Submitted:
22 July 2024
Posted:
24 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
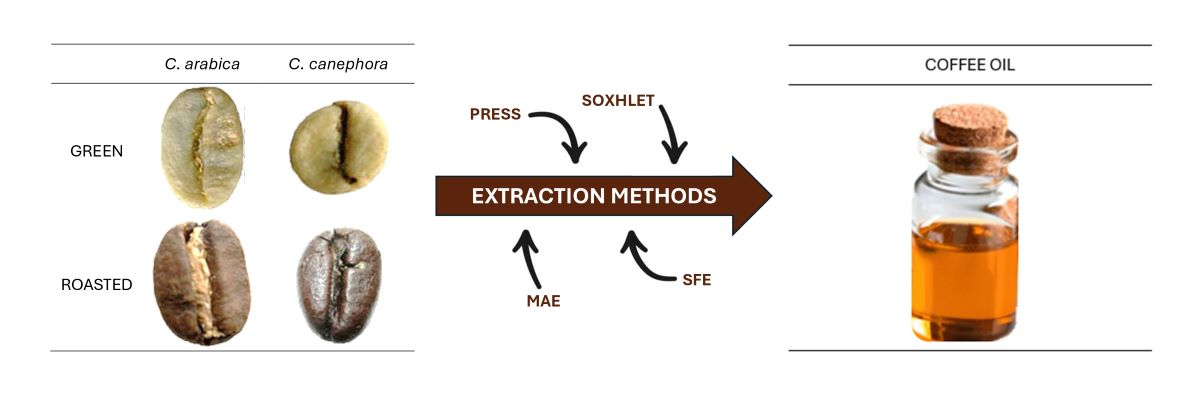
2. Green and Roasted Coffee Oils
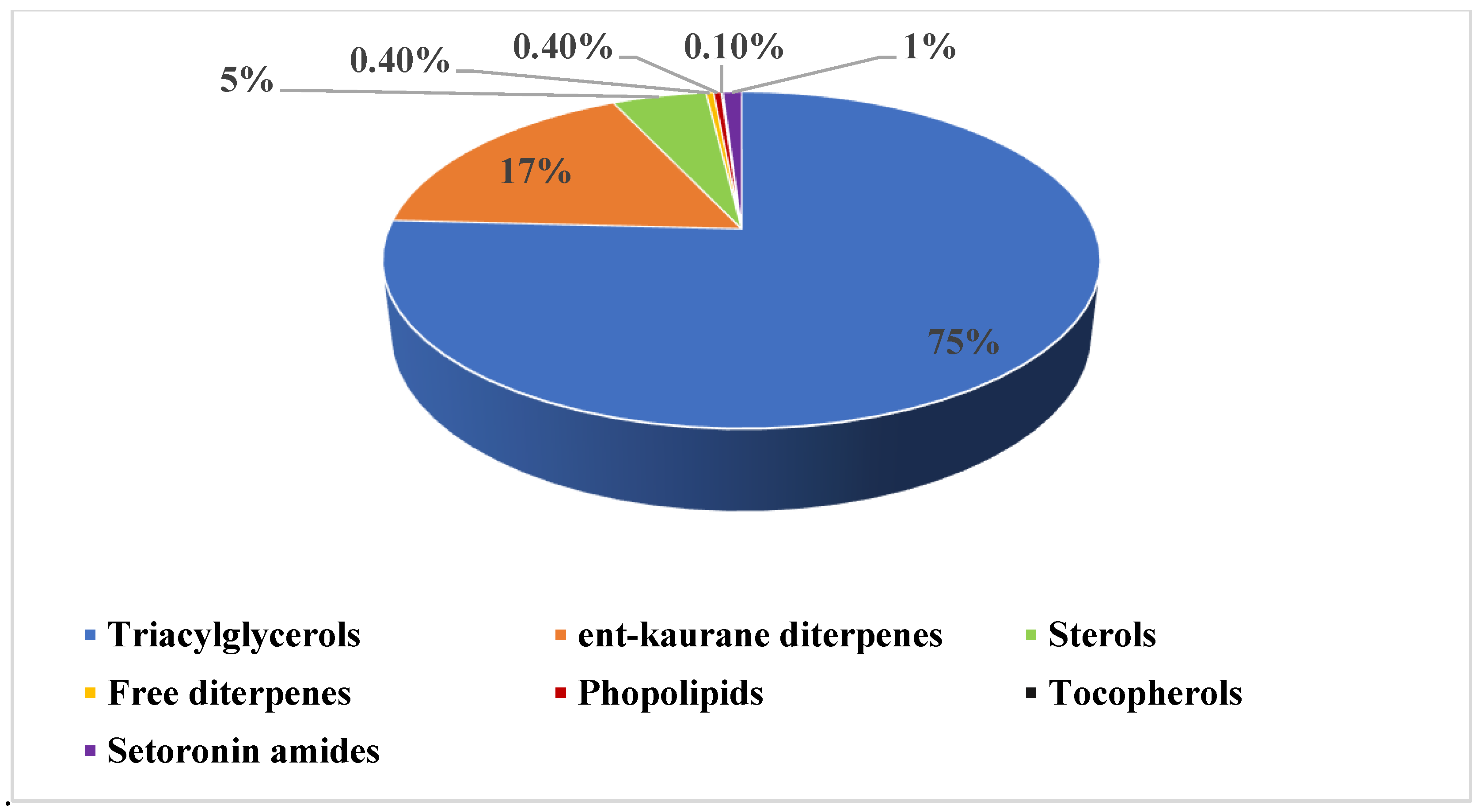
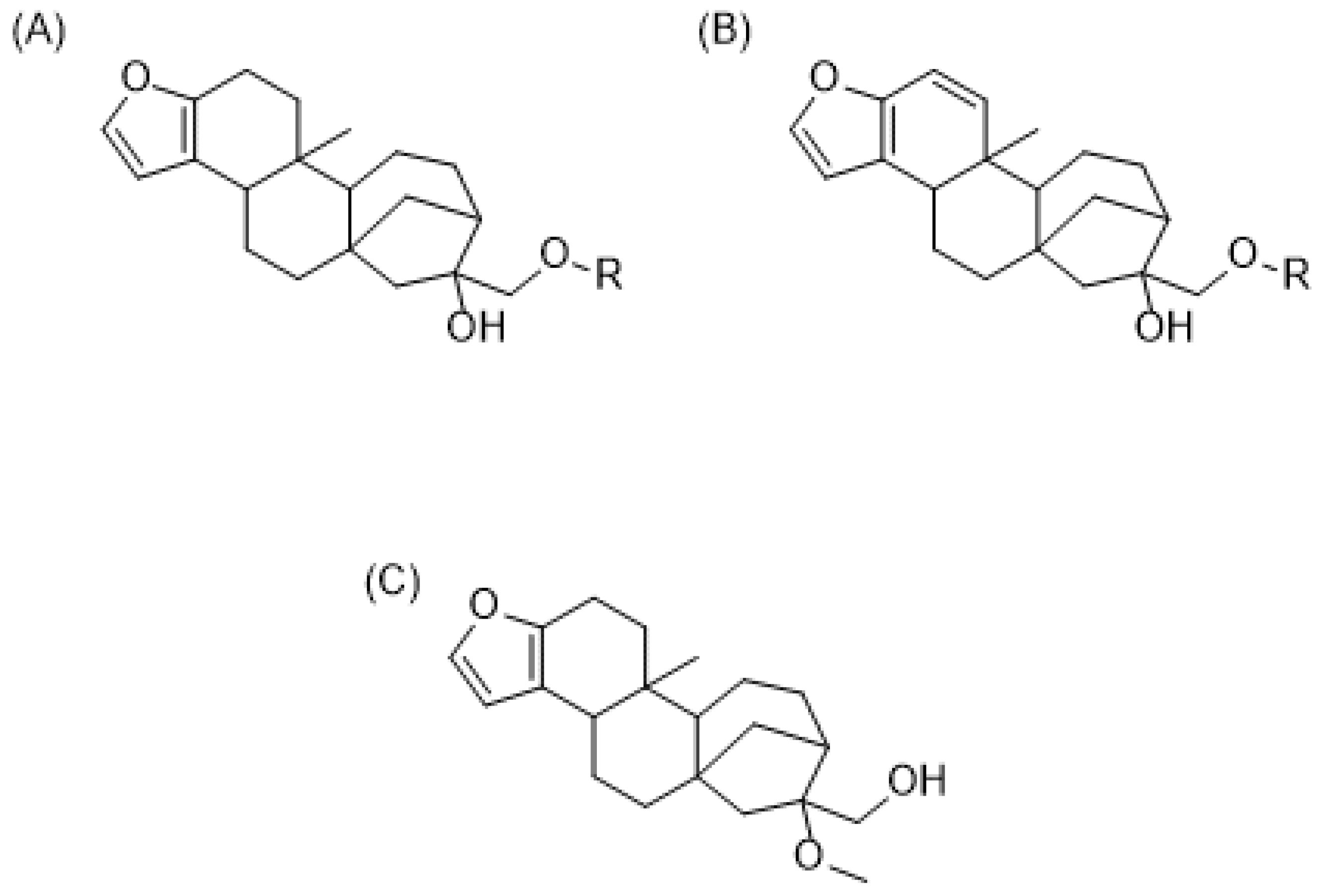
2.1. Chemical Composition of Green Coffee Oil
2.2. Chemical Composition of Roasted Coffee Oil
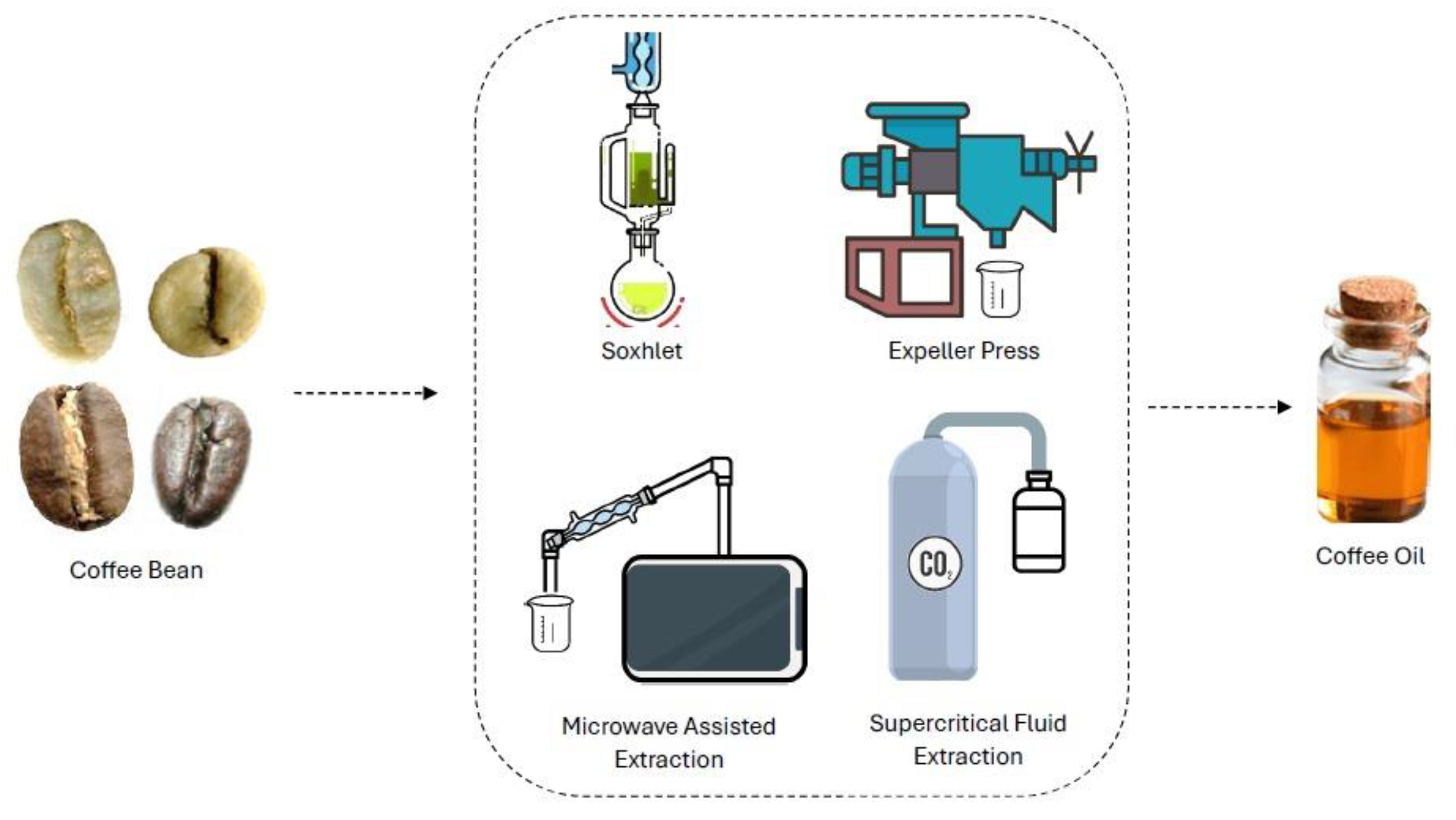
3. Coffee Oil Extraction Methods
3.1. Mechanical Extraction
3.1.1. Hydraulic Press
3.1.2. Expeller Press
3.2. Soxhlet Extraction and Its Comparison to Supercritical Fluid and Microwave Extraction Techniques
3.4. Supercritical Fluid Extraction (SFE)
3.5. Other Techniques
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- 1. U.S. Departament of Agriculture (USDA). Accessed July 16, 2024. https://fas.usda.gov/data/coffee-world-markets-and-trade-06202024.
- Clarke, R.J.; Macrae, R. Coffee: volume 1: Chemistry. Elsevier Applied Science, 1985. [Google Scholar]
- Gunning, Y.; Defernez, M.; Watson, A.D.; Beadman, N.; Colquhoun, I.J.; Le Gall, G.; Philo, M.; Garwood, H.; Williamson, D.; Davis, A.P.; Kemsley, E.K. 16-O-methylcafestol is present in ground roast Arabica coffees: Implications for authenticity testing. Food Chem. 2018, 248, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Kurzrock, T.; Speer, K. Diterpenes and diterpene esters in coffee. Food Rev Int. 2001, 17, 433–450. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, C.; Xu, J.; Wang, S. Cafestol and kahweol: A review on their bioactivities and pharmacological properties. Int J Mol Sci. 2019, 20, 4238. [Google Scholar] [CrossRef] [PubMed]
