1. Introduction
Bluetongue virus (BTV) was first detected at the end of the 18. century, when sheep were imported into South Africa and became infected showing different clinical signs of hemorrhagic disease [
1]. BTV belongs to
Orbivirus genus within the family
Reoviridae and has a double-stranded RNA genome that encodes four non-structural (NS1–NS4) and seven structural proteins (VP1–VP7) [
2]. The viral genome is highly susceptible to reassortment due to its segmenattion, and so far, more than 36 serotypes of the BT virus have been described [
3]. BTV1-28 are considered significant for animal health. Though BTVs share common group antigens, they can be distinguished by serotype-specific virus neutralization test (VNT) [
4,
5] due to the no cross reactivity. Furter more, there is no cross BTV causes bluetongue disease which is a highly infectious and non-contagious, arthropod vector-borne disease in ruminants worldwide [
4]. The virus is mainly transmitted to by
Culicoides spp. Other transmission routes such as oral transmission and transplacental transmission, have also been described [
4]. The disease affects domestic and wild ruminants, and causes significant economic losses for livestock production [
4]. Eradication of the disease is based on culling of infected animals and vaccination with the serotype-specific vaccine [
5]. Live attenuated vaccines are still used today in the countries where the use of live vaccines is not prohibited. However, inactivated vaccines against bluetongue disease are used the most due to their safety. Recent research has led to the development of the DISA (Disabled Infectious Single Animal) vaccine which is a attenuated vaccine with deleted VP3 gene responsible for viremia, but this new generation of live vaccines is not yet in use [
6].
BTV4 occurred in Serbia first time in 2014, and later on in 2016 when 414 outbreaks were reported [
7]. The disease was successfully put under control by implementing strict restrictive measures, stamping out and vaccination with monovalent inactivated BTV4 vaccine in infected and high risk areas determined by the Ministry of Agriculture, Forestry and Water Economy of the Republic of Serbia, Veterinary Directorate. Vaccination was practiced until 2022. The last outbreak of BTV4 in was reported in 2020.
Due to the large scale epidemics and the shortage of vaccines available, the company Veterinarski Zavod Subotica (VZS) developed a novel candidate vaccine against BTV4. The aim of this is to present preliminary results of our examination of the quality of starting substances of biological origin, active substance - virus, laboratory serological analyzes of the humoral immune response of individuals vaccinated with the novel vaccine. In addition to our results, the preliminary results of the safety and efficacy tests of the new vaccine, which were performed in other institutions, were presented.
2. Materials and Methods
The novel BTV4 vaccine was inactivated oil-based vaccine, with Montanide ISA 61 (produced by SEPPIC, France) used as the adjuvant [
8,
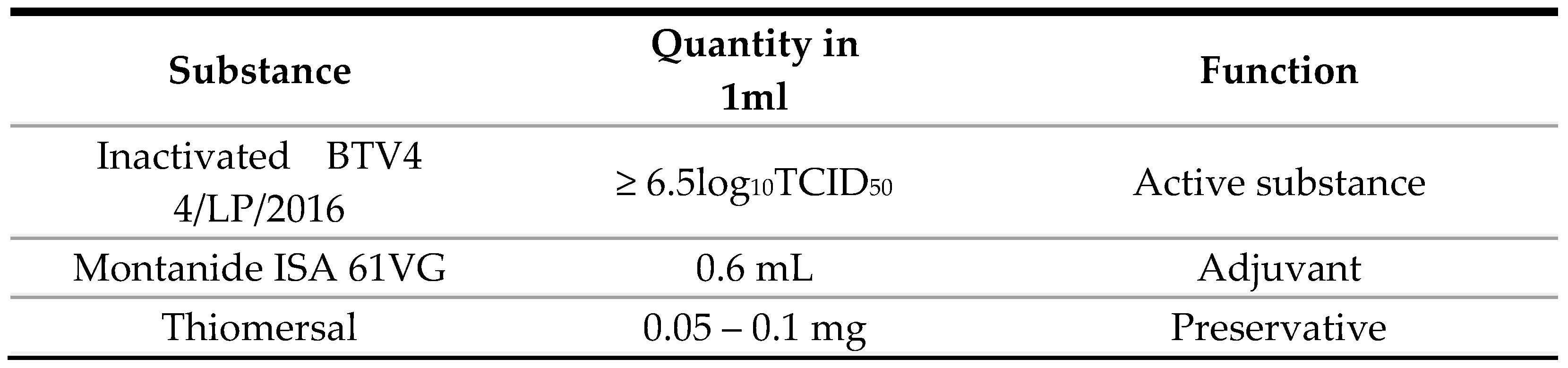
9]. The master seed virus (MSV) was produced on master cell line (MCL) “cell line BHK 21 clone 13” obtained from ATCC animal cell line collection (CCL 10). Master seed virus BT serotype 4 was isolated from blood of infected sheep from the Kraljevo district (Serbia). The composition of one dose of the novel test vaccine is shown in Table 1.
Table 1.
Composition of examined candidate novel vaccine against BTV 4.
Table 1.
Composition of examined candidate novel vaccine against BTV 4.
2.1. BTV4 Characterization in MVS
2.1.1. q Real Time RT-PCR
The lyophilized MVS was reconstituted with 1 ml of distilled water, vortexed, and after complete dissolution MVS was used for nucleic acid extraction. Viral nucleic acid was extracted using the IndiSpin Pathogen Kit (Indical Bioscience GmbH, Leipzig, Germany). Extraction was carried out using automatic extraction device QIA cube connect produced by Qiagen (GmbH Germany). Real time RT-PCR amplification was completed using Luna Universal Probe One Step RT-PCR (produced by New England, BioLabs. USA) and previously published primers/probe [
10].
2.1.2. Virus Isolation
Virus growth was confirmed by virus isolation on BHK21 clone 13 [
9] and subsequent real timeRT-PCR.
