1. Introduction
The prevalence of limb loss is increasing globally due to various factors such as road accidents, diseases like diabetes, and conflicts, with the United States alone accounting for approximately 185 thousand amputees [
1]. Limb loss significantly impacts individuals' ability to perform daily tasks, affecting their overall quality of life [
2]. The limb amputees have been expecting the high-performance artificial upper limbs to restore the motion functions involved in their lost arms. Therefore, it is crucial to explore advanced technologies, algorithms, and surgical interventions to restore motor and sensory functions in individuals with limb loss [
3,
4,
5,
6,
7]
Traditionally, body-powered mechanical prostheses were developed and used for amputees to restore their lost limb functions. With the limited dexterity and indirect control mechanisms, the body-powered limb prostheses are limited in utility, frustratingly slow to operate, which pose challenges in achieving intuitive functionality [
8]. To improve the prosthetic performance, a lot of progress has been made in the development of motorized limb prostheses that are primarily controlled by electromyographic (EMG) signals detected from the skin surface overlying the residual muscles. The most significant improvement for myoelectric prostheses should be the EMG pattern recognition based control strategy [
9], in which the distinguishing characteristics of EMG patterns can be used to identify different intended movements with a trained classifier. While EMG pattern recognition based control would allow amputees to easily and intuitively operate their myoelectric prostheses, this control strategy will be inapplicable for people with above-elbow amputations because few muscles remain in their residual arm from which to extract myoelectric control signals. To address this challenge, a new neural machine interfacing technology called targeted muscle reinnervation (TMR) have been proposed and developed at Rehabilitation Institute of Chicago, which has the ability to improve control performance of multifunctional myoelectric upper-limb prostheses [
10] by redirecting stump motor nerves into specific muscles to provide richer EMG signals. The previous studies have showed that the approach enabled more intuitive and parallel control of advanced multifunctional myoelectric prostheses [
10,
11,
12]. Recently, our investigation revealed that TMR could potentially restore motor function in cases of transected median nerves, even when there was a delay in transferring them to their targets. Interestingly, we observed no significant electrophysiological disparities between immediate and delayed transfers [
13]. However, it's worth noting that despite these advancements, the subject did not experience restoration of proprioceptive feedback, such as limb position and velocity.
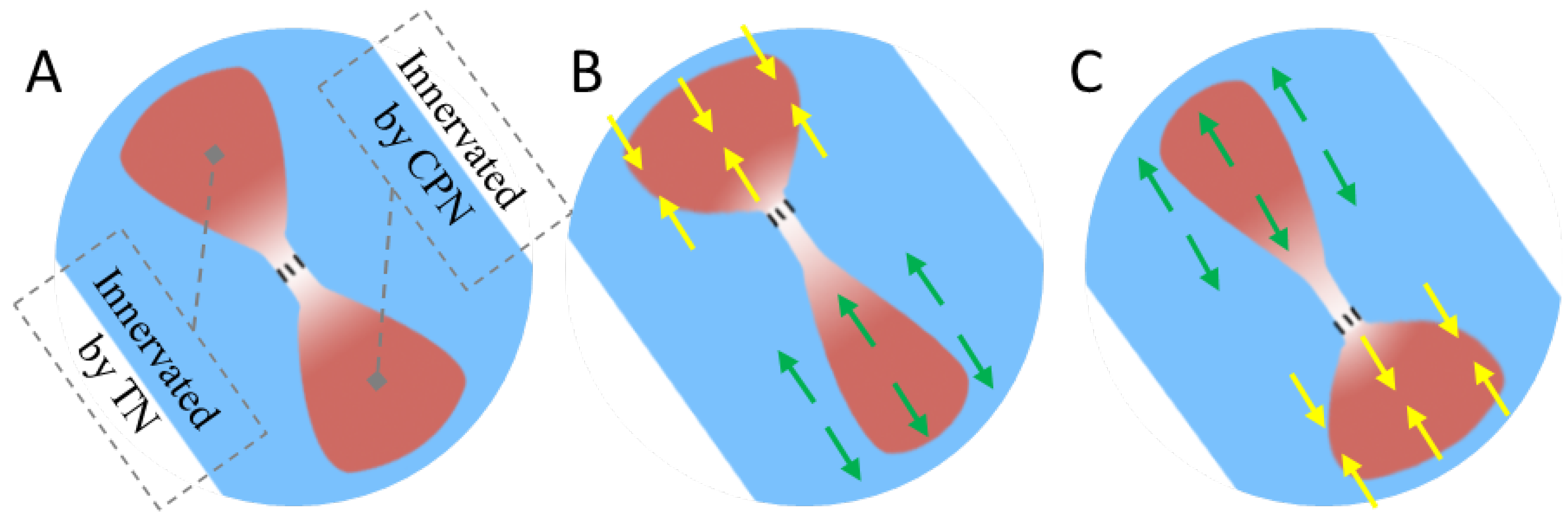
Recently, an innovative approach known as the agonist-antagonist myoneural interface (AMI) has emerged as a potential method to provide proprioceptive feedback. This technique leverages muscle spindle receptors located in both an agonist and antagonist muscle pair connected by a tendon [
14]. In their pioneering work, Clites et al. established a surgical model for AMI by implanting muscle donors innervated by the tibial nerve (TN) and common peroneus nerve (CPN), and subsequently connecting them via their distal tendons. Following a period of rehabilitation, recordings of compound action potentials (CAPs) from the nerve supplying the antagonist muscle during agonist contraction revealed the induction of proprioceptive sensations mediated by muscle spindle receptors within the artificial AMI construct [
15].
Figure 1 shows an example of the AMI concept where when the agonist contracts, the antagonist is triggered to stretch. While the AMI is regarded as a promising technique in both motor and proprioception function reconstruction for amputated subjects [
16,
17], it encounters several challenges. Firstly, the process of implanted nerves reinnervating targeted muscles is prolonged, contributing to delays in functional recovery [
18,
19]. Moreover, preventing denervation and atrophy in the artificial agonist-antagonist system due to lack of neural nutrition and adequate blood circulation present a significant challenge [
20], which would weaken the reinnervation efficacy of AMI.
