Submitted:
23 July 2024
Posted:
24 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Result and Analysis
3.1. Experiment 1
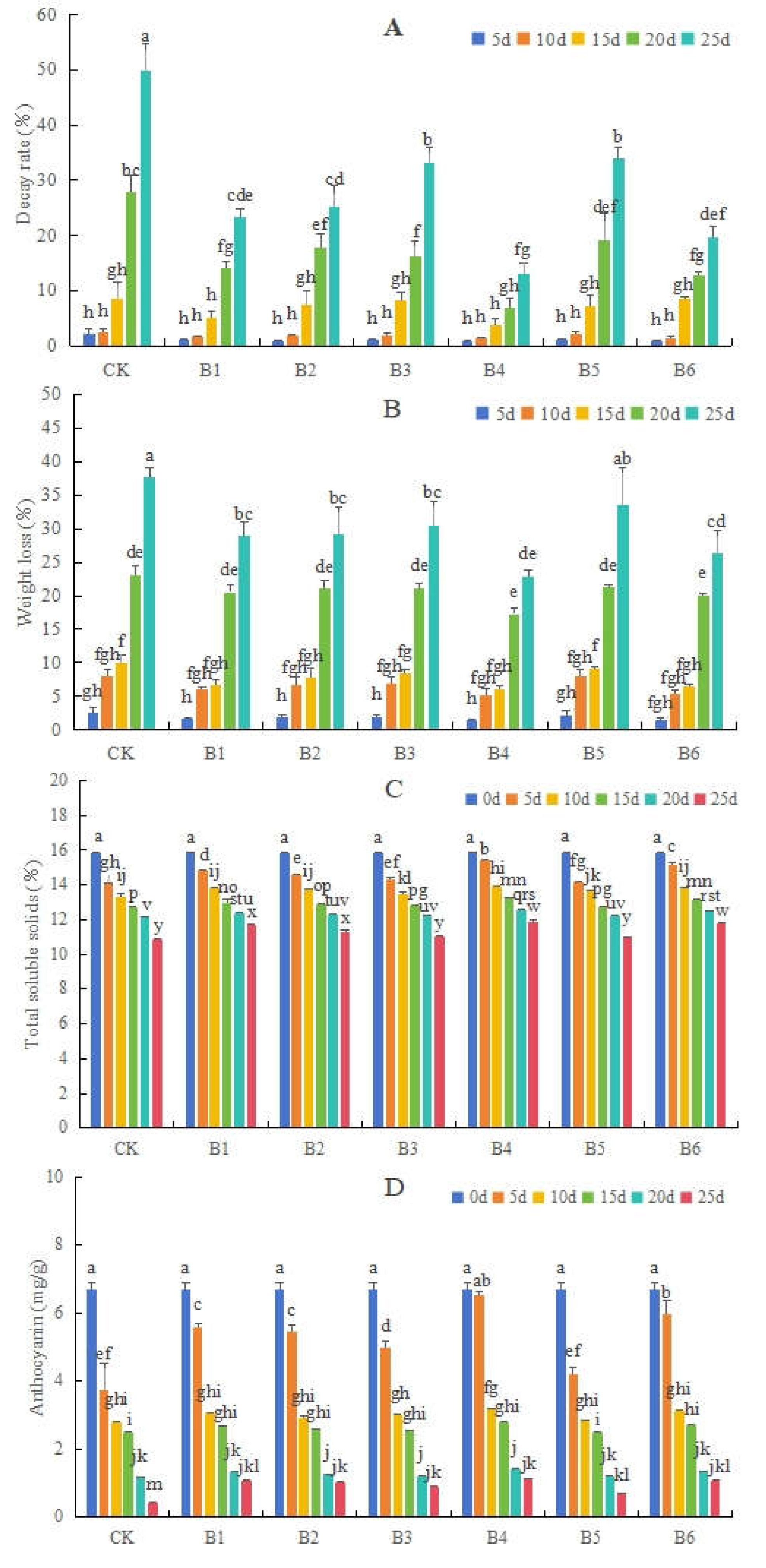
3.1.1. Effects of Different Bacillus on Decay and Weight Loss Rates, as Well as TSS and Anthocyanin Contents, in Blueberries
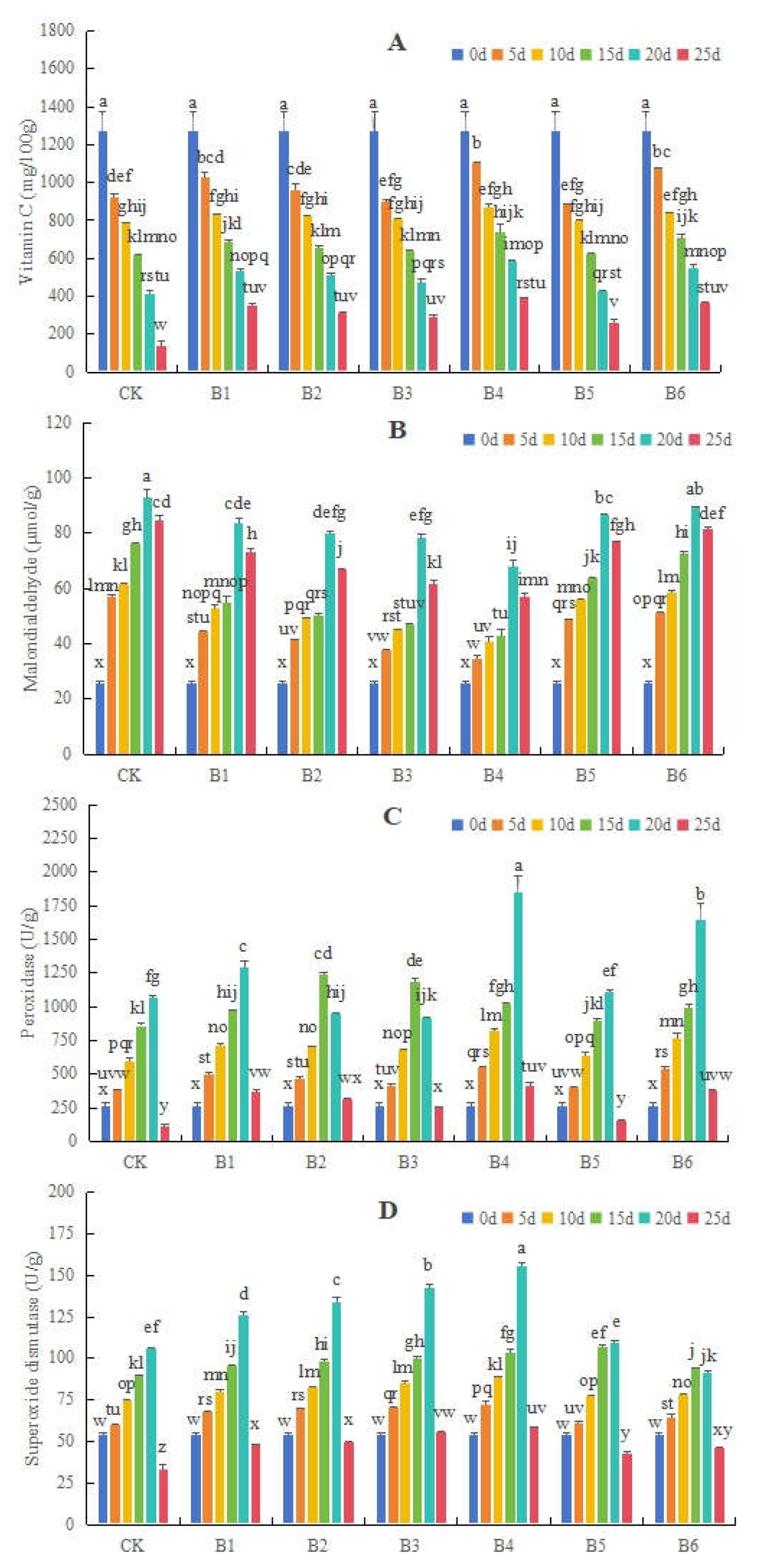
3.1.2. Effects of Different Bacillus on Vc and MDA Contents, and POD and SOD Activity Levels, in Blueberries
3.2. Experiment 2
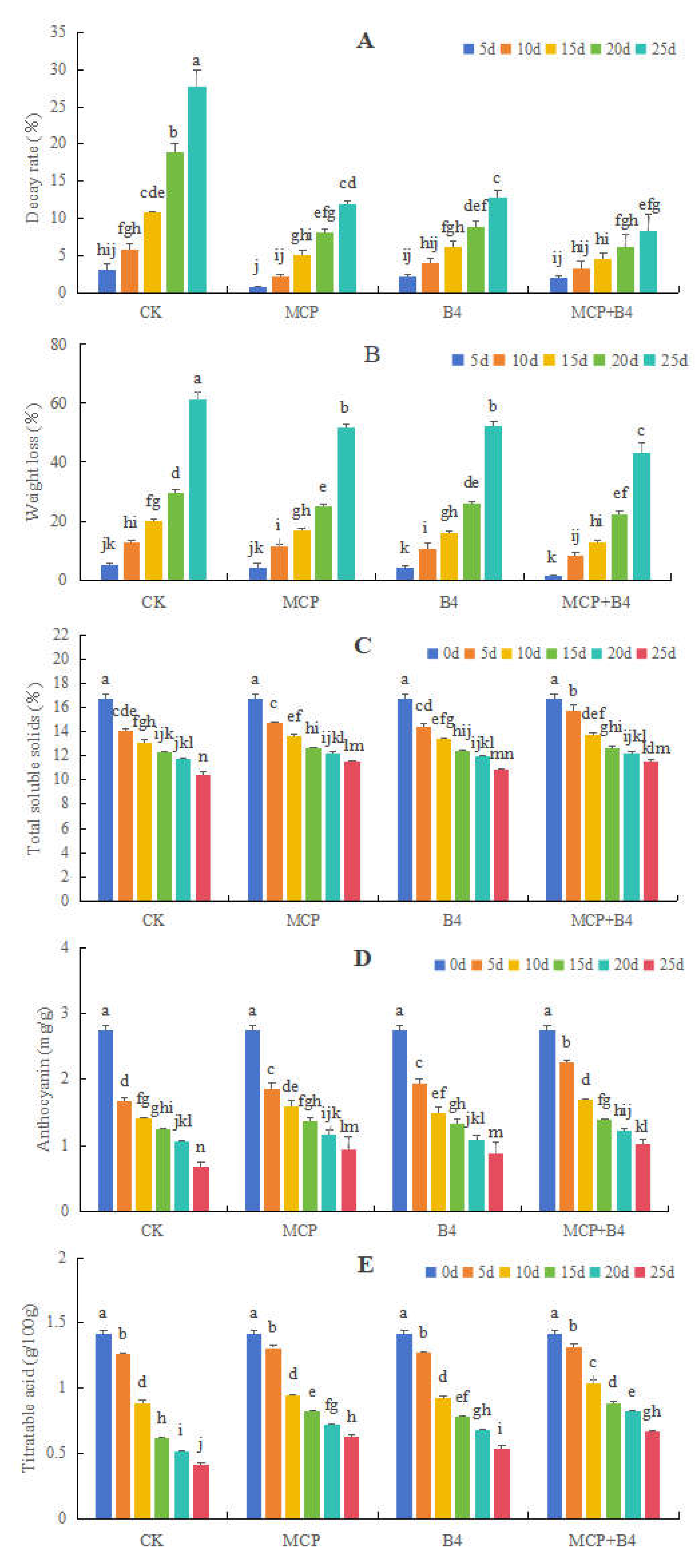
3.2.1. Effects of MCP and B4 on the Decay and Weight Loss Rates, as Well as the TSS, Anthocyanin, and TA Contents, in Blueberries
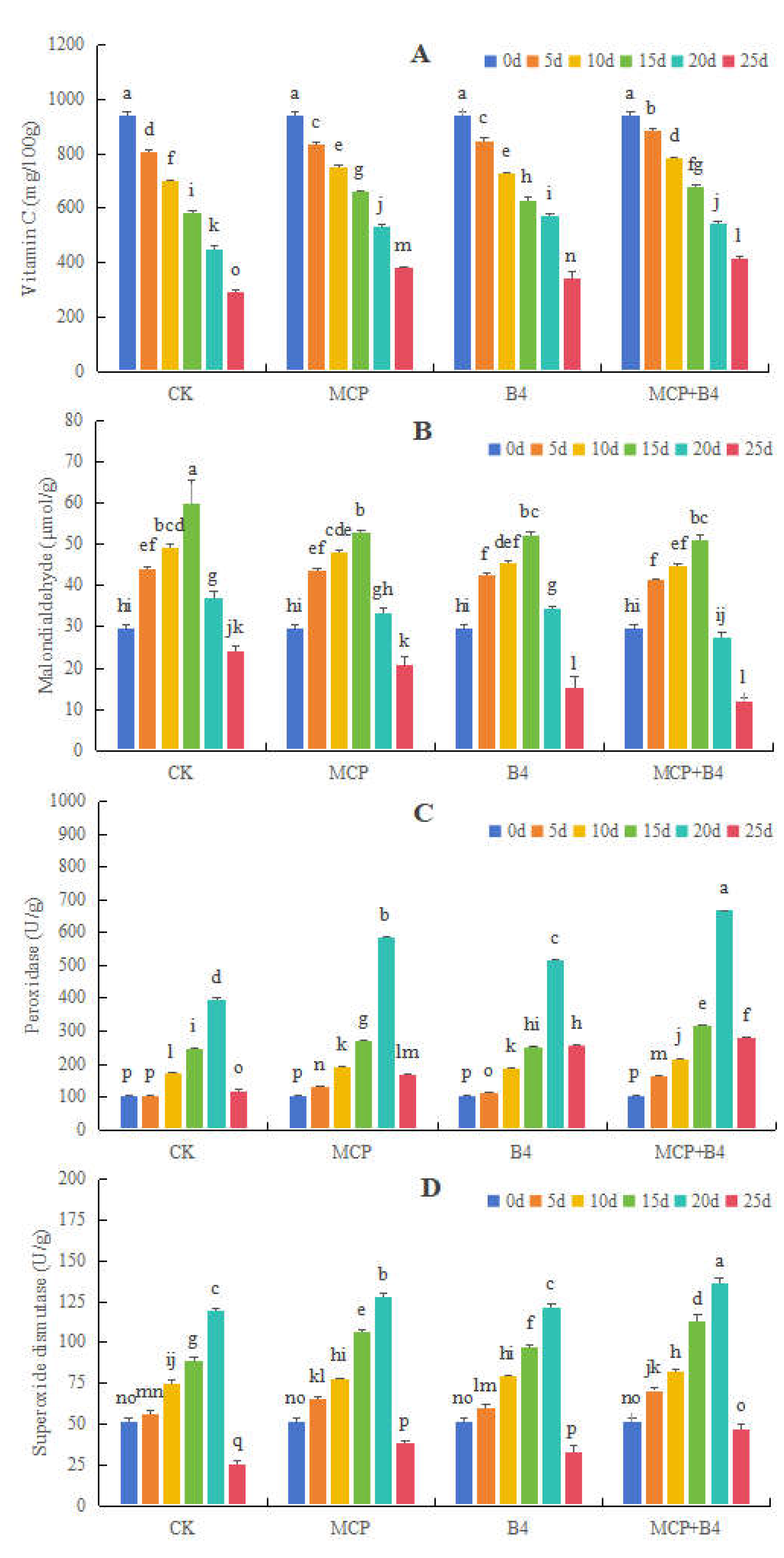
3.2.2. Effects of MCP and B4 on Vc and MDA Contents, as Well as POD and SOD Activity Levels, in Blueberries
