Introduction
The Vestibular System
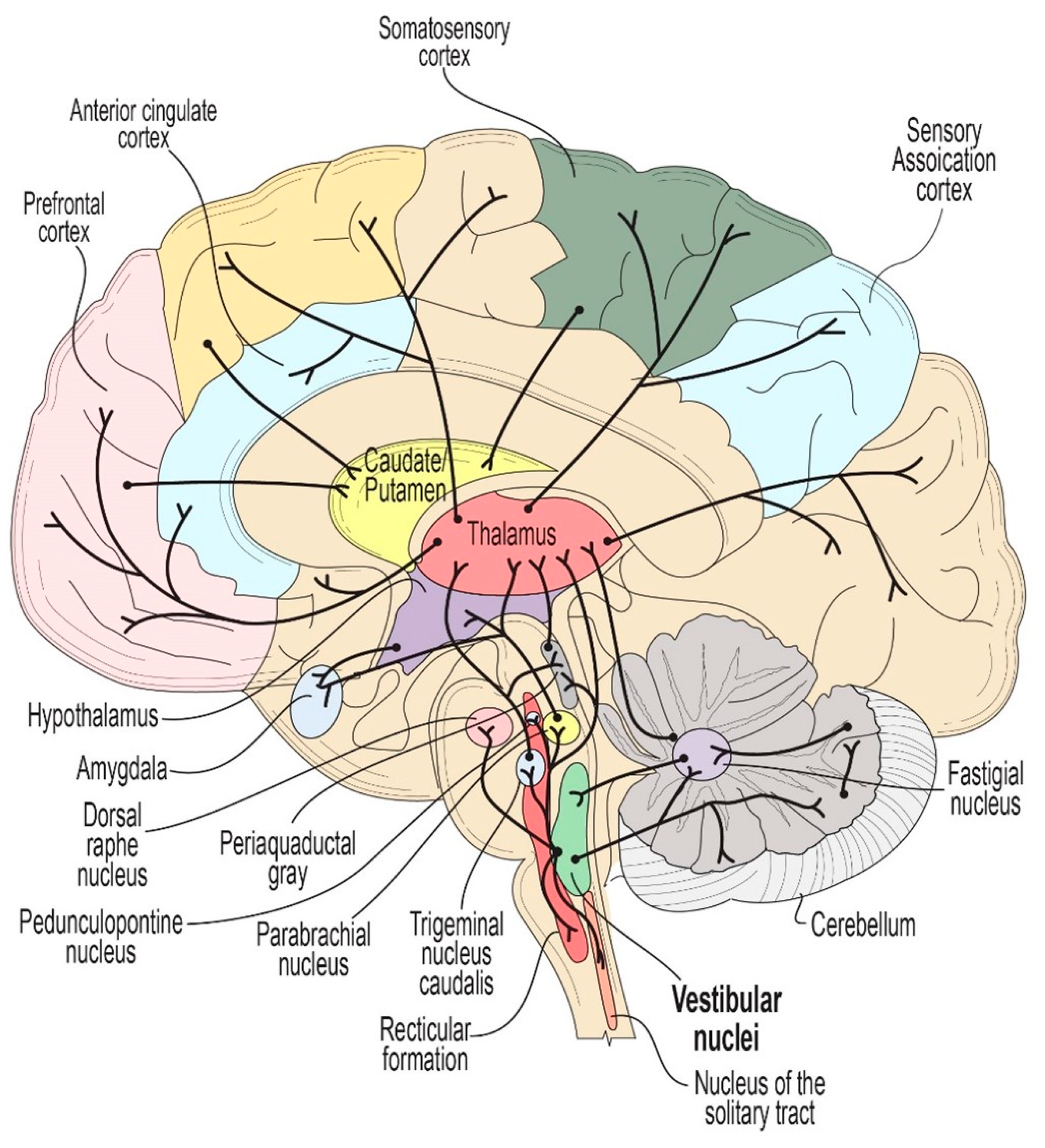
The vestibular system (VS) is a sensory network made up of both peripheral and central nervous system components, responsible for maintaining balance and coordinating movement. The VS has extensive connections throughout the brainstem and also regulates activity in both midbrain and higher corticial regions via a vast network of polysynaptic relays. Motion-induced stimulation of the VS initially involves the fluid within the three semi-circular canals in each ear and two otolith structures (utricle & saccule). When the head moves, the resultant movement of the fluid within the canals will lead to the displacement of hair cells within the cupola and otolithic organs, which translate mechanical movements to electrical signals [
1]. This process modulates the tonic firing rate of the vestibulocochlear nerve, leading to an increase or decrease in firing rate depending on the direction of the fluid motion. These changes in firing rate subsequently modulate the activity of neurons in brainstem vestibular nuclei, as well as a multitude of connected regions in the brainstem, cerebellum, thalamus and cortex via extensive upward and lateral projections (
Figure 1).
