1. Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), remains a public health problem, even though the pandemic has been controlled effectively across the world. Since the first case of COVID-19 was reported in Kenya in March 13, 2020, a total of 344,130 COVID-19 cases, 5,689 deaths, and 337,309 recoveries have been confirmed, with approximately 1,132 ongoing infections reported as of April 12, 2024 [
1]. Researchers have acknowledged that the reported numbers are a gross underestimation of the actual figures [
2]. To inform infection control strategies, such as vaccination, studying the seroprevalence of anti-SARS-CoV-2 IgG and IgM could help to better define the epidemiology of COVID-19 [
3]. Anti-SARS-CoV-2 IgM antibodies are produced in the early phase of the immune response and are indicators of current or recent infection [
4]. In contrast, the IgG antibodies emerge later after IgM antibodies in a primary infection or vaccination and persist in the body for months or years. Therefore, the anti-SASR-CoV-2 IgG and IgM antibodies can provide important insights necessary for understanding immunity and progression of COVID-19.
COVID-19 in Kenya has shown a dual epidemiological pattern, with cities like Nairobi recording higher seroprevalence of anti-SARS-CoV-2 antibodies compared to rural areas [
5]. In the study conducted by Etyang et al. from December 2020 to May 2021 in Kenya, the seroprevalence of anti-SARS-CoV-2 IgG was 36%, 32.4%, and 14.5% in Kisumu (sub-urban), Nairobi (urban), and Kilifi (rural), respectively, at the beginning of the study [
6]. The seroprevalence rose to 42%, 50.2%, and 24.7% in the three areas at the end of the study. By the end of 2022, the seroprevalence of anti-SARS-CoV-2 IgG had risen to 92.2% in Nairobi and 77.4% in Kilifi [
7]. The rise in seroprevalence could be due to natural infection by SARS-CoV-2 or COVID-19 vaccination, which was actively rolled out from March 2021 to the end of 2022. On May 5, 2023, the World Health Organization (WHO) downgraded COVID-19 from a public health emergency of international concern, but maintained that a public health risk remained, especially because of the possible emergence of new variants [
8].
Vaccination has been instrumental to the control of the COVID-19 pandemic worldwide. In Kenya, as of May 2022, more than 32 million individuals had received at least one dose of COVID-19 vaccine, and as of August 2022, 9.3 million adults had been fully vaccinated. Following the approval of COVID-19 vaccines, 36.5% of the population in Kenya expressed reluctance toward getting vaccinated against COVID-19 [
9]. This hesitancy may impede efforts to achieve herd immunity and control the spread of the virus. Additionally, the effectiveness of the vaccination drives in Kenya has been further complicated by the emergence of multiple SARS-CoV-2 variants of concern [
10]. This raises questions about the ability of certain populations to maintain protective levels of anti-SARS-CoV-2 antibodies and the efficacy of the rolled-out COVID-19 vaccines.
Studies on the epidemiological patterns of COVID-19 in Kenya were mainly conducted during the early days of the COVID-19 pandemic and vaccine roll out, and the seroprevalence of anti-SARS-CoV-2 antibodies post-vaccination remains unclear. With COVID-19 now establishing endemicity with seasonal outbreaks, determining the level of antibody protection is an important public health priority. Therefore, this study aimed to determine the seroprevalence of anti-SARS-CoV-2 IgM and IgG and evaluate the COVID-19 vaccination uptake in a university community in Nairobi, Kenya.
2. Materials and Methods
2.1. Study Design and Setting
We conducted a cross-sectional, population-based study to assess the seroprevalence of anti-SARS-CoV-2 IgM and IgG antibodies among healthy volunteers from the Kenyatta University community. The study was carried out from April to July 2023. Ethical approval was obtained from the Kenyatta University Ethics Review Committee (protocol code: PKU/2379/11516; February 28, 2022). Further approval was granted by the National Commission for Science, Technology, and Innovation. All volunteers provided written informed consent to participate in this study.
2.2. Recuitment of Participants and Eligibility Criteria
A call for participation was made via posters, staff meetings, and after-class announcements. Volunteers were considered eligible if they were 1) students or staff at Kenyatta University, 2) aged ≥18 years, 3) consented to provide nasopharyngeal swab and blood specimen, and 4) consented to complete the structured questionnaire.
2.3. Data Collection, Research Instruments, and Procedures
2.3.1. Collection of Clinical and Demographic Data
The study utilized a structured questionnaire to gather clinical and demographic data. The demographic data included age, sex, level of education, and occupation. Clinical data encompassed history of COVID-19 diagnosis, COVID-19 vaccination status, type of vaccine administered, administration of booster vaccine, and occurrences of breakthrough infection.
2.3.2. SARS-CoV-2 Testing
Nasopharyngeal swabs were collected from each participant and labeled with a unique identifier. The nasopharyngeal swabs underwent on-site testing for SARS-CoV-2 using a rapid COVID-19 test kit (Panbio™ COVID-19 Ag Rapid Test Device, Abbott, Jena, Germany).