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, H.Y.; Xu, L.; Chen, H.J.; Fang, X.J. Research progress on postharvest quality control and antioxidant activity of blueberry. Chinese J. Food Sci. 2013, 13, 1–8. [Google Scholar]
- Zhang Jia. Research on inner quality evaluation of Beigaocong Blueberry. Chinese Academy of Agricultural Sciences, Beijing, 2020. [CrossRef]
- Hu, Y.; Zhang, F.S.; Xu, H.; Tao, L. Preliminary report on preservation technology of original base in fresh blueberries. Agric. Products Process. 2009, (4), 12-13. [CrossRef]
- Zhu, L.; Ling, J.G. Research progress of preservation technology of blueberry at home and abroad. Food and Fermentation Industry. 2011, 37, 173–176. [Google Scholar] [CrossRef]
- Fang, X.J.; Wu, W.J.; Mu, H.L.; Chen, H.J.; Zheng, X.L.; Gao, H.Y. Effects of exogenous abscisic acid treatment on physiological response of blueberry under low temperature stress. Chinese J. Food Sci. 2023, 23, 232–242. [Google Scholar] [CrossRef]
- Jiang, L.Q.; Hu, T.J.; Zheng, J.X. Experiment、application and research of cold-storage and fruit technologyfor fruit and vegetable. Grain and Oil Process. Food Machinery. 2003, 0, 65–66. [Google Scholar]
- Zhang, H. Effects of chitosan and ultraviolet treatment on storage tolerance of blueberry fruit and its physiological mechanism. Anhui Normal University, Wuhu, Anhui province, 2017.
- Zhang, J.; Ma, Y.; Dong, C.; Terry, L.A.; Watkins, C.B.; Yu, Z.; Cheng, Z.M. Meta-analysis of the effects of 1-methylcyclopropene (1-MCP) treatment on climacteric fruit ripening. Hortic. Res. 2020, 7, 208. [Google Scholar] [CrossRef] [PubMed]
- Satekge, T.K.; Magwaza, L.S. Postharvest application of 1-methylcyclopropene (1-mcp) on climacteric fruits: factors affecting efficacy. Int. J. Fruit Sci. 2022, 22, 595–607. [Google Scholar] [CrossRef]
