Submitted:
22 July 2024
Posted:
24 July 2024
You are already at the latest version
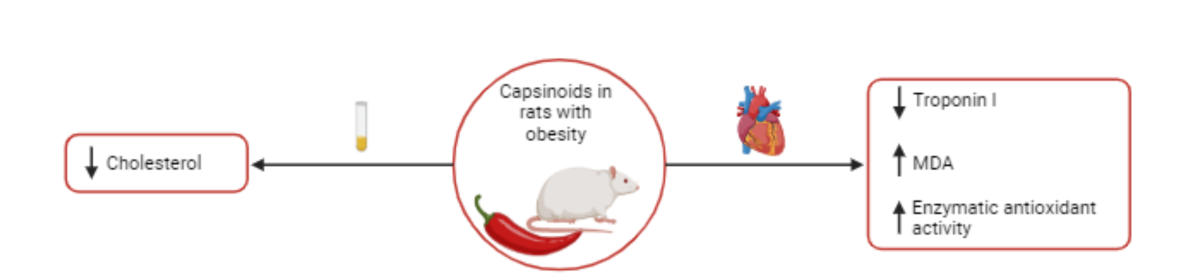
Abstract
Keywords:
1. Introduction
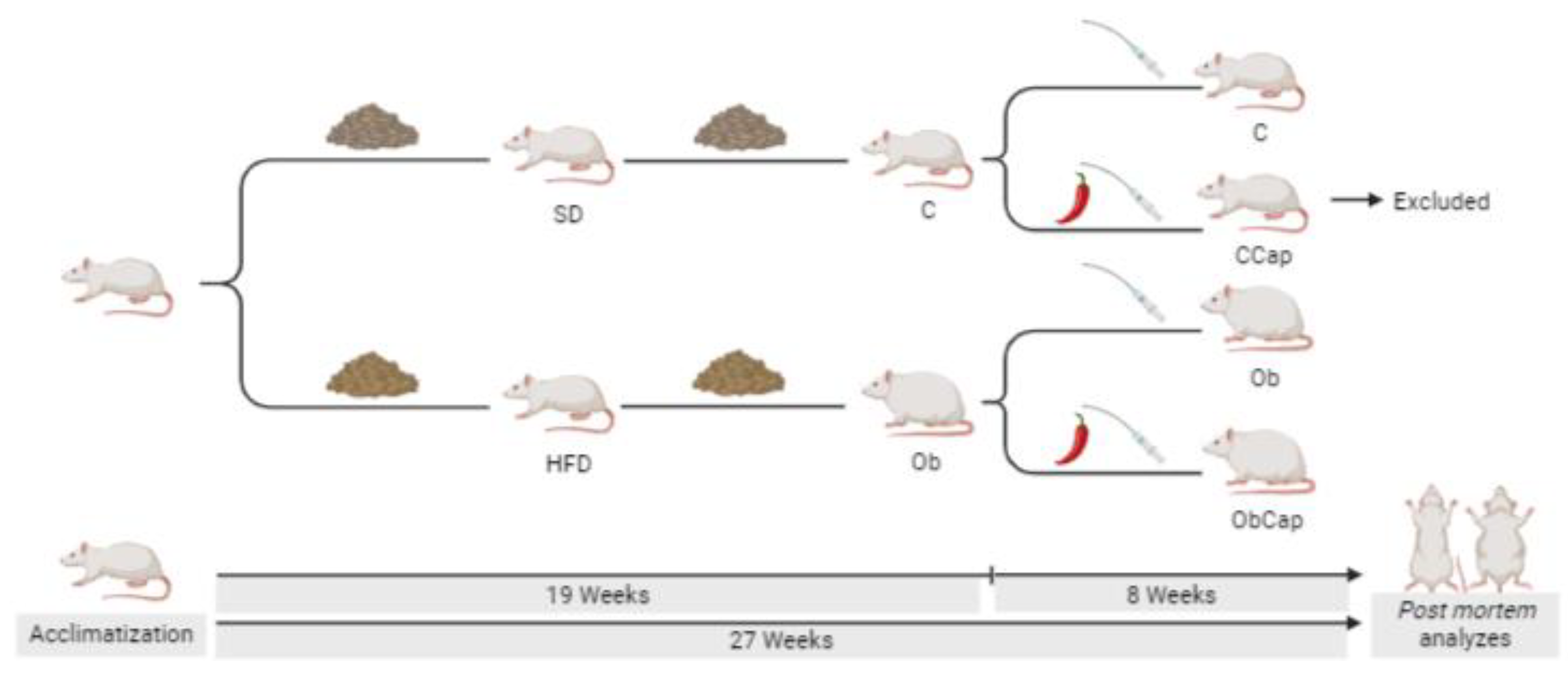
2. Materials and Methods
3. Results
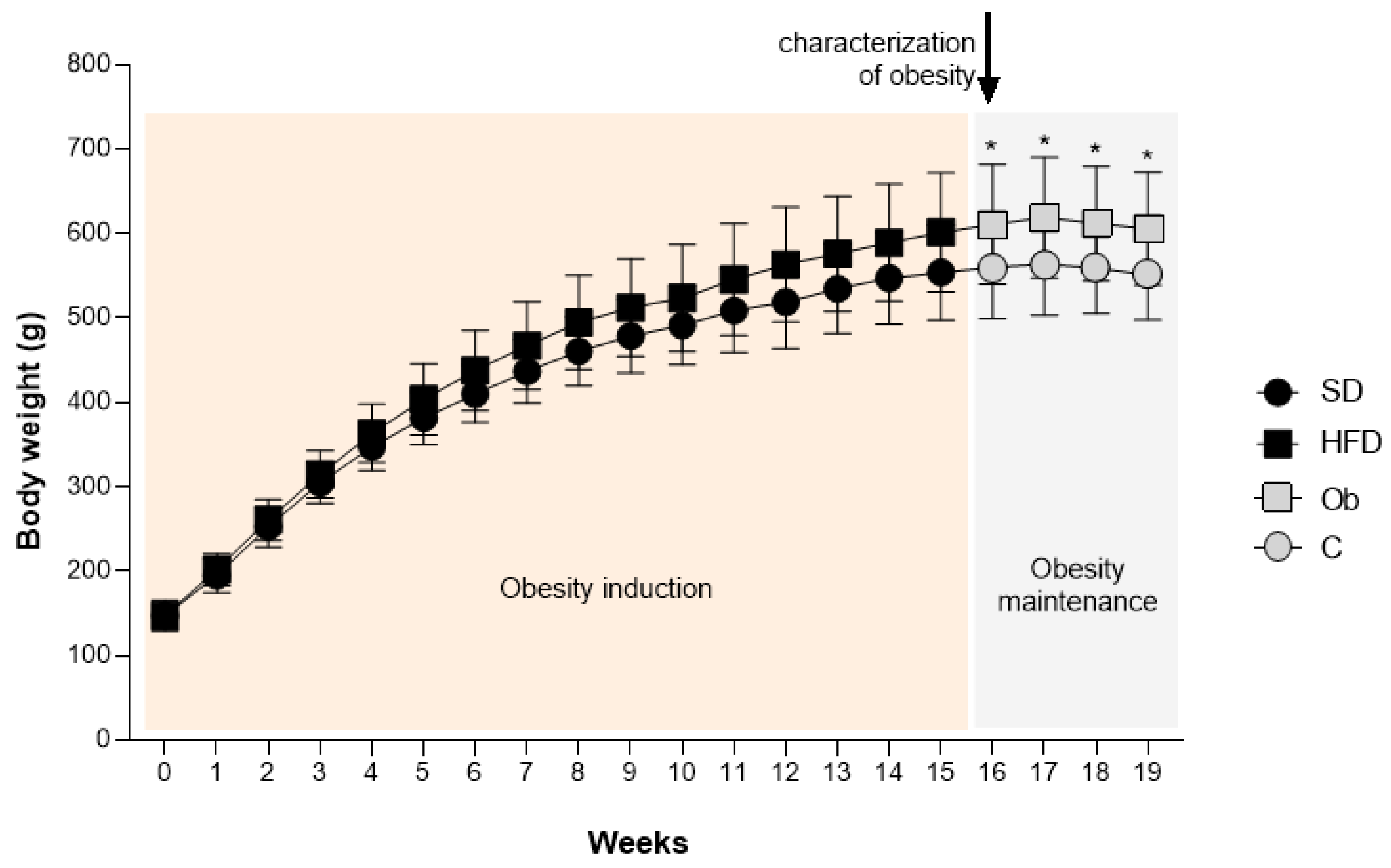
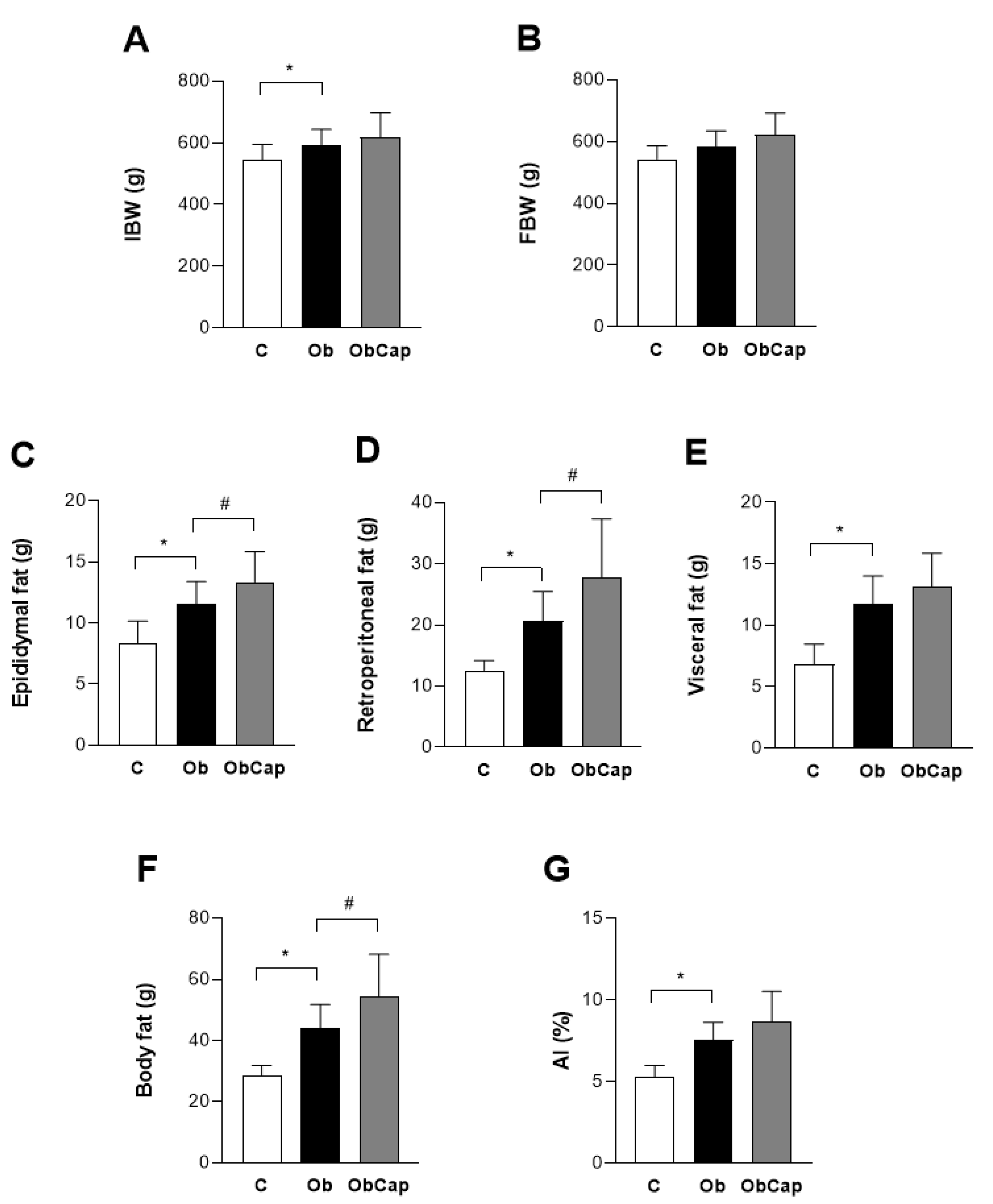
3.1. Exposure to Experimental Diets
3.2. Exposure to Capsinoids Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Čolak, E.; Pap, D. The role of oxidative stress in the development of obesity and obesity-related metabolic disorders. J. Med. Biochem. .40. [CrossRef]
- Hawkes, C.; Fanzo, J.; Udomkesmalee, E. Nourishing the SDGs: Global Nutrition Report 2017. Bristol: Development Initiatives Poverty Research Ltd. 2017.
- Mosqueda-Solís, A.; Sánchez, J.; Reynés, B.; Palou, M.; Portillo, M.P.; Palou, A.; Picó, C. Hesperidin and capsaicin, but not the combination, prevent hepatic steatosis and other metabolic syndrome-related alterations in western diet-fed rats. Sci. Rep. 1510; .8. [Google Scholar] [CrossRef]
- Zheng, J.; Zheng, S.; Feng, Q.; Zhang, Q.; Xiao, X. Dietary capsaicin and its anti-obesity potency: from mechanism to clinical implications. Biosci. Rep. 2017; .37. [Google Scholar] [CrossRef]
