Submitted:
21 July 2024
Posted:
22 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
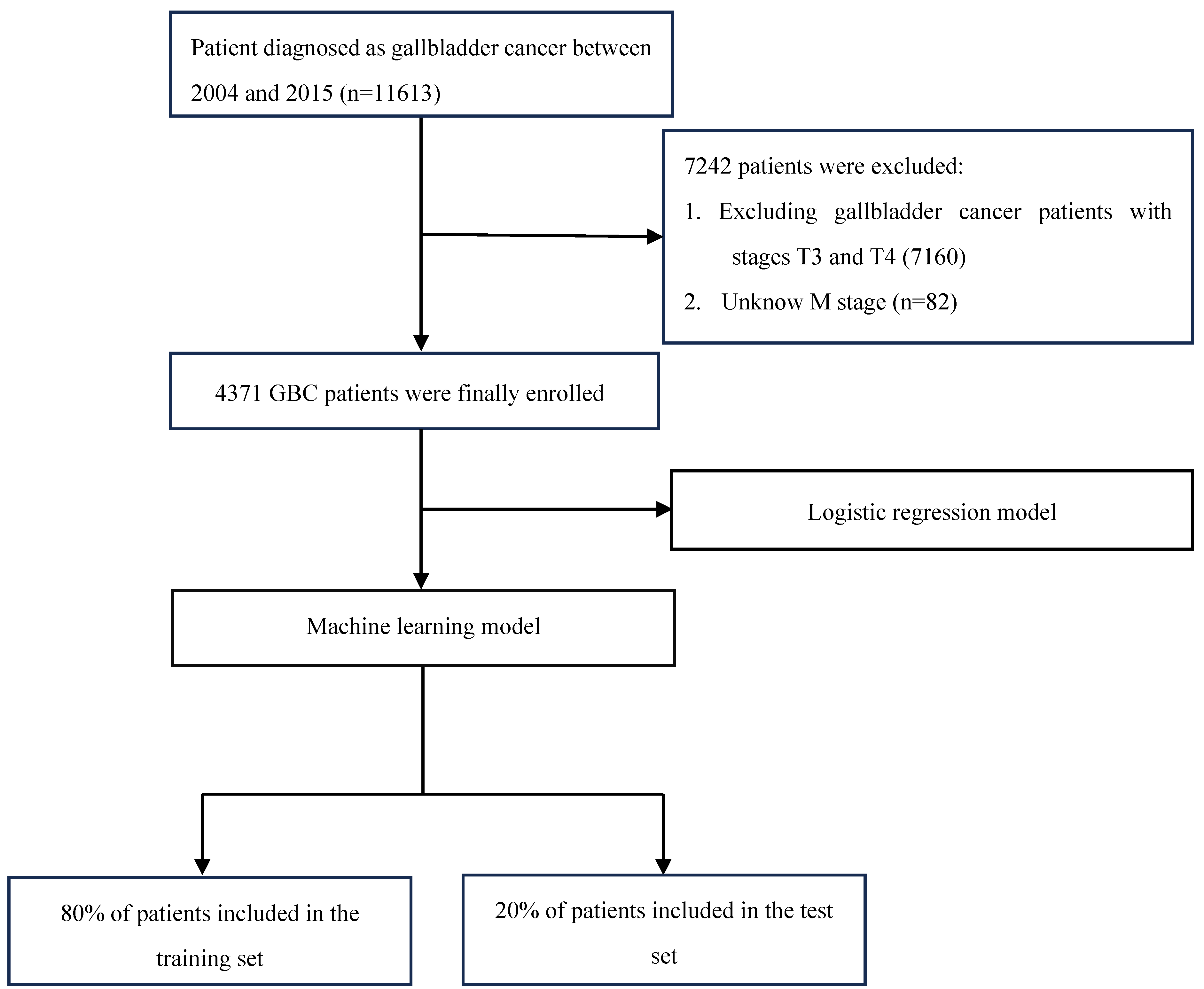
2.1. Data Sources and Study Population
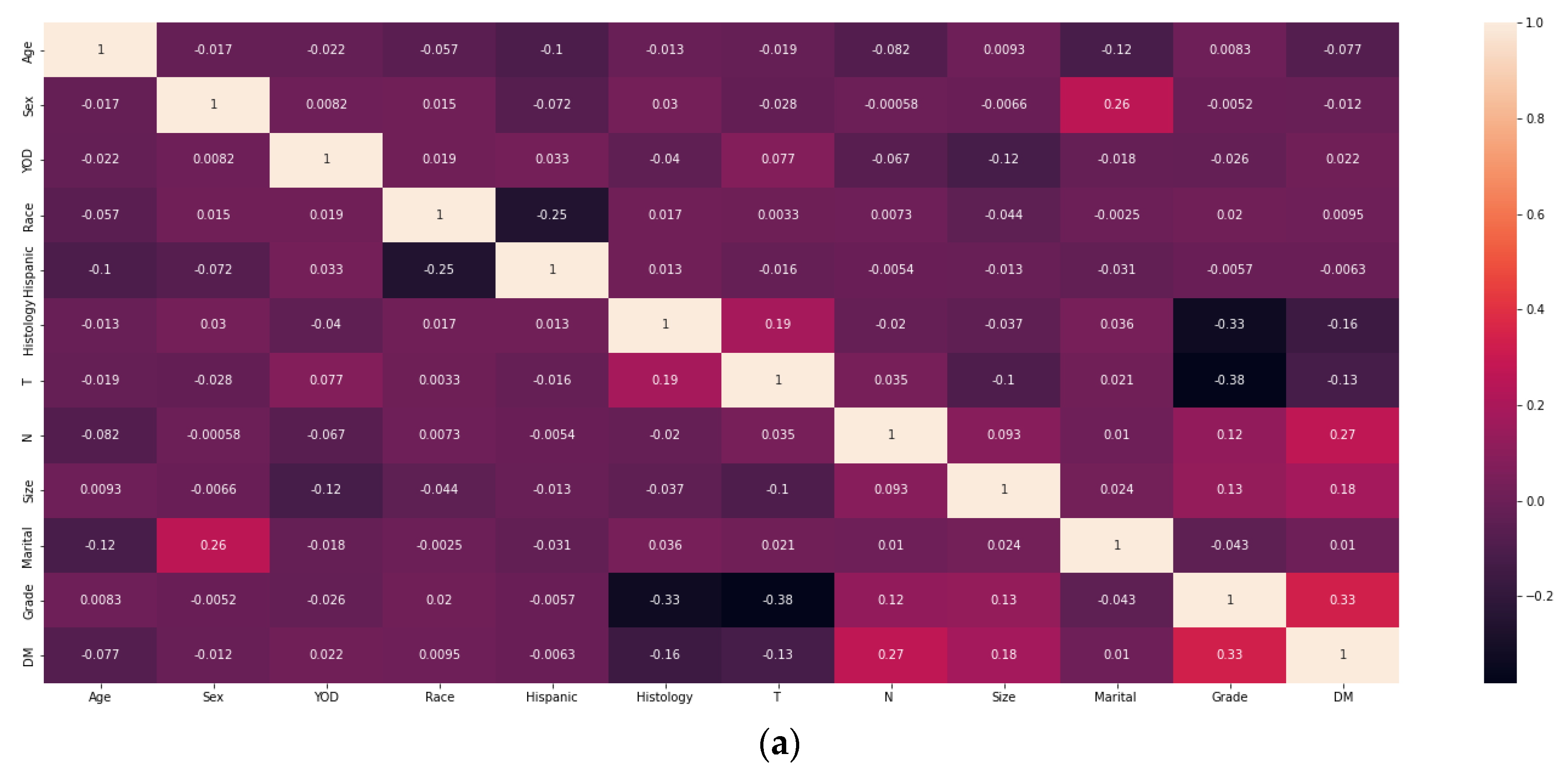
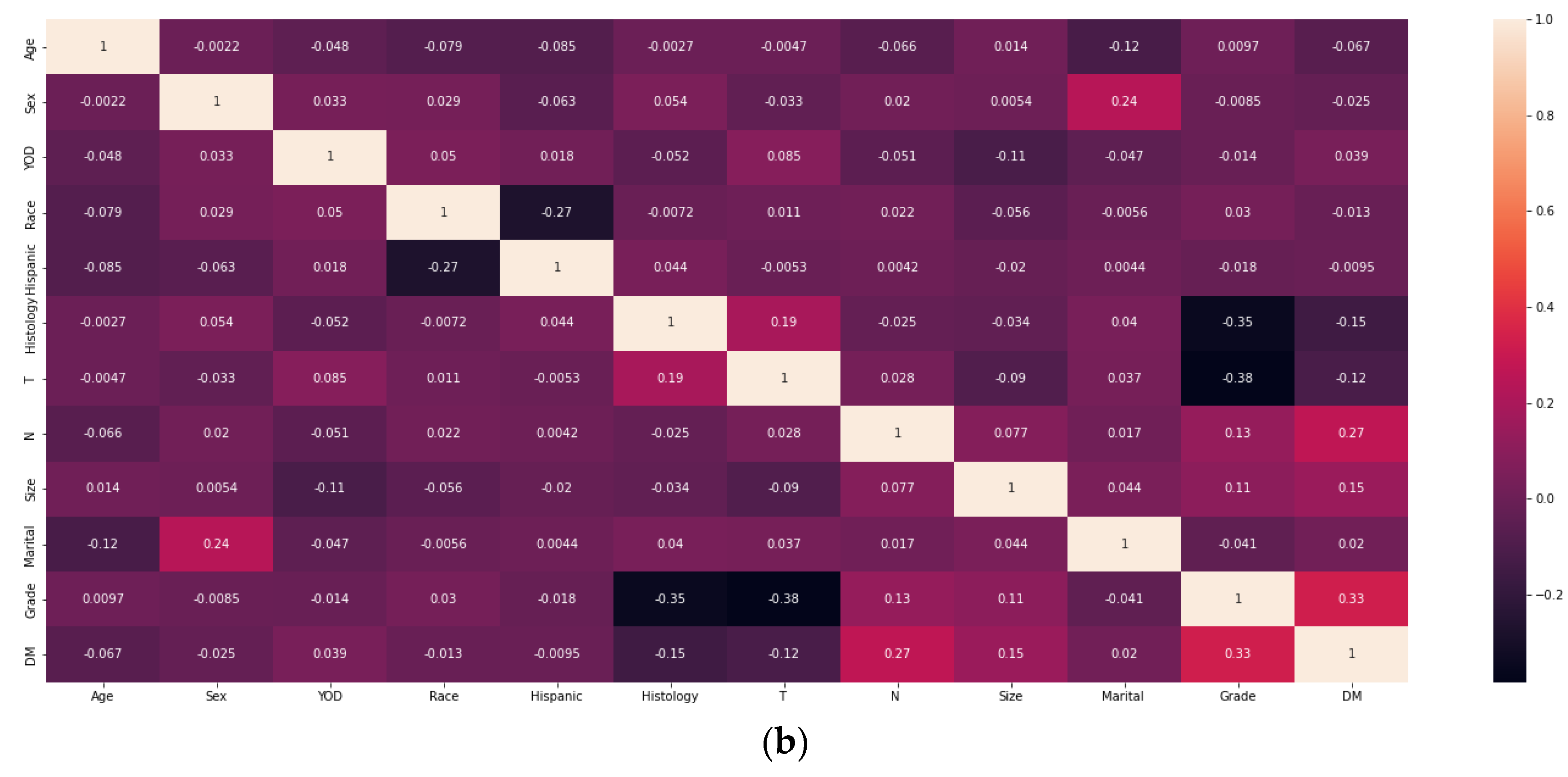
2.2. Screening for Risk Factors and Model Construction
3. Results
3.1. Analysis of Patient Information
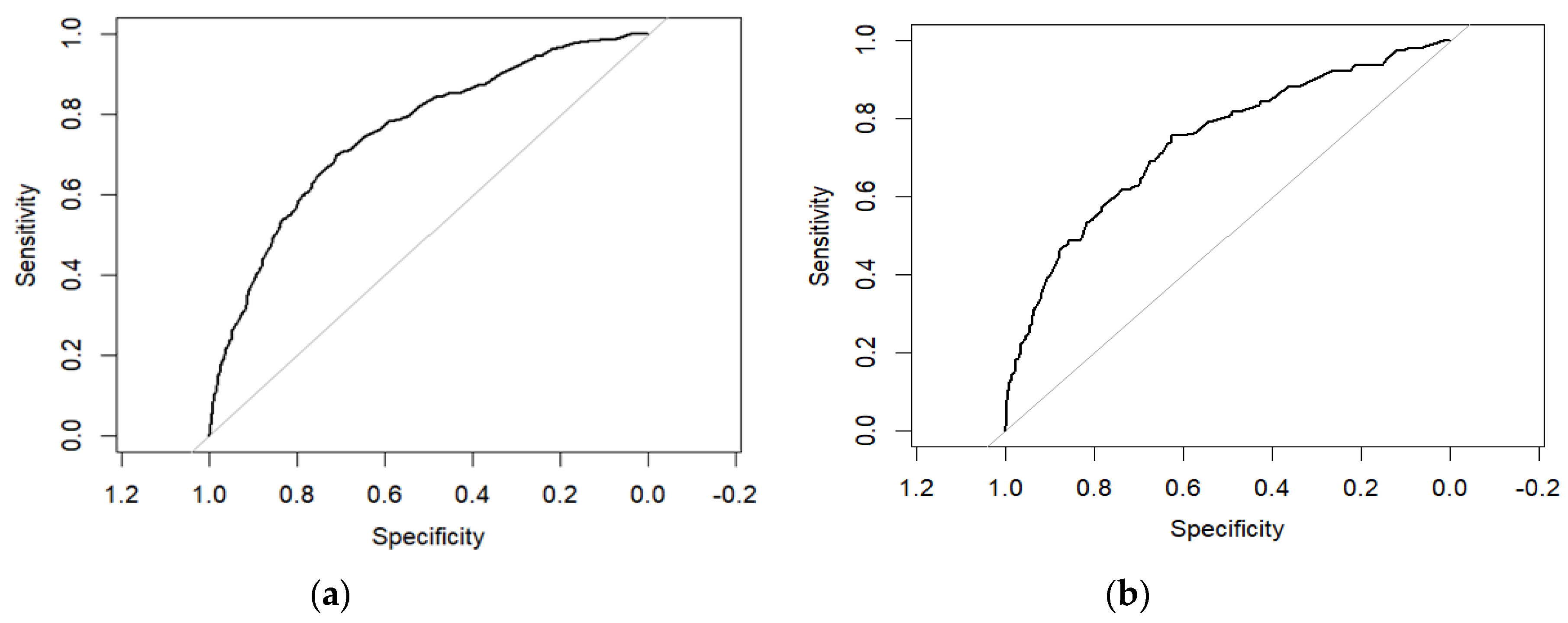
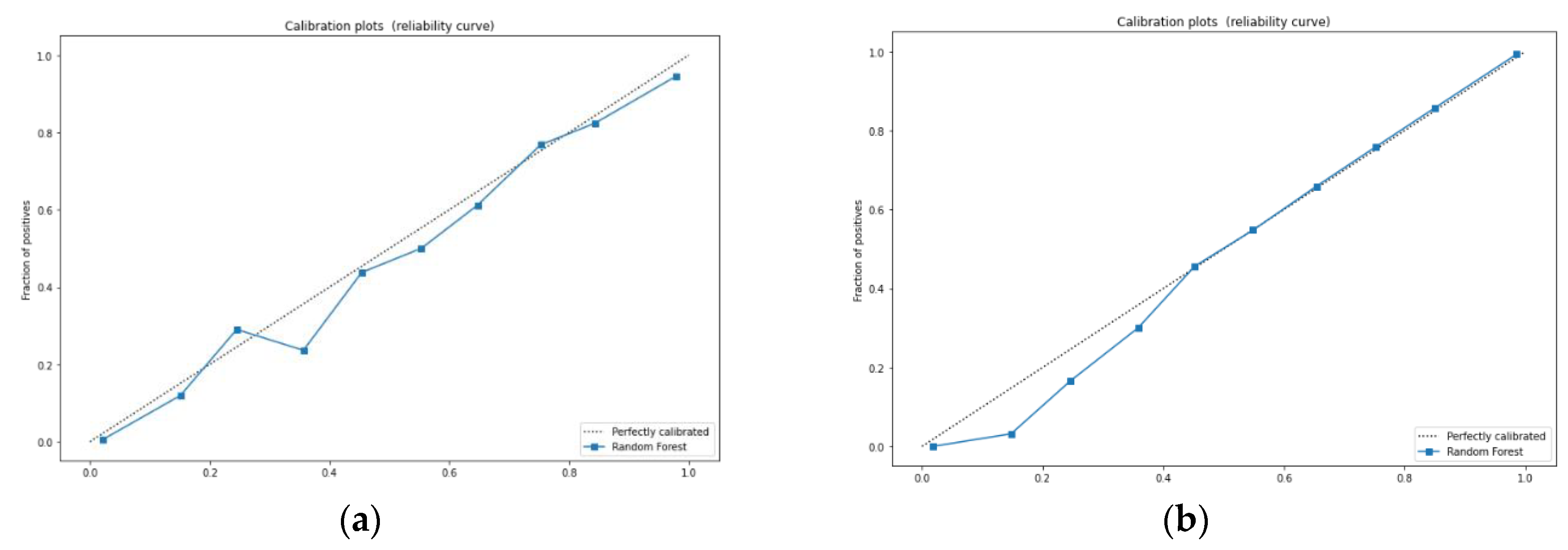
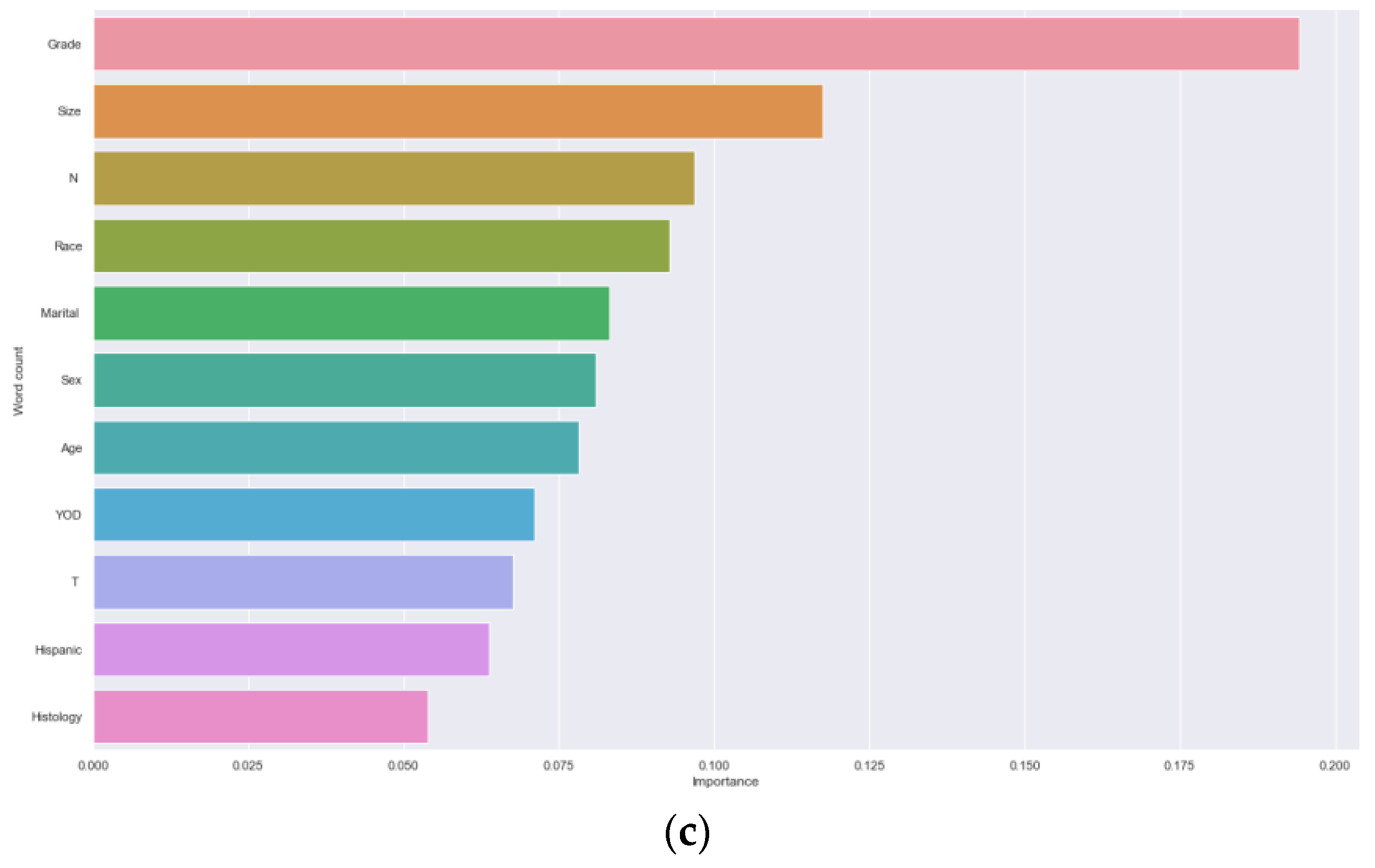
3.2. Analysis of Machine Learning Algorithm Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ji, Z.; Ren, L.; Liu, F.; Liu, L.; Song, J.; Zhu, J.; Ji, G.; Huang, G. Effect of different surgical options on the long-term survival of stage I gallbladder cancer: a retrospective study based on SEER database and Chinese Multi-institutional Registry. Journal of cancer research and clinical oncology 2023, 149, 12297–12313. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Patel, H.K.; Boakye, D.; Chandrasekar, V.T.; Koulaouzidis, A.; Lucero-Prisno Iii, D.E.; Ngai, C.H.; Pun, C.N.; Bai, Y.; Lok, V.; et al. Worldwide distribution, associated factors, and trends of gallbladder cancer: A global country-level analysis. Cancer letters 2021, 521, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Torres, O.J.M.; Alikhanov, R.; Li, J.; Serrablo, A.; Chan, A.C.; de Souza, M.F.E. Extended liver surgery for gallbladder cancer revisited: Is there a role for hepatopancreatoduodenectomy? International journal of surgery (London, England) 2020, 82s, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Seo, D.W.; Park, D.H.; Lee, S.S.; Lee, S.K.; Kim, M.H.; Hwang, S. Prognostic factors in patients with