1. Introduction
Improving road safety is a priority concern of all governments. United Nations Resolution 74/299, adopted in 2020, establishes a target of reducing road traffic fatalities and injuries by 50% within the decade spanning from 2021 to 2030 (General Assembly United Nations, 2020). Road crashes can be attributed to various factors, including environmental conditions, vehicle specifications, and human behaviours. Research suggests that drivers cause up to 94% of accidents (Singh, 2018).
Elander et al. (1993) proposed a distinction between driving skills and driving styles, with the former encompassing experiential and attentional factors, while the latter involves choices such as driving speed, adherence to traffic regulations, and gap acceptance. Several questionnaires have been developed to assess driving styles (French et al., 1993; Gulian et al., 1989; Reason et al., 1990; Taubmen-Ben-Ari et al., 2004). The Multidimensional Driving Style Inventory (MDSI) has emerged as an effective tool for examining both adaptive and maladaptive driving behaviours (Taubman-Ben-Ari & Skvirsky, 2016).
Research shows that drivers with maladaptive driving styles tend to have difficulty regulating their emotions, compared to drivers with adaptive driving styles (Trogolo et al., 2014; Navon et al., 2020). Negative emotional states have been shown to reduce the perception of danger (Trick et al., 2012) and increase driving errors (Jeon & Zhang, 2013), thereby promoting risky driving behaviours (Seibokaite et al., 2017). Conversely, positive emotional states have also been linked to risky driving tendencies (Eherenfreund-Hager et al., 2017). The difficulty in regulating emotions appears to be a contributing factor in the manifestation of maladaptive driving styles, particularly among young individuals (Navon–Eyal & Taubman–Ben-Ari, 2020). The adoption of emotion regulation techniques, particularly reappraisal, has been shown to promote more adaptive driving styles (Holman & Popusoi, 2020).
The prefrontal cortex plays a crucial role in the reappraisal process. The ventral areas of the prefrontal cortex evaluate the emotional characteristics of stimuli, while the dorsal areas regulate these emotional responses (Ochsner & Gross, 2005; Mitchel, 2011; Ochsner et al., 2012; Kohn et al., 2014). Electrophysiological studies support the involvement of the prefrontal cortex in emotional regulation. When exposed to unpleasant images accompanied by a tone, activation in the orbicularis oculi muscle, which produces the startle response, is increased (Balada et al., 2014; Aluja et al., 2015; Aluja et al., 2020). Emotional regulation has been found to attenuate this response (Ray et al., 2010), particularly in the left prefrontal areas (Jackson et al., 2003). Several studies have shown gender differences in the brain's response to emotions. It is noticeable that a greater lateralization is observed in males (Hamann & Canli, 2004).
In recent years, there has been growing interest in functional near-infrared spectroscopy (fNIRS) studies. This technique allows imaging in a more user-friendly environment, with good temporal resolution and low cost. However, it has limitations, such as the detection of surface structures and low spatial resolution (Quaresima et al., 2012). Recent systematic reviews of fNIRS studies measuring neural activation during emotion processing in healthy individuals have shown contradictory findings. Westgarth et al. (2022), in a recent systematic review, found increased prefrontal activity during emotional regulation in 9 out of 11 studies. In in two studies, it was only observed during the regulation of negative stimuli, while studies related to perception or emotional experience showed more inconsistent results. Similar results can be found in other reviews (Doi et al., 2013; Bendall et al., 2016).
Several factors may contribute to these inconsistent results, including sample characteristics, stimulus type and task demands (Westgarth et al., 2022). In the extensive review by Westgarth et al. (2022), most of the evaluated studies focused on young adults (53/72; Mean age = 23.4 years). Among the 16 studies involving middle-aged adults (mean age = 35.9 years), the maximum sample size was 28 participants, and only in three studies was there a balanced participation by gender. Moreover, the majority (6/16) of these 16 studies with middle-aged populations used face stimuli, and only a few utilized emotionally regulated imageries. Several studies have indicated diminished resting prefrontal brain activity in younger adults (Yeung & Chang, 2021). However, differences between adults and younger individuals diminish in scenarios such as driving (Harada et al., 2007). Conversely, older adults exhibit lower prefrontal activity during emotional reappraisal tasks (Opitz et al., 2012). Given these findings, it seems pertinent to investigate the response of the prefrontal cortex to negative emotional stimuli, particularly images related to traffic accidents, in a large sample of drivers of different ages and both genders. In addition to this exploratory study, we intend to analyse how this response correlates with their driving behaviour. Based on data in the literature, we expect that drivers with more maladaptive styles (characterized by high scores on the Recklessness, Anger, Anxiety and Dissociation scales and low scores on the Carefulness and Distress Reduction scales) will present lower prefrontal activation after exposure to imagery, especially in the left hemisphere of the prefrontal cortex. This lateralization might be more evident in the case of male and older participants.
2. Method
2.1. Participants and Procedure
One hundred and eighteen healthy males (44.38 ± 12.98 years) and eighty-four healthy females (38.89 ± 10.60 years) participated in this study. Participants were recruited from a collective email addressed to teaching and administration staff of the university. They received an incentive of 25 euros. The participants had a driving license and stated that they drive regularly. The study was conducted in a midsize city in a rural area where daily displacements are frequent. Participants declared no history of substance abuse. The phase of the menstrual cycle and oral contraceptive intake in women were monitored, as well as the hours of sleep (mean sleep < 7.1 ± 1.0) of each participant. They were also instructed not to consume stimulants or tobacco in the previous 12 hours. In total, four blocks of 6 images each were presented in a pseudo-random manner. Two of these blocks corresponded to neutral pictures, and the other two to pictures related to traffic accidents. Before the presentation of each block, the word "Look" appeared for 2 s, and then the different images were presented for 6 s each, with an ITI varying between 0.5 and 1 s (mean 0.75). The presentation of the pictures in the same block was randomised. The total duration of each block was 40.5 seconds. After the end of the image presentation, a screen appeared that allowed the valence and arousal characteristics of the block to be rated on a scale of 0 to 100. Afterwards, a period of 10 s was established to return to basal brain activation levels before the presentation of the next block. The study was authorized by the ethics committee. All participants signed an informed consent.
2.2. Material
2.2.1. Multidimensional Driving Styles Inventory (MDSI)
The MDSI was developed to evaluate different driving styles (Taubman-Ben-Ari et al., 2004) and validated in Spain by Padilla et al., (2020) (for details, see Padilla Garcia et al., 2020). This questionnaire has 34 items and considers adaptive and maladaptive driving styles. The response format is a 6-point Liker type ranging from “not at all” (1) to “very much” (6). Careful and Distress reduction are the adaptive styles, whereas Reckless, Angry, Anxious, and Dissociative are the maladaptive styles. In the original article, internal consistency ranged from 0.72 to 0.86, and in the Spanish version between 0.65 and 0.80.
