Submitted:
23 July 2024
Posted:
23 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
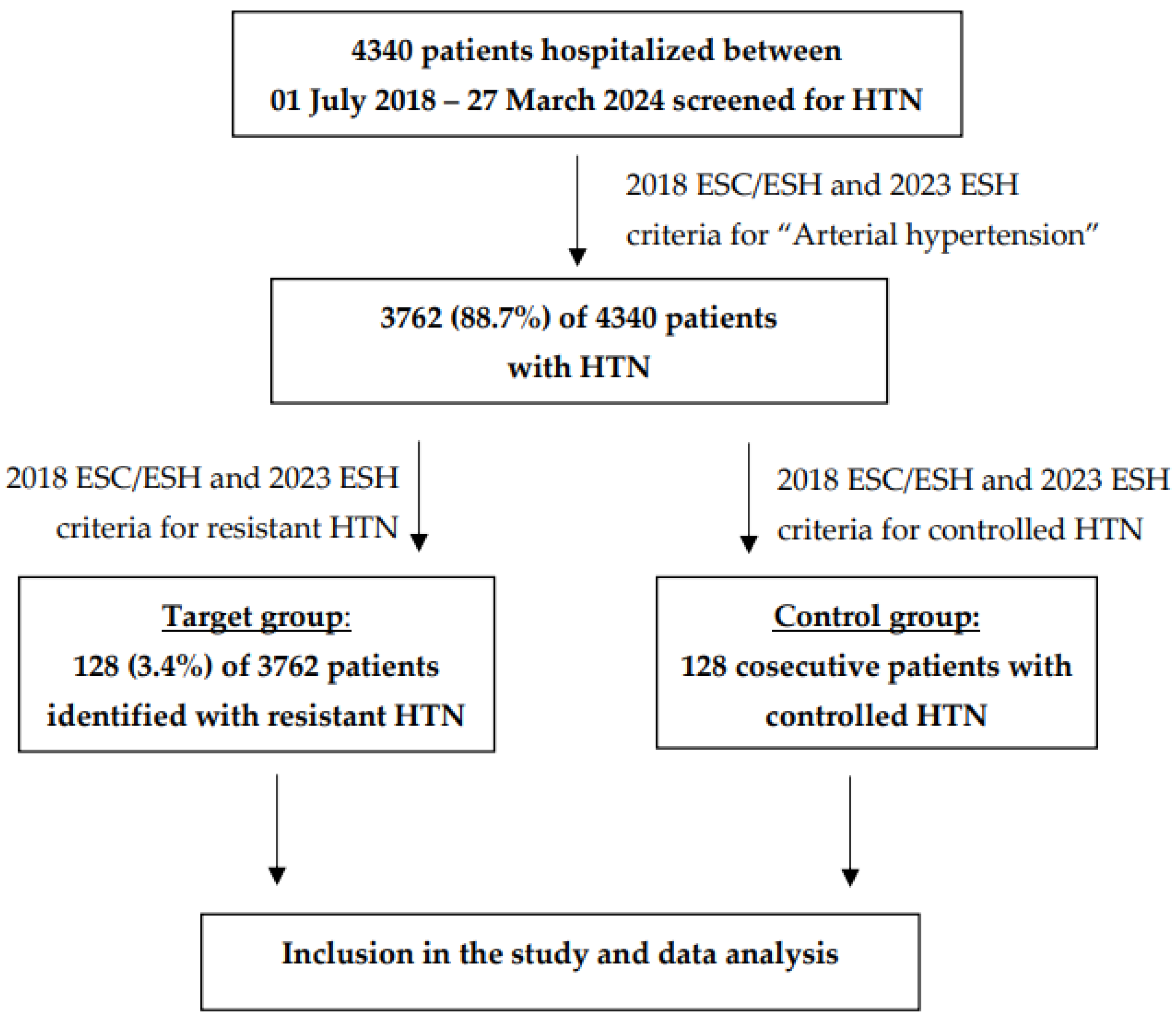
2. Materials and Methods
Statistical Analysis
3. Results
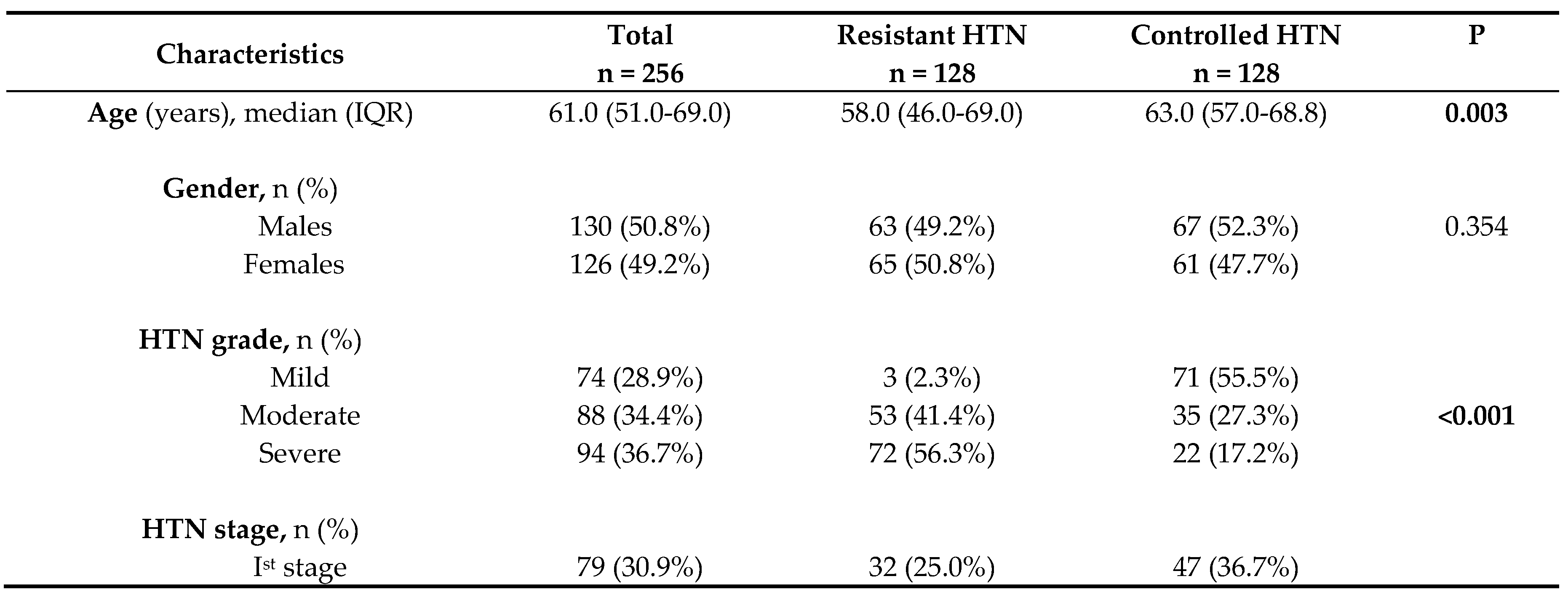