gallbladder cancer after surgical resection: analysis of 279 operated patients. Journal of clinical gastroenterology 2013, 47, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sharma, K.L.; Gupta, A.; Yadav, A.; Kumar, A. Gallbladder cancer epidemiology, pathogenesis and molecular genetics: Recent update. World journal of gastroenterology 2017, 23, 3978–3998. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.L.; Lin, Y.X.; Jiang, L.S.; Ye, H.; Li, F.Y.; Cheng, N.S. A Novel Nomogram Predicting Distant Metastasis in T1 and T2 Gallbladder Cancer: A SEER-based Study. International journal of medical sciences 2020, 17, 1704–1712. [Google Scholar] [CrossRef]
- Yang, Y.; Tu, Z.; Ye, C.; Cai, H.; Yang, S.; Chen, X.; Tu, J. Site-specific metastases of gallbladder adenocarcinoma and their prognostic value for survival: a SEER-based study. BMC surgery 2021, 21, 59. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, G.Y.; Chen, M.; Wei, R.; Chen, J.; Wang, Z. Pattern of distant metastases in primary extrahepatic bile-duct cancer: A SEER-based study. Cancer medicine 2018, 7, 5006–5014. [Google Scholar] [CrossRef] [PubMed]
- Mady, M.; Prasai, K.; Tella, S.H.; Yadav, S.; Hallemeier, C.L.; Rakshit, S.; Roberts, L.; Borad, M.; Mahipal, A. Neutrophil to lymphocyte ratio as a prognostic marker in metastatic gallbladder cancer. HPB : the official journal of the International Hepato Pancreato Biliary Association 2020, 22, 1490–1495. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, X.; Hu, X.; Ren, H.; Wu, S.; Wu, J.; Wu, G.; Si, X.; Wang, B. Survival analysis of patients with primary gallbladder cancer from 2010 to 2015: A retrospective study based on SEER data. Medicine 2020, 99, e22292. [Google Scholar] [CrossRef]