In recent decades, electrical muscle stimulation (EMS) has emerged as a dependable modality for enhancing rehabilitation following various surgeries, including nerve repair [
21,
22] and muscle atrophy [
23,
24]. These inspired us to know if the EMS treatment on the AMI muscles can significantly improve the electrophysiological performance of AMI. In this study, we aimed to investigate the potential of EMS in improving the reinnervation outcomes of AMI in animal models, along with exploring any associated electrophysiological changes.
2. Methods
2.1. Surgery
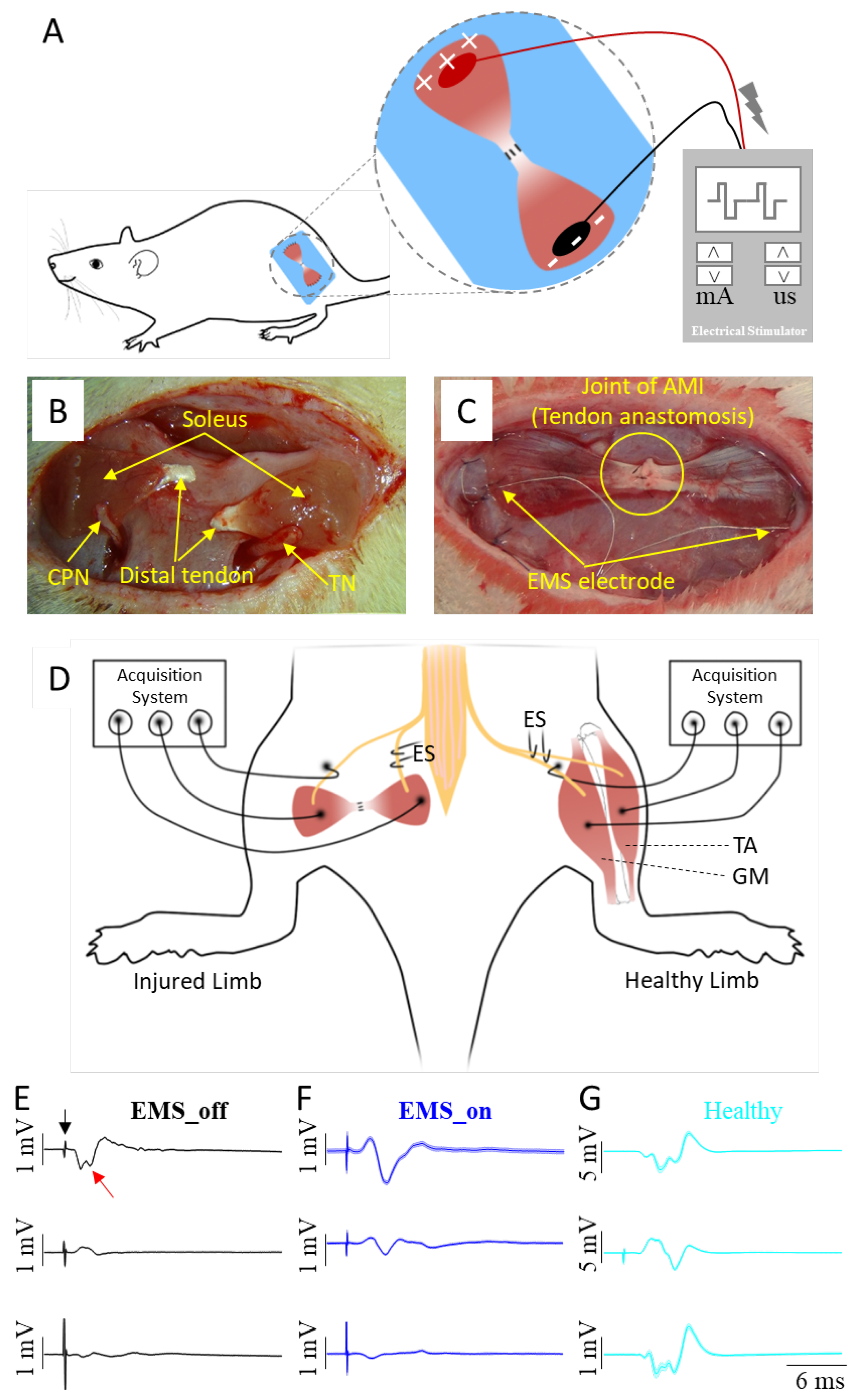
A total of 14 adult male Sprague Dawley rats, bred under Specific Pathogen-Free (SPF) conditions and weighing approximately 260 grams each, were used in this study. Prior to surgery, the rats were anesthetized using 5% isoflurane in an enclosed chamber, followed by maintenance anesthesia at 2% isoflurane on an operation table equipped with a heating pad. The fur covering the bilateral hind limbs was shaved to prevent contamination during surgery. To minimize the risk of bacterial infection, the skin was sterilized using 75% medical alcohol and povidone-iodine solution. The experimental procedures were conducted in accordance with the guidelines provided by the Committee on Use and Care of Animals of the Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences (SIAT-IACUC-210907-JCS-HJP-A2050).
Figure 2A illustrates the EMS paradigm, while
Figure 2B outlines the key steps involved in the AMI surgery, which includes nerve implantation and tendon anastomosis. Specifically, the surgical procedure involved four incisions made on the sterilized skin. The first two incisions were made along the lateral tibia to expose and harvest the distal parts of the donor soleus muscles. The third incision was made along the left femur to expose the biceps femoris, where the two donor solei were respectively implanted with the ipsilateral tibial nerve (TN) and the common peroneal nerve (CPN), and connected by tendon anastomosis to construct an artificial AMI. The two ends of the artificial AMI were sutured onto the surface of the biceps femoris with appropriate tension. The fourth incision was made on the scalp to expose the parietal bone. At the third incision site, a connector containing two insulated stainless steel electrodes was subcutaneously pulled to the fourth incision and secured to the surface of the parietal bone using dental cement. Two stainless steel stimulating electrode ends with a 5 mm length of insulating layer peeled off were affixed onto the ends of the agonist and antagonist muscles, respectively, as depicted in
Figure 2C. Meanwhile, the contralateral healthy TN and its GM, as well as the CPN and its TA, served as the natural AMI.