2.3.3. Enzyme-Linked Immunosorbent Assays (ELISA) for Anti-SARS-CoV-2 IgM and IgG
Blood samples (5 ml) were collected from each participant and labeled with a unique identifier. The blood samples were centrifugated at 1000 x g for 5 min to extract serum and immediately refrigerated at -200C until testing. As described by the manufacturer, the levels of anti-SARS-CoV-2 IgM and IgG in the serum were quantified using human SARS-CoV-2 Spike (Trimer) IgM and IgG enzyme-linked immunosorbent assays (ELISA), respectively (Thermo Fisher Invitrogen, Waltham, MA, USA). The assays were based on sandwich ELISA in which wells were precoated with a trimerized spike protein. For the respective assays, the serum was diluted 1000-fold and 10 µL of the diluted sample added to the wells. Biotin-conjugated IgM and IgG antibodies were used to detect the captured anti-SARS-CoV-2 IgM and IgG antibodies, and the absorbances were read at a wavelength of 450 nm using a spectrophotometer (RT-2100C, Rayto Life and Analytical Sciences, Guangdong, China).
2.4. Statistical Analysis
The absorbance readings of the standards, controls, and test samples were obtained from the spectrophotometer. Qualitative results were presented by calculating the ratio of the test optical density (OD) to the OD of the plate medium control. Samples with OD ratios >1.3 were classified as positive for SARS-CoV-2 IgG and IgM, while those with ratios <1 were considered negative. For quantitative analysis, absorbance data were used to construct a standard curve via the Excel Curve Fitting algorithm, leveraging a four-parameter algorithm for optimal curve fitting. Unknown sample concentrations were extrapolated from the standard curve.
The obtained concentrations of anti-SARS-CoV-2 IgG and IgM underwent the Kolmogorov–Smirnov test and were found to have a non-normal distribution. As such, these data are summarized using median and interquartile range. Categorical data are expressed as counts and percentages. Descriptive statistics were used to analyze the characteristics of the participants, including age, sex, and COVID-19 vaccination status. Statistical comparisons were conducted using the Mann–Whitney test (U) for binary comparisons and the Kruskal–Wallis Test (H) for multigroup comparisons. The quantitative analyses were conducted using SPSS (version 18) software (IBM Corp, Armonk, NY), with significance set at p < 0.05.
Qualitative data on vaccine hesitancy were grouped into three categories according to the 3C model of vaccine hesitancy by the WHO’s Strategic Advisory Group of Experts on Immunization (SAGE) working group [
11]. The 3Cs include confidence, complacency, and convenience. Then, a thematic analysis of participants’ responses was performed based on the three categories.
3. Results
3.1. Clinical and Demographic Characteristics
A total of 189 participants (female, 50.8%; median age, 21 years) were recruited to participate in this study. Participants aged 20–29 years were the majority (n = 135, 71.4%), whereas those above 50 years were the minority (n = 5, 2.6%). The overall rate of vaccination was 56.6% (n = 107), with females (n = 61, 63.5%) having a higher vaccination rate than males (n = 46, 49.5%). All the participants had college/university education. The majority of the study participants were students (n = 170, 89.9%) while the rest were teaching (n = 10, 5.3%) and non-teaching staff (n = 9, 4.8%) (
Table 1).
3.2. Previous and Current Status of COVID-19
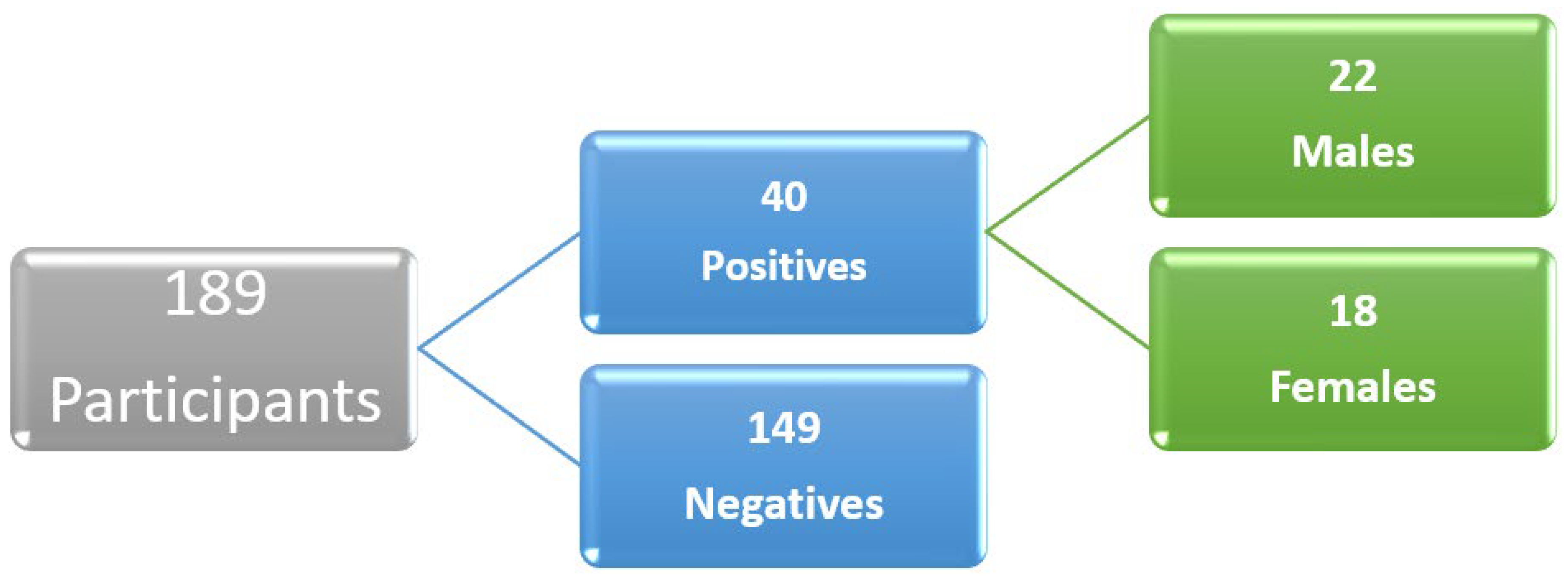
A total of 40 participants (21.2%) self-reported that they were positive for COVID-19 in the past based on laboratory testing or clinical diagnosis. Among these, males were 22 (23.7%) and females were 18 (18.8%) (
Figure 1).