- Jiang, Q,J,; Xie, W.; Chen, Y.Z.; Wang, C.T.; Zhang, X.X. Research progress of 1-MCP on berry quality and postharvest physiology. Farm Products Process. 2017, 441, 64-65.
- Blankenship, S.M.; Dole, J.M. 1-Methylcyclopropene: a review. Postharvest Biol. Tec. 2003, 28, 1–25. [Google Scholar] [CrossRef]
- Xu, F.; Liu, Y.; Dong, S.; Wang, S. Effect of 1-methylcyclopropene (1-MCP) on ripening and volatile compounds of blueberry fruit. J. Food Process. Pres. 2020. [Google Scholar] [CrossRef]
- Kwon, J.-G.; Yoo, J.; Win, N.M.; Maung, T.-T.; Naing, A.H.; Kang, I.-K. Fruit Quality Attributes of ‘Arisoo’ and ‘Picnic’ Apples as Influenced by 1-Methylcyclopropene Concentration and Its Application Frequency during Cold Storage. Horticulturae 2021, 7, 477. [Google Scholar] [CrossRef]
- Ji, S.J.; Zhou, Q.; Ma, C.; Cheng, S.C. Effect of 1-MCP treatment on shelf quality change of blueberry at room temperature. Food Sci. 2014, 35, 322–327. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; He, X.X.; Xiong, J.L.; Peng, M.Y.; Huang, M.; Liu, R.P.; Guan, W.Q. Identification of pathogenic bacteria of brown rot of brittle plum in Wushan and its control effect by 1-MCP. Southwest Chinese J. Agric. Sci. 2023, 36, 84–90. [Google Scholar] [CrossRef]
- Francesco, A.D.; Martini, C.; Mari, M. Biological control of postharvest diseases by microbial antagonists: how many mechanisms of action? European J. Plant Pathology. 2016, 145, 711–717. [Google Scholar] [CrossRef]
- Qin, S.W. Isolation, identification and biological control of pathogenic fungi latent infection of blueberry fruit. Dalian University of Technology, Dalian, Liaoning Province, 2017.
