Submitted:
22 July 2024
Posted:
22 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Epidemiology and pathogenesis of Co-M
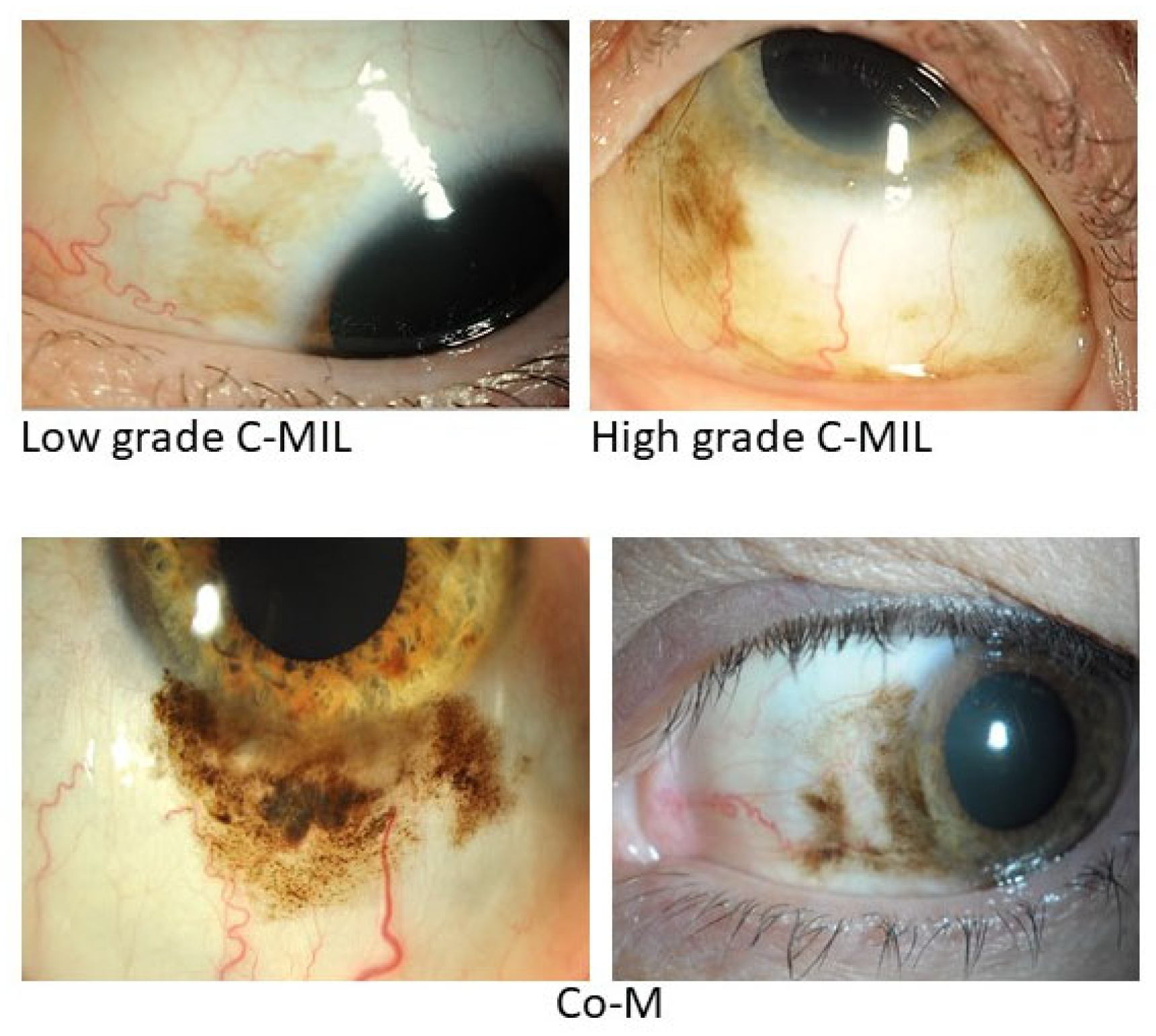
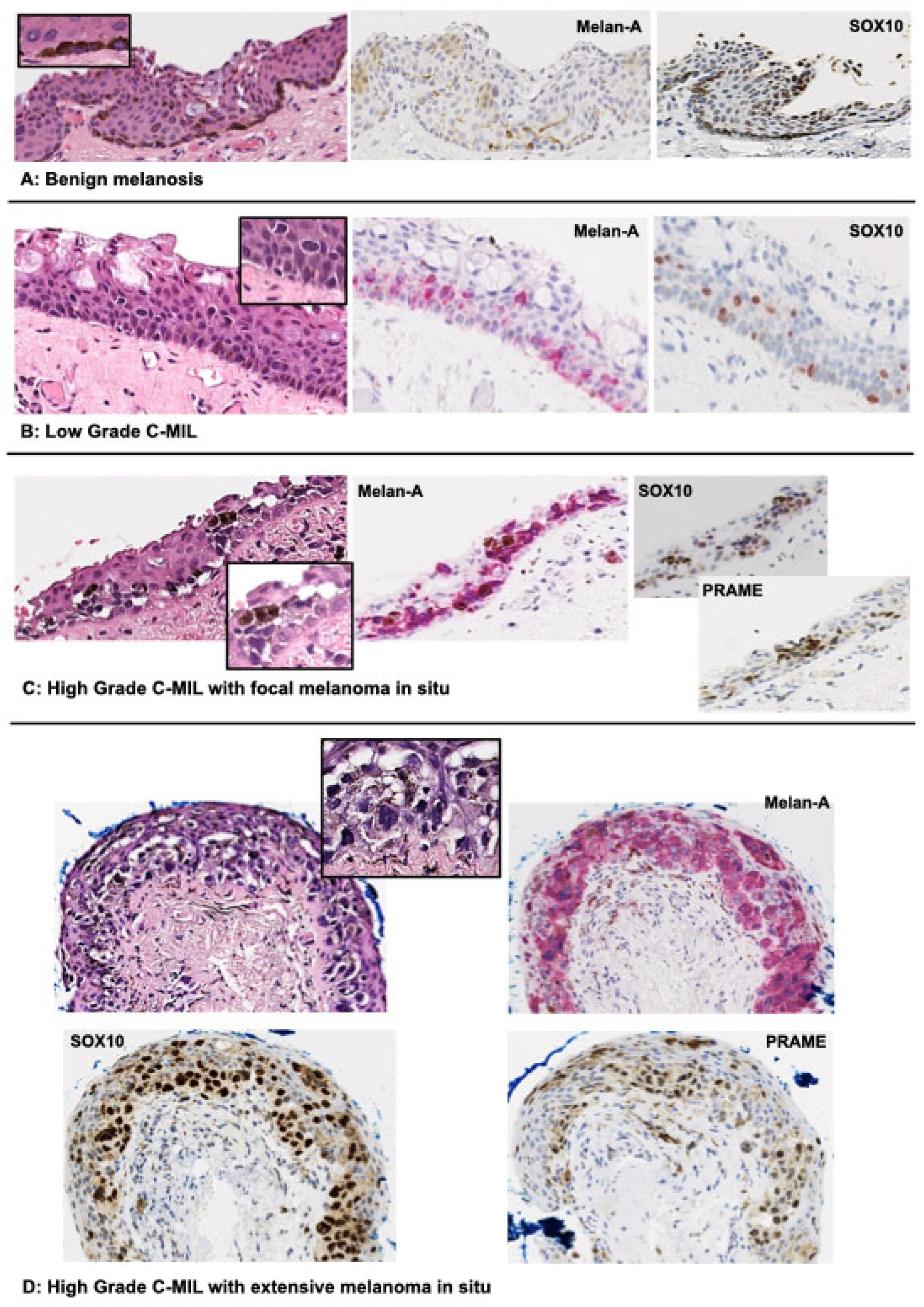
3. Precursor lesions (C-MIL)
4. Clinical presentation and assessment
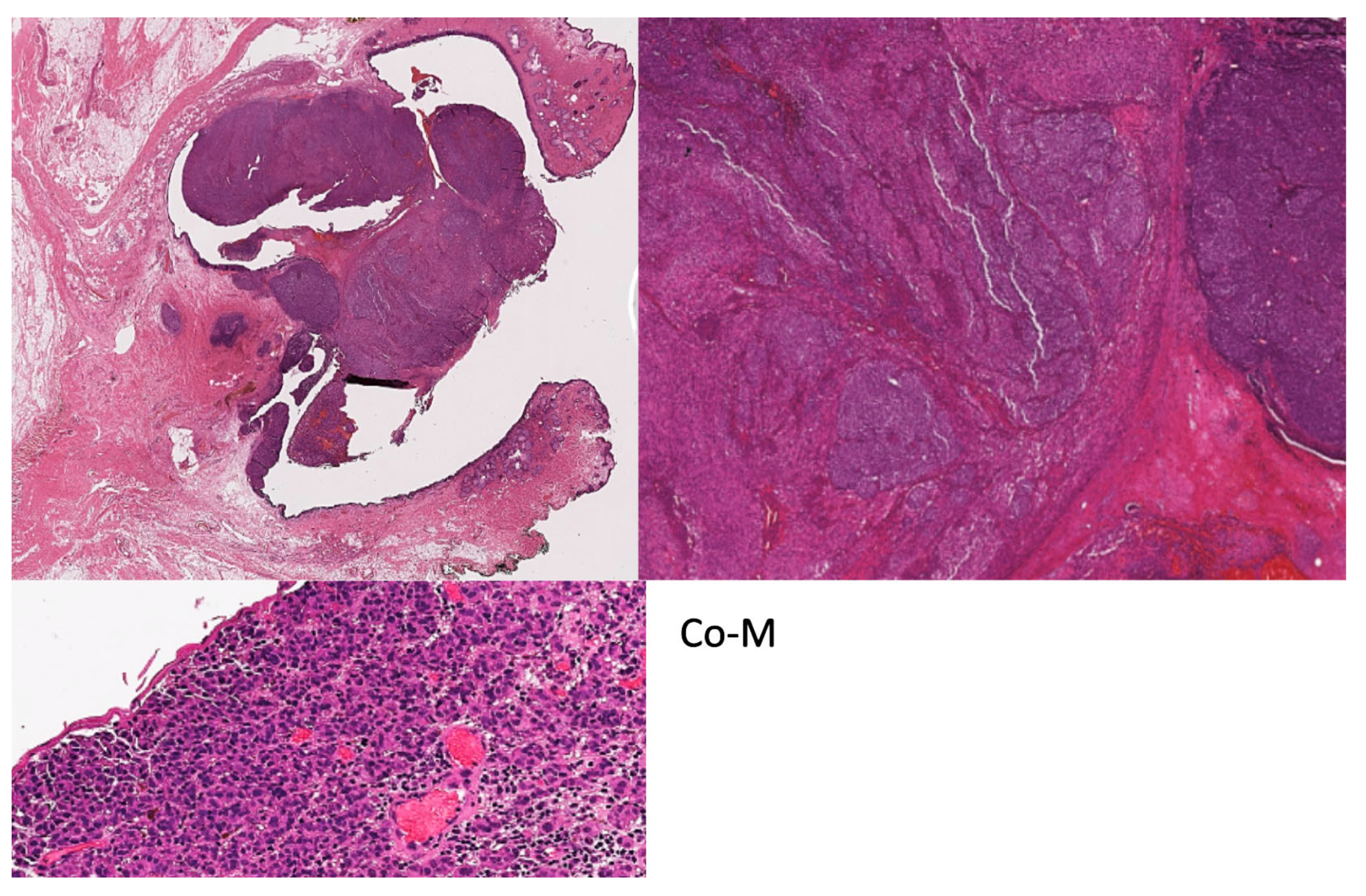
5. Histomorphological features
6. Treatment and prognosis
7. Conclusions and future directions
Author Contributions
Funding
Conflicts of Interest
References
- E. Triay, L. Bergman, B. Nilsson, C. All-Ericsson, and S. Seregard, "Time trends in the incidence of conjunctival melanoma in Sweden," (in eng), Br J Ophthalmol, vol. 93, no. 11, pp. 1524-8, Nov 2009. [CrossRef]
- G. Virgili et al., "Incidence and Survival of Patients With Conjunctival Melanoma in Europe," (in eng), JAMA Ophthalmol, vol. 138, no. 6, pp. 601-608, Jun 01 2020. [CrossRef]
- C. Larsen et al., "A Retrospective Review of Conjunctival Melanoma Presentation, Treatment, and Outcome and an Investigation of Features Associated With BRAF Mutations," (in eng), JAMA Ophthalmol, vol. 133, no. 11, pp. 1295-303, Nov 2015. [CrossRef]
- L. Shields et al., "Conjunctival melanoma: outcomes based on tumor origin in 382 consecutive cases," (in eng), Ophthalmology, vol. 118, no. 2, pp. 389-95.e1-2, Feb 2011. [CrossRef]
- H. E. Spendlove, B. E. Damato, J. Humphreys, K. T. Barker, P. S. Hiscott, and R. S. Houlston, "BRAF mutations are detectable in conjunctival but not uveal melanomas," (in eng), Melanoma Res, vol. 14, no. 6, pp. 449-52, Dec 2004. [CrossRef]
- N. Kenawy et al., "Conjunctival melanoma copy number alterations and correlation with mutation status, tumor features, and clinical outcome," (in eng), Pigment Cell Melanoma Res, vol. 32, no. 4, pp. 564-575, Jul 2019. [CrossRef]
- S. L. Lake, F. Jmor, J. Dopierala, A. F. Taktak, S. E. Coupland, and B. E. Damato, "Multiplex ligation-dependent probe amplification of conjunctival melanoma reveals common BRAF V600E gene mutation and gene copy number changes," (in eng), Invest Ophthalmol Vis Sci, vol. 52, no. 8, pp. 5598-604, Jul 29 2011. [CrossRef]
- K. N. Smit, M. J. Jager, A. de Klein, and E. Kiliҫ, "Uveal melanoma: Towards a molecular understanding," (in eng), Prog Retin Eye Res, vol. 75, p. 100800, Mar 2020. [CrossRef]
- M. J. Jager et al., "Uveal melanoma," (in eng), Nat Rev Dis Primers, vol. 6, no. 1, p. 24, Apr 09 2020. [CrossRef]
- M. Rodrigues et al., "So Close, yet so Far: Discrepancies between Uveal and Other Melanomas. A Position Paper from UM Cure 2020," (in eng), Cancers (Basel), vol. 11, no. 7, Jul 22 2019. [CrossRef]