Non-invasive techniques can also be used to modulate activity of the vestibular system through either electrical galvanic vestibular stimulation (GVS) or thermal stimulation (caloric vestibular stimulation or CVS) [
2]. Both GVS and CVS can modulate activity propagating through endogenous networks of several brainstem nuclei with projections to the midbrain, thalamus, and cortex [
3] . As a result, noninvasive vestibular stimulation is capable of modulating neuronal activity broadly throughout the brain, as has been evidenced through several fMRI studies of GVS and CVS [
4,
5]. The means to modulate activity across broad regions of the brain separates vestibular stimulation from other conventional forms of non-invasive neurostimulation like transcranial magnetic stimulation (TMS) and transcranial direct or alternating current stimulation (tDCS and tACS, respectively), which offer a top-down approach and stimulate a focused and narrow target, primarily limited to the neocortex [
6]. Furthermore, because GVS and CVS act through a sensory system, the applied stimulus is modified by the sensory organ in a way that is matched to the native neuronal dynamics [
3,
4,
7] whereas with other forms of non-invasive neurostimulation including TMS, tDCS, and tACS, there is a mismatch between an applied tACS signal and endogenous neuronal network frequencies [
2].
Effects of vestibular stimulation may also involve several neurotransmitters including serotonin, histamine, acetylcholine and gamma aminobutyric acid (GABA) [
8,
9,
10,
11]. These neurotransmitters are closely involved in the pathophysiology of cognition and neuropsychiatric dysfunction, as well as a number of other nonmotor symptoms (NMS) of Parkinson’s disease (PD) and recently described nonmotor subtypes of PD as well as the stepped care for PD [
12,
13,
14,
15,
16]. Importantly, previous tracing studies have evidenced monosynaptic or polysynaptic connectivity of the vestibular nuclei to brain regions implicated in the motor symptoms of PD including the dorsolateral striatum (involved in bradykinesia), the pedunculopontine nucleus (PPN; involved in gait and postural stability), and the cerebellum (involved in modulating resting tremor and levodopa-induced dyskinesia) [
17,
18,
19,
20].
Moreover, the vestibular nuclei have direct and/or indirect inputs into brain regions that have been implicated in NMS of PD, including (1) depression and anxiety via the corticolimbic network (consisting of the anterior cingulate cortex, dorsolateral prefrontal cortex, amygdala, and hippocampus), the dorsal raphe nucleus and the parabrachial nucleus; (2) memory deficits and cognitive impairment via the sensory association cortices, temporal-parietal regions, peri-sylvia, PPN, and hippocampal structures; (3) autonomic dysfunction affecting both blood pressure and bladder control via the periaqueductal gray, hypothalamus, cerebellum, basal ganglia and frontal cortex; and (4) disruptions in sleep, arousal, and the experience of visual hallucinations via the PPN, dorsal raphe nucleus, and thalamus [
21]. Also of note, the vestibular nuclei have direct neural inputs to the thalamus, which regulates the functional connectivity of multiple cortical regions, contributes to most aspects of brain function, and has been implicated in several non-motor symptoms of PD [
22].