Following the surgical procedures, all incisions were closed, and each rat received intraperitoneal injections of penicillin sodium (dose: 100,000 IU/rat) and meloxicam (dose: 2 mg/kg) for three consecutive days to prevent infection and alleviate pain. Subsequently, each rat was housed individually in a cage with a 12-hour light-dark cycle in a SPF room, with free access to food and water. One day post-surgery, all rats were randomly assigned to either the EMS_on group (undergoing electrical muscle stimulation) or the EMS_off group (not subjected to electrical muscle stimulation).
2.2. Electrical Muscle Stimulation
Electrical muscle stimulation (EMS) was initiated following the implantation of the electrical stimulating electrodes. A tetanic contraction movement could be induced and perceived by palpation of the skin covering each artificial AMI in all rats. Beginning one day after surgery, rats in the EMS_on group received EMS treatments using a NeuroTrac electrical stimulating equipment (Verity Medical, Inc.), while rats in the EMS_off group received sham treatments with the power of the stimulating equipment turned off. The parameters of the electrical muscle stimulation were set as follows: 100 Hz frequency, 200 μs pulse width, 600 tetanic contractions per day, administered for 60 minutes per day, six days per week, with a current intensity ranging from 2 mA to 6 mA until a proper contraction could be sensed [
23]. Following the four-week EMS treatment period, an additional four-week period was allotted to allow for complete and natural recovery.
2.3. Neural Data Acquisition
In a healthy neuromuscular system, when the agonist muscle contracts, the relative antagonist muscle is simultaneously stretched. This stretching activates stretch-sensitive mechanoreceptors in the muscle and tendon, which then transmit impulses to the brain via ascending pathways. Consequently, an action potential related to proprioception sensation can be detected by a sensor on the peripheral nerve during this excitatory conduction process. To assess the efficacy of the constructed AMI, the study recorded the CAP of the nerve innervating the antagonist muscle during excitation of the agonist muscle by electrical stimulation. This CAP was considered a manifestation of proprioceptive sensation and may serve as a valuable additional signal for the recipient to perceive parameters such as prosthetic limb velocity and joint rotation angle.
Eight weeks post-surgery, all animals were anaesthetized with pentobarbital sodium (50 mg/kg body weight) via intraperitoneal injection. The hair covering the bilateral hind limbs was then shaved off, and the animals were placed in a prone position on a heating pad. An incision was made to expose both the artificial AMI and the natural AMI. Mineral oil was applied to prevent tissue desiccation, and the nerves supplying the muscles were carefully dissected from the surrounding tissue. During the procedure, as depicted in
Figure 2D, either TNs or CPNs were electrically stimulated. Three recording electrodes were placed in contact with the agonist muscle, antagonist muscle, and the nerve of the antagonist muscle, respectively. To minimize the impact of stimulating currents on the compound action potential (CAP), the ground electrode was intentionally positioned 3 mm distally from the stimulating electrode. Additionally, to reduce the influence of muscle compound action potentials on nerve compound action potentials, the reference electrode was placed closer to the recorded nerve but farther from the muscles.
Two additional verification steps were implemented to ensure the integrity of the collected CAPs. Firstly, the tendon junction was severed to induce CAPs exclusively in the agonist muscle. Secondly, the stimulated nerve was completely crushed to verify the absence of CAPs in all sites. In this study, neural data acquisition was facilitated using the OmniPlex Express system (Plexon USA, Inc.) to record the evoked CAPs, while an electrical stimulator (StimuPlex Express, Plexon USA, Inc.) was employed to perform electrical stimulation. The stimulating current was gradually increased until a maximal action potential was observed, after which the stimulus intensity was set at 120% for recording CAPs of both the muscle and nerve. The constant parameters for evoking CAPs included a pulse width of 20 μs and a bipolar rectangle wave. Examples of recorded CAP signals from the agonist-antagonist myoneural interface in both EMS_on and EMS_off, and the healthy limb are illustrated in
Figure 2E–G.
Motor unit number estimation (MUNE) is a technique used to assess the physiological status of the neuromuscular system. Arnold et al. and Gooch et al. conducted a comprehensive experimental investigation to derive a numerical estimation of MUNE [
25,
26]. They recorded ten grades of CAPs from the muscle and calculated the average amplitude increment to estimate the average single motor unit potential (SMUP) amplitude. The CAP of the muscle was defined as the maximal value attained when a stronger current stimulus failed to elicit a response. Subsequently, MUNE was calculated by dividing the CAP amplitude of the muscle by the SMUP amplitude.
When a transferred TN or CPN successfully reinnervates its targeted muscle, it can transmit motor impulses and evoke muscle contraction. CAPs can be recorded from the muscle when a supraliminal electrical stimulus is applied to its nerve. In this context, the propagation time refers to the duration from the onset of the electrical stimulus to the onset of the CAP, while the propagation distance is measured from the stimulus position at the nerve to the recording location at the muscle. Therefore, NCV is defined as the ratio of the propagation distance to the propagation time.
2.4. Statistical Analysis
To assess the statistical significance of the effect of electrical muscle stimulation (EMS) on improving the agonist-antagonist myoneural interface in the experimental group and compare it with the control group, we conducted a one-way analysis of variance (ANOVA) followed by Bonferroni post-hoc test, with a significance level p < 0.05. The statistical analysis was performed using the SPSS software (version 21.0, IBM Corp., Chicago, IL).
