Submitted:
23 July 2024
Posted:
23 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Collection
2.3. Sub-Sampling of Raw Materials
2.4. Sample Preparation
2.5. LC-MS/MS Analysis
2.6. Quality Assurance and Quality Control
2.7. Statistical Analysis
3. Results
3.1. Occurrence of Mycotoxins in Raw Materials
3.2. Occurrence of Mycotoxins in Infant Flours
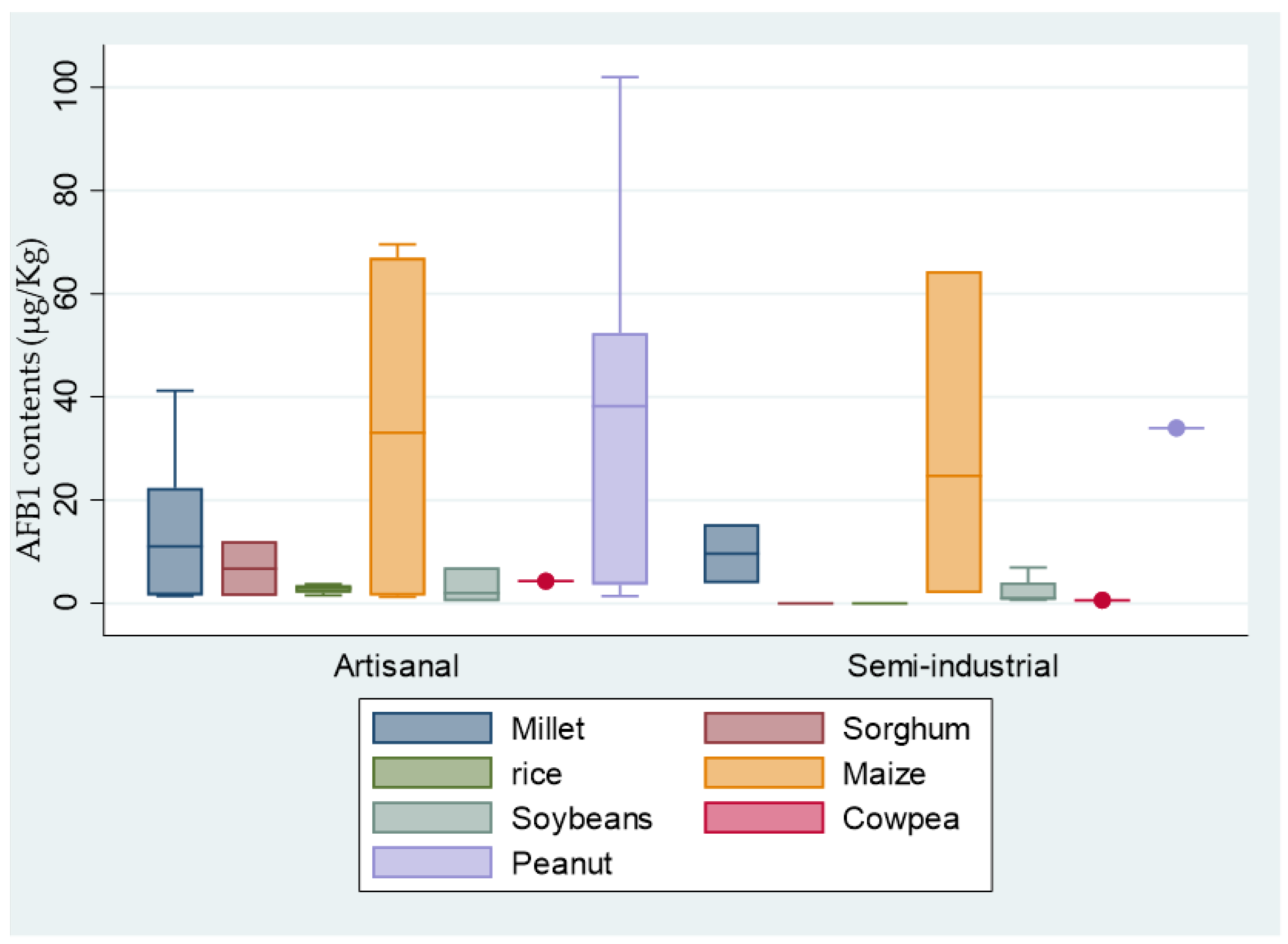
3.3. Occurrence of AFB1 in Raw Materials according to the Types of Production Units
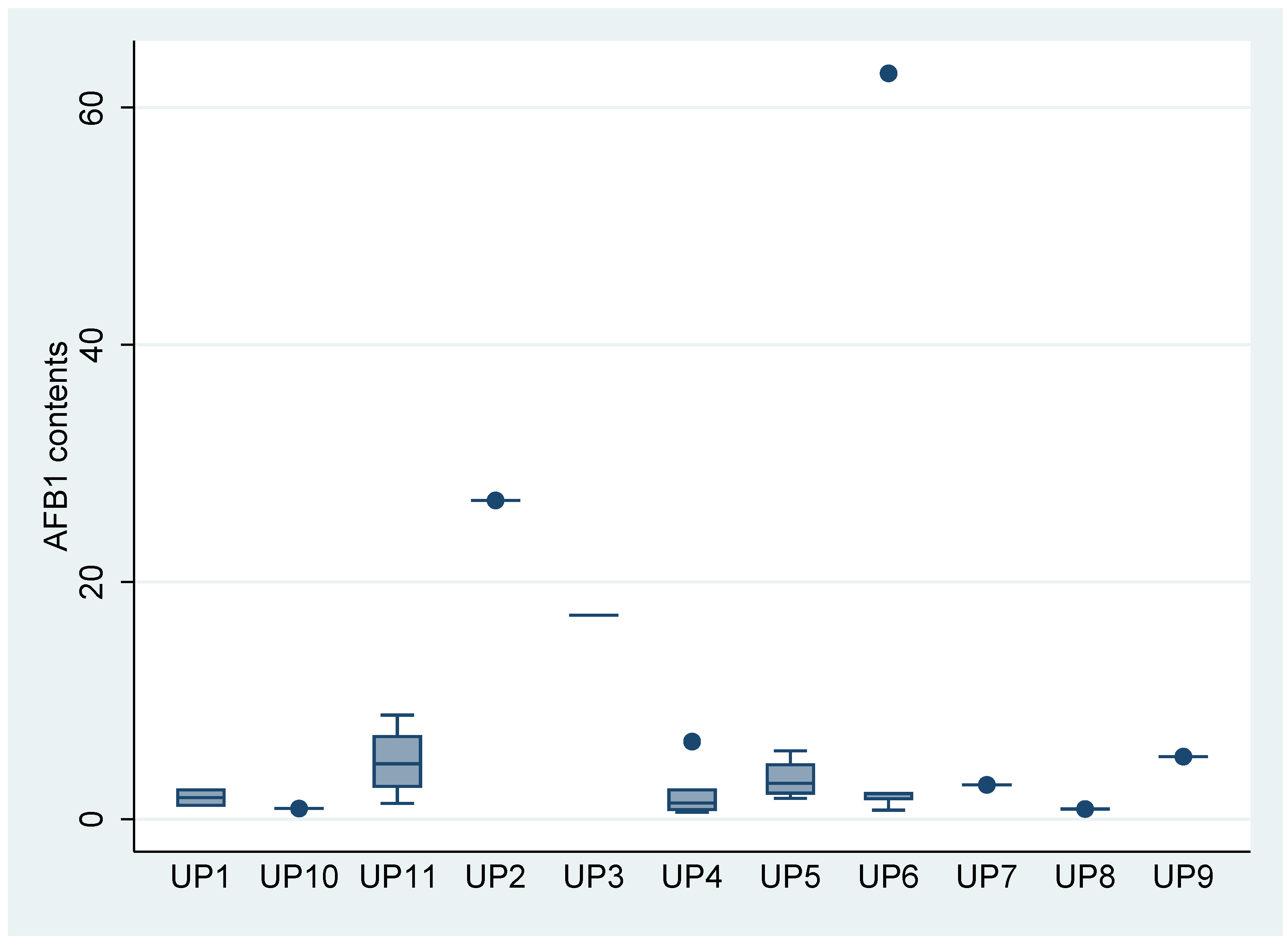
3.4. Occurrence of AFB1 in Infant Flour according to The Production Units
4. Discussion
4.1. Occurrence of Mycotoxins in Raw Materials
4.2. Occurrence of Mycotoxins in Infant Flours
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tozlovanu, M. Évaluation Du Risque de Contamination Alimentaire En Mycotoxines Néphrotoxiques et Cancérogènes (Notamment l’ochratoxine A): Validation de Biomarqueurs d’exposition et d’effet. PhD Thesis, 2008. [Google Scholar]
- Moretti, A.; Logrieco, A.F.; Susca, A. Mycotoxins: An Underhand Food Problem. Mycotoxigenic Fungi Methods Protoc. 2017, 3–12. [Google Scholar] [CrossRef]
- Huybrechts, B.; Tangni, E.K.; Debongnie, P.; Geys, J.; Callebaut, A. Méthodes Analytiques de Détermination Des Mycotoxines Dans Les Produits Agricoles: Une Revue. Cah. Agric. 2013, 22, 202–215. [Google Scholar] [CrossRef]
- JARD, G. Etude de Différents Modes d’élimination Biologique de La Zéaralénone, Mycotoxine Présente Dans Les Céréales: Adsorption et Biotransformation, Université de Toulouse: Toulouse, 2009.