- Speer, K.; Kölling-Speer, I. The lipid fraction of the coffee bean. Brazilian J Plant Physiol. 2006, 18, 201–216. [Google Scholar] [CrossRef]
- Folstar, P. Lipids. In Coffee; Clarke, R.J, Macrae, R., Eds.; Elsevier Applied Science, 1985; pp. 203–222. [Google Scholar]
- Hall, R.D.; Trevisan, F.; de Vos, R.C.H. Coffee berry and green bean chemistry – Opportunities for improving cup quality and crop circularity. Food Res Int. 2022, 151, 110825. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M. de S.G.; Scholz, M.B.S.; Kitzberger, C.S.G.; Benassi, M.T. Correlation between the composition of green Arabica coffee beans and the sensory quality of coffee brews. Food Chem. 2019, 292, 275–280. [CrossRef] [PubMed]
- Caporaso, N.; Whitworth, M.B.; Cui, C.; Fisk, I.D. Variability of single bean coffee volatile compounds of Arabica and robusta roasted coffees analysed by SPME-GC-MS. Food Res Int. 2018, 108, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.J.; Jeong, H.J.; Moon, H.; Kim, H.N. Assessment of green coffee bean metabolites dependent on coffee quality using a 1H NMR-based metabolomics approach. Food Res Int. 2015, 67, 175–182. [Google Scholar] [CrossRef]
- Turatti, M. Extração e caracterização de óleo de café. In II simpósio Pesquisa dos cafés do Brasil; Vitória, 2001; pp. 1533–1539. [Google Scholar]
