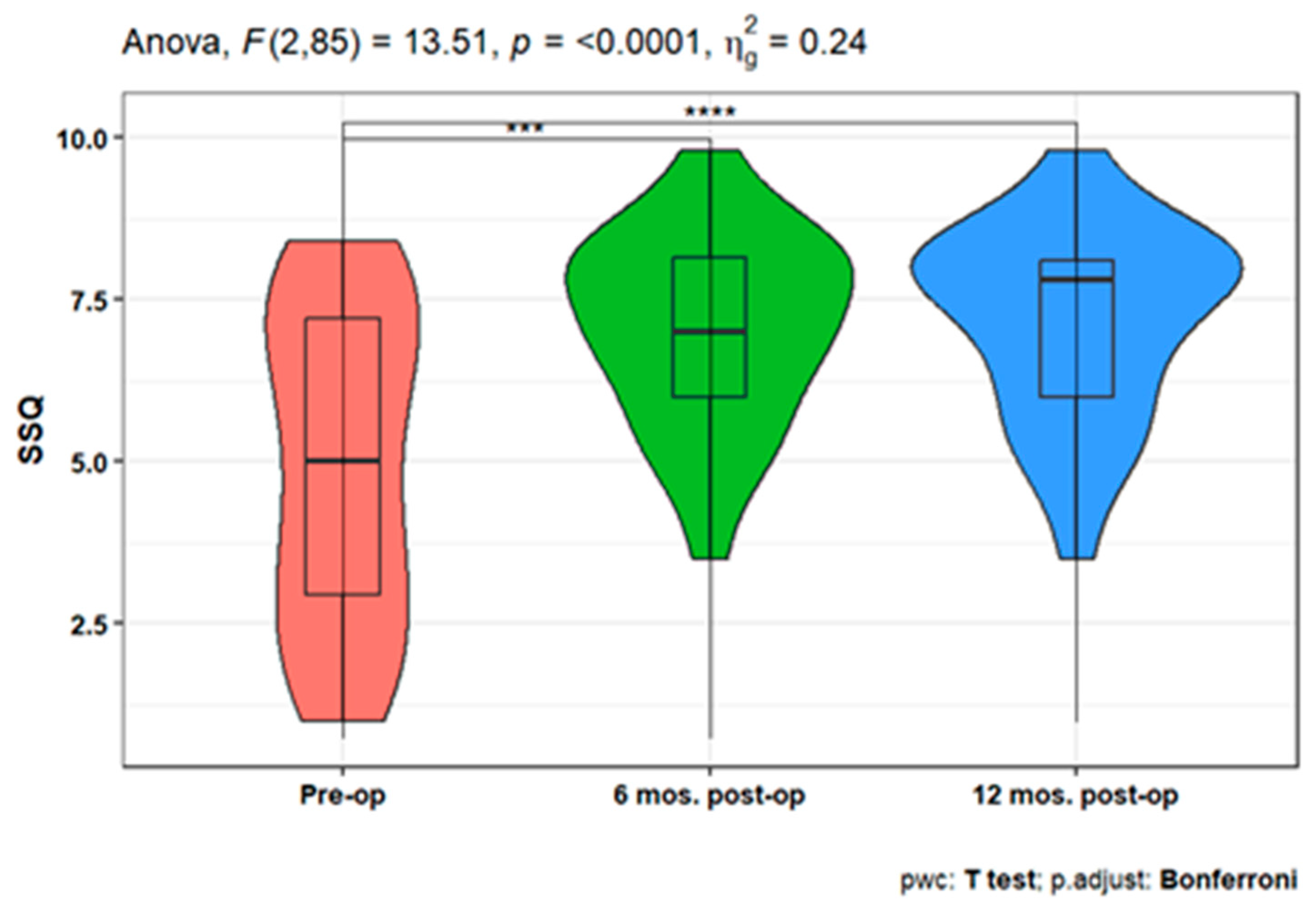
Figure 6). When studying the variation in SSQ), the analysis showed a statistically significant increase after 6-, and 12-months post-operation compared to the pre-operative scale (p <0.001 for both). However, there is no significant difference between the SSQ at 6 and 12 post-op (
Figure 7).
4. Discussion
Numerous clinical studies have been carried out since the AMEI device was first introduced to show the advantages of the device for hearing-impaired patients [
12,
13,
14]. AMEI use is known to benefit patients with all three categories of hearing loss, as established by Luetje et al. [
15] for sensorineural hearing loss and Baumgartner et al. [
16] for conductive and mixed hearing loss.