- Pleadin, J.; Frece, J.; Markov, K. Mycotoxins in Food and Feed. Adv. Food Nutr. Res. 2019, 89, 297–345. [Google Scholar] [CrossRef]
- Nikiema, P.A. Etude des aflatoxines au Burkina Faso. Détermination quantitative et qualitative des aflatoxines de l’arachide par des tests biochimiques et immunologiques, Univresité de Ouagadougou: Ouagadougou, 1993.
- Ouattara-Sourabie, P.B.; Nikiema, P.A.; Traore, A.S. Caractérisation de Souches d’Aspergillus Spp Isolées Des Graines d’arachides Cultivées Au Burkina Faso, Afrique de l’Ouest. Int. J. Biol. Chem. Sci. 2011, 5. [Google Scholar] [CrossRef]
- Bationo, J.F.; Nikiéma, P.A.; Koudougou, K.; Ouédraogo, M.; Bazié, S.R.; Sanou, E.; Barro, N. Assessment of Aflatoxin B1 and Ochratoxin A Levels in Sorghum Malts and Beer in Ouagadougou. Afr. J. Food Sci. 2015, 9, 417–420. [Google Scholar] [CrossRef]
- Turner, P.C.; Nikiema, P.; Wild, C.P. Fumonisin Contamination of Food: Progress in Development of Biomarkers to Better Assess Human Health Risks. Mutat. Res. Toxicol. Environ. Mutagen. 1999, 443, 81–93. [Google Scholar] [CrossRef]
- Nikiema, P.N.; Worrillow, L.; Traore, A.S.; Wild, C.P.; Turner*, P.C. Fumonisin Contamination of Maize in Burkina Faso, West Africa. Food Addit. Contam. 2004, 21, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Ware, L.Y.; Durand, N.; Nikiema, P.A.; Alter, P.; Fontana, A.; Montet, D.; Barro, N. Occurrence of Mycotoxins in Commercial Infant Formulas Locally Produced in Ouagadougou (Burkina Faso). Food Control 2017, 73, 518–523. [Google Scholar] [CrossRef]