- L. H. Mikkelsen, A. C. Larsen, C. von Buchwald, K. T. Drzewiecki, J. U. Prause, and S. Heegaard, "Mucosal malignant melanoma - a clinical, oncological, pathological and genetic survey," (in eng), APMIS, vol. 124, no. 6, pp. 475-86, Jun 2016. [CrossRef]
- K. Cisarova et al., "Genomic and transcriptomic landscape of conjunctival melanoma," (in eng), PLoS Genet, vol. 16, no. 12, p. e1009201, Dec 2020. [CrossRef]
- S. E. Lally et al., "Mutational Landscape and Outcomes of Conjunctival Melanoma in 101 Patients," (in eng), Ophthalmology, vol. 129, no. 6, pp. 679-693, Jun 2022. [CrossRef]
- C. Larsen et al., "BRAF mutations in conjunctival melanoma: investigation of incidence, clinicopathological features, prognosis and paired premalignant lesions," (in eng), Acta Ophthalmol, vol. 94, no. 5, pp. 463-70, Aug 2016. [CrossRef]
- S. L. Scholz et al., "NF1 mutations in conjunctival melanoma," (in eng), Br J Cancer, vol. 118, no. 9, pp. 1243-1247, May 2018. [CrossRef]
- S. Gardrat et al., "Definition of Biologically Distinct Groups of Conjunctival Melanomas According to Etiological Factors and Implications for Precision Medicine," (in eng), Cancers (Basel), vol. 13, no. 15, Jul 30 2021. [CrossRef]
- N. M. van Poppelen et al., "Molecular Genetics of Conjunctival Melanoma and Prognostic Value of," (in eng), Int J Mol Sci, vol. 22, no. 11, May 28 2021. [CrossRef]
- N. J. Brouwer, R. M. Verdijk, S. Heegaard, M. Marinkovic, B. Esmaeli, and M. J. Jager, "Conjunctival melanoma: New insights in tumour genetics and immunology, leading to new therapeutic options," (in eng), Prog Retin Eye Res, vol. 86, p. 100971, Jan 2022. [CrossRef]
- M. Krauthammer et al., "Exome sequencing identifies recurrent mutations in NF1 and RASopathy genes in sun-exposed melanomas," (in eng), Nat Genet, vol. 47, no. 9, pp. 996-1002, Sep 2015. [CrossRef]
- J. Wolf et al., "Transcriptional characterization of conjunctival melanoma identifies the cellular tumor microenvironment and prognostic gene signatures," (in eng), Sci Rep, vol. 10, no. 1, p. 17022, Oct 12 2020. [CrossRef]
- L. H. Mikkelsen et al., "The molecular profile of mucosal melanoma," (in eng), Melanoma Res, vol. 30, no. 6, pp. 533-542, Dec 2020. [CrossRef]
- B. Damato and S. E. Coupland, "Conjunctival melanoma and melanosis: a reappraisal of terminology, classification and staging," (in eng), Clin Exp Ophthalmol, vol. 36, no. 8, pp. 786-95, Nov 2008. [CrossRef]
- J. A. Shields et al., "Primary acquired melanosis of the conjunctiva: risks for progression to melanoma in 311 eyes. The 2006 Lorenz E. Zimmerman lecture," (in eng), Ophthalmology, vol. 115, no. 3, pp. 511-519.e2, Mar 2008. [CrossRef]