Further support for vestibular regulation of these motor and nonmotor symptoms can be derived from observations of comorbidities associated with vestibular dysfunction. For instance, one study investigating patients with chronic neurotological dysfunction, including vestibular migraine, reported that symptoms of anxiety, depression, fatigue, and daytime sleepiness frequently occurred concurrently [
23]. Additionally, a U.S. survey of patients with vestibular vertigo highlighted significant cognitive and neuropsychiatric issues [
24]. Furthermore, recent evidence suggests that vestibular dysfunction may contribute to motor-related deficits in PD, including postural instability, gait dysfunction motor phenotype, and freezing of gait [
25].
Methods for Vestibular Stimulation
Galvanic Vestibular Stimulation
Electrical stimulation, or GVS, is delivered through the application of low amplitude (< ~2 mA) transcutaneous current applied to electrodes placed on the bilateral mastoid processes to stimulate vestibular hair cells and irregularly firing vestibular afferents [
26]. There are several possible electrode configurations that can be used for GVS, including bilateral monopolar, unilateral monopolar, or bilateral bipolar configurations, though the latter is most common in clinical practice [
2]. The current applied can be direct current, alternating current, or band-limited noise waveforms. GVS works by creating a voltage bias between the two sets of vestibular organs. It is generally agreed that GVS acts on a subset of afferent neurons (about 25% of all vestibular afferents) that respond more strongly to acceleration, though the specific sites of action are still debated within the literature. Some claim that GVS primarily acts on the otoliths, while others have reported effects on the semicircular canals [
7]. Recent work by Forbes et al. indicates that both structures are likely involved [
27].
Caloric Vestibular Stimulation
Thermal stimulation or CVS is an established technique that is commonly used to clinically diagnose disorders of balance and evaluate brainstem function in the assessment of brain death [
28]. The process traditionally uses water or air irrigators to warm or cool the external auditory canal of patients, changing the density of the endolymphatic fluid in the semicircular canals and stimulating the vestibulocochlear nerves via the cupola. CVS primarily affects the horizontal semicircular canal, which protrudes into the middle ear space, and thereby, is the first structure to intersect a thermal wave formed by CVS. However, there is evidence that the other canals as well as the utricle also respond to CVS. There is some debate about the path that heat takes from the external ear canal to the inner ear, but the preponderance of evidence confirms that conduction along the petrous bone (part of the temporal bone) dominates [
28]. The warming temperature stimulation increases while cooling temperatures decrease the tonic firing rate of the vestibulocochlear nerves, which then has a “cascading” effect on all areas of the brainstem and its projections to the cortex. Despite the long-standing history of safe use in the diagnostic setting, the therapeutic potential of traditional water or air irrigation CVS methods has been hampered by challenges associated with modulating temperatures specifically or rapidly, difficulties associated with controlling dosing, and adaptation that results in response to constant-temperature CVS.
Recent advancements in CVS technology have led to the development of solid-state devices capable of delivering time-varying CVS (tvCVS) through small metal earpieces that sit inside the ear canals. The solid-state tvCVS devices allow for a continuous change of temperature for sustained modulation of vestibulocochlear nerve activity, avoiding the ciliary adaptation that occurs within minutes of constant-temperature irrigation CVS [
4]. It is also possible to modulate the time rate of change of the thermal stimulus with the solid-state device, which could limit common side effects of irrigation-based CVS, such as dizziness, nausea, and vomiting. Furthermore, the portable, headset-like design of the solid-state tvCVS device allows for at-home application of tvCVS therapy. Although early human studies with tvCVS devices have demonstrated promising outcomes for both motor symptoms and NMS of PD these benefits have yet to be evaluated in rigorous, large-scale clinical trials [
29].
Physiological Effects of Vestibular Stimulation
Several studies have demonstrated broad physiological effects of vestibular stimulation related to neurological processes throughout the brain. The use of GVS in rat models has been shown to induce hippocampal cell proliferation as well as striatal reduction in c-Fos expression and the release of serine and threonine [
30,
31,
32]. In humans, GVS treatment has been shown to effect changes in EEG patterns such as po wer spectra and P300 morphology as well as balance and postural sway [
7,
33]. Previous reports have indicated alterations in blood flow and the induction of oscillations in cerebrovascular resistance with tvCVS [
28]. It has been hypothesised that the changes in cerebral blood flow that result from coordinated tvCVS neurostimulation may enhance neuronal and cerebrovascular integrity; however, these hypotheses have not yet been tested experimentally, and the mechanism(s) of action have yet to be determined. Functional magnetic resonance imaging (fMRI ) studies also indicate that tvCVS modulates activity in several brain regions implicated in memory and cognition, including the hippocampus, cerebellum, operculum, and frontal and parietal lobules following both irrigation-based CVS and tvCVS, and therefore, may be of relevance to cognitive decline and dementia in PD [
5,
34,
35,
36].
