Submitted:
22 July 2024
Posted:
22 July 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Methods
Study Population
Primary Exposure and Outcome
Primary Outcome
Covariates
Analysis
Results
Discussion
Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Saigal CS, Joyce G, Timilsina AR. Direct and indirect costs of nephrolithiasis in an employed population: opportunity for disease management? Kidney Int 2005;68:1808-14.
- Borghi L, Meschi T, Amato F, Briganti A, Novarini A, Giannini A. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: a 5-year randomized prospective study. J Urol 1996;155:839-43.
- Curhan GC, Willett WC, Rimm EB, Spiegelman D, Stampfer MJ. Prospective study of beverage use and the risk of kidney stones. Am J Epidemiol 1996;143:240-7.
- Xu C, Zhang C, Wang XL, et al. Self-Fluid Management in Prevention of Kidney Stones: A PRISMA-Compliant Systematic Review and Dose-Response Meta-Analysis of Observational Studies. Medicine (Baltimore) 2015;94:e1042.
- García-Sanchez A, Gonzalez-Calvin JL, Diez-Ruiz A, Casals JL, Gallego-Rojo F, Salvatierra D. Effect of acute alcohol ingestion on mineral metabolism and osteoblastic function. Alcohol Alcohol 1995;30:449-53.
- Perry HM, 3rd, Horowitz M, Fleming S, et al. The effects of season and alcohol intake on mineral metabolism in men. Alcohol Clin Exp Res 1999;23:214-9.
- De Marchi S, Cecchin E, Basile A, Bertotti A, Nardini R, Bartoli E. Renal tubular dysfunction in chronic alcohol abuse--effects of abstinence. N Engl J Med 1993;329:1927-34.
- Lieber CS, Jones DP, Losowsky MS, Davidson CS. Interrelation of uric acid and ethanol metabolism in man. J Clin Invest 1962;41:1863-70.
- Maclachlan MJ, Rodnan GP. Effect of food, fast and alcohol on serum uric acid and acute attacks of gout. Am J Med 1967;42:38-57.
- Faller J, Fox IH. Ethanol-induced hyperuricemia: evidence for increased urate production by activation of adenine nucleotide turnover. N Engl J Med 1982;307:1598-602.
- Gibson T, Rodgers AV, Simmonds HA, Toseland P. Beer drinking and its effect on uric acid. Br J Rheumatol 1984;23:203-9.
- McCollister RJ, Flink EB, Lewis MD. Urinary Excretion of Magnesium in Man Following the Ingestion of Ethanol. The American Journal of Clinical Nutrition 1963;12:415-20.
- Rylander R, Mégevand Y, Lasserre B, Amstutz W, Granbom S. Moderate alcohol consumption and urinary excretion of magnesium and calcium. Scand J Clin Lab Invest 2001;61:401-5.
- Barker ES, Elkinton JR, Clark JK. Studies of the renal excretion of magnesium in man. J Clin Invest 1959;38:1733-45.
- Kalbfleisch JM, Lindeman RD, Ginn HE, Smith WO. EFFECTS OF ETHANOL ADMINISTRATION ON URINARY EXCRETION OF MAGNESIUM AND OTHER ELECTROLYTES IN ALCOHOLIC AND NORMAL SUBJECTS. J Clin Invest 1963;42:1471-5.
- Eggleton, MG. The diuretic action of alcohol in man. J Physiol 1942;101:172-91.
- Strauss MB, Rosenbaum JD, Nelson WP, 3rd. The effect of alcohol on the renal excretion of water and electrolyte. J Clin Invest 1950;29:1053-8.
- Rubini ME, Kleeman CR, Lamdin E. Studies on alcohol diuresis. I. The effect of ethyl alcohol ingestion on water, electrolyte and acid-base metabolism. J Clin Invest 1955;34:439-47.
- Jones, AW. Excretion of alcohol in urine and diuresis in healthy men in relation to their age, the dose administered and the time after drinking. Forensic Sci Int 1990;45:217-24.
- Hirvonen T, Pietinen P, Virtanen M, Albanes D, Virtamo J. Nutrient intake and use of beverages and the risk of kidney stones among male smokers. Am J Epidemiol 1999;150:187-94.
