1. Introduction
Hercynite (FeAl
2O
4) is a dark-colored, blue-green, yellow, or brown mineral formed under Earth's conditions of relatively high temperature and pressure, and a characteristic rock-former, commonly associated with feldspars, annite, sekaninaite, corundum fayalite, and ilmenite [
1,
2,
3,
4]. Nevertheless, hercynite has been found in iron rich argillaceous metamorphic rocks as well as igneous mafic and ultramafic rocks, and even has been found as a component in meteorites [
3,
4]. Hercynite is a material with good corrosion resistance, great structural flexibility, alkali resistance, and outstanding coating adherence, which allow its integration into the refractory devices for the burning zone of content rotary kilns [
5,
6]. In fact, thanks to its low thermal expansion coefficient, hercynite presents a high thermal shock resistance [
3,
5]. The iron spinel, or hercynite, is used also as pigment in the production of porcelain withstanding the burning temperature of decorated products. Furthermore, the hercynite mineral also is used in a very promising technology for producing renewable hydrogen in a redox cycle in the solar thermochemical water splitting [
7].
Because hercynite is rare in nature, it is obtained by artificial synthesis, either by the plasma arc method [
8,
9], molten salt method, mechanochemical synthesis method [
8,
9], electrofusion method, and the sintering method [
11,
12,
13,
14], among others. Nevertheless, each of these artificial synthesis methods presents some difficulties because they require high energy consumption to raise high temperatures as its melting point of 1780°C, expensive equipment, or complicated operation. The sintering method requires strict control of the atmosphere and high reaction temperature. Although the molten salt method, dominated by the “dissolution-precipitation” mechanism widely used to obtain hercynite, reduces the reaction sintering temperature and the reaction time, avoiding the agglomeration between the precursor powders [
6,
15,
16,
17]. It has also synthesized hercynite using flake α-Al
2O
3 and Fe
2O
3 as precursor materials, graphite as a reducing agent, and KCl-K2SO4 as a molten salt medium at 1000°C. However, the size distribution of particles synthesized, using the molten salt method, was not homogeneous because were obtained some small particles of hercynite due to the incomplete development of the sample in the growth stage [
6]. On the other hand, the mechanochemical synthesis method is susceptible to thermal reactions affecting the production of hercynite. The arc plasma synthesis method, widely used to synthesize hercynite, uses electrofusion that demands a high energy consumption, and usually, the precursor powders have poor surface activity [
6]. Nevertheless, there are other similar procedures followed by various work teams around the world that have synthetized hercynite, sometimes with partial good results.
Moreover, Hercynite is a ferrimagnetic material belonging to the ferrite spinel category with tunable and extraordinary properties such as high permeability, high resistivity, and high saturation magnetization. Ferrites can be classified into four categories depending on the metal cations and oxygen anions arrange themselves in the crystal lattice to generate different geometric configurations: garnets, ortho-ferrites, hexagonal ferrites, and spinel ferrites or cubic ferrites [
18,
19]. Spinel ferrites, which get their name due to the similarity of their structure with the mineral MgAl
2O
4 named spinel, are the most relevant category of ferrites. Spinels are a closely packed cubic structures with a general formula of AB
2O
4 where divalent (A) and trivalent (B) metal cations with oxygen anions generate arrangements with different geometric configurations in the crystal lattice [
18,
20,
21]. These ferrites, also called ferro-spinels, of semiconducting nature have a widespread occurrence in nature and are of considerable importance in understanding the genesis of crystal rocks [
21,
22]. For this reason, spinels have been widely studied in theoretical and experimental research to understand igneous and metamorphic rocks [
4].
In the present work, we have performed a study of the structural properties and chemical composition of natural origin hercynite mineral recollected from Yepomera, Chihuahua, Mexico. The main objective of this research is to show how and in what percentage can coexist other phases in ferrite natural crystals that contain mainly hercynite. While it is true that there have been many attempts to synthesize hercynite artificially, and it has developed its structural and other characterizations, it is worth doing a similar study on natural hercynite rocks. To carry out this research, we have used the following experimental techniques: X-ray diffraction (XRD) with semiquantitative elemental detection, scanning electron microscopy (SEM), quantitative analysis with inductively coupled plasma mass spectrometry (ICP-MS), and mineralographic/petrographic microscopy studies.
The results obtained in this work show the existence of various crystallographic phases in Hercynite-spinel (FeAl2O4) long crystals up to 3.5 cm length and in its powders that, as far as we know, has not previously been reported in similar samples. The samples studied show a high content of Hercynite with Neumann bands/Widmanstätten patterns, which indicates isometric cleavage of spinel filled with Hematite (Fe2O3). Although, we found other phases present in the Hercynite natural mineral studied, such as Esseneite, Kamacite, Hedenbergite, Pseudobrookite, Spinel (MgAl2O4), Ulvospinel, and Corundum.
2. Materials and Methods
2.1. Mineralographic/Petrographic Microscopy
This research was performed using five pieces of hercynite mineral shown in
Figure 1. All the pieces collected have an irregular shape, with a greater length of around 3.5 cm, having pieces with characteristic sizes around 2.5, 3.5, 1.5, 3.3, and 2.8 cm, labeled as SM1, SM2, SM3, SM4, and SM5, respectively. These representative samples are black or dark gray and present pores of a few microns. Two samples (SM1 and SM2) were cut and polished with 1200-grade abrasive grit and analyzed to determine their mineral composition and texture. The reflectivity and light transmission of some of the minerals studied were observed using mineralographic/petrographic microscopy with an Olympus PROVIS model AX70 microscope, and using also an EA-300 VHX Keyence digital microscope.
2.2. Energy Dispersive Spectroscopy (EDS)
The composition of the hercynite minerals studied was obtained by energy dispersive spectroscopy (EDS) on a scanning electron microscope (SEM) Hitachi SU3500 SEM at 20 kV and low vacuum (60 Pa) at room temperature. It was obtained a semi-quantitative elemental analysis and a mapping of chemical elements present in each sample.
2.3. X-Ray Diffraction
Before the identification of the crystallographic phases that conform to the five samples of hercynite studied, two of these were powdered using an agate mortar and pestle to grind them down to a fine powder. Next, X-ray diffraction patterns (XRD) for both one of the powdered and one solid sample were obtained at room temperature using an X’Pert PRO PANalytical diffractometer in Bragg-Brentano geometry with Cu Ka (l = 1.540 Å) radiation as an X-ray source with an accelerating voltage of 40 kV and a beam current of 30 mA. The XRD patterns were collected in an X´Celerator detector considering a step scanning in a dispersion angle domain from 15o to 80o with increments of 0.03o and a counting time of 20 seconds. Phase identification was achieved using the ICDD PDF2 database in the X’Pert Highscore Plus software from PANalytical.
