1. Introduction
Eggshell translucency is the appearance of moisture accumulation that causes transparent spots to appear on the shell, especially when light penetrates the egg [
1]. This phenomenon varies considerably between breeds and even between individual eggs [
2,
3]. Translucent eggs not only increase the risk of bacterial contamination [
4], but also reduce consumer demand, ultimately leading to a decrease in economic profitability for producers [
5].
The intestinal tract is a crucial component of the avian digestive apparatus, responsible for nutrients digestion and absorption [
6,
7], lipid metabolism, detoxification, neuroendocrine function, as well as immune and defensive functions against external pathogens [
8,
9]. Numerous studies have demonstrated a significant correlation between the capacity of the intestine to absorb nutrients, particularly calcium ion, and egg production, egg quality, and eggshell quality [
10,
11,
12,
13,
14]. One crucial reason for the hens laying translucent eggs may be attributed to a corresponding impairment of their intestinal functional state [
15]. To date, comprehensive molecular mechanism differences of intestinal functions of laying hens producing distinctively different quality eggs remain poorly understood.
The intestinal segments exhibit a range of absorptive capacities for various nutrients and minerals, including calcium [
16]. Furthermore, calcium constitutes approximately half of the elemental composition of eggshells. Consequently, the quality of eggshells is directly correlated with the calcium utilization by laying hens [
17]. Calcium forming eggshell mainly comes from feed, and calcium ions occur in the intestine mainly through transcellular processes of active absorption and paracellular processes of passive diffusion [
18,
19,
20,
21]. Following absorption, calcium is transported into the bloodstream, where it forms blood calcium. Subsequently, the calcium travels to the uterine portion of the ovary, where the eggshell is formed. A series of chemical reactions results in the combination of calcium ions into calcium carbonate, which is subsequently deposited on the eggshell membrane to form the eggshell.
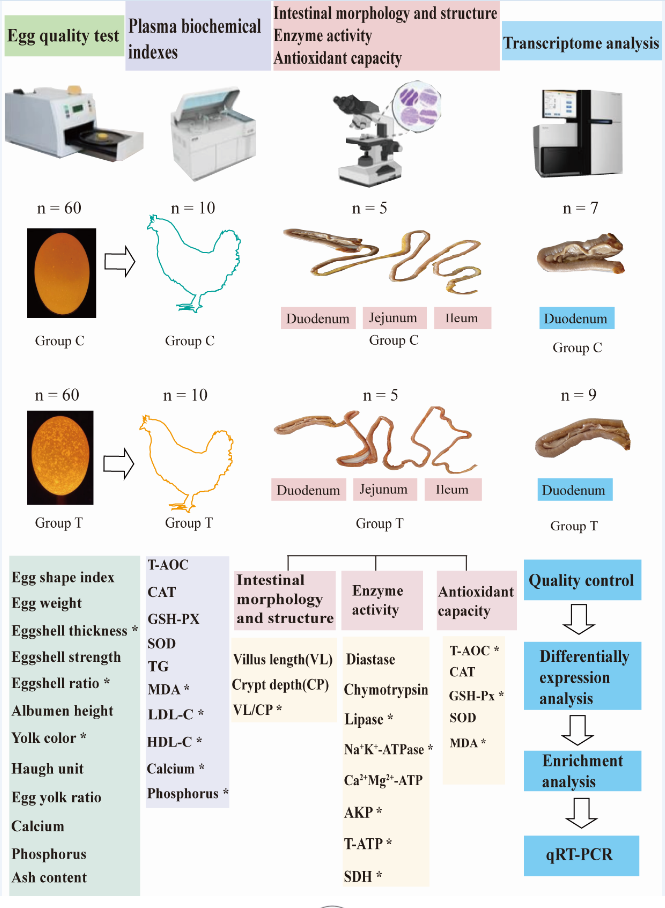
It was postulated that alterations in intestinal morphology, structure, and function would influence the absorption and transport of calcium ions, resulting in reduced calcium ion deposition and the formation of translucent eggs. To verify this, we conducted egg quality test, plasma biochemical indexes, intestinal morphology and structure, intestinal enzyme activity, intestinal antioxidant capacity as well as the duodenum transcriptome analyses of laying hens who produced translucent and normal eggs (
Figure 1). Our study will provide a theoretical foundation for future research into the production of less translucent eggs.
2. Materials and Methods
2.1. Ethics Statement
The study was carried out in compliance with the ARRIVE guidelines. The care and use of experimental animals were carried out in accordance with the Directory Proposals on the Ethical Treatment of Experimental Animals, established by the Ministry of Science and Technology (Beijing, China). All study animal procedures were approved, and supervised by the Animal Ethics committee of Shandong Academy of Agricultural Science (Permit No: 2018412). All methods were performed in accordance with relevant guidelines and regulations.