- C. V. Miller et al., "Clinical outcome of advanced squamous cell carcinoma of the conjunctiva," (in eng), Eye (Lond), vol. 28, no. 8, pp. 962-7, Aug 2014. [CrossRef]
- N. Kenawy, A. Garrick, H. Heimann, S. E. Coupland, and B. E. Damato, "Conjunctival squamous cell neoplasia: the Liverpool Ocular Oncology Centre experience," (in eng), Graefes Arch Clin Exp Ophthalmol, vol. 253, no. 1, pp. 143-50, Jan 2015. [CrossRef]
- J. Yang and C. S. Foster, "Squamous cell carcinoma of the conjunctiva," (in eng), Int Ophthalmol Clin, vol. 37, no. 4, pp. 73-85, 1997. [CrossRef]
- Y. A. Yousef and P. T. Finger, "Squamous carcinoma and dysplasia of the conjunctiva and cornea: an analysis of 101 cases," (in eng), Ophthalmology, vol. 119, no. 2, pp. 233-40, Feb 2012. [CrossRef]
- G. K. Vora, H. Demirci, B. Marr, and P. Mruthyunjaya, "Advances in the management of conjunctival melanoma," (in eng), Surv Ophthalmol, vol. 62, no. 1, pp. 26-42, 2017. [CrossRef]
- S. Kaštelan, A. Gverović Antunica, L. Beketić Orešković, J. Salopek Rabatić, B. Kasun, and I. Bakija, "Conjunctival Melanoma - Epidemiological Trends and Features," (in eng), Pathol Oncol Res, vol. 24, no. 4, pp. 787-796, Oct 2018. [CrossRef]
- D. Paridaens, D. C. Minassian, A. C. McCartney, and J. L. Hungerford, "Prognostic factors in primary malignant melanoma of the conjunctiva: a clinicopathological study of 256 cases," (in eng), Br J Ophthalmol, vol. 78, no. 4, pp. 252-9, Apr 1994. [CrossRef]
- P. De Potter, C. L. Shields, J. A. Shields, and H. Menduke, "Clinical predictive factors for development of recurrence and metastasis in conjunctival melanoma: a review of 68 cases," (in eng), Br J Ophthalmol, vol. 77, no. 10, pp. 624-30, Oct 1993. [CrossRef]
- N. J. Brouwer, M. Marinkovic, S. G. van Duinen, J. C. Bleeker, M. J. Jager, and G. P. M. Luyten, "Treatment of conjunctival melanoma in a Dutch referral centre," (in eng), Br J Ophthalmol, vol. 102, no. 9, pp. 1277-1282, Sep 2018. [CrossRef]
- B. Esmaeli, "Patterns of regional and distant metastasis in patients with conjunctival melanoma: experience a cancer centre over four decades," vol. 11713086, no. 108, pp. 2101-5, 2001. [CrossRef]
- Sagiv et al., "Immunotherapy With Programmed Cell Death 1 Inhibitors for 5 Patients With Conjunctival Melanoma," (in eng), JAMA Ophthalmol, vol. 136, no. 11, pp. 1236-1241, Nov 01 2018. [CrossRef]
- P. T. Finger and A. C. Pavlick, "Checkpoint inhibition immunotherapy for advanced local and systemic conjunctival melanoma: a clinical case series," (in eng), J Immunother Cancer, vol. 7, no. 1, p. 83, Mar 25 2019. [CrossRef]