3. Results
On average, the duration of the 14 AMI surgeries was approximately 2 hours and 15 minutes. Furthermore, all rats successfully survived the surgery and demonstrated a gradual improvement in their physiological status throughout the rehabilitation process.
3.1. Evaluation Based on Compound Action Potential (CAP)
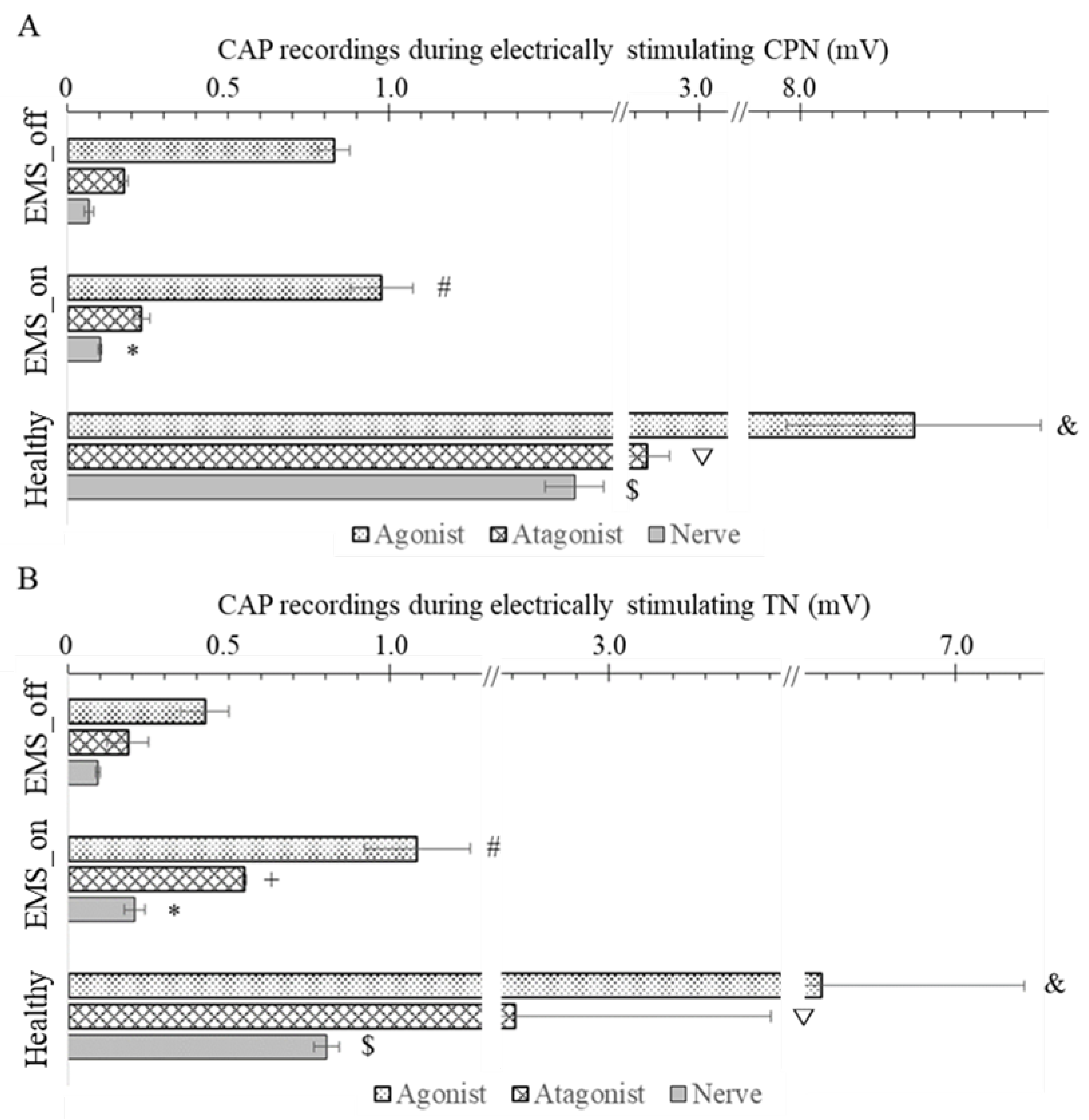
The experimental observations depicted in
Figure 3A and 3B reveal distinctive patterns in the CAP among healthy and artificial agonist-antagonist systems. In a healthy system, the highest CAP amplitude occurred in the agonist muscle directly evoked by electrical impulses, followed by the stretched antagonist muscle, and finally, the nerve supplying the antagonist muscle exhibited the lowest CAP amplitude. Similarly, the artificial agonist-antagonist system displayed a comparable trend, but with lower CAP amplitudes overall. However, in the artificial system, both the EMS_on and EMS_off groups exhibited lower CAP values compared to the healthy system. Notably, within the artificial system, the CAP amplitudes of both the agonist and antagonist muscles, as well as the nerve, were significantly higher in the EMS_on group compared to the EMS_off group.
Upon close examination of the results presented in
Figure 3A, focusing on the stimulation of the CPN, several noteworthy observations emerge. In the EMS_on group, the CAP amplitude of the agonist muscle was significantly higher (p = 0.04) compared to the EMS_off group, with respective values of 1.03 ± 0.10 mV and 0.83 ± 0.05 mV. However, both groups exhibited CAP amplitudes lower than those observed in the healthy group (8.35 ± 0.40 mV). Conversely, for the antagonist muscle, the CAP amplitudes in the EMS_on and EMS_off groups were 0.23 ± 0.02 mV and 0.17 ± 0.02 mV, respectively. Notably, there was no significant difference (p = 0.113) between the EMS_on and EMS_off groups in terms of antagonist muscle CAP amplitude, yet both groups displayed significantly lower values than the healthy group (2.84 ± 0.07 mV).Additionally, the CAP amplitude of the nerve in the EMS_on group was 0.10 ± 0.01 mV, which was significantly higher than that of the EMS_off group (0.07 ± 0.01 mV). However, both EMS groups exhibited CAP amplitudes markedly lower than those observed in the healthy group (1.58 ± 0.09 mV).