2.2. Animal and Sample Collection
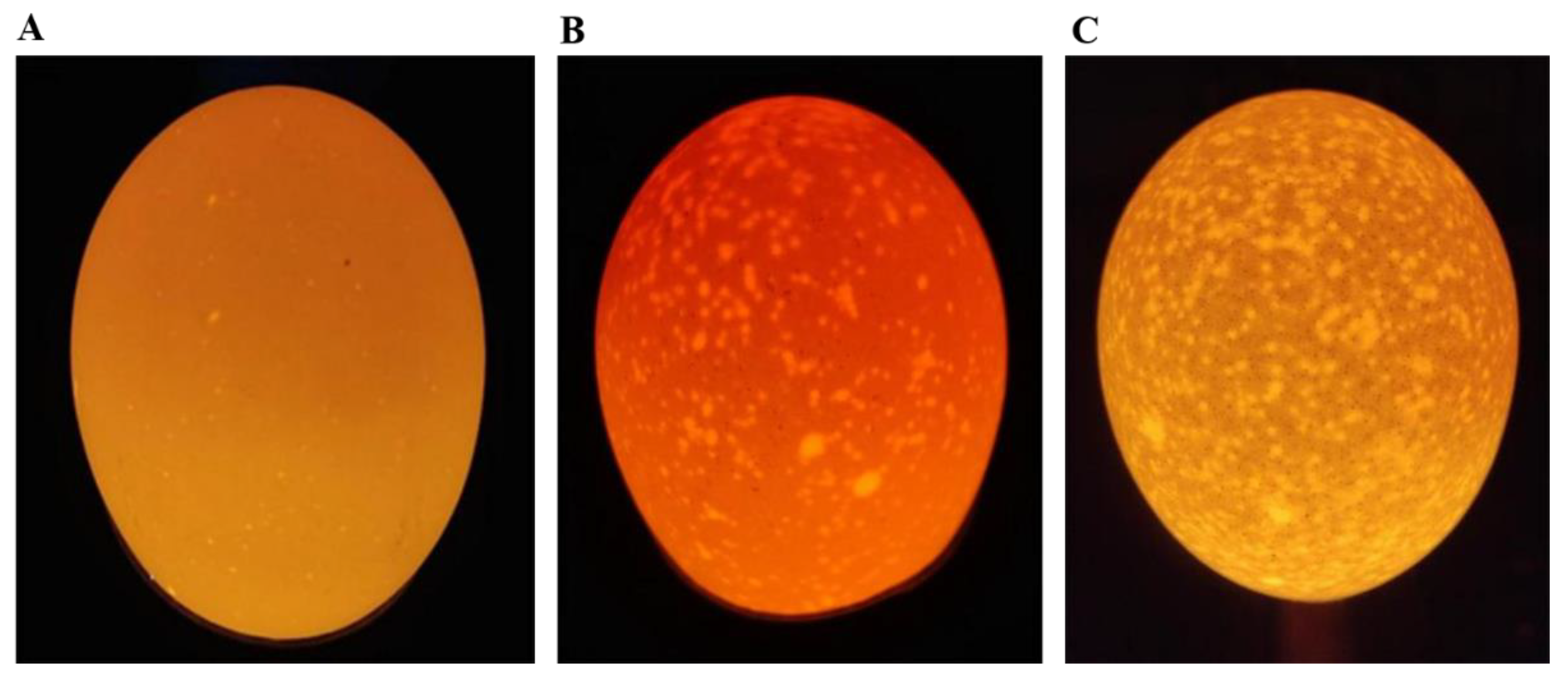
One hundred 276-day-old Jining Bairi hens were randomly selected from Poultry Institute of Shandong Academy of Agricultural Sciences. All the experimental chickens were fed and watered freely, under the same management conditions, with the same feeding environment and production performance. The laying hens were raised in single cages after numbering. We collected eggs at 09:00 every day and stored them in a constant environment (temperature 20 to 25°C, RH 50 to 60%) for 1 days. All eggs were identified using a conventional source of illumination (Feisheng electronic technology co., LTD, shanghai, China) and classified into 3 grades according to the degree of shell translucence [
1]. First-class: opaque, second-level: semi-translucent, and third-level: translucent (
Figure 2). Ten laying hens with a high rate of grade 1 eggshell translucence were selected as the control group (group C), and ten laying hens with a high rate of grade 3 eggshell translucence were selected as the eggshell translucence group (group T). The chickens in the control group and the eggshell translucence group were euthanized. The middle section of the duodenum, jejunum and ileum tissues were taken and fixed in 4% neutral formaldehyde solution, and tissues were frozen in liquid nitrogen and stored in -80°C refrigerator.
We selected 60 normal eggs and 60 translucent eggs for egg quality testing. Measurements of egg length (L) and width (W) were taken with a digital calliper to the nearest 0.01mm. The egg shape index (SI) was determined from the measurements according to the formula from Anderson et al [
22].
To weight the yolk weight (YW) after using the separator to get it, and compare it with the egg weight (EW) to get the egg yolk ratio (EYR). Egg yolk ratio was calculated by the following formula:
Remove the attachments inside the egg shell, using an electronic tray balance to get the egg shell weight (ESW), and calculate the egg shell ratio based on the following formula:
The blunt, middle and sharp shell thickness were tested by the egg shell thickness gauge (Fujihira Industry co., LTD, Tokyo, Japan). Eggshell thickness was calculated by the average of blunt, middle and sharp shell thickness. We used eggshell strength measuring instrument (Tenovo International Co., Limited, Beijing, China) to record the eggshell strength, and the egg quality analyzer to measure the egg weight (Robotmation Co., LTD, Tokyo, Japan). We broke the eggs and poured the egg contents into specific position with the yolk as the center to measure the eggs quality, albumen height, and yolk color as well as the Haugh unit. The eggshell calcium, phosphorus and ash content were tested by Shandong Runda testing technology Co., Ltd (Shandong, China).
2.3. Intestinal Tissue Morphology
The measurements of villus height and crypt depth were taken exclusively from sections where the place of section ran vertically from the tip of villus to the base of an adjacent crypt by using a light microscope. Ten well-oriented villus sections from three different locations on each hen were used to determine these indices. The duodenum, jejunum and ileum tissues were fixed with 4% formaldehyde and subsequently sent to Wuhan Servicebio for paraffin sectioning and hematoxylin and eosin (HE) staining. After returning the finished products, we observed 5 straight and intact villi of each section sample using an inverted microscope (Mack Photoelectric Instrument Co., Chongqing, China). The villi height and crypt depth were then measured. The ratio of villus height to crypt depth was calculated.
2.4. Plasma Biochemical Index
We tested the plasma biochemical index including total antioxidant capacity (T-AOC), catalase (CAT), glutathione (GSH-Px), superoxide dismutase (SOD), triglyceride (TG), malondialdehyde (MDA), Low-density lipoprotein cholesterol (LDL-C), High density lipoprotein cholesterol (HDL-C), phosphorus and calcium. All the indexes were tested by the commercially available kit purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
2.5. Intestinal Biochemical Indexes
We tested three intestinal digestive enzymes activity, four intestinal energy metabolism enzymes activity and five antioxidant factors in duodenum, jejunum and ileum. The three intestinal digestive enzymes activities including amylase, lipase and chymotryps in each intestinal segment. The energy metabolism enzymes including natrium potassium ATPase (Na+K+-ATPase) activity, calcium and magnesium ATPase (Ca2+Mg2+-ATPase) activity, ATPase, alkaline phosphatase (AKP) activity and succinate dehydrogenase (SDH) activity were also measured by colorimetric method following the manufacturer’s instructions. The five antioxidant capacity indexes including T-AOC, MDA, CAT, GSH-Px, and SOD were also tested by commercially available kit. All the assay kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Each intestinal segment was homogenized (1:4, weight[g]/volume[mL]) with ice-cold sodium chloride solution (154 mmol/L) using mechanically homogenize (Shanghai, China) to make 20% tissue homogenate, centrifuge at 2500 rpm for 10 min, then taken the supernatant to measure.