- E. Chang, H. Demirci, and F. Y. Demirci, "Genetic Aspects of Conjunctival Melanoma: A Review," (in eng), Genes (Basel), vol. 14, no. 9, Aug 23 2023. [CrossRef]
- B. Esmaeli and O. Sagiv, "Targeted Biological Drugs and Immune Check Point Inhibitors for Locally Advanced or Metastatic Cancers of the Conjunctiva, Eyelid, and Orbit," (in eng), Int Ophthalmol Clin, vol. 59, no. 2, pp. 13-26, 2019. [CrossRef]
- E. Rossi et al., "Dabrafenib and Trametinib in BRAF Mutant Metastatic Conjunctival Melanoma," (in eng), Front Oncol, vol. 9, p. 232, 2019. [CrossRef]
- P. Isager et al., "Uveal and conjunctival malignant melanoma in Denmark, 1943-97: incidence and validation study," (in eng), Ophthalmic Epidemiol, vol. 12, no. 4, pp. 223-32, Aug 2005. [CrossRef]
- S. Tuomaala, S. Eskelin, A. Tarkkanen, and T. Kivelä, "Population-based assessment of clinical characteristics predicting outcome of conjunctival melanoma in whites," (in eng), Invest Ophthalmol Vis Sci, vol. 43, no. 11, pp. 3399-408, Nov 2002.
- F. M. Ghazawi et al., "Incidence trends of conjunctival malignant melanoma in Canada," (in eng), Br J Ophthalmol, vol. 104, no. 1, pp. 23-25, Jan 2020. [CrossRef]
- D. N. Hu, G. Yu, S. A. McCormick, and P. T. Finger, "Population-based incidence of conjunctival melanoma in various races and ethnic groups and comparison with other melanomas," (in eng), Am J Ophthalmol, vol. 145, no. 3, pp. 418-423, Mar 2008. [CrossRef]
- C. L. Shields, J. L. Chien, T. Surakiatchanukul, K. Sioufi, S. E. Lally, and J. A. Shields, "Conjunctival Tumors: Review of Clinical Features, Risks, Biomarkers, and Outcomes--The 2017 J. Donald M. Gass Lecture," (in eng), Asia Pac J Ophthalmol (Phila), vol. 6, no. 2, pp. 109-120, 2017. [CrossRef]
- T. A. Weppelmann, K. T. Zimmerman, and V. Rashidi, "Trends in Incidence of Conjunctival Melanoma in the US," (in eng), JAMA Netw Open, vol. 5, no. 10, p. e2237229, Oct 03 2022. [CrossRef]
- G. P. Yu, D. N. Hu, S. McCormick, and P. T. Finger, "Conjunctival melanoma: is it increasing in the United States?," (in eng), Am J Ophthalmol, vol. 135, no. 6, pp. 800-6, Jun 2003. [CrossRef]
- B. W. R. Balzer, S. Cherepanoff, A. M. Joshua, M. Giblin, R. M. Conway, and A. C. Anazodo, "Conjunctival Melanoma in Childhood and Adolescence: A Systematic Review," (in eng), Ocul Oncol Pathol, vol. 5, no. 6, pp. 387-395, Oct 2019. [CrossRef]
- C. C. McLaughlin, X. C. Wu, A. Jemal, H. J. Martin, L. M. Roche, and V. W. Chen, "Incidence of noncutaneous melanomas in the U.S," (in eng), Cancer, vol. 103, no. 5, pp. 1000-7, Mar 01 2005. [CrossRef]
- M. Waugh, "Roxburgh's Common Skin Diseases," (in eng), Skinmed, vol. 20, no. 4, p. 320, 2022.