2.4. Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
Complementary quantitative analysis of the elements not detected by SEM was performed on a powder sample (sample SM3, obtained previously by pulverizing the mineral fragments) using inductively coupled plasma mass spectroscopy (ICP-MS).
3. Results
3.1. Mineralographic/Petrographic Microscopyubsection
One of the samples, labeled as sample SM5, was polished to observe the texture of its surface in an optical microscope, using both reflected and transmitted non-polarized white light and linear polarized light at a magnification of 100X. Although it also used a materials identifier digital microscope.
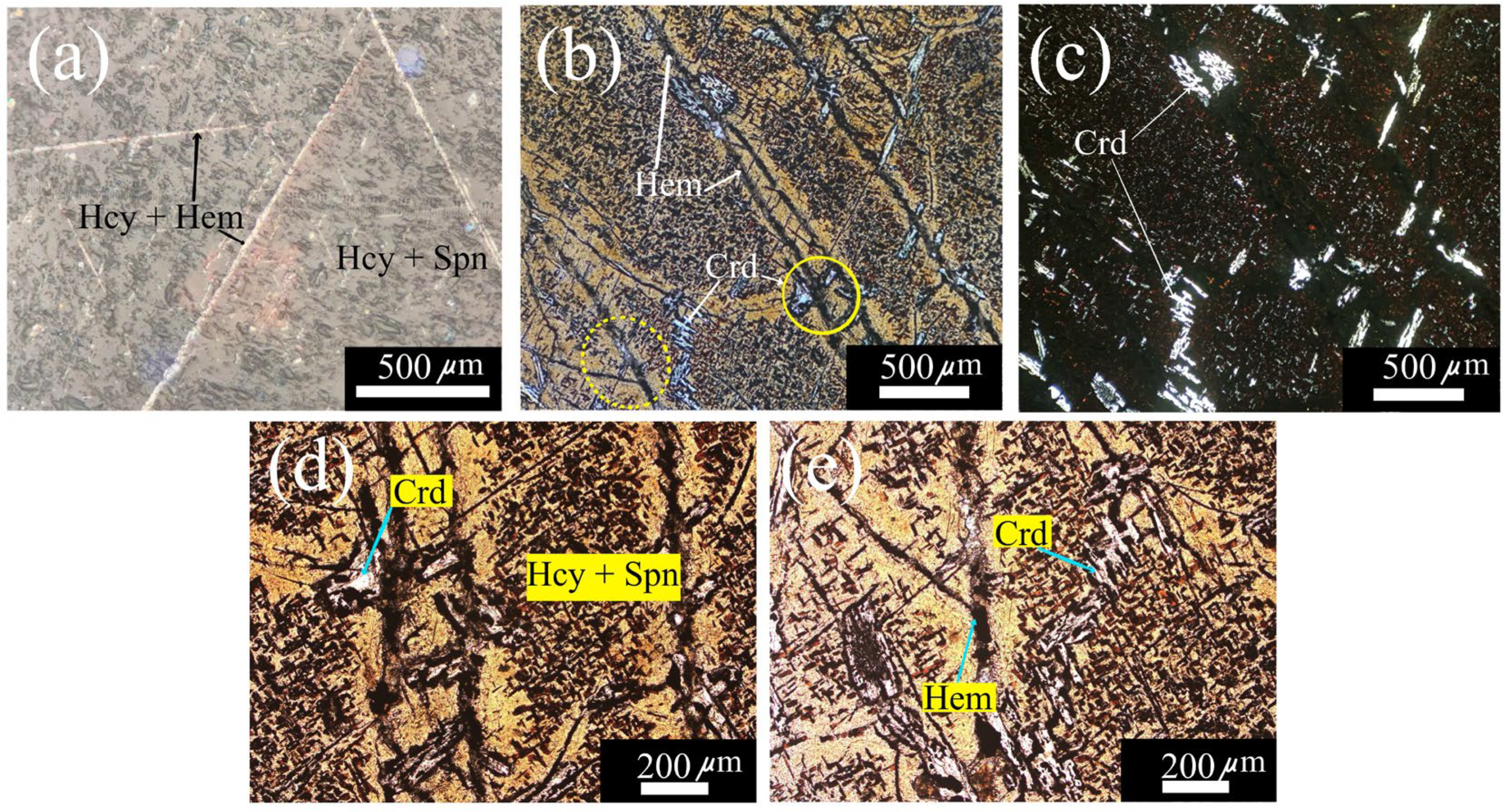
Figure 2(a) shows the hercynite-spinel matrix where clearly can be appreciated the Neumann bands/Widmanstätten pattern composed of a hercynite-hematite mix (FeAl
2O
4 + MgAl
2O
4).
Figure 2(b) shows a photograph of another zone of the same sample SM5, obtained at a magnification of 100 X with reflected polarized white light that allows us to observe with more detail the texture of the surface that shows porosity and fractures. Indeed, these fractures caused by thermal shock running along the observed area generate cleavage planes, which give texture and orientation to hematite crystals.
Figure 2(c) shows a photograph of the same zone shown in
Figure 2(b), except now was used incoherent white light transmitted through crossed linear polarizers to reveal the high light reflection of corundum inclusions over the background of the hercynite-spinel matrix. Figures 2(d) and 2(e) show a magnification of two zones of
Figure 2(b), marked with continue and dashed yellow circles, respectively.
Figure 2(d) shows the abundant corundum (Crd) and hematite (Hem) dispersed over the hercynite-spinel matrix (Hcy-Spn).
Figure 2(e) shows an amplification of a fracture filled with hematite (Hem) shown in
Figure 2(b) and also observed corundum inclusions as in
Figure 2(c).
