Submitted:
22 July 2024
Posted:
22 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Patients and Bacterial Strains
2.2. Antimicrobial Susceptibility Testing
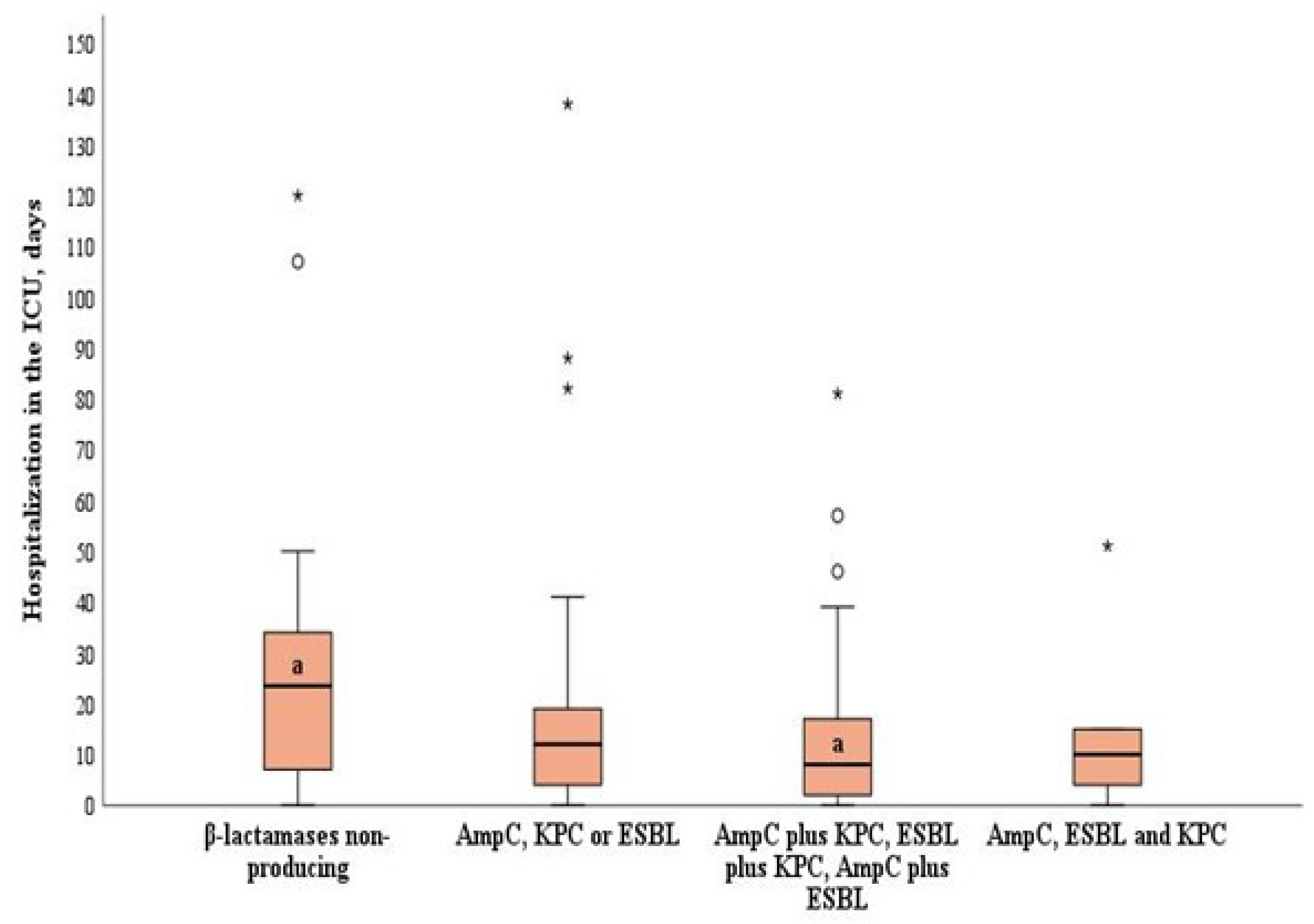
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Huang, H.; et al. A multi-center study on the risk factors of infection caused by multi-drug resistant Acinetobacter baumannii. BMC Infectious Diseases 2018, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; et al. Clinical and Pathophysiological Overview of Acinetobacter Infections: a Century of Challenges. Clin Microbiol Rev 2017, 30, 409–447. [Google Scholar] [CrossRef] [PubMed]
- Dickstein, Y.; et al. Treatment Outcomes of Colistin- and Carbapenem-resistant Acinetobacter baumannii Infections: An Exploratory Subgroup Analysis of a Randomized Clinical Trial. Clin Infect Dis 2019, 69, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Diao, H.; et al. Risk factors for multidrug-resistant and extensively drug-resistant Acinetobacter baumannii infection of patients admitted in intensive care unit: a systematic review and meta-analysis. J Hosp Infect 2024, 149, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; et al. Risk factors and drug resistance of the MDR Acinetobacter baumannii in pneumonia patients in ICU. Open Medicine 2019, 14, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Ye, H.; Liu, S. Risk factors for extensive drug-resistance and mortality in geriatric inpatients with bacteremia caused by Acinetobacter baumannii. American Journal of Infection Control 2015, 43, 857–860. [Google Scholar] [PubMed]
- Freire, M.P.; et al. Bloodstream infection caused by extensively drug-resistant Acinetobacter baumannii in cancer patients: high mortality associated with delayed treatment rather than with the degree of neutropenia. Clinical Microbiology and Infection 2016, 22, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Muntean, D.; et al. Extensively drug-resistant Acinetobacter baumannii and Proteeae association in a Romanian intensive care unit: risk factors for acquisition. Infect Drug Resist 2018, 11, 2187–2197. [Google Scholar] [CrossRef]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN surveillance definition of health care–associated infection and criteria for specific types of infections in the acute care setting. American journal of infection control 2008, 36, 309–332. [Google Scholar]
- Giske, C.G.; et al. Update from the European Committee on Antimicrobial Susceptibility Testing (EUCAST). J Clin Microbiol 2022, 60, e0027621. [Google Scholar] [CrossRef]
- Černiauskienė, K.; Dambrauskienė, A.; Vitkauskienė, A. Associations between β-Lactamase Types of Acinetobacter baumannii and Antimicrobial Resistance. Medicina (Kaunas) 2023, 59. [Google Scholar]
- Gonzalez-Villoria, A.M.; Valverde-Garduno, V. Antibiotic-Resistant<i> Acinetobacter baumannii</i> Increasing Success Remains a Challenge as a Nosocomial Pathogen. Journal of Pathogens 2016, 2016, 7318075. [Google Scholar]
- Benaissa, E.; et al. Risk factors for acquiring Acinetobacter baumannii infection in the intensive care unit: experience from a Moroccan hospital. Access Microbiol 2023, 5. [Google Scholar] [CrossRef] [PubMed]
