Keywords light pollution; seabird; LED; vessel; interactions; artificial light at night
Introduction
It is well known that artificial light at night (ALAN) acts as a beacon that attracts many species, including birds, insects, and other taxonomic groups. Many bird species are drawn to lights, which can cause disorientation, groundings, and collisions with structures (Holmes 2017). The impacts of lighting attraction on various species have been well documented (Arthur 2020; Cieraad & Farnworth 2023; Heswall et al. 2023; Mitkus et al. 2016). Although diurnal species are also affected, nocturnal species are particularly sensitive to disruption of natural darkness. Nocturnality in many species, including seabirds, is believed to be a trait that developed as a defence mechanism against predation (McNeil et al. 1993; Mougeot & Bretagnolle 2000) and evolved independently of the lighting pressures that are now persistent in natural environments. This adaptive behaviour is commonly referred to as a predator avoidance response.
The impacts of light at night are determined by species physiological and ecological traits, combined with the characteristics of light emissions (including colour, intensity, and timing). Spectral sensitivity is species-specific (Longcore 2023; Syposz et al. 2021). Generally, seabirds are more attracted to blue, violet, and ultraviolet wavelengths and less so to warmer colours like amber or red wavelengths (Syposz et al. 2021). Most petrels (Procellariiformes), including fairy prions (Tītī wainui, Pachyptila turtur), are nocturnal. They differ from other seabird species in that they are predominantly adapted to flying at night during colony attendance (Mougeot & Bretagnolle 2000; Rodríguez et al. 2019). However, the current body of evidence is divided. For example, some Procellariiformes are attracted to light (e.g., fairy prions), whilst others (e.g. Manx shearwaters Puffinus puffinus) are repelled (Syposz et al. 2021).
Most studies determining lighting impacts at night have been land-based, however, they can also occur at sea. For example, lighting from marine vessels can result in unintended consequences of seabirds flocking towards the light source (positive phototaxis) and subsequently colliding with the vessel and/or its superstructure. Known as deck or vessel strikes it is a widespread phenomenon internationally and of growing conservation concern (Rodríguez et al. 2017b). Marine vessels differ in the types of lighting used. The health and safety of staff and the need to perform various tasks at night (e.g., deployment and recovery of fishing gear and or cleaning of decks) is the primary consideration in the selection and use of vessel-mounted lights for fishing vessels (pers. comms. D.G). In New Zealand, the Department of Conservation (DOC) has played a key role in raising awareness within the New Zealand fishing and cruise ship industries of the need to reduce lighting impacts. Mitigation Standards (DOC & FNZ 2023) include eliminating non-essential operational lights, minimising and shielding all other lighting (e.g. using window shading), and avoiding high-risk areas to seabirds such as breeding colonies.
Technological advances, such as the availability of energy-efficient LED light sources in many colours and lumen outputs, provide opportunities to tailor lighting requirements in the outdoor environment. However, while there are measures in place to help reduce at-sea light attraction to seabirds, little research has focused on how LED colour and the amount of light being emitted can be used in vessel-related mitigation strategies (Rodríguez et al. 2017a). Our study aims to address that knowledge shortfall, focussing on fairy prions, a species with a history of attraction to, and interaction with, fishing and cruise ship vessels at night, including in the Marlborough Sounds (Te Tau Ihu o Te Waka-a-Maui), New Zealand (Aotearoa). Specifically, we investigated the effects of LED colour and lumen output on the abundance and behaviour of adult fairy prions at a colony during the breeding season using an experimental setup with six different light treatments. Generalised linear-mixed-effects models allowed us to account for environmental conditions. We also conducted a desktop study to predict response rankings based on each light treatment’s colour spectrum and lumen outputs, and a related species’ spectral response.
Materials and Methods
Study location and timing
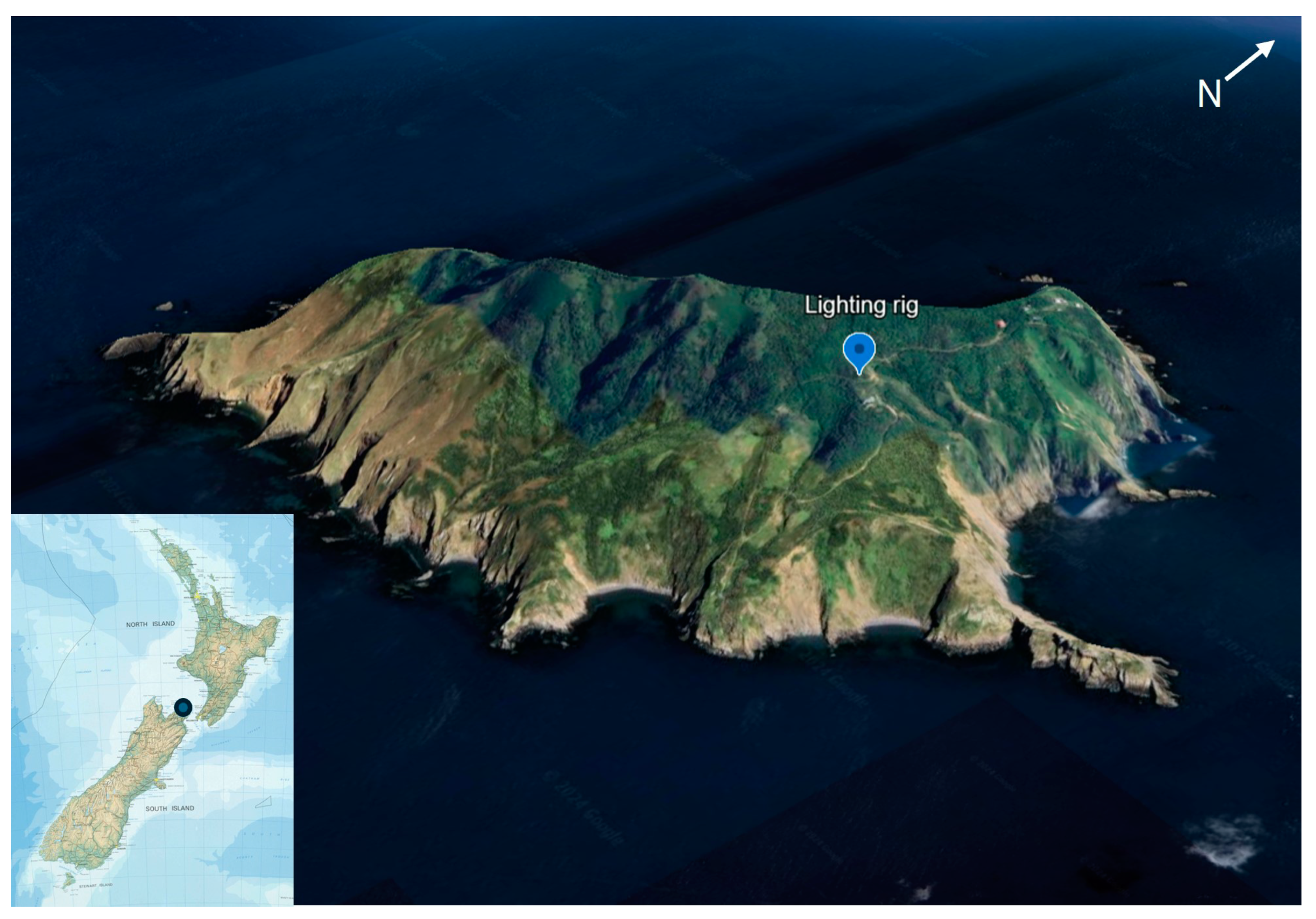
Light exposure experiments were undertaken on Stephen’s Island (Takapourewa), an uninhabited remote island at the north of the Marlborough Sounds, Cook Strait, New Zealand (Latitude -40.6693817, Longitude 173.9999533). Stephen’s Island has a land area of 1.5 square kilometres and rises 283 metres above sea level. Trials were conducted along the eastern side of the island at an altitude of 173 m above sea level (