- Wang H, Fan J, Yu C, et al. Consumption of Tea, Alcohol, and Fruits and Risk of Kidney Stones: A Prospective Cohort Study in 0.5 Million Chinese Adults. Nutrients 2021;13:1119.
- Krieger JN, Kronmal RA, Coxon V, Wortley P, Thompson L, Sherrard DJ. Dietary and behavioral risk factors for urolithiasis: potential implications for prevention. Am J Kidney Dis 1996;28:195-201.
- Shuster J, Finlayson B, Scheaffer RL, Sierakowski R, Zoltek J, Dzegede S. Primary liquid intake and urinary stone disease. J Chronic Dis 1985;38:907-14.
- Ferraro PM, Taylor EN, Gambaro G, Curhan GC. Soda and other beverages and the risk of kidney stones. Clin J Am Soc Nephrol 2013;8:1389-95.
- Goldfarb DS, Fischer ME, Keich Y, Goldberg J. A twin study of genetic and dietary influences on nephrolithiasis: a report from the Vietnam Era Twin (VET) Registry. Kidney Int 2005;67:1053-61.
- Liu CC, Huang SP, Wu WJ, et al. The impact of cigarette smoking, alcohol drinking and betel quid chewing on the risk of calcium urolithiasis. Ann Epidemiol 2009;19:539-45.
- Zhou Z, Huang Z, Ai G, Guo X, Zeng G, Zhu W. Association between alcohol consumption and kidney stones in American adults: 2007-2016 NHANES. Front Public Health 2023;11:1156097.
- CDC Alcohol Use-About Standard Drink Sizes. 2024. (Accessed 6/27/24, 2024, at https://www.cdc.gov/alcohol/standard-drink-sizes/index.html.
- Orsini N, Greenland S. A procedure to tabulate and plot results after flexible modeling of a quantitative covariate. Stata Journal 2011;11:1-29.
- Royston P, Sauerbrei W, Becher H. Modelling continuous exposures with a 'spike' at zero: a new procedure based on fractional polynomials. Stat Med 2010;29:1219-27.
- Grases F, Rodriguez A, Costa-Bauza A. Efficacy of Mixtures of Magnesium, Citrate and Phytate as Calcium Oxalate Crystallization Inhibitors in Urine. J Urol 2015;194:812-9.
- Lieske JC, Farell G, Deganello S. The effect of ions at the surface of calcium oxalate monohydrate crystals on cell-crystal interactions. Urol Res 2004;32:117-23.
- Shringi S, Raker CA, Tang J. Dietary Magnesium Intake and Kidney Stone: The National Health and Nutrition Examination Survey 2011-2018. R I Med J (2013) 2023;106:20-5.
- Bird ED, Thomas WC. Effect of Various Metals on Mineralization in vitro. Proceedings of the Society for Experimental Biology and Medicine 1963;112:640-3.
- Sutor, DJ. Growth studies of calcium oxalate in the presence of various ions and compounds. Br J Urol 1969;41:171-8.
- François B, Cahen R, Pascal B. Inhibitors of urinary stone formation in 40 recurrent stone formers. Br J Urol 1986;58:479-83.
- Atakan IH, Kaplan M, Seren G, Aktoz T, Gül H, Inci O. Serum, urinary and stone zinc, iron, magnesium and copper levels in idiopathic calcium oxalate stone patients. Int Urol Nephrol 2007;39:351-6.
- Tang J, McFann K, Chonchol M. Dietary Zinc Intake and Kidney Stone Formation: Evaluation of NHANES III. American Journal of Nephrology 2012;36:549-53.
- Chavassieux P, Serre CM, Vergnaud P, Delmas PD, Meunier PJ. In vitro evaluation of dose-effects of ethanol on human osteoblastic cells. Bone Miner 1993;22:95-103.
- Dai J, Lin D, Zhang J, et al. Chronic alcohol ingestion induces osteoclastogenesis and bone loss through IL-6 in mice. J Clin Invest 2000;106:887-95.
- Laitinen K, Tähtelä R, Välimäki M. The dose-dependency of alcohol-induced hypoparathyroidism, hypercalciuria, and hypermagnesuria. Bone Miner 1992;19:75-83.
- Ka T, Yamamoto T, Moriwaki Y, et al. Effect of exercise and beer on the plasma concentration and urinary excretion of purine bases. J Rheumatol 2003;30:1036-42.