Clinical Implications in Parkinson’s Disease and other Conditions
Galvanic Vestibular Stimulation and Parkinson’s Disease
GVS has been reported to provide a therapeutic benefit for motor aspects of PD in some animal studies and human case studies. Samoudi et al. demonstrated that noisy GVS improved rotarod performance in hemi-parkinsonian rats. They also found that GABA release was enhanced in the substantia nigra of treated rats [
37]. In humans, Kataoka et al. reported a reduction in postural instability in 3 out of 5 patients after providing a single session of direct current GVS in a small case study [
38]. In another study, Pal et al. examined anterior-posterior and side-to-side sway in PD and reported a minor benefit after subsensory, noisy GVS [
39]. A later study evaluating responses to the “pull test” in 10 PD subjects demonstrated that subsensory, noisy GVS was associated with shorter postural corrections and quicker regain of posture when pulled back unexpectedly [
11]. Other reported motor benefits of GVS include improvements in finger tapping speed and the Timed Up and Go test upon administration with direct current GVS [
40]. Case studies also suggest improved visuo-motor tracking and rapid rates of rest to active motor transitions with GVS [
41]. GVS treatments have been shown to normalize functional connectivity of the PPN in the brainstem as well as the pallidum and the inferior parietal and cerebellar cortices [
42]. These observations in motor-related neural pathways suggest that GVS treatment may lead to improvements in motor behaviour outcomes for neurodegenerative conditions such as PD. However, to date, GVS has only been delivered in clinical laboratory settings to date, limiting the translatability of GVS as a longitudinal therapy
Caloric Vestibular Stimulation and Parkinson’s Disease
The bulk of the clinical data related to vestibular stimulation as a potential treatment for PD relates to tvCVS. The first evidence to support potential efficacy of tvCVS to treat motor and nonmotor symptoms of PD came from a case study reporting that a single participant showed approximately 50% reduction in both motor symptoms and NMS associated with PD following 8 weeks of twice-daily tvCVS treatments self-administered in the home setting with a solid state device (
Figure 2).
The investigators followed up on this finding by performing a follow-on, single-site randomized controlled trial in which 46 people diagnosed with PD and taking stable doses of medications self-administered tvCVS, twice daily over a period of 8 weeks. Participants in the active tvCVS group demonstrated reduced ON-state motor signs, based upon the International Parkinson’s and Movement Disorder Society -Unified Parkinson’s Disease Rating Scale part III (95% CI for therapeutic gains between the active and control group: -13.2 to -1.8 points). Additionally, NMS burden according to the Non Motor Symptom Scale was reduced (95% CI for therapeutic gains between the active and control group: -45.9 to -13.5 points). This reduction in non-motor symptoms was driven by reductions across a broad spectrum of NMS domains including sleep/fatigue, mood/cognition, attention/memory, gastrointestinal function, urinary function, sexual function and miscellaneous NMS [
43].
Notably, these clinical benefits in both motor and non-motor domains showed evidence of persistence one month post-treatment. Based on these observations, a large-scale multicentre sham-controlled tvCVS trial called the STEM-PD trial (NCT02991430) is currently under way. Other ongoing early stage clinical trials in people with Parkinson’s are further exploring tvCVS treatments with the solid state device as possible treatments for postural instability and gait difficulties (NCT04768647) and Parkinson’s disease dementia (NCT05987540).
Conclusions
To date, all vestibular stimulation devices remain limited to investigational use for PD indications, and it is too early to draw any specific clinical conclusions pending data reporting from trials such as the STEM_PD trial.