- Warth, B.; Parich, A.; Atehnkeng, J.; Bandyopadhyay, R.; Schuhmacher, R.; Sulyok, M.; Krska, R. Quantitation of Mycotoxins in Food and Feed from Burkina Faso and Mozambique Using a Modern LC-MS/MS Multitoxin Method. J. Agric. Food Chem. 2012, 60, 9352–9363. [Google Scholar] [CrossRef]
- Afolabi, C.G.; Ezekiel, C.N.; Kehinde, I.A.; Olaolu, A.W.; Ogunsanya, O.M. Contamination of Groundnut in South-Western Nigeria by Aflatoxigenic Fungi and Aflatoxins in Relation to Processing. J. Phytopathol. 2015, 163, 279–286. [Google Scholar] [CrossRef]
- Ruppol, P.; Delfosse, P.; Hornick, J.-L. La Contamination de La Filière Laitière Par Les Mycotoxines: Un Risque Pour La Santé Publique En Afrique Subsaharienne. In Proceedings of the Annales de Médécine Vétérinaire; 2004; Vol. 2, pp. 141–146. [Google Scholar]
- Shabeer, S.; Asad, S.; Jamal, A.; Ali, A. Aflatoxin Contamination, Its Impact and Management Strategies: An Updated Review. Toxins 2022, 14, 307. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Zhang, W.; Jiang, H.; Liu, D.; Liu, X.; Li, L.; Li, C.; Xiao, X.; Tang, S.; Li, D. Food-Origin Mycotoxin-Induced Neurotoxicity: Intend to Break the Rules of Neuroglia Cells. Oxid. Med. Cell. Longev. 2021, 2021, 1–14. [Google Scholar] [CrossRef]
- Amirahmadi, M.; Shoeibi, S.; Rastegar, H.; Elmi, M.; Mousavi Khaneghah, A. Simultaneous Analysis of Mycotoxins in Corn Flour Using LC/MS-MS Combined with a Modified QuEChERS Procedure. Toxin Rev. 2018, 37, 187–195. [Google Scholar] [CrossRef]