- Wei, Y.Y. Study on pathogens of postharvest diseases of blueberry and the yeast HMQAUSZ01 for biocontrol of postharvest diseases of fruits and vegetables. Qingdao Agricultural University, Qingdao, Shandong Province, 2017.
- Kurniawan, O.; Wilson, K.; Mohamed, R.; Avis, T.J. Bacillus and Pseudomonas spp. provide antifungal activity against gray mold and Alternaria rot on blueberry fruit. Biological Control:Theory and application in pest management. 2018, 126, 136–141. [Google Scholar] [CrossRef]
- Chan, Z.L.; Qin, G.Z.; Xu, X.B.; Li, B.Q.; Tian, S.P. Proteome approach to characterize proteins induced by antagonist yeast and salicylic acid in peach fruit. J Proteome Res. 2007, 6, 1677–1688. [Google Scholar] [CrossRef]
- Yan, Q.C.; Jiang, X.M. Determination of total acid and amino nitrogen in fruit juice by continuous titration with acidometer. Shandong Animal Husbandry and Veterinary. 2012, 33, 8–9. [Google Scholar] [CrossRef]
- Feng, G.T. Extraction, purification, component identification and antioxidant of blueberry anthocyanins. Guizhou University, Guiyang, Guizhou Province, 2016.
- Song, D.Q.; Meng, X.J.; Wang, C.Y.; Wang, G.Q.; Lv, C.M. Determination of blueberry anthocyanins by pH differential method. J. Shenyang Agric. Univ. 2013, 44, 231–233. [Google Scholar] [CrossRef]
- Wang, H.J. Study on the stability of vitamin C and determination of its content in fruits, vegetables and fruit juices by ultraviolet spectrophotometry. Shanxi Medical University, Taiyuan, Shanxi Province, 2015. [CrossRef]
- Li, H.S. Experimental principles and techniques of plant physiology and biochemistry. Beijing: Higher Education Press, 2003; pp. 167-169.
- Wang, X.R. Burkholderia contaminans biological control and mechanism of postharcrop gray mold of strawberry. Shanxi Agricultural University, Taiyuan, Shanxi Province, 2019. https://link.cnki.net/doi/10.27285/d.cnki.gsxnu.2019.000024.
- Zheng, X.X. Study on the control and bacteriostatic effect of Bacillus against postharvest Streptospora of apricot and peach. Tianjin University of Commerce, Tianjin, China, 2019.
- Zhang, Y.M.; Niu, X.X.; Zou, Q.; Yang, H.M.; Chu, M.; Shi, Y.W. Effects of Bacillus Velez on storage quality of postharvest grape fruit. Storage and Process. 2022, 22, 1–8. [Google Scholar]
- Wang, C.H. Effects of biocontrol bacterium(Bacillus amyloliquefaciens)LY1 on preservation and postharvest disease control of longan fruit and its mechanism. Fujian Agriculture and Forestry University, Fuzhou, Fujian Province, 2012. https://cdmd.cnki.com.cn/Article/CDMD-10389-1017279329.htm.