- Zulqarnain, Ayoub M.; Yusoff, M.H.M.; Nazir, M.H.; Zahid, I.; Ameen, M.; Sher, F.; Floresyona, D.; Budi Nursanto, E. A. A comprehensive review on oil extraction and biodiesel production technologies. Sustainability 2021, 13, 1–28.
- Mwaurah, P.W.; Kumar, S.; Kumar, N.; Attkan, A.K.; Panghal, A.; Singh, V.K.; Garg, M.K. Novel oil extraction technologies: Process conditions, quality parameters, and optimization. Compr Rev Food Sci Food Saf. 2020, 19, 3–20. [Google Scholar] [CrossRef]
- Restrepo-Serna, D.L.; Cardona, A.C.A. Economic pre-feasibility of supercritical fluid extraction of antioxidants from fruit residues. Sustain Chem Pharm. 2022, 25, 100600. [Google Scholar] [CrossRef]
- Guerra, A.F.; Santos, J.F.; Ferreira, L.T.; Rocha, O.C. Land-saving technologies. In: Coffees of Brazil. Research, sustainability and innovation. Telhado SFP, Capdeville G de, eds. Consórcio Pesquisa Café. Embrapa. 2021; 62–74. [Google Scholar]
- Speer, K.; Koölling-Speer, I. Chemistry I: Non-Volatile Compounds. In Coffee; Wiley, 2001; pp. 33–49. [Google Scholar]
- Boekschoten, M.V.; Hofman, M.K.; Buytenhek, R.; Schouten, E.G.; Princen, H. .MG.; Katan, M.B. Coffee oil consumption increases plasma levels of 7α-hydroxy-4- cholesten-3-one in humans. J Nutr. 2005, 135, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Rubach, M.; Lang, R.; Seebach, E.; Somoza, M.M.; Hofmann, T.; Somoza, V. Multi-parametric approach to identify coffee components that regulate mechanisms of gastric acid secretion. Mol Nutr Food Res. 2012, 56, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Tsukui, A.; Oigman, S.S.; Rezende, C.M. Oil green coffee beans: Diterpenes cafestol and kahweol. Rev Virtual Quim. 2014, 6, 16–33. [Google Scholar] [CrossRef]