Figure 1). An active lighthouse is located at the northern end of the island about 1 km away from the study site and could not be seen due to the geography of the island. Stephen’s Island is exposed to vessel traffic passing en route to and beyond the Cook Strait and Tasman Bay (Te Tai-o-Aorere) areas. The study took place over 17 non-consecutive nights during the period from 8
th December 2022 to 18
th January 2023.
Study species
Adult fairy prions (Procellariidae) were chosen as the study subjects as they are known to be attracted to vessel lights. They are among New Zealand’s most abundant and smallest petrels, weighing up to 160 grams. Protected under the New Zealand Wildlife Act (1953), they breed in large numbers, predominantly at colonies south of Cook Strait (Te Moana-o-Raukawa) to the sub-Antarctic islands. The largest population of fairy prions is on Stephen’s Island, in the Marlborough Sounds, where colony numbers are in excess of one million breeding pairs (Taylor 2000). Spending most of the year at sea, they return to small offshore islands to breed in caves or burrows. They do so under the cover of darkness and depart land before dawn each day. The breeding season is typically from October to February, with egg laying taking place in November. Experiments in the current study were conducted from December to January, before fledging. Adults and juveniles exhibit different responses to light emissions and DOC staff closely monitored the population to ensure fledglings were not included in the dataset.
Equipment
Lighting rig
A lighting rig was fitted with red, amber, and white light sources for the experiment (
Figure 2C). Red light was provided by six 24 V DC Bee Calm LED luminaires. Each was rated at 800 lumens, providing a total of 4,800 lumens for this treatment. Marine grade long range HELLA HypaLUME LED luminaires with twin light bars were used to provide 2500 K amber and 5700 K white light (1GJ 958 334-121 and 1GJ 011 872-511, respectively). The amber light at full power was rated at 19,000 lumens and powered with 24V DC (“amber high”), and the white at full power with 240 V AC was rated at 28,000 lumens (“white high”). A shade was used to cover one of the amber light bars to provide a half-setting (amber-low). The white-low setting was provided by switching off one of the white light bars. Lights were chosen based on current use in New Zealand commercial fisheries.
All lights were powered by a Honda eu20i petrol generator, which ran continually throughout experimental periods. The lights were attached to a vertical pole and positioned perpendicular to the earth’s surface (
Figure 2). The lighting rig was positioned facing seawards, to the south-east, and the ground sloped downwards toward the sea at an approximately 50° angle.
Thermal imaging camera
A Pulsar Helion XP38 thermal imaging camera was used for data extraction on abundance, attraction, and disorientation (
Figure 2C) (objective lens: F38/1.2, field of view:11.4° x 8.6° (horizontal * vertical)).
Experimental design
A total of 17-nights were sampled between 22:00 and 00:00 hours, with each night divided into three treatment blocks. Each block contained six 1-minute light treatments (light on), with each treatment separated by a 5-minute dark interval (light off). Treatments included a control (“dark”, no anthropogenic light), red light, high/low lumen amber light, and a high/low lumen white light. Treatments were run in a randomly assigned order (using a phone app random number generator) for each treatment block. In addition to recording bird abundance and behaviour using the thermal scope, we also used a recording sheet for each night to annotate the number of grounded birds, collisions with the lighting rig, maximum bird count visible with the naked eye, and data on environmental covariates.
Environmental data
Environmental data was sourced from the Meteorological Service of New Zealand weather station located at the northern end of Stephen’s Island, approximately one kilometre from the study site. Variables included wind speed, wind direction, relative humidity, and precipitation (no precipitation occurred on any of the 17 nights). In addition, local on-the-night data was recorded during the experiment nights at the beginning and end of each treatment, including the percentage of cloud cover and whether the moon was visible in the sky. We used moon illumination as a proxy of moon phase. This measure represents the closeness to full moon (but does not distinguish between waxing and waning moon) and was estimated using date and location from
https://www.timeanddate.com/moon/phases/@2182351). The variable of moon brightness was included as this same measure of moon illumination if the moon was visible and zero if it was not (the moon was below the horizon or obscured by the surrounding hills or clouds).