3.2. EDS Spectroscopy
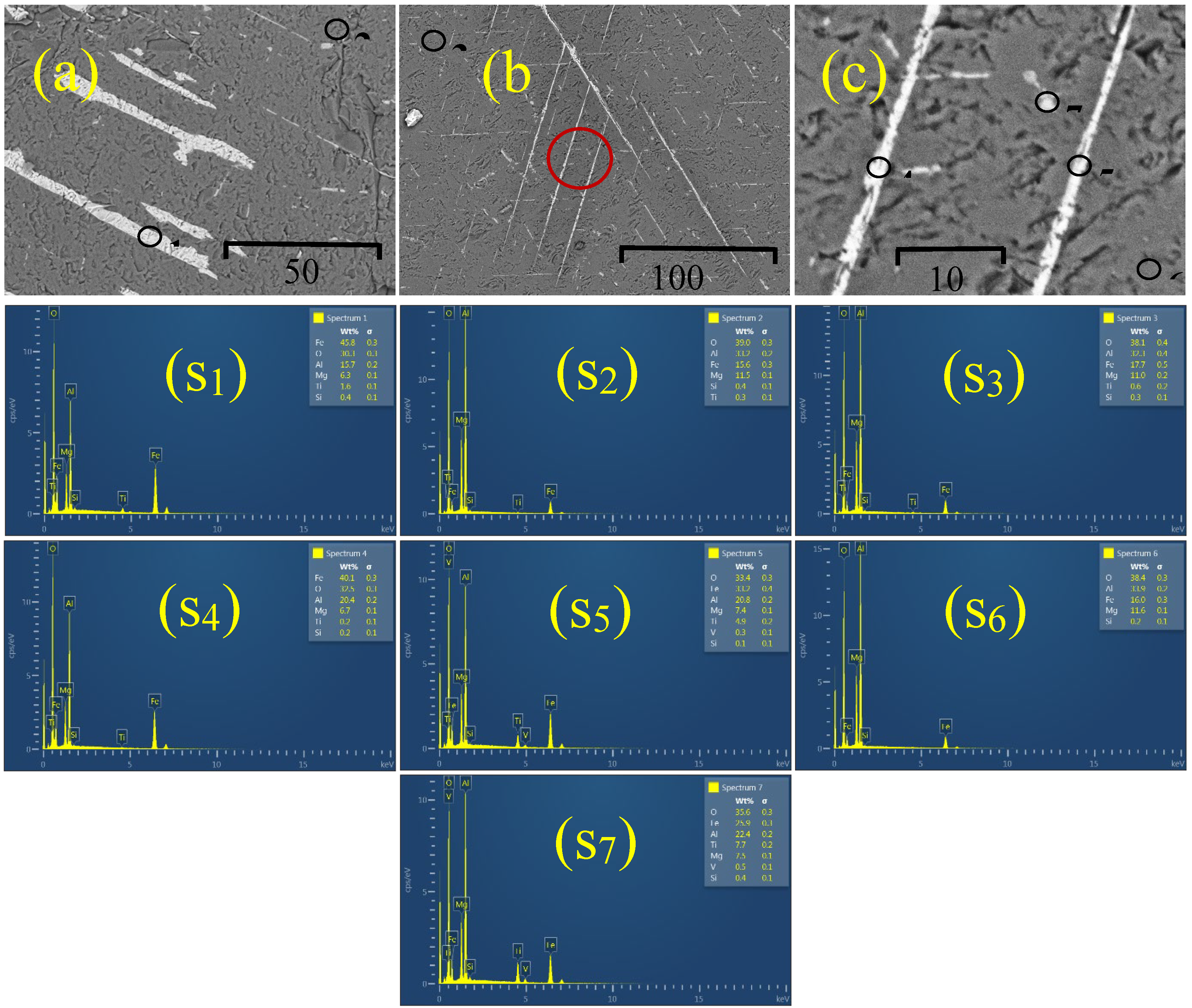
Chemical elements present in two of the studied samples (SM1 and SM2) were determined in several of their representative points by EDS in an SEM. Figures 3(a) - 3(c) show three representative SEM micrographs corresponding to the SM1 and SM2 samples studied, which were encapsulated in a bakelite holder to sand them and get a flat polished surface for their analysis. The elements detected by the EDS analysis were O, Mg, Al, Si, Ti, and Fe, as shown in Figs. 3 (S
1) - (S
7). Nevertheless, the Fe, Al, and O appear in a higher content than any other material existing in the samples, resalting the hercynite presence. The semi-quantitative results of the chemical elements contained in the two representative hercynite samples SM1, and SM2 obtained by EDS are summarized in
Table 1.
As can be observed in Figs. 3(a) and 3(b), the samples analyzed have dendritic/skeletal textures resulting from relatively rapid growth due to a supersaturation high degree of the Fe/Ni relation. As is known, the grain shape is controlled by the ratio D/G of the diffusion rate (D) of a chemical component governing the growth of the crystal in the liquid mix and the crystal growth rate (G) [
23]. The semi-quantitative results of the chemical elements in the two representative hercynite samples, SM1 and SM2, obtained by EDS are shown in
Table 1. These results allow us to confirm the detection of relatively high concentrations of Fe, O, and Al.
Figure 3.
SEM micrographs of samples SM1 and SM2 encapsulated in a bakelite holder. The micrograph (a) corresponds to the sample SM1, and the micrographs (b) and (c) correspond to the sample SM2. Figure (c) is the amplified zone denoted by the red circle in Figure (b). The black circles shown in Figs. (a), (b), and (c) denote the analyzed points by EDS in each sample. In the sample, SM1 the points marked with 1 and 2 have the chemical composition shown in the spectrums depicted in pictures S1 and S2, respectively. While in the analysis of sample SM2, points 3, 4, 5, 6, and 7 have the chemical composition shown in the spectrums depicted in pictures S3, S4, S5, S6, and S7.
Figure 3.
SEM micrographs of samples SM1 and SM2 encapsulated in a bakelite holder. The micrograph (a) corresponds to the sample SM1, and the micrographs (b) and (c) correspond to the sample SM2. Figure (c) is the amplified zone denoted by the red circle in Figure (b). The black circles shown in Figs. (a), (b), and (c) denote the analyzed points by EDS in each sample. In the sample, SM1 the points marked with 1 and 2 have the chemical composition shown in the spectrums depicted in pictures S1 and S2, respectively. While in the analysis of sample SM2, points 3, 4, 5, 6, and 7 have the chemical composition shown in the spectrums depicted in pictures S3, S4, S5, S6, and S7.