- Brand, A.L.M.; Silva, A.C.R.; Garrett, R.; Rezende, C.M. Quantitative lipidomics in green robusta coffees from the Brazilian Amazon by LC-HRMS. Food Biosci. 2024, 57, 103472. [Google Scholar] [CrossRef]
- Wermelinger, T.; D’Ambrosio, L.; Klopprogge, B.; Yeretzian, C. Quantification of the robusta fraction in a coffee blend via raman spectroscopy: Proof of principle. J Agric Food Chem. 2011, 59, 9074–9079. [Google Scholar] [CrossRef]
- Moeenfard, M.; Alves, A. New trends in coffee diterpenes research from technological to health aspects. Food Res Int. 2020, 134, 109207. [Google Scholar] [CrossRef]
- Silva, M.A.E.; Brand, A.L.M.; Novaes, F..JM.; Rezende, C.M. Cafestol, Kahweol and Their Acylated Derivatives: Antitumor Potential, Pharmacokinetics, and Chemopreventive Profile. Food Rev Int. 2023, 39, 7048–7080. [Google Scholar] [CrossRef]
- Kusumah, J.; Gonzalez de Mejia, E. Coffee constituents with antiadipogenic and antidiabetic potentials: A narrative review. Food Chem Toxicol. 2022, 161, 112821. [Google Scholar] [CrossRef]
- Eldesouki, S.; Qadri, R.; Abu Helwa, R.; Barqawi, H.; Bustanji, Y.; Abu-Gharbieh, E.; El-Huneidi, W. Recent Updates on the Functional Impact of Kahweol and Cafestol on Cancer. Molecules 2022, 27, 1–15. [Google Scholar] [CrossRef]
- Thelle, D.S.; Arnesen, E.; Førde, O.H. The Tromsø Heart Study. N Engl J Med. 1983, 308, 1454–1457. [Google Scholar] [CrossRef] [PubMed]