2.6. Total RNA Isolation and cDNA Library Construction and Sequencing
RNA was extracted and purified from duodenum according to the manufacturer’s protocols using TRizol (Invitrogen, CA, USA). RNA quality and purity were determined by using a NanoDrop ND-1000 (ThermoFisher, MA, USA). RNA integrity was assessed using the Bioanalyzer 2100 (Agilent, CA, USA). Next, specific mRNA was captured by PolyA using oligo dT (Invitrogen, CA, USA), then the captured polyA mRNA was fragmented at high temperature using the NEBNext® Magnesium RNA Fragmentation Module (NEB, MA, USA). First strand cDNA was synthesized from segmented RNA using the Invitrogen SuperScript™ II Reverse Transcriptase kit (Invitrogen, CA, USA). RNA was removed from the cDNA-RNA hybrid to generate double-stranded cDNA using E. coli DNA polymerase I (NEB, MA, USA) and RNase H (NEB, MA, USA). After that, dUTP solution (NEB, MA, USA) was added to generate blunt ends on double-stranded cDNA. A base was ligated to the T base adaptor at both ends of the cDNA, with fragment size screened and purified using oligo dT. Uracil-DNA glycocasylase (NEB, MA, US) was used to digest the second-strand cDNA and a library of 300 base pair (bp) ± 50 bp fragments was formed by PCR amplification. Finally, Illumina Novaseq™ 6000 (Illumina, CA, USA) was used to conduct paired-end sequencing following standard operating procedures using the PE150 sequencing.
2.7. Real-time Fluorescence Quantitative PCR
Seven DEGs including microsomal glutathione S-transferase 1 gene (
MGST1),
MGST2, ATP binding cassette subfamily G member 8 (
ABCG8),
ABCG5, fatty acid 2-hydroxylase (
FA2H), NAD(P)H quinone dehydrogenase 1 gene (
NQO1) and coagulation factor II thrombin receptor (
F2R) were randomly selected to confirm the accuracy of sequencing results by qRT-PCR using
β-Actin as a reference gene. The primers were designed by using Primer Express 3.0 software according to the mRNA sequence from GenBank. Sangon Biotech (Shanghai, China) helped to synthesize the primers. All the primers are shown in
Table S1. The Evo M-MLV reverse transcription premix kit (Accurate Biology, Hunan, China) was used for synthesized cDNA. The SYBR Green Pro Taq HS premix qPCR kit (Accurate Biology, Hunan, China) was used on a real-time fluorescent quantitative PCR apparatus (Roche, Switzerland). The reaction conditions were as follows: pre-denaturation 95°C, 30 s, 95°C, 5 s, 60°C 34 s, 40 cycles. The 2
-△△Ct method was used to calculate relative gene expression levels which normalized to the expression of
β-Actin.
2.8. Bioinformatics Analysis
Raw data were stored with Fasta format. We used CUTADAPT software (version 1.9) [
23] to generate high quality data,Hisat2 (version 2.0.4) [
24] to compare clean reads with the chicken genome reference. Stringtie software (version 1.3.4d) [
24] was used to reconstruct transcripts and calculate gene expression levels in samples. To quantitate gene expression, Fragments Per Kilobase of transcript sequence per Millions base pairs sequenced (FPKM = [total exon fragments/mapped reads (millions)x exon length (kb)]) was used as a measurable indicator in different samples. DESeq2 (version 1.42.1) [
25] was used for differential expression analysis. The Gene Ontology (GO) terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment were analyzed by R package
clusterProfiler (version 4.0) [
26] based on Benjamina-Hochberg method with statistical significance at adjusted
P-value less than 0.05. R package
enrichplot (version1.15.2) was used for visualization of functional enrichment result [
27]
2.9. Data Statistics and analysis
SPSS 20.0 software was used for statistical analysis and independent sample T-test was used for comparison between two groups. The result is expressed as mean ± standard error. P < 0.05 means significant difference, P < 0.01 means very significant difference.
3. Results
3.1. Egg Quality Parameters and Hens Plasma Biochemical Index
Table 1 presents a comprehensive overview of the egg quality parameters and the plasma biochemical indices of the laying hens. The eggshell thickness and ratio significantly increased (P < 0.01) in group T, while the egg yolk color significantly decreased (P < 0.05) in group T. The egg weight was slightly increased, whereas the eggshell index, eggshell color, Haugh unit, and albumen height tended to decrease in group T (
Table S2). The calcium content was slightly increased in group T, however the phosphorus and ash content in the eggshell was slightly decreased in translucent eggs. The plasma biochemical indexes, including LDL-C, HDL-C, phosphorus, and calcium exhibited a significant decrease, whereas the MDA demonstrated an increase (P < 0.05) in group T (
Table 1). The T-AOC, CAT, GSH-PX, and SOD were found to be slightly reduced in the hens that laid translucent eggs, while the TG value exhibited a tendency towards to elevation (
Table S2).
3.2. Intestinal Morphology and Structure, Enzyme Activity and Antioxidant Capacity
To obtain a comprehensive understanding of the digestive and absorptive capabilities within the small intestine, we analyzed the intestinal morphology, structure, digestive enzyme and energy metabolism enzyme activity, as well as the antioxidant capacity (
Table 2). Our findings demonstrated a decline in intestinal villus length and an upward trajectory in crypt depth (
Table S2), which result in the ratio of villus length to crypt depth in the duodenum exhibited a decline in group T (P < 0.05). Subsequently, the intestinal digestive enzymes activity and energy metabolism enzymes activity results showed that chymotrypsin activity diminished (P < 0.05) in the duodenum of group T, lipase activity in the jejunum extremely decreased (P < 0.01). Moreover, diastase activity in the ileum decreased (P < 0.05) in the group T. In the duodenum tissue of group T, the SDH activity decreased (P < 0.01), as the total ATPase and AKP activity (P < 0.05). In contrast, only the Na
+K
+-ATPase activity was decreased in jejunum. Similarly, the Ca
2+Mg
2+-ATPase activity exhibited a reduction (P < 0.05) within the same group in ileum. Significant reductions in intestinal antioxidant capacity, such as GSH-Px activity in the duodenum and jejunum (P < 0.05) were also found. In contrast, MDA content exhibited an increase in the duodenum (P < 0.05).
3.3. Quality Control and Statistics Alignment for RNA Sequencing Data
In order to reveal the molecular mechanism of intestinal effects on the formation of translucent egg, we used transcriptome analysis to detect the differentially expressed genes in duodenum from the two groups. Transcriptome sequencing of the duodenal tissues from the hens laid translucent eggs and normal eggs are shown in
Table S4. Total sequencing data for each sample were 40,786,044 to 55,668,976. The average GC content was 51.13%, the percentage of Q30 base was more than 97.00%. After filter, the number of clean reads per sample ranged from 38,508,396 to 52,293,940. The total mapped read was above 82%, the unique mapped reads was between 61.62% to 69.28% when compared with the chicken reference genome (
Table S5). The higher mapped rate indicated that the that RNA-Seq data quality meets the requirements for analyzing.