- Zhong, X.; Lin, Y.; Zhang, W.; Bi, Q. Predicting diagnosis and survival of bone metastasis in breast cancer using machine learning. Scientific reports 2023, 13, 18301. [Google Scholar] [CrossRef]
- Liu, W.C.; Li, Z.Q.; Luo, Z.W.; Liao, W.J.; Liu, Z.L.; Liu, J.M. Machine learning for the prediction of bone metastasis in patients with newly diagnosed thyroid cancer. Cancer medicine 2021, 10, 2802–2811. [Google Scholar] [CrossRef]
- Mao, Y.; Lan, H.; Lin, W.; Liang, J.; Huang, H.; Li, L.; Wen, J.; Chen, G. Machine learning algorithms are comparable to conventional regression models in predicting distant metastasis of follicular thyroid carcinoma. Clinical endocrinology 2023, 98, 98–109. [Google Scholar] [CrossRef]
- Wernberg, J.A.; Lucarelli, D.D. Gallbladder cancer. The Surgical clinics of North America 2014, 94, 343–360. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Wu, X.; Li, Q.; Ge, X.; Wang, F.; Wu, P.; Deng, X.; Miao, L. Long noncoding RNAs as potential biomarkers and therapeutic targets in gallbladder cancer: a systematic review and meta-analysis. Cancer cell international 2019, 19, 169. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; He, M.; Lin, R.; Zhan, M.; Xu, S.; Huang, X.; Xu, C.; Chen, W.; Yao, Y.; Mohan, M.; et al. PLEK2 promotes gallbladder cancer invasion and metastasis through EGFR/CCL2 pathway. Journal of experimental & clinical cancer research : CR 2019, 38, 247. [Google Scholar] [CrossRef]