2.2.2. Pictures
We selected 24 authorized pictures from International Affective Picture System (IAPS
1) (Lang et al., 2008) and Nencki Affective Picture System (NAPS
2) (Marchewka et al., 2014) databases (
Figure 1B). Pictures were grouped into four blocks of six images each. Two of these blocks corresponded to neutral images, while the other two blocks were related to traffic accidents. With the aim of evaluating the valence and arousal of the images used, at the end of each block, the participants were asked to indicate using semantic differential scales whether the images presented in that block had caused them feelings of being happy-sad, angry-glad, exalted-calm or indifferent-impressed.
2.2.3. fNIRs Recording and Data Analysis
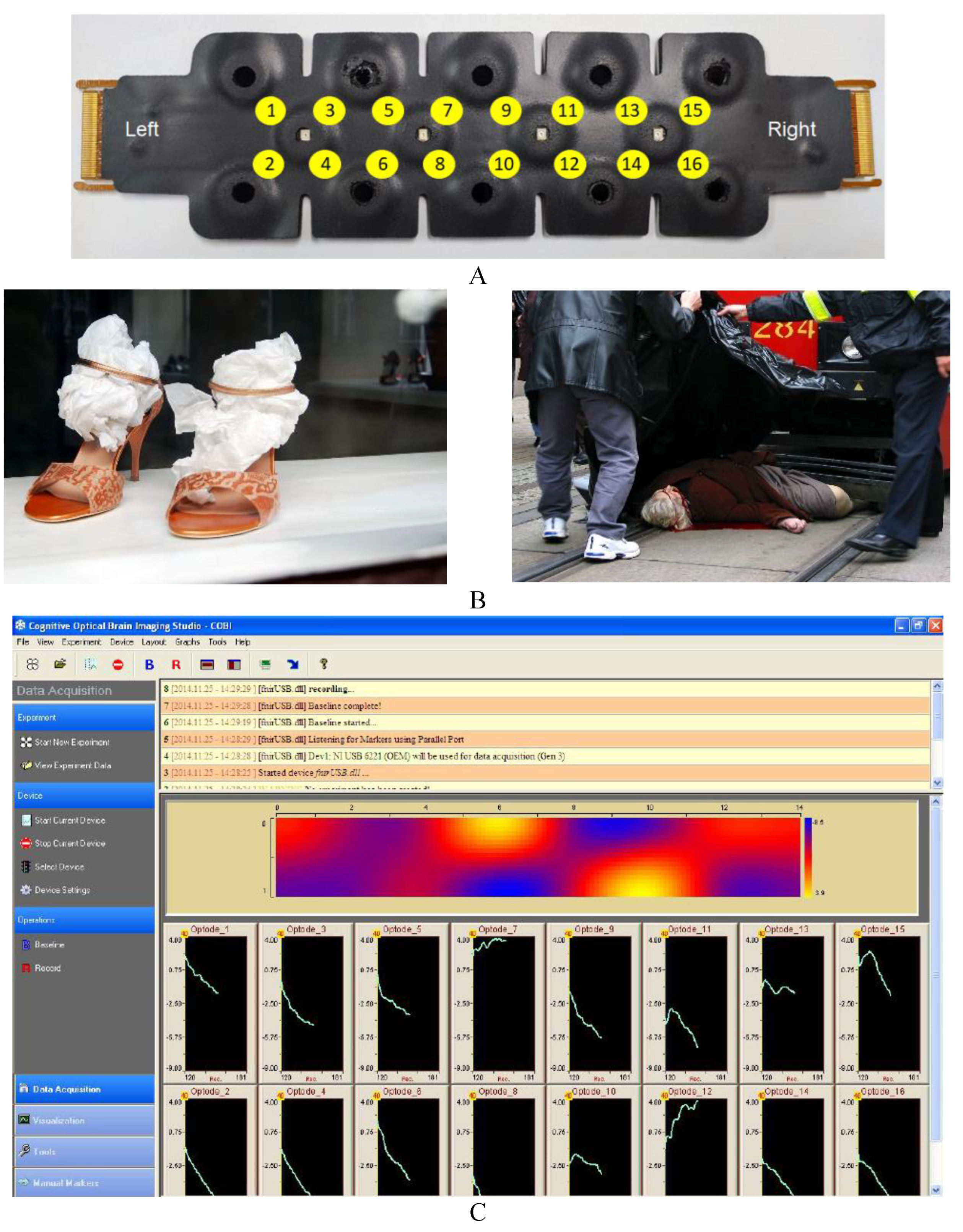
The fNIRs module used allows us to monitor changes in prefrontal blood oxygenation during picture presentation. These changes are detected by means of a sensor featuring 4 LED light sources and 10 photodetectors that allow us to obtain the changes in oxygenated (O2Hb) and deoxygenated (HHb) haemoglobin (measured in μmol/L) in 16 channels by combining the signals captured by the photodetectors (
Figure 1A). The difference between oxygenated and deoxygenated haemoglobin indicates the oxygenation (Oxy) of that area. Sensor placement is according to electrode positions F7, FP1, FP2 and F8, International EEG System 10-20, corresponding to Brodmann areas 9, 10, 45, 46 and 47. Before the start of the task, the light intensity and amplification of each channel were calibrated to avoid weak or saturated signals. Recording was performed using the COBI data collection suite (Biopac System Inc.;
Figure 1C), as described in Ayaz et al. (2011). The fNIRSoft software was used to analyse the data obtained (Ayaz et al., 2018) (
Figure 1 C).
2.2.4. Statistical Analysis
The values of skewness and kurtosis of the variables were analysed. Skewness values ranged from -1.36 to 1.03. Kurtosis values ranged from -.91 to 1.52, except for the careful scale where a value of 4.25 was obtained. A principal components analysis of an unrotated factor was carried out using all MDSI scales to have a single value for driving styles. This total score can be computed as the overall driving style. Note that angry, reckless, dissociative, and anxious scales load positive (all > .50), and careful loads negative (-.48), while distress reduction scores virtually zero on this factor. The factor score showed a strong positive correlation (.35; p <.001) with the number of traffic fines. Outlier detection was performed using Tukey's nonparametric fence method (Tukey, 1977), which is based on the interquartile range. The formula Q3 + k (Q3 - Q1) was used to calculate the outer fence, where k corresponds to a constant value of 3.