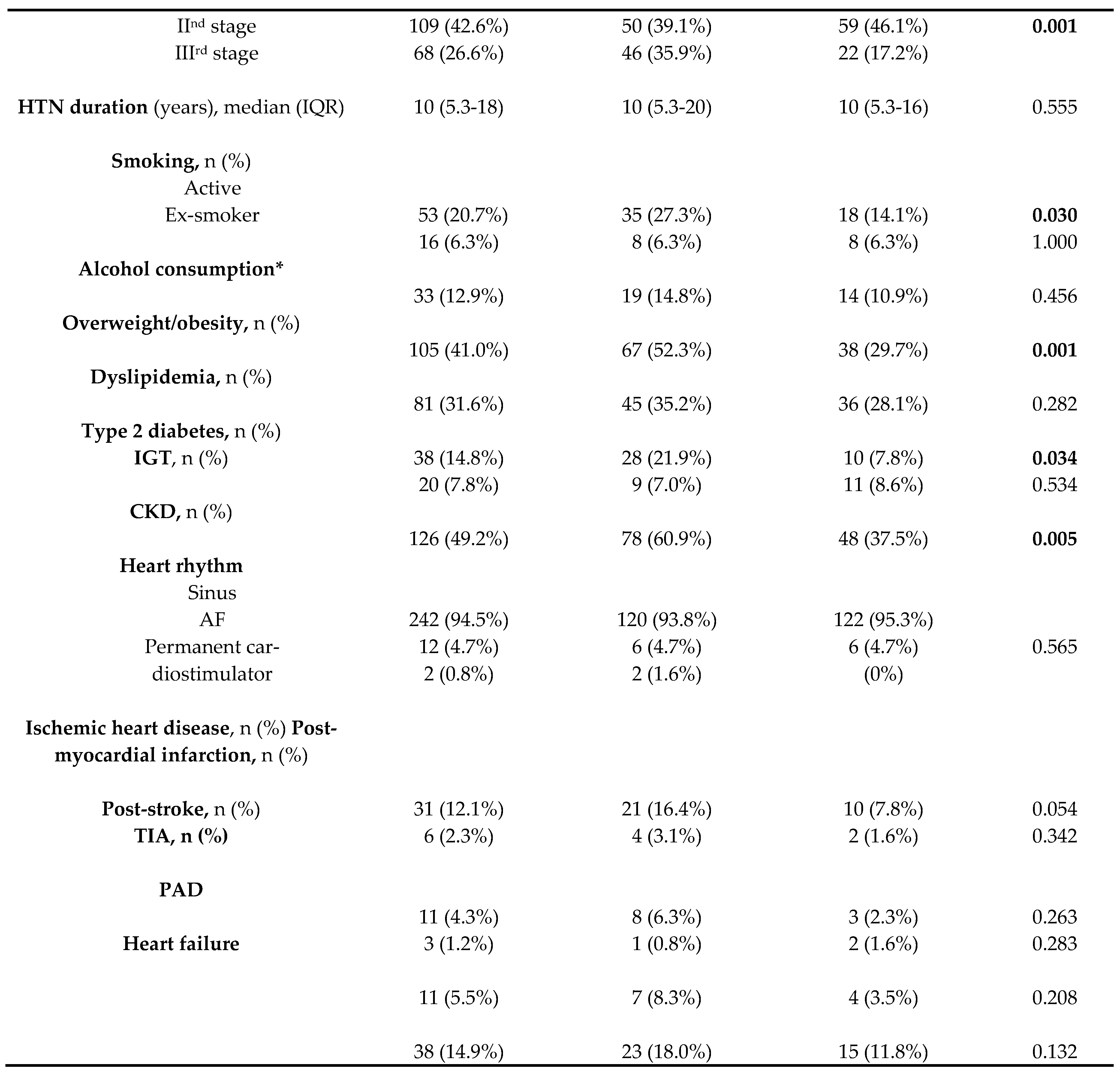
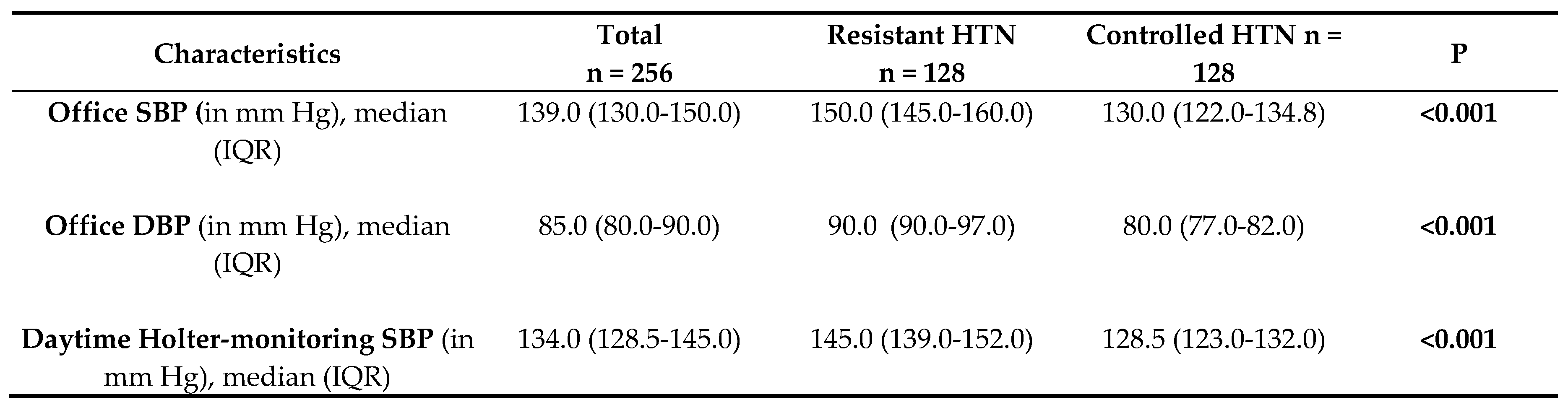
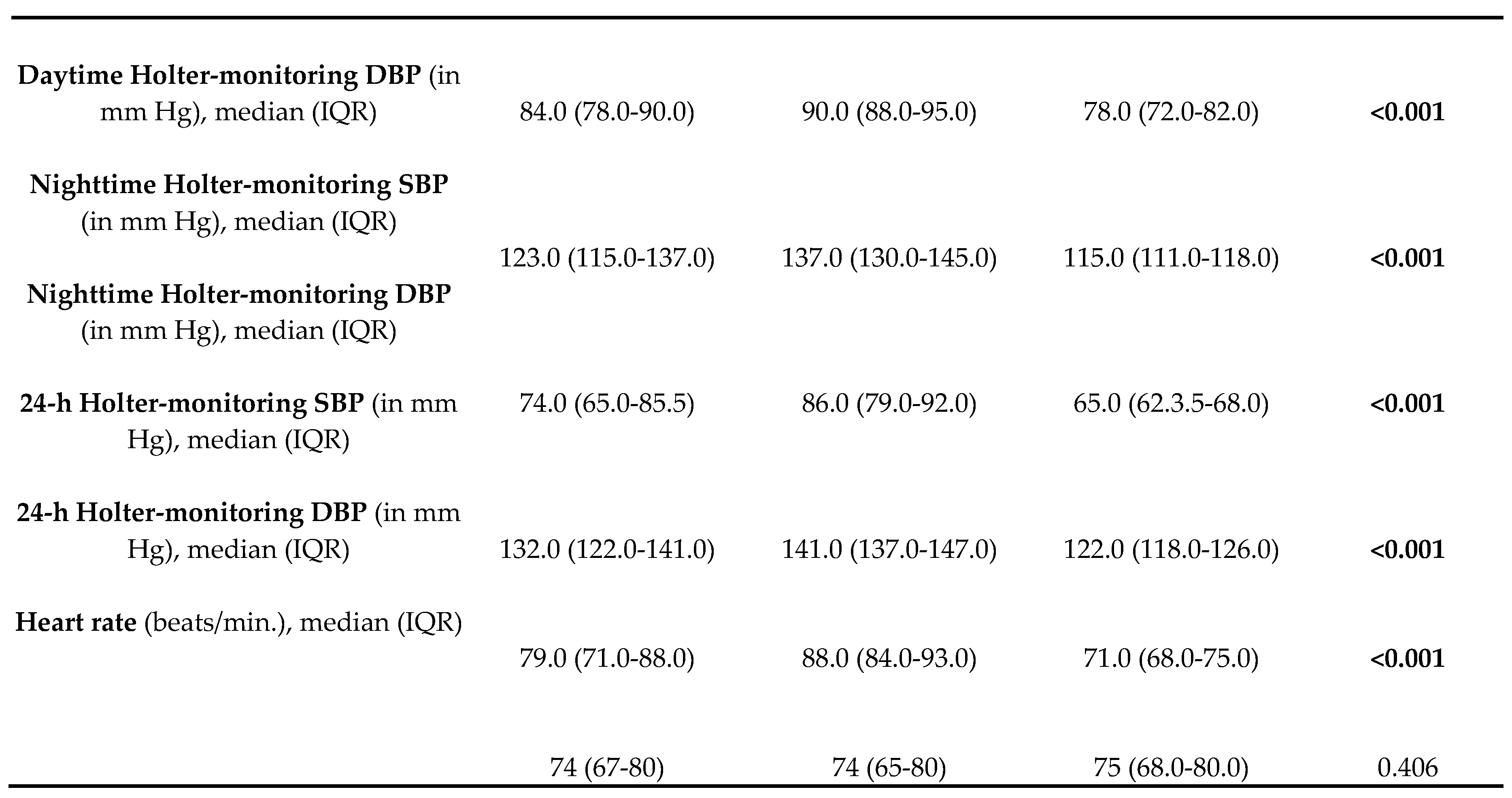
3.1. Patient’s Demographic and Clinical Characteristics
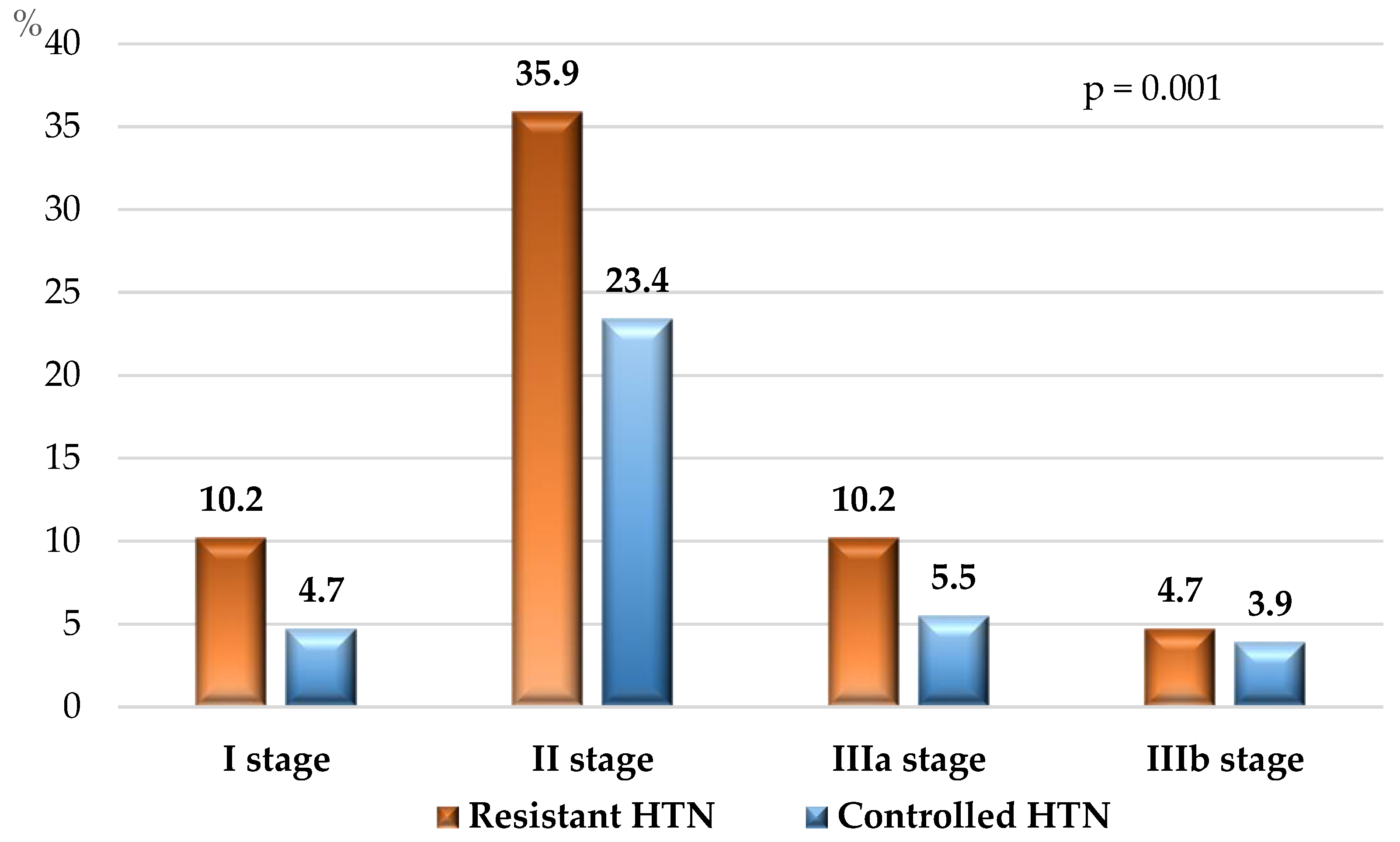