- Magill, S.S.; et al. Multistate Point-Prevalence Survey of Health Care–Associated Infections. New England Journal of Medicine 2014, 370, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Piperaki, E.T.; et al. Carbapenem-resistant Acinetobacter baumannii: in pursuit of an effective treatment. Clinical Microbiology and Infection 2019, 25, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Calò, F.; et al. Outcome of patients with carbapenem-resistant Acinetobacter baumannii infections treated with cefiderocol: A multicenter observational study. Journal of Infection and Public Health 2023, 16, 1485–1491. [Google Scholar] [CrossRef]
- Uwingabiye, J.; et al. Intensive care unit-acquired Acinetobacter baumannii infections in a Moroccan teaching hospital: epidemiology, risk factors and outcome. Germs 2017, 7, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Appaneal, H.J.; et al. Treatment, clinical outcomes, and predictors of mortality among a national cohort of admitted patients with Acinetobacter baumannii infection. Antimicrobial Agents and Chemotherapy 2022, 66, e01975–21. [Google Scholar] [CrossRef]
- Arefian, H.; et al. Extra length of stay and costs because of health care-associated infections at a German university hospital. Am J Infect Control 2016, 44, 160–166. [Google Scholar] [CrossRef]
- Torres, A.; et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT). Eur Respir J 2017, 50. [Google Scholar]
- Papanikolopoulou, A.; et al. Ventilator-Associated Pneumonia, Multidrug-Resistant Bacteremia and Infection Control Interventions in an Intensive Care Unit: Analysis of Six-Year Time-Series Data. Antibiotics (Basel) 2022, 11. [Google Scholar] [CrossRef]
- Hafiz, T.A.; et al. A two-year retrospective study of multidrug-resistant Acinetobacter baumannii respiratory infections in critically Ill patients: Clinical and microbiological findings. Journal of Infection and Public Health 2023, 16, 313–319. [Google Scholar] [CrossRef]
- Blanco, N.; et al. Risk Factors and Outcomes Associated with Multidrug-Resistant Acinetobacter baumannii upon Intensive Care Unit Admission. Antimicrob Agents Chemother 2018, 62. [Google Scholar] [CrossRef]
- Martín-Aspas, A.; et al. Differential characteristics of Acinetobacter baumannii colonization and infection: risk factors, clinical picture, and mortality. Infection and Drug Resistance, 2018, 11, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; et al. Risk and Prognostic Factors for Multidrug-Resistant Acinetobacter Baumannii Complex Bacteremia: A Retrospective Study in a Tertiary Hospital of West China. PLoS One 2015, 10, e0130701. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; et al. Clinical features and risk factors for development of breakthrough gram-negative bacteremia during carbapenem therapy. Antimicrobial agents and chemotherapy 2016, 60, 6673–6678. [Google Scholar] [CrossRef]
- Zhou, H.; et al. Risk factors for acquisition and mortality of multidrug-resistant Acinetobacter baumannii bacteremia: A retrospective study from a Chinese hospital. Medicine (Baltimore) 2019, 98, e14937. [Google Scholar]
- López-Cortés, L.E.; et al. Monotherapy versus combination therapy for sepsis due to multidrug-resistant Acinetobacter baumannii: analysis of a multicentre prospective cohort. J Antimicrob Chemother 2014, 69, 3119–3126. [Google Scholar]