The participants were divided into three groups (low, medium, and high maladaptive driving style), using the 33rd (-.545) and 66th (.300) percentiles of the factor obtained as cut-off points. In addition, the total sample was also divided into three groups according to the age of the participants. The age ranges for the three groups were 20-35; 36-50 and 51-70. fNIRs measures recorded from the optodes were grouped into four separate quadrants: lateral left (optodes 1, 2, 3 and 4), rostral left (optodes 5, 6, 7 and 8), rostral right (optodes 9, 10, 11 and 12) and lateral right (optodes 13, 14, 15 and 16). Each area was obtained from the mean of the four corresponding channels (
Figure 1A). Pearson’s correlation analysis was performed to analyse the correlation between psychometric variables and prefrontal oxygenation. ANOVA and T-test analyses were performed to compare the levels of prefrontal oxygenation during viewing of neutral and traffic accident-related pictures in function of age, driving styles or gender. Cohen's d and eta square were calculated to assess effect size. According to the cut-off points indicated by Cohen (1988), a Cohen's
d value of .20 would be a small effect, .50 would be medium, and .80 would be large, whereas
η2 = 0.01 indicates a small effect,
η2 = 0.06 indicates a medium effect, and
η2 = 0.14 indicates a large effect.
3. Results
The analysis of the semantic differentials corresponding to the image blocks used revealed that the blocks containing images of traffic accidents had a valence rating of 28.56 points on a scale from 0 to 100, and an arousal rating of 68.73 points. On the other hand, the blocks with neutral images had a valence rating of 51.87 points and an arousal rating of 30.84 points.
Table 1 presents the means and standard deviations of age, MDSI scales, the factor obtained from the MDSI, and the oxygenation of the four quadrants of the prefrontal cortex for each gender. It also includes the observed differences between males and females, which were significant only for age (
t-test = 3.3;
p < .001; Cohen’s
d = .46) and the Reckless scale (
t-test = 2.28;
p < .024; Cohen’s
d = .32). Additionally, the table displays the values of Cronbach's alpha for the MDSI scales. Women rated traffic accident images (
t-test = 2.43;
p < .016; Cohen’s
d = .35) and neutral images (
t-test = 2.19;
p < .030; Cohen’s
d = .30) with lower valence than men, while differences in arousal were only observed for traffic accident images (
t-test = -3.06;
p < .002; Cohen’s
d = -.44).
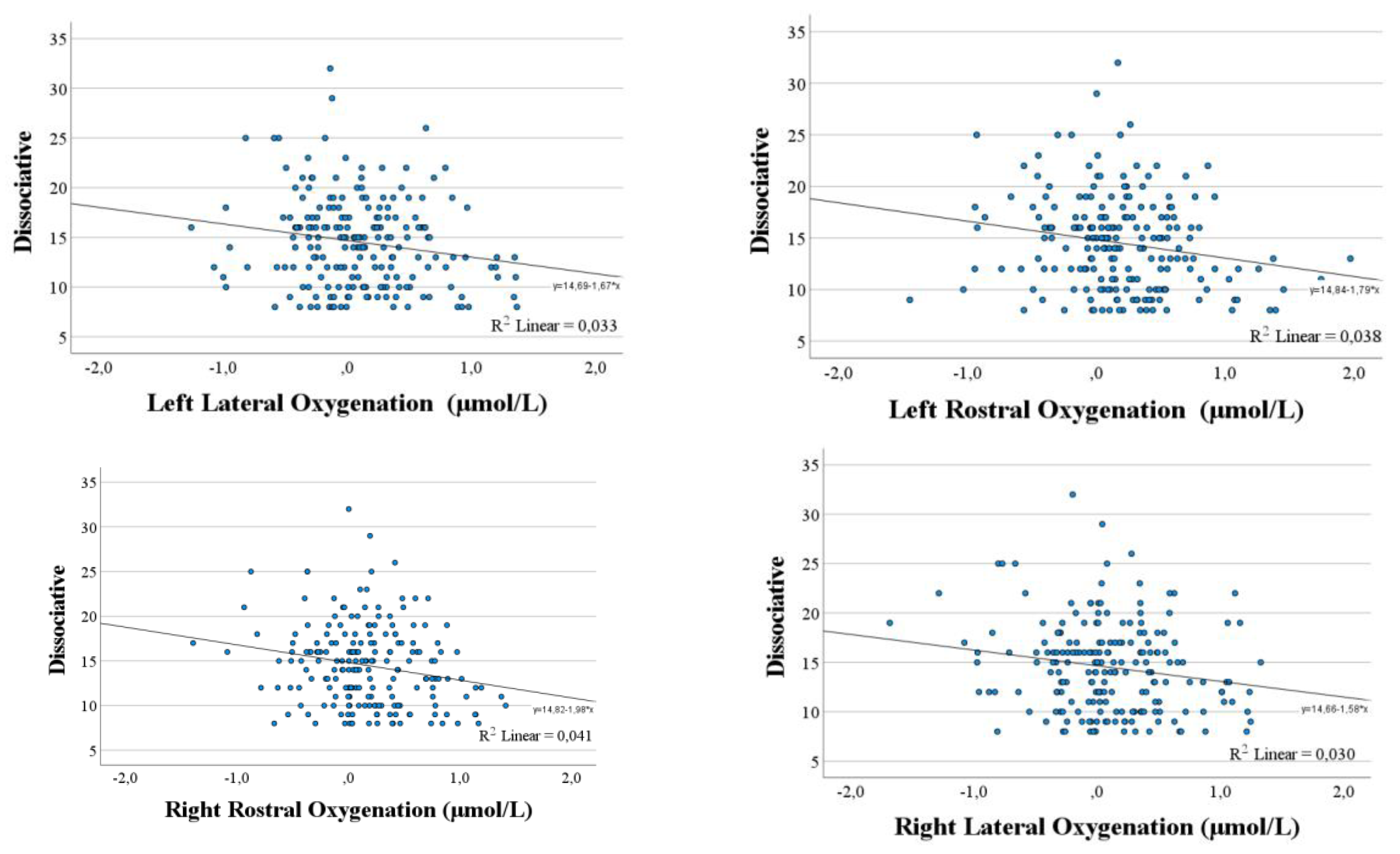
Correlational analysis between psychometric variables and total oxygenation levels in each quadrant indicated an inverse relationship between Dissociative scale scores and oxygenation in all quadrants analysed (all
p ≤ .013;
Figure 2). Moreover, a tendency for an inverse relationship was also observed between Anxious scale score and oxygenation in the right rostral area (
r = -.12;
p < .09), although the other correlations were negative, they were not significant. No further significant correlations were found, although Careful scores were positively correlated with oxygenation levels, and Distress Reduction scores were also positively correlated, except for the right lateral area. In contrast, the Reckless scale showed negative correlations in all four quadrants.