In multiple instances of auditory atresia and fibrous dysplasia of the temporal bone (FDTB), AMEI has been successfully used as one of the treatment options for individuals with conductive or mixed hearing loss, with outcomes that greatly improved PTA, SRT, and SDS [
17,
18].
According to preliminary evidence from existing patients, AMEI is a safe and effective therapeutic option for people with all three types of hearing loss etiologies. There were no major intraoperative or postoperative complications. In this retrospective analysis, patients implanted with AMEI were evaluated for audiological outcomes, quality of life, and complications in a single-center study.
As analysis showed that patients’ perceptions of the AMEI benefits for PTA, SRT, and SDS were clearly improved, most patients in this study reported being generally satisfied or extremely satisfied with the device. For the group of 31 implanted patients, the audiological follow-up data revealed a significant improvement in hearing thresholds overtime at 250 Hz, 500 Hz, 1000 Hz, 2000 Hz, 4000 Hz, and 6000 Hz. Additionally, PTA 4 showed a statistically significant decline post-operatively as compared to pre-operatively. However, there were no discernible variations across the postoperative time points in pairwise comparisons.
In terms of speech recognition outcomes, an audiologic benefit for AMEI revealed a statistically significant decrease in 3, 6, and 12-month post-operative measurements when compared to pre-operative SRT. Furthermore, despite the non-significant differences found between the three post-operative time points, Alzahrani et al. [
19] report that SRT measurements with AMEI were significantly better in all patients compared to preoperative measurements. Ernst et al. [
20] and Sprinzl et al. [
21], reported speech recognition improvement of 52% to 81% after at least 6 months of use with the Freiburger monosyllabic words, which is similar to what our patients demonstrated.
Lassaletta et al.[
22] observed that after 1 year postoperatively, word recognition (SDS at 65 dB) greatly improved, also Alzahrani et al.[
19] report the mean SDS in quiet conditions was 51% in the unaided condition and 94.60 % in the aided condition for monosyllables at 65 dB HL. our results indicated a significant increase in post-operative speech in either quiet or noise at 3, 6, and 12 months compared to pre-operative. In addition to a statistically significant rise in SDS at quiet after 12 months compared to 3 months post-operative scores (p = 0.021). As a result, we infer that the AMEI improves speech understanding outcomes significantly.
In this study, assessing patient benefit following AMEI implantation with the SSQ revealed a significant increase after 6- and 12-months post-operation compared to the pre-operative scale, but there was no significant difference between the 6 and 12 post-operative SSQ. This supports the concept that patients who improve their speech understanding with AMEI also have higher hearing quality, at least in the categories addressed by the SSQ.
According to these results, the patient’s subjective reports of an improvement in their hearing and overall quality of life may be supported by actual improvement. The long-term postoperative outcomes of their AMEI users were published by Rameh et al. [
23] in their study. Although there was a lot of variety among the devices, patients were generally happy with their implants. Positive effects on patients’ social interactions are seen when they can hear and speak clearly. Due to communication challenges, people who have hearing loss frequently withdraw from social situations, particularly ones with background noise.
In addition to the audiologic findings, we discovered no differences in perioperative and postoperative complications, which is consistent with the literature [
12,
14,
24]. However, only one patient experienced postoperative secondary facial nerve paralysis, which could have been caused by a late reactivated viral infection such as herpes simplex virus 1 or varicella-zoster virus and resolved spontaneously. In our investigation, all patients exhibited good, aided hearing thresholds with the AMEI following surgery, demonstrating that the surgical and fitting methods were adequate for the patient’s demands, as Wolf-Magele et al., [
25] study.
An average postoperative complication rate of 16.3% was found in a systematic review of the use of the AMEI for treating conductive and mixed hearing loss [
20]. The reported explanation percentage for AMEI, however, in long-term follow-up studies of AMEI use in individuals with mixed hearing loss ranged from 10.17% to 18.5% [
26,
27]. Colletti et al. [
28] described two explanations that were required due to misdiagnosed significant hearing loss. Brkic et al. [
26] reported a 10.2% explanation rate.