2.1.3. Sequencing of Master Seed Strain Bluetongue Virus Serotype 4, Strain 4/LP/2016
Virus Propagation
For the propagation of the BTV strain, the BHK-21 cell line was used. The strain was inoculated on a confluent monolayer using DMEM (Gibco, Grand Island, NY, USA) supplemented with 5% heat-inactivated fetal bovine serum (Gibco, Grand Island, NY, USA). Additionally, the DMEM was supplemented with a 0.5 mL MycoZap ampule containing a combination of antibiotics and antimetabolic agents, according to the manufacturer's instructions (Lonza, Basel, Switzerland). The virus was incubated at 37 °C for seven days and monitored daily for the appearance of a cytopathic effect. After the incubation period, the remaining cells were detached by gentle shaking, and the fluid containing cell debris and the virus was decanted. Further, the sample was centrifuged at 11000 RPM for 5 min in order to reduce potential environmental contamination, then filtered through 0.45 mm filters (Sigma-Aldrich, Missouri, USA) and stored at -80 °C until further use.
MinION Sequencing
Metagenomic sequencing was performed utilising the Sequence-independent single-primer amplification (SISPA) which was previously described by [
11]. The sequencing was performed on the MinION Nanopore sequencing platform. The SISPA approach uses two Rounds of amplification. In Round A there are two reaction mixes. The first one includes 11 μl of the extracted nucleic acid, 1 μl of 10 mM dNTP Mix (Invitrogen, Waltham, MA, USA), and 1 μl of a single primer FR26RV-N primer (5′-GCCGGAGCTCTGCAGATATCNNNNNN-3′) in order to obtain cDNA. The thermal profile heating of the master mix at 65 °C for 5 min, and cooling on ice for 1 min. The second mixture contains 4 μL of SSIV Buffer, 1 μL of SuperScript IV Reverse Transcriptase (200 U/μL), 1 μL of 100 mM DTT, and 1 μL of RNase OUT Recombinant RNase Inhibitor (Invitrogen, Waltham, USA). The obtained cDNA from the first mix and the second mix were combined and incubated at 23 °C, 50 min at 50 °C and 10 min at 80 °C, followed by an addition of 1 μl of Klenow polymerase (NEB, Ipswich, MA, USA) and a new incubation at 60 min at 37 °C and 10 min at 75 °C.
Round B included the amplification of the Round A cDNA with the FR20RV primer (5′-GCCGGAGCTCTGCAGATATC-3′). The mix included 25 µL of PfuUltra II Hotstart PCR Master Mix (Agilent Technologies, Santa Clara, CA, USA), 1 µL of 10 µM primer, 21 µL of RNase-free water and 3 µL of the obtained cDNA. The temperature profile included an initial activation at 98°C for 30 seconds, followed by 25-35 cycles of denaturation at 98°C for 10 seconds, annealing at 65°C for 30 seconds, and extension at 72°C for 40 seconds, with a final extension at 72°C for 2 minutes. The obtained products were quantified on Qubit dsDNA High Sensitivity Assay (Thermo Fisher Scientific, Waltham, MA, USA) for Qubit 4 fluorometer. Sequencing was done on a MinION Mk1-c device using the R 10.4.1. flow cell and the Rapid PCR Barcoding Kit 24 V14 (SQK-RPB114.24) according to the manufacturer's instructions.
Sequencing lasted for 12h, the base-calling was done with Guppy v. 24.02.16 in MinKNOW GUI – v. 5.9.18., and the initial quality score was set to 9. SISPA primers were removed, and the obtained reads were mapped using the following tools minmap2 v2.24-r1122
https://github.com/lh3/minimap2/releases) was used for mapping the sequence to the reference genome, then the samtools 1.19.2 (
https://github.com/samtools/samtools/releases/) was used for checking and indexing the obtained file, and at the end, iVar version 1.4.2 (
https://github.com/andersen-lab/ivar) was used for the creation of the consensus sequence. The reads were mapped to strains described in a previous study (
https://doi.org/10.1155/2023/3112126). The obtained consensus sequences were further annotated in the Geneious Prime software (Dotmatics, Boston, MA, USA), and submitted to the NCBI.
2.2. Extraneous Agent Detection in MSV and MCL
2.2.1. Cytopathic Virus Isolation Test for MSV and MCL and Hemagglutination Tests
Lyophilized MSV was reconstituted with 1 ml of sterile distilled water and after short vortexed, MSV was inoculated in a cell line. MCL was prepared by triple freezing and thawing cycles, centrifuged for 5 minutes at 134 g rpm to remove cellular debris and inoculated on confluent monolayers of cell lines in 2 flasks of 75cm2. One flask of each cell lines served as a negative control of cell line.
The MSV and MCL were inoculated on a confluent monolayer of MDBK (CCLV-RIE 15), MDCK (CCLV-RIE 83), PK-15 (CCLV-RIE 56-1), VERO (CCLV-RIE 15) and BHK21 (CCLV-RIE 164) cell lines (procured from Friedrich Loeffler Institute , Department of Experimental Animal Facilities and Biorisk Management, Collection of cell lines in Veterinary Medicine (CCLV)), using DMEM (Gibco, Grand Island, NY, USA) supplemented with 10% heat-inactivated foetal bovine serum (Gibco, Grand Island, NY, USA). The inoculated cell cultures were incubated at 37 °C for seven days and monitored daily for the appearance of a cytopathic effect (CPE). After the 7 days, inoculated monolayers were frozen and thawed three times than inoculated again on fresh cell lines. Procedures were repeated four times and virus isolation was monitored in 4 passages of 7 days each. Hemagglutination tests of MVS and MCL were performed with all 4 passages after virus isolation.