- Li, W.X. Study on the bacteriostatic effects of spoilage bacteria and Bacillus on kiwi fruit during storage. Jiamusi University, Jiamusi, Heilongjiang Province, 2022. [CrossRef]
- Arrebola, E.; Jacobs, R.; Korsten, L. Iturin a is the principal inhibitor in the biocontrol activity of Bacillus amyloliquefaciens PPCB004 against postharvest fungal pathogens. J. Appl. Microbiol. 2010, 108, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Alvindia, D.G.; Natsuaki, K.T. Biocontrol activities of Bacillus amyloliquefaciens DGA14 isolated from banana fruit surface against banana crown rot-causing pathogens. Crop Prot. 2009, 28, 236–242. [Google Scholar] [CrossRef]
- Shi, Hao.; Ding, R.H.; Luo, Q.L,; Fu, J.; He, X.E. Study on storage tolerance of winter jujube fruit treated with natamycin combined with 1-MCP. Storage and Process. 2021, 21, 26-33.
- Mohamed, S.O.; Dharini, S.; Lise, K. Effect of bioinhibitor Bacillus amylolytica combined with 1-methylcyclopropylene treatment on postharvest disease control and quality maintenance of fruits. Storage and Process. 2011, 2, 56–56. [Google Scholar]
- Liu, K.; Zhao, H.L.; Zong, N.; Li, H.H.; Liu, Y.S.; Miao, M. Evaluation of inhibition and preservation effect of Bacillus subtilis BS-1 strain on postharvest soft rot of kiwi fruit. Storage and Process. 2021, 21, 40–49. [Google Scholar] [CrossRef]
- Horzum, Ö.; Güneş, N.T. Influence of storage technology and 1-methylcyclopropene on postharvest behavior of ‘Ankara’ pear. Tur. J. Agric. and Forestry. 2024, 48, 81–94. [Google Scholar] [CrossRef]
- Zuo, C.G.; Wang, J.Y.; Niu, X.X.; Guan, L.H.; Liu, Ping.; Yang, H.M.; Chu, M.; Wang, N.; Lin, Q.; Shi, Y.W. Effects of Bacillus Belez-BG-2 on storage quality and defense enzyme activity of Muskmelon with thick skin. Micr. Bulletin. 2022, 49, 4171-4185. [CrossRef]
- Zheng, J.R.; Li, Y.Z. Research progress of induced resistance in plants. J. Mountain Agric. Biology. 2022, 41, 51–58. [Google Scholar] [CrossRef]
- Chen, J.Q.; Zhang, Y.M.; Zhang, L.M.; Han, H.G.; Shi, J.Y. Effects of different packaging methods on storage quality and antioxidant activity of fresh garlic. Food Res. and Dev. 2021, 42, 28–34. [Google Scholar]
- Lv, Y.; Fu, A.; Song, X.; Wang, Y.; Chen, G.; Jiang, Y. 1-Methylcyclopropene and UV-C treatment effect on storage quality and antioxidant activity of ‘Xiaobai’ apricot fruit. Foods 2023, 12, 1296. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Wang, C.; Yi, P.; Li, L.; Wu, G.; Huang, F.; Huang, M.; Gan, T. The Effects of Combined 1-Methylcyclopropene and Melatonin Treatment on the Quality Characteristics and Active Oxygen Metabolism of Mango Fruit during Storage. Foods 2023, 12, 1979. [Google Scholar] [CrossRef]
- Guo, S.; Li, T.; Wu, C.; Fan, G.; Wang, H.; Shen, D. Melatonin and 1-methylcyclopropene treatments on delay senescence of apricots during postharvest cold storage by enhancing antioxidant system activity. J. Food Process. Pres. 2021, 45. [Google Scholar] [CrossRef]
- Krupa, T.; Kistechok, A.; Tomala, K. Estimating the Physicochemical and Antioxidant Properties of Hardy Kiwi (Actinidia arguta) Treated with 1-Methylcyclopropene during Storage. Agriculture 2023, 13, 1665. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