Based on the connectivity of the vestibular nuclei with several region implicated in the emergence of NMS and some motor symptoms of PD, further investigations are invisaged for vestibular stimulation approaches such as tvCVS in the management of PD. For instance, the regulation of sensory cortices, the temporal-parietal area, the perisylvia, the PPN, and hippocampal structures by vestibular signaling suggest plausibility to evaluate vestibular stimulation as pential treatment for memory and cognitive dysfunction. Modulation of neuropsychiatric problems such as depression and anxiety can also be investigated using tvCVS as the corticolimbic network (anterior cingulate cortex, dorsolateral prefrontal cortex, amygdala, and hippocampus), the dorsal raphe nucleus, and the parabrachial nucleus are all modulated by vestibular signaling.
Via synaptic and polysynaptic relays, the vestibular nuclei are interconnected with a range of brainstem nuclei, including the serotonergic raphe nuclei and the noradrenergic locus coeruleus [
43]. These nuclei are the main structures affected by the neurodegenerative processes in PD and are thought to be involved in the recently described noradrenergic subtype of PD and both noradrenaline (NA) and 5-hydroxytryptamine (5-HT) are involved in descending pain modulatory pathway functionality [
13]. The role of tvCVS in management of central pain is therefore worthy of investigation and a proof-of-concept trial called the PavePark study is currently planned to evaluate the effect of tvCVS on descending pain pathways in PD patients with pain. Vestibular regulation of activity in the periaqueductal gray, a key area for autonomic regulation of blood pressure as well as the pathogenesis of orthostatic hypotension and urinary incontinence, suggests plausibility in investigating tvCVS to address these features of autonomic dysfunction of PD.
Author Contributions
Author contributions are as per CrediT authorship contribution statement. KPD, KRC with KA conceptualised and designed the review. MQ, LB, AP, NL did literature search and compilation of table and review of literature and references. VM, LB and all authors reviewed paper, content and provided critique.
Institutional Review Board Statement
Not required, this is a review of published data.
Data Availability Statement
All data are publicly available in published papers referenced in the paper.
Acknowledgments
Kelsey McLaughlin, Robert Black for advice and reading manuscript. Olabisi Awogbemila, Alexandra Rizos and Kings Parkinson’s centre CRN research delivery team.
Conflicts of Interest
KA is employed by Scion NeuroStim, Inc., manufacturer of a time-varying caloric vestibular stimulation device mentioned in the paper. The rest of the authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Khan, S.; Chang, R. Anatomy of the vestibular system: A review. NeuroRehabilitation. 2013, 32, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D. Caloric and galvanic vestibular stimulation for the treatment of Parkinson’s disease: rationale and prospects. Expert Rev. Med. Devices. 2021, 18, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Black, R.D.; Rogers, L.L. Sensory Neuromodulation. Front. Syst. Neurosci. 2020, 14. [Google Scholar] [CrossRef] [PubMed]
- Black, R.D.; Bell, R.P.; Riska, K.M.; et al. The Acute Effects of Time-Varying Caloric Vestibular Stimulation as Assessed With fMRI. Front. Syst. Neurosci. 2021, 15. [Google Scholar] [CrossRef] [PubMed]
- Klingner, C.M.; Volk, G.F.; Flatz, C.; et al. Components of vestibular cortical function. Behav. Brain Res. 2013, 236, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Danilov, Y.; Kaczmarek, K.; Skinner, K.; Tyler, M. Cranial Nerve Noninvasive Neuromodulation: New Approach to Neurorehabilitation. In: Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. CRC Press/Taylor & Francis, Boca Raton (FL); 201. [PubMed]