- Moriwaki Y, Ka T, Takahashi S, Tsutsumi Z, Yamamoto T. Effect of Beer Ingestion on the Plasma Concentrations and Urinary Excretion of Purine Bases: One-Month Study. Nucleosides, Nucleotides & Nucleic Acids 2006;25:1083-5.
- Ka T, Moriwaki Y, Inokuchi T, et al. Effects of allopurinol on beer-induced increases in plasma concentrations and urinary excretion of purine bases (uric acid, hypoxanthine, and xanthine). Horm Metab Res 2006;38:188-92.
- Li Y, Pan J, Zhang Y, et al. Effects of small molecules water that may retard kidney stone formation. Int Urol Nephrol 2018;50:225-30.
- Ferraro PM, Baccaro R, Baroni S, et al. Effect of water composition and timing of ingestion on urinary lithogenic profile in healthy volunteers: a randomized crossover trial. J Nephrol 2021;34:875-81.
- Wang JS, Chiang HY, Chen HL, Flores M, Navas-Acien A, Kuo CC. Association of water intake and hydration status with risk of kidney stone formation based on NHANES 2009-2012 cycles. Public Health Nutr 2022;25:2403-14.
- Bao Y, Tu X, Wei Q. Water for preventing urinary stones. Cochrane Database of Systematic Reviews 2020.
- Díaz AB, Durán-Guerrero E, Lasanta C, Castro R. From the Raw Materials to the Bottled Product: Influence of the Entire Production Process on the Organoleptic Profile of Industrial Beers. Foods 2022;11.