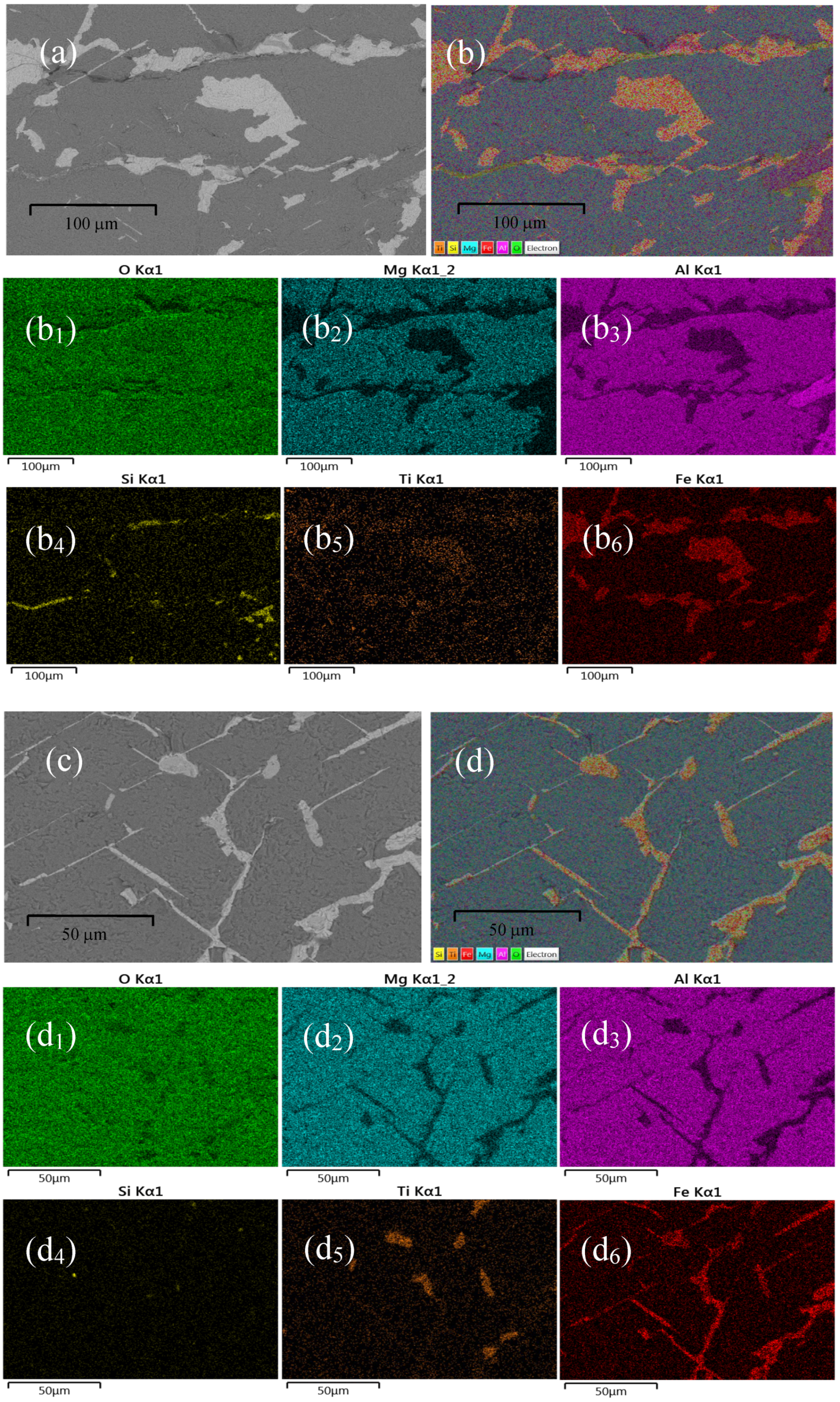
On the other hand, to reveal the spatial distribution of the chemical elements detected in the two samples analyzed, SM1 and SM2, was carried out an elemental mapping in representative zones of each of them. As it is well known, the elements detected by EDS in a material are associated with the radiated X-ray corresponding to the electronic transitions of that certain element. The O, Mg, Al, Si, Ti, and Fe contained in the samples were detected through the X-rays corresponding to the K-shell electronic transitions. Figures 4(a) and 4(c) show a grey-scale micrograph of a selected zone for the elemental mapping analysis in the representative samples SM1 and SM2, respectively. Figures4(b) and 4(d) are the full-color mapping version of Figs. 4(a) and 4(c), respectively. Figures 4(b1) - 4(b6) and 4 (d1) - 4 (d
6) show the mapping of O, Mg, Al, Si, Ti, and Fe with the K-shell transition through a high-contrast colored spectrum that allows visualizing their presence in the hercynite samples. The brighter regions describe the presence of a certain element in that specific region, whereas the darker region indicates the absence of that certain element [
24]. A chemical element present in a sample is associated with the radiated X-rays resulting from the electronic transitions in that element. As can be observed in Figs. 4(a) and 4(c), the most brilliant regions correspond to a Fe distribution revealed in Figs. 4(b
6) and 4(d
6), respectively. Certainly, the O and Al are distributed throughout the entire pictures 4(b) and 4(d), revealing the possible presence of Hercynite mineral. Although it was also detected Mg, contained practically throughout the entire zone depicted as can be observed in Figs. 4(b
2) and 4(d
2), and some few segregations of Si as shown in Figs. 4(b
4) and 4(d
4), respectively. A few quantities of titanium are present in the same zones as the Fe distribution, as shown in Figs. 4(b
5) and 4(d
5), which reveal the presence of the pseudobrookite phase in both analyzed samples SM1 and SM2.
3.3. X-Ray Diffraction
3.3.1. X-Diffraction Patterns of Two Representative Samples
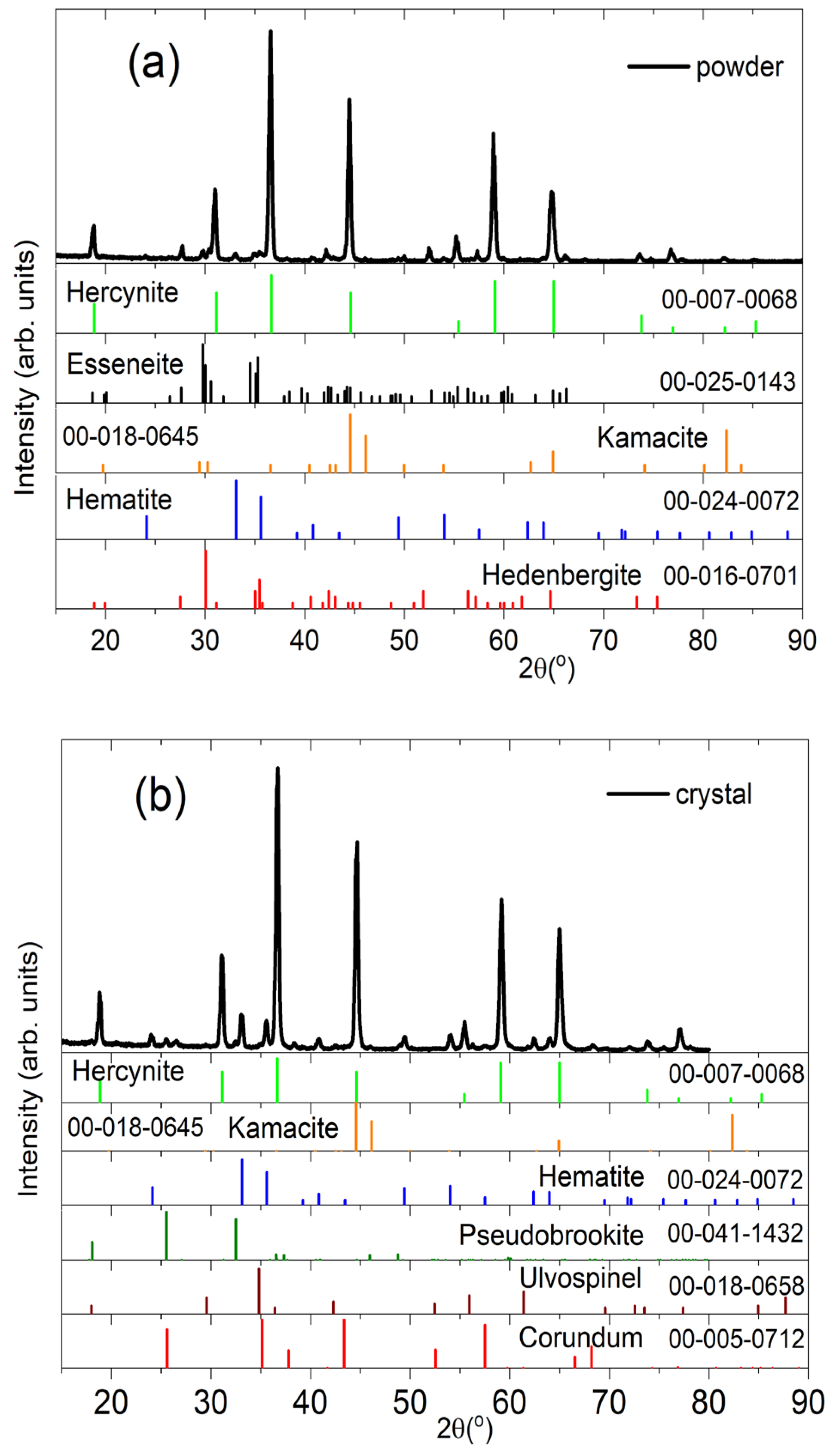
It is well known that a rapid growth around a limited number of nuclei or along intergranular boundaries under circumstances of unfavorable nucleation can induce the formation of additional secondary phases in metamorphic rocks [
25]. For this reason, the XRD patterns obtained were used to identify the minerals and their phases present in the samples studied in this work. The main diffraction peaks showed in the XRD patterns of Figs. 5(a) for a powder sample and 5(b) for a single crystal-spinel are attributed to a met-asomatism-Fe association of Hercynite (cubic, Fd3m, PDF 00-007-0068) abundant with other secondary phases attributed to the presence of minerals such as kamacite (Ka, cubic, Fm3m, PDF 00-018-0645), esseneite (Es, monoclinic, C2/c, PDF 00-025-0143), hedenbergite (Hd, monoclinic, B2/b, PDF 00-016-0701), pseudobrookite (Ps, orthorhombic, Bbmm, PDF 00-041-1432), and ulvospinel (Ul, cubic, Fd3m, PDF 00-018-0658). The detected kamacite (FeNi) mineral by XRD patterns of Figs. 5(a) and 5(b) exhibits the characteristic evidence of metallic Fe-Ni formed by the decomposition of iron carbide (Fe3C cementite) [