- Fitzpatrick, R.C.; Day, B.L. Probing the human vestibular system with galvanic stimulation. J. Appl. Physiol. 2004, 96, 2301–2316. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.R.; Liu, J.X.; Li, X.P.; et al. Effects of caloric vestibular stimulation on serotoninergic system in the media vestibular nuclei of guinea pigs. Chin. Med. J. (Engl) 2007, 120, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Horii, A.; Takeda, N.; Mochizuki, T.; et al. Effects of vestibular stimulation on acetylcholine release from rat hippocampus: an in vivo microdialysis study. J. Neurophysiol. 1994, 72, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Horii, A.; Takeda, N.; Matsunaga, T.; et al. Effect of Unilateral Vestibular Stimulation on Histamine Release From the Hypothalamus of Rats In Vivo. J. Neurophysiol. 1993, 70. [Google Scholar] [CrossRef]
- Samoudi, G.; Jivegard, M.; Mulavara, A.P.; Bergquist, F. Effects of stochastic vestibular galvanic stimulation and LDOPA on balance and motor symptoms in patients with Parkinson’s disease. Brain Stimul. 2015, 8, 474–480. [Google Scholar] [CrossRef]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Ray Chaudhuri, K.; Leta, V.; Bannister, K.; et al. The noradrenergic subtype of Parkinson disease: from animal models to clinical practice. Nat. Rev. Neurol. 2023, 19, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Sauerbier, A.; Jenner, P.; Todorova, A.; Chaudhuri, K.R. Non motor subtypes and Parkinson’s disease. Parkinsonism Relat. Disord. 2016, 22, S41–S46. [Google Scholar] [CrossRef] [PubMed]
- Tall, P.; Qamar, M.A.; Rosenzweig, I.; et al. The Park Sleep subtype in Parkinson’s disease: from concept to clinic. Expert Opin. Pharmacother. 2023, 24, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Popławska-Domaszewicz, K.; Falup-Pecurariu, C.; Chaudhuri, K.R. An Overview of a Stepped-care Approach to Modern Holistic and Subtype-driven Care for Parkinson’s Disease in the Clinic. TouchRev Neurol. 2024, 20, 27–32. [Google Scholar]
- Balaban, C.D.; Jacob, R.G.; Furman, J.M. Neurologic bases for comorbidity of balance disorders, anxiety disorders and migraine: Neurotherapeutic implications. Expert Rev. Neurother. 2011, 11, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.E.; Card, J.P.; Yates, B.J. Polysynaptic pathways from the vestibular nuclei to the lateral mammillary nucleus of the rat: Substrates for vestibular input to head direction cells. Exp. Brain Res. 2005, 161, 47–61. [Google Scholar] [CrossRef]
- Horowitz, S.S.; Blanchard, J.; Morin, L.P. Medial vestibular connections with the hypocretin (orexin) system. J. Comp. Neurol. 2005, 487, 127–146. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, V.; Keeser, D.; Hergenroeder, T.; et al. Structural and functional connectivity mapping of the vestibular circuitry from human brainstem to cortex. Brain Struct. Funct. 2016, 221, 1291–1308. [Google Scholar] [CrossRef]
- Hitier, M.; Besnard, S.; Smith, P.F. Vestibular pathways involved in cognition. Front. Integr. Neurosci. 2014, 8. [Google Scholar] [CrossRef]
- Dalrymple-Alford, J.; Anderson, T.; Melzer, T. Thalamus, Thalamocortical Networks, and Non-Motor Symptoms in Parkinson’s Disease. In The Cerebral Cortex and Thalamus. Mitchell, A.S., Usrey, W.M., Sherman, S.M., Oxford University Press. 2023, 722-734. [CrossRef]
- Smith, L.; Wilkinson, D.; Bodani, M.; et al. Short-term memory impairment in vestibular patients can arise independently of psychiatric impairment, fatigue, and sleeplessness. J. Neuropsychol. 2019, 13, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Bigelow, R.T.; Semenov, Y.R.; Du Lac, S.; et al. Vestibular vertigo and comorbid cognitive and psychiatric impairment: The 2008 National Health Interview Survey. J. Neurol. Neurosurg. Psychiatry. 2016, 87, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Bohnen, N.I.; Kanel, P.; van Emde Boas, M.; et al. Vestibular Sensory Conflict During Postural Control, Freezing of Gait, and Falls in Parkinson’s Disease. Mov. Disord. 2022, 37, 2257–2262. [Google Scholar] [CrossRef] [PubMed]