- Urgert, R.; van der Weg, G.; Kosmeijer-Schuil, T.G.; van de Bovenkamp, P.; Hovenier, R.; Katan, M.B. Levels of the Cholesterol-Elevating Diterpenes Cafestol and Kahweol in Various Coffee Brews. J Agric Food Chem. 1995, 43, 2167–2172. [Google Scholar] [CrossRef]
- Weusten-Van der Wouw, M.P.M.E.; Katan, M.B.; Viani, R.; Huggett, A.C.; Liardon, R.; Lund-Larsen, P.G.; Thelle, D.S.; Ahola, I.; Aro, A. Identity of the cholesterol-raising factor from boiled coffee and its effects on liver function enzymes. J Lipid Res. 1994, 35, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Novaes, F.J.M.; Oigman, S.S.; De Souza, R.O.M.A.; Rezende, C.M.; De Aquino Neto, F.R. New approaches on the analyses of thermolabile coffee diterpenes by gas chromatography and its relationship with cup quality. Talanta. 2015, 139, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Novaes, F.J.M.; Kulsing, C.; Bizzo, H.R.; De Aquino Neto, F.R.; Rezende, C.M.; Marriott, P.J. Analysis of underivatised low volatility compounds by comprehensive two-dimensional gas chromatography with a short primary column. J Chromatogr A. 2018, 1536, 75–81. [Google Scholar] [CrossRef] [PubMed]
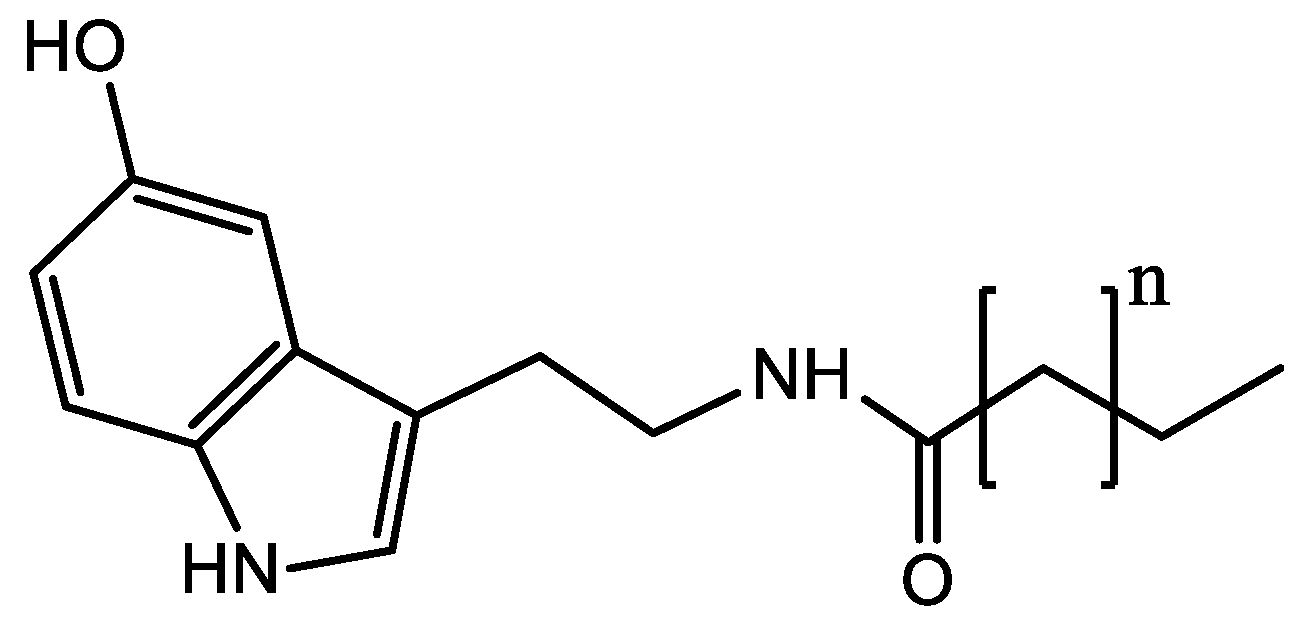
- Brand, A.L.M.; Lima, F.A.; Tinoco, N.A.B.; Mota, J.C.; Moreira, I.G.S.; Novaes, F.J.M.; Garrett, R.; Giorno, I.B.S.; Fernandes, P.D.; Rezende, C.M. βN-Alkanoyl-5-Hydroxytryptamines (Cn-5HTs) in Coffee: A Review. Food Rev Int. 2022, 39, 4761–4780. [Google Scholar] [CrossRef]