Similarly, when the TN was stimulated, as depicted in
Figure 3B, notable differences emerged. In the EMS_on group, the CAP amplitude of the agonist muscle was significantly higher (p = 0.037) compared to the EMS_off group, with respective values of 1.09 ± 0.17 mV and 0.43 ± 0.08 mV. However, both groups exhibited CAP amplitudes lower than those observed in the healthy group (6.58 ± 0.63 mV).Turning to the results for the antagonistic muscle, the CAP amplitudes in the EMS_on and EMS_off groups were 0.55 ± 0.01 mV and 0.19 ± 0.06 mV (p = 0.017). Additionally, both groups displayed CAP amplitudes significantly lower than the healthy group (2.71 ± 0.80 mV).Furthermore, the CAP amplitude of the nerve in the EMS_on group was 0.21 ± 0.03 mV, significantly higher than that of the EMS_off group (0.09 ± 0.01 mV). However, both EMS groups exhibited CAP amplitudes markedly lower than those observed in the healthy group (0.80 ± 0.04 mV).
3.2. Evaluation Based on Motor Unit Number
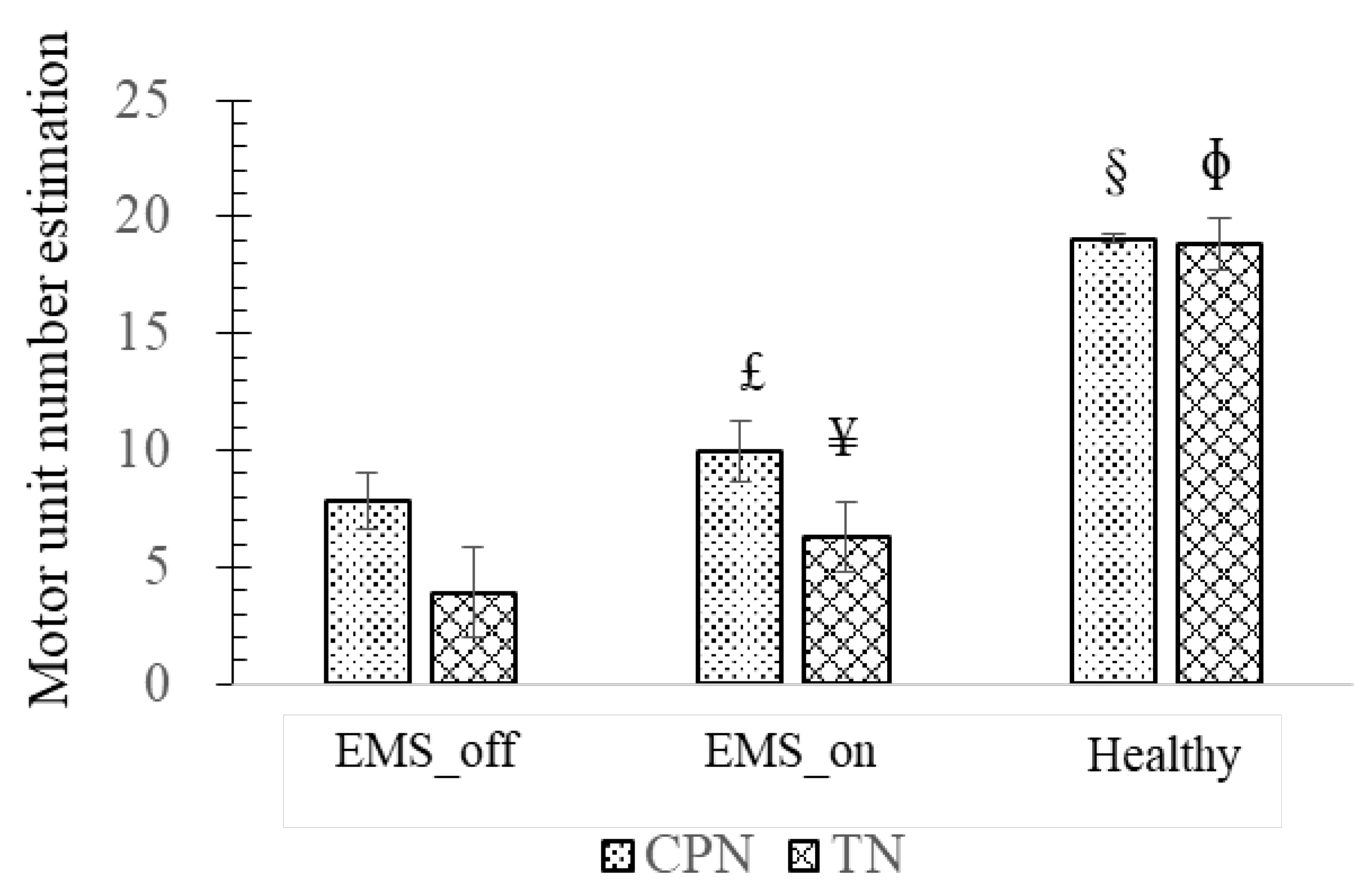
Analyzing the experimental findings presented in
Figure 4, it becomes evident that the highest MUNE is observed in the healthy agonist-antagonist system, while the lowest MUNE is noted in the EMS_off group. Focusing on the observations related to the CPN and its associated muscle, the EMS_on group exhibited a significantly higher MUNE value of 10.04 ± 1.34 motor units (p = 0.048) compared to the EMS_off group, which recorded 7.85 ± 1.21 motor units. However, both EMS groups demonstrated lower MUNE values than those observed in the healthy agonist-antagonist system (19.06 ± 0.21, p = 0).