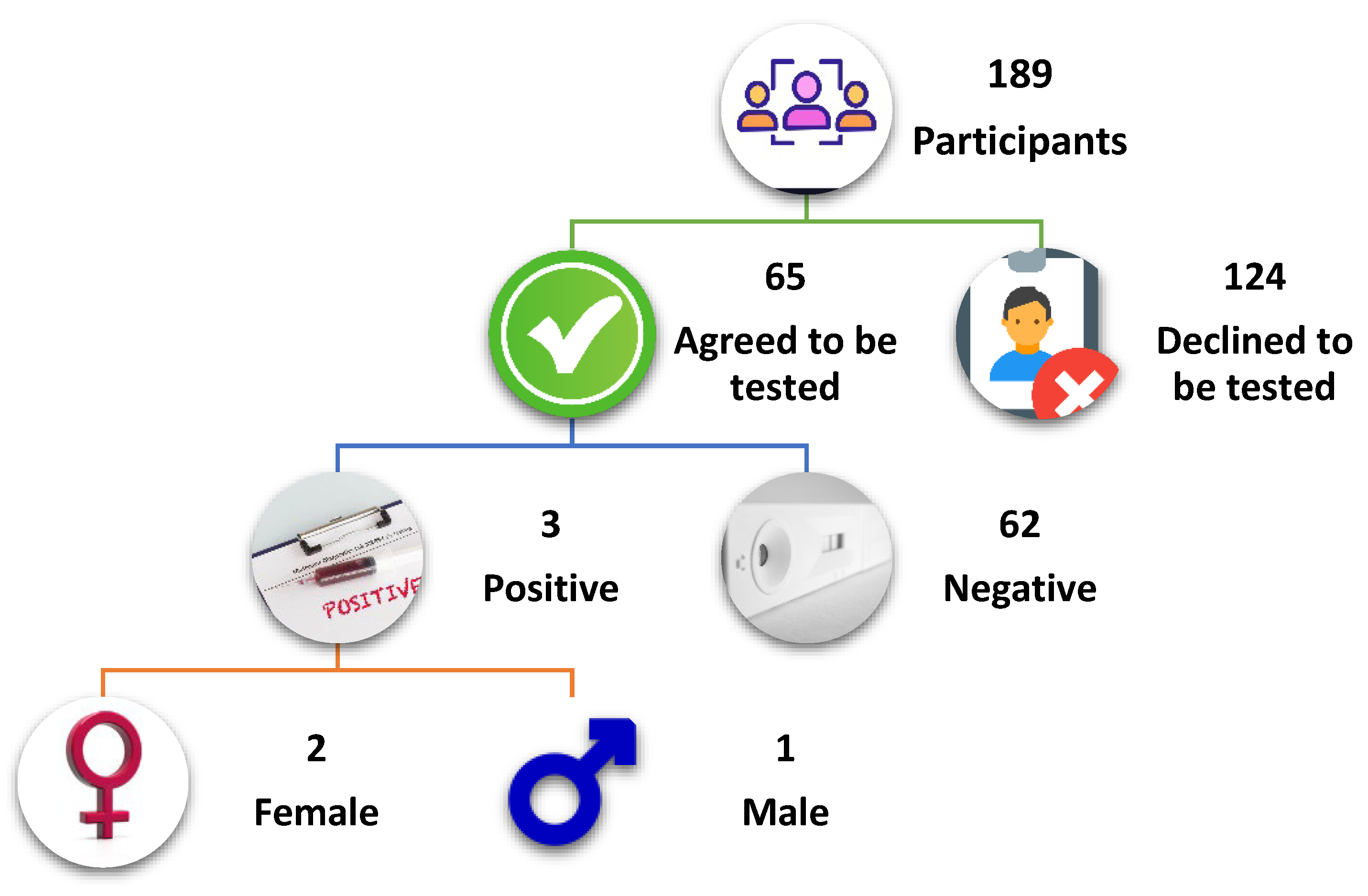
A test for anti-SARS-CoV-2 antigen positivity during the study, which was performed in only 65 consenting participants, revealed a positivity rate of 4.6% (n = 3) (
Figure 2). The study reported breakthrough infections in 9.3% (n = 10) of the vaccinated individuals.