- Park, S.Y.; et al. Survival of carbapenem-resistant Acinetobacter baumannii bacteremia: colistin monotherapy versus colistin plus meropenem. J Int Med Res 2019, 47, 5977–5985. [Google Scholar]
- Schmid, A.; et al. Monotherapy versus combination therapy for multidrug-resistant Gram-negative infections: Systematic Review and Meta-Analysis. Sci Rep 2019, 9, 15290. [Google Scholar] [CrossRef]
- Alrahmany, D.; et al. Acinetobacter baumannii Infection-Related Mortality in Hospitalized Patients: Risk Factors and Potential Targets for Clinical and Antimicrobial Stewardship Interventions. Antibiotics (Basel) 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; et al. Prognosis of patients with Acinetobacter baumannii infection in the intensive care unit: A retrospective analysis. Exp Ther Med 2017, 13, 1630–1633. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; et al. Risk factors for early mortality in patients with carbapenem-resistant Acinetobacter baumannii bacteraemia. J Glob Antimicrob Resist 2022, 31, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Itani, R.; et al. Acinetobacter baumannii: assessing susceptibility patterns, management practices, and mortality predictors in a tertiary teaching hospital in Lebanon. Antimicrobial Resistance & Infection Control 2023, 12, 136. [Google Scholar]
- Du, X.; et al. Predictors of mortality in patients infected with carbapenem-resistant Acinetobacter baumannii: a systematic review and meta-analysis. American journal of infection control 2019, 47, 1140–1145. [Google Scholar] [CrossRef]
- Russo, A.; et al. ISGRI-SITA (Italian Study Group on Resistant Infections of the Società Italiana Terapia Antinfettiva). Bloodstream infections caused by carbapenem-resistant Acinetobacter baumannii: Clinical features, therapy and outcome from a multicenter study. J. Infect 2019, 79, 130–138. [Google Scholar]
| Variable | A. baumannii strains producing different types and numbers of β-lactamases | p | ||
|---|---|---|---|---|
| One type* (n = 53) | Two different types** (n = 115) | All three types*** (n = 9) | ||
| Gender, n (%) Female |
16 (30.2) |
31 (27.0) |
7 (77.8)a |
0.006a vs. b |
| Male | 37 (69.8) | 84 (73.0) | 2 (22.2)b | |
| Age, mean (SD), years | 61.6 (17.5) | 62 (16.1) | 63.6 (16.8) | 0.949 |
| Cause of hospitalization, n (%) | ||||
| Surgery | 40 (75.5) | 83 (72.2) | 6 (66.7) | 0.825 |
| Trauma | 7 (13.2) | 7 (6.1) | 0 (0) | 0.188 |
| Comorbid disease, n (%) | 41 (77.4) | 87 (75.7) | 7 (77.8) | 0.965 |
| Antibiotic treatment before infection (class), n (%) | ||||
| Cephalosporin | 41 (77.4) | 86 (74.8) | 6 (66.7) | 0.781 |
| Penicillin + BLI | 31 (58.5) | 69 (60.0) | 4 (44.4) | 0.658 |
| Carbapenem | 22 (41.5) | 39 (33.9) | 5 (55.6) | 0.325 |
| Antifungal | 8 (15.1) | 22 (19.1) | 0 (0) | 0.308 |
| Quinolone | 9 (17.0) | 10 (8.7) | 0 (0) | 0.154 |
| Antibiotic treatment after infection, n (%) | ||||
| Monotherapy | 18 (40.0)a | 20 (21.1)b | 1 (14.3) | 0.019a vs. b |
| Combination therapy | 27 (60.0)a | 75 (78.9)b | 6 (85.7) | 0.019a vs. b |
| Length of stay before A. baumannii infection, days | 17 (10–30) | 13 (6–21) | 14 (8–33) | 0.353 |
| Length of ICU stay before A. baumannii infection, days | 6 (2–11) | 5 (2–10) | 4 (2–13) | 0.908 |