- McDonald JT, Margen S. Wine versus ethanol in human nutrition. III. Calcium, phosphorous, and magnesium balance. Am J Clin Nutr 1979;32:823-33.
- McDonald JT, Margen S. Wine versus ethanol in human nutrition. IV. Zinc balance. Am J Clin Nutr 1980;33:1096-102.
- Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Beverage use and risk for kidney stones in women. Ann Intern Med 1998;128:534-40.
- Serio F, Imbriani G, Acito M, et al. Moderate red wine intake and cardiovascular health protection: a literature review. Food Funct 2023;14:6346-62.
- Fukui S, Okada M, Rahman M, et al. Differences in the Association Between Alcoholic Beverage Type and Serum Urate Levels Using Standardized Ethanol Content. JAMA Netw Open 2023;6:e233398.
- Taivainen H, Laitinen K, Tähtelä R, Kilanmaa K, Välimäki MJ. Role of plasma vasopressin in changes of water balance accompanying acute alcohol intoxication. Alcohol Clin Exp Res 1995;19:759-62.
- Helderman JH, Vestal RE, Rowe JW, Tobin JD, Andres R, Robertson GL. The response of arginine vasopressin to intravenous ethanol and hypertonic saline in man: the impact of aging. J Gerontol 1978;33:39-47.

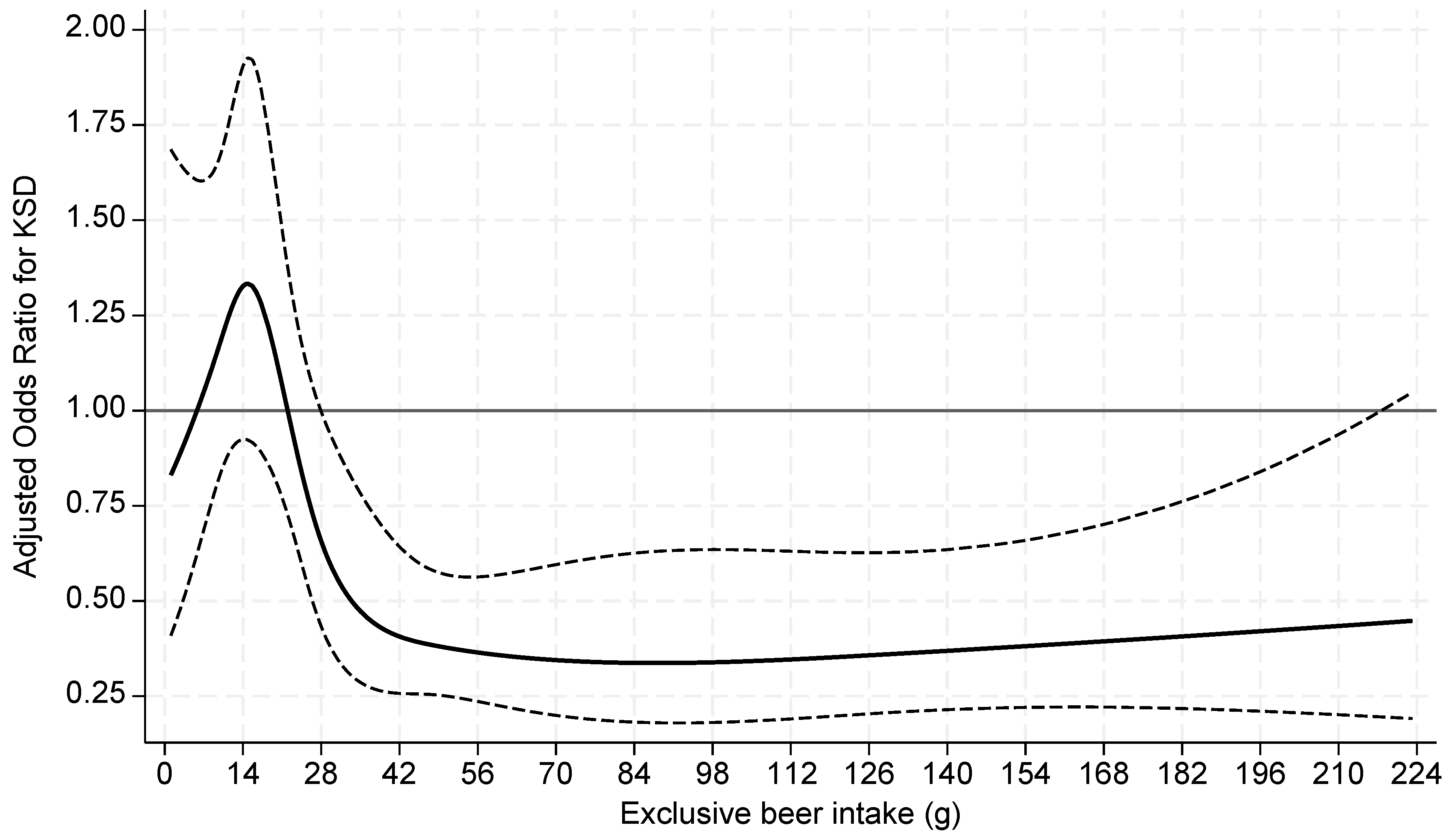
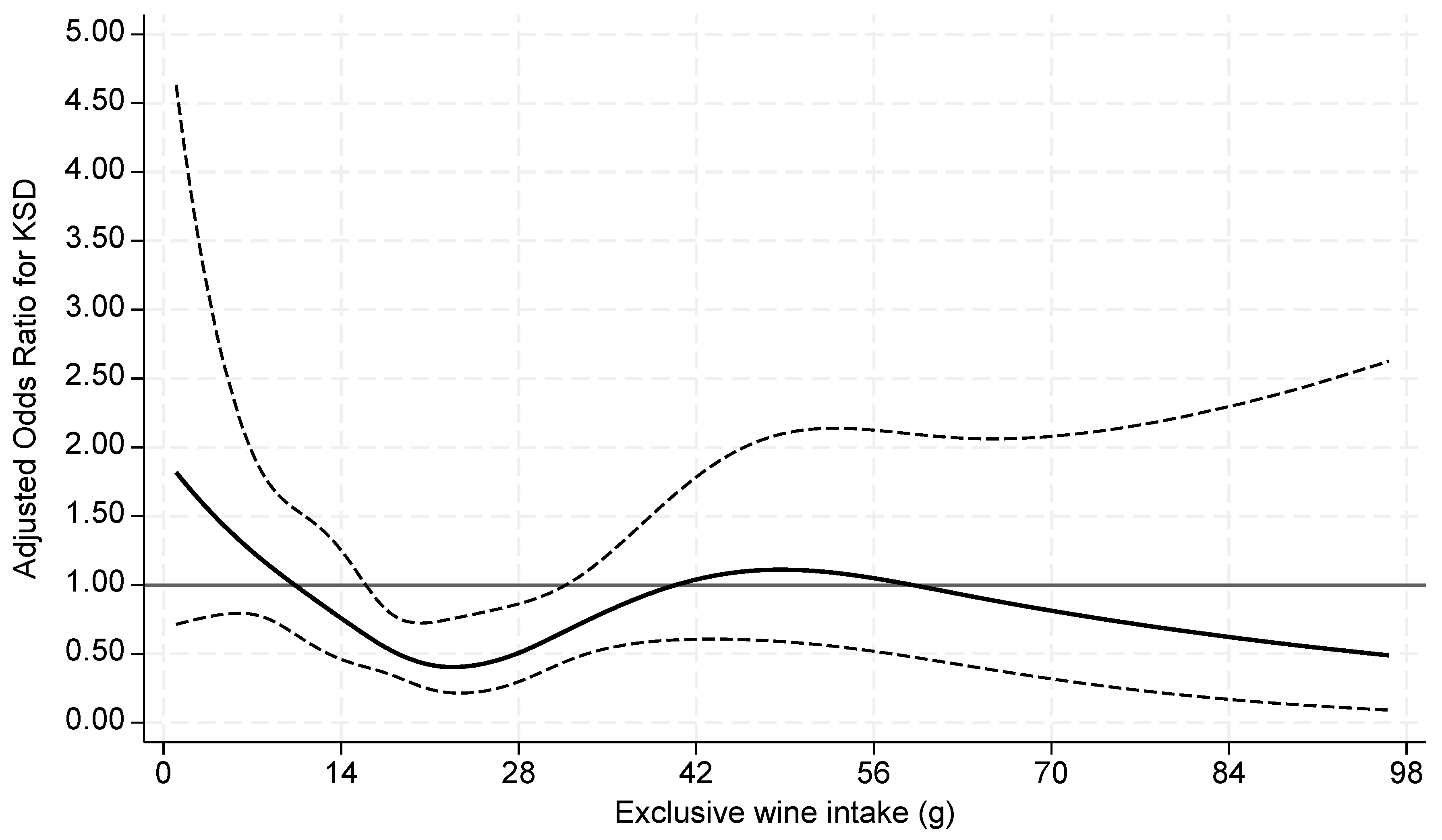
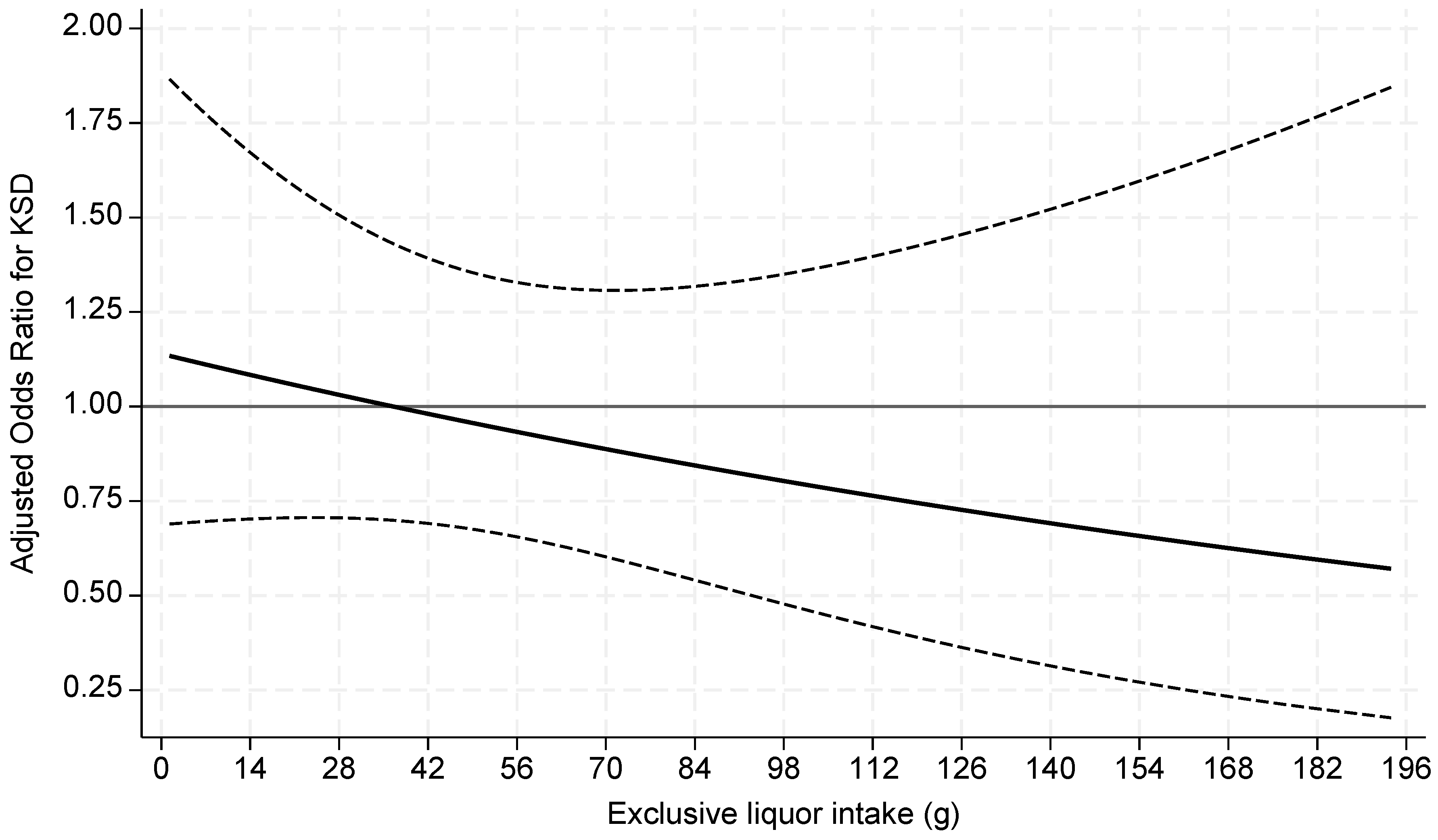
| KS Former | Non-KS Former | p value | |
|---|---|---|---|
| Total n, unweighted | 9.7 (2,840) | 90.3 (26,844) | |