3.3. Seroprevalence of Anti-SARS-CoV-2 IgM and IgG and Comparison of IgG Titers
Of the 189 participants, 24 were positive for anti-SARS-CoV-2 IgM (12.7%), whereas 166 were positive for anti-SARS-CoV-2 IgG (87.8%) (
Table 2). Female participants had a slightly higher seroprevalence (IgM, 15.6%; IgG, 92.7%) compared to males (IgM, 9.7%; IgG, 83.8%).
The anti-SARS-CoV-2 IgG levels were significantly higher in females than in males (
p = 0.024,
U = 3616) and in vaccinated individuals than in the non-vaccinated individuals (
p < 0.001,
U = 2817.5). No significant age differences in the anti-SARS-CoV-2 IgG levels were observed (
Table 3).
3.4 Anti-SARS CoV-2 IgG Levels according to Duration Since Vaccination, Type of Vaccine, and Booster Doses
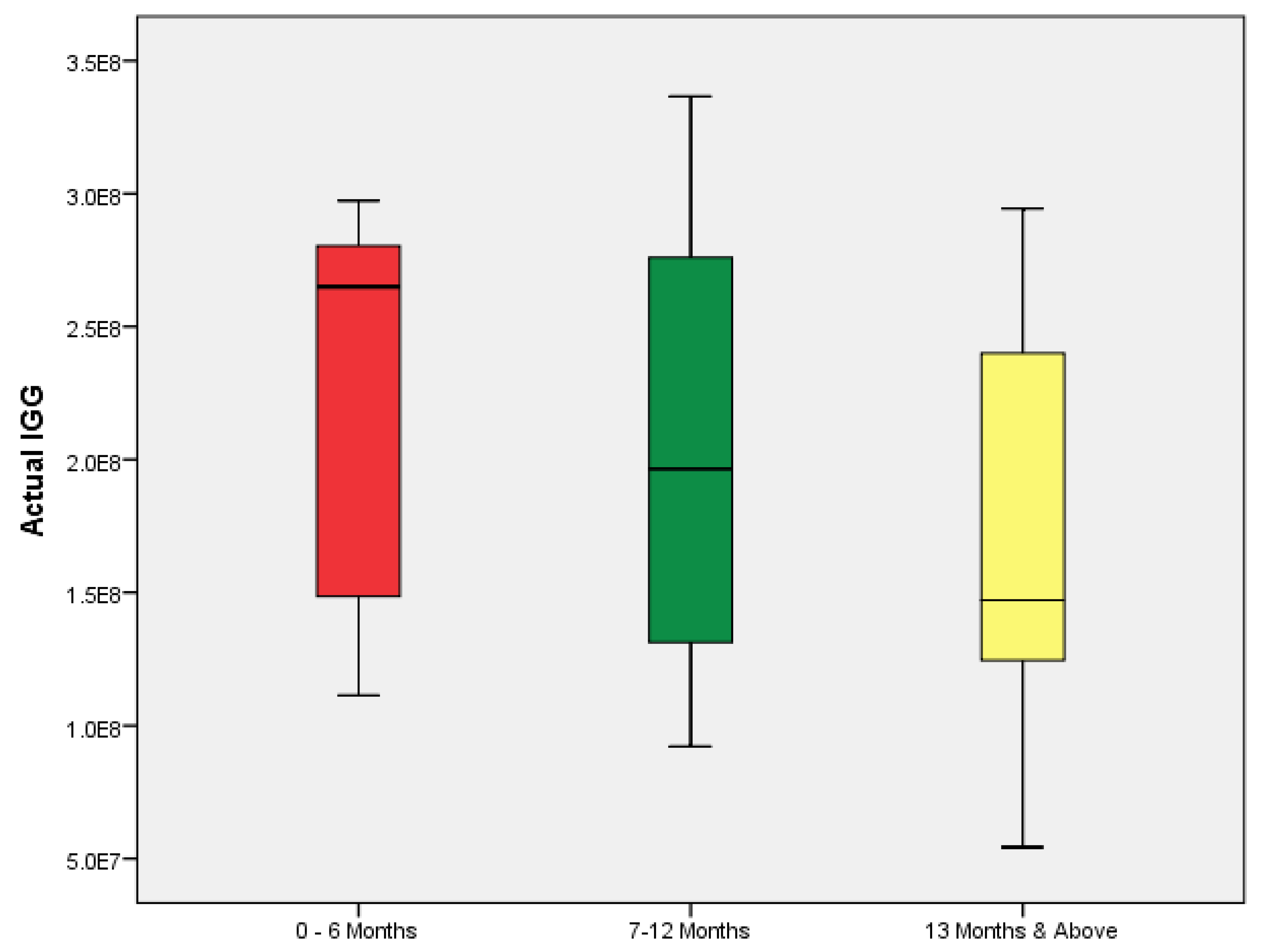
A comparison of anti-SARS-CoV-2 IgG levels and duration since vaccination showed that participants vaccinated 6 months or less prior to the study had significantly higher anti-SARS-CoV-2 IgG levels than those vaccinated more than 1 year earlier (
p = 0.002,
H = 12.359) (
Figure 3).