Revision surgery was reported in two of our patients, one patient with a history of CWD who was presented with electrode protrusion into the external auditory meatus 2 years after the primary surgery. The second patient presented with decreased in the performance one year post implantation. During the revision surgery, the FMT was found to have limited movement due to mastoid bone re-growth around it. Device failure was reported by Zwartenkot et al.[
27] (7% technical failure rate), Brkic et al. [
26] (4.0%), Sprinzl et al. [
21], and Schmuziger et al. [
29], however, none of our patients experienced device failure.
The main limitation of the study was that all questionnaires were administered only after treatment (i.e., after AMEI). The second limitation, we the inability to compare the AMEI outcome with conventional Hearing Aids. Despite possible limitations, retrospective studies generally reflect clinical practice in terms of patient selection, assessment, and surgical techniques and are therefore generalizable to routine clinical care.
After analyzing the temporal improvement in outcome measures, We found that no significant differences were found in PTA and SRT between the 3-, 6-, and 12-month visits. Consequently, it is reasonable to suggest that these outcomes could be assessed once or twice per year. Additionally, the SDS exhibited a significant increase only after 12 months, compared to the 3-month time point, which further supports the notion that measuring the SDS at 6 months may be of lesser importance. However, satisfaction levels did not significantly differ between the 6-month and 12-month measurements following surgery. As a result, these findings warrant further investigation to establish the necessity of a 6-month visit after AMEI, or if it suffices to have patients visit the clinic at 3- and 12-month intervals post-surgery. That helps patients with time, especially patients who stay far away from the hospital.
5. Conclusion
This retrospective study showed that AMEI can improve subjective satisfaction scores and audiological test scores in patients with different types of hearing loss. AMEI has a low risk of medical or surgical complications, the ease of using a hearing implant, and the social benefits of good hearing and communication, we think that AMEI should be regularly offered to patients with hearing loss if it is audiological and surgically indicated.
References
- “Ageing and health.” https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed Jul. 06, 2023).
- A.M. Goman and F. R. Lin, “Prevalence of hearing loss by severity in the United States,” Am J Public Health, vol. 106, no. 10, pp. 1820–1822, Oct. 2016. [CrossRef]
- S. H. Lim et al., “Audiologic outcomes and complications of active middle ear implantation in older adults,” Acta Otolaryngol, vol. 141, no. 7, pp. 702–706, 2021. [CrossRef]
- G. M. Sprinzl et al., “Long-Term Stability and Safety of the Soundbridge Coupled to the Round Window,” Laryngoscope, vol. 131, no. 5, pp. E1434–E1442, May 2021. [CrossRef]
- T. Chen et al., “A comparative study of MED-EL FMT attachment to the long process of the incus in intact middle ears and its attachment to disarticulated stapes head,” Hear Res, vol. 353, pp. 97–103, Sep. 2017. [CrossRef]
- R. Perez, C. Adelman, S. Chordekar, M. A. De Jong, and H. Sohmer, “The mechanism of direct stimulation of the cochlea by vibrating the round window,” J Basic Clin Physiol Pharmacol, vol. 25, no. 3, pp. 273–276, Sep. 2014. [CrossRef]
- A.Khan, T. Hillman, and D. Chen, “Vibrant soundbridge rehabilitation of sensorineural hearing loss,” Otolaryngologic Clinics of North America, vol. 47, no. 6. W.B. Saunders, pp. 927–939, 2014. [CrossRef]
- Ernst, I. Todt, and J. Wagner, “Safety and effectiveness of the Vibrant Soundbridge in treating conductive and mixed hearing loss: A systematic review,” Laryngoscope, vol. 126, no. 6. John Wiley and Sons Inc., pp. 1451–1457, Jun. 01, 2016. [CrossRef]
- Grégoire, J. P. Van Damme, C. Gilain, B. Bihin, and P. Garin, “Our auditory results using the Vibrant Soundbridge on the long process of the incus: 20 years of data,” Auris Nasus Larynx, vol. 45, no. 1, pp. 66–72, Feb. 2018. [CrossRef]
- T. Rahne et al., “A retrospective European multicenter analysis of the functional outcomes after active middle ear implant surgery using the third generation vibroplasty couplers,” European Archives of Oto-Rhino-Laryngology, vol. 278, no. 1, pp. 67–75, Jan. 2021. [CrossRef]
- M. B. Alkhodair, T. A. Mesallam, A. Hagr, and M. F. Yousef, “Arabic version of short form of the speech, spatial, and qualities of hearing scale (SSQ12),” Saudi Med J, vol. 42, no. 11, pp. 1180–1185, Nov. 2021. [CrossRef]
- U. Fisch et al., “Clinical Experience with the Vibrant Soundbridge Implant Device,” 2001.