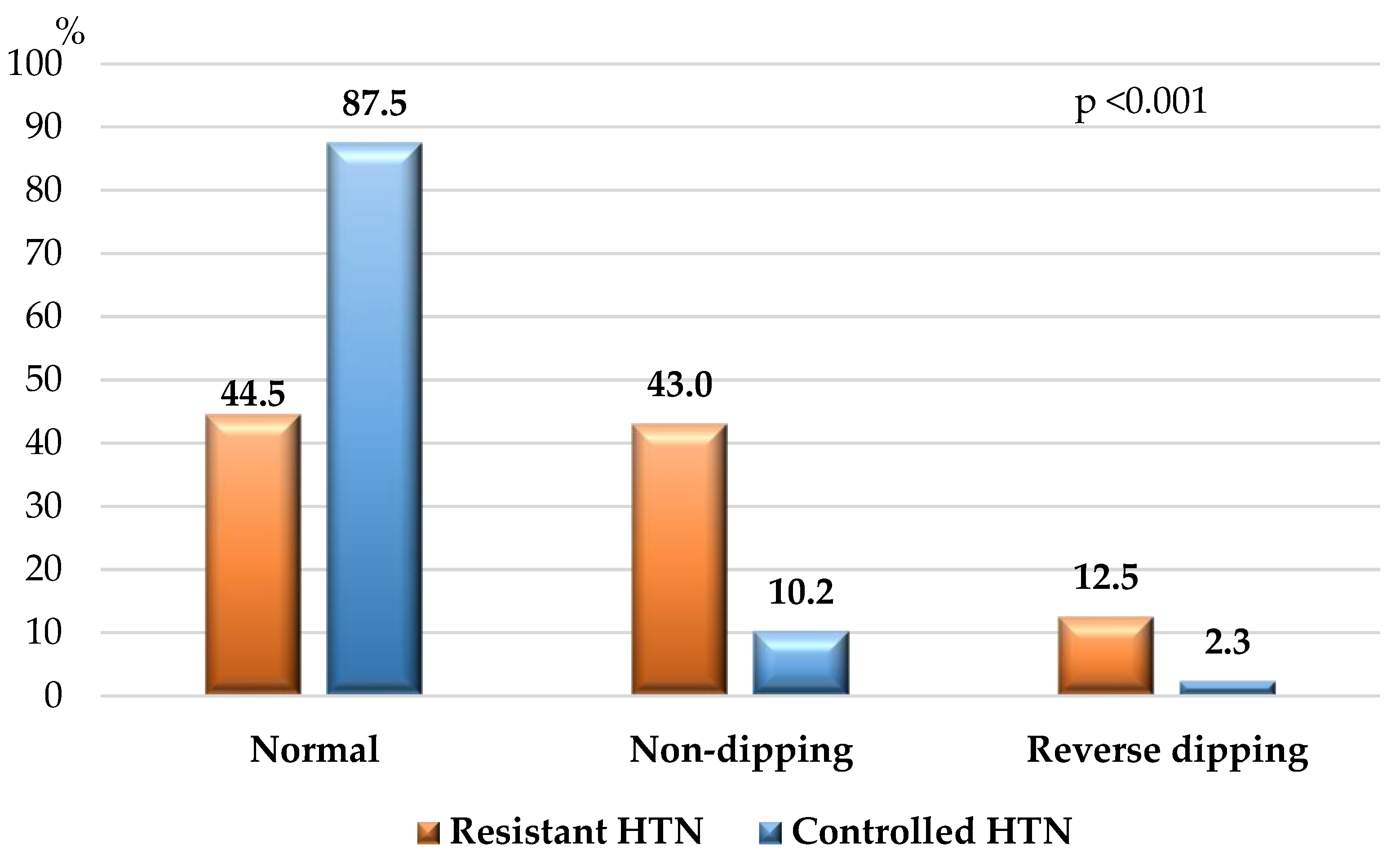
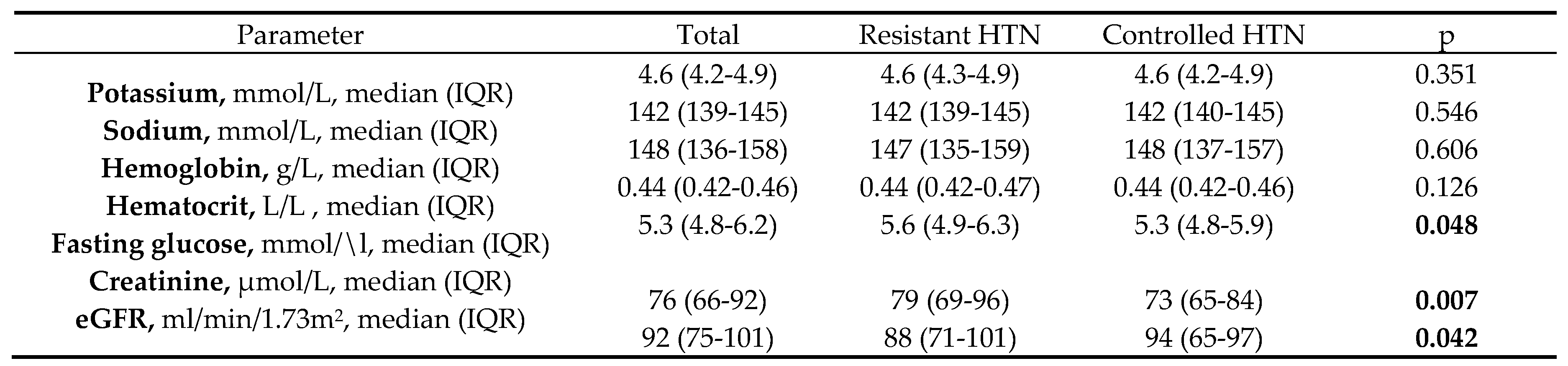
3.2. Laboratory and Instrumental Investigations
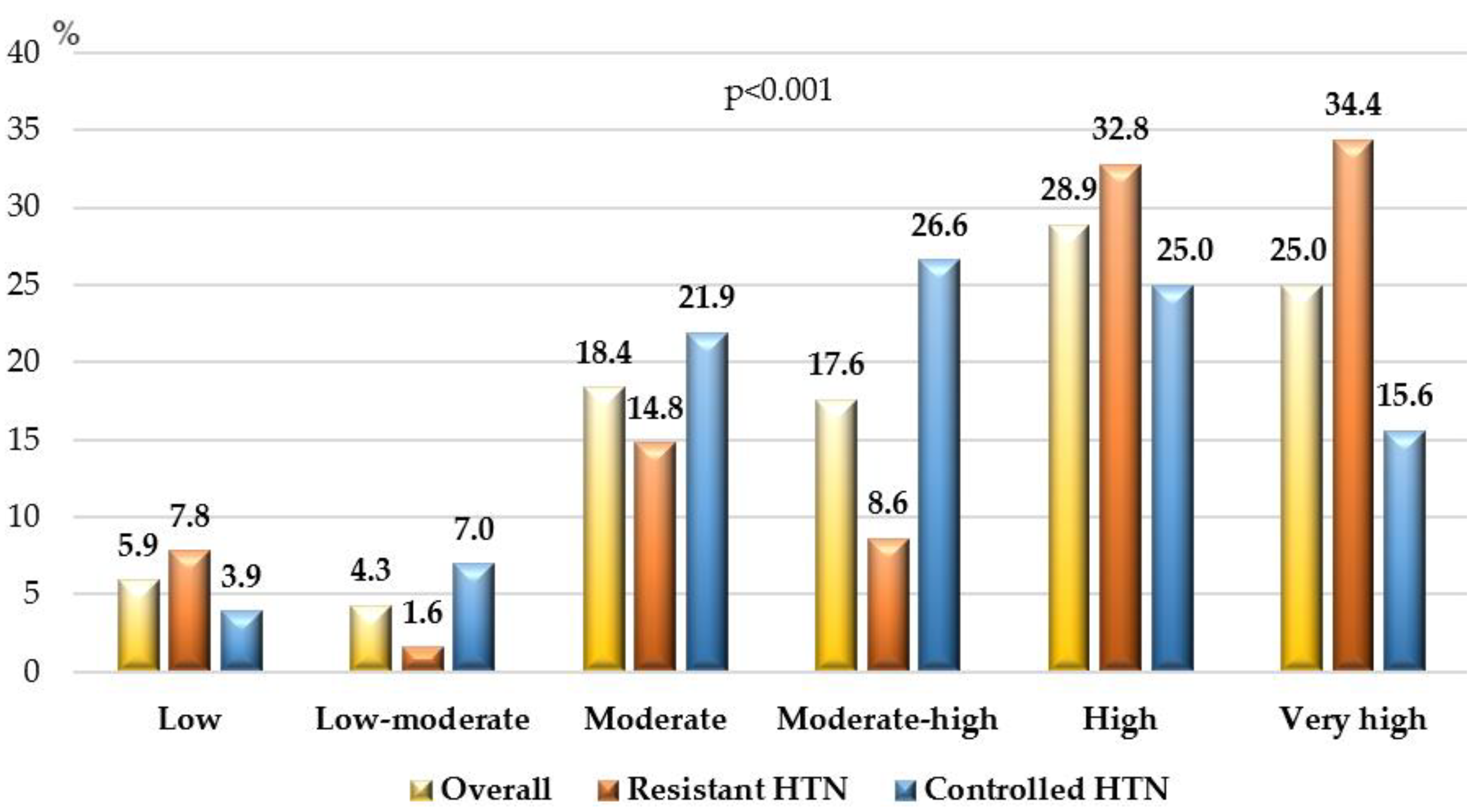
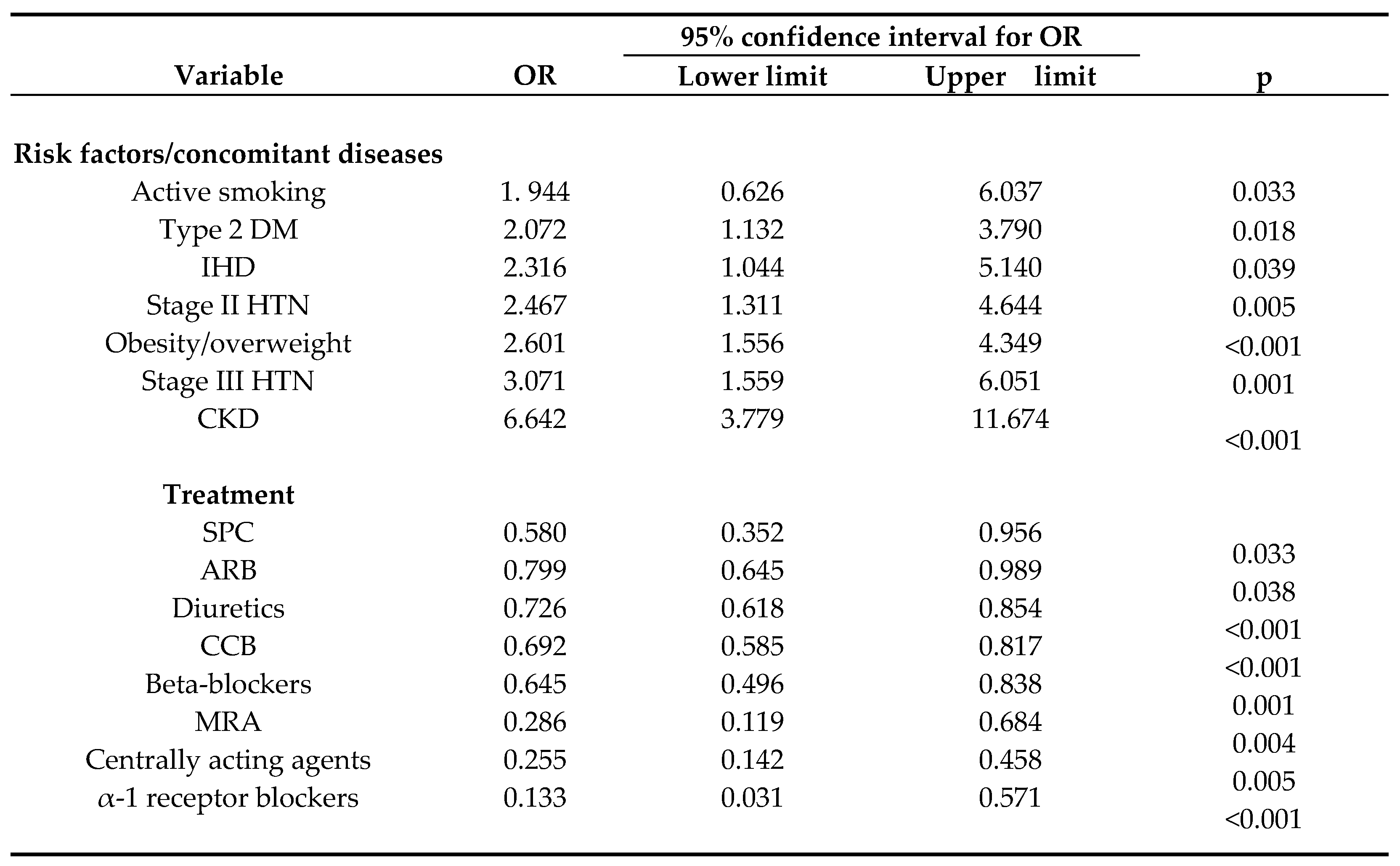