Video review
Consecutive video clips were stitched together for each night’s footage, without rendering, using open source Avidemux software. Clips of each treatment minute, and the dark period 1-minute before each treatment were reviewed twice to establish bird abundance and bird attraction and disorientation separately. To estimate bird abundance, footage was reviewed at two-thirds speed, and the number of birds visible in the frame were counted at 10, 20, 30, 40, and 50 seconds into each treatment. The second review was conducted at half speed to count the number of birds over the whole minute that flew towards the camera/light (which we assumed to represent attraction to the light, i.e., a proxy for positive phototaxis) and those birds that turned within the video frame (which we assumed to represent birds that were confused, disoriented and/or trapped by the light).
Spectral response
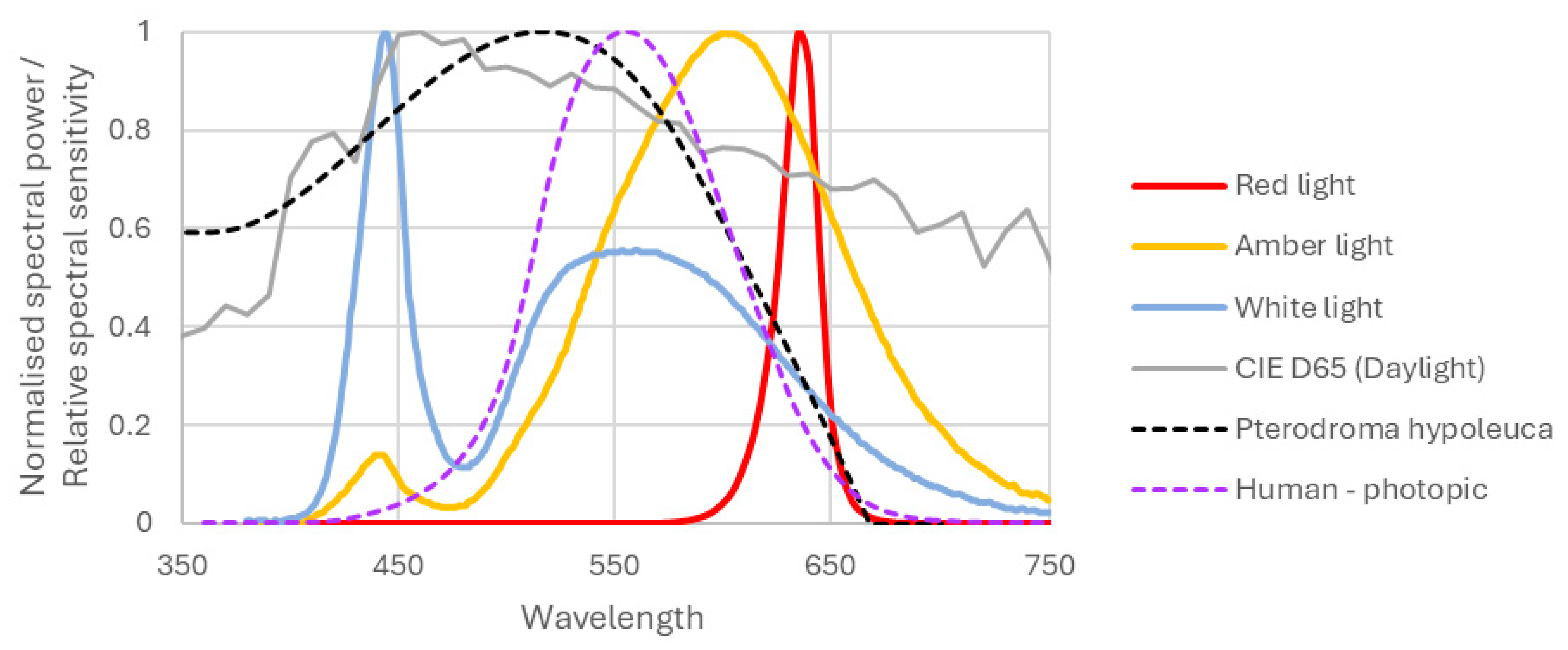
Following the approach of (Aubé et al. 2013) and (Longcore et al. 2018), we computed the spectral response index (SRI) for each light treatment. This index represents the degree of overlap of our light sources’ spectral power distribution (SPD) curves with the spectral response curve for our species, with reference to the D65 standard illuminant for daylight from the International Commission on Illumination (CIE). The SPDs were obtained for the amber and white light from the manufacturer, and the red lamp was tested by the lighting laboratory at Massey University. The wavelengths ranged from 380 nm to 750 nm to match the outer limits of the light sources’ response. The SPD curves were resampled to 5 m increments to ensure consistency for all light treatments. The curves were then normalised for a constant lumen output using the CIE photopic spectral sensitivity function
(Longcore et al. 2018). This normalisation was chosen to ensure that the light sources provided equal visual stimulus to humans, as the primary purpose of electric light on marine vessels is to illuminate human activity. The normalisation method to find
is given in Equation 1, where
is the maximum value of the spectral luminous efficacy (≈ 683 lm∙W
-1), a mathematical constant that is needed to convert data to lumens (CIE 2014).
SPD curves for the light treatments are shown in
Figure 3, together with other relevant curves that will be discussed in the following paragraphs.
Due to a lack of published research on vision in fairy prion and/or close relatives, we used the spectral response curve for Bonin petrel (
Pterodroma hypoleuca) from (Longcore 2023), original data from Reed (1986). Bonin petrels have a similar ecology to fairy prions with birds of both species returning to their colonies at night during the breeding season, which we expect results in a similar eyesight adaptation for low-light conditions. We normalised the spectral response curve to 1 at its maximal value and annotated this as
(refer to
Figure 3).