| Male sex | 54.6 (1,571) | 47.4 (12,865) | <0.001 |
| Age (y) | 53.7 ± 0.38 | 46.8 ± 0.26 | <0.001 |
| Race | <0.001 | ||
| Non-Hispanic White | 76.2 (1,553) | 65.3 (10,851) | |
| Non-Hispanic Black | 5.9 (376) | 11.8 (5,995) | |
| Hispanic/Latino | 11.7 (690) | 14.7 (6,844) | |
| Non-Hispanic other | 6.1 (221) | 8.2 (3,154) | |
| BMI (kg/m2) | <0.001 | ||
| <25.0 | 19.8 (542) | 30.2 (7,803) | |
| 25.0-<30.0 | 32.9 (962) | 32.8 (8,784) | |
| 30.0+ | 47.2 (1,336) | 37.0 (10,257) | |
| History of diabetes | 22.4 (736) | 10.8 (3,895) | <0.001 |
| History of hypertension | 48.7 (1,487) | 32.3 (9,841) | <0.001 |
| Thiazide diuretic use | 12.6 (377) | 7.8 (2,498) | <0.001 |
| Smoking status | <0.001 | ||
| Never | 49.8 (1,392) | 56.2 (15,104) | |
| Former | 30.6 (884) | 24.0 (6,267) | |
| Current | 19.6 (564) | 19.8 (5,473) | |
| Total calories (kcal) | 2,122.9 ± 28.7 | 2,142.9 ± 8.9 | 0.5 |
| Protein intake (g) | 80.8 ± 1.4 | 82.8 ± 0.42 | 0.16 |
| Dietary sodium (mg) | 3,534.0 ± 54.6 | 3,539 ± 15.9 | 0.93 |
| Dietary potassium (mg) | 2,644.0 ± 38.3 | 2,690.2 ± 15.5 | 0.22 |
| Dietary calcium (mg) | 934.3 ± 15.3 | 973.7 ± 6.6 | 0.02 |
| Total fluid intake, excluding alcohol (g) | 2,905.0 ± 35.4 | 2,885.9 ± 20.0 | 0.58 |
| Alcohol drinking status | 0.002 | ||
| Never | 12.3 (442) | 13.6 (4,638) | |