- M. A. Watsky, M. M. Jablonski, and H. F. Edelhauser, "Comparison of conjunctival and corneal surface areas in rabbit and human," (in eng), Curr Eye Res, vol. 7, no. 5, pp. 483-6, May 1988. [CrossRef]
- N. Goldenberg-Cohen et al., "T1799A BRAF mutations in conjunctival melanocytic lesions," (in eng), Invest Ophthalmol Vis Sci, vol. 46, no. 9, pp. 3027-30, Sep 2005. [CrossRef]
- J. Cao et al., "Targeting of the MAPK and AKT pathways in conjunctival melanoma shows potential synergy," (in eng), Oncotarget, vol. 8, no. 35, pp. 58021-58036, Aug 29 2017. [CrossRef]
- K. G. Griewank et al., "Conjunctival melanomas harbor BRAF and NRAS mutations and copy number changes similar to cutaneous and mucosal melanomas," (in eng), Clin Cancer Res, vol. 19, no. 12, pp. 3143-52, Jun 15 2013. [CrossRef]
- El Zaoui et al., "Conjunctival Melanoma Targeted Therapy: MAPK and PI3K/mTOR Pathways Inhibition," (in eng), Invest Ophthalmol Vis Sci, vol. 60, no. 7, pp. 2764-2772, Jun 03 2019. [CrossRef]
- H. Francis, H. E. Grossniklaus, L. A. Habib, B. Marr, D. H. Abramson, and K. J. Busam, "BRAF, NRAS, and GNAQ Mutations in Conjunctival Melanocytic Nevi," (in eng), Invest Ophthalmol Vis Sci, vol. 59, no. 1, pp. 117-121, Jan 01 2018. [CrossRef]
- van Ipenburg et al., "Prognostic value of," (in eng), Br J Ophthalmol, vol. 105, no. 10, pp. 1454-1461, Oct 2021. [CrossRef]
- G. Griewank et al., "TERT promoter mutations in ocular melanoma distinguish between conjunctival and uveal tumours," (in eng), Br J Cancer, vol. 109, no. 2, pp. 497-501, Jul 23 2013. [CrossRef]
- E. Koopmans et al., "Prevalence and implications of TERT promoter mutation in uveal and conjunctival melanoma and in benign and premalignant conjunctival melanocytic lesions," (in eng), Invest Ophthalmol Vis Sci, vol. 55, no. 9, pp. 6024-30, Aug 26 2014. [CrossRef]
- Alessandrini et al., "C-Kit SCF receptor (CD117) expression and KIT gene mutation in conjunctival pigmented lesions," (in eng), Acta Ophthalmol, vol. 91, no. 8, pp. e641-5, Dec 2013. [CrossRef]
- Beadling et al., "KIT gene mutations and copy number in melanoma subtypes," (in eng), Clin Cancer Res, vol. 14, no. 21, pp. 6821-8, Nov 01 2008. [CrossRef]
- X. Sheng et al., "Prognostic factors for conjunctival melanoma: a study in ethnic Chinese patients," (in eng), Br J Ophthalmol, vol. 99, no. 7, pp. 990-6, Jul 2015. [CrossRef]
- G. S. Missotten, S. Keijser, R. J. De Keizer, and D. De Wolff-Rouendaal, "Conjunctival melanoma in the Netherlands: a nationwide study," (in eng), Invest Ophthalmol Vis Sci, vol. 46, no. 1, pp. 75-82, Jan 2005. [CrossRef]
- C. L. Shields, S. Kaliki, S. A. Al-Dahmash, S. E. Lally, and J. A. Shields, "American Joint Committee on Cancer (AJCC) clinical classification predicts conjunctival melanoma outcomes," (in eng), Ophthalmic Plast Reconstr Surg, vol. 28, no. 5, pp. 313-23, 2012. [CrossRef]
- S. E. Coupland, T. Milman, R. M. Verdict, and N. J. Brouwer, Conjunctival Melanocytic Intraepithelial Lesions In: WHO Classification of Tumours Editorial Board. Eye tumours [Internet; beta version ahead of print] 5th ed. (WHO classification of tumours). Lyon (France): International Agency for Research on Cancer, 2023.
- H. Demirci et al., "Integrative Exome and Transcriptome Analysis of Conjunctival Melanoma and Its Potential Application for Personalized Therapy," (in eng), JAMA Ophthalmol, vol. 137, no. 12, pp. 1444-1448, Dec 01 2019. [CrossRef]
- P. A. Mundra et al., "Ultraviolet radiation drives mutations in a subset of mucosal melanomas," (in eng), Nat Commun, vol. 12, no. 1, p. 259, Jan 11 2021. [CrossRef]
- C. Rivolta et al., "UV light signature in conjunctival melanoma; not only skin should be protected from solar radiation," (in eng), J Hum Genet, vol. 61, no. 4, pp. 361-2, Apr 2016. [CrossRef]
- Y. J. Lee, C. Lee, M. K. Kim, S. I. Khwarg, and J. Y. Oh, "Conjunctival pigmented lesion: Clinicopathological analysis of 85 cases in Korean population," (in eng), Sci Rep, vol. 9, no. 1, p. 18204, Dec 03 2019. [CrossRef]
- J. Brouwer, M. Marinkovic, G. P. M. Luyten, C. L. Shields, and M. J. Jager, "Lack of tumour pigmentation in conjunctival melanoma is associated with light iris colour and worse prognosis," (in eng), Br J Ophthalmol, vol. 103, no. 3, pp. 332-337, Mar 2019. [CrossRef]
- C. L. Shields et al., "Conjunctival melanoma: risk factors for recurrence, exenteration, metastasis, and death in 150 consecutive patients," (in eng), Arch Ophthalmol, vol. 118, no. 11, pp. 1497-507, Nov 2000. [CrossRef]
- J. R. Wong, A. A. Nanji, A. Galor, and C. L. Karp, "Management of conjunctival malignant melanoma: a review and update," (in eng), Expert Rev Ophthalmol, vol. 9, no. 3, pp. 185-204, Jun 2014. [CrossRef]
- C. L. Shields et al., "Clinical Features Differentiating Benign From Malignant Conjunctival Tumors in Children," (in eng), JAMA Ophthalmol, vol. 135, no. 3, pp. 215-224, Mar 01 2017. [CrossRef]
- F. S. D. Cruz, S. F. S. D. Cruz, D. H. Ishigai, K. S. Santos, and S. Felberg, "Conjunctival tattoo: report on an emerging body modification trend," (in eng), Arq Bras Oftalmol, vol. 80, no. 6, pp. 399-400, 2017. [CrossRef]
- N. Kenawy, S. L. Lake, S. E. Coupland, and B. E. Damato, "Conjunctival melanoma and melanocytic intra-epithelial neoplasia," (in eng), Eye (Lond), vol. 27, no. 2, pp. 142-52, Feb 2013. [CrossRef]
- C. L. Shields, A. Manchandia, R. Subbiah, R. C. Eagle, and J. A. Shields, "Pigmented squamous cell carcinoma in situ of the conjunctiva in 5 cases," (in eng), Ophthalmology, vol. 115, no. 10, pp. 1673-8, Oct 2008. [CrossRef]
- E. Vizvári, Á. Skribek, N. Polgár, A. Vörös, P. Sziklai, and E. Tóth-Molnár, "Conjunctival melanocytic naevus: Diagnostic value of anterior segment optical coherence tomography and ultrasound biomicroscopy," (in eng), PLoS One, vol. 13, no. 2, p. e0192908, 2018. [CrossRef]
- N. J. Brouwer, M. Marinkovic, J. C. Bleeker, G. P. M. Luyten, and M. J. Jager, "Anterior Segment OCTA of Melanocytic Lesions of the Conjunctiva and Iris," (in eng), Am J Ophthalmol, vol. 222, pp. 137-147, Feb 2021. [CrossRef]
- E. Cinotti et al., "Handheld In Vivo Reflectance Confocal Microscopy for the Diagnosis of Eyelid Margin and Conjunctival Tumors," (in eng), JAMA Ophthalmol, vol. 135, no. 8, pp. 845-851, Aug 01 2017. [CrossRef]
- T. A. Ferreira et al., "MR and CT Imaging of the Normal Eyelid and its Application in Eyelid Tumors," (in eng), Cancers (Basel), vol. 12, no. 3, Mar 12 2020. [CrossRef]
- Zembowicz, R. V. Mandal, and P. Choopong, "Melanocytic lesions of the conjunctiva," (in eng), Arch Pathol Lab Med, vol. 134, no. 12, pp. 1785-92, Dec 2010. [CrossRef]
- Esmaeli et al., "Greater Tumor Thickness, Ulceration, and Positive Sentinel Lymph Node Are Associated With Worse Prognosis in Patients With Conjunctival Melanoma: Implications for Future AJCC Classifications," (in eng), Am J Surg Pathol, vol. 43, no. 12, pp. 1701-1710, Dec 2019. [CrossRef]
- Esmaeli et al., "Histologic features of conjunctival melanoma predictive of metastasis and death (an American Ophthalmological thesis)," (in eng), Trans Am Ophthalmol Soc, vol. 110, pp. 64-73, Dec 2012.