As noted above, we chose to reference all calculations to the standard SPD for daylight, which is the CIE D65 standard illuminant. Using Equation 1, the D65 SPD was normalised to provide
. To find the SRI we first computed the lamp SPD as weighted by the spectral response curve. We then used the same methodology to calculate the weighted D65 SPD. The ratio of the weighted lamp SPD over the weighted D65 SPD was then used to provide the SRI, per Equation 2.
As the spectral response index is normalised for constant lumen output, the impact of a particular treatment can be found by multiplying the SRI by the light output in lumens. This allows us to scale the results and determine the combined effects of the different light treatments’ spectrum and intensity.
Statistical Analysis
Statistical analysis was carried out in R version 4.2.2 (Team 2021). To evaluate differences in the number of birds observed between treatments, generalised linear mixed-effects models were used within the glmmTMB package (Brooks et al. 2017). The response variables were bird abundance (the number of birds observed in the field of view, repeated five times within each treatment period) and the number of attracted and disoriented birds (the number of birds observed to move towards the camera/light and those that turn in the video frame). Response variables were fitted with a negative binomial distribution to account for overdispersion. To account for a variation in numbers of birds through the night in the model, an offset of the mean count during a 1-minute dark period immediately prior to the treatment was included, or if the mean was zero, a count of one was used. Both response variables are thus relative measures.
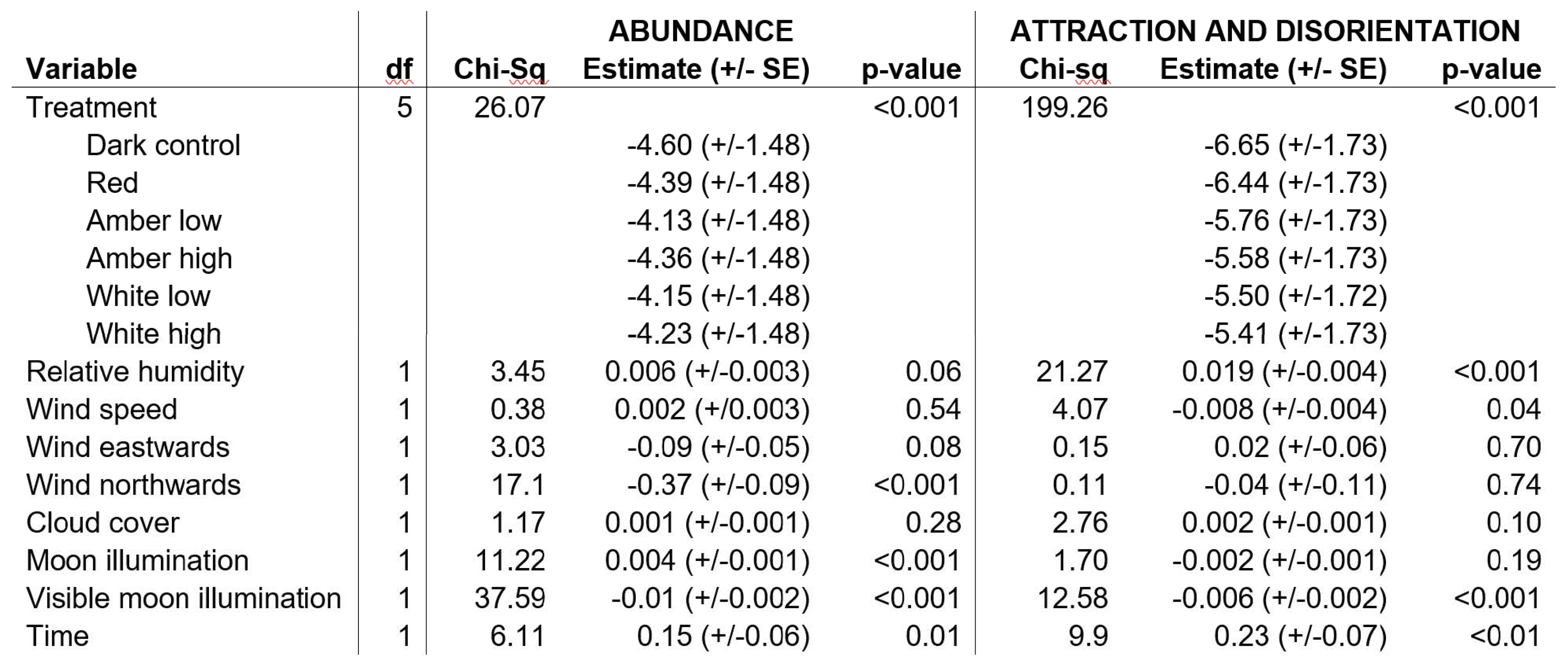
A random effect of treatment blocks nested within nights was included to account for differences in the number of birds between nights and segments of each night. For the abundance measure, multiple measurements (5 x 10 s counts) per treatment were included by adding treatment to this nested random effect. Fixed-effects included treatment (five treatment light colours and a dark control), as well as relative humidity, cloud cover, windspeed, moon illumination, moon brightness and the time of night. The extent of eastward and northward wind direction was included as a fixed-effect as the sine and cosine of the wind direction, respectively. For each response variable, a full model that included all fixed-effects (as well as the random-effects and offset) was constructed. Simplified models were tested by dropping variables; a simplified model (i.e. were a variable was dropped) was accepted if it received considerable support from the data (with lower AIC (Akaike information criterion) than the full model).
The DHARMa package (Dharm 2023) was used to check model diagnostics, including distribution assumptions, heteroscedasticity, and outliers. For the abundance model there was a very slight pattern in the second and third quantile residuals, while in the attraction/disorientation model there was a significant hump shape in the residuals which was somewhat similar to the residuals related to the time of night variable; this patterning could not be fully alleviated with various model forms. Potential implications are described in the Discussion section. Differences between treatments were assessed using the emmeans package (Lenth 2023).