- Commission, E. Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006. J Eur Union 2023, 119, 103–157. [Google Scholar]
- Ezekiel, C.N.; Udom, I.E.; Frisvad, J.C.; Adetunji, M.C.; Houbraken, J.; Fapohunda, S.O.; Samson, R.A.; Atanda, O.O.; Agi-Otto, M.C.; Onashile, O.A. Assessment of Aflatoxigenic Aspergillus and Other Fungi in Millet and Sesame from Plateau State, Nigeria. Mycology 2014, 5, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Bayala-Yaï, L.K.A.; Nikièma, P.A.; Ouédraogo, W.A.; Dembélé, I.; Nébié, Z.; Simpore, J. Management of Infant Flour Production in the City of Ouagadougou: A Survey Study. PAMJ - One Health 2024, 14. [Google Scholar] [CrossRef]
- Bruce, A.C.C.; Kayode, A.P. Aptitude Au Stockage de Quelques Farines Infantiles à Base de Ressources Alimentaires Locales Du BENIN. 2017, 90. [Google Scholar]
- Dieme, E.; Fall, R.; Sarr, I.; Sarr, F.; Traore, D.; Seydi, M. Contamination Des Céréales Par l’aflatoxine En Afrique : Revue Des Méthodes de Lutte Existantes. Int. J. Biol. Chem. Sci. 2016, 10, 2285–2299. [Google Scholar] [CrossRef]
- Brochard, G.; Le Bacle, C. Mycotoxines En Milieu de Travail. Orig. Propr. Toxiques Princ. Mycotoxines INRS Doc. Pour Med. Trav. 2009, 119. [Google Scholar]
- Nikiéma, P.A. Etude des aflatoxines au Burkina Faso: Détermination quantitative et qualitative des aflatoxines de l’arachide par des tests biochimiques et immunologiques. 1993.
- Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene: This Publication Represents the Views and Expert Opinions of an IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Which Met in Lyon, 12 - 19 February 2002; International Agency for Research on Cancer, Ed.; IARC monographs on the evaluation of carcinogenic risks to humans; IARC: Lyon, 2002; ISBN 978-92-832-1282-9.
- Hadjeba-Medjdoub, K. Risque de Multicontaminations En Mycotoxines et Moyens de Désactivation Par Les Parois de Levures et Levures Enrichies En Glutathion Ou Sélénométhionine. PhD Thesis, 2012. [Google Scholar]
- GALTIER, P.; LOISEAU, N.; OSWALD, I.P.; PUEL, O. Toxicology of mycotoxins, hazards and risks in human and animal food. [CrossRef]
- MAHNINE, N. Etude de La Contamination Des Produits Céréaliers Par Les Mycotoxines: Cas Des Aflatoxines, de l’ochratoxine A, Des Fumonisines et Des Mycotoxines Émergentes, Université Mohammed V: Rabat, 2017.
- Ouattara–Sourabie, P.B.; Nikiema, P.A.; Traore, A. Antifungal Activity of Hyptis Spicigera (Lamiaceae) Extracts and Essential Oils of Cymbopogon Citratus (Poaceae) and Cymbopogon Giganteus against the Growth of Aspergillus Strains Isolated in Bur Ina Faso. J Pharm Pharmacol 2017, 7, 17–27. [Google Scholar]
- Sankara, F.; Sanou, A.G.; Waongo, A.; Somda, M.; Toé, P.; Somda, I. Pratique Paysanne Post Récolte Du Maïs Dans La Région Des Hauts-Bassins Du Burkina Faso. J. Anim. Plant Sci. 2017, 33, 5274–5288. [Google Scholar]
- Amoin, A.; Agbo, E.A.; Dago, A.G.; Gbogouri, A.G.; Brou, D.K.; Dago, G. Comparaison Des Caractéristiques Nutritionnelles et Rhéologiques Des Bouillies Infantiles Préparées Par Les Techniques de Germination et de Fermentation. Int. J. Biol. Chem. Sci. 2015, 9, 944–953. [Google Scholar] [CrossRef]
- Olive, F.; Mouquet-Rivier, C.; Fioroni, N.; Bichard, A.; Boulle-Martinaud, N.; Kaboré, C.; Denizeau, M.; Zagré, N.; Le Dain, A.; Ndiaye, N. La Filière Des Farines Infantiles Produites Localement Dans 6 Pays Sahéliens 2020.
- Panisset, J.-C.; Dewailly, É.; Doucet-Leduc, H. Contamination Alimentaire. Environ. Santé Publique Fond. Prat. Éditions TEC DOC Edisen 2003. [Google Scholar]