- Hundal, R.; Shaffer, E.A. Gallbladder cancer: epidemiology and outcome. Clinical epidemiology 2014, 6, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Shindoh, J.; de Aretxabala, X.; Aloia, T.A.; Roa, J.C.; Roa, I.; Zimmitti, G.; Javle, M.; Conrad, C.; Maru, D.M.; Aoki, T.; et al. Tumor location is a strong predictor of tumor progression and survival in T2 gallbladder cancer: an international multicenter study. Annals of surgery 2015, 261, 733–739. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Z.; Sa, B. Construction and validation of the predictive model for gallbladder cancer liver metastasis patients: a SEER-based study. European journal of gastroenterology & hepatology 2024, 36, 129–134. [Google Scholar] [CrossRef]
- Fang, C.; Li, W.; Wang, Q.; Wang, R.; Dong, H.; Chen, J.; Chen, Y. Risk factors and prognosis of liver metastasis in gallbladder cancer patients: A SEER-based study. Frontiers in surgery 2022, 9, 899896. [Google Scholar] [CrossRef]
- Leonard, G.; South, C.; Balentine, C.; Porembka, M.; Mansour, J.; Wang, S.; Yopp, A.; Polanco, P.; Zeh, H.; Augustine, M. Machine Learning Improves Prediction Over Logistic Regression on Resected Colon Cancer Patients. The Journal of surgical research 2022, 275, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.T.; Tian, K.; Xie, X.Y.; Zhang, Y.H.; Fang, D.B. Machine Learning for Predicting Distant Metastasis of Medullary Thyroid Carcinoma Using the SEER Database. International journal of endocrinology 2023, 2023, 9965578. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Zhu, J.; Chen, X.; Chen, R.; Jiang, Y.; Wang, S.; Xu, D.; Shen, G.; Zheng, J.; Xu, C. Application of artificial intelligence in a real-world research for predicting the risk of liver metastasis in T1 colorectal cancer. Cancer cell international 2022, 22, 28. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Kwak, M.S.; Lee, H.H.; Cha, J.M.; Shin, H.P.; Jeon, J.W.; Yoon, J.Y. Development of a Novel Prognostic Model for Predicting Lymph Node Metastasis in Early Colorectal Cancer: Analysis Based on the Surveillance, Epidemiology, and End Results Database. Frontiers in oncology 2021, 11, 614398. [Google Scholar] [CrossRef]
- Osório, F.M.; Vidigal, P.V.; Ferrari, T.C.; Lima, A.S.; Lauar, G.M.; Couto, C.A. Histologic Grade and Mitotic Index as Predictors of Microvascular Invasion in Hepatocellular Carcinoma. Experimental and clinical transplantation : official journal of the Middle East Society for Organ Transplantation 2015, 13, 421–425. [Google Scholar] [CrossRef]
- Butte, J.M.; Gönen, M.; Allen, P.J.; D'Angelica, M.I.; Kingham, T.P.; Fong, Y.; Dematteo, R.P.; Blumgart, L.; Jarnagin, W.R. The role of laparoscopic staging in patients with incidental gallbladder cancer. HPB : the official journal of the International Hepato Pancreato Biliary Association 2011, 13, 463–472. [Google Scholar] [CrossRef]
- Shirai, Y.; Sakata, J.; Wakai, T.; Ohashi, T.; Ajioka, Y.; Hatakeyama, K. Assessment of lymph node status in gallbladder cancer: location, number, or ratio of positive nodes. World journal of surgical oncology 2012, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Su, X.H.; Qin, X.; Wang, Q. Application of machine learning techniques in real-world research to predict the risk of liver metastasis in rectal cancer. Frontiers in oncology 2022, 12, 1065468. [Google Scholar] [CrossRef]
- Sakata, J.; Shirai, Y.; Wakai, T.; Ajioka, Y.; Hatakeyama, K. Number of positive lymph nodes independently determines the prognosis after resection in patients with gallbladder carcinoma. Annals of surgical oncology 2010, 17, 1831–1840. [Google Scholar] [CrossRef]