The present study found no significant differences in the levels of anti-SARS-CoV-2 IgG among the participants who received vaccines from different manufacturers (Moderna, AstraZeneca, Pfizer, and Johnson & Johnson,
p = 0.170,
H = 5.027) (
Table 4). Similarly, no significant differences in anti-SARS CoV-2 IgG levels were observed between those who had received a booster dose compared to those who had not (
p = 0.758,
U = 1087).
3.5. COVID-19 Vaccine Hesitancy
A total of 82 (43.4%) participants were not vaccinated against COVID-19. Of these, 10 did not indicate the reasons for non-vaccination. The rate of vaccine hesitancy was higher in males than in females (50.5% vs. 36.5%,
p = 0.05). An analysis for the reasons for non-vaccination was conducted on the remaining 72 participants. Under the confidence category, the identified themes, which comprise the reasons for non-vaccination, were mistrust in the vaccine effectiveness and production process (22.4%), health concerns/side effects (19.7%), lack of information about the vaccines (18.4%), and religious and cultural reasons (3.9%). The complacency category comprised no reason (14.5%), lack of interest (11.8%), and procrastination (2.6%). In addition, the convenience category included vaccine unavailability (2.6%) and lack of time (3.9%) (
Table 5).
4. Discussion
COVID-19 morbidity and mortality remain significant public health concerns, necessitating robust serosurveillance to elucidate disease epidemiology and evaluate the efficacy of control strategies. This study found an overall seroprevalence of 12.7% for anti-SARS-CoV-2 IgM and 87.8% for anti-SARS-CoV-2 IgG. We observed significantly higher levels of anti-SARS-CoV-2 IgG in females than in males (p = 0.024), among the vaccinated than among the non-vaccinated individuals (p < 0.001), and among those vaccinated 6 months or less prior to the study compared to those vaccinated more than 1 year earlier (p = 0.002). However, no significant differences in anti-SARS-CoV-2 IgG levels were observed across the various brands of vaccines and after the administration of booster doses. The present study showed a vaccine hesitancy rate of 43.4%, and the primary reasons for non-vaccination were mistrust in the vaccine effectiveness and production process, health concerns/side effects, and lack of information about the vaccines.
The reduced burden of COVID-19 in the community was evident in the present study, as indicated by the low seroprevalence of anti-SARS-CoV-2 IgM (12.7%), an indication of current or recent infection. Also, among the 65 participants tested for COVID-19, only three cases were positive (4.6%). The low rate of current SARS-CoV-2 infection was also reported in Central African Republic, in which only 3.6% of the surveyed population tested positive for anti-SARS-CoV-2 IgM in May 2022 [
12]. Similarly, in Cameroon, the seroprevalence of antiSARS-CoV-2 IgM in September 2022 was 6.8% [
13]. Therefore, the results of our study and those of similar surveys corroborate the decision by the WHO to downgrade COVID-19 from a public health emergency to an ongoing health issue.
The high seroprevalence of anti-SARS-CoV-2 IgG in the present study (87.8%) is consistent with that reported by Kagucia et al. among randomly selected, age-stratified population samples from the Health and Demographic Surveillance System in Nairobi (92.2%) [
7]. Similarly, a hospital-based study in a rural region in Kenya conducted from January 2022 to December 2022 reported a seroprevalence of anti-SARS-CoV-2 IgG of 97.8% [
14]. These findings allude to widespread exposure to SARS-CoV-2 through natural infection or vaccination.
Among the factors affecting the seroprevalence of anti-SARS-CoV-2 IgG, sex and vaccination status were significantly associated with the titers of anti-SARS-CoV-2 IgG. Females had significantly higher titers of anti-SARS-CoV-2 IgG than males, likely because of the higher rate of vaccination, which was approaching statistical significance. Vaccination is an effective strategy of strengthening the immunity against COVID-19. In the present study, 56.6% of the participants (female, 63.5%; male, 49.5%) were vaccinated against COVID-19 and had higher anti-SARS-CoV-2 IgG titers compared to the non-vaccinated individuals. Similarly, an increase in anti-SARS-CoV-2 IgG titers among the vaccinated individuals has been reported in previous studies [
15,
16]. However, the occurrence of breakthrough infections has been acknowledged, as the vaccines do not necessarily prevent infection, but are mainly beneficial for protection against severe disease. In addition, the vaccines may not offer protection against new variants of SARS-CoV-2.
In addition, the duration since vaccination significantly affected the anti-SARS-CoV-2 IgG titers. The individuals vaccinated 6 months or less prior to the study had significantly higher levels of anti-SARS-CoV-2 IgG than those who were vaccinated more than 1 year before the study. Consistent with previous studies, the anti-SARS-CoV-2 IgG titers peak within 6 months after vaccination, followed by a rapid decline [
17,
18]. The administration of first, second, or third booster doses is reported to prolong the high titers of anti-SARS-CoV-2 IgG. Matsumoto et al. found a longitudinal increase in anti-SARS-CoV-2 levels with administration of each booster dose [
19]. However, in the present study, no difference in anti-SARS-CoV-2 IgG titers was found between individuals who received a booster dose and those who did not. The necessity for booster doses in augmenting vaccine efficacy and strengthening immune response has been demonstrated by reduced rates of COVID-19-related hospitalization and mortality risk in previous studies [
20,
21]. The lack of a significant difference in antibody titers according to the administration of booster vaccines in the present study might be due to the low number of booster dose recipients (29 out of 107), which precludes definitive conclusions regarding the association between booster administration and enhanced antibody response.