Similarly, upon analyzing the results concerning the TN and its muscle, it is observed that the EMS_on group displayed a significantly higher MUNE (p = 0.039) than the EMS_off group. However, both EMS groups exhibited MUNE values lower than those observed in the healthy group (p = 0).
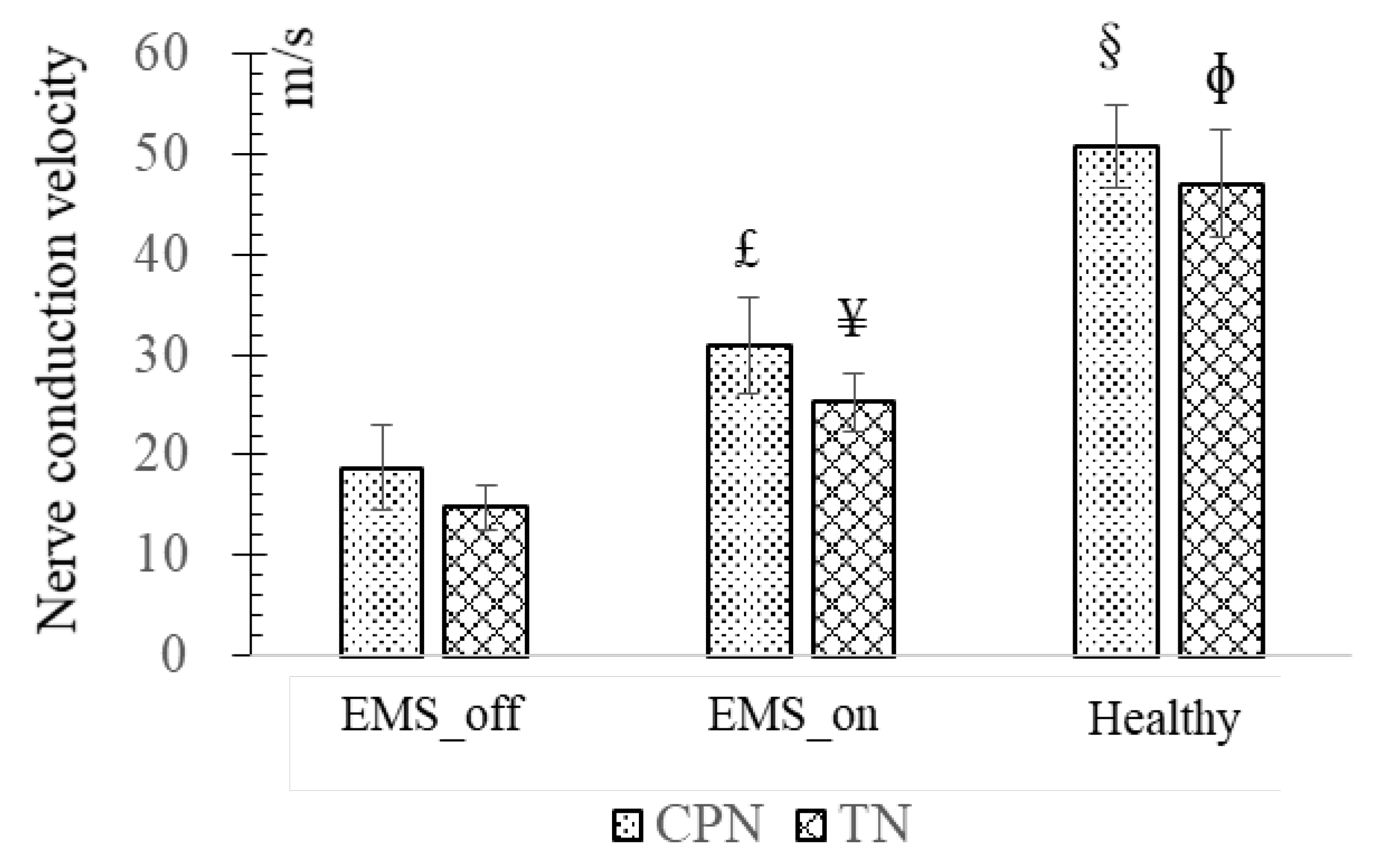
3.3. Evaluation Based on Nerve conduction Velocity (NCV)
Upon the experimental data depicted in
Figure 5, delineating the NCV across the EMS_off, EMS_on, and Healthy groups reveals distinct trends. The EMS_off group exhibited the lowest NCV values, whereas the Healthy group recorded the highest. Specifically, the average NCV of the CPN in the EMS_on group was significantly higher (30.93 ± 4.89 m/s, p = 0.026) compared to the EMS_off group (18.76 ± 4.14 m/s), although both were notably lower than the healthy counterparts. This trend was similarly observed in the TN.
5. Discussion
Limb amputation stands as the primary cause of motor and sensory impairment, and leads significant disability. Subsequent to amputation, individuals contend with the absence of motor and sensory function in the amputated limb segments, originating from the excision of terminal muscles innervated by peripheral motor nerves, thus inducing motor deficits. Nonetheless, notwithstanding this loss, the central nervous system and the majority of peripheral motor nerve pathways remain functionally intact. Additionally, the severed peripheral motor nerve within the stump retains the capacity to reinnervate skeletal muscle, endowing it with renewed purpose [
27,
28]. To harness this potential, Kuiken et al. pioneered a TMR surgical approach, aimed at augmenting EMG signal reservoirs to enable intuitive control of myoelectric prosthetic limbs [
10].
In the absence of an agonist-antagonist system post-amputation, muscle spindles in the antagonist, which are sensitive to stretch, remain inactive during agonist contraction. Consequently, amputees experience impaired proprioception, rendering them unable to recognize changes in speed, position, and posture of the prosthetic limb [
29]. Clites et al. posited that AMI surgery, based on TMR principles, could restore bidirectional efferent motor signals and afferent proprioception signals concurrently. However, challenges such as muscle disuse atrophy and nerve degeneration impede AMI surgery's ability to fully reconstruct satisfactory efferent motor and afferent somatosensory signals.