3.3. Risk Profile of the Study Population
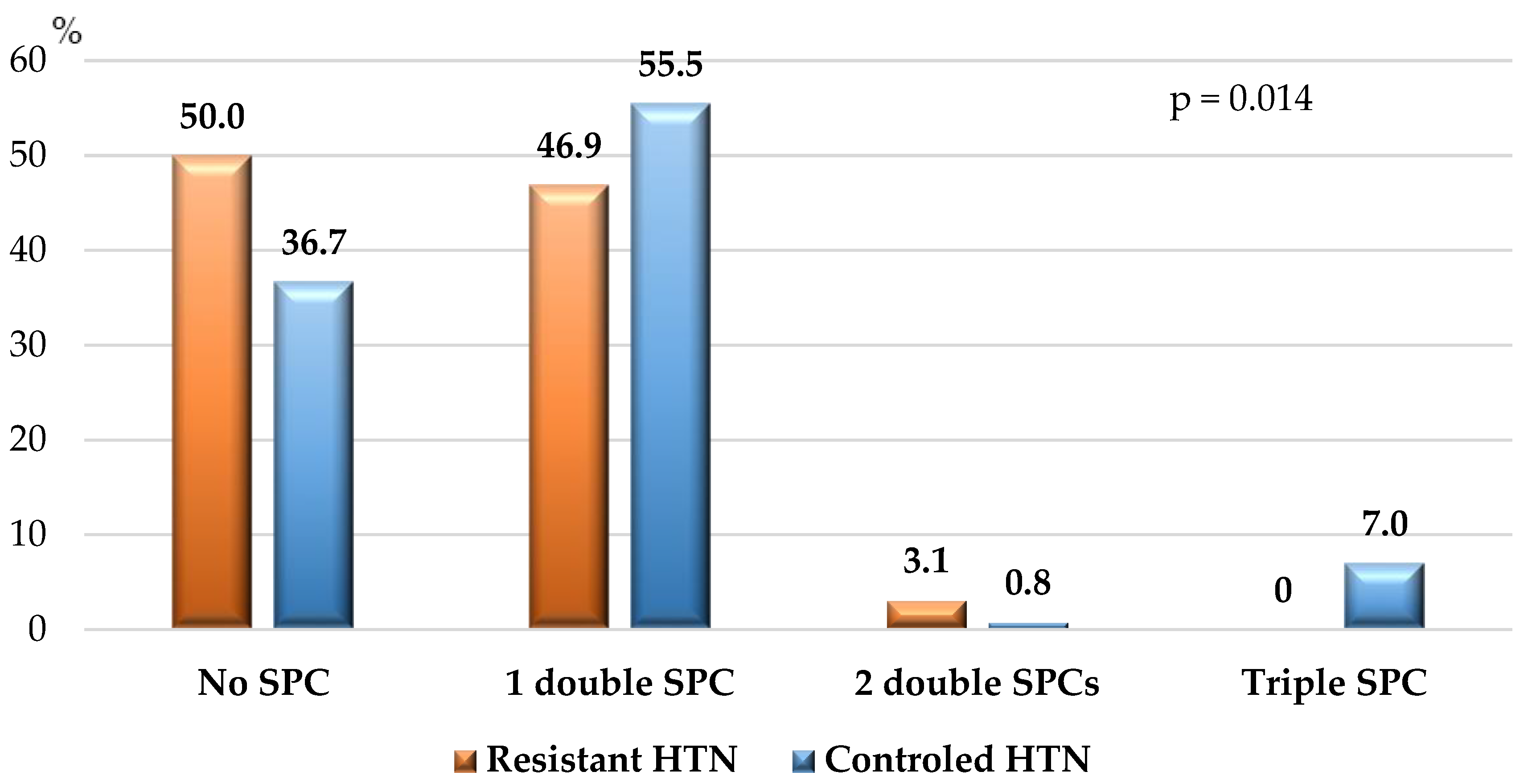
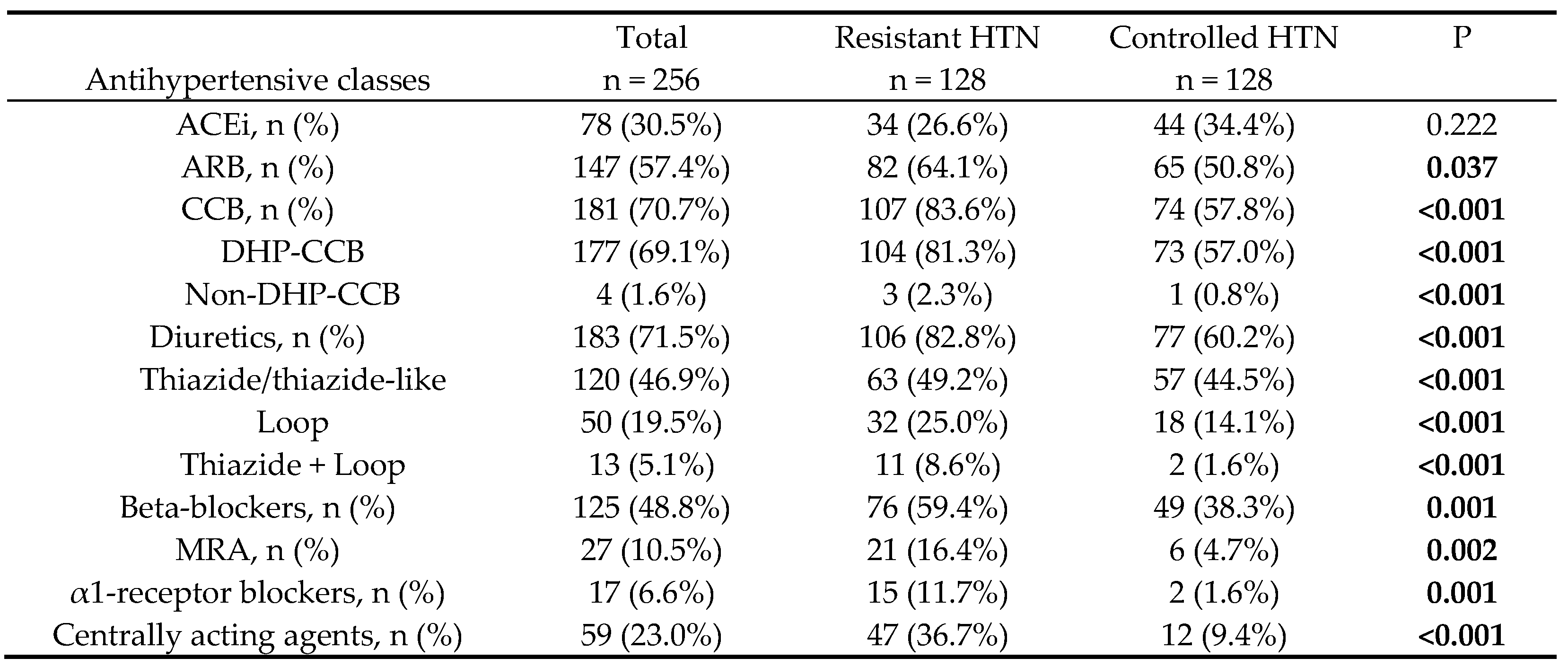
3.4. Treatment of the Study Population
- Perindopril in 34 (43.6%) of 78 patients on ACEi: 15 (44.1%) of 34 patients with resistant HTN and 19 (43.2%) of 44 patients with controlled HTN, p = 0.637;
- Valsartan in 87 (59.2%) of 147 patients on ARB: 54 (65.9%) of 82 patients with resistant HTN and 33 (50.8%) of 65 patients with controlled HTN, p = 0.072;
- Amlodipine in 80 (44.2%) of 181 patients on CCB: 37 (34.6%) of 107 patients with resistant HTN and 43 (58.1%) of 74 patients with controlled HTN, p < 0.001.