26]. The additional phases presented in the powder sample include hematite (Fe2O3, rhombohedral, R3), pseudobrookite (Fe
2TiO
5) and ulvospinel (Fe
2TiO
4). The last ones are the associated minerals that differentiated the matrix crystallographic association with the presence of titanium linking with silicon, aluminum, and iron. While the few traces of corundum (aluminum oxide (Al
2O
3)) also observed in the single crystal-spinel sample, with traces of iron, titanium, and chromium, is the surplus material of the aluminum that did not link to other elements [
27]. The corundum (Co, rhombohedral, R3c, PDF 00-005-0712) presence in the XRD patterns of the single crystal-spinel sample indicates a crystallographic continuity between the Fe spinel (hercynite), Mg, and Ti (ulvospinel) [
28].
Figure 4.
SEM images of a selected zone in samples SM1 and SM2, (a), (c) gray-scale, and (b), (d) full color mapping, respectively. (b1) - (b6), (d1) -(d6) Elemental mapping analysis of O, Mg, Al, Si, Ti, and Fe, in SM1 and SM2 samples, respectively, with the K-shell transition in a high-contrast colored spectrum that allows visualizing their presence in each sample.
Figure 4.
SEM images of a selected zone in samples SM1 and SM2, (a), (c) gray-scale, and (b), (d) full color mapping, respectively. (b1) - (b6), (d1) -(d6) Elemental mapping analysis of O, Mg, Al, Si, Ti, and Fe, in SM1 and SM2 samples, respectively, with the K-shell transition in a high-contrast colored spectrum that allows visualizing their presence in each sample.
Figure 5.
XRD patterns of: (a) a powder sample SM3, and (b) a single crystal-spinel SM4. Phases present in (a): Hercynite (FeAl2O4), Esseneite (AlCaFeO6Si), Hematite (Fe2O3), Kamacite (FeNi), and Hedenbergite (CaFeO6Si2); in (b): Hercynite, Hematite, Kamacite, Pseudobrookite (Fe2TiO5), Spinel (MgAl2O4), Ulvospinel (Fe2TiO4), and Corundum (Al2O3).
Figure 5.
XRD patterns of: (a) a powder sample SM3, and (b) a single crystal-spinel SM4. Phases present in (a): Hercynite (FeAl2O4), Esseneite (AlCaFeO6Si), Hematite (Fe2O3), Kamacite (FeNi), and Hedenbergite (CaFeO6Si2); in (b): Hercynite, Hematite, Kamacite, Pseudobrookite (Fe2TiO5), Spinel (MgAl2O4), Ulvospinel (Fe2TiO4), and Corundum (Al2O3).
As can be observed from the diffractograms shown in Figures 5(a) and 5(b), the diffraction peaks of the powdered sample SM3 and the bulk sample SM4 have an extraordinary definition and sharpness that denote a high degree of crystallinity. The samples studied also have a mixture of phases identified with the help of the ICDD PDF2 2013 database. Thus, the natural samples studied have a base or matrix of hercynite that embeds other phases that constitute the sample of interest. It is relevant to comment that this degree of crystallinity is not obtained or is hard to attain in samples of hercynite synthesized by artificial methods [
9,
11,
12,
13,
14]. It is also important to highlight that the artificially synthesized hercynite is usually much more porous than the natural samples studied in the present research. In natural hercynite samples, as shown in
Figure 3(c), their pores have average typical sizes around 1-3 µm. In comparison, in synthesized hercynite samples, their pores have a size between 1 µm and several tens of microns [
9,
12,
14].