- Itoh, T.; Tamura, T.; Matsumoto, T. Sterol composition of 19 vegetable oils. J Am Oil Chem Soc. 1973, 50, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Valdenebro, M.S.; León-Camacho, M.; Pablos, F.; González, A.G.; Martín, M.J. Determination of the arabica/robusta composition of roasted coffee according to their sterolic content. Analyst. 1999, 124, 999–1002. [Google Scholar]
- Basurto-Islas, G.; Blanchard, J.; Tung, Y.C.; Fernandez, J.R.; Voronkov, M.; Stock, M.; Zhang, S.; Stock, J.B.; Iqbal, K. Therapeutic benefits of a component of coffee in a rat model of Alzheimer’s disease. Neurobiol Aging. 2014, 35, 2701–2712. [Google Scholar] [CrossRef]
- Giorno, T.B.S.; Brand, A.L.M.; Lima, F.A.; de Oliveira, C.M.; Rezende, C.M.; Fernandes, P.D. Characterization of βN-octadecanoyl-5-hydroxytryptamide anti-inflammatory effect. Molecules. 2021, 26, 1–13. [Google Scholar] [CrossRef]
- Lee, K.W.; Im, J.Y.; Woo, J.M.; Grosso, H.; Kim, Y.S.; Cristovao, A.C.; Sonsalla, P.K.; Schuster, D.S.; Jalbut, M.M.; Fernandez, J.R.; Voronkov, M.; Junn, E.; Braithwaite, S.P.; Stock, J.B.; Mouradian, M.M. Neuroprotective and Anti-inflammatory Properties of a Coffee Component in the MPTP Model of Parkinson’s Disease. Neurotherapeutics. 2013, 10, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.; Bardelmeier, I.; Weiss, C.; Rubach, M.; Somoza, V.; Hofmann, T. Quantitation of βN-alkanoyl-5-hydroxytryptamides in coffee by means of LC-MS/MS-SIDA and assessment of their gastric acid