Results
Bird abundance and behaviour (attraction/disorientation) counts
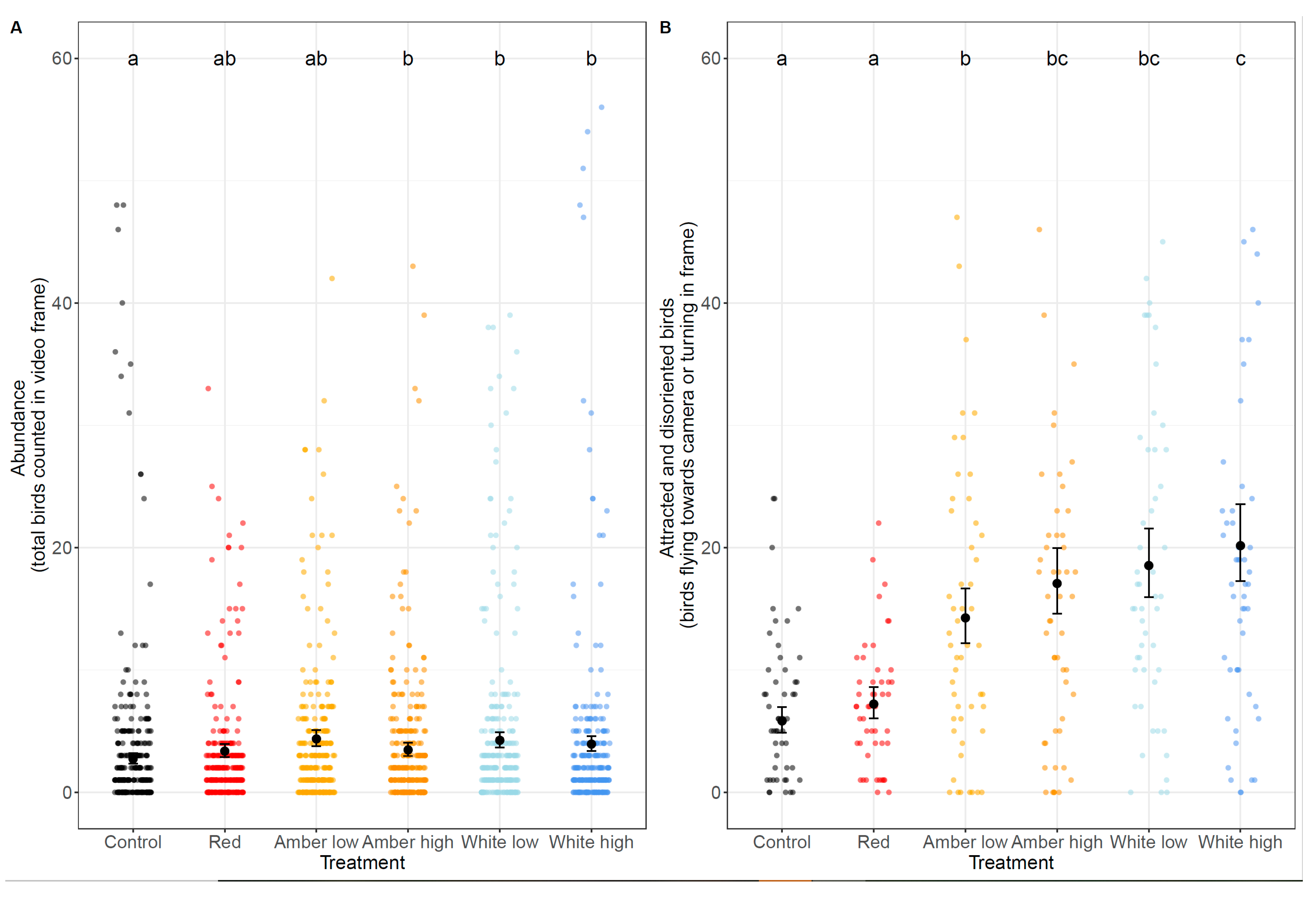
Bird abundance showed considerable variation between treatments. Typically, fewer than 20 individuals were counted in the video frame during any 10-second period. Model results showed significant differences between some treatments (
p < 0.001), with higher counts in the treatments with a higher lumen output and whiter lights (
Figure 4A,
Table 1). Higher counts were correlated with less northerly wind, days closer to full moon (higher illumination), less moon visibility, and later time at night (
Table 1).
Counts of birds flying towards the camera/light and those turning within the frame during the 1-minute treatment were used as a proxy for birds attracted to and disoriented by the light. Clear differences were shown between light treatments and the numbers of individuals exhibiting attraction/disorientation behaviours (
Figure 4B). The number of birds displaying these behaviours did not significantly differ between the dark control and red light, and all other treatments resulted in significantly more attracted and disoriented birds (
p < 0.05). In general, treatments with a higher lumen output and whiter light resulted in higher counts (
Figure 4B). Later at night, higher humidity and lower wind speeds were associated with more birds flying towards the camera or turning in the video frame (
Table 1). In the final model for both response variables, all environmental variables were retained because they improved the model fit, as determined by the AIC.
Across experimental nights, seven low-speed collisions occurred with the lighting rig (pers. obs. KM): one during a red treatment, three during amber low treatments, one during an amber high treatment, and two during a white low treatment. A total of n=34 birds were grounded during treatments with higher numbers grounded in treatments with higher lumen output and whiter light (
Figure 5). No birds were harmed, and all were retrieved and released immediately.
Laboratory-based spectral sensitivity of study species
The spectral response indices for the light treatments, normalised against the CIE D65 standard illuminant, are provided in
Table 2. Using the Bonin petrel as a proxy spectral response curve, amber light is predicted to have the least effect per lumen (0.562), followed by red (0.667) and white light (0.808). As the experimental lamp treatments have different intensities, it is vital to include the effects of lumen output rather than considering spectrum alone. Column 4 of
Table 2 predicts the combined effect of spectrum and lumen output and shows that the 4,800 lm red light has the least impact. At the other extreme, the 28,000 lm white high treatment is predicted to have over seven times the effect of red light. Falling between these two figures, the amber low treatment has nearly double the impact of red, while amber high and white low have similar responses of 10,678 and 11,312, respectively. The ranking of the predicted spectral response indices for the rated output of our treatments conformed with the observed ranking of the impact of the treatments (cf.