3.3.2. Estimation of the Crystallites with a Size of Two Representative Samples
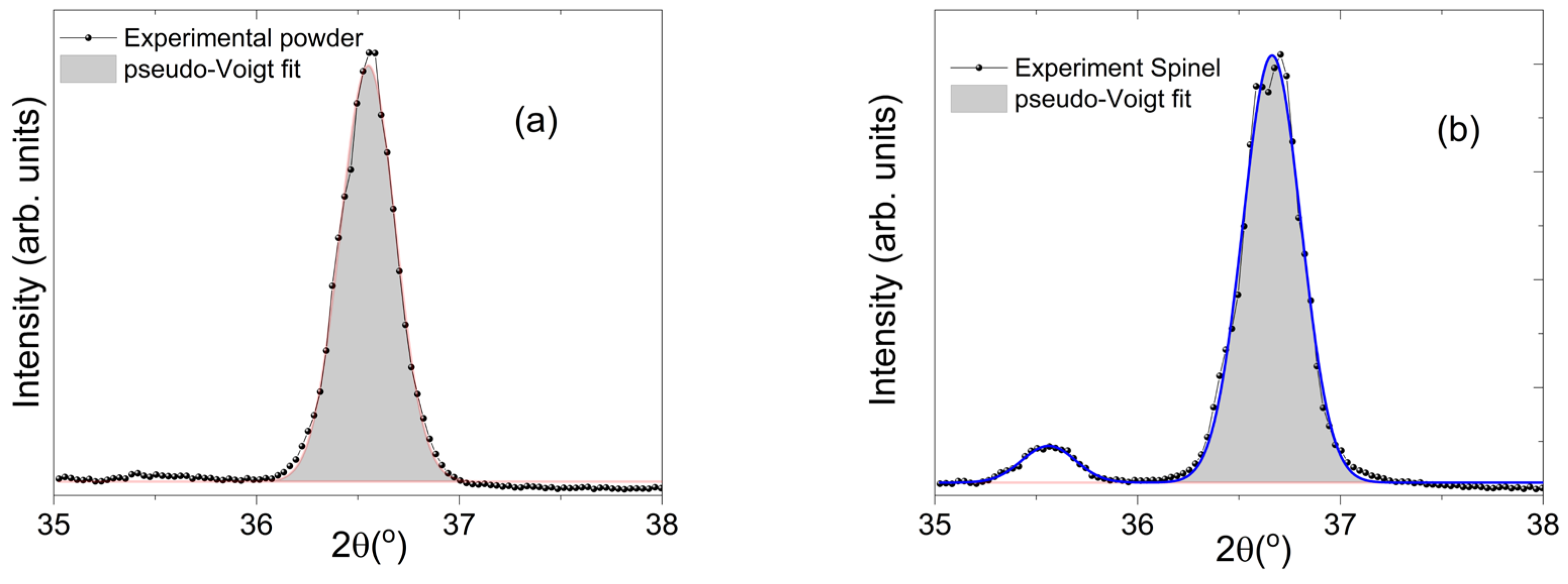
In order to contribute to the characterization performed by XRD, was also determined the apparent crystallite size
L for the powder sample SM3 and for the Hercynite-spinel SM4 sample by the Scherrer equation,
L=Kλ/βcosθ. This equation considers the XRD radiation of wavelength
λ (
CuKα= 0.15405 nm) and the corrected full width at half maximum of diffraction peaks (
β, FWHM, pseudo-Voigt function) in radians located at 2θ ~ 36.55
o (powder) and ~ 35.56
o (crystal) in their respective pattern. The Scherrer constant (shape factor)
K for this calculation was taken as 0.89.
Figure 6(a) and 6(b) depict the pseudo-Voigt approximation used to determine the corrected FWHM of diffraction peaks associated to the hkl (311) of Hercynite-spinel
Summarizing the results, the powders were constituted of crystallites with a size of about 27 nm, while the crystal reveals a value about 28 nm.
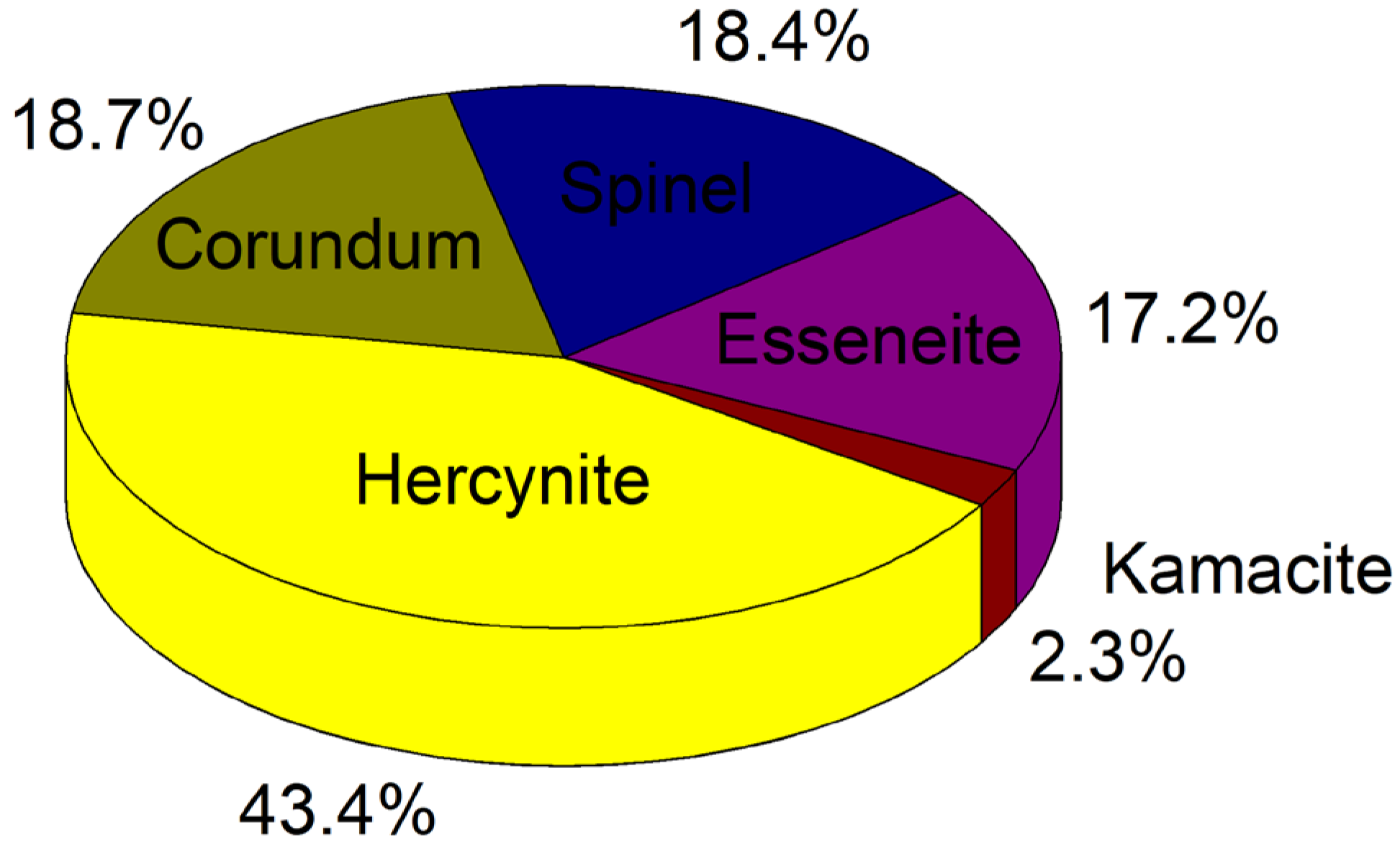
3.3.3. Estimation of the Percentage of the Main Phases Present in a Representative Sample
On the other hand, from the x-ray diffraction studies, the percentage of the main phases present in one of the pulverized crystalline samples, the SM3, was also estimated by calculating the total area under the diffraction peaks after adjusting pseudo-Voigt functions as in Refs. [
29,
30]. Once identified the phases that match the X-ray diffraction pattern, shown in
Figure 5(a), by indexation using the database ICDD PDF2 2013 was estimated their corresponding contribution. The atomic positions of each compound in the spinel sample were obtained using the Malvern Panalytical software employing the COD open-access collection of crystal structures database. This estimation is one of the main objectives of this research, as was commented in the introduction section.