| Former (0 drinks in past year) | 9.7 (311) | 6.9 (2,221) | |
| Current (>0 drinks in past year) | 78.1 (2,087) | 79.6 (19,985) | |
| Type of alcohol, if any | 0.01 | ||
| Beer only | 43.8 (235) | 43.5 (2,828) | |
| Wine only | 22.5 (103) | 23.3 (1,209) | |
| Liquor only | 24.8 (110) | 17.9 (1,103) | |
| Other/combination | 8.9 (69) | 15.3 (946) |
| Unadjusted Model | Adjusted Model 1 | Adjusted Model 2 | Adjusted Model 3 | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Never/Currently none | REF | REF | REF | REF | ||||
| Beer only | 0.79 (0.64-0.97) | 0.02 | 0.76 (0.62-0.94) | 0.01 | 0.79 (0.64-0.97) | 0.03 | 0.76 (0.61-0.94) | 0.01 |
| Wine only | 0.75 (0.58-0.99) | 0.04 | 0.64 (0.49-0.84) | 0.001 | 0.74 (0.57-0.96) | 0.02 | 0.75 (0.59-0.96) | 0.03 |
| Liquor only | 1.08 (0.77-1.52) | 0.63 | 1.04 (0.74-1.47) | 0.82 | 1.05 (0.75-1.49) | 0.76 | 0.99 (0.69-1.42) | 0.97 |
| Unadjusted Model | Adjusted Model 1 | Adjusted Model 2 | Adjusted Model 3 | |||||
|---|---|---|---|---|---|---|---|---|
| Beer | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value |
| 0-<1g | REF | REF | REF | REF | ||||
| 1-≤ 14g | 1.46 (1.00-2.15) | 0.05 | 1.35 (0.93-1.96) | 0.11 | 1.45 (1.00-2.12) | 0.05 | 1.41 (0.97-2.05) | 0.07 |
| >14-28g | 0.67 (0.44-1.01) | 0.06 | 0.65 (0.43-0.99) | 0.04 | 0.66 (0.43-1.02) | 0.06 | 0.65 (0.42-1.00) | 0.05 |