- Negi, S.S.; Singh, A.; Chaudhary, A. Lymph nodal involvement as prognostic factor in gallbladder cancer: location, count or ratio? Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract 2011, 15, 1017–1025. [Google Scholar] [CrossRef]
- Feng, S.; Wang, J.; Wang, L.; Qiu, Q.; Chen, D.; Su, H.; Li, X.; Xiao, Y.; Lin, C. Current Status and Analysis of Machine Learning in Hepatocellular Carcinoma. Journal of clinical and translational hepatology 2023, 11, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Bhinder, B.; Gilvary, C.; Madhukar, N.S.; Elemento, O. Artificial Intelligence in Cancer Research and Precision Medicine. Cancer discovery 2021, 11, 900–915. [Google Scholar] [CrossRef] [PubMed]
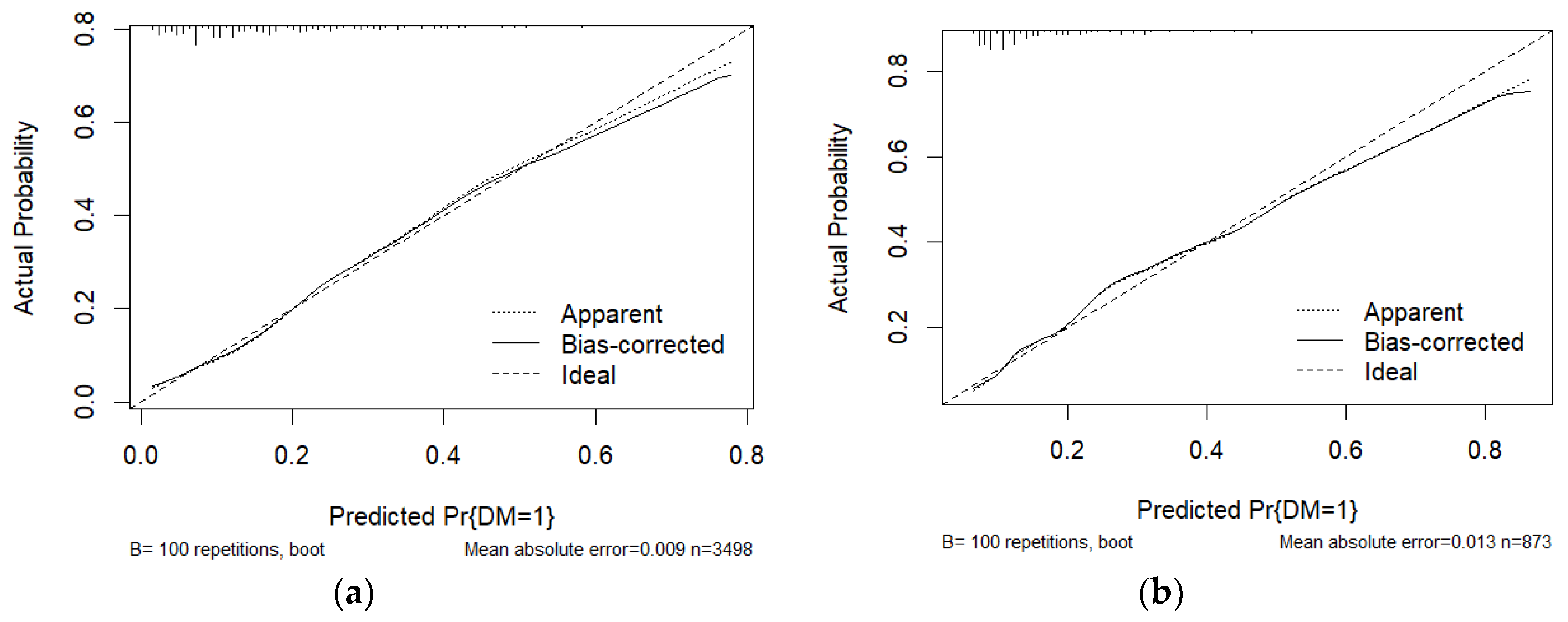
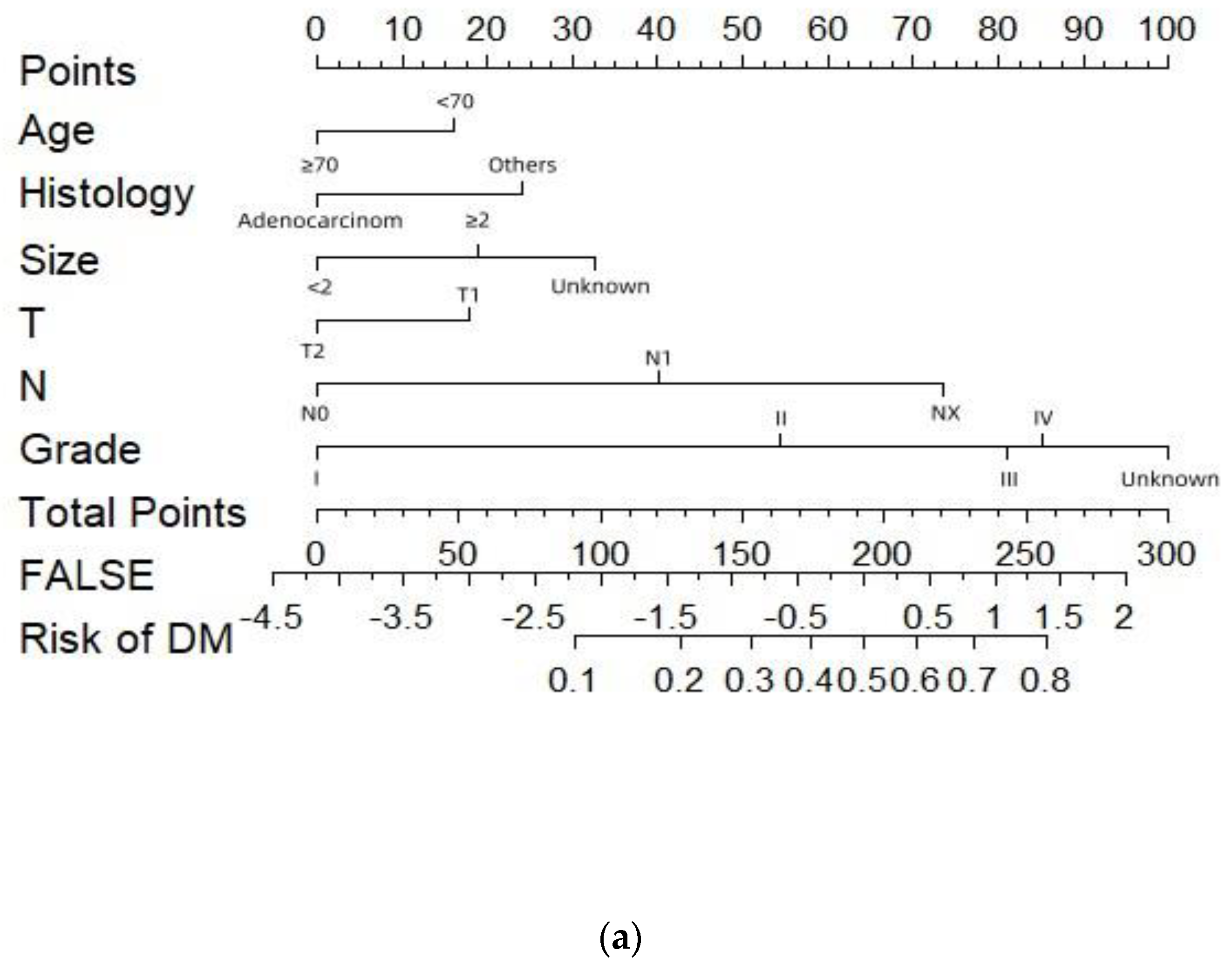
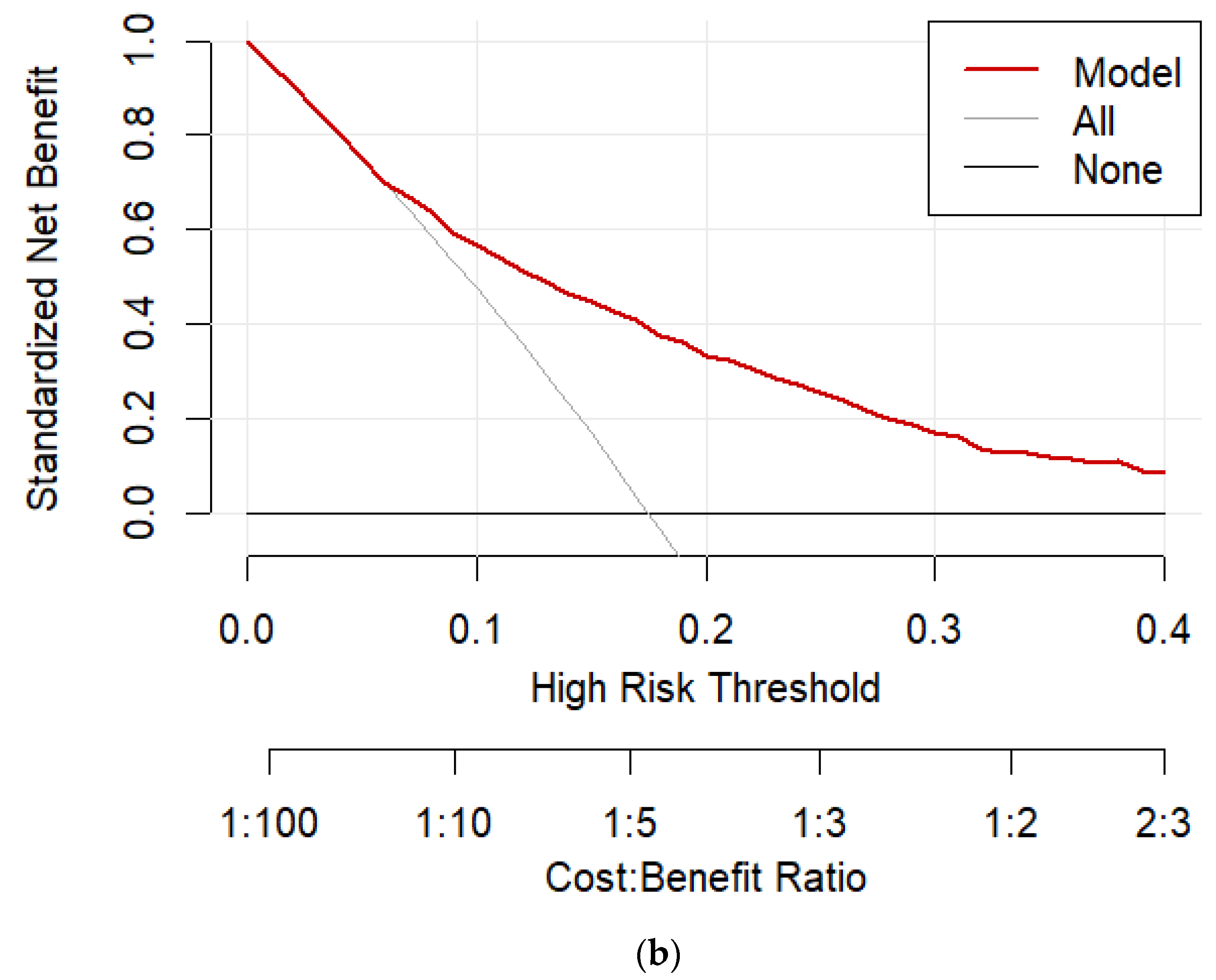
| Characteristic | Without DM (N=3607) |
With DM (N=764) |
p-value |
|---|---|---|---|
| Age(year) | <0.001 | ||
| <70 | 1508 (41.8%) | 374 (49.0%) | |
| ≥70 | 2099 (58.2%) | 390 (51.0%) | |
| Gender | 0.181 | ||
| Female | 2523 (69.9%) | 553 (72.4%) | |
| Male | 1084 (30.1%) | 211 (27.6%) | |
| Race | 0.599 | ||
| white | 2770 (76.8%) | 578 (75.7%) | |
| black | 400 (11.1%) | 97 (12.7%) | |
| other | 437 (12.1%) | 89 (11.6%) | |
| Hispanic | 0.572 | ||
| YES | 808 (22.4%) | 164 (21.5%) | |
| NO | 2799 (77.6) | 600 (78.5%) | |
| Histology | <0.001 | ||
| Adenocarcinom | 3308 (91.7%) | 611 (80.0%) | |
| Others | 299 (8.3%) | 153 (20.0%) | |
| Year of diagnosis | 0.262 | ||
| 2004-2009 | 1624 (45.0%) | 327 (42.8%) | |
| 2010-2015 | 1983 (55.0%) | 437 (57.2%) | |