3.4. Screening and Validation of Differentially Expressed Genes Between Groups
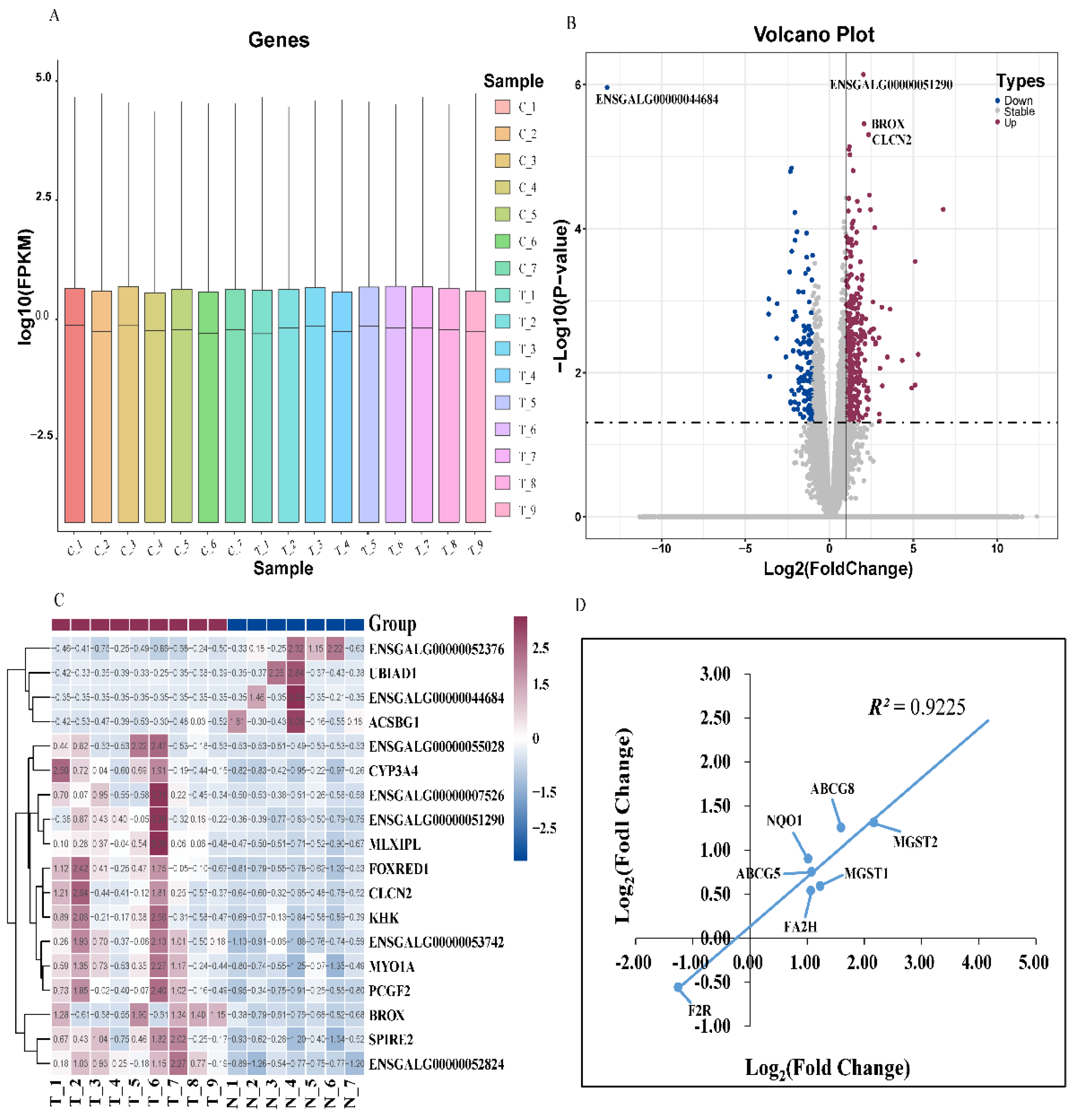
The gene density distribution according to FPKM did not shown difference among the samples (
Figure 3A). A total of 23910 genes were identified, and 471 DEGs were obtained (P ≤ 0.05 and |Log
2 Fold Change| ≥ 1). Among the DEGs, 327 genes were up-regulated and 144 genes were down-regulated (
Figure 3B). Furthermore, the hierarchical clustering analysis indicated that the expression trends of the replicates in DEGs (P ≤ 0.05 and |log
2 fold change| ≥ 1) were basically consistent, which further proving that our sequencing data had good biological repeatability (
Figure 3C). The correlation coefficient between the RNA-Seq and the qRT-PCR of the seven DEGs was 0.9225 (
Figure 3D), indicating that the sequencing results were reliable.
3.5. Functional Enrichment Analysis of Differentially Expressed Genes
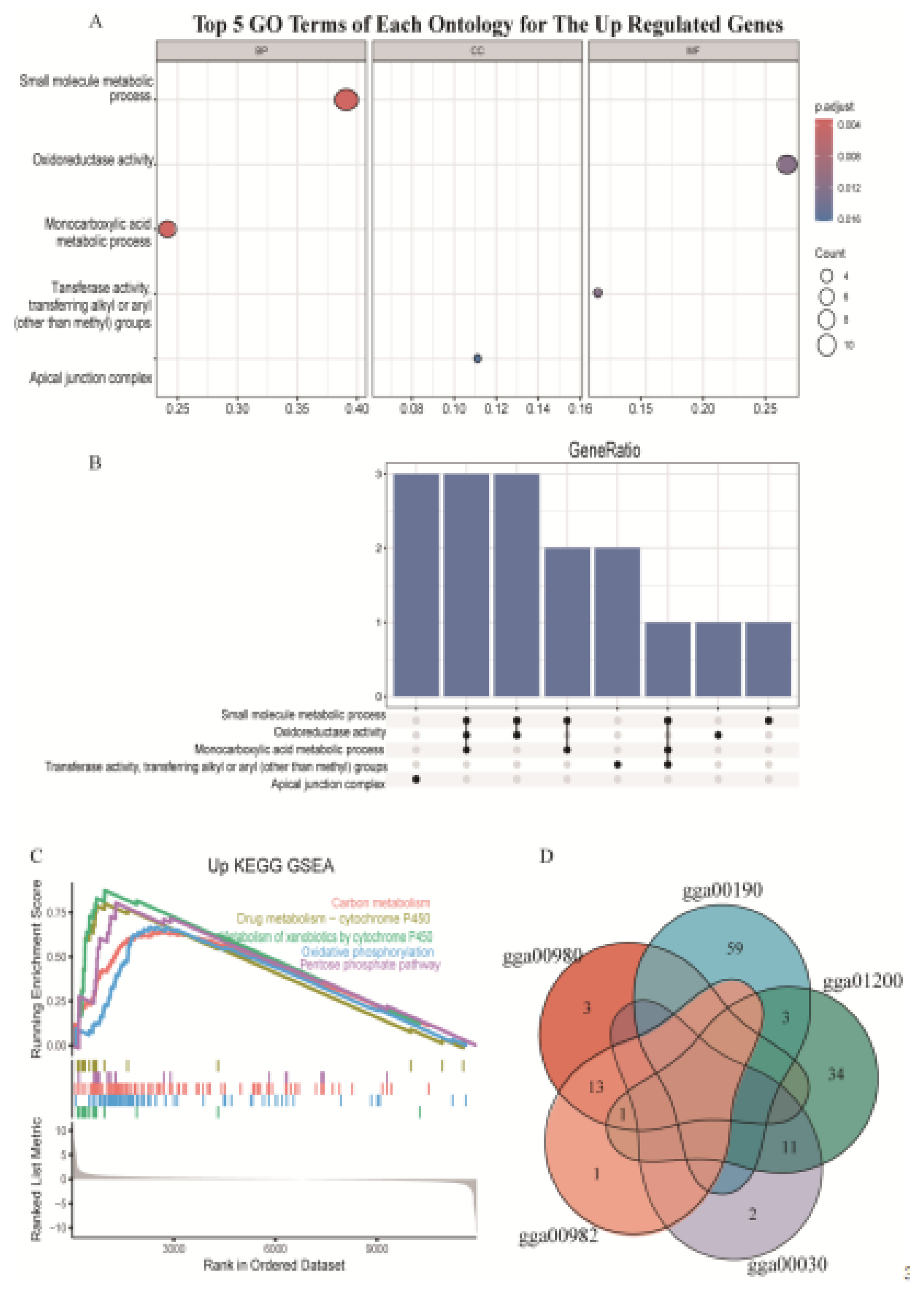
We conducted GO and KEGG pathway analysis. The GO analysis showed that up-regulated genes were mainly enriched in small molecule metabolic process (GO:0044281), oxidoreductase activity (GO:0016491), and monocarboxylic acid metabolic process (GO:0032787), but the down-regulated genes were not significantly enriched in any GO terms (
Figure 4A). Three up-regulated genes including acyl-CoA dehydrogenase family member 11 gene (ACAD11), alcohol dehydrogenase 1C (class I), gamma polypeptide gene (ADH1C), aldehyde dehydrogenase 1 family member A1 gene (ALDH1A1) enriched in three different GO terms (
Figure 4B). KEGG enrichment analysis showed that up regulated genes were mainly enriched in the carbon metabolism (gga01200), drug metabolism-cytochrome P450 (gga00982), metabolism of xenobiotics by cytochrome P450 (gga00980), oxidative phosphorylation (gga00190) and pentose phosphate pathway (gga00030) (
Figure 4C), but the down-regulated genes were not significantly enriched in any KEGG pathways. Eleven up-regulated genes were enriched in pentose phosphate pathway (gga00030) and carbon metabolism (gga01200). Fourteen up-regulated genes, including ADH4, ADH6, ADH1C, GSTA3, GSTA4, GSTK1, GSTM2, GSTO2, GSTT1, HPGDS, MGST1, MGST2, MGST3, UGT1A1 both enriched in gga00982 and in gga00980 (
Figure 4D).
4. Discussion
The formation of translucent eggs is a complex process. Eggshell translucency is regulated by genetic factors, which the heritability has been estimated to be 0.18 to 0.22 [
2]. Other factors, such as the temperature and humidity of the environment [
28], the diet [
29], as well as the age of laying hens [
30], could also lead to eggshell translucency. In this study, we first separated the eggs into control group and translucent egg group, and then tested the plasma biochemical indexes, the intestinal morphology and structure, as well as intestinal enzyme activity and antioxidant capacity of the laying hens. Transcriptome analysis of the duodenum from the laying hens who produced translucent and normal eggs was used to explore the DE genes and functional enrichment analysis.