- World Obesity Federation (WOF). World obesity atlas 2024. Disponível em: https://data.worldobesity.org/publications/?cat=22. (Accessed in March, 2024).
- World Health Organization (WHO). Obesity and overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed in May, 2023).
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, Oxidative Stress, and Obesity. Int. J. Mol. Sci. 3117; .12. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. .3. [CrossRef]
- Koenen, M.; Hill, M.A.; Cohen, P.; Sowers, J.R. Obesity, adipose tissue and vascular dysfunction. Circ. Res. 7. [CrossRef]
- Salehi, B.; Martorell, M.; Arbiser, J.L.; Sureda, A.; Martins, N.; Maurya, P.K.; Sharifi-Rad, M.; Kumar, P.; Sharifi-Rad, J. Antioxidants: positive or negative actors? Biomolecules. .8. [CrossRef]
- Martelli, F.; Nunes, F.M.F. Radicais livres: em busca do equilíbrio. Ci. e Cult, .66. [CrossRef]
- Ferreira, L.G.; Lunz, W. Tópicos em fisiologia e bioquímica com ênfase no exercício e treinamento físico, 1st ed.; Editora da Universidade Federal do Espírito Santo: Vitória, Brazil, 2022; pp. 53–67. [Google Scholar]
- Prasad, S.; Srivastava, S.K. Oxidative Stress and Cancer: chemopreventive and therapeutic role of triphala. Antioxidants, .9. [CrossRef]
- Jones, D.P. Redefining Oxidative Stress. Antioxid. Redox Signal. 1865; .8. [Google Scholar] [CrossRef]
- Niemann, B.; Rohrbach, S.; Miller, M.R.; Newby, D.E.; Fuster, V.; Kovacic, J.C. Oxidative Stress and Cardiovascular Risk: obesity, diabetes, smoking, and pollution: Part 3 of a 3-Part Series. J. Am. Coll. Cardiol. .70. [CrossRef]
- Münzel, T.; Camici, G.G.; Maack, C.; Bonetti, N.R.; Fuster, V.; Kovacic, J.C. Impact of Oxidative Stress on the Heart and Vasculature: Part 2 of a 3-Part Series. J. Am. Coll. Cardiol. 70. [CrossRef]
- Cote, B.; Elbarbry, F.; Bui, F.; Su, J.W.; Seo, K.; Nguyen, A.; Lee, M.; Rao, D.A. Mechanistic Basis for the Role of Phytochemicals in Inflammation-Associated Chronic Diseases. Molecules, .27. [CrossRef]
- Tran, N.; Pham, B.; LE, L. Bioactive Compounds in Anti-Diabetic Plants: from herbal medicine to modern drug discovery. Biology, .9. [CrossRef]
- Cansian, A.C.C. Efeitos da ingestão de capsinóides sobre a adiposidade corporal em ratos Wistar. Dissertação (Mestrado em Clínica Médica) - Programa de Pós-Graduação em Clínica Médica, Universidade de São Paulo, Ribeirão Preto, 2016.