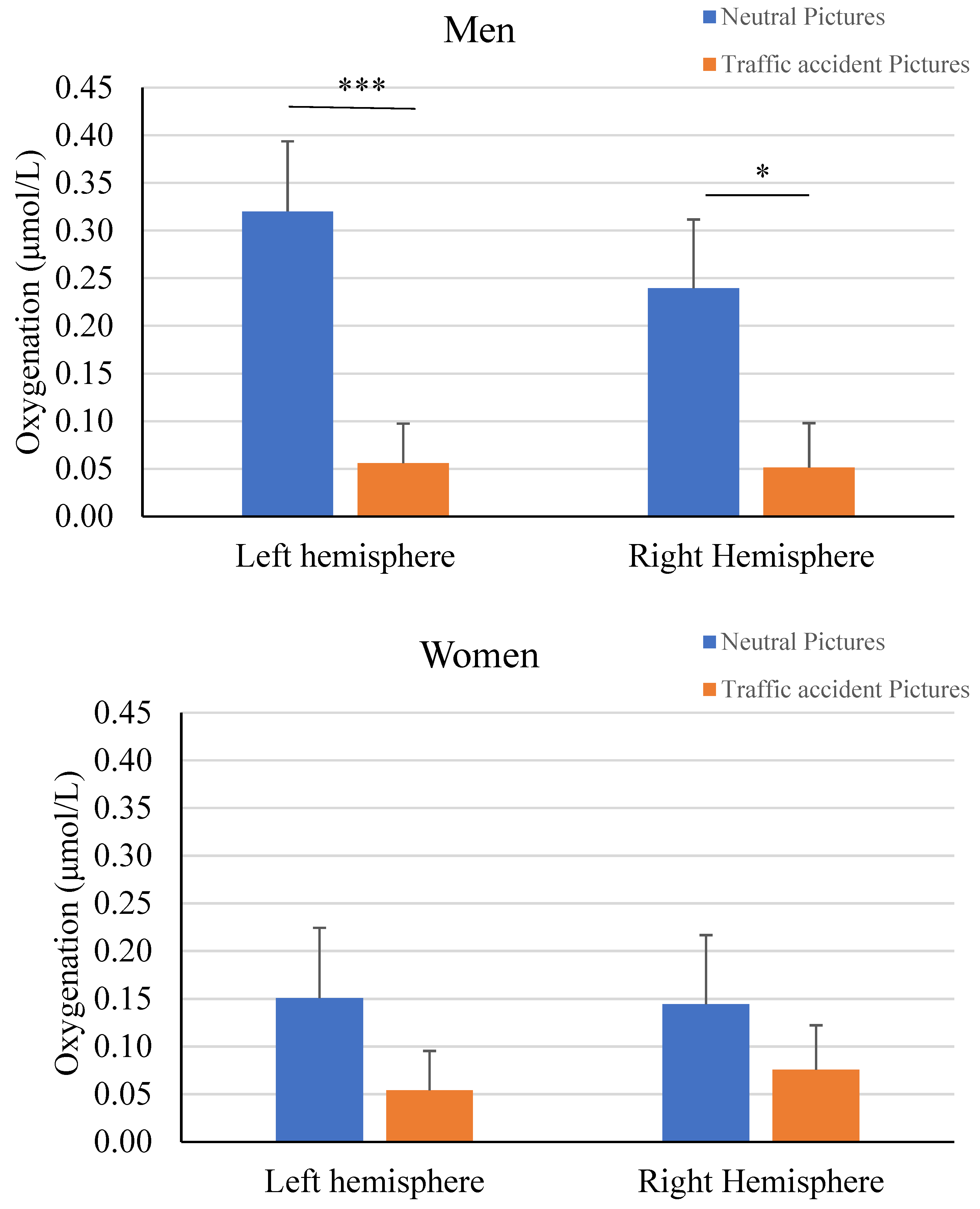
When viewing neutral images, greater prefrontal oxygenation was observed compared to viewing images of traffic accidents. The left hemisphere (
t-test = 3.23;
p < .001; Cohen’s
d = .23) showed greater differences than the right hemisphere (
t-test = 2.46;
p < .015; Cohen’s
d = .17). Additionally, the left (
t-test = 3.29;
p < .001; Cohen’s
d = .24) and right (
t-test = 2.50;
p < .013; Cohen’s
d = .18) lateral areas exhibited greater differences than the left (
t-test = 2.80;
p < .006; Cohen’s
d = .20) and right (
t-test = 1.96;
p < .052; Cohen’s
d = .14) rostral areas. Separate analysis by gender indicated that these differences were attributable to the response of male participants (
Figure 3). Once again, the differences were more significant in the lateral (
t-test = 3.02;
p < .003; Cohen’s
d = .28) and rostral (
t-test = 3.04;
p < .003; Cohen’s
d = .28) areas of the left hemisphere compared to the right rostral (
t-test = 1.88;
p < .063; Cohen’s
d = .18) and lateral (
t-test = 2.35;
p < .021; Cohen’s
d = .22) areas. In contrast, the analysis in the female sample showed no significant differences in either the left (
t-test = 1.14;
p < .26) or right (
t-test = .95;
p < .36) hemisphere.