Figure 7 shows the percentages of the crystallographic phases present in sample SM3. The phases with the highest percentage present, in order of importance, in the spinel sample SM3 are Hercynite (43.4%), Corundum 18.7%, Spinel (MgAl
2O
4) 18.4%, Esseneite 17.2%, and Kamacite with a 2.3 %.
3.4. Inductively coupled plasma mass spectrometry (ICP-MS)
The chemical elements present in the representative sample SM3 un-identified by SEM due to the limit sensing were detected using ICP-MS once the sample was powdered.
Table 2 shows the weight percentage obtained by ICP-MS of Co, V, Cr, Mg, and Ca that suggests the presence of rich spinels of these elements. Although Al, Fe, Cr, Si, Mg, Ti, and Ca were the materials found in the highest percentage. Because of the existence of Fe, Ni, Mg, and Al are expected to find kamacite (Fe
2O
3) and spinels (MgAl
2O
4). Although, as can be observed in
Table 2, there is a significative presence of Al, Fe, Cr, and Si. However, there are also Si, Ca, Ti, Zn, and Mn in the sample that can rise to the presence of inhomogeneous Mg-Al-spinel grains when dominating Mg and Al, and Cr-spinel when dominating Fe and Cr, or there may even be pyroxene grains [
31].
It is relevant to remark that due to the low concentration of Ni in the samples studied in this research, they are not classified as meteorite material as reported in the literature [
31,
32,
33]. However, there is a plausible doubt about this statement because the temperatures required to synthesize the compounds present in the samples studied in this research are very high, and on the earth's surface by natural sources, there are few possibilities to reach them.
4. Conclusions
This work reports a study of the structural properties and the chemical composition of natural origin hercynite mineral samples recollected from Yepomera, Chihuahua, Mexico. The structural characterization was performed using X-ray diffraction with semiquantitative elemental detection, scanning electron microscopy, quantitative analysis with inductively coupled plasma mass spectrometry, and mineralographic/petrographic microscopy studies. The results show the coexistence of various crystallographic phases in the hercynite-spinel (FeAl2O4) long crystals and in its powders studied. The samples examined show a high content of hercynite with Neumann bands/Widmanstätten patterns, which indicates isometric cleavage of spinel filled with hematite (Fe2O3). The other existent phases in the hercynite natural mineral studied are Esseneite, Kamacite, Hedenbergite, Pseudobrookite, Spinel (MgAl2O4), Ulvospinel, and Corundum. The diffractograms of two samples (SM3 and SM4) allowed us to observe an extraordinary sharpness of the corresponding diffraction peaks, which denotes a high degree of crystallinity that differs from the hercynite synthesized by artificial methods. In addition, the artificially synthesized hercynite is usually much more porous than the natural samples studied in the present research. From the X-ray diffraction pattern of one of the pulverized crystalline samples (the SM3), it was possible to estimate the percentage of the main phases present using the Malvern Panalytical software and the COD collection of crystal structures database. The crystallographic existent phases with the highest participation in the spinel sample SM3 were hercynite (43.4%), corundum 18.7%, spinel (MgAl2O4) 18.4%, esseneite 17.2%, and kamacite with a 2.3 %. These results can help to understand the processes that led to the formation of this interesting and unique material.
Author Contributions
Conceptualization, visualization, project administration, Andrés I. González-Jacquez, and José G. Murillo-Ramírez; methodology, validation, formal analysis, data curation, writing—original draft preparation, José G. Murillo-Ramírez, Andrés I. González-Jacquez, Guillermo Herrera-Perez; software, Andrés I. González-Jacquez, Guillermo Herrera-Perez ; investigation, Andrés I. González-Jacquez, Jose G. Murillo-Ramírez, Cesar Modesto-Acosta, Guillermo Herrera-Perez, Karla Campos-Venegas, and Cesar Leyva-Porras; resources, Cesar Modesto-Acosta, and Andrés I. González-Jacquez; writing—review and editing, José G. Murillo-Ramírez; supervision, José G. Murillo-Ramírez ; funding acquisition, Cesar Modesto-Acosta. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding, and the APC was funded by Instituto de Innovación y Competitividad de la Secretaría de Innovación y Desarrollo Económico del Estado de Chihuahua, México.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank, Alejandro Benavides Montoya for obtaining the ICP results, and José Trinidad Holguín Momaca, for the samples preparation required for EDS elemental mapping, and the XRD patterns, from the Laboratorio Nacional de Nanotecnología (NaNoTeCh) at Centro de Investigación en Materiales Avanzados S. C. G. Herrera-Pérez would like to thank for the complimentary support SNI II-CONACyT and Cátedra Grant No. 2563 of CONACyT México.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results”.
References
- Yakovleva, O. S.; Pekov, I. V.; Horváth, L.; Bryzgalov, I. A.; Yapaskurt, V. O.; Guseva, E. V. Mineralogy, geochemistry, and genesis of high-alumina fenites of the Mont Saint-Hilaire alkaline pluton, Geol. Ore Deposits, Québec, Canada 2010, 52, 725-735. [CrossRef]
- Mikhailova, J. A.; Pakhomovsky, Y. A.; Konopleva, N. G.; Kalashnikov, A.O.; Yakovenchuk, V. N.; Fluorine Controls Mineral Assemblages of Alkaline Metasomatites. Minerals 2022, 12, 9, 1076. [CrossRef]
- Bernier, L. R. Vanadiferous zincian-chromian hercynite in a metamorphosed basalt-hosted alternation zone, Atik Lake, Manitoba. Can. Mineral 1990, 28, 37–50.