- Gupta, R.; Kapoor, B.; Gulati, M.; Kumar, B.; Gupta, M.; Singh, S.K.; Awasthi, A. Sweet pepper and its principle constituent capsiate: functional properties and health benefits. Crit. Rev. Food Sci. Nutr, 7370; .62. [Google Scholar] [CrossRef]
- Snitker, S.; Fujishima, Y.; Shen, H.; Ott, S.; Pi-Sunyer, X.; Furuhata, Y.; Sato, H.; Takahashi, M. Effects of novel capsinoid treatment of fatness and energy metabolism in humans: possible pharmacogenetic implications. Am. J. Clin. Nutr, 89. [CrossRef]
- Galgani, J.E.; Ryan, D.H.; Ravussin, E. Effect of capsinoids on energy metabolism in humans. Br. J. Nutr. .1. [CrossRef]
- Sasahara, I.; Furuhata, Y.; Iwasaki, Y.; Inue, N.; Sato, H.; Watanabe, T.; Takahashi, M. Assessment of the biological similarity of three capsaicin analogs (Capsinoids) found in non-pungent Chilli Pepper (CH-Sweet) fruits. Biosc. Biotechnol. Biochem, .74. [CrossRef]
- Koch, C.E.; Lowe, C.; Pretz, D.; Steger, J.; Williamns, L.M.; Tups, A. High-fat diet induces leptin resistance in leptin-deficient mice. J. Neuroendocrinol, .26. [CrossRef]
- Irving, B.A.; Weltman, J.Y.; Patrie, J.T.; Davis, C.K.; Brock, D.W.; Swift, D.; Barret, E.J.; Gaesser, G.A.; Weltman, A. Effects of exercise training intensity on nocturnal growth hormone secretion in obese adults with the metabolic syndrome. J. Clin. Endocrinol. Metab. 1979; .94. [Google Scholar] [CrossRef]
- Kim, C.H.; Youn, J.H.; Park, J.Y.; Hong, S.K.; Park, K.S.; Park, S.W.; Suh, K.I.; Lee, K.U. Effects of high-fat diet and exercise training on intracellular glucose metabolism in rats. Am. J. Physiol. Endocrinol. Metab. 6. [CrossRef]
- Kobi, J.B.B.S.; Matias, A.M.; Gasparini, P.V.F.; Torezani-Sales, S.T.; Madureira, A.R.; Silva, D.S.; Corrêa, C.R.; Garcia, J.L.; Haese, D.; Nogueira, B.V.; Assis, A.L.E.M.; Lima-Leopoldo, A.P. ; Leopoldo A,S. High-fat, high-sucrose, and combined high-fat/high-sucrose diets effects in oxidative stress and inflammation in male rats under presence or absence of obesity. Physiol. Rep. e: v.11, n.7, 1563; .11. [Google Scholar] [CrossRef]
- Matias, A.M.; Coelho, P.M.; Marques, V.B.; Santos, L.; Assis, A.L.E.M.; Nogueira, B.V.; Lima-Leopoldo, A.P.; Leopoldo, A.S. Hypercaloric diet models do not develop heart failure, but the excess sucrose promotes contractility dysfunction. Plos One, 0228; .15. [Google Scholar] [CrossRef]
- Universidade Federal de São Paulo (UNIFESP). Guia de eutanásia para animais de ensino e pesquisa, 2019.
- Rolls, B.L.; Shide, D.J. The influence of dietary fat on food intake and body weight. Nutr. Rev. 50. [CrossRef]
- Taylor, B.A.; Phillips, S.J. Detection of obesity QTLs on mouse chromosomes 1 and 7 by selective DNA pooling. Genomics, .34. [CrossRef]
- Mendes, B.F.; Costa-Pereira, L.V.; Andrade, J.A.; Magalhães, C.O.D.; Pereira, R.R.S.; Esteves, E.A.; Cassilhas, R.C.; Andrade, E.F.; Gripp, F.; Magalhães, F.C.; Sampaio, K.H.; Amorim, F.T.; Dias-Peixoto, M.F. Superior cardiometabolic and cellular adaptive responses to multiple versus single daily sessions of high-intensity interval training in Wistar rats. Sci. Rep. 2118; 12. [Google Scholar] [CrossRef]
- Sociedade Brasileira de Diabetes (SBD). Diretrizes da Sociedade Brasileira de Diabetes 2019-2020. Clannad Editora Científica, 2019.