| Tumor size(cm) | <0.001 | ||
| <2 | 2270 (76.8%) | 578 (75.7%) | |
| ≥2 | 400 (11.1%) | 97 (12.7%) | |
| Unknown | 437 (12.1%) | 89 (11.6%) | |
| T stage | <0.001 | ||
| T1 | 1259 (34.9%) | 361 (47.3%) | |
| T2 | 2348 (65.1%) | 403 (52.7%) | |
| N stage | <0.001 | ||
| N0 | 2871 (79.6%) | 422 (55.2%) | |
| N1 | 644 (17.8%) | 257 (33.7%) | |
| NX | 92 (2.6%) | 85 (11.1%) | |
| Marital status | 0.531 | ||
| Single | 1839 (51.0%) | 380 (49.7%) | |
| Married | 1768 (49.0%) | 384 (50.3%) | |
| Grade | <0.001. | ||
| Grade I | 737 (20.4%) | 39 (5.1%) | |
| Grade II | 1536 (42.6%) | 219 (28.6%) | |
| Grade III | 894 (24.8%) | 255 (33.4%) | |
| Grade IV | 55 (1.5%) | 18 (2.4%) | |
| Unknown | 385 (10.7) | 233 (30.5%) |
| Univariable analysis | Multivariable analysis | |||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Age(year) | ||||||
| <70 | Ref | Ref | ||||
| ≥70 | 0.723 | 0.607-0.861 | <0.001 | 0.705 | 0.583-0.852 | <0.001 |
| Gender | ||||||
| Female | Ref | |||||
| male | 0.881 | 0.726-1.069 | 0.200 | |||
| Race | ||||||
| white | Ref | |||||
| black | 1.116 | 0.850-1.464 | 0.431 | |||
| other | 0.980 | 0.746-1.287 | 0.885 | |||
| Hispanic | ||||||
| YES | 0.997 | 0.810-1.228 | 0.977 | |||
| NO | Ref | |||||
| Histology | ||||||
| Adenocarcinom | 0.345 | 0.274-0.436 | <0.001 | 0.595 | 0.456-0.777 | <0.001 |
| Others | Ref | Ref | ||||
| Year of diagnosis | ||||||
| 2004-2009 | Ref | |||||
| 2010-2015 | 1.151 | 0.965-1.374 | 0.117 | |||
| Tumor size(cm) | ||||||
| <2 | Ref | Ref | ||||
| ≥2 | 1.916 | 1.449-2.534 | <0.001 | 1.507 | 1.121-2.027 | 0.007 |
| Unknown | 2.729 | 2.067-3.602 | <0.001 | 2.023 | 1.509-2.714 | <0.001 |