4.1. Translucent Eggs Showed Thicker Eggshell and Lower Egg Yolk Color
Eggshell thickness is one of the most important indices of the external characteristics of eggs. The damage rate of eggshells is directly related to small changes in eggshell thickness. Previous researchers have confirmed that translucent eggs have higher eggshell thickness [
31], which is consistent with our finding. Previously, researchers have found that translucent egg grade was positively associated with eggshell structure including mastoid space height, mastoid space width, and mastoid space area [
28]. We speculated that the larger the mastoid space, the thicker the eggshell, and the higher the translucent score. The eggshell ratio is the ratio of eggshell weight to egg weight. Eggshell weight is mainly affected by eggshell thickness and eggshell density. In our study, there was no significant difference in egg weight between the group T and group C, but the eggshell thickness increased significantly, so it is speculated that the increase in eggshell thickness leads to a corresponding increase in eggshell ratio. Egg yolk color is often used as an important indicator to evaluate the internal characteristics of eggs, and to influence egg prices. Carotenoids could not be synthesized by hens and were extensively used in the diets of laying hens to improve the internal quality of eggs [
32]. The decrease in egg yolk color may be related to the intestinal absorption and transformation ability of carotenoids [
33].
4.2. Plasma Lipid Metabolism Disorder Result in Hens Laying Translucent Eggs
TC, TG, HDL-C and LDL-C in serum are closely related to lipid metabolisms [
34]. Compared with the control group, the HDL-C and LDL-C content in the plasma of the T group was significantly reduced, but the MDA content was significantly increased, which indicated the plasma lipid metabolism of the laying hens who laying translucent eggs is disordered. which in turn increases the production of lipid peroxides and reduces the antioxidant capacity.
4.3. Calcium and Phosphorus Content Decreased in Plasma of the Hens Laying Translucent Eggs
The main sources of calcium ions in the blood are from the intestines to absorb nutrients from the diet, then blood calcium is transported to the uterus, where the eggshell is formed when the calcium ions combine to form calcium carbonate, and are deposited on the eggshell membrane to form the eggshell [
35]. Researchers also found that calcium and phosphorus are two main elements for eggshell formation, and dietary calcium and phosphorus contents are associated with egg translucency [
28,
36,
37]. In our study, the plasma calcium and phosphorus contents of the translucent group were significantly reduced, which also indicated that the laying hens’ plasma calcium and phosphorus contents were associated with the formation of translucent eggs.