secretion potential using the HGT-1 cell assay. J Agric Food Chem. 2010, 58, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Rubach, M.; Lang, R.; Skupin, C.; Hofmann, T.; Somoza, V. Activity-guided fractionation to characterize a coffee beverage that effectively down-regulates mechanisms of gastric acid secretion as compared to regular coffee. J Agric Food Chem. 2010, 58, 4153–4161. [Google Scholar] [CrossRef] [PubMed]
- Tinoco, N.A.B.; Pacheco, S.; Godoy, R.L.O.; Bizzo, H.R.; de Aguiar, P.F.; Leite, S.G.F.; Rezende, C.M. Reduction of βN-alkanoyl-5-hydroxytryptamides and diterpenes by yeast supplementation to green coffee during wet processing. Food Res Int. 2019, 115, 487–492. [Google Scholar] [CrossRef]
- Bosso, H.; Barbalho, S.M.; de Alvares Goulart, R.; Otoboni, A.M.M.B. Green coffee: economic relevance and a systematic review of the effects on human health. Crit Rev Food Sci Nutr. 2023, 63, 394–410. [Google Scholar] [CrossRef] [PubMed]
- Del Carmen Velazquez Pereda, M.; de Campos Dieamant, G.; Eberlin, S.; Nogueira, C.; Colombi, D.; Di Stasi, L.C.; de Souza Queiroz, M.L. Effect of green Coffea arabica L. seed oil on extracellular matrix components and water-channel expression in in vitro and ex vivo human skin models. J Cosmet Dermatol 2009, 8, 56–62. [Google Scholar] [CrossRef]
- Wagemaker, T.A.L.; Fernandes, A.S.; Maia Campos, P.; Monteiro Rodrigues, L.; Rijo, P. Evaluation of antioxidant and antimicrobial activities of green coffee oil in cosmetic formulations. J Biomed Biopharm Res. 2012, 9, 207–214. [Google Scholar] [CrossRef]