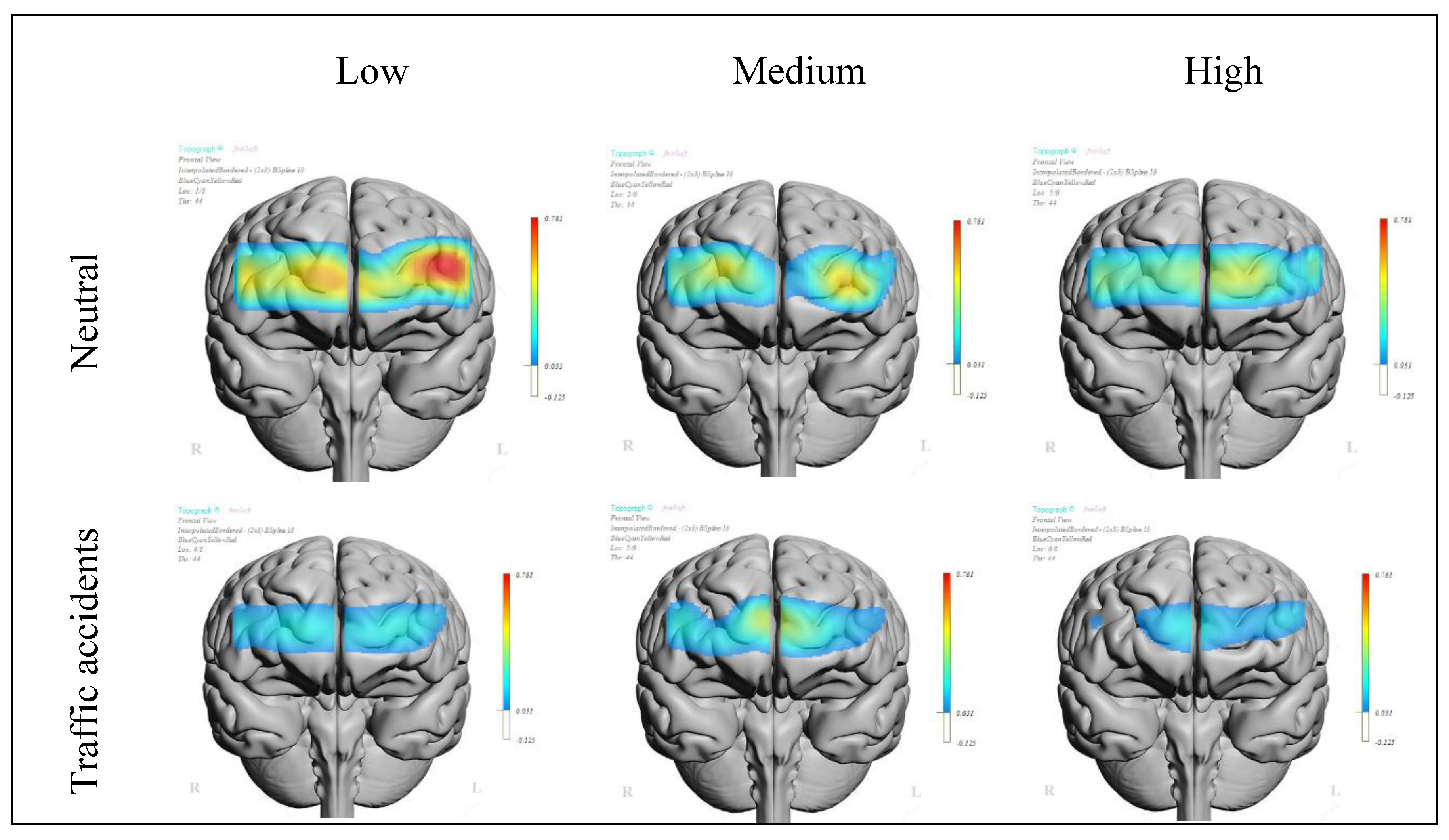
Figure 4 presents the topographic representation of the mean oxygenation levels for the sixteen channels of the neutral image blocks and the traffic accident image blocks for each of the three groups obtained from the single factor score of MDSI. A gradation in oxygenation levels can be observed. There is a noticeable variation in oxygenation levels among the groups. Individuals with a lower driving style factor score, indicating a more adaptive driving style, tended to exhibit higher oxygenation levels (.17 μmol/L), during both the neutral and accident image blocks. Those with a medium driving style factor score showed a lower oxygenation level (.14 μmol/L), and individuals with a higher driving style factor score, indicating a more maladaptive driving style, demonstrated the lowest oxygenation levels (.11 μmol/L). Nevertheless, these differences between groups are not statistically significant. In contrast, when comparing oxygenation levels during the neutral and traffic accident imaging blocks within each group, significant differences are observed. Among drivers with more adaptive styles, the differences in oxygenation levels between neutral and accident blocks are evident in both the left (
t-test = 2.72,
p < .009; Cohen’s
d = .34) and right hemispheres (
t-test = 2.22
p < .030; Cohen’s
d = .28). Similarly, drivers with a more maladaptive style display greater differences in oxygenation levels between the neutral and accident pictures in the rostral quadrants, reaching significance only in the left rostral area (
t-test = 2.25,
p < .028; Cohen’s
d = .28). No significant differences were found regarding the assessment of valence and arousal between any of the three driving style subgroups (all
p > .50). On the other hand, the comparison of means according to the scores on the dissociative scale (low ≤12; medium 13-16; high ≥17) shows the existence of significant differences in men in the right rostral area
(F = 3.28;
p < .041;
η2 = .054). A tendency towards significance appeared in the right lateral area
(F = 2. 51;
p < .086;
η2 = .042). The highest levels of oxygenation were found in all quadrants in participants with lower scores on the dissociative scale, while the lowest levels of oxygenation were observed in participants with higher scores on the dissociative scale. No significant differences were found in females. Separate analysis of neutral imagery in men shows significant differences for neutral imagery in the right rostral area
(F = 4.19;
p < .018;
η2 = .07). In contrast, the analysis of the prefrontal response to accident images shows the existence of significant effects in the left
(F = 4.23;
p < .017;
η2 = .069) and right
(F = 4.57;
p < .012;
η2 = .074) ventral areas, but on this occasion, the highest levels of oxygenation are observed in those participants with average scores on the dissociative scale.
Furthermore, we found an inverse relationship between age and scores obtained on the Anxious scale (r = -.15; p < .035), Angry scale (r = -.20; p < .005), and the factor derived from the MDSI scales (r = -.15; p < .031). Nevertheless, we did not find any significant association between age and prefrontal oxygenation levels (all p > .42) or image assessment (all p > .49).
4. Discussion
This study investigated changes in prefrontal oxygenation during the viewing of neutral and traffic accident-related images among a heterogeneous cohort of drivers, examining their driving styles. Findings revealed that male drivers exhibiting a dissociative driving pattern displayed diminished prefrontal oxygenation levels during both neutral and accident-related imagery. Furthermore, prefrontal oxygenation was higher during neutral image viewing compared to accident images.