- Shulters, J.C.; Bohlen, S.R. The Stability of Hercynite and Hercynite-Gahnite Spinels in Corundum- or Quartz-Bearing Assemblages, J. Petrol. 1989, 30, 4, 1017-1031. [CrossRef]
- Jastrzębska, I.; Bodnar, W.; Witte, K.; Burkel, E.; Stoch, P.; Szczerba J. Structural properties of Mn-substituted hercynite. Nukleonika 2017, 62, 2, 95-100. [CrossRef]
- Tan, W.; Guo, X.; She, Y.; Li, H.; Lei, Y.; You, J.; Zhang, X.; Luo, X. Effects of molten salt temperature and holding time on synthesis of hercynite by molten salt method, Ceram. Int. 2022, 48, 8, 11555-11560. [CrossRef]
- Al-Shankiti, I. A.; Bayon, A.; Weimer A. W. Reduction kinetics of hercynite redox materials for solar thermochemical water splitting. J. Chem. Eng. 2020, 389, 124429. [CrossRef]
- Jastrzębska, I.; Szczerba, J.; Błachowski, A.; Stoch P. Structure and microstructure evolution of hercynite spinel (Fe2+Al2O4) after annealing treatment, Eur. J. Mineral 2017, 28, 1, 63-72. [CrossRef]
- Padhi, L.N.; Sahu, P.; Sahoo, N.; Singh, S.K.; Tripathy, J.K. A novel process for synthesis of iron-alumina spinel and its application in refractory for cement rotary kiln, Trans. Indian Ceram. Soc. 2017, 76, 3, 196–201. [CrossRef]
- Alcala, M.D.; Matteazzi, P. Mechanochemical synthesis of iron-alumina (75Vol%) nanocermets, J. Radioanal. Nucl. Chem. 1995, 190, 2, 341–346. [CrossRef]
- Botta, P.M.; Aglietti, E.F.; Porto, J.M. Mechanochemical synthesis of hercynite, Mater. Chem. Phys. 2002, 76, 1, 104–109. [CrossRef]
- Schicker, S.; Erny, T.; Garcıa, D.E.; Janssen, R.; Claussen, N. Microstructure and mechanical properties of Al-assisted sintered Fe/Al2O3 cermets, J. Eur. Ceram. Soc. 1999, 19, 13-14, 2455–2463. [CrossRef]
- Daghetta, M.; Dapiaggi, M.; Pellegrino, L.; Pastore, B. Synthesis of hercynite at very mild condition, Chem. Eng. Trans. 2015, 43, 1741–1746. [CrossRef]
- Chen, J.; Yu, L.; Sun, J.; Li, Y.; Xue, W. Synthesis of hercynite by reaction sintering, J. Eur. Ceram. Soc. 2011, 31, 3, 259–263. [CrossRef]
- Ye, L.; Zhao, L.; Liang, F.; He, X.; Fang, W.; Chen, H.; Zhang, S.; An S. Facile synthesis of hexagonal boron nitride nanoplates via molten-salt-mediated Magnesiothermic reduction, Ceram. Int. 2015, 41, 10, 14941–14948. [CrossRef]
- Huang, Z.; Li, F.; Jiao, C.; Liu, J.; Huang, J.; Lu, L.; Zhang, H.; Zhang, S. Molten salt synthesis of La2Zr2O7 ultrafine powders, Ceram. Int. 2016, 42, 5, 6221–6227. [CrossRef]
- Li, Z.; Zhang, S.; Lee, W.E. Molten salt synthesis of LaAlO3 powder at low temperatures, J. Eur. Ceram. Soc. 2007, 27, 3201–3205. [CrossRef]
- Pardavi-Horvath, M. Microwave applications of soft ferrites, J. Magn. Magn. Mater. 2000, 215–216, 171–183. [CrossRef]
- Tolani, S.C.; Golhar, A.R.; Rewatkar, K.G. A review of morphological, structural behaviour and technological applications of ferrites, AIP Conf. Proc. 2019, 2104, 030032. [CrossRef]
- Atiq, S.; Majeed, M.; Ahmad, A.; Abbaas, S.K.; Saleem, M.; Riaz, S.; Naseem, S. Synthesis and investigation of structural, morphological, magnetic, dielectric and impedance spectroscopic characteristics of Ni-Zn ferrite nanoparticles. Ceram. Int. 2017, 43, 2, 2486 - 2494. [CrossRef]
- Ditta, A.; Khan, M.A.; Junaid, M.; Khalil, R.M.A.; Warsi, M.F. Structural, magnetic and spectral properties of Gd and Dy co-doped dielectrically modified Co-Ni (Ni0.4Co0.6Fe2O4) ferrites, Physica B 2017, 507, 27-34. [CrossRef]
- Jalaiah, K.; Babu, K.V. Structural, magnetic and electrical properties of nickel doped Mn-Zn spinel ferrite synthesized by sol-gel method, J. Magn. Magn. Mater. 2017, 423, 275-280. [CrossRef]
- Vernon, R.H. A Practical Guide to Rock Microstructure, Microstructures of Igneous Rocks, Cambridge University Press 2004, 10.1017/9781108654609, 3, 28 -134. [CrossRef]
- Khan, M.A.; Shahbaz, K.; Mustafa, G.M.; Ramay S.M.; Naseem, S.; Atiq, S. Optimization of magnetoelectric coupling in BiFeO3-BaTiO3-MnFe2O4 tri-phase composites for ultra-sensitive devices, J. Alloys Compd. 2023, 947, 169571. [CrossRef]
- Vernon, R.H. A Practical Guide to Rock Microstructure, Microstructures of Metamorphic Rocks. Cambridge University Press 2018, 2nd Ed. 135-227. [CrossRef]
- Rubin, A.E. Mineralogy of meteorite groups. Meteorit. Planet. Sci. 1997, 32, 231-247. [CrossRef]
- Howie R. A.; Anthony, J.W.; Bideaux, R.A.; Bladh, K.W.; Nichols, M.C. Handbook of mineralogy. Volume III: Halides, hydroxides, oxides mineral Mag., Mineralogical Society of America 1997, 62, 3, 432-433, ISSN: 1945-3027.
- Needham, A. W.; Messenger, S.; Han, J.; Keller, L. P. Corundum–hibonite inclusions and the environments of high temperature processing in the early Solar System. Geochim. Cosmochim. Acta 2017, 196, 18–35. [CrossRef]
- Guinebretière René, X-ray Diffraction by Polycrystalline Materials, John Wiley & Sons, 2013.
- Ladd Mark, Palmer Rex, Structure Determination by X-ray Crystallography, Analysis by X-rays and Neutrons. Springer New York, NY 2014, Fifth Ed. [CrossRef]
- Bjärnborg, K.; Schmitz, B. Large spinel grains in a CM chondrite (Acfer 331): Implications for reconstructions of ancient meteorite fluxes. Meteorit. Planet. Sci. 2013, 48, 2, 180-194. [CrossRef]
- Petrovic, J. J. Review Mechanical properties of meteorites and their constituents. J. Mater. Sci. 2001, 36, 1579–1583. [CrossRef]
- Nabawy, B. S.; Rochette, P. A Preliminary Study On the Electrical Signatures of Some Iron and Stony Meteorites and Their Dependence On Nickel Content. Acta Geophys. 2016, 64, 1942–1969. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).