In recent decades, various treatments have emerged aimed at mitigating muscle atrophy and facilitating nerve regeneration [
21,
22,
24]. Among these interventions, EMS has gained prominence in clinical practice, demonstrating significant advancements in addressing muscle disuse atrophy and promoting nerve regeneration. Consequently, this study explores the potential of EMS as a therapeutic modality to augment the rehabilitation outcomes of individuals with AMI.
Based on the systematic experimental protocol employed in our study, we successfully reconstructed the proprioceptive conduction pathway within the AMI. Our results revealed that the agonist muscle exhibited the highest CAP amplitudes, as it was directly stimulated, followed by the antagonist muscle, while the nerve innervating the antagonist muscle demonstrated the lowest CAP amplitude. These findings align with previous research by Clites et al. [
15], which observed differential EMG amplitudes between agonist and antagonist muscles in the context of dorsiflexion and plantar flexion following AMI surgery.
Following the introduction of EMS, a discernible enhancement in nerve regeneration was observed, as evidenced by the CAP of nerves recorded in both the CPN and the TN among the EMS_on and EMS_off groups, with the EMS_on group exhibiting the highest CAP. Similarly, in terms of muscle response, a pronounced increase in CAP was noted in the agonist muscle of the EMS_on group following electrical stimulation of the TN or CPN. These findings underscore the efficacy of EMS in augmenting nerve reinnervation and facilitating proprioception restoration, thereby promoting the rehabilitation of AMI recipients. This observation aligns with the study by Fu et al. which demonstrated that adjunctive EMS application to muscles significantly improves the electrophysiological performance of denervated muscles, resulting in a stronger CAP of muscle [
30].
Furthermore, similar comparative outcomes were observed in the MUNE and NCV of the CPN and TN among the EMS_on and EMS_off groups. The MUNE of the TN and CPN in the healthy group exceeded that of the TN and CPN in the EMS_on group, while the results for the TN and CPN in the EMS_on group surpassed those of the TN and CPN in the EMS_off group. Increased MUNE levels are indicative of enhanced motor function recovery, underscoring the significance of motor units in motor function rehabilitation.
This study highlights the pivotal role of EMS in the rehabilitation of AMI subjects. By applying EMS to an artificial AMI, the ischemic agonist-antagonist system experienced forced contractions, thereby decelerating its disuse atrophy process [
23,
25]. Additionally, Amaral et al. demonstrated that EMS could significantly enhance vessel density [
31]. Elevated vessel density signifies improved physiological conditions for artificial AMI, potentially contributing to more favorable outcomes.
While this study showcased the efficacy of EMS in enhancing AMI surgery rehabilitation, achieving a state of full health remains elusive. Hence, further exploration of alternative treatments is warranted to optimize rehabilitation outcomes. Additionally, although stronger proprioception signals were recorded in the peripheral nerves of EMS_on subjects, limited investigation into central nervous system changes was conducted. Therefore, future research endeavors will focus on elucidating alterations in the central nervous system and delineating the correlation between central nervous system changes and the artificial agonist-antagonist system treated with EMS.
5. Conclusions
This study presents the initial evidence of the beneficial impact of EMS on the electrophysiological performance of rats undergoing AMI surgery. Specifically, the CAP amplitudes of the agonist muscle and the nerve were notably higher in the EMS_on group compared to the EMS_off group. Moreover, both nerve conduction velocity and motor unit number estimation were significantly elevated in the EMS_on group relative to the EMS_off group. By inducing passive muscle contractions, EMS effectively ameliorated the physiological state of the muscles and attenuated muscle degeneration resulting from neurotrophic nutrient deficiency.
References
- Owings, M.; Kozak, L.J. Ambulatory and inpatient procedures in the United States, 1996; US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics, 1998. [Google Scholar]
- Sinha, R.; Heuvel, W.J.v.D.; Arokiasamy, P. Factors affecting quality of life in lower limb amputees. Prosthetics Orthot. Int. 2011, 35, 90–96. [Google Scholar] [CrossRef]
- Roche, A.D.; Rehbaum, H.; Farina, D.; Aszmann, O.C. Prosthetic Myoelectric Control Strategies: A Clinical Perspective. Curr. Surg. Rep. 2014, 2, 1–11. [Google Scholar] [CrossRef]
- Raichle, K.A. Prosthesis use in persons with lower- and upper-limb amputation. J. Rehabilitation Res. Dev. 2008, 45, 961–972. [Google Scholar] [CrossRef]
- Raspopovic, S.; Valle, G.; Petrini, F.M. Sensory feedback for limb prostheses in amputees. Nat. Mater. 2021, 20, 925–939. [Google Scholar] [CrossRef]
- Farina, D.; Vujaklija, I.; Sartori, M.; Kapelner, T.; Negro, F.; Jiang, N.; Bergmeister, K.; Andalib, A.; Principe, J.; Aszmann, O.C. Man/machine interface based on the discharge timings of spinal motor neurons after targeted muscle reinnervation. Nat. Biomed. Eng. 2017, 1, 0025. [Google Scholar] [CrossRef]
- Resnik, L.; Borgia, M.; Clark, M. Function and Quality of Life of Unilateral Major Upper Limb Amputees: Effect of Prosthesis Use and Type. Arch. Phys. Med. Rehabilitation 2020, 101, 1396–1406. [Google Scholar] [CrossRef]
- Geethanjali, P. Myoelectric control of prosthetic hands: state-of-the-art review. Med. Devices 2016, 2016, 247–255. [Google Scholar] [CrossRef]
- Hudgins, B.; Parker, P.; Scott, R.N. A new strategy for multifunction myoelectric control. IEEE Trans. Biomed. Eng. 1993, 40, 82–94. [Google Scholar] [CrossRef]
- Kuiken, T.A.; Li, G.; Lock, B.A.; Lipschutz, R.D.; Miller, L.A.; Stubblefield, K.A.; Englehart, K.B. Targeted Muscle Reinnervation for Real-time Myoelectric Control of Multifunction Artificial Arms. JAMA 2009, 301, 619–628. [Google Scholar] [CrossRef]
- Kuiken, T.A.; Dumanian, G.A.; Lipschutz, R.D.; Miller, L.A.; Stubblefield, K.A. The use of targeted muscle reinnervation for improved myoelectric prosthesis control in a bilateral shoulder disarticulation amputee. Prosthetics Orthot. Int. 2004, 28, 245–253. [Google Scholar] [CrossRef]
- Kuiken, T.; et al. Targeted muscle reinnervation for improved myoelectric prosthesis control. in Conference Proceedings. 2nd International IEEE EMBS Conference on Neural Engineering, 2005. 2005. IEEE.