A notable observation in our study is the presence of gender disparities in prefrontal activation. Extensive research has delineated differences between males and females in prefrontal cortex development and function (Reber & Tranel, 2017; Drzewiecki & Juraska, 2020). Our study revealed no significant effects in the analysis of female participants. Conversely, analysis of male participants revealed significant effects, albeit of modest to moderate magnitude. No gender discrepancies were observed either in driving styles, except for the reckless scale, or in oxygenation levels. Several factors may account for the absence of significant differences in women, among which the effect of hormonal fluctuations characteristic of the menstrual cycle (Dreher et al., 2007; Dan et al., 2019), and variances in the degree of cerebral lateralization (Reber & Tranel, 2017; Li et al., 2021) are noteworthy. On the basis of the results obtained, it was conjectured that drivers with maladaptive driving styles or elevated scores on related scales would display diminished prefrontal oxygenation. While our results supported this hypothesis, it was primarily associated with scores on the Dissociative scale and manifested variances by gender.
During the development of the MDSI, the Dissociative scale accounted for a greater percentage of the variance (21%) and characterized drivers with high scores as having a tendency “to be easily distracted during driving, to commit driving errors due to this distraction, and to display cognitive gaps and dissociations during driving" (Taubman-Ben-Ari et al., 2004). This conduct might stem from an inability to regulate attention. Three networks implicated in the attentional system have been identified: alerting, orienting and executive (Fan et al., 2005; Petersen & Posner, 2012). The executive attentional system entails prefrontal cortex activation through a cognitive network or a dual mechanism. In the former case, the dorsolateral prefrontal cortex would exert top-down inhibition on the basis of performance information provided by the medial areas, whereas in dual networks, these systems would operate independently (Petersen & Posner, 2012). Participants with higher dissociative scores exhibited reduced activity in the right prefrontal cortex during neutral image viewing, potentially indicating poorer attentional control. Indeed, activation of the right prefrontal cortex is associated with vigilance and sustained attention (De Joux et al., 2013) and its stimulation improves attentional control (Li et al., 2017). It has also been theorised that poor right prefrontal functioning could be responsible for the inability to discriminate between neutral and emotional images in patients with depression (Manelis et al., 2019). Conversely, greater right prefrontal activation in men suggested higher attentional capacity and emotional discrimination, contributing to a more adaptive driving style.
Regarding the response of ventral areas to accident images, an inverted U-shaped effect was observed, with average dissociative scorers showing higher oxygenation levels. This scale would have been linked to anxious personality traits or neuroticism (Poo & Ledesma, 2013; Wang et al., 2018), which is associated with the response to emotional arousal (Eysenck, 1994), presenting an inverted U-shaped relationship with cognitive performance (Eysenck, 1967). Functional connectivity studies highlighted negative correlations between neuroticism and both the amygdala and the left lateral orbitofrontal and ventrolateral PFC (Madsen et al., 2016). A greater sensitivity of the ventral prefrontal cortex to the intensity of the emotional stimulus than to its valence has been shown (Hu, 2020). Additionally, lateral and medial prefrontal areas demonstrated sensitivity to external and internal emotional changes, respectively (Yamasaki et al., 2002).
Another noteworthy aspect is the differences in prefrontal oxygenation levels as a function of image valence. The findings indicated higher oxygenation levels during neutral image viewing in all prefrontal areas. Different relationships between image valence and neural activity have been observed. In dorsal prefrontal areas, an inverted U-shaped relationship between image valence and neural activity has been described (Viinikainen et al., 2010). This implies that the highest levels of neuronal activation would occur with images with neutral valence, as we can observe in our study. Although significant differences were detected in all prefrontal areas analysed, the most notable effects emerged in the left hemisphere. This may be attributed to hemispheric differences in emotional response (Cheng et al., 2022). Lastly, despite finding no differences based on age, an inverse relationship between age and maladaptive driving style was observed. This finding aligns with prior research (Foy et al. 2016).
Nonetheless, this study is not without limitations. Firstly, although the driving style factor correlated well with the number of reported complaints, additional measures such as years of experience or annual kilometres driven might have been useful. Moreover, the use of driving simulators could provide us with more direct information on the participant's driving style. Also, bearing in mind the importance of personality characteristics in the emotional impact of the images, the availability of personality measures could have been helpful. Furthermore, while menstrual cycle information was gathered from female participants, further control of the phase of the menstrual cycle phase, including hormonal assessments, could enhance the study’s findings.
Notwithstanding its limitations, this study boasts several strengths. It was conducted with a sizable sample size, encompassing both genders and a wide age range. Additionally, the utilization of traffic accident-related images allowed for the assessment of participants’ emotional responses directly related to the characteristic being studied. Lastly, the use of spectroscopy facilitated the evaluation of prefrontal cortex response in a comfortable setting for participants, circumventing interference from discomfort prevalent in other neuroimaging techniques.
In conclusion, our study suggests lower prefrontal oxygenation levels in male drivers with maladaptive driving styles, particularly dissociative ones, in response to neutral and traffic accident images, whereas this response was not evident in female drivers, nor did significant differences emerge based on age.
Ethical approval
All procedures performed were in accordance with the ethical standards of the institutional research Ethical Committee of the University, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflicts of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Ayaz, H.; Shewokis, P.A.; Curtin, A.; Izzetoglu, M.; Izzetoglu, K.; Onaral, B. Using MazeSuite and functional near infrared spectroscopy to study learning in spatial navigation. JoVE (Journal of Visualized Experiments), 2011; e3443. [Google Scholar] [CrossRef]
- Ayaz, H.; Shewokis, P.A.; Bunce, S.C.; Onaral, B. Functional near infrared spectroscopy-based brain computer interface (United States Patent US9946344B2). 2018. https://patents.google.
- Aluja, A.; Balada, F.; Blanco, E.; Lucas, I.; Blanch, A. Startle reflex modulation by affective face “Emoji” pictographs. Psychological Research 2020, 84, 15–22. [Google Scholar] [CrossRef]
- Aluja, A.; Blanch, A.; Blanco, E.; Balada, F. Affective modulation of the startle reflex and the reinforcement sensitivity theory of personality: the role of sensitivity to reward. Physiology & Behavior 2015, 138, 332–339. [Google Scholar] [CrossRef]
- Balada, F.; Blanch, A.; Aluja, A. Arousal and habituation effects (excitability) on startle responses to the International Affective Picture Systems (IAPS). Journal of Psychophysiology 2014, 28, 233–241. [Google Scholar] [CrossRef]
- Bendall, R.C.; Eachus, P.; Thompson, C. A brief review of research using near-infrared spectroscopy to measure activation of the prefrontal cortex during emotional processing: the importance of experimental design. Frontiers in Human Neuroscience 2016, 10, 529. [Google Scholar] [CrossRef]
- Cheng, S.; Qiu, X.; Li, S.; Mo, L.; Xu, F.; Zhang, D. Different Roles of the Left and Right Ventrolateral Prefrontal Cortex in Cognitive Reappraisal: An Online Transcranial Magnetic Stimulation Study. Frontiers in Human Neuroscience 2022, 16, 928077. [Google Scholar] [CrossRef]
- Cohen, J. Statistical power analysis for the behavioral sciences (2nd ed). L. Erlbaum Associates. 1988.