- Abbariki, F.; Mikhail, Y.; Hamadjida, A.; et al. Effect of galvanic vestibular stimulation applied at the onset of stance on muscular activity and gait cycle duration in healthy individuals. Front. Neural Circuits. 2023, 16, 1065647. [Google Scholar] [CrossRef] [PubMed]
- Forbes, P.A.; Kwan, A.; Mitchell, D.E.; et al. The Neural Basis for Biased Behavioral Responses Evoked by Galvanic Vestibular Stimulation in Primates. J. Neurosci. 2023, 43, 1905–1919. [Google Scholar] [CrossRef] [PubMed]
- Black, R.D.; Rogers, L.L.; Ade, K.K.; et al. Non-invasive neuromodulation using time-varying caloric vestibular stimulation. IEEE J. Transl. Eng. Health Med. 2016, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.; Podlewska, A.; Banducci, S.E.; et al. Caloric vestibular stimulation for the management of motor and non-motor symptoms in Parkinson’s disease. Parkinsonism Relat. Disord. 2019, 65, 261–266. [Google Scholar] [CrossRef]
- Shaabani, M.; Lotfi, Y.; Karimian, S.M.; et al. Short-term galvanic vestibular stimulation promotes functional recovery and neurogenesis in unilaterally labyrinthectomized rats. Brain Res. 2016, 1648, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Stiles, L.; Reynolds, J.N.; Napper, R.; et al. Single neuron activity and c-Fos expression in the rat striatum following electrical stimulation of the peripheral vestibular system. Physiol. Rep. 2018, 6. [Google Scholar] [CrossRef]
- Stiles, L.; Zheng, Y.; Smith, P.F. The effects of electrical stimulation of the peripheral vestibular system on neurochemical release in the rat striatum. PLoS ONE. 2018, 13. [Google Scholar] [CrossRef]
- Schmidt-Kassow, M.; Wilkinson, D.; Denby, E.; Ferguson, H. Synchronised vestibular signals increase the P300 event-related potential elicited by auditory oddballs. Brain Res. 2016, 1648, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, M.; Brandt, T. Functional brain imaging of peripheral and central vestibular disorders. Brain. 2008, 131, 2538–2552. [Google Scholar] [CrossRef]
- Karnath, H.O.; Dieterich, M. Spatial neglect - A vestibular disorder? Brain. 2006, 129, 293–305. [Google Scholar] [CrossRef]
- Lopez, C.; Blanke, O.; Mast, F.W. The human vestibular cortex revealed by coordinate-based activation likelihood estimation meta-analysis. Neuroscience. 2012, 212, 159–179. [Google Scholar] [CrossRef]
- Samoudi, G.; Nissbrandt, H.; Dutia, M.B.; Bergquist, F. Noisy galvanic vestibular stimulation promotes GABA release in the substantia nigra and improves locomotion in Hemiparkinsonian rats. PLoS ONE. 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Okada, Y.; Kiriyama, T.; et al. Can Postural Instability Respond to Galvanic Vestibular Stimulation in Patients with Parkinson’s Disease? J. Mov. Disord. 2016, 9, 40–43. [Google Scholar] [CrossRef]
- Pal, S.; Rosengren, S.M.; Colebatch, J.G. Stochastic galvanic vestibular stimulation produces a small reduction in sway in Parkinson's disease. J. Vestib. Res. 2009, 19, 137–142. [Google Scholar] [CrossRef]
- Khoshnam, M.; Häner, D.M.C.; Kuatsjah, E.; et al. Effects of galvanic vestibular stimulation on upper and lower extremities motor symptoms in parkinson’s disease. Front. Neurosci. 2018, 12. [Google Scholar] [CrossRef]
- Lee, S.; Smith, P.F.; Lee, W.H.; McKeown, M.J. Frequency-Specific Effects of Galvanic Vestibular Stimulation on Response-Time Performance in Parkinson’s Disease. Front. Neurol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Lee, S.; Ba, F.; et al. Galvanic Vestibular Stimulation (GVS) Augments Deficient Pedunculopontine Nucleus (PPN) Connectivity in Mild Parkinson's Disease: fMRI Effects of Different Stimuli. Front. Neurosci. 2018, 12, 101. [Google Scholar] [CrossRef]
- Wilkinson, D.; Ade, K.K.; Rogers, L.L.; et al. Preventing Episodic Migraine with Caloric Vestibular Stimulation: A Randomized Controlled Trial. Headache. 2017, 57, 1065–1087. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).