| Mycotoxins | Precursor ion (M/Z) | Qualifier ion (M/Z) | Quantifier ion (M/Z) |
|---|---|---|---|
| Aflatoxin B1 | 313 | 285.1 | 241.0 |
| Aflatoxin B2 | 315 | 287.1 | 259.1 |
| Aflatoxin G1 | 329 | 233 | 311 |
| Aflatoxin G2 | 331 | 313 | 245 |
| Fumonisin B1 | 722.2 | 334.2 | 252.2 |
| OTA | 404 | 239 | 221 |
| Sample level | AFB1 | AFB2 | AFG1 | AFG2 | AFs | FB1 | OTA |
|---|---|---|---|---|---|---|---|
| Mi 6 | 1.54±0.44 | 0.76±0.21 | 8.04±0.92 | 3.77±0.58 | 14.10±2.15 | 9.68±16.76 | 0.00 |
| Mi MSL | 1.38±1.37 | 0.81±0.36 | 3.62±2.13 | 4.91±1.31 | 10.72±5.18 | 58.44±101.21 | 0.00 |
| Mi MRJ | 6.69±1.08 | 2.18±0.34 | 6.69±2.10 | 11.50±1.73 | 27.06±5.24 | 0.00 | 0.00 |
| Mi BSR | 22.37±1.35 | 2.07±0.91 | 2.07±1.01 | 4.31±1.18 | 30.81±4.45 | 0.00 | 0.00 |
| Mi KSN | 15.31±1.91 | 0.77±0.15 | 2.61±1.56 | 3.93±2.03 | 22.62±5.65 | 0.00 | 0.00 |
| Mi BAM | 41.21±4.55 | 3.13±0.24 | 6.58±4.00 | 3.87±2.38 | 54.80±11.17 | 0.00 | 0.00 |
| Mi SDP | 3.87±1.25 | 1.54±1.33 | 5.80±0.79 | 10.98±2.47 | 22.18±5.84 | 0.00 | 0.00 |
| So BSR | 12.04±0.84 | 6.35±0.34 | 1.71±0.94 | 1.80±1.13 | 21.90±3.25 | 0.00 | 0.00 |
| So MRJ | 1.40±0.10 | 0.34±0.58 | 1.96±0.98 | 0.52±0.09 | 4.22±1.76 | 0.00 | 0.00 |
| So 6 | 6.67±0.25 | 2.87±0.15 | 2.20±1.48 | 2.55±0.46 | 14.29±2.34 | 0.00 | 0.00 |
| Ri 6 | 3.69±1.38 | 0.40±0.49 | 0.70±0.66 | 1.64±1.18 | 6.43±3.71 | 0.00 | 5.79±1.22 |
| Ri RSL | 3.37±0.15 | 1.02±0.57 | 2.32±1.40 | 1.76±0.53 | 8.47±2.65 | 0.00 | 6.75±1.24 |
| Ri MRJ | 1.51±0.53 | 0.26±0.25 | 2.53±0.37 | 2.24±0.91 | 6.54±2.05 | 0.00 | 2.74±1.33 |
| Ri SC | 2.46±1.00 | 0.49±0.44 | 1.23±1.53 | 1.84±0.85 | 6.02±3.82 | 0.00 | 0.00 |
| Ma G | 1.26±0.24 | 0.22±0.20 | 1.71±1.32 | 0.50±0.53 | 3.69±2.28 | 0.00 | 0.00 |
| Ma FTZ | 1.96±0.35 | 0.78±0.73 | 0.25±1.32 | 0.28±0.49 | 3.27±1.84 | 0.00 | 0.00 |
| Ma RSL | 64.36±8.40 | 12.12±0.36 | 1.15±0.53 | 1.37±1.19 | 79.00±10.47 | 0.00 | 0.52±0.89 |
| Ma 6 | 1.76±0.11 | 0.30±0.27 | 0.45±0.79 | 0.00 | 2.51±1.16 | 0.00 | 0.00 |
| Ma BSR | 69.56±8.95 | 13.87±0.84 | 2.82±0.62 | 1.53±0.37 | 87.78±10.78 | 0.00 | 0.00 |
| Ma SDP | 24.67±1.36 | 6.35±0.53 | 1.96±0.97 | 1.37±0.82 | 34.35±3.69 | 0.00 | 0.00 |