- Dan, R.; Canetti, L.; Keadan, T.; Segman, R.; Weinstock, M.; Bonne, O.; Reuveni, I.; Goelman, G. Sex differences during emotion processing are dependent on the menstrual cycle phase. Psychoneuroendocrinology 2019, 100, 85–95. [Google Scholar] [CrossRef]
- De Joux, N.; Russell, P.N.; Helton, W.S. A functional near-infrared spectroscopy study of sustained attention to local and global target features. Brain and Cognition 2013, 81, 370–375. [Google Scholar] [CrossRef]
- Doi, H.; Nishitani, S.; Shinohara, K. NIRS as a tool for assaying emotional function in the prefrontal cortex. Frontiers in Human Neuroscience 2013, 7, 770. [Google Scholar] [CrossRef]
- Dreher, J.-C.; Schmidt, P.J.; Kohn, P.; Furman, D.; Rubinow, D.; Berman, K.F. Menstrual cycle phase modulates reward-related neural function in women. Proceedings of the National Academy of Sciences 2007, 104, 2465–2470. [Google Scholar] [CrossRef] [PubMed]
- Drzewiecki, C.M.; Juraska, J.M. The structural reorganization of the prefrontal cortex during adolescence as a framework for vulnerability to the environment. Pharmacology Biochemistry and Behavior 2020, 199, 173044. [Google Scholar] [CrossRef]
- Eherenfreund-Hager, A.; Taubman–Ben-Ari, O.; Toledo, T.; Farah, H. The effect of positive and negative emotions on young drivers: A simulator study. Transportation Research Part F: Traffic Psychology and Behaviour 2017, 49, 236–243. [Google Scholar] [CrossRef]
- Elander, J.; West, R.; French, D. Behavioral correlates of individual differences in road-traffic crash risk: An examination of methods and findings. Psychological Bulletin 1993, 113, 279–294. [Google Scholar] [CrossRef]
- Eysenck, H.J. The biological basis of personality; Transaction Publ.; p. 1967.
- Eysenck, H.J. Personality. In The Neuropsychology of Individual Differences (p. 151-207). Elsevier. 1994. [CrossRef]
- Fan, J.; McCandliss, B.D.; Fossella, J.; Flombaum, J.I.; Posner, M.I. The activation of attentional networks. Neuroimage 2005, 26, 471–479. [Google Scholar] [CrossRef]
- Foy H., J.; Runham, P.; Chapman, P. Prefrontal cortex activation and young driver behaviour: a fNIRS study. PLoS one 2016, 11, e0156512. [Google Scholar] [CrossRef]
- French, D.J.; West, R.J.; Elander, J.; Wilding, J.M. Decision-making style, driving style, and self-reported involvement in road traffic accidents. Ergonomics 1993, 36, 627–644. [Google Scholar] [CrossRef] [PubMed]
- G.A. Res. 74/299, ¶ 12, U.N. GAOR, 74th Sess., Supp. No. 49, (Vol. I), U.N. Doc. A/74/49 (Vol. III), at 26-33 (Aug. 2020; 31.
- Gulian, E.; Matthews, G.; Glendon, A.I.; Davies, D.R.; Debney, L.M. Dimensions of driver stress. Ergonomics 1989, 32, 585–602. [Google Scholar] [CrossRef]
- Hamann, S.; Canli, T. Individual differences in emotion processing. Current Opinion in Neurobiology 2004, 14, 233–238. [Google Scholar] [CrossRef]
- Harada, H.; Nashihara, H.; Morozumi, K.; Ota, H.; Hatakeyama, E. A comparison of cerebral activity in the prefrontal region between young adults and the elderly while driving. Journal of Physiological Anthropology 2007, 26, 409–414. [Google Scholar] [CrossRef]
- Holman, A.C.; Popușoi, S.A. How you deal with your emotions is how you drive. Emotion regulation strategies, traffic offenses, and the mediating role of driving styles. Sustainability 2020, 12, 4929. [Google Scholar] [CrossRef]
- Hu, K. Investigations into ventral prefrontal cortex using mediation models. Journal of Neuroscience Research 2020, 98, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.C.; Mueller, C.J.; Dolski, I.; Dalton, K.M.; Nitschke, J.B.; Urry, H.L. . & Davidson, R.J. Now you feel it, now you don't: Frontal brain electrical asymmetry and individual differences in emotion regulation. Psychological Science 2003, 14, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.; Zhang, W. (2013). Sadder but wiser? Effects of negative emotions on risk perception, driving performance, No. 1, and perceived workload. In Proceedings of the Human Factors and Ergonomics Society Annual Meeting (Vol. 57; pp. 1849–1853. [CrossRef]
- Kohn, N.; Eickhoff, S.B.; Scheller, M.; Laird, A.R.; Fox, P.T.; Habel, U. Neural network of cognitive emotion regulation—an ALE meta-analysis and MACM analysis. Neuroimage 2014, 87, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.J.; Bradley, M.M.; Cuthbert, B.N. (2008) International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8. University of Florida, Gainesville, FL. 2008.