Figure 4,
Table 2).
Discussion
Using an experiment assessing lighting impacts from different LED colours (red, amber and white) and lumen outputs (brightness), we show that fairy prions are most impacted by white lights and high lumen output. The control (“dark”, no anthropogenic light) and red light resulted in the lowest abundance and fewest attracted/disoriented birds. Medium responses were elicited by amber low/high and white low, while high lumen white prompted the highest abundance and attraction/disorientation response. Hence, specifically, warmer-coloured (red or amber) LEDs at reduced lumen outputs have the potential to help minimise seabird-vessel interactions, which supports previous work in other seabird systems (Syposz et al. 2021). Importantly, these findings will help inform seabird conservation efforts and highlight the need for mitigation measures including the use of warmer LED colours operating at reduced lumen output to decrease vessel strikes.
In addition to treatment effects, bird abundance and attraction/disorientation were also affected by environmental variables, including wind direction and speed, relative humidity, and moon illumination. This is consistent with other research on petrel species (Brown et al. 2023). More fairy prions were seen when there was a less northerly wind direction, presumably because birds preferred to approach the island downwind. Attraction/disorientation increased with lower wind speed. This is likely due to aerodynamics and increased precision with which birds can manoeuvre in lower wind speeds requiring oscillation of the wings for propulsion as opposed to being wind assisted. Relative humidity also strongly correlated with increased abundance and attraction/disorientation. Greater illumination of water particles in the air increasing visibility may have played a role in this. Similarly, assessing light-induced seabird-vessel interactions, Merkel and Johansen (2011) also found a strong correlation with high humidity, cloudy and foggy conditions, and poor visibility.
Lunar cycles drive animal behaviour (Bastos et al. 2022), which was supported by the inclusion of moon illumination in our models, where a brighter moon (closer to full moon) resulted in higher bird abundance. Interestingly, moon illumination of a visible moon (i.e., not obscured by horizon, cloud, or hills) was a more important variable than moon illumination itself; that is, a brighter moon resulted in a lower abundance and fewer attracted/distracted birds. This supports a seabird rescue programme review by Rodríguez et al. (2023), including fledgling Procellariiformes, which hypothesised that artificial lighting from sources such as vessels is less attractive or disorientating in clear sky conditions when the moon is visible and bright. Our model included moon illumination (an index of closeness to full moon), rather than moon phase per se. Given these findings, we recommend that future research investigate the lunar effect in more detail (including running such experiments across waxing and waning phases) to better understand the combined effect of natural and anthropogenic light attraction on seabirds.
Our two different response measures showed qualitatively similar differences between light treatments however, compared with the abundance counts of birds, patterns were stronger for the attraction/disorientation. Behavioural metrics like these can be subject to observer bias (Freeberg et al. 2024). The video footage used to extract behaviour and abundance data in the current study was performed by a single reviewer, who had not been present during the experiment. Therefore, the authors consider that observer bias was not a factor in our study. When considering impacts of anthropogenic light at night, more direct measures of attraction/disorientation (such as interaction with the light beam) are likely more representative for assessing the risk of seabird-vessel interactions (i.e., seabird injury and/or mortality). We recommend that future work uses a camera with a field of view wider than the one used in the current study, as both of the above measures may have been underestimated, although we have no reason to believe that this would have resulted in biased estimated between treatments.
Counts of grounded birds and collisions with the lighting rig showed a similar pattern to the modelled results, with increasing collisions and groundings for higher lumen outputs and whiter lights, albeit with a small sample size. Compared to other research investigating the impacts of lighting on seabirds (Rodríguez et al. 2017a), our study had low numbers of groundings and collisions. Since groundings are more common for fledglings (Rodríguez et al. 2017a), our study was designed to take place prior to chicks fledging, and hence only adults were included in the counts. It has been recognised that it is important to incorporate species-specific responses in assessing impacts of anthropogenic light at night (Longcore 2023; Longcore et al. 2018), as this is the biologically relevant information for the species, rather than the colour spectrum perceived by humans. Less recognised is that the effects of lumen output are also incorporated, rather than spectrum alone. Our study is one of the first to incorporate both. Using data from a closely related species in phylogeny and ecology, we calculated the response in terms of an increase in brightness as likely experienced by the birds. Our results showed that the response of fairy prion to different light treatments is a combination of spectral response and intensity.
Although we used an offset of bird abundance from the dark period immediately prior to treatment, the incorporation of time of night as a fixed-effect still improved model fit for both response measures. Little is known about impacts from lighting on nocturnal attendance rates to colonies. However, research by Austad et al. (2023) on Yelkouan shearwaters (Puffinus yelkouan) showed that natural behaviour associated with nocturnal colony attendance was altered and hourly return rates reduced by around 20% in the presence of anthropogenic lighting. Given fairy prion are attracted to vessel lights at night, we had expected a high rate of attraction at the colony. It is commonly acknowledged that most nocturnal seabird species return to their burrows shortly after sunset to avoid predators (Austad et al. 2023). However, relatively more birds (compared to the dark period immediately prior) were present and attracted/disoriented as the experimental night progressed (from 10 pm to midnight). It should be noted there was a 9-minute difference in sunset start time over the entire period of the current study and this is not considered to have impacted colony return rates so wasn’t included in the model. We propose three theories to account for the observed return rates.