- R. Folberg, I. W. McLean, and L. E. Zimmerman, "Primary acquired melanosis of the conjunctiva," (in eng), Hum Pathol, vol. 16, no. 2, pp. 129-35, Feb 1985. [CrossRef]
- T. Milman et al., "Validation of the Newly Proposed World Health Organization Classification System for Conjunctival Melanocytic Intraepithelial Lesions: A Comparison with the C-MIN and PAM Classification Schemes," (in eng), Am J Ophthalmol, vol. 223, pp. 60-74, Mar 2021. [CrossRef]
- Maly, D. Epstein, K. Meir, and J. Pe'er, "Histological criteria for grading of atypia in melanocytic conjunctival lesions," (in eng), Pathology, vol. 40, no. 7, pp. 676-81, Dec 2008. [CrossRef]
- M. Sugiura, K. A. Colby, M. C. Mihm, and A. Zembowicz, "Low-risk and high-risk histologic features in conjunctival primary acquired melanosis with atypia: Clinicopathologic analysis of 29 cases," (in eng), Am J Surg Pathol, vol. 31, no. 2, pp. 185-92, Feb 2007. [CrossRef]
- H. S. Mudhar et al., "A multicenter study validates the WHO 2022 classification for conjunctival melanocytic intraepithelial lesions with clinical and prognostic relevance," (in eng), Lab Invest, p. 100281, Nov 02 2023. [CrossRef]
- B. Damato and S. E. Coupland, "Management of conjunctival melanoma," (in eng), Expert Rev Anticancer Ther, vol. 9, no. 9, pp. 1227-39, Sep 2009. [CrossRef]
- F. A. Jakobiec, "Conjunctival Primary Acquired Melanosis: Is It Time for a New Terminology?," (in eng), Am J Ophthalmol, vol. 162, pp. 3-19.e1, Feb 2016. [CrossRef]
- F. A. Jakobiec, "Clinicopathologic characteristics of premalignant and malignant melanocytic lesions of the conjunctiva," vol. 96, R. Folberg and T. Iwamato, Eds., ed. Ophthalmology 1989, 1989, pp. 147-66.
- 90. C. G. Eberhart, S. E. Coupland, R. Folberg, C. Margo, and N. Rao, Conjunctival Melanocytic Intraepithelial Neoplasia In: WHO Classification of Tumours of the Eye 4th ed. Lyon, France: International Agency for Research on Cancer, 2018.
- D. Šekoranja, G. Hawlina, and J. Pižem, "PRAME expression in melanocytic lesions of the conjunctiva," (in eng), Histopathology, vol. 79, no. 6, pp. 989-996, Dec 2021. [CrossRef]
- R. E. LeBlanc, D. M. Miller, and M. E. Zegans, "PRAME immunohistochemistry is useful in the evaluation of conjunctival melanomas, nevi, and primary acquired melanosis," (in eng), J Cutan Pathol, vol. 48, no. 12, pp. 1442-1448, Dec 2021. [CrossRef]
- H. S. Mudhar, S. S. Salvi, D. Pissaloux, and A. de La Fouchardiere, "Single Time Frame Overview of the Genetic Changes in Conjunctival Melanoma from Intraepithelial Disease to Invasive Melanoma: A Study of 4 Exenteration Specimens Illustrating the Potential Role of Cyclin D1," (in eng), Ocul Oncol Pathol, vol. 8, no. 1, pp. 52-63, Feb 2022. [CrossRef]
- H. S. Mudhar et al., "PRAME expression by immunohistochemistry and reverse transcription quantitative PCR in conjunctival melanocytic lesions-a comprehensive clinicopathologic study of 202 cases and correlation of cytogenetics with PRAME expression in challenging conjunctival melanocytic lesions," (in eng), Hum Pathol, vol. 134, pp. 1-18, Apr 2023. [CrossRef]
- S. Lassalle et al., "PD-L1 Expression in 65 Conjunctival Melanomas and Its Association with Clinical Outcome," (in eng), Int J Mol Sci, vol. 21, no. 23, Nov 30 2020. [CrossRef]
- Jain et al., "Multicenter, International Assessment of the Eighth Edition of the American Joint Committee on Cancer Cancer Staging Manual for Conjunctival Melanoma," (in eng), JAMA Ophthalmol, vol. 137, no. 8, pp. 905-911, Aug 01 2019. [CrossRef]
- S. Jia et al., "American Joint Committee on Cancer Tumor Staging System Predicts the Outcome and Metastasis Pattern in Conjunctival Melanoma," (in eng), Ophthalmology, vol. 129, no. 7, pp. 771-780, Jul 2022. [CrossRef]
- TNM Classification of Malignant Tumours, 8th ed. Oxford, UK: Wiley-Blackwell, 2017, p. 272.
- V. M. Cohen, M. Tsimpida, J. L. Hungerford, H. Jan, R. Cerio, and G. Moir, "Prospective study of sentinel lymph node biopsy for conjunctival melanoma," (in eng), Br J Ophthalmol, vol. 97, no. 12, pp. 1525-9, Dec 2013. [CrossRef]
- M. Lim, T. Tatla, D. Hersh, and J. Hungerford, "Patterns of regional head and neck lymph node metastasis in primary conjunctival malignant melanoma," (in eng), Br J Ophthalmol, vol. 90, no. 12, pp. 1468-71, Dec 2006. [CrossRef]
- G. Anastassiou, A. Heiligenhaus, N. Bechrakis, E. Bader, N. Bornfeld, and K. P. Steuhl, "Prognostic value of clinical and histopathological parameters in conjunctival melanomas: a retrospective study," (in eng), Br J Ophthalmol, vol. 86, no. 2, pp. 163-7, Feb 2002. [CrossRef]
- J. M. Grimes, N. V. Shah, F. H. Samie, R. D. Carvajal, and B. P. Marr, "Conjunctival Melanoma: Current Treatments and Future Options," (in eng), Am J Clin Dermatol, vol. 21, no. 3, pp. 371-381, Jun 2020. [CrossRef]
- M. B. Pahlitzsch, EckartMai, Christian, "Conjunctival Melanoma and BRAF Inhibitor Therapy," Journal of Clinical and Experimental Ophthalmology, vol. Volume 5, no. 1, 2014.