| T stage | ||||||
| T1 | Ref | |||||
| T2 | 0.594 | 0.498-0.708 | <0.001 | 0.679 | 0.547-0.843 | <0.001 |
| N stage | ||||||
| N0 | Ref | Ref | ||||
| N1 | 2.656 | 2.155-3.197 | <0.000 | 2.377 | 1.920-2.944 | <0.001 |
| NX | 6.067 | 4.299-8.563 | <0.000 | 4.913 | 3.398-7.105 | <0.001 |
| Marital status | ||||||
| Single | Ref | |||||
| Married | 1.096 | 0.920-1.305 | 0.304 | |||
| Grade | ||||||
| Grade I | Ref | Ref | ||||
| Grade II | 3.507 | 2.281-5.391 | <0.001 | 3.236 | 2.090-5.010 | <0.001 |
| Grade III | 6.835 | 4.453-10.489 | <0.001 | 5.776 | 3.721-8.966 | <0.001 |
| Grade IV | 8.990 | 4.316-18.725 | <0.001 | 6.316 | 2.932-13.605 | <0.001 |
| Unknown | 13.936 | 8.977-21.635 | <0.001 | 8.684 | 5.475-13.774 | <0.001 |
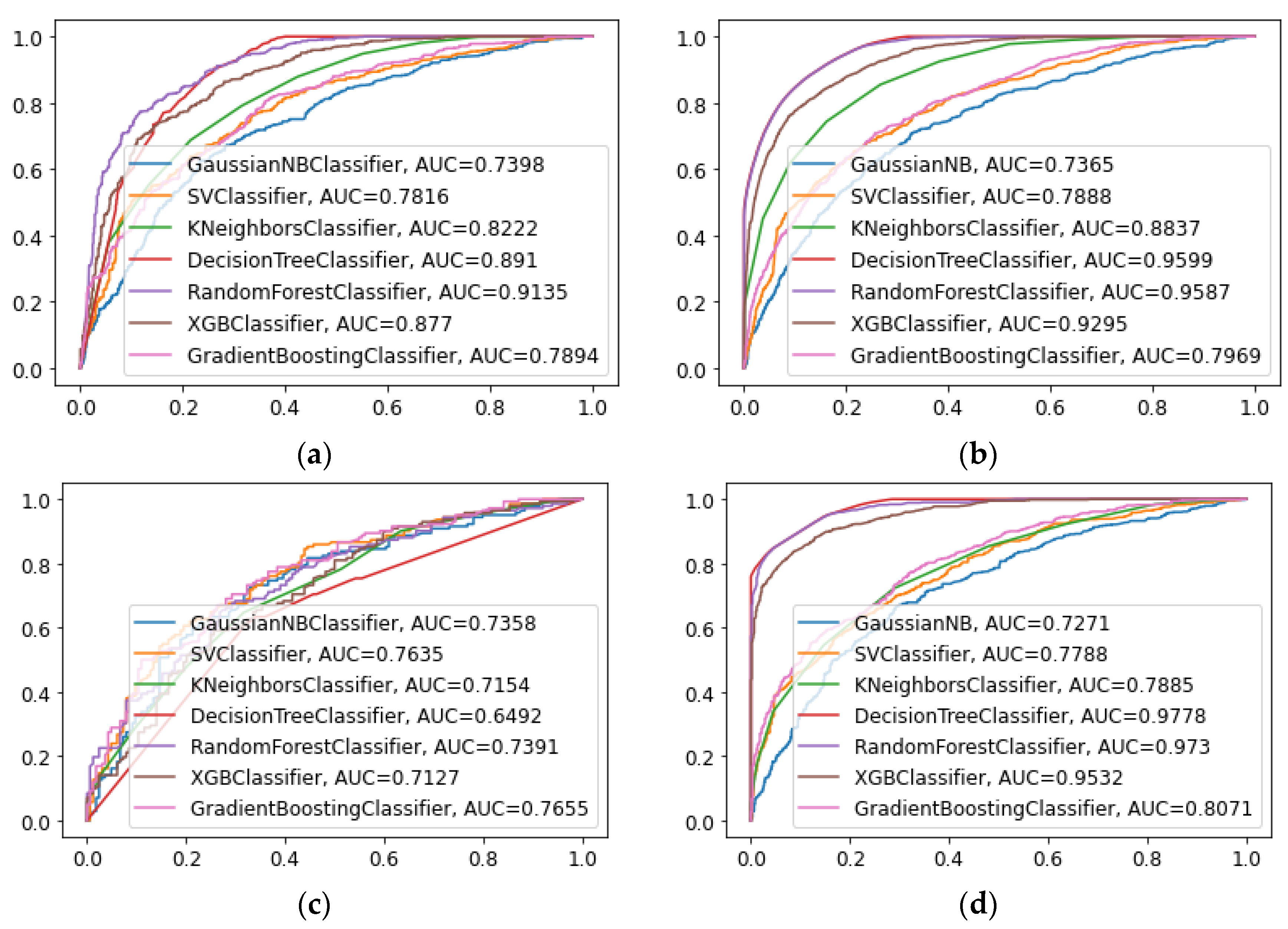
| Model | Accuracy | AUC | Precision | Recall rate | F1-score |
|---|---|---|---|---|---|
| NB | 0.681 | 0.739 | 0.734 | 0.587 | 0.652 |
| SVC | 0.707 | 0.781 | 0.722 | 0.690 | 0.706 |
| KNN | 0.738 | 0.822 | 0.721 | 0.791 | 0.761 |
| DT | 0.681 | 0.891 | 0.686 | 0.688 | 0.687 |
| RF | 0.828 | 0.913 | 0.811 | 0.862 | 0.836 |
| XGBoost | 0.784 | 0.877 | 0.781 | 0.799 | 0.790 |
| GBM | 0.704 | 0.789 | 0.711 | 0.704 | 0.707 |
| Model | Accuracy | AUC | Precision | Recall rate | F1-score |
|---|---|---|---|---|---|
| NB | 0.689 | 0.735 | 0.715 | 0.549 | 0.621 |
| SVC | 0.702 | 0.763 | 0.691 | 0.647 | 0.669 |
| KNN | 0.604 | 0.715 | 0.562 | 0.661 | 0.687 |
| DT | 0.699 | 0.649 | 0.676 | 0.676 | 0.676 |
| RF | 0.686 | 0.739 | 0.643 | 0.725 | 0.682 |
| XGBoost | 0.656 | 0.712 | 0.624 | 0.654 | 0.639 |
| GBM | 0.702 | 0.765 | 0.683 | 0.669 | 0.676 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