- Yin, F.C.; Spurgeon, H.A.; Rakusan, K.; Weisfeldt, M.L.; Lakatta, E.G. Use of tibial length to quantify cardiac hypertrophy: application in the aging rat. Am. J. Physiol. Heart Circ. Physiol, 6. [CrossRef]
- Doulberis, M.; Papaefthymiou, A.; Polyzos, S.A.; Katsinelos, P.; Grigoriadis, N.; Srivastava, D.S.; Kountouras, J. Rodent models of obesity. Minerva Endocrinol, 2020; .45. [Google Scholar] [CrossRef]
- Hariri, N.; Thibault, L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev.. 2010; v. 23, n.2, p. 270-299. [CrossRef]
- Moura e Dias, M.; Reis, S.A.; Conceição, L.L.; Sediyama, C.M.N.O.; Pereira, S.S.; Oliveira, L.L.; Peluzio, M.C.G.; Martinez, J.A.; Milagro, F.I. Diet-induced obesity in animal models: points to consider and influence on metabolic markers. Diabetol. Metab. Syndr. .13. [CrossRef]
- Feriani, A.; Bizzarri, M.; Tir, M.; Aldawood, N.; Alobaid, H.; Allagui, M.S.; Dahmash, W.; Tlili, N.; Alwasel, S.; Harrath, A.H. High-fat diet-induced aggravation of cardiovascular impairment in permethrin-treated Wistar rats. Exotoxicol. Environ. Saf. 1124. [Google Scholar] [CrossRef]
- Michicoti-Meneses, M.M.; Thompson-Bonilla, M.R.; Reyes-López, C.A.; García-Pérez, B.E.; López-Tenorio, I.I.; Ordaz-Pichardo, C.; Jaramillo-Flores, M.E. Inflammation Markers in Adipose Tissue and Cardiovascular Risk Reduction by Pomegranate Juice in Obesity Induced by a Hypercaloric Diet in Wistar Rats. Nutrients, 2577; 13. [Google Scholar] [CrossRef]
- Chapa-Oliver, A.; Mejía-Teniente, L. Capsaicin: from plants to a cancer-suppressing agent. Molecules, 21. [CrossRef]
- Hong, Q.; Xia, C.; Xiangying, H.; Quan, Y. Capsinoids supress fat accumulation via lipid metabolism. Mol. Med. Rep. 1669; 11. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, S.; Li, D.; Zhang, Y.; Tang, B.; Qiu, C.; Yang, Y.; Yang, D. Dietary Capsaicin Ameliorates Pressure Overload-Induced Cardiac Hypertrophy and Fibrosis Through the Transient Receptor Potential Vanilloid Type 1. Am. J. Hypertens. 1521; .27. [Google Scholar] [CrossRef]