The hemagglutination test was performed in 96-well plates with a V bottom. We added 25μl PBS solution to each well in two rows (well 2 to well 12 in a row) than added 50μl of appropriate samples of MSV and MCL to well 1 of each row, then double dilute 25μl across the plate from well 1 to 12 and discard the final 25μl from well 12 of each row. Allowed plate to stand at room temperature (18˚C - 24˚C) for 30mins (+/- 2mins). After that 50μl of 1% avian red blood cells was added to all wells and allowed plate to stand at room temperature (18˚C - 24˚C) for 45 mins (+/- 2mins). After that microplate was ready for estimation of the results. Sedimentation of erythrocytes in one point at the bottom of the well is a sign of a negative reaction and the absence of hemagglutination. The appearance of a net of erythrocytes on the walls without sedimentation on the bottom is a sign of positive hemagglutination.
2.2.2. Fluorescent Antibody Test (FAT) Rabies Virus Detection in MSV and MCL
MSV and MCL prepared as previously described were simultaneously inoculated into flasks, and on monolayer of cell line BKH 21 in 96-well microplates for rabies virus isolation. The last column of wells was inoculated with positive and negative control for rabies. Each passage of inoculated MSV and MCL was tested for the presence of rabies virus by direct immunofluorescence. After incubation, the microplates were washed, dried, fixed and stained with a FITC conjugate monoclonal ant Rabies antibodies manufactured by SIFIN (Berlin GmBH-Germany). The working solution of the conjugate was directly applied to the wells on fixed cells in a volume of 50 microliters. Microplates with the conjugate were incubated according to the manufacturer's instructions for up to 30 min at 37 °C in a dark humid chamber. The microplate wells were washed 2 times with PBS pH 7,2 for up to 5 minutes and finally washed with distilled water. Cell monolayers in the microplate wells were observed under a fluorescent microscope in order to prove the presence or absence of rabies virus in the tested material. Direct immunofluorescence test was performed after each of four 4 passages of the test material MSV and MCL. The appearance of green fluorescent granules in the inoculated cells is a sign of a positive result. The absence of fluorescein in the wells with cells is a sign of a negative result for the presence of the rabies virus.
2.2.3. Nucleic Acid Extraction and (RT) PCR Tests
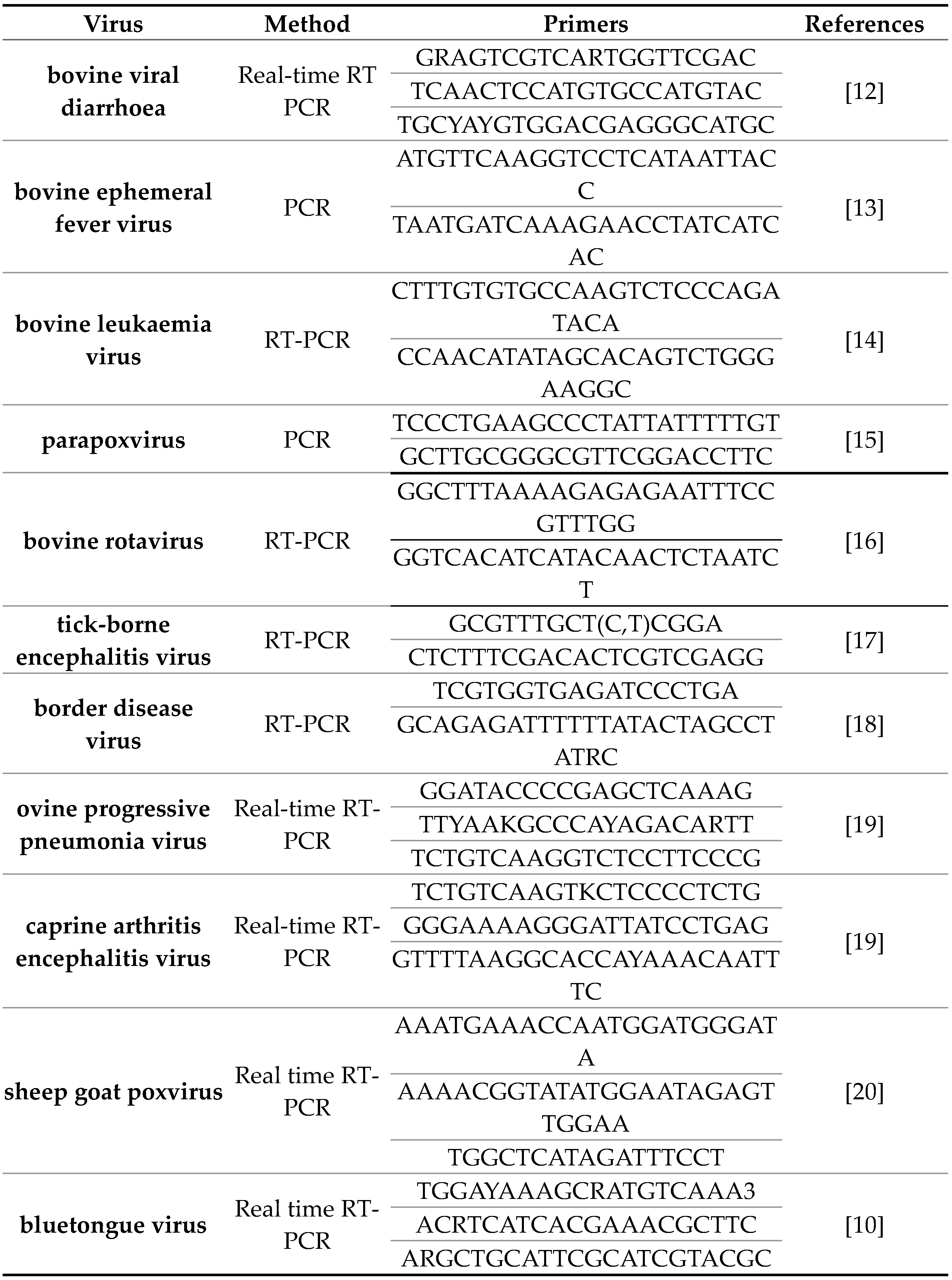
Viral nucleic acid was extracted using the IndiSpin Pathogen Kit (Indical Bioscience GmbH, Leipzig, Germany) according to the manufacturer's instructions. Nucleic acid extraction was performed by automatic extraction device QIA cube Connect (produced by Qiagen GmbH Germany) according to operation manual. QIAGEN one step RT-PCR (Qiagen, GmbH, Germany), Luna Universal Probe qPCR master mix (New England, BioLabs Ipswich, MA, USA)), Luna Univeral Probe One Step RT-PCR (New England BioLabs Ipswich, MA, USA), OneTaq HotStart 2X Master Mix with Buffer (produced by New England Biolabs, USA) and primers shown in Table 2 were used for specific genomes amplifications.
Table 2.
List of primers for PCR methods used in tests for external agents of MSV and MCL.
Table 2.
List of primers for PCR methods used in tests for external agents of MSV and MCL.
2.3. Sterility Test
Sterility test was done by direct inoculation test [
21] and culture method for Mycoplasma spp. test [
22].
2.4. Safety Test
2.4.1. Safety Test in Sheep
Safety trials on sheep was done on Faculty of Veterinary Medicine of Belgrade, Belgrade University (Serbia). Three groups of 8 lambs were selected to test the safety of single-dose and double-dose vaccine applications according to the manufacturer's instructions. The lambs were 4 to 6 months old. The first group A was the control group. The second group B of 8 lambs received 1 ml of vaccine (one dose) and were revaccinated after 21 days with 1 ml of vaccine (one dose). The third group C received 2 ml of vaccine (double dose) and were revaccinated after 21 days with two milliliters of vaccine (double dose of vaccine). The vaccine was applied subcutaneously (s.c.) in the area of the left side of the neck. The second application was injected subcutaneously on the right side of the neck. All applications are given in the middle third of the neck. The control A group received 1 ml of saline solution at intervals of up to 21 days on the same dates when the vaccine was applied on group B and C. General and local side effects were monitored from the first application day up to 21 days after second application.
2.4.2. Safety Test in Pregnant Ewes
Two group of 8 pregnant ewes of the Württemberg breed were selected for safety test. All ewes were in the second half of pregnancy (on the end of third months of pregnancy), which was confirmed by ultrasonography. Upon arrival in the facilities, the sheep were in good general health. The sheep were left in quarantine for seven days during which they adjusted to the local conditions. The first group (group A) of 8 pregnant ewes received the single dose of vaccine and boosterised after 21 days with the second single dose of 1 ml. All applications are given in the middle third of the neck, subcutaneously (s.c.). The 8 pregnant ewes of control group B received a saline solution in a dose of 1 ml. in the same interval of 21 days as a placebo. General and local side effects on the application site, were monitored until the end of pregnancy, during parturition, as well as later, in the case of an impact on the vitality of the ewes and offspring
2.5. Efficacy Test
Vaccine efficacy was tested in two trials. Trial 1 was detection of seroconversion test in the sera of vaccinated and revaccinated sheep by ELISA test. Trials 1 were done on Institute of Veterinary Medicine of Serbia. The trial 2 challenge test of vaccinated animals was done in Hungarian company” Prophyl “LTD Immunolab.
2.5.1. Efficacy Test, Trial No.1
The first laboratory efficacy test served to estimate detection of specific antibodies against BTV in vaccinated sheep, to assess the onset of immunity and duration of immunity. First group of 8 healthy individuals aged between 6 months and 1 year were vaccinated and revaccinated with the novel vaccine against BTV4 in doses of 1 ml. Control group of 8 sheep with same age received a sterile saline solution in the same volume and the same time interval. ELISA tests for specific antibodies against BT virus were done at Institute of Veterinary Medicine of Serbia (NRL for bluetongue disease). Serology tests had been repeated up to 382. day post-vaccination by ELISA test kit “INGEZIM BTV DR 12. BTV.K.O “ produced by Ingenasa (Spain). All tests were performed according to the original manual instruction for use of ELISA kit by producer.
2.5.2. Efficacy Test, Trial No. 2
Challenge test, was carried out by the Hungarian company” Prophyl “LTD Immunolab”. Twenty lambs 6-8 months old were included in the challenge test. Ten lambs (group1) were the control group that received a saline solution and ten lambs (group 2) were vaccinated on 0 day of experiment with novel candidate vaccine against BTV4 with single dose of 1 ml. The application was performed subcutaneously in the middle third of the neck on left side. After 21 days, ten vaccinated lambs in group 2 received second dose of the vaccine, and the control group 1 received saline solution on the same way and volume. On day 42. all lambs were challenged with 0.5 ml of pathogen strain BTV4 subcutaneously at a dose of 6.50 log10 TCID50. All lambs were observed until the end of 63 days from the beginning of the trial (21 days after the challenge). All 20 lambs were monitored daily after challenge for the presence of blue tongue disease clinical sings such as inappetence, facial oedema, hyperemia of the gums, nasal discharge and laboured breathing.
Interventionary studies involving animals or humans, and other studies that require ethical approval, must list the authority that provided approval and the corresponding ethical approval code.
3. Results and Discusion
3.1. Results of BTV4 Characterization
Genom of BTV4 was confirmed in MSV by real time RT-PCR. High-quality sequences were obtained for all segments besides segments 6 and 7. The obtained sequences were deposited in the NCBI under the following accession numbers PP982459-PP982466. The highest similarity for segment 1 was recorded with serotype four sequences from France, and Greece at 99.82% (MG944817.1, MT879211.1), for segment 2 with serotype four sequences from France, and Kosovo* (MG944818.1, OP186407.1) at 99.79%, for segment 3 with serotype four sequences from France and Kosovo* (MG944819.1, OP186418.1) at 99.67%, segment 4 with serotype four sequences from Kosovo* (OP186409.1) at 99.9%, segment 5 with serotype four sequences from Kosovo* (OP186410.1, and OP186400.1) at 99.82%, segment 8 with serotype four sequences from Kosovo* (OP186423.1) at 99.9%, segment 9 with serotype four sequences from Kosovo* (OP186424.1) at 99.6%, and segment 10 with serotype four sequences from France (MG944826.1) at 99.8%.
3.2. Results of Extraneous Agent Detection in MSV and MCL
MSV was positive only for the presence of BTV, causing a cytopathogenic effect on the BHK21 cell line which was confirmed later by real time RT-PCR test. After four passages of virus isolation on the other test cell lines, no CPE was observed. MSL gave negative hemagglutination test results after each of four passages. All PCR test for detection of genomes of non-cytopathogenic viruses and direct immunofluorescence for detection of rabies virus were negative.
MVS passed the conditions considering that the sterility test for the presence of bacteria and the test for the presence of mycoplasma gave negative results.
The results of the master seed test gave satisfactory results. The complete absence of external virus agents from MSV as well as its sterility are the basic prerequisites for the use of MSV in the production of vaccines. The presence of any external virus agents other than the production vaccine virus is not allowed in such a master seed that could be used for production purposes [
23].
MCL was negative for the presence of cytopathogenic viruses tested on 5 cell lines MDBK, BHK21, MDCK, VERO and PK-15 cell lines during four consecutive passages lasting 7 days each. MCL was also negative on presence of hemagglutinating viruses, rabies virus and bovine viral diarrhea viruses as well. Master cell line was sterile according the findings in direct inoculation test and culture method for Mycoplasma spp. tests.
Trials of the presence of external agents end sterility of the master cell line passed the test and gave satisfactory results. The absence of external agents is a prerequisite for vaccine production on the tested cell line and this tested cell line BHK 21 clone 13 meets the conditions for vaccine production based on the test results. [
23] .
3.3. Safety Test Results
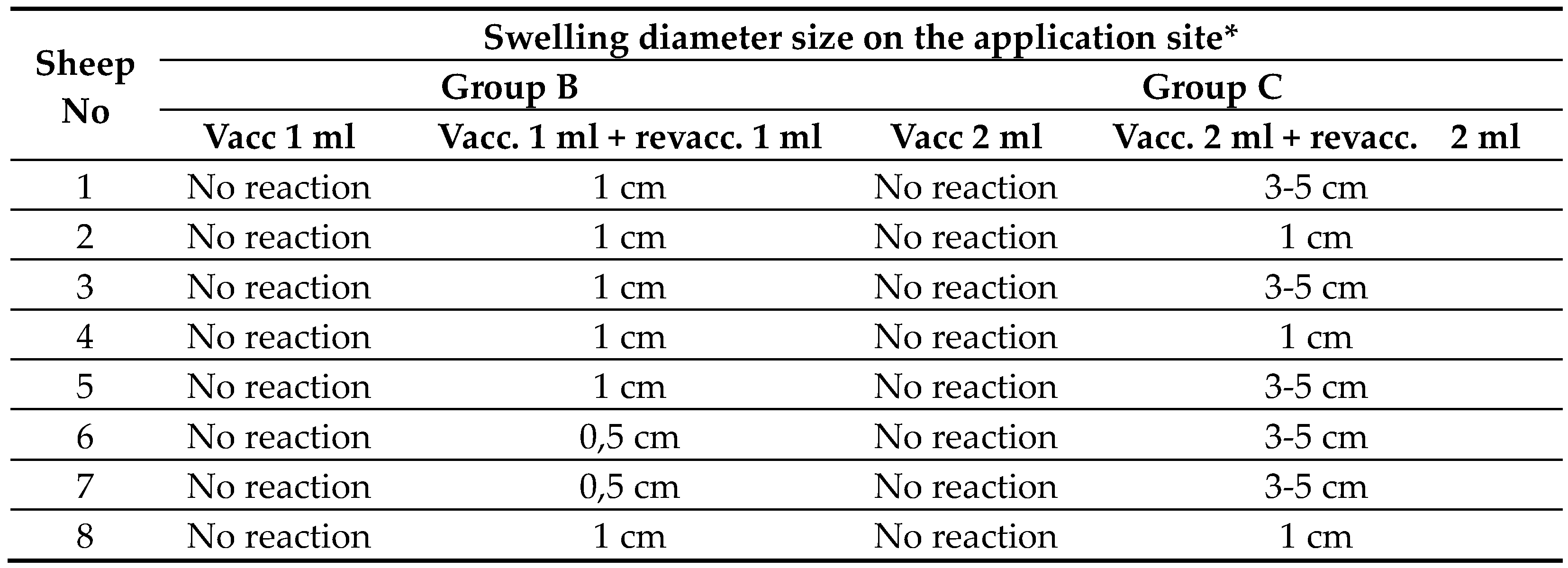
After a double blind application of 1 ml of saline solution, none of the 8 lambs in the control group A showed changes in the application site. Results of side effects (local reaction on the application site) of group B (vaccination and revaccination with single dose) and group C (vaccination and revaccination with double dose) are presented in Table 3.
After administration of the first single dose of novel vaccine on 8 lambs in group B, there were no changes at the application site. After revaccination with 1 ml of vaccine on 8 lambs of group B a swelling the size of 1 cm in diameter appeared in 6 out of 8 (75%) of vaccinated lambs, and spontaneously subsided in a few days without consequences for the general state of health. Two vaccinated lambs in group B (25%) got swelling the size less than 1 cm in diameter.
After administration of the first double dose (2 ml) of novel vaccine on 8 lambs in group C, there were no changes at the application site. After the second administration with 2 ml of vaccine on 8 lambs in group C, local swellings the size of 3-5 cm in diameter appeared at the application site on 5 lambs (62.5%) and local swelling the size of 1 cm appeared on 3 lambs (37.5%). All swellings in group C spontaneously subsided until 42 days of buster without treatment and consequences. There were no side reactions with general disorder of health status.
The results of testing the efficacy and safety of the vaccine after application were performed in accordance with the regulations on the safety of inactivated vaccines [
24]. The safety of each inactivated vaccine must be tested with administration of single dose and a double dose. If the manufacturer stated that the vaccine is applied twice with one dose in a certain time interval, the safety of the vaccine must be tested after two administrations of a double dose in the same prescribed time interval. Safety has been tested by applying the recommended method of vaccine application (subcutaneous administration, s.c.) [
23]. The results of the side effects of the vaccination gave satisfactory results and they are similar or milder compared to those occurring after the application of already registered vaccines against BTV [
25,
26].
Safety tests of novel vaccine against BTV4 should be done on calves and kids as well and bearing in mind that the tests should be performed on the youngest age category of all indicated animal species for the vaccine to ensure the parameters for the worst-case scenario.
Table 3.
Side effects (local reaction on the application site) of vaccination after the vaccination and revaccination with 1 ml (single dose) and 2 ml (double dose).
Table 3.
Side effects (local reaction on the application site) of vaccination after the vaccination and revaccination with 1 ml (single dose) and 2 ml (double dose).
The general state of health of the vaccinated sheep in in the control and vaccinated groups during pregnancy and after parturition did not change. No significant differences between the control and experimental groups were detected. All 16 pregnant ewes, 8 in the experimental group (group A) and 8 ewes in the control group (group B) went through parturition without difficulty. Lambs originating from pregnant ewes from the experimental and control groups were vital and no negative impact of the vaccine on the health of the ewes during pregnancy and newborn lambs was detected.
The experiment gave very well safety results of the vaccine for use in sheep during the second half of pregnancy. So far, the safety results in pregnant sheep are promising. Similar experiment should be performed on pregnant heifers and, if necessary, on pregnant goats. Parallel testing on other indicated species would complete the results of the vaccine's safety in full taking into account that three animal species are indicated for vaccination against BTV.
3.4. Efficacy Tests Results
3.4.1. Efficacy Results of Humoral Response by ELISA Test
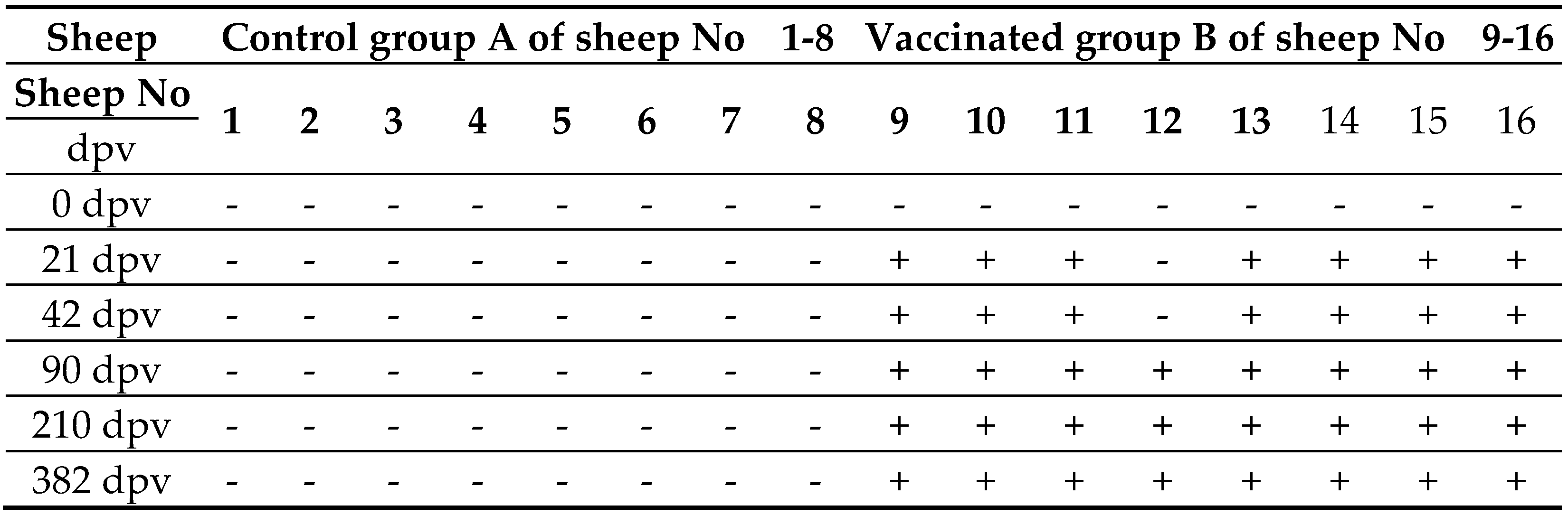
Eight sheep in control group A remained seronegative throughout the study period. Results of ELISA tests for detection of specific antibodies against the BT virus, indicate a high degree of vaccine efficacy after the vaccination of sheep in group B. The onset of immunity was 28 days after vaccination or earlier. As many as 87.5% of vaccinated individuals had detected antibodies against the BT virus, 4 weeks after revaccination. The results of ELISA tests are shown in Table 6. The ELISA test confirmed the presence of antibodies against the bluetongue virus at 28. day after vaccination in (87.5%) cases, i.e. in 7 out of 8 vaccinated sheep in group B. After 42 days post vaccination, all vaccinated sheep from group B had antibodies against BTV. Sera of vaccinated individuals were tested for the last time on day 382 after vaccination. All 8 sheep from the control group that received the placebo remained negative for the presence of antibodies against BTV throughout the testing period.
Table 4.
ELISA test results for the presence of specific antibodies against BTV in sheep sera.
Table 4.
ELISA test results for the presence of specific antibodies against BTV in sheep sera.
The laboratory efficacy serological tests on the humoral immunity in vaccinated sheep gave quite good results. A similar vaccination test and serological tests for the presence of specific antibodies against BTV should be performed on large ruminants, on the youngest age category with the least potency of novel vaccine (6.50 log10 TCID50/ml before inactivation), as large ruminants are also indicated for vaccination.
3.4.2. Result of Challenge Test
After the challenge test, all 10 sheep from the blind control group developed bluetongue disease and showed moderate and typical clinical symptoms such as inappetence, facial oedema, hyperemia of the gums, nasal discharge and laboured breathing. Four of the 10 sheep (40%) that received two doses of the novel vaccine BTV4 had not shown clinical signs typical for bluetongue disease. The other 6 out of 10 vaccinated individuals (60%) from the same group showed barely detectable clinical signs of Bluetongue disease in the form of mild inappetence, facial oedema and nasal discharge which disappeared within 48 to 72 hours without treatment.
The challenge test showed that the new vaccine against BTV protects vaccinated individuals from bluetongue or significantly reduces symptoms that disappear quickly without treatment. It would be helpful if the efficacy were performed in the future trials with the least potent vaccine lot, on the youngest prescribed age categories of all species indicated for vaccination. In that case, the worst-case scenario would be fulfilled as a prerequisite for vaccine registration. This would be a guarantee that every other batch of vaccine would induce at least the same or higher level of immunity as the least potent vaccine lot. [
27].
3.5. Duration of Immunity against Infection with Novel Candidate Vaccine Against BTV4
According to the results of ELISA for eight vaccinated sheep (group B) in Table 6, immunity against BTV lasts at least 382 days after revaccination. Based on the obtained results of the ELISA test, the manufacturer declared the duration of immunity at least one year after vaccination. Montanid ISA 61 VG proved to be a good adjuvant and considering the obtained results of duration of immunity in vaccinated and revaccinated individuals with the new vaccine.
Results of serology trials of the duration of immunity are promising so far. It is necessary to do a challenge test on vaccinated sheep and large ruminants after 12 months, to justify the claim that immunity lasts a minimum of one year after vaccination with the vaccine close to expiration date [
27] In order to complete vaccine documentation, the vaccine should be tested on youngest category indicated for vaccination of calves and kids [
23]. Duration of the presence of antibodies in serum must be at least 3 months longer than the declared duration of immunity from the manufacturer.
4. Conclusions
Preliminary results of the candidate vaccine against bluetongue disease serotype 4 have shown satisfactory results in tested parameters so far.
Sterility of MSV and MCL was confirmed during this trial as well as the absence of external agents
The safety test of the novel vaccine after application gave very good results without the occurrence of general adverse effects on the health of vaccinated lambs. Side effects are limited to a local reaction on the application site that subsided after few weeks without treatment and therapy.
The efficacy of the tested new vaccine against BTV is at a satisfactory level, taking into account the results of the humoral response of the vaccinated sheep as well as the results of the challenge test that proved that the vaccine protects (40% vaccinated) against infection or at least significantly reduces the intensity and duration of the clinical signs (60%) after vaccination of lambs.
Duration of the immunity lasted at least one year.
Taking into account the current preliminary results of the purity, onset and duration of immunity, results of safety and efficacy tests of the candidate novel BTV4 vaccine, we could reasonably be optimistic that a new vaccine against bluetongue virus serotype 4 is on the horizon.
Author Contributions
Conceptualization, LJ.V. and V.M.; methodology, M.K.; software, D.G.; validation, V.J., V.M. and D.G..; formal analysis, LJ.V.; resources, M.K..; writing—original draft preparation, LJ.V..; writing review and editing, X.X.; visualization, V.M..; supervision, V.M.; All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by the Serbian Ministry of Science, Technological Development and Innovation (Contract No 451-03-66/2024-03/ 200030 and 451-03-66/2024-03/ 200178).
Institutional Review Board Statement
Vaccine safety tests were performed at the Faculty of Veterinary Medicine in Belgrade. The Faculty of Veterinary Medicine (according to agreement with producer “Veterinarski Zavod Subotica”) licensed for experiments on animals in vivo, approved by Ministry of Agriculture and environment protection of Republic of Serbia. Document number: 323-70-04611/2016-05/3. Date of approval: October 24. 2022. Clinical study was carried out in accordance with Serbian animal welfare law and was approved by the “Ministry for agriculture and environmental Protection” of Republic of Serbia under the registration number: 323-07 -04611/2016.05/3 from 24.10.2022. Efficacy challenge test was performed in: Hungarian National Institute for Pharmacy and Nutrition – OGYEI (Protocol code 39-7/2020, date of approval, January 29. 2020.).” for studies involving animals” in vivo experiments.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results”.
References
- Saminathan M, Singh KP, Khorajiya JH, Dinesh M, Vineetha S, Maity M, Rahman AF, Misri J, Malik YS, Gupta VK, Singh RK, Dhama K. An updated review on bluetongue virus: epidemiology, pathobiology, and advances in diagnosis and control with special reference to India. Vet Q. 2020 Dec;40(1):258-321. [CrossRef]
- Marín-López A, Poza F, Menaya-Vargas R, Calvo-Pinilla E, Benavente J , Martínez-Costas J, Ortego J; (2014.), VP2, VP7, and NS1 proteins of bluetongue virus targeted in avian reovirus muNS-Mi microspheres elicit a protective immune response in IFNAR(-/-) mice ; Antiviral Res 2014 Oct:110:42-51. [CrossRef]
- Ries C, Vögtlin A, Hüssy D, Jandt T, Gobet H, Hilbe M, Burgener C, Schweizer L, Häfliger-Speiser S, Beer M, Hoffmann B.(2021.) Putative Novel Atypical BTV Serotype '36' Identified in Small Ruminants in Switzerland. Viruses. 2021 Apr 21;13(5):721. [CrossRef]
- Rojas J M, Martín V, and Sevilla N; (2021.) Vaccination as a Strategy to Prevent Bluetongue Virus Vertical Transmission; Pathogens. 2021 Nov; 10(11): 1528. [CrossRef]
- Savini D N, MacLachlan J, Sánchez-Vizcaino J.M, Zientara S. (2008.) Vaccines against bluetongue in Europe; Comparative Immunology, Microbiology and Infectious Diseases, Volume 31, Issues 2–3, March 2008, Pages 101-120. [CrossRef]
- Ranjan K, Prasad M, Brar B, Lambe U, Kumar G, Ghosh M, Prasad G; (2019.); Bluetongue virus vaccine: conventional to modern approach; Acta virologica 63: 3 – 18, 2019;. [CrossRef]
- https://empres-i.apps.fao.org/diseases.
- Ibrahim E E, Mossad Gamal W, Ismail Hassan A, Mahdy E, Zakria Hegazy S.A.; (2015.) Comparative study on the immunopotentiator effect of ISA 201, ISA 61, ISA 50, ISA 206 used in trivalent foot and mouth disease vaccine; Veterinary World, EISSN: 2231-0916;.
- Bhat Shabir A. and Dar, Kondabattula P, Sharma G, Kumar G, Gupta G, Vikas and Khorajiya, Jaynudin H, Singh H, Karam Pal, Tiwari A, Kumar A , Nandi, Sukdeb N; (2023.) Enhanced Immune Responses and Protection Against Viral Challenge in Sheep with a Novel Nanoemulsion-Based Inactivated Bluetongue Virus Vaccine. Available at SSRN: https://ssrn.com/abstract=4448591 or. [CrossRef]
- WOAH, Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (2021) Chapter: 3.1.3 point: 1.3.1.1.
- Chrzastek, K, Lee, D, Smith, D, Sharma P, Suarez D.L.; Pantin-Jackwood, M.; Kapczynski, D.R. Use of Sequence-Independent, Single-Primer-Amplification (SISPA) for Rapid Detection, Identification, and Characterization of Avian RNA Viruses. Virology 2017, 509, 159–166.
- WOAH, Manual of Diagnostic Tests and Vaccines for Terrestrial Animals Chapter 3.4.7, point 1.2.1.
- Wang I F, Hsu A M, Huang K J; (2001.), Bovine ephemeral fever in Taiwan; J Vet Diagn Invest, 2001 Nov;13(6):462-7. [CrossRef]
- Rulka J, Kubis P, Deren W, Buzala E; (2001.) Evaluation of the nested-PCR meth-d for the diagnosis of bovine leukaemia virus [BLV] infection Bulletin of the Veterinary Institute in Puławy 2001 | 45.1.
- Abrahão JS, Lima LS, Assis FL, Alves PA, Silva-Fernandes AT, Cota M, Ferreira MV, Campos R.K, Mazur C, Lobato Z, Trindade GS and Kroon E; (2009) Nested-multiplex PCR detection of Orthopoxvirus and Parapoxvirus directly from exanthematic clinical samples, Virol J. 2009; 6: 140. [CrossRef]
- Falcone E, Tarantino M, Di Trani L, Cordioli P, Lavazza A, Tollis M; (1999) Determination of bovine rotavirus G and P serotypes in Italy by PCR; Journal of Clinical Microbiology, 1999, Am Soc Microbiol.
- Rudenko N; (2004.) Tick-borne encephalitis virus-specific rt-pcr – a rapid Test for detection of the pathogen without viral RNA Purification. 2004, Acta virologica 48: 167 – 171.
- 18. Vilcek S. Paton D J; (2000.) RT-PCR assay for the rapid recognition of border disease virus; Vet. Res, Volume 31, Number 4, 2000, 437 – 445 . [CrossRef]
- Kuhar U, Barlič-Maganja D and Grom J; (2013.) Development and validation of TaqMan probe based real time PCR assays for the specific detection of genotype A and B small ruminant lentivirus strains BMC Vet Res. 2013; 9: 172. Published online 2013 Sep 3. [CrossRef]
- Bowden TR, Babiuk SL, Parkyn GR, Copps JS, Boyle DB. Capripoxvirus tissue tropism and shedding: A quantitative study in experimentally infected sheep and goats. Virology. 2008 Feb 20;371(2):380-93. [CrossRef]
- European Pharmacopoeia 10.0, Volume 1, chapter 2.6.1.
- European Pharmacopoeia 10.0, Volume 1, chapter 2.6.7.
- European Pharmacopoeia 10.0; 07/2018:0062, “Vaccines for veterinary use”.
- European Pharmacopoeia 10.0, chapter 5.2.6. “Evaluation of safety of veterinary vaccines and immunosera.
- Gethmann J, Hüttner K, Heyne H, Probst C, Ziller M, Beer M, Hoffmann B, Mettenleiter TC, Conraths FJ. Comparative safety study of three inactivated BTV-8 vaccines in sheep and cattle under field conditions. Vaccine. 2009 Jun 24;27(31):4118-26. [CrossRef]
- Macedo L B, Portela Lobato Z I , FialhoS L , de Marco Viott, Carvalho Guedes M R and Silva-Cunha A ; (2013.) Evaluation of Different Adjuvants Formulations for Bluetongue Vaccine; Braz. Arch. Biol. Technol. v.56 n.6: pp. 932-941, Nov/Dec 2013, ISSN 1516-8913 Printed in Brazil.
- European Pharmacopoeia 10.0; 04/2008:50207; chapter: 5.2.7. “Evaluation of efficacy of veterinary vaccines and immunosera.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).