4.4. Intestinal Digestion, Absorption and Metabolism Affected Hens Laying Translucent Eggs
The structure of the avian small intestine serves as the basis for the digestive and absorptive processes. Intestinal villus length and crypt depth provide a comprehensive reflection of the small intestine functional status. Shorter villus length and greater crypt depth decrease the number of mature epithelial cells and reduce the contact area with chyme, which can lead to poor intestinal nutrient absorption [
38,
39]. Decreased villus to crypt ratio in the duodenum indicated damaged mucosa and reduced digestibility in hens laying translucent eggs group. In addition, the activity of intestinal digestive enzymes, including amylase, chymotrypsin, and lipase, plays a central role in nutrient digestion and absorption and has been considered to be a valuable parameter for feed utilization efficiency and performance in livestock [
13,
40]. We found that the activity of intestinal digestive enzymes decreased in the hens laying translucent eggs, especially chymotrypsin in the duodenum, lipase in the jejunum and amylase in the ileum, indicating that the hens have lower intestinal digestibility and absorptive capacity.
Intestinal AKP plays a pivotal role in maintaining intestinal homeostasis and protection [
41]. Our finding demonstrated that AKP activity was significantly diminished in the duodenum which may result in the inhibition of duodenum fat absorption and energy intake in hens laying translucent eggs. The Na
+-K
+-ATPase and Ca
2+-Mg
2+-ATPase are the primary ion pumps in cells, responsible for maintaining intracellular and intercellular ion concentration, and osmotic balance, transmembrane electrochemical potential, and cellular energy metabolism [
42]. The results demonstrated a significant reduction in Na
+-K
+-ATPase activity in the jejunum and Ca
2+-Mg
2+-ATPase activity in the ileum. This indicates that the energy production and utilization of the intestinal tract of laying hens with eggshell translucence are impaired, which affects the energy dissipation processes such as the active transport of feed nutrients. Consequently, the formation of translucent eggs may be associated with a reduction in duodenal mucosal integrity, as well as a diminished capacity for intestinal nutrient digestion, absorption and energy metabolism.
4.5. Intestinal Antioxidant Capacity Decreased Influenced Hens Laying Translucent Eggs
The dynamic balance of production and utilization of reactive oxygen species (ROS) will break when in a pathological or stress condition, and lead to ROS accumulation. T-AOC can reflect the comprehensive antioxidant capacity under the co-regulation of CAT, SOD, and GSH-Px. MDA is a product of toxic lipid peroxidation product formed by unsaturated fatty acids and ROS could indirectly reflect the organism damage [
43]. The GSH-Px in duodenum significantly decreased, but MDA significantly increased in the group T indicate that the intestinal antioxidant capacity decreased, which may trigger intestinal oxidative stress, resulted in inhibiting intestinal Ca
2+ absorption [
44], and influenced hens laying translucent eggs.
4.6. Transcriptome Analysis in Duodenum
Transcriptome analysis has emerged as one of the most frequently utilized methods among recent research activities, due to the rapid and inexpensive Next Generation Sequencing (NGS) technology which offers high throughput gene expression profiling [
45]. Our results indicated that the upregulated genes most enriched in the metabolism related pathways. Such as oxidative phosphorylation pathway, the process of oxidative phosphorylation mainly occurs in intracellular mitochondria [
46], and mitochondrial dysfunction in duodenum could restrict nutrient or Ca
2+ absorption, therefore causing the hens laying translucent egg.
Glutathione-S transferases (GSTs) have functions in modulating glutathione metabolism.
MGST1,
GSTA3, and
GSTT1 are three different types of glutathione S-transfer. Specifically,
MGST1 has anti-inflammatory and antioxidant effects [
47].
GSTA3 is found to exist in the mitochondria and capable to clear various peroxidation products [
48]. The up-regulation of
GSTA3,
GSTT1 and
GSTO2 genes is aimed at clearing peroxide products and alleviating oxidative stress, which indicated that the altered intestinal metabolism capabilities will lead to translucent eggs production.
5. Conclusions
Our study found that translucent eggs showed thicker eggshell and lower egg yolk color, which may be caused by the laying hens plasma lipid metabolism disorder, decreased intestinal antioxidant capacity, reduced digestion and absorption as well as metabolism capabilities. Our study is also the first to use RNA-seq to identify and annotate DEGs in the duodenal tissues of hens laying translucent eggs and normal eggs. We identified 327 DEGs associated with the production of translucent eggs producing, which mainly enriched in the pathways of metabolism, oxidative phosphorylation, and energy homeostasis, including GSTA3, GSTT1 and GSTO2 gene. Nevertheless, further investigation is required to elucidate the intestinal homeostasis function and the interactions between the microbiota, the intestinal epithelium, and the host immune system to translucent egg production.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org. Table S1. The gene primers sequences for quantitative real-time reverse-transcription PCR. Table S2. Egg quality parameters, eggshell calcium, phosphorus, ash content and the chicken plasma biochemical indexes. Table S3. Small intestinal morphology and structure, enzyme activity and antioxidant capacity index. Table S4. Overview of sequencing data quality control. Table S5. Overview of sequencing data mapping rate to the reference genome. Table S6 Statistical interval distribution of FPKM value in samples
Author Contributions
Conceptualization, Y.JD, D.H and Y.Y; methodology, D.H; software, D.H.; validation, Y.JD, WL, WL,; formal analysis, DH.; investigation, DPL, QXL,; resources, WL, WL, DPL, QXL, YZ, JL, and DGC; data curation, DH.; writing—original draft preparation, YJD and DH.; writing—review and editing, YJD and DH.; visualization, DH.; supervision, FWL.; project administration, HXH; funding acquisition, FC,FWL and HXH. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by China Agriculture Research System (CARS-40), Poultry Industry Technology System Nutrition and Feed Post Project (SDAIT-11-07), Agricultural Breed Project of Shandong Province (2022LZGC013), Central Leading Local Science and Technology Development Special Project (YDZX2023034), Science and Technology Innovation 2030-Major Project (2023ZD04052), Taishan Industry Experts Program (TSCX202312057).
Institutional Review Board Statement
The animal study protocol was approved by the Animal Ethics committee of Shandong Academy of Agricultural Science (Permit No: 2018412) The study was carried out in compliance with the ARRIVE guidelines. The care and use of experimental animals were carried out in accordance with the Directory Proposals on the Ethical Treatment of Experimental Animals, established by the Ministry of Science and Technology (Beijing, China). All study animal procedures were approved, and supervised by the Animal Ethics committee of Shandong Academy of Agricultural Science (Permit No: 2018412). All methods were performed in accordance with relevant guidelines and regulations.
Data Availability Statement
Raw sequence data were deposited in the Genome Sequence Archive in the BIG Data Center, Beijing Institute of Genomics, Chinese Academy of Sciences, and is publicly accessible at
http://bigd.big.ac.cn/gsa (accession no. CRA009523).