- Ohnuki, K.; Haramizu, S.; Oki, K.; Watanabe, T.; Yazawa, S.; Fushiki, T. Administration of Capsiate, a Non-Pungent Capsaicin Analog, Promotes Energy Metabolism and Suppresses Body Fat Accumulation in Mice. Biosci. Biothecnol. Biochem. 2735; .65. [Google Scholar] [CrossRef]
- Vilariño-García, T.; Polonio-González, M.L.; Pérez-Pérez, A.; Ribalta, J.; Arrieta, F.; Aguilar, M.; Obaya, J.C.; Gimeno-Orna, J.A.; Iglesias, P.; Navarro, J.; Durán, S.; Pedro-Botet, J.; Sánchez-Margalet, V. Role of Leptin in Obesity, Cardiovascular Disease, and Type 2 Diabetes. Int. J. Mol. Sci, 2338; .25. [Google Scholar] [CrossRef]
- Bays, H.E.; Kirkpatrick, C.F.; Maki, K.C.; Toth, P.P.; Morgan, R.T.; Tondt, J.; Christensen, S.M.; Dixon, D.L.; Jacobson, T.A. Obesity, Dyslipidemia, and Cardiovascular Disease: A joint expert review from the Obesity Medicine Association and the National Lipid Association 2024. J. Clin. Lipidol, .18. [CrossRef]
- Tani, Y.; Fujioka, T.; Sumioka, M.; Furuichi, Y.; Hamada, H.; Watanabe, T. Effects of capsinoid on serum and liver lipids in hyperlipidemic rats. J. Nutr. Sci. Vitaminol, .50. [CrossRef]
- Zhang, L.; Fang, G.; Zheng, L.; Chen, Z.; Liu, X. The hypocholesterolemic effect of capsaicinoids in ovariectomized rats fed with a cholesterol-free diet was mediated by inhibition of hepatic cholesterol synthesis. Food Funct. 4. [CrossRef]
- Pereira-Lancha, L.O.; Campos-Ferraz, P.L.; Lancha Junior, A.H. Obesity: considerations about etiology, metabolism, and the use of experimental models. Diabetes Metab. Syndr. Obes. .5. [CrossRef]
- Han, Y.; Sun, Q.; Chen, W.; Gao, Y.; Ye, J.; Chen, Y.; Wang, T.; Gao, L.; Liu, Y.; Yang, Y. New advances of adiponectin in regulating obesity and related metabolic syndromes. J. Pharm. Anal, 1009; .14. [Google Scholar] [CrossRef]
- Cavalera, M.; Wang, J.; Frangogiannis, N.G. Obesity, metabolic dysfunction and cardiac fibrosis: pathophysiologic pathways, molecular mechanisms and therapeutic opportunities. Transl. Res. .4. [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; St-Onge, M.P. ; American Heart Association Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation. 1010; .21. [Google Scholar] [CrossRef]
- Ilkun, O.; Boudina, S. Cardiac dysfunction and oxidative stress in the metabolic syndrome: an update on antioxidant therapies. Curr. Pharm. Des, 4806; .19. [Google Scholar] [CrossRef]