The present study also found no significant difference in anti-SARS-CoV-2 IgG titers according to the type of vaccine. This finding contrasts that of Karl et al. who found higher levels of anti-SARS-CoV-2 in individuals who received two doses of the Comirnaty® vaccine and one dose of the Spikevax® vaccine compared to those who received three doses of the Spikevax® vaccine [
15]. Another study found significantly higher titers of anti-SARS-CoV-2 IgG in those who received three doses of the Pfizer/BioNTech® vaccine compared to those who received two doses of the AstraZeneca® vaccine and a third dose of the Pfizer/BioNTech® vaccine [
22].
Vaccine hesitancy is a critical barrier to achieving herd immunity against COVID-19. In the present study, 43.4% of the participants did not receive the COVID-19 vaccine, which is higher than that reported in an earlier study in Kenya, in which 19% of the participants were reluctant to get vaccinated [
23]. Our study comprised mainly young people, and the high vaccine hesitancy in young adults has been reported previously. Rajshekhar et al. reported that 59.5% of young adults in informal settlements in Kenya showed COVID-19 vaccine hesitancy [
24]. In the United States, 37.2% of young adults aged 18–24 years were not vaccinated against COVID-19 [
25]. These findings show a high rate of vaccine hesitancy in the youthful and well-educated population of mostly university students in the case of the current study, which is suggested to be due to the distrust of the government and pharmaceutical companies and fear of adverse health effects of the vaccines [
26]. Similarly, in the present study, most non-vaccinated individuals cited mistrust in the vaccine effectiveness and production process, health concerns/side effects, and lack of information about the vaccines. According to the 3Cs model by SAGE, these factors relate to a lack of confidence in the vaccine [
11]. The quick development of the COVID-19 vaccines for emergency use may have heightened suspicion and eroded the confidence that people had in the pharmaceutical industry, given that vaccines usually undergo many years of basic research and clinical trials for safety and efficacy before regulatory approval. In addition, the lack of adequate knowledge about the vaccine was also a major barrier to enhanced vaccination. For instance, some participants thought that the COVID-19 vaccine was meant for only those with a previous infection. Given the public health need at the height of the pandemic, less effort was made to engage the public on the new vaccines, compounding the effect of widespread misinformation and conspiracy theories. Knowledge enables people to make informed decisions about the need for vaccination and helps address unfounded fears about vaccines [
27].
Further, complacency was an important driver of vaccine hesitancy in the present study, as indicated by participants who were not interested in vaccination and those who reported no reason for non-vaccination. This complacent attitude is reported to arise from low perceived risk of COVID-19 among young adults, who mainly had mild disease [
24]. The other factor is convenience, which was a concern for only a few participants, suggesting that vaccine availability was not a major problem. As emphasized by the WHO, these factors need to be considered when formulating vaccination programs in the population.
This study has a few limitations. First, the study comprised mainly young adults aged 18–29 years, and hence we could not compare anti-SARS-CoV-2 seroprevalence across different age groups. Nevertheless, the inclusion of a homogenously young and educated population has helped characterize the seroprevalence of this population and understand the reasons for vaccination hesitancy, which could be unique to this population. Second, the majority of participants were not tested for COVID-19 during the study, and hence the current prevalence of COVID-19 could be underestimated. Nonetheless, the assay for IgM helped identify possible cases of ongoing infection.
5. Conclusions
The current study revealed widespread exposure to SARS-CoV-2 through vaccination or natural infection, as indicated by the high seroprevalence of anti-SARS-CoV-2 IgG. IgG antibody responses were more robust in vaccinated compared to non-vaccinated participants, underscoring the importance of vaccination in controlling the spread of COVID-19. The high vaccine hesitancy rate highlights the need for intensified community engagement and educational efforts to bolster vaccine uptake rates within Kenyan communities. These findings underscore the importance of understanding the interplay between vaccination status, immune response, and seroprevalence rates in shaping effective public health strategies post-COVID-19 pandemic.
Author Contributions
Conceptualization, E.M.N., P.T., J.O., L.O., J.K.M., V.O., S.G., G.M., and P.O.; methodology, E.M.N., P.T., J.O., L.O., G.M., and P.O.; formal analysis, A.O.M., E.M.N., and M.T.; investigation, A.O.M., E.M.N., M.T., and J.O.; resources, E.M.N., J.O., P.O., and G.M.; data curation, A.O.M., E.M.N., M.T.; writing—original draft preparation, A.O.M., M.T., E.M.N., and P.T.; writing—review and editing, M.T., A.O.M., E.M.N, M.M., J.O., S.G., G.M., and P.T.; visualization, A.O.M., E.M.N., M.T., P.T., J.O., L.O., J.K.M., V.O., S.G., G.M.; supervision, E.M.N., P.T., J.O., M.M., P.O., and G.M.; project administration, E.M.N., P.T., J.O., M.M., G.M., and P.O.; funding acquisition, E.M.N., J.O., P.T., L.O., P.O., and G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Foundation (NRF) South Africa, grant number COV19200617532972.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Kenyatta University (protocol code: PKU/2379/11516; February 28, 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We express our gratitude to the research sponsors and the study team for their assistance in administrative and technical matters. We acknowledge the support provided by the Kenyatta University staff throughout the study procedures. Also, we are thankful to the study participants for actively taking part in the research.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Worldometer Kenya COVID - Coronavirus Statistics - Worldometer. Available online: https://www.worldometers.info/coronavirus/country/kenya/ (accessed on 4 July 2024).