- J. L. Weber, K. S. Smalley, V. K. Sondak, and G. T. Gibney, "Conjunctival melanomas harbor BRAF and NRAS mutations--Letter," (in eng), Clin Cancer Res, vol. 19, no. 22, pp. 6329-30, Nov 15 2013. [CrossRef]
- Maleka, G. Åström, P. Byström, and G. J. Ullenhag, "A case report of a patient with metastatic ocular melanoma who experienced a response to treatment with the BRAF inhibitor vemurafenib," (in eng), BMC Cancer, vol. 16, p. 634, Aug 12 2016. [CrossRef]
- S. Pinto Torres, T. André, E. Gouveia, L. Costa, and M. J. Passos, "Systemic Treatment of Metastatic Conjunctival Melanoma," (in eng), Case Rep Oncol Med, vol. 2017, p. 4623964, 2017. [CrossRef]
- B. Y. Hong et al., "Immune Checkpoint Inhibitor Therapy as an Eye-Preserving Treatment for Locally Advanced Conjunctival Melanoma," (in eng), Ophthalmic Plast Reconstr Surg, vol. 37, no. 1, pp. e9-e13, 2021 Jan-Feb 01 2021. [CrossRef]
- T. Kiyohara et al., "Two cases of BRAF-mutated, bulbar conjunctival melanoma, and review of the published literature," (in eng), Clin Exp Dermatol, vol. 45, no. 2, pp. 207-211, Mar 2020. [CrossRef]
- L. J. Chaves, B. Huth, J. J. Augsburger, and Z. M. Correa, "Eye-Sparing Treatment for Diffuse Invasive Conjunctival Melanoma," (in eng), Ocul Oncol Pathol, vol. 4, no. 4, pp. 261-266, Jun 2018. [CrossRef]
| Conjunctival nevi (%) | PAM without atypia (%) | PAM with atypia (%) | Prevalence in Co-M (%) | |
|---|---|---|---|---|
| BRAF | 14/28 (50%) (Goldenberg-Cohen et al., 2005) 13-23 (56%) (Francis et al., 2018) 7/37 (19%) (Cao et al., 2017) 9/12 (75%) (Larsen et al., 2016) 15/35 (43%) (El Zaoui et al., 2019) |
0/11 (0%) (Goldenberg-Cohen et al., 2005) 0/17 (0%) (Cao et al., 2017) |
0/4 (0%) (Goldenberg-Cohen et al., 2005) 0/13 (0%) (Cao et al., 2017) 2/8 (25%) (Larsen et al., 2016) |
4/15 (27%) (Beadling et al., 2008) 3/21 (14%) (Spendlove et al., 2004) 23/78 (29%) (Griewank, Westekemper, et al., 2013) 2/5 (40%) (Goldenberg-Cohen et al., 2005) 10/39 (26%) (Cao et al., 2017) 39/111 (35%) (Larsen et al., 2016) 4/53 (8%) (Sheng et al., 2015) 31/101 (31%) (Lally et al., 2022) 16/47 (34%) (Gardrat et al., 2021) 13/28 (46%) (van Poppelen et al., 2021) 4/14 (29%) (Cisarova et al., 2020) 23/78 (29%) (van Ipenburg et al., 2021) 11/31 (35%) (El Zaoui et al., 2019) |
| NRAS | 9/23 (39%) (Francis et al., 2018) | NA | NA | 0/11 (0%) (Beadling et al., 2008) 14/78 (18%) (Griewank, Westekemper, et al., 2013) 25/95 (26%) (Lally et al., 2022) 5/47 (11%) (Gardrat et al., 2021) 6/28 (21%) (van Poppelen et al., 2021) 1/14 (7%) (Cisarova et al., 2020) |
| KIT | 0/5 (0%) (Alessandrini et al., 2013) | NA | 1/3 (33%) (Alessandrini et al., 2013) | 1/13 (8%) (Beadling et al., 2008) 0/42 (0%) (Griewank, Westekemper, et al., 2013) 0/8 (0%) (Alessandrini et al., 2013) 6/53 (11%) (Sheng et al., 2015) 2/47 (4%) (Gardrat et al., 2021) 2/28 (7%) (van Poppelen et al., 2021) |
| TERT | 0/56 (0%) (Koopmans et al., 2014) | 0/14 (0%) (Koopmans et al., 2014) | 2/25 (8%) (Koopmans et al., 2014) | 12/38 (32%) (Griewank, Murali, et al., 2013) 16/39 (41%) (Koopmans et al., 2014) 20/47 (43%) (van Ipenburg et al., 2021) 15/24 (54%) (van Poppelen et al., 2021) 9/14 (64%) (Cisarova et al., 2020) 34/78 (43%) (van Ipenburg et al., 2021) |
| NF1 | NA | NA | NA | 21/63 (33%) (Scholz et al., 2018) 29/74 (39%) (Lally et al., 2022) 7/14 (50%) (Cisarova et al., 2020) |
| WHO | Acceptable alternative terminology | Increased cellularity | Histologic features | Risk of progression to invasive melanoma |
|---|---|---|---|---|
| Not applicable | Benign melanosis C-MIN (grades (0-1) PAM without atypia |
No/minimal | Conjunctival hypermelanosis (increased pigment in epithelial cells without melanocytic hyperplasia or atypia). Slight or focal melanocytic hyperplasia without atypia (parabasal melanocytes with condensed round nuclei, smaller than basal epithelial cell, inconspicuous nucleoli, and inconspicuous cytoplasm) may be seen. | None |
| Low-grade C-MIL | PAM with mild atypia C-MIN (grades 2-4) |
Yes | Predominantly basilar melanocytic proliferation with low-grade atypia (dendritic or small to moderate size polyhedral, usually non-epithelioid melanocytes with round to irregular nuclear contours, often nuclear hyperchromasia, inconspicuous nucleoli, and inconspicuous or scant cytoplasm). | Lower |
| High-grade C-MIL | PAM with moderate to severe atypia C-MIN (grade 5-10) |
Yes | More confluent basilar and significant non-basilar proliferation of melanocytes with high-grade atypia (moderate to severe), evidence of intraepithelial nested and/or pagetoid growth, and epithelioid cell cytomorphology. | Higher |
| High-grade C-MIL | Melanoma in situ | Yes | The term melanoma in situ may be used for (1) the most atypical high-grade C-MILs involving close to full thickness of the epithelium, (2) histologically obvious melanomas without documented evidence of subepithelial invasion. | Highest |
| Study | Patient | Co-M | Primary treatment | Agent used | Dosage | Outcome | Adverse reactions |
|---|---|---|---|---|---|---|---|
| Indicated for primary CoM | |||||||
| (Pahlitzsch, 2014) | 80y, Female | BRAF mutation | Exenteration (rejected) | Vemurafenib | Successful tumour response Tumour decreased in size |
8kg weight loss Nausea vomiting, headaches |
|
| Indicated for metastatic disease | |||||||
| (Weber et al., 2013) | 45y, Male | Metastatic Co-M (nodal, subcutaneous, pulmonary, osseous) BRAF mutation v600e |