- Hydrochlorothiazide in 90 (49.7%) of 181 patients on diuretics: 45 (42.5%) of 106 patients with resistant HTN and 45 (58.4%) of 77 patients with controlled HTN, p = 0.001;
- Bisoprolol in 82 (65.6%) of 125 patients on beta-blockers: 50 (65.8%) of 76 patients with resistant HTN and 32 (65.3%) of 49 patients with controlled HTN, p = 0.537;
- Spironolactone in 23 (85.2%) of 27 patients on MRAs: 17 (80.9%) of 21 patients with resistant HTN and 6 (100.0%) of 6 patients with controlled HTN, p = 0.006
- Doxazosin in 11 (64.7%) of 17 patients on α1-receptor blockers: 10 (66.7%) of 15 patients with resistant HTN and 1 (50.0%) of 2 patients with controlled HTN, p = 0.012;
- Moxonidine in 52 (88.1%) of 59 patients on centrally acting agents: 40 (85.1%) of 47 patients with resistant HTN and 12 (100.0%) of 12 patients with controlled HTN, p <0.001.
- Oral anticoagulants – in 34 (13.3%) of 256 patients (mostly direct oral anticoagulants - 33 of 256, 12.9%): 21 (16.4%) of patients with resistant and 13 (10.2%) with controlled HTN, p = 0.250;
- Antiplatelet drugs – in 20 (7.9%) of 256 patients: 12 (9.4%) with resistant HTN and 8 (6.3%) with controlled HTN, p = 0.381;
- Lipid-lowering drugs – in 68 (26.6%) of 256 patients (mostly a statin - 61 of 256, 23.8%): 41 (32.0%) with resistant and 27 (21.1%) with controlled HTN, p = 0.045;
- Sodium-glucose cotransporter-2 (SGLT2) inhibitors – in 17 (6.6%) of 256 patients: 8 (6.2%) of patients with resistant and 9 (7.0%) with controlled HTN, p = 0.761.
3.5. Impact of Concomitant Risk Factors/Diseases and Drug Treatment on HTN Control
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D. L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Cardiology and the European Society of Hypertension. Journal of Hypertension 2018, 36, 1953–2041. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M. L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E. A. E.; et al. 2023 ESH Guidelines for the Management of Arterial Hypertension The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). Journal of Hypertension 2023, 41, 1874–2071. [Google Scholar] [CrossRef] [PubMed]
- Parasher, A.; Jhamb, R. Resistant Hypertension: A Review. Int J Adv Med 2021, 8(9), 1433. [Google Scholar] [CrossRef]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N. A.; Poulter, N. R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G. S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef] [PubMed]
- Hypertension, Third edition. ; Nadar, S., Lip, G. Y. H., Eds.; Oxford cardiology library; Oxford University Press: Oxford, United Kingdom ; New York, NY, 2023. [Google Scholar]
- Matanes, F.; Khan, M. B.; Siddiqui, M.; Dudenbostel, T.; Calhoun, D.; Oparil, S. An Update on Refractory Hypertension. Curr Hypertens Rep 2022, 24, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.; Dubourg, J.; Blanchard, A.; Bergerot, D.; Courand, P.-Y.; Forni, V.; Frank, M.; Bobrie, G.; Menard, J.; Azizi, M. Copeptin Is Increased in Resistant Hypertension. Journal of Hypertension 2016, 34, 2458–2464. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, D. A. Hyperaldosteronism as a Common Cause of Resistant Hypertension. Annu. Rev. Med. 2013, 64, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Seravalle, G.; Brambilla, G.; Pini, C.; Alimento, M.; Facchetti, R.; Spaziani, D.; Cuspidi, C.; Mancia, G. Marked Sympathetic Activation and Baroreflex Dysfunction in True Resistant Hypertension. International Journal of Cardiology 2014, 177, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Velasco, A.; Siddiqui, M.; Kreps, E.; Kolakalapudi, P.; Dudenbostel, T.; Arora, G.; Judd, E. K.; Prabhu, S. D.; Lloyd, S. G.; Oparil, S.; Calhoun, D. A. Refractory Hypertension Is Not Attributable to Intravascular Fluid Retention as Determined by Intracardiac Volumes. Hypertension 2018, 72, 343–349. [Google Scholar] [CrossRef]
- Dudenbostel, T.; Acelajado, M. C.; Pisoni, R.; Li, P.; Oparil, S.; Calhoun, D. A. Refractory Hypertension: Evidence of Heightened Sympathetic Activity as a Cause of Antihypertensive Treatment Failure. Hypertension 2015, 66, 126–133. [Google Scholar] [CrossRef]
- Visseren, F. L. J.; Mach, F.; Smulders, Y. M.; Carballo, D.; Koskinas, K. C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice. European Heart Journal 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Charles, K.; Lewis, M. J.; Montgomery, E.; Reid, M. The 2021 Chronic Kidney Disease Epidemiology Collaboration Race-Free Estimated Glomerular Filtration Rate Equations in Kidney Disease: Leading the Way in Ending Disparities. Health Equity 2024, 8, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Noubiap, J. J.; Nansseu, J. R.; Nyaga, U. F.; Sime, P. S.; Francis, I.; Bigna, J. J. Global Prevalence of Resistant Hypertension: A Meta-Analysis of Data from 3.2 Million Patients. Heart 2019, 105, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Sinnott, S.-J.; Smeeth, L.; Williamson, E.; Douglas, I. J. Trends for Prevalence and Incidence of Resistant Hypertension: Population Based Cohort Study in the UK 1995-2015. BMJ 2017, j3984. [Google Scholar] [CrossRef] [PubMed]
- Koracevic, G.; Micic, S.; Stojanovic, M.; Zdravkovic, M. A Need for Improvement in the Definition of Resistant Arterial Hypertension. Medicina 2023, 59(4), 803. [Google Scholar] [CrossRef] [PubMed]
- Banga, S.; Mungee, S.; Patel, A. R.; Singh, S.; Kizhakekuttu, T. J. Management of Resistant Hypertension Based on Recommendations from Different Guidelines and the Systolic Blood Pressure Intervention Trial. Cureus 2019. [Google Scholar] [CrossRef] [PubMed]
- Carey, R. M.; Calhoun, D. A.; Bakris, G. L.; Brook, R. D.; Daugherty, S. L.; Dennison-Himmelfarb, C. R.; Egan, B. M.; Flack, J. M.; Gidding, S. S.; Judd, E.; et al. Resistant Hypertension: Detection, Evaluation, and Management: A Scientific Statement From the American Heart Association. Hypertension 2018, 72(5). [Google Scholar] [CrossRef]