- Bwire, G.; Ario, A.R.; Eyu, P.; Ocom, F.; Wamala, J.F.; Kusi, K.A.; Ndeketa, L.; Jambo, K.C.; Wanyenze, R.K.; Talisuna, A.O. The COVID-19 Pandemic in the African Continent. BMC Med. 2022, 20, 167. [Google Scholar] [CrossRef] [PubMed]
- Mohammad Hossein, Z.J.; Mehran, D. Seroprevalence of Anti-SARS-CoV-2 IgG and IgM Antibodies among Government Employees in Iran. Arch. Razi Inst. 2023, 78, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, L.; Li, F.; He, A. Long-Term Evaluation of the Seroprevalence of SARS-CoV-2 IgG and IgM Antibodies in Recovered Patients: A Meta-Analysis. BMC Infect. Dis. 2023, 23, 444. [Google Scholar] [CrossRef]
- Adetifa, I.M.O.; Uyoga, S.; Gitonga, J.N.; Mugo, D.; Otiende, M.; Nyagwange, J.; Karanja, H.K.; Tuju, J.; Wanjiku, P.; Aman, R.; et al. Temporal Trends of SARS-CoV-2 Seroprevalence during the First Wave of the COVID-19 Epidemic in Kenya. Nat. Commun. 2021, 12, 3966. [Google Scholar] [CrossRef] [PubMed]
- Etyang, A.O.; Adetifa, I.; Omore, R.; Misore, T.; Ziraba, A.K.; Ng’oda, M.A.; Gitau, E.; Gitonga, J.; Mugo, D.; Kutima, B.; et al. SARS-CoV-2 Seroprevalence in Three Kenyan Health and Demographic Surveillance Sites, December 2020-May 2021. PLOS Glob. Public Health 2022, 2, e0000883. [Google Scholar] [CrossRef] [PubMed]
- Kagucia, E.W.; Ziraba, A.K.; Nyagwange, J.; Kutima, B.; Kimani, M.; Akech, D.; Ng’oda, M.; Sigilai, A.; Mugo, D.; Karanja, H.; et al. SARS-CoV-2 Seroprevalence and Implications for Population Immunity: Evidence from Two Health and Demographic Surveillance System Sites in Kenya, February-December 2022. Influenza Other Respir. Viruses 2023, 17, e13173. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Statement on the Fifteenth Meeting of the IHR (2005) Emergency Committee on the COVID-19 Pandemic. 2005. Available online: https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations-.
- Orangi, S.; Mbuthia, D.; Chondo, E.; Ngunu, C.; Kabia, E.; Ojal, J.; Barasa, E. A Qualitative Inquiry on Drivers of COVID-19 Vaccine Hesitancy among Adults in Kenya. PLOS Glob. Public Health 2024, 4, e0002986. [Google Scholar] [CrossRef]
- Khamadi, S.A.; Opanda, S.; Symekher, S.L.; Limbaso, S.K.; Langat, S.; Cirindi, J.K.; Mwangi, M.; Mwikwabe, N.; Okeyo, S.; Koskei, E.; et al. Whole-Genome Sequence Analysis Reveals the Circulation of Multiple SARS-CoV-2 Variants of Concern in Nairobi and Neighboring Counties, Kenya between March and July 2021. Virol. J. 2022, 19, 178. [Google Scholar] [CrossRef]
- Gerretsen, P.; Kim, J.; Caravaggio, F.; Quilty, L.; Sanches, M.; Wells, S.; Brown, E.E.; Agic, B.; Pollock, B.G.; Graff-Guerrero, A. Individual Determinants of COVID-19 Vaccine Hesitancy. PLoS ONE 2021, 16, e0258462. [Google Scholar] [CrossRef]
- Manirakiza, A.; Malaka, C.; Mossoro-Kpinde, H.D.; Yambiyo, B.M.; Mossoro-Kpinde, C.D.; Fandema, E.; Yakola, C.N.; Doyama-Woza, R.; Kangale-Wando, I.M.; Komba, J.E.K.; et al. Seroprevalence of Anti-SARS-CoV-2 Antibodies before and after Implementation of Anti-COVID-19 Vaccination among Hospital Staff in Bangui, Central African Republic. PLOS Glob. Public Health 2023, 3, e0001497. [Google Scholar] [CrossRef]
- Sorel, B.K.D.; Didiane, Y.M.; Samuel, M.M.; Pierre, F.C.S.; Caroline, D.T.N.; Richard, N.; Nicolas, N.Y. Seroprevalence of SARS-CoV-2 Antibodies among the University Population of Ngaoundere-Cameroon.