| SJ KSN | 6.93±0.77 | 1.46±0.56 | 0.85±0.81 | 0.80±0.54 | 10.05±2.68 | 58.40±34.15 | 0.00 |
| SJ SDP | 0.68±0.60 | 0.51±0.13 | 0.59±0.20 | 0.56±0.48 | 2.34±1.41 | 0.00 | 21.77±18.85 |
| SJ G | 0.67±0.12 | 0.30±0.37 | 1.35±0.61 | 1.66±0.74 | 3.97±1.84 | 0.00 | 34.42±1.99 |
| SJ FTZ | 1.15±1.98 | 0.44±0.39 | 2.15±2.68 | 10.94±18.16 | 14.68±23.22 | 0.00 | 34.17±34.72 |
| SJ MSL | 0.39±0.35 | 0.71±0.12 | 1.04±0.95 | 1.64±1.06 | 3.78±2.49 | 6.30±10.92 | 36.73±4.22 |
| SJ BAM | 2.00±1.79 | 0.19±0.17 | 0.84±0.33 | 0.76±0.38 | 3.79±2.66 | 0.00 | 33.43±2.08 |
| N FTZ | 0.60±0.59 | 1.23±0.35 | 3.25±2.70 | 5.82±1.53 | 10.89±5.18 | 0.00 | 56.49±19.06 |
| H MRJ | 4.29±0.99 | 0.32±0.29 | 0.51±0.45 | 1.49±1.08 | 6.61±2.81 | 0.00 | 0.00 |
| Ar BAM | 0.00 | 0.36±0.39 | 0.79±0.38 | 1.34±0.72 | 2.50±1.49 | 0.00 | 12.75±22.08 |
| Ar MSL | 1.38±0.32 | 0.54±0.19 | 1.16±0.65 | 2.79±0.97 | 5.87±2.13 | 7.79±13.49 | 22.59±19.64 |
| Ar G | 101.96±2.90 | 22.52±0.84 | 8.78±1.08 | 6.72±1.89 | 139.99±6.72 | 0.00 | 12.08±20.91 |
| Ar BSR | 24.07±0.72 | 3.34±0.72 | 0.76±0.66 | 1.38±0.89 | 29.55±2.99 | 0.00 | 0.00 |
| Ar FTZ | 33.96±6.36 | 7.96±0.59 | 0.77±0.80 | 1.54±0.39 | 44.23±8.14 | 0.00 | 0.00 |
| Ar KSN | 52.36±4.33 | 13.51±1.03 | 2.81±1.56 | 3.48±1.73 | 72.16±8.65 | 7.28±12.61 | 0.00 |
| Ar MRJ | 3.62±0.36 | 0.64±0.12 | 1.14±0.58 | 1.54±1.48 | 6.94±2.55 | 0.00 | 10.94±18.95 |
| Mi CRL | 15.31±1.91 | 0.77±0.15 | 2.61±1.56 | 3.93±2.03 | 22.62±5.65 | 0.00 | 0.00 |
| Ma CRL | 64.36±8.40 | 12.12±0.36 | 1.15±0.53 | 1.37±1.19 | 79.00±10.47 | 0.00 | 0.51±0.89 |
| SJ CRL | 6.93±0.77 | 1.46±0.56 | 0.85±0.81 | 0.80±0.54 | 10.05±2.68 | 58.40±34.15 | 0.00 |
| Ar CRL | 52.36±4.33 | 13.51±1.03 | 2.81±1.56 | 3.48±1.73 | 72.16±8.65 | 7.28±12.61 | 0.00 |
| Infant flours | AFB1 | AFB2 | AFG1 | AFG2 | AFs | FB1 | OTA |
|---|---|---|---|---|---|---|---|
| FSo6 | 2.40±1.69 | 1.98±0.42 | 21.91±6.81 | 2.57±1.78 | 28.87±10.70 | 82.50±27.90 | 2.88±2.62 |
| BRPE1 | 5.36±0.30 | 2.71±0.24 | 11.82±3.41 | 1.58±0.33 | 21.47±4.28 | 37.48±3.89 | 2.80±1.03 |
| Fri | 2.58±0.84 | 0.51±0.24 | 3.66±0.85 | 1.75±1.49 | 8.51±3.42 | 32.60±11.56 | 0.36±0.62 |