Firstly, whilst not measured in the current study, we theorise that non-breeders are effectively more active later at night and that it was these birds that were represented by increased counts. This theory is supported by a previous study by Fischer et al. (2021) on light-sensitive seabird species and would need further research to confirm in fairy prion, also considering the minor patterns in the model residuals that related to time of night in our experiment (10 pm – midnight). Secondly, and more likely, our results supported a combination of the predator avoidance hypothesis (McNeil et al. 1993; Mougeot & Bretagnolle 2000) and adaptive behavioural plasticity (behavioural change in response to an external stimulus) (Mery & Burns 2010). Raw abundance counts were high when the treatments started at 10 pm during the first three experimental nights. Patterns from experimental day 5 onwards showed lower fairy prion numbers at 10 pm. Perhaps this resulted from birds maintaining a holding pattern out to sea with the repeated presence of anthropogenic light near burrows, and then as energy resources were depleted, predation risk was overridden by the need to feed chicks and to conserve energy by landing. This is an important consideration because it highlights that light disturbance can impact natural behaviour of breeding birds. Thirdly, our study was consistent with findings in other Procellariiformes (Bourgeois et al. 2008) where higher humidity and lower wind speeds resulted in relatively more birds flying towards the camera or turning in the video frame, therefore it may be a combination of any or all three factors.
Overall, our results with fairy prion support findings in other published literature stating that reducing the lumen output and using non-white lights has the potential to help minimise seabird-vessel interactions (Syposz et al. 2021). Specifically, warmer-coloured LEDs at reduced lumen outputs may be beneficial. Mitigation Standards to reduce light-induced vessel interactions of seabirds with New Zealand commercial fishing vessels (DOC & FNZ 2023) are supported by our results. We recommend that the following mitigation measures are implemented via vessel lighting management plans:
Undertake vessel-specific audits to determine lighting needs for different operations. Light should only be provided when and where needed.
Use shielded luminaires, shaded windows, and non-reflective surfaces to minimise light spill and glare.
Ensure that the quality and level of light enables the vessel crew to operate safely. Factors such as colour rendering index (the ability to accurately show colours) may need to be considered.
Use low-lumen warm LEDs to minimise ecological impacts, especially in at-sea areas where seabird-vessel interaction is considered high-risk.
Include breeding colony locations and timing of fledging periods within the vessel’s area of operation in vessel lighting management plans, to identify areas where and times of year when it is desirable to run a ‘dark ship’, especially under conditions of poor visibility.
We recommend that future studies investigate red, amber, and white light with the same lumen output to cross-validate our data. Further work should also consider whether flicker from LED electronics impacts bird response (Inger et al. 2014). Additional land and sea-based studies will deepen our understanding of LED colour, lumen output, and time of night required to inform selection of ecologically appropriate vessel lighting.
Acknowledgements
The authors would like to acknowledge and thank Department of Conservation (DOC) rangers Kiara Duke, Hugo Bell, Marc Le Lec, and Marissa Le Lec for their enthusiasm in the face of lugging heavy lighting equipment up and down Stephen’s Island via a long, steep walking track, for helping set up and dismantling equipment, and for many nights spent after dark sitting on the side of a hill collecting data. We also thank DOC (Picton Ops) for providing logistical support, including boat transport of people and gear to and from the remote Stephen’s Island (David Hayes, Siobain Finlow-Bates, Dan Palmer, Gus Johnston, and Bart Mehrtens) and in sometimes challenging sea conditions. We especially thank Pene Gieger (Ngāti Koata) for iwi support to carry out research on Stephen’s Island (Takapourewa). Graeme Taylor (DOC) and Dr. Airam Rodríguez (Estación Biológica de Doñana, Spain) provided valuable insight in the initial methodology scoping session, and the Meteorological Service of New Zealand kindly supplied the Stephen’s Island weather station data.
Author Contributions
Project inception JF. Methodology development KM, DG, & JF. Lighting rig engineering DG and assembly KM. Stephens’s Island data collection KM. Laboratory spectral analyses SM. Data analyses EC and DG. Manuscript writing and project management KM with important input from EC, DG, JF & SM.
Funding
The research was administered through the DOC Conservation Services Programme against project CSP MIT2022-06 and cost recovered from the New Zealand Commercial Fishing Industry. Spectral measurements were funded by Nelson-Marlborough Institute of Technology and open access funding was enabled by Leiden University.
Data Availability
The datasets analysed in the current study are held by DOC, and will be deposited in an online repository upon acceptance.
Declarations
Experiments comply with the current laws of New Zealand. All research was led by the Department of Conservation (DOC), New Zealand, who hold statutory responsibilities for protected species conservation under the Wildlife Act (1953) and Conservation Act (1987).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest and have no competing interests to declare that are relevant to the content of this article.
References
- Arthur, K. (2020). National Light Pollution Guidelines for Wildlife-Including marine turtles, seabirds and migratory shorebirds. The Australian Government department of the environment and energy 107pp: CMS: Convention on the Conservation of Migratory Species of Wild Animals.
- Aubé, M., Roby, J., & Kocifaj, M. (2013). Evaluating potential spectral impacts of various artificial lights on melatonin suppression, photosynthesis, and star visibility. Plos one, 8(7), e67798. [CrossRef]
- Austad, M., Oppel, S., Crymble, J., Greetham, H.R., Sahin, D., Lago, P., . . . Quillfeldt, P. (2023). The effects of temporally distinct light pollution from ships on nocturnal colony attendance in a threatened seabird. Journal of Ornithology, 164(3), 527-536. [CrossRef]
- Bastos, R., Martins, B., Ramos, J., Paiva, V., Pereira, J., Ceia, F., . . . Cabral, J. (2022). Shearwaters’ nest attendance patterns throughout the lunar cycle: Are oceanographic conditions decisive for timing of nest arrival? Journal of Experimental Marine Biology and Ecology, 549, 151698. [CrossRef]
- Bourgeois, K., Dromzée, S., Vidal, É., & Legrand, J. (2008). Yelkouan shearwater Puffinus yelkouan presence and behaviour at colonies: not only a moonlight question. Comptes rendus. Biologies, 331(1), 88-97. [CrossRef]
- Brooks, M.E., Kristensen, K., Van Benthem, K.J., Magnusson, A., Berg, C.W., Nielsen, A., . . . Bolker, B.M. (2017). glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R journal, 9(2), 378-400. [CrossRef]
- Brown, T.M., Wilhelm, S.I., Mastromonaco, G.F., & Burness, G. (2023). A path forward in the investigation of seabird strandings attributed to light attraction. Conservation Science and Practice, 5(1), e12852. [CrossRef]
- CIE. (2014). Relating photochemical and photobiological quantities to photometric quantities. CIE TN 02:2014. In. Austria: International Commission on Illumination.