Acknowledgments
We would like to express our gratitude to our colleagues who contributed to this article and to the faculty members who reviewed the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Holst, W.F.; Almquist, H.J.; Lorenz, F.W. A Study of Shell Texture of the Hen’s Egg. Poult Sci 1932, 11, 144–149. [Google Scholar] [CrossRef]
- Zhang, H.D.; Zhao, X.F.; Ren, Z.Z.; Tong, M.Q.; Chen, J.N.; Li, S.Y.; Chen, H.; Wang, D.H. Comparison between Different Breeds of Laying Hens in Terms of Eggshell Translucency and Its Distribution in Various Ends of the Eggshell. Poult Sci 2021, 100, 1–8. [Google Scholar] [CrossRef]
- Baker, R.C.; Curtiss, R. Individual Hen Differences in Egg Shell Mottling and the Relationship of Shell Mottling to Clutch Size, Internal Quality and Weight Loss. Poult Sci 1957, 36, 904–908. [Google Scholar] [CrossRef]
- Chousalkar, K.K.; Flynn, P.; Sutherland, M.; Roberts, J.R.; Cheetham, B.F. Recovery of Salmonella and Escherichia Coli from Commercial Egg Shells and Effect of Translucency on Bacterial Penetration in Eggs. Int J Food Microbiol 2010, 142, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.H.; Chen, H.; Zhou, R.Y.; Huang, C.X.; Gao, H.X.; Fan, B.L.; Liu, G.J.; Ning, Z.H. Study of Measurement Methods on Phenotype of Translucent Eggs. Poult Sci 2019, 98, 15853–15869. [Google Scholar] [CrossRef]
- Noy, Y.; Sklan, D. Digestion and Absorption in the Young Chick. Poult Sci 1995, 74, 366–373. [Google Scholar] [CrossRef]
- Oviedo-Rondón, E.O. Holistic View of Intestinal Health in Poultry. Anim Feed Sci Technol 2019, 250, 1–8. [Google Scholar] [CrossRef]
- Johnson, C.D.; Kudsk, K.A. Nutrition and Intestinal Mucosal Immunity. Clinical Nutrition 1999, 18, 337–344. [Google Scholar] [CrossRef]
- Ravindran, V.; Reza Abdollahi, M. Nutrition and Digestive Physiology of the Broiler Chick: State of the Art and Outlook. Animals 2021, 11, 1–23. [Google Scholar] [CrossRef]
- Zhu, L.P.; Wang, J.P.; Ding, X.M.; Bai, S.P.; Zeng, Q.F.; Su, Z.W.; Xuan, Y.; Applegate, T.J.; Zhang, K.Y. The Effects of Varieties and Levels of Rapeseed Expeller Cake on Egg Production Performance, Egg Quality, Nutrient Digestibility, and Duodenum Morphology in Laying Hens. Poult Sci 2019, 98, 4942–4953. [Google Scholar] [CrossRef]
- Zhao, Y.; Hou, Y.; Xu, Y.; Luan, Y.; Zhou, H.; Qi, X.; Hu, M.; Wang, D.; Wang, Z.; Fu, Y.; et al. A Compendium and Comparative Epigenomics Analysis of Cis-Regulatory Elements in the Pig Genome. Nat Commun 2021, 12, 1–17. [Google Scholar] [CrossRef]
- Chen, J.F.; Xu, M.M.; Kang, K.L.; Tang, S.G.; He, C.Q.; Qu, X.Y.; Guo, S.C. The Effects and Combinational Effects of Bacillus Subtilis and Montmorillonite on the Intestinal Health Status in Laying Hens. Poult Sci 2020, 99, 1311–1319. [Google Scholar] [CrossRef]
- Gu, Y.F.; Chen, Y.P.; Jin, R.; Wang, C.; Wen, C.; Zhou, Y.M. A Comparison of Intestinal Integrity, Digestive Function, and Egg Quality in Laying Hens with Different Ages. Poult Sci 2021, 100, 1–8. [Google Scholar] [CrossRef]
- Shalaei, M.; Hosseini, S.M.; Zergani, E. Effect of Different Supplements on Eggshell Quality, Some Characteristics of Gastrointestinal Tract and Performance of Laying Hens. Vet Res Forum 2014, 5, 277–286. [Google Scholar]
- Cheng, X.; Ning, Z. Research Progress on Bird Eggshell Quality Defects: A Review. Poult Sci 2023, 102, 1–10. [Google Scholar] [CrossRef]
- Wongdee, K.; Chanpaisaeng, K.; Teerapornpuntakit, J.; Charoenphandhu, N. Intestinal Calcium Absorption. Compr Physiol 2021, 11, 2047–2073. [Google Scholar] [CrossRef] [PubMed]
- Kebreab, E.; France, J.; Kwakkel, R.P.; Leeson, S.; Darmani Kuhi, H.; Dijkstra, J. Development and Evaluation of a Dynamic Model of Calcium and Phosphorus Flows in Layers. Poult Sci 2009, 88, 680–689. [Google Scholar] [CrossRef]
- Peng, J.B.; Chen, X.Z.; Berger, U.V.; Vassilev, P.M.; Tsukaguchi, H.; Brown, E.M.; Hediger, M.A. Molecular Cloning and Characterization of a Channel-like Transporter Mediating Intestinal Calcium Absorption. Journal of Biological Chemistry 1999, 274, 22739–22746. [Google Scholar] [CrossRef]
- Bronner, F. Recent Developments in Intestinal Calcium Absorption. Nutr Rev 2009, 67, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Fleet, J.C.; Schoch, R.D. Molecular Mechanisms for Regulation of Intestinal Calcium Absorption by Vitamin D and Other Factors. Crit Rev Clin Lab Sci 2010, 47, 181–195. [Google Scholar] [CrossRef]
- Hoenderop, J.G.J.; Nilius, B.; Bindels, R.J.M. Calcium Absorption Across Epithelia. Physiol Rev 2005, 85, 373–422. [Google Scholar] [CrossRef]
- Anderson, K.E.; Tharrington, J.B.; Curtis, P.A.; Jones, F.T. Shell Characteristics of Eggs from Historic Strains of Single Comb White Leghorn Chickens and the Relationship of Egg Shape to Shell Strength. Int J Poult Sci 2004, 3, 17–19. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet J 2011, 17, 1–10. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-Level Expression Analysis of RNA-Seq Experiments with HISAT, StringTie and Ballgown. Nat Protoc 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol 2014, 15, 1–21. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. ClusterProfiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. Innovation 2021, 2, 1–10. [Google Scholar] [CrossRef]
- Yu, G. Enrichplot: Visualization of Functional Enrichment Result. R package version 1.12.3 2021.