| BRPE4 | 8.77±0.81 | 1.16±0.31 | 9.38±3.99 | 1.84±0.64 | 21.15±5.76 | 28.33±7.67 | 1.87±0.63 |
| FMa6 | 1.75±0.33 | 1.12±0.24 | 4.40±2.32 | 1.52±0.93 | 8.78±3.82 | 27.68±7.68 | 1.94±0.62 |
| BRPE2 | 1.33±0.44 | 1.35±0.26 | 2.76±1.87 | 2.55±2.52 | 7.99±5.09 | 27.21±26.26 | 0.84±0.75 |
| BRPE3 | 3.97±0.57 | 3.42±0.66 | 3.60±1.87 | 3.61±1.88 | 14.59±4.97 | 4.98±8.63 | 0.00 |
| FSoMRJ | 2.27±0.33 | 1.49±0.87 | 12.09±3.76 | 3.90±1.79 | 19.74±6.74 | 2.57±4.45 | 2.42±4.19 |
| VCS Inst | 6.54±0.34 | 4.41±0.47 | 2.23±1.27 | 2.84±1.51 | 16.03±3.59 | 0.00 | 0.00 |
| VTL Inst | 0.59±0.38 | 0.84±0.52 | 0.84±0.40 | 0.55±0.32 | 2.82±1.62 | 0.00 | 0.00 |
| FRi6 | 3.65±0.62 | 0.42±0.16 | 5.51±2.35 | 1.62±1.33 | 11.20±4.46 | 20.45±6.79 | 1.85±0.23 |
| CRL | 0.85±0.38 | 0.79±0.49 | 5.29±3.66 | 1.84±0.65 | 8.76±5.17 | 0.00 | 2.65±0.34 |
| VTL Lc | 1.37±0.37 | 1.46±0.33 | 8.12±6.66 | 2.27±1.11 | 13.22±8.48 | 0.00 | 0.00 |
| FTZ | 17.19±0.81 | 6.81±0.86 | 7.99±0.82 | 3.10±0.85 | 35.09±3.33 | 0.00 | 1.06±0.95 |
| VCS Lc | 0.68±0.19 | 0.65±0.24 | 4.34±1.07 | 1.87±1.80 | 7.54±3.31 | 0.00 | 0.26±0.45 |
| NTV | 5.27±0.20 | 1.29±0.79 | 5.71±2.57 | 4.56±3.29 | 16.84±6.86 | 1.53±2.66 | 0.00 |
| FMi MRJ | 2.17±0.36 | 2.73±1.54 | 3.42±1.66 | 7.89±5.02 | 16.22±8.58 | 0.00 | 0.00 |
| SL | 2.59±0.28 | 1.08±0.47 | 2.35±2.10 | 1.79±1.94 | 7.81±4.78 | 1.73±3.00 | 0.00 |
| FMi 6 | 5.76±0.74 | 1.63±1.48 | 3.47±1.17 | 9.22±12.93 | 20.07±16.31 | 0.00 | 0.00 |
| MSL | 1.05±0.61 | 1.20±0.80 | 7.13±2.16 | 1.84±0.43 | 11.22±4.00 | 0.00 | 0.33±0.57 |
| BAM | 26.87±4.70 | 3.91±0.69 | 6.59±1.60 | 3.63±2.30 | 41.00±9.29 | 3.16±5.47 | 0.23±0.40 |
| FH MRJ | 1.59±0.34 | 0.51±0.24 | 2.38±0.97 | 2.83±0.29 | 7.31±1.84 | 8.80±0.54 | 0.00 |
| FRi MRJ | 0.75±0.29 | 0.37±0.32 | 5.44±1.95 | 0.96±1.19 | 7.53±3.76 | 2.64±4.57 | 0.88±0.76 |
| KSN | 0.89±0.12 | 1.17±0.63 | 5.09±1.19 | 1.85±0.93 | 8.99±2.87 | 0.00 | 0.00 |
| PG Ma | 2.89±0.56 | 2.27±0.51 | 1.73±0.86 | 1.50±1.43 | 8.40±3.36 | 0.00 | 0.24±0.41 |
| Tar MRJ | 62.88±4.49 | 17.17±1.41 | 16.63±7.11 | 6.74±3.65 | 103.43±16.66 | 417.09±117.15 | 37.43±8.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