- Georgianos, P. I.; Agarwal, R. Hypertension in Chronic Kidney Disease (CKD): Diagnosis, Classification, and Therapeutic Targets. American Journal of Hypertension 2021, 34, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Frąk, W.; Wojtasińska, A.; Lisińska, W.; Młynarska, E.; Franczyk, B.; Rysz, J. Pathophysiology of Cardiovascular Diseases: New Insights into Molecular Mechanisms of Atherosclerosis, Arterial Hypertension, and Coronary Artery Disease. Biomedicines 2022, 10(8), 1938. [Google Scholar] [CrossRef]
- Weitzman, D.; Chodick, G.; Shalev, V.; Grossman, C.; Grossman, E. Prevalence and Factors Associated With Resistant Hypertension in a Large Health Maintenance Organization in Israel. Hypertension 2014, 64, 501–507. [Google Scholar] [CrossRef]
- Mahapatra, R.; Kaliyappan, A.; Chinnakali, P.; Hanumanthappa, N.; Govindarajalou, R.; Bammigatti, C. Prevalence and Risk Factors for Resistant Hypertension: Cross-Sectional Study From a Tertiary Care Referral Hospital in South India. Cureus 2021. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, D. M.; Kilfiger, H.; Apan, B.; Roman, G.; Veresiu, I. A. RESISTANT HYPERTENSION IN TYPE 2 DIABETES: PREVALENCE AND PATIENT CHARACTERISTICS. Medicine and Pharmacy Reports 2015, 88, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, A.; Abujbara, M.; Alshraideh, J.; Jaddou, H.; Ajlouni, K. The Prevalence of Resistant Hypertension Among Type 2 Diabetic Patients Attending the National Center for Diabetes, Endocrinology, and Genetics. J Endocrinol Metab 2017, 7, 153–158. [Google Scholar] [CrossRef]
- Smith, S. M.; Gong, Y.; Handberg, E.; Messerli, F. H.; Bakris, G. L.; Ahmed, A.; Bavry, A. A.; Pepine, C. J.; Cooper-DeHoff, R. M. Predictors and Outcomes of Resistant Hypertension among Patients with Coronary Artery Disease and Hypertension. Journal of Hypertension 2014, 32, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Cai, A.; Calhoun, D. A. Resistant Hypertension: An Update of Experimental and Clinical Findings. Hypertension 2017, 70, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; MacDonald, T. M.; Morant, S.; Webb, D. J.; Sever, P.; McInnes, G.; Ford, I.; Cruickshank, J. K.; Caulfield, M. J.; Salsbury, J.; et al. Spironolactone versus Placebo, Bisoprolol, and Doxazosin to Determine the Optimal Treatment for Drug-Resistant Hypertension (PATHWAY-2): A Randomised, Double-Blind, Crossover Trial. The Lancet 2015, 386, 2059–2068. [Google Scholar] [CrossRef] [PubMed]
- Smith, S. M.; Gurka, M. J.; Winterstein, A. G.; Pepine, C. J.; Cooper-DeHoff, R. M. Incidence, Prevalence, and Predictors of Treatment-resistant Hypertension with Intensive Blood Pressure Lowering. J of Clinical Hypertension 2019, 21, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Bendzala, M.; Kruzliak, P.; Gaspar, L.; Soucek, M.; Mrdovic, I.; Sabaka, P.; Dukat, A.; Gasparova, I.; Malan, L.; Takazawa, K. Prognostic Significance of Dipping in Older Hypertensive Patients. Blood Pressure 2015, 24, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Ingabire, P. M.; Ojji, D. B.; Rayner, B.; Ogola, E.; Damasceno, A.; Jones, E.; Dzudie, A.; Ogah, O. S.; Poulter, N.; Sani, M. U.; et al. High Prevalence of Non-Dipping Patterns among Black Africans with Uncontrolled Hypertension: A Secondary Analysis of the CREOLE Trial. BMC Cardiovasc Disord 2021, 21, 254. [Google Scholar] [CrossRef]
- Joo, H. J.; Yum, Y.; Kim, Y. H.; Son, J.-W.; Kim, S. H.; Choi, S.; Han, S.; Shin, M.-S.; Jeong, J.-O.; Kim, E. J.; Working Group on Hypertension Complication. Gender Difference of Blood Pressure Control Rate and Clinical Prognosis in Patients With Resistant Hypertension: Real-World Observation Study. J Korean Med Sci 2023, 38, e124. [Google Scholar] [CrossRef]
- Daugherty, S. L.; Powers, J. D.; Magid, D. J.; Tavel, H. M.; Masoudi, F. A.; Margolis, K. L.; O’Connor, P. J.; Selby, J. V.; Ho, P. M. Incidence and Prognosis of Resistant Hypertension in Hypertensive Patients. Circulation 2012, 125, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Mosca, L.; Barrett-Connor, E.; Kass Wenger, N. Sex/Gender Differences in Cardiovascular Disease Prevention: What a Difference a Decade Makes. Circulation 2011, 124, 2145–2154. [Google Scholar] [CrossRef]
- Brant, L. C. C.; Passaglia, L. G.; Pinto-Filho, M. M.; De Castilho, F. M.; Ribeiro, A. L. P.; Nascimento, B. R. The Burden of Resistant Hypertension Across the World. Curr Hypertens Rep 2022, 24, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Kollias, A.; Foukarakis, E.; Karakousis, K.; The HYPEDIA Study Group; Adamopoulos, E. ; Afaras, G.; Aggelopoulos, G.; Alexandropoulos, T.; Alexiadis, S.; Alexoudis, A. et al.; Implementation of the 2018 ESC/ESH Guidelines for the Management of Hypertension in Primary Care: The HYPEDIA Study. J Hum Hypertens 2022, 37, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, A.; Drame, K.; Geutjens, S.; Airaksinen, M. Does the Polypill Improve Patient Adherence Compared to Its Individual Formulations? A Systematic Review. Pharmaceutics 2020, 12, 190. [Google Scholar] [CrossRef] [PubMed]
- Tsioufis, K.; Kreutz, R.; Sykara, G.; Van Vugt, J.; Hassan, T. Impact of Single-Pill Combination Therapy on Adherence, Blood Pressure Control, and Clinical Outcomes: A Rapid Evidence Assessment of Recent Literature. Journal of Hypertension 2020, 38, 1016–1028. [Google Scholar] [CrossRef] [PubMed]
- Corrao, G.; Parodi, A.; Zambon, A.; Heiman, F.; Filippi, A.; Cricelli, C.; Merlino, L.; Mancia, G. Reduced Discontinuation of Antihypertensive Treatment by Two-Drug Combination as First Step. Evidence from Daily Life Practice. Journal of Hypertension 2010, 28, 1584–1590. [Google Scholar] [CrossRef] [PubMed]
- Izzo Jr, J. L.; Weir, M. R. Angiotensin-Converting Enzyme Inhibitors: Angiotensin-Converting Enzyme Inhibitors. The Journal of Clinical Hypertension 2011, 13, 667–675. [Google Scholar] [CrossRef]
- Telmisartan, Ramipril, or Both in Patients at High Risk for Vascular Events. N Engl J Med 2008, 358, 1547–1559. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