- Wagemaker, T.A.L.; Carvalho, C.R.L.; Maia, N.B.; Baggio, S.R.; Guerreiro Filho, O. Sun protection factor, content and composition of lipid fraction of green coffee beans. Ind Crops Prod. 2011, 33, 469–473. [Google Scholar] [CrossRef]
- Wagemaker, T.A.L.; Silva, S.A.M.; Leonardi, G.R.; Maia Campos, P.M.B.G. Green Coffea arabica L: Seed oil influences the stability and protective effects of topical formulations. Ind Crops Prod. 2015, 63, 34–40. [Google Scholar] [CrossRef]
- Wagemaker, T.A.L.; Rijo, P.; Rodrigues, L.M.; Maia Campos, P.M.B.G.; Fernandes, A.S.; Rosado, C. Integrated approach in the assessment of skin compatibility of cosmetic formulations with green coffee oil. Int J Cosmet Sci. 2015, 37, 506–510. [Google Scholar] [CrossRef]
- Wagemaker, T.A.L.; Maia Campos, P.M.B.G.; Fernandes, A.S.; Rijo, P.; Nicolai, M.; Roberto, A.; Reis, C.; Rodrigues, L.M.; Carvalho, C.R.; Maia, N.B.; Guerreiro Filho, O. Unsaponifiable matter from oil of green coffee beans: cosmetic properties and safety evaluation. Drug Dev Ind Pharm. 2016, 42, 1695–1699. [Google Scholar] [CrossRef] [PubMed]
- Voytena, A.P.N.; Affonso, R.C.L.; Pitz, H.S.; Ramlov, F.; Alberti, T.; Coelho, D.S.; Pereira, A.; Navarro, B.B.; Fanan, S.; Marcelo Casagrande, M.; Ribeiro-do-Valle, R.M.; Maraschin, M. Phytochemical profile and in vitro assessment of the cytotoxicity of green and roasted coffee oils (Coffea arabica L.) and their polar fractions. Rec Nat Prod. 2017, 