Resection | Vemurafenib | 960mg twice daily | Improvement in pain and subjective tumour regression after 1 month | Disease progression 2 months into treatment. Enlarged paraspinal mass. |
| (Maleka et al., 2016) | 53y, Female | Metastatic Co-M (orbit, parotid gland, lung, brain) BRAF mutation v600e |
Excision Cryotherapy Mitomycin eye drops Enucleation |
Vemurafenib | 960mg twice daily | Initially good response and reduction of mets, after 4 months reappearance of mets and death, | Skin rash (dose reduced to 720mg twice daily) |
| (Rossi et al., 2019) | 70y, Male | Metastatic Co-M (parotid gland and lymph node) BRAF mutation v600e |
Excisional biopsy | Dabrafenib Trametinib |
Dabrafenib (150mg twice daily) Trametinib (2mg daily) |
Reduction of lymph node metastasis activity | Fever |
| (Pinto Torres et al., 2017) | 59y, Female | Metastatic Co-M (Oropharyngeal wall) BRAF mutation v600 |
Excision | Vemurafenib | 960mg twice daily | Full symptomatic recovery after 1 month | Arthralgia, diarrhoea, skin rash (dose was reduced to 480mg twice daily) |
| Study | Patient | Co-M | Primary treatment | Agent used | Dosage | Outcome | Adverse reactons |
|---|---|---|---|---|---|---|---|
| Indicated for primary CoM | |||||||
| (Finger & Pavlick, 2019) | 94y, Female | Bulbar to eyelid | None (rejected exenteration) | First – Pembrolizumab Second – Pembrolizumab and ipilimumab |
Pembrolizumab – 200mg Ipilimumab – 1mg/kg |
Progression | None reported |
| (Finger & Pavlick, 2019) | 76y, Male | Recurrence. Cornea to eyelid |
Local treatments and topical interferon-alpha chemotherapy | First – ipilimumab Second – Pembrolizumab Third – Pembrolizumab and IFN-alpha |
Pembrolizumab – 2mg/kg | Ipilimumab – no response Pembrolizumab – minimal response then complete with IFN-alpha |
Ipilimumab - Adrenal insufficiency Pembrolizumab – Dermatitis |
| (Finger & Pavlick, 2019) | 84y, Female | Recurrence. Cornea to eyelid | Excision Cryotherapy Topical mitomycin Eye plaque brachytherapy |
First – Pembrolizumab Second – Pembrolizumab and ipilimumab Third – Pembrolizumab and ipilimumab and IFN-alpha |
Pembrolizumab – 200mg Ipilimumab – 1mg/kg IFN-alpha – 3 million units per eyelid |
Pembrolizumab – minimal success Pembrolizumab and ipilimumab – progression |
None reported |
| (Hong et al., 2021) | 53y, Female | Bulbar to tarsal | None | Pembrolizumab | 200mg | Complete reduction o pigment and disease free 12 months of follow up | Cutaneous pruritus |
| Indicated for metastatic disease | |||||||
| (Pinto Torres et al., 2017) | 51y, Male | Co-M recurrence with metastasis (lymph) No BRAF mutation |
Excision Lymphadenectomy |
Pembrolizumab | 2mg/kg every 3 weeks | Complete resolution of subcutaneous lesions | None noted, patient on complete remission |
| (Sagiv et al., 2018) | 68y, Female | Co-M recurrence Metastasis – lung BRAF v600e mutation |
Resection Topical mitomycin C Exenteration, sentinel lymph node biopsy |
First – Pembrolizumab Second – Ipilimumab and dacarbazine |
Pembrolizumab 2mg/kg every 3 weeks Ipilimumab – 3mg/kg Dacarbazine – 800-1000mg/m^2 |
Pembrolizumab – stable at 6 months | Ipilimumab and dacarbazine - hepatotoxicity |
| (Sagiv et al., 2018) | 58y, Female | Co-M recurrence to orbit Metastasis – lung and liver |
Multiple resections Orbital exenteration |
nivolumab | 3mg/kg every 2 weeks | Complete resolution or orbit and metastasis lesions | Elevated liver enzymes |
| (Sagiv et al., 2018) | 28y, Female | Co-M recurrence Metastasis – breast, lung and bone |
Excision Cryotherapy Topical mitomycin C |
nivolumab | 3mg/kg every 2 weeks | Complete resolution | None reported |
| (Sagiv et al., 2018) | 47y, Female | Co-M recurrence Metastasis – lung |
Excision Cryotherapy Radiotherapy Topical interferon Mitomycin C |
nivolumab | 3mg/kg every 2 weeks | Resolution of lung metastasis and free from disease 7 months after nivolumab | Diarrhoea |
| (Sagiv et al., 2018) | 74y, Male | Co-M recurrence Metastasis – lung |
Multiple excision | nivolumab | 3mg/kg every 2 weeks | Decrease in tumour size Disease free 1 month after nivolumab |
Colitis |
| (Chaves et al., 2018) | 72y, Male | Recurrent Co-M Metastasis – Lung |
Debulking and sentinel lymph node biopsy Radioactive iodine 125 |
Ipilimumab | 3mg/kg every 3 weeks | Satisfactory response to treatment and excellent local tumour control | Mild fatigue |
| (Finger & Pavlick, 2019) | 72y, Female | Epibulbar BRAF v600k Metastasis – liver, lung, bone, skin, lymph node |
Local excision and topical chemotherapy | Ipilimumab and nivolumab | Ipilimumab – 3mg/kg Nivolumab – 1mg/kg |
Resolution of subcutaneous nodules Reduction of systemic tumour burden |
Hepatotoxicity Colitis |
| (Finger & Pavlick, 2019) | 76y, Female | NRAS mutation Metastasis – lymph, skin |
Excision Cryotherapy Topical mitomycin chemotherapy |
First – ipilimumab Second – ipilimumab Third - Pembrolizumab |
Ipilimumab – 3mg/kg Pembrolizumab – 200mg |
Ipilimumab – new skin metastases and lymph metastases | None reported |
| (Kiyohara et al., 2020) | 71y, Male | Co-M recurrence BRAF v600e Metastasis – bone and liver |
Excision Cryotherapy Vemurafenib |
Nivolumab | Died 24 months after combined therapy | None reported | |
| (Hong et al., 2021) | 66y, Male | Fornix and orbit Metastasis – lung and liver |
None | Ipilimumab and nivolumab | No dose mentioned | Resolution of lesion and good response to mets | Pituitary failure |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