- Ruttoh, V.K.; Kipkoech, B.; Tonui, R.; John, R.O.; Mwangi, I.N.; Gicho, R.W.; Kamau, S.W.; Njihia, M.W.; Symekher, S.L.; Mwanzia, T.M.; et al. Seroprevalence of Anti-SARS-Cov-2 Antibodies among Patients Visiting Hospital-Based Sentinel Sites in the Rift Valley Region, Kenya 2024.
- Karl, T.; Schuster, A.; Stangassinger, L.M.; Stiboller, T.; Cadamuro, J.; Oostingh, G.J. Factors Affecting SARS-CoV-2 IgG Production after Vaccination and/or Disease: A Large-Scale Seroprevalence Study. Vaccines 2023, 11, 1615. [Google Scholar] [CrossRef] [PubMed]
- Bullock, J.L.; Hickey, T.E.; Kemp, T.J.; Metz, J.; Loftus, S.; Haynesworth, K.; Castro, N.; Luke, B.T.; Lowy, D.R.; Pinto, L.A. Longitudinal Assessment of BNT162b2- and mRNA-1273-Induced Anti-SARS-CoV-2 Spike IgG Levels and Avidity Following Three Doses of Vaccination. Vaccines 2024, 12, 516. [Google Scholar] [CrossRef] [PubMed]
- Pegu, A.; O’Connell, S.E.; Schmidt, S.D.; O’Dell, S.; Talana, C.A.; Lai, L.; Albert, J.; Anderson, E.; Bennett, H.; Corbett, K.S.; et al. Durability of mRNA-1273 Vaccine-Induced Antibodies against SARS-CoV-2 Variants. Science 2021, 373, 1372–1377. [Google Scholar] [CrossRef]
- Collier, A.-R.Y.; Yu, J.; McMahan, K.; Liu, J.; Chandrashekar, A.; Maron, J.S.; Atyeo, C.; Martinez, D.R.; Ansel, J.L.; Aguayo, R.; et al. Differential Kinetics of Immune Responses Elicited by Covid-19 Vaccines. N. Engl. J. Med. 2021, 385, 2010–2012. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, N.; Sasaki, A.; Kadowaki, T.; Mitsuhashi, T.; Takao, S.; Yorifuji, T. Longitudinal Antibody Dynamics after COVID-19 Vaccine Boosters Based on Prior Infection Status and Booster Doses. Sci. Rep. 2024, 14, 4564. [Google Scholar] [CrossRef] [PubMed]
- Kislaya, I.; Machado, A.; Magalhães, S.; Rodrigues, A.P.; Franco, R.; Leite, P.P.; Dias, C.M.; Nunes, B. COVID-19 mRNA Vaccine Effectiveness (Second and First Booster Dose) against Hospitalisation and Death during Omicron BA.5 Circulation: Cohort Study Based on Electronic Health Records, Portugal, May to July 2022. Eurosurveillance 2022, 27, 2200697. [Google Scholar] [CrossRef] [PubMed]
- Mattiuzzi, C.; Lippi, G. Efficacy of the Second COVID-19 Vaccine Booster Dose in the Elderly. Vaccines 2023, 11, 213. [Google Scholar] [CrossRef] [PubMed]
- Biedrzycka, B.W.; Bieńkowska, A.; Smolińska-Fijołek, E.; Biedrzycki, G.; Dorf, J. The Influence of Two Priming Doses of Different Anti-COVID-19 Vaccines on the Production of Anti-SARS-CoV-2 Antibodies After the Administration of the Pfizer/BioNTech Booster. Infect. Drug Resist. 2022, 15, 7811–7821. [Google Scholar] [CrossRef]
- Osur, J.; Muinga, E.; Carter, J.; Kuria, S.; Hussein, S.; Ireri, E.M. COVID-19 Vaccine Hesitancy: Vaccination Intention and Attitudes of Community Health Volunteers in Kenya. PLOS Glob. Public Health 2022, 2. [Google Scholar] [CrossRef]
- Rajshekhar, N.; Pinchoff, J.; Boyer, C.B.; Barasa, E.; Abuya, T.; Muluve, E.; Mwanga, D.; Mbushi, F.; Austrian, K. Exploring COVID-19 Vaccine Hesitancy and Uptake in Nairobi’s Urban Informal Settlements: An Unsupervised Machine Learning Analysis of a Longitudinal Prospective Cohort Study from 2021 to 2022. BMJ Open 2023, 13, e071032. [Google Scholar] [CrossRef]
- CDC Archive: COVID-19 Vaccination and Case Trends by Age Group, United States | Data | Centers for Disease Control and Prevention. Available online: https://data.cdc.gov/Vaccinations/Archive-COVID-19-Vaccination-and-Case-Trends-by-Ag/gxj9-t96f/about_data (accessed on 4 July 2024).
- Kim, S.; Willis, E.; Wehlage, S.; Scheffer-Wentz, H.; Dulitz, M. COVID-19 Vaccine Hesitancy and Short-Term and Long-Term Intentions among Unvaccinated Young Adults: A Mixed-Method Approach. BMC Public Health 2022, 22, 2030. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Chisty, M.A.; Alam, M.A.; Sakib, M.S.; Quader, M.A.; Shobuj, I.A.; Halim, M.A.; Rahman, F. Knowledge, Attitude, and Hesitancy towards COVID-19 Vaccine among University Students of Bangladesh. PLOS ONE 2022, 17, e0270684. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).