| MV duration before A. baumannii infection, days | 5 (2–10) | 4 (2–9) | 5 (2–19) | 0.497 |
| Inflammatory markers on A. baumannii infection onset | ||||
| WBC, ×109/L | 12.3 (7.3–16.2) | 11.2 (7.8–15.7) | 12.5 (4.9–16.6) | 0.988 |
| CRP, mg/L | 126.5 (76.0–250.3) | 108 (88.8–272.6) | 236.9 (86.8–295.9) | 0.294 |
| Treatment groups |
A. baumannii strains producing different types and numbers of β-lactamases | ||
|---|---|---|---|
| One type* (n = 53) | Two different types** (n = 115) | All three types*** (n = 9) |
|
| Treatment unadjusted for colonization and lethal outcome (n = 46) | 16 (34.8) | 27 (58.7) | 3 (6.7) |
| Continuation of empirical treatment (n = 15) | 3 (20.0) | 10 (66.7) | 2 (13.3) |
| Monotherapy with BLI (n = 23) | 11 (47.8) | 12 (52.2) | 0 (0.0) |
| Combination of colistin with BLI (n = 68) | 8 (11.8)a | 57 (83.8)b | 3 (4.4) |
| Combination of colistin and carbapenems (n = 14) | 8 (57.1)a | 5 (35.7)b | 1 (7.1) |
|
Other combinations#
(n = 12) |
7 (63.6)a | 4 (36.4)b | 0 (0.0) |
| Variable | Survivors (n = 81) | Non-survivors (n = 115) | p |
|---|---|---|---|
| Gender, n (%) | |||
| Female | 23 (28.4) | 37 (32.2) | 0.572 |
| Male | 58 (71.6) | 78 (67.8) | |
| Age, mean (SD), years | 55.9 (17.9) | 65.9 (14.2) | <0.001 |
| Comorbid diseases, n (%) | |||
| Cardiovascular | 40 (32.8) | 82 (67.2) | 0.002 |
| Respiratory | 53 (65.4) | 105 (91.3) | <0.001 |
| Renal | 7 (8.6) | 16 (13.9) | 0.259 |
| Liver | 4 (21.1) | 15 (78.9) | 0.085 |
| Diabetes mellitus | 14 (17.3) | 25 (22.2) | 0.591 |
| Cancer | 20 (24.7) | 34 (29.6) | 0.452 |
| At least one comorbid disease | 55 (67.9) | 95 (82.6) | 0.017 |
| Cause of hospitalization, n (%) | |||
| Surgery | 68 (84.0) | 73 (63.5) | 0.002 |
| Trauma | 10 (12.3) | 5 (4.3) | 0.038 |
| Length of hospital stay before ICU, days | 10 (1 –19.7) | 10 (4–21) | 0.146 |
| Length of ICU stay before A. baumannii infection, days | 5 (1 –12) | 5 (2–10) | 0.450 |
| Length of hospital stay after A. baumannii infection, days | 21 (12.5–41.5) | 6 (1–21) | <0.001 |
| Duration of MV before A. baumannii infection, days | 3.0 (2.3–10) | 3.5 (2–9) | 0.212 |
| Duration of antibiotic treatment before A. baumannii infection, median (IQR), days | 15.0 (8–24.5) | 14.0 (8–23.0) | 0.649 |
| Antibiotic treatment after infection, n (%) | |||
| Monotherapy | 33 (40.7) | 13 (16.0) | 0.045 |
| Combination therapy | 48 (59.3) | 68 (84.0) | |
| Co-infection with other bacteria, n (%) | |||
| No | 29 (30.9) | 65 (69.1) | < 0.001 |
| Yes | 52 (51.0) | 50 (49.0) | |
| A. baumannii strains producing different types and numbers of β-lactamases, n (%) | |||
| One type* | 26 (32.1) | 27 (23.5) | 0.407 |
| Two different types** | 46 (56.8) | 69 (60.0) | |
| All three types*** | 2 (2.5) | 7 (6.1) | |
| Risk factor | OR (95% CI) | p |
|---|---|---|
| Combination therapy | 6.11 (2.47–15.10) | < 0.001 |
| Hospitalization of < 10 days after A. baumannii infection | 4.26 (1.79–10.09) | < 0.001 |
| Age of > 58 years | 1.06 (1.03–1.09) | <0.001 |
| No co-infection | 2.55 (1.15–5.65) | 0.021 |
| At least one chronic disease | 0.621 (0.23–1.71) | 0.358 |
| Surgery | 0.68 (0.26–1.75) | 0.442 |
| Trauma | 0.37 (0.08–1.77) | 0.215 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