| >28-56g | 0.60 (0.40-0.91) | 0.02 | 0.61 (0.40-0.92) | 0.02 | 0.64 (0.41-0.98) | 0.04 | 0.60 (0.39-0.93) | 0.02 |
| >56g | 0.39 (0.24-0.63) | <0.001 | 0.38 (0.24-0.62) | <0.001 | 0.38 (0.24-0.62) | <0.001 | 0.34 (0.20-0.57) | <0.001 |
| Unadjusted Model | Adjusted Model 1 | Adjusted Model 2 | Adjusted Model 3 | |||||
|---|---|---|---|---|---|---|---|---|
| Wine | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value |
| 0-<1g | REF | REF | REF | REF | ||||
| 1-≤ 14g | 0.98 (0.62-1.53) | 0.91 | 0.90 (0.56-1.44) | 0.66 | 1.10 (0.69-1.77) | 0.68 | 1.14 (0.72-1.83) | 0.57 |
| >14-28g | 0.52 (0.35-0.77) | 0.001 | 0.43 (0.29-0.64) | <0.001 | 0.53 (0.36-0.78) | 0.002 | 0.54 (0.36-0.81) | 0.003 |
| >28g | 0.86 (0.52-1.44) | 0.56 | 0.77 (0.47-1.27) | 0.31 | 0.87 (0.54-1.4) | 0.56 | 0.85 (0.54-1.33) | 0.47 |
| Unadjusted Model | Adjusted Model 1 | Adjusted Model 2 | Adjusted Model 3 | |||||
|---|---|---|---|---|---|---|---|---|
| Liquor | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value |
| 0-<1g | REF | REF | REF | REF | ||||
| 1-≤ 28g | 1.18 (0.70-1.99) | 0.53 | 1.15 (0.67-1.96) | 0.62 | 1.20 (0.71-2.02) | 0.49 | 1.16 (0.69-1.97) | 0.58 |
| >28g | 0.97 (0.65-1.44) | 0.87 | 0.94 (0.62-1.40) | 0.75 | 0.94 (0.62-1.43) | 0.79 | 0.85 (0.56-1.30) | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