- Gasparini, P.V.F.; Matias, A.M.; Torezani-Sales, S.; Kobi, J.B.B.S.; Siqueira, J.S.; Corrêa, C.R.; Leopoldo, A.P.L.; Leopoldo, A.S. High-Fat and Combined High-Fat and Sucrose Diets Promote Cardiac Oxidative Stress Independent of Nox2 Redox Regulation and Obesity in Rats. Cell. Physiol. Biochem, .55. [CrossRef]
- Wang, Q.; Ma, S.; Li, D.; Zhang, Y.; Tang, B.; Qiu, C.; Yang, Y.; Yang, D. Dietary Capsaicin Ameliorates Pressure Overload-Induced Cardiac Hypertrophy and Fibrosis Through the Transient Receptor Potential Vanilloid Type 1. Am. J. Hypertens. 1521; .27. [Google Scholar] [CrossRef]
- Gao, F.; Liang, Y.; Lu, Z.; Li, L.; Zhu, S.; Liu, D.; Yan, Z.; Zhu, Z. TRPV1 Activation Attenuates High-Salt Diet-Induced Cardiac Hypertrophy and Fibrosis through PPAR-δ Upregulation. PPAR Res, 2014. [Google Scholar] [CrossRef]
- Ohyama, K.; Suzuky, K. Dihydrocapsiate improved age-associated impairments in mice by increasing energy expenditure. Am. J. Physiol. Endocrinol. Metab, .5. [CrossRef]
- Bhardwaj, A.; Baran, D.A. Biomarkers in HF: How Does the “Weight” Weigh in? J. Card. Fail. 1132; .29. [Google Scholar] [CrossRef]
- Horiuchi, Y.; Wettersten, N.; Vanveldhuisen, D.J.; Mueller, C.; Nowak, R.; Hogan, C.; Contas, M.C.; Cannon, C.M.; Birkhahn, R.; Vilke, G.M.; Mahon, N.; Nuñez, J.; Briguori, C.; Duff, S.; Murray, P.T.; Maisel, A. The Influence of Body Mass Index on Clinical Interpretation of Established and Novel Biomarkers in Acute Heart Failure. J. Card. Fail, 1121; .29. [Google Scholar] [CrossRef]
- Huang, W.; Rubistein, J.; Prieto, A.R.; Thang, L.V.; Wang, D.H. TRPV1 gene deletion exacerbates inflammation and atypical cardiac remodeling after myocardial infarction. Hypertension, .53. [CrossRef]
- Panth, N.; Paudel, K.R.; Parajuli, K. Reactive Oxygen Species: a key hallmark of cardiovascular disease. Advances In Medicine, 2016. [Google Scholar] [CrossRef]
- Rosa, A.; Deiana, M.; Corona, G.; Atzeri, A.; Incani, A.; Appendino, G.; Dessi, M.A. Protective effect of capsinoid on lipid peroxidation in rat tissues induced by Fe-NTA. Free Radic. Res. 1155; 39. [Google Scholar] [CrossRef]