- Cieraad, E., & Farnworth, B. (2023). Lighting trends reveal state of the dark sky cloak: light at night and its ecological impacts in Aotearoa New Zealand. New Zealand Journal of Ecology, 47(1), 3559. [CrossRef]
- Dharm, H.F. (2023). Residual diagnostics for hierarchical (multi-level/mixed) regression models. 2020. R package version. 320, from http://florianhartig.github.io/DHARMa/.
- Fischer, J.H., Debski, I., Taylor, G.A., & Wittmer, H.U. (2021). Consistent offshore artificial light at night near the last breeding colony of a critically endangered seabird. Conservation Science and Practice, 3(9), e481. [CrossRef]
- DOC & FNZ (2023). Mitigation Standards to Reduce Light-induced Vessel Strikes of Seabirds with New Zealand Commercial Fishing Vessels. Wellington, New Zealand: The Department of Conservation and Fisheries New Zealand. Retrieved from https://www.mpi.govt.nz/dmsdocument/56320. /.
- Freeberg, T.M., Benson, S.A., & Burghardt, G.M. (2024). Minimizing observer bias in animal behavior studies revisited: Improvement, but a long way to go. Ethology, 130(6), e13446. [CrossRef]
- Heswall, A.M., DOMINGUEZ, A., WIJAYA, B., MILLER, L., CAIN, K., FRIESEN, M., & GASKETT, A. (2023). Why did they die? Analysing the cause of death of grounded seabirds lodged at an avian rescue centre in Auckland, New Zealand. Notornis, 70(3), 124-134.
- Holmes, M. (2017). Characterising deck strikes. https://www.doc.govt.nz/globalassets/documents/conservation/marine-and-coastal/marine-conservation-services/reports/pre-2019-annual-plans/characterising-deck-strikes-2017.pdf Department of Conservation.
- Inger, R., Bennie, J., Davies, T.W., & Gaston, K.J. (2014). Potential biological and ecological effects of flickering artificial light. Plos one, 9(5), e98631. [CrossRef]
- Lenth, R. (2023). Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.8. 1–1 (2022). Available at https://CRAN.R-project.org/package=emmeans.
- Longcore, T. (2023). A compendium of photopigment peak sensitivities and visual spectral response curves of terrestrial wildlife to guide design of outdoor nighttime lighting. Basic and Applied Ecology, 73, 40-50. [CrossRef]
- Longcore, T., Rodríguez, A., Witherington, B., Penniman, J.F., Herf, L., & Herf, M. (2018). Rapid assessment of lamp spectrum to quantify ecological effects of light at night. Journal of Experimental Zoology Part A: Ecological and Integrative Physiology, 329(8-9), 511-521. [CrossRef]
- McNeil, R., Drapeau, P., & Pierotti, R. (1993). Nocturnality in colonial waterbirds: occurrence, special adaptations, and suspected benefits. In Power, D.M. (Ed.), Current Ornithology (Vol. 10). New York, USA Plenum Press.
- Merkel, F.R., & Johansen, K.L. (2011). Light-induced bird strikes on vessels in Southwest Greenland. Marine Pollution Bulletin, 62(11), 2330-2336. [CrossRef]
- Mery, F., & Burns, J.G. (2010). Behavioural plasticity: an interaction between evolution and experience. Evolutionary Ecology, 24, 571-583. [CrossRef]
- Mitkus, M., Nevitt, G.A., Danielsen, J., & Kelber, A. (2016). Vision on the high seas: spatial resolution and optical sensitivity in two procellariiform seabirds with different foraging strategies. Journal of Experimental Biology, 219(21), 3329-3338. [CrossRef]
- Mougeot, F., & Bretagnolle, V. (2000). Predation risk and moonlight avoidance in nocturnal seabirds. Journal of Avian Biology, 31(3), 376-386.
- Reed, J.R. (1986). Seabird vision: Spectral sensitivity and light-attraction behavior. Dissertation from The University of Wisconsin-Madison.
- Rodríguez, A., Arcos, J.M., Bretagnolle, V., Dias, M.P., Holmes, N.D., Louzao, M., . . . Chiaradia, A. (2019). Future Directions in Conservation Research on Petrels and Shearwaters. Frontiers in Marine Science, 6. [CrossRef]
- Rodríguez, A., Atchoi, E., Rodríguez, B., Pipa, T., Le Corre, M., & Ainley, D.G. (2023). Moonlight diminishes seabird attraction to artificial light. Conservation Science and Practice, 5(10), e13014. [CrossRef]
- Rodríguez, A., Dann, P., & Chiaradia, A. (2017a). Reducing light-induced mortality of seabirds: high pressure sodium lights decrease the fatal attraction of shearwaters. Journal for Nature Conservation, 39, 68-72. [CrossRef]
- Rodríguez, A., Holmes, N.D., Ryan, P.G., Wilson, K.J., Faulquier, L., Murillo, Y., . . . Rodríguez, B. (2017b). Seabird mortality induced by land-based artificial lights. Conservation Biology, 31(5), 986-1001. [CrossRef]
- Syposz, M., Padget, O., Willis, J., Van Doren, B.M., Gillies, N., Fayet, A.L., . . . Guilford, T. (2021). Avoidance of different durations, colours and intensities of artificial light by adult seabirds. Scientific Reports, 11(1), 18941. [CrossRef]
- Taylor, G. (2000). Action Plan for Seabird Conservation in New Zealand Part b: Non-Threatened Seabirds. Department of Conservation, Wellington. Retrieved from https://www.doc.govt.nz/documents/science-and-technical/tsop17.pdf.
- R Core Team (2021). R: A language and environment for statistical computing: R Foundation for Statistical Computing, Vienna, Austria. Retrieved from https://www.R-project.org/.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).