- Shi, X.; Liang, Q.; Wang, E.; Jiang, C.; Zeng, L.; Chen, R.; Li, J.; Xu, G.; Zheng, J. A Method to Reduce the Occurrence of Egg Translucency and Its Effect on Bacterial Invasion. Foods 2023, 12, 1–15. [Google Scholar] [CrossRef]
- Bouvarel, I.; Nys, Y.; Lescoat, P. Hen Nutrition for Sustained Egg Quality. In Improving the Safety and Quality of Eggs and Egg Products: Egg Chemistry, Production and Consumption; Yves Nys, Maureen Bain, Filip Van Immerseel, Ed.; Woodhead Publishing: Brussels Belgium, 2011; pp. 261–299. [Google Scholar]
- Ren, H.L.; Zhao, X.Y.; Di, K.Q.; Li, L.H.; Hao, E.Y.; Chen, H.; Zhou, R.Y.; Nie, C.S.; Wang, D.H. Eggshell Translucency in Late-Phase Laying Hens and Its Effect on Egg Quality and Physiological Indicators. Front Vet Sci 2023, 10, 1–10. [Google Scholar] [CrossRef]
- Wang, D.-H.; Li, Y.-J.; Liu, L.; Liu, J.-S.; Bao, M.; Yang, N.; Zhuo-Cheng, H.; Ning, Z.-H. Traits of Eggshells and Shell Membranes of Translucent Eggs. Poult Sci 2017, 96, 351–358. [Google Scholar] [CrossRef]
- Yunitasari, F.; Jayanegara, A.; Ulupi, N. Performance, Egg Quality, and Immunity of Laying Hens Due to Natural Carotenoid Supplementation: A Meta-Analysis. Food Sci Anim Resour 2023, 43, 282–304. [Google Scholar] [CrossRef]
- Dansou, D.M.; Zhang, H.; Yu, Y.; Wang, H.; Tang, C.; Zhao, Q.; Qin, Y.; Zhang, J. Carotenoid Enrichment in Eggs: From Biochemistry Perspective. Animal Nutrition 2023, 14, 315–333. [Google Scholar] [CrossRef]
- Li, Q.P.; Gooneratne, S.R.; Wang, R.L.; Zhang, R.; An, L.L.; Chen, J.J.; Pan, W. Effect of Different Molecular Weight of Chitosans on Performance and Lipid Metabolism in Chicken. Anim Feed Sci Technol 2016, 211, 174–180. [Google Scholar] [CrossRef]
- Stapane, L.; Le Roy, N.; Ezagal, J.; Rodriguez-Navarro, A.B.; Labas, V.; Combes-Soia, L.; Hincke, M.T.; Gautron, J. Avian Eggshell Formation Reveals a New Paradigm for Vertebrate Mineralization via Vesicular Amorphous Calcium Carbonate. Journal of Biological Chemistry 2020, 295, 15853–15869. [Google Scholar] [CrossRef]
- Nie, W.; Yang, Y.; Yuan, J.; Wang, Z.; Guo, Y. Effect of Dietary Nonphytate Phosphorus on Laying Performance and Small Intestinal Epithelial Phosphate Transporter Expression in Dwarf Pink-Shell Laying Hens. J Anim Sci Biotechnol 2013, 4, 1–7. [Google Scholar] [CrossRef]
- Cusack, M.; Fraser, A.C.; Stachel, T. Magnesium and Phosphorus Distribution in the Avian Eggshell. Comparative Biochemistry and Physiology - B Biochemistry and Molecular Biology 2003, 134, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.R.; Hu, C.H.; Xia, M.S.; Zhan, X.A.; Wang, M.Q. Effects of Dietary Fructooligosaccharide on Digestive Enzyme Activities, Intestinal Microflora and Morphology of Male Broilers. Poult Sci 2003, 82, 1030–1036. [Google Scholar] [CrossRef]
- Yason, C.V.; Summers, B.A.; Schat, K.A. Pathogenesis of Rotavirus Infection in Various Age Groups of Chickens and Turkeys: Pathology. Am J Vet Res 1987, 48, 927–938. [Google Scholar] [PubMed]
- Giannenas, I.; Tsalie, E.; Triantafillou, E.; Hessenberger, S.; Teichmann, K.; Mohnl, M.; Tontis, D. Assessment of Probiotics Supplementation via Feed or Water on the Growth Performance, Intestinal Morphology and Microflora of Chickens after Experimental Infection with Eimeria Acervulina, Eimeria Maxima and Eimeria Tenella. Avian Pathology 2014, 43, 209–216. [Google Scholar] [CrossRef]
- Lallès, J.P. Intestinal Alkaline Phosphatase: Multiple Biological Roles in Maintenance of Intestinal Homeostasis and Modulation by Diet. Nutr Rev 2010, 68, 323–332. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, J.; Wang, B.; Tang, J. Effect of γ-Aminobutyric Acid on Digestive Enzymes, Absorption Function, and Immune Function of Intestinal Mucosa in Heat-Stressed Chicken. Poult Sci 2014, 93, 2490–2500. [Google Scholar] [CrossRef]
- Zhu, Y.W.; Lu, L.; Li, W.X.; Zhang, L.Y.; Ji, C.; Lin, X.; Liu, H.C.; Odle, J.; Luo, X.G. Effect of Dietary Manganese on Antioxidant Status and Expressions of Heat Shock Proteins and Factors in Tissues of Laying Broiler Breeders under Normal and High Environmental Temperatures. British Journal of Nutrition 2016, 116, 1851–1860. [Google Scholar] [CrossRef] [PubMed]
- Diaz De Barboza, G.; Guizzardi, S.; Tolosa De Talamoni, N. Molecular Aspects of Intestinal Calcium Absorption. World J Gastroenterol 2015, 21, 7142–7154. [Google Scholar] [CrossRef]
- Mutz, K.O.; Heilkenbrinker, A.; Lönne, M.; Walter, J.G.; Stahl, F. Transcriptome Analysis Using Next-Generation Sequencing. Curr Opin Biotechnol 2013, 24, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Guerbette, T.; Boudry, G.; Lan, A. Mitochondrial Function in Intestinal Epithelium Homeostasis and Modulation in Diet-Induced Obesity. Mol Metab 2022, 63, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Lu, X. MGST1 Alleviates the Oxidative Stress of Trophoblast Cells Induced by Hypoxia/Reoxygenation and Promotes Cell Proliferation, Migration, and Invasion by Activating the PI3K/AKT/MTOR Pathway. Open Medicine (Poland) 2022, 17, 2062–2071. [Google Scholar] [CrossRef]
- Ma, K.Y.; Yang, N.; Jiao, R.; Peng, C.; Guan, L.; Huang, Y.; Chen, Z.Y. Dietary Calcium Decreases Plasma Cholesterol by Down-Regulation of Intestinal Niemann-Pick C1 like 1 and Microsomal Triacylglycerol Transport Protein and up-Regulation of CYP7A1 and ABCG 5/8 in Hamsters. Mol Nutr Food Res 2011, 55, 247–258. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).