- Li, S.; Cai, Y.; Liu, J.; Li, D.; Feng, Z.; Chen, C.; Xue, G. Dissociated roles of the parietal and frontal cortices in the scope and control of attention during visual working memory. NeuroImage 2017, 149, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Jin, X.; Niu, D.; Zhang, L.; Jiang, S.Y.; Ruan, H.D.; Ho, G.W. Sex differences in hemispheric lateralization of attentional networks. Psychological Research 2021, 85, 2697–2709. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.K.; Mc Mahon, B.; Andersen, S.B.; Siebner, H.R.; Knudsen, G.M.; Fisher, P.M. Threat-related amygdala functional connectivity is associated with 5-HTTLPR genotype and neuroticism. Social Cognitive and Affective Neuroscience 2016, 11, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Manelis, A.; Huppert, T.J.; Rodgers, E.; Swartz, H.A.; Phillips, M.L. The role of the right prefrontal cortex in recognition of facial emotional expressions in depressed individuals: fNIRS study. Journal of Affective Disorders 2019, 258, 151–158. [Google Scholar] [CrossRef]
- Marchewka, A.; Żurawski, Ł.; Jednoróg, K.; Grabowska, A. The Nencki Affective Picture System (NAPS): introduction to a novel, standardized, wide-range, high-quality, realistic picture database. Behavior Research Methods 2014, 46, 596–610. [Google Scholar] [CrossRef]
- Mitchell, D.G. The nexus between decision making and emotion regulation: a review of convergent neurocognitive substrates. Behavioural Brain Research 2011, 217, 215–231. [Google Scholar] [CrossRef]
- Navon–Eyal, M.; Taubman–Ben-Ari, O. Can emotion regulation explain the association between age and driving styles? Transportation Research Part F: Traffic Psychology and Behaviour 2020, 74, 439–445. [Google Scholar] [CrossRef]
- Ochsner, K.N.; Gross, J.J. The cognitive control of emotion. Trends in Cognitive sciences 2005, 9, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Ochsner, K.N.; Silvers, J.A.; Buhle, J.T. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences 2012, 1251, E1–E24. [Google Scholar] [CrossRef] [PubMed]
- Opitz, P.C.; Rauch, L.C.; Terry, D.P.; Urry, H.L. Prefrontal mediation of age differences in cognitive reappraisal. Neurobiology of Aging 2012, 33, 645–655. [Google Scholar] [CrossRef]
- Padilla García J., L.; Castro Ramírez, C.; Doncel, P.; Ben-Ari, O.T. Adaptation of the multidimensional driving styles inventory for Spanish drivers: Convergent and predictive validity evidence for detecting safe and unsafe driving styles. Accident Analysis & Prevention 2020, 136, 105413. [Google Scholar] [CrossRef]
- Petersen, S.E.; Posner, M.I. "The attention system of the human brain: 20 years after. " Annual review of neuroscience 2012, 35, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Poó, F.M.; Ledesma, R.D. A Study on the Relationship Between Personality and Driving Styles. Traffic Injury Prevention 2013, 14, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Quaresima, V.; Bisconti, S.; Ferrari, M. A brief review on the use of functional near-infrared spectroscopy (fNIRS) for language imaging studies in human newborns and adults. Brain and Language 2012, 121, 79–89. [Google Scholar] [CrossRef]
- Ray, R.D.; McRae, K.; Ochsner, K.N.; Gross, J.J. Cognitive reappraisal of negative affect: converging evidence from EMG and self-report. Emotion 2010, 10, 587–592. [Google Scholar] [CrossRef]
- Reason, J.; Manstead, A.; Stradling, S.; Baxter, J.; Campbell, K. Errors and violations on the roads: a real distinction? Ergonomics 1990, 33, 1315–1332. [Google Scholar] [CrossRef]
- Reber, J.; Tranel, D. Sex differences in the functional lateralization of emotion and decision making in the human brain. Journal of Neuroscience Research 2017, 95, 270–278. [Google Scholar] [CrossRef]
- Šeibokaitė, L.; Endriulaitienė, A.; Sullman, M.J.; Markšaitytė, R.; Žardeckaitė-Matulaitienė, K. Difficulties in emotion regulation and risky driving among Lithuanian drivers. Traffic Injury Prevention 2017, 18, 688–693. [Google Scholar] [CrossRef]
- Singh, S. Critical reasons for crashes investigated in the National Motor Vehicle Crash Causation Survey. (Traffic Safety Facts Crash Stats. Report No. DOT HS 812 506). Washington, DC: National Highway Traffic Safety Administration. 2018.
- Taubman-Ben-Ari, O.; Skvirsky, V. The multidimensional driving style inventory a decade later: Review of the literature and re-evaluation of the scale. Accident Analysis & Prevention 2016, 93, 179–188. [Google Scholar] [CrossRef]
- Taubman-Ben-Ari, O.; Mikulincer, M.; Gillath, O. The multidimensional driving style inventory—scale construct and validation. Accident Analysis & Prevention 2004, 36, 323–332. [Google Scholar]
- Trick, L.M.; Brandigampola, S.; Enns, J.T. How fleeting emotions affect hazard perception and steering while driving: The impact of image arousal and valence. Accident Analysis & Prevention 2012, 45, 222–229. [Google Scholar] [CrossRef]
- Trógolo, M.A.; Melchior, F.; Medrano, L.A. The role of difficulties in emotion regulation on driving behavior. Journal of Behavior, Health & Social Issues 2014, 6, 107–117. [Google Scholar]
- Tukey, J.W. (1977) Exploratory data analysis. Addison-Wesley Publishing Company Reading, Mass. — Menlo Park, Cal., London, Amsterdam, Don Mills, Ontario, Sydney, XVI, 688 S. 1977.
- Viinikainen, M.; Jääskeläinen, I.P.; Alexandrov, Y.; Balk, M.H.; Autti, T.; Sams, M. Nonlinear relationship between emotional valence and brain activity: evidence of separate negative and positive valence dimensions. Human brain mapping 2010, 31, 1030–1040. [Google Scholar] [CrossRef]
- Wang, Y.; Qu, W.; Ge, Y.; Sun, X.; Zhang, K. Effect of personality traits on driving style: Psychometric adaption of the multidimensional driving style inventory in a Chinese sample. PLOS ONE 2018, 13, e0202126. [Google Scholar] [CrossRef]
- Westgarth, M.M.; Hogan, C.A.; Neumann, D.L.; Shum, D.H. A systematic review of studies that used NIRS to measure neural activation during emotion processing in healthy individuals. Social Cognitive and Affective Neuroscience 2021, 16, 345–369. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, H.; LaBar, K.S.; McCarthy, G. Dissociable prefrontal brain systems for attention and emotion. Proceedings of the National Academy of Sciences 2002, 99, 11447–11451. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.K.; Chan, A.S. A systematic review of the application of functional near-infrared spectroscopy to the study of cerebral hemodynamics in healthy aging. Neuropsychology Review 2021, 31, 139–166. [Google Scholar] [CrossRef]
| 1 |
The IAPS labels and codes for the stimuli used in the first neutral block were: Spoon (7004), Stool (7025), Clock (7211), File cabinets (7224), chair (7235) and Cabinet (7705). |
| 2 |
The block of neutral images was composed of Landscapes_045_h, Landscapes_091_h, Objects_166_h, Objects_204_h, Objects_237_h and Objects_307_v. The first block of accidents was composed of the following images: People_004_h, People_007_h, People_009_h, People_010_h, People_013_v and People_016_h. Finally, the other block of accident images included People_020_h, People_021_h, People_022_h, People_226_h, Objects_003_h and Objects_149_h. (NAPS). |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).