- Li, Y.; Huang, J.; Chen, Y.; Zhu, S.; Huang, Z.; Yang, L.; Li, G. Nerve function restoration following targeted muscle reinnervation after varying delayed periods. Neural Regen. Res. 2023, 18, 2762–2766. [Google Scholar] [CrossRef] [PubMed]
- Clites, T.R.; Carty, M.J.; Srinivasan, S.; Zorzos, A.N.; Herr, H.M. A murine model of a novel surgical architecture for proprioceptive muscle feedback and its potential application to control of advanced limb prostheses. J. Neural Eng. 2017, 14, 036002. [Google Scholar] [CrossRef] [PubMed]
- Clites, T.R.; Herr, H.M.; Srinivasan, S.S.; Zorzos, A.N.; Carty, M.J. The Ewing Amputation: The First Human Implementation of the Agonist-Antagonist Myoneural Interface. Plast. Reconstr. Surg. - Glob. Open 2018, 6, e1997. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.S.; Diaz, M.; Carty, M.; Herr, H.M. Towards functional restoration for persons with limb amputation: A dual-stage implementation of regenerative agonist-antagonist myoneural interfaces. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Herr, H. and M. Carty. The Agonist-antagonist Myoneural Interface. Techniques in Orthopaedics, 2021. Publish Ahead of Print.
- Langer, H.T.; Senden, J.M.G.; Gijsen, A.P.; Kempa, S.; van Loon, L.J.C.; Spuler, S. Muscle Atrophy Due to Nerve Damage Is Accompanied by Elevated Myofibrillar Protein Synthesis Rates. Front. Physiol. 2018, 9, 1220. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, W.; Gordon, T. Neurobiology of peripheral nerve injury, regeneration, and functional recovery: from bench top research to bedside application. Ochsner Journal 2013, 13, 100–8. [Google Scholar] [PubMed]
- Zhang, D.-Y.; Jiang, B.-G.; Weng, J.; Wang, Y.-H.; Li, M. GSK3β inhibitor promotes myelination and mitigates muscle atrophy after peripheral nerve injury. Neural Regen. Res. 2018, 13, 324–330. [Google Scholar] [CrossRef]
- Willand, M.P.; Nguyen, M.-A.; Borschel, G.H.; Gordon, T. Electrical Stimulation to Promote Peripheral Nerve Regeneration. Neurorehabilit. Neural Repair 2015, 30, 490–496. [Google Scholar] [CrossRef]
- Gordon, T. Electrical Stimulation to Enhance Axon Regeneration After Peripheral Nerve Injuries in Animal Models and Humans. Neurotherapeutics 2016, 13, 295–310. [Google Scholar] [CrossRef]
- Willand, M.P.; Chiang, C.D.; Zhang, J.J.; Kemp, S.W.P.; Borschel, G.H.; Gordon, T. Daily Electrical Muscle Stimulation Enhances Functional Recovery Following Nerve Transection and Repair in Rats. Neurorehabilit. Neural Repair 2014, 29, 690–700. [Google Scholar] [CrossRef]
- Nakagawa, K.; Tamaki, H.; Hayao, K.; Yotani, K.; Ogita, F.; Yamamoto, N.; Onishi, H. Electrical Stimulation of Denervated Rat Skeletal Muscle Retards Capillary and Muscle Loss in Early Stages of Disuse Atrophy. BioMed Res. Int. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Arnold, W.D.; et al. Electrophysiological motor unit number estimation (MUNE) measuring compound muscle action potential (CMAP) in mouse hindlimb muscles. JoVE (Journal of Visualized Experiments) 2015, e52899. [Google Scholar]
- Gooch, C.L.; Doherty, T.J.; Chan, K.M.; Bromberg, M.B.; Lewis, R.A.; Stashuk, D.W.; Berger, M.J.; Andary, M.T.; Daube, J.R. Motor unit number estimation: A technology and literature review. Muscle Nerve 2014, 50, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Cheesborough, J.E.; Smith, L.H.; Kuiken, T.A.; Dumanian, G.A. Targeted Muscle Reinnervation and Advanced Prosthetic Arms. Semin. Plast. Surg. 2015, 29, 062–072. [Google Scholar] [CrossRef]
- Huang, J.; et al. A pilot study of nerve function reinnervation on a transhumeral amputee. J Integr Technol 2016, 5, 30–37. [Google Scholar]
- Gerwin, L.; Haupt, C.; Wilkinson, K.A.; Kröger, S. Acetylcholine receptors in the equatorial region of intrafusal muscle fibres modulate mouse muscle spindle sensitivity. J. Physiol. 2019, 597, 1993–2006. [Google Scholar] [CrossRef]
- Fu, T.; Jiang, L.; Peng, Y.; Li, Z.; Liu, S.; Lu, J.; Zhang, F.; Zhang, J. Electrical Muscle Stimulation Accelerates Functional Recovery After Nerve Injury. Neuroscience 2019, 426, 179–188. [Google Scholar] [CrossRef]
- Amaral, S.L.; et al. Angiogenesis induced by electrical stimulation is mediated by angiotensin II and VEGF. Microcirculation 2001, 8, 57–67. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).