- Fraysse et al., “A Multicenter Study of the Vibrant Soundbridge Middle Ear Implant: Early Clinical Results and Experience,” 2001.
- A. Sterkers et al., “A Middle Ear Implant, the Symphonix Vibrant Soundbridge: Retrospective Study of the First 125 Patients Implanted in France,” 2003.
- M. Luetje et al., “Phase III clinical trial results with the Vibrant Soundbridge implantable middle ear hearing device: A prospective controlled multicenter study,” Otolaryngology - Head and Neck Surgery, vol. 126, no. 2, pp. 97–107, 2002. [CrossRef]
- “Baumgartner”.
- F. Alzhrani, R. Halawani, and M. Yousef, “Feasibility and Efficacy of Vibrant Soundbridge Short Process Coupler in Patients With Aural Atresia,” Otol Neurotol, vol. 41, no. 10, pp. e1219–e1223, Dec. 2020. [CrossRef]
- Y. Al-shawi, L. Alsughayer, A. Alradhi, and F. Alzhrani, “Middle Ear Implant in a Patient With Fibrous Dysplasia: An Alternative for Hearing Restoration,” Ear Nose Throat J, vol. 100, no. 3_suppl, pp. 207S-211S, Jun. 2021. [CrossRef]
- F. Alzhrani, S. F. Alhabib, and M. Yousef, “Speech performance and subjective satisfaction of middle ear implant in congenital aural atresia,” Acta Otorhinolaryngologica Italica, vol. 42, no. 2, pp. 182–188, Apr. 2022. [CrossRef]
- A. Ernst, I. Todt, and J. Wagner, “Safety and effectiveness of the Vibrant Soundbridge in treating conductive and mixed hearing loss: A systematic review,” Laryngoscope, vol. 126, no. 6. John Wiley and Sons Inc., pp. 1451–1457, Jun. 01, 2016. [CrossRef]
- G. M. Sprinzl et al., “Long-Term Stability and Safety of the Soundbridge Coupled to the Round Window,” Laryngoscope, vol. 131, no. 5, pp. E1434–E1442, May 2021. [CrossRef]
- L. Lassaletta, M. Calvino, I. Sánchez-Cuadrado, R. M. Pérez-Mora, E. Muñoz, and J. Gavilán, “Pros and Cons of Round Window Vibroplasty in Open Cavities: Audiological, Surgical, and Quality of Life Outcomes,” Otology & Neurotology, Inc, 2015.
- Rameh, R. Meller, J.-P. Lavieille, A. Deveze, and J. Magnan, “Long-Term Patient Satisfaction With Different Middle Ear Hearing Implants in Sensorineural Hearing Loss.”.
- Vincent, B. Fraysse, J. P. Lavieille, E. Truy, O. Sterkers, and F. M. Vaneecloo, “A longitudinal study on postoperative hearing thresholds with the Vibrant Soundbridge device,” European Archives of Oto-Rhino-Laryngology, vol. 261, no. 9, pp. 493–496, Oct. 2004. [CrossRef]
- A. Wolf-Magele, J. Schnabl, T. Woellner, V. Koci, H. Riechelmann, and G. M. Sprinzl, “Active Middle Ear Implantation in Elderly People: A Retrospective Study,” 2011.
- F. Brkic et al., “Long-Term Outcome of Hearing Rehabilitation With An Active Middle Ear Implant,” Laryngoscope, vol. 129, no. 2, pp. 477–481, Feb. 2019. [CrossRef]
- J. W. Zwartenkot, J. J. S. Mulder, A. F. M. Snik, C. W. R. J. Cremers, and E. A. M. Mylanus, “Active Middle Ear Implantation: Long-term Medical and Technical Follow-up, Implant Survival, and Complications,” 2016. [Online]. Available: http://links.lww.com/MAO/A382.
- L. Colletti, M. Mandalà, and V. Colletti, “Long-term outcome of round window vibrant soundbridge implantation in extensive ossicular chain defects,” Otolaryngology - Head and Neck Surgery (United States), vol. 149, no. 1, pp. 134–141, Jul. 2013. [CrossRef]
- †nicolas Schmuziger, F. Schimmann, ‘ Wengen, †jochen Patscheke, and R. Probst, “Long-Term Assessment after Implantation of the Vibrant Soundbridge Device,” 2006.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).