- McCarty, M.F.; Dinicolantonio, J.J.; O'keefe, J.H. Capsaicin may have important potential for promoting vascular and metabolic health. Open Heart, 0002; .2. [Google Scholar] [CrossRef]
- Näpänkangas, J.P.; Liimatta, E.V.; Joensuu, P.; Bergmann, U.; Ylitalo, K.; Hassinen, I.E. Superoxide production during ischemia–reperfusion in the perfused rat heart: A comparison of two methods of measurement. J. Mol. Cell. Cardiol. 53. [CrossRef]
- Demirci-Çekiç, S.; Özkan, G.; Avan, C.N.; Uzunboy, S.; Çapanoğlu, E.; Apak, R. Biomarkers of Oxidative Stress and Antioxidant Defense. J. Pharm. Biomed. Anal. 1144. [Google Scholar] [CrossRef]
- Vona, R.; Gambardella, L.; Cittadini, C.; Straface, E.; Pietraforte, D. Biomarkers of oxidative stress in metabolic syndrome and associated diseases. Oxid. Med. Cell. Longev, 2019. [Google Scholar] [CrossRef]
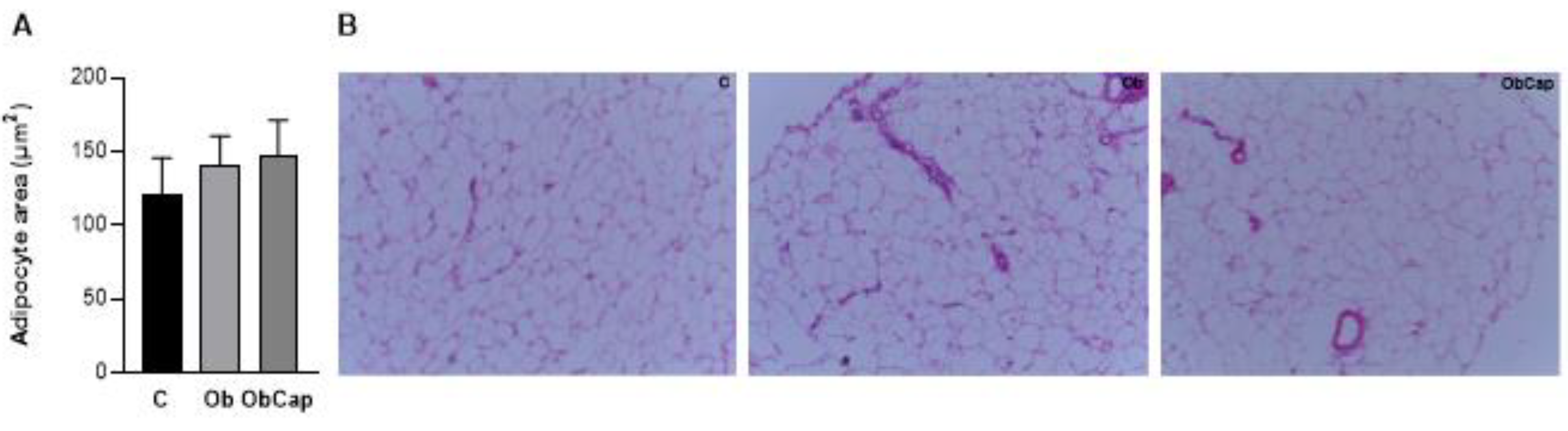
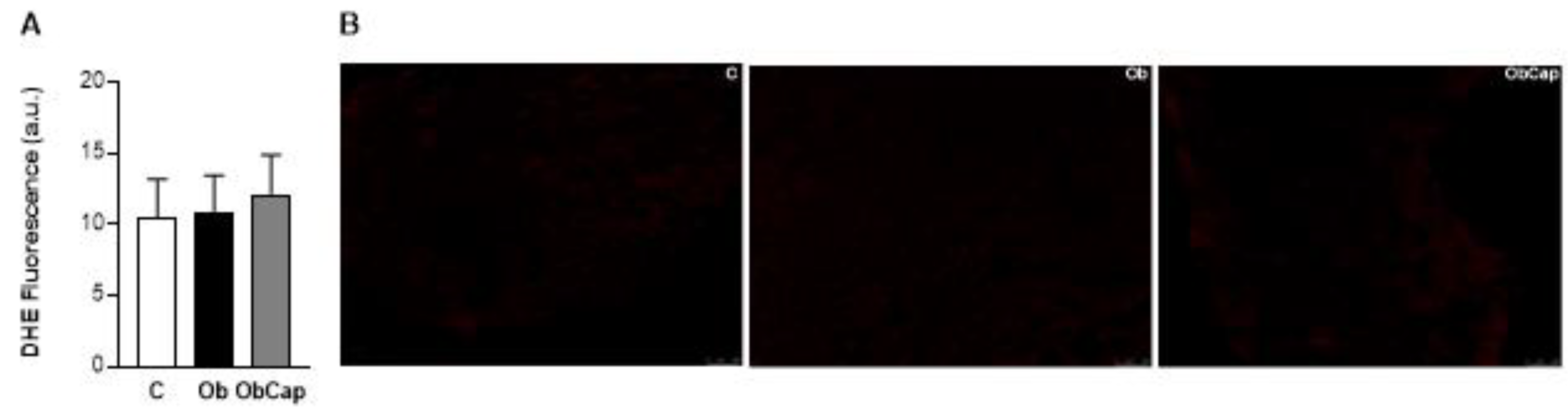
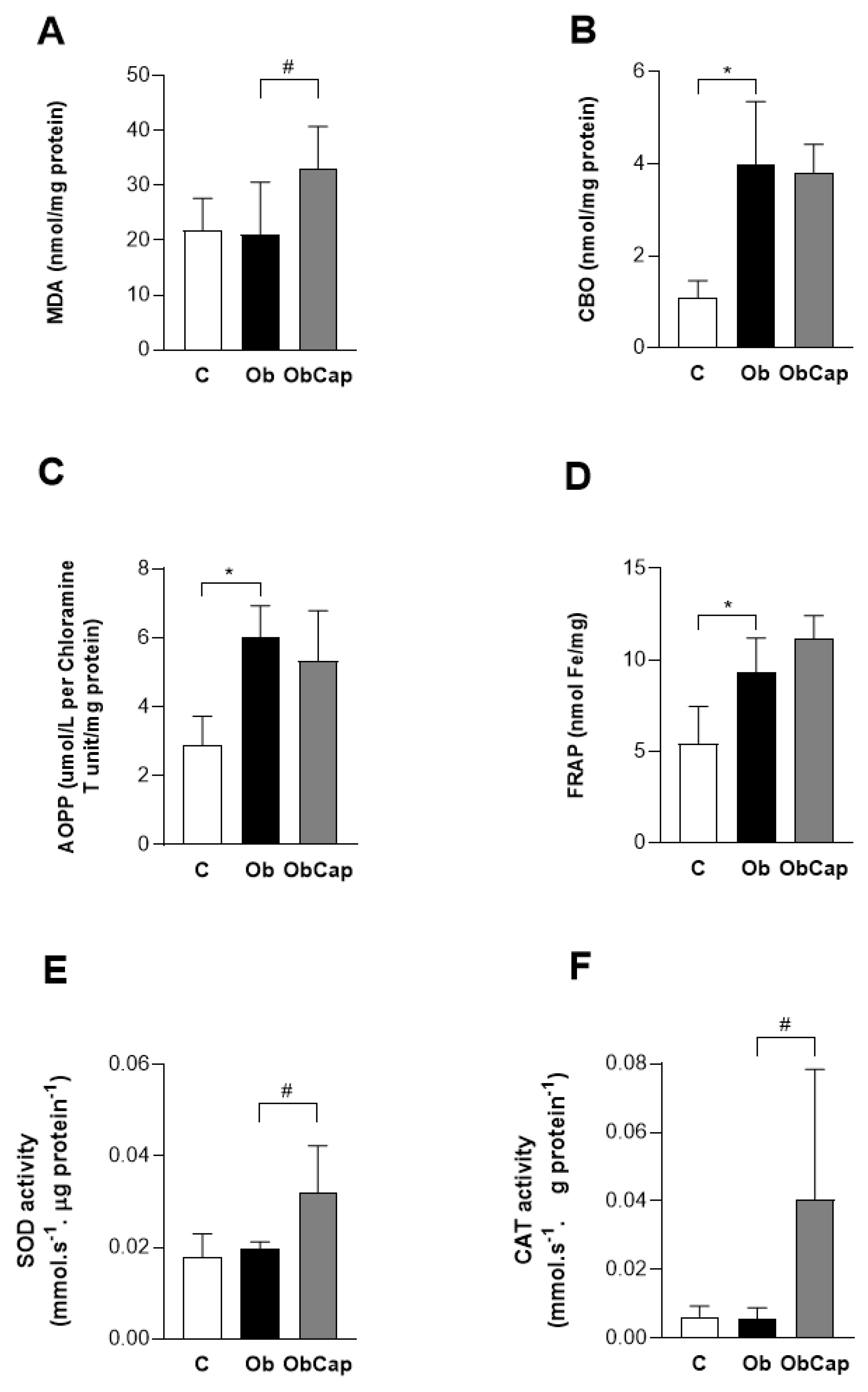
| Variable | Groups | ||
|---|---|---|---|
| C | Ob | ObCap | |
| Cholesterol (mg/dL) | 41.8 ± 9.0 | 70.8 ± 6.8* | 57.5 ± 18.2# |
| Triglycerides (mg/dL) | 25.5 ± 9.2 | 32.1 ± 17,.1 | 19.5 ± 6.4 |
| HDL (mg/dL) | 15.9 ± 3.3 | 18.2 ± 3.3 | 17.5 ± 5.3 |
| LDL (mg/dL) | 14.9 ± 5.4 | 13.1 ± 2.7 | 12.9 ± 3.3 |
| Glucose (mg/dL) | 90.2 ± 12,2 | 102 ± 8 | 118 ± 7# |
| AUC for glucose (mg/dL.min) | 936 ± 144 | 943 ± 106 | 1071 ± 178 |
| Insulin (pg/mL) | 35.4 ± 9.0 | 80.8 ± 19.9* | 73.1 ± 11.3 |
| HOMA-IR | 0.23 ± 0.07 | 0.57 ± 0.14* | 0.62 ± 0.09 |
| Leptin (ng/mL) | 1.81 ± 0.60 | 2.90 ± 0.30* | 3.03 ± 0,38 |
| Glucagon (ng/mL) | 0.14 ± 0.02 | 0.12 ± 0.04 | 0.11 ± 0,01 |
| Adiponectin (ng/mL) | 37.7 ± 11.5 | 43.5 ± 10.1 | 45.5 ± 5.5 |
| Variable | Groups | ||
|---|---|---|---|
| C | Ob | ObCap | |
| Heart (g) | 1.28 ± 0.13 | 1.51 ± 0.17* | 1.47 ± 1.10 |
| Hearth/tibia length (g/cm) | 0.30 ± 0.03 | 0.37 ± 0.03 | 0.33 ± 0.02 |
| LV (g) | 0.72 ± 0.12 | 0.99 ± 0.22* | 1.06 ± 0.07 |
| LV/tibia length (g/cm) | 0.17 ± 0.03 | 0.22 ± 0.05 | 0.24 ± 0.01 |
| Troponin I (pg/mL) | 15.32 ± 3.24 | 18.46 ± 3.28 | 12.37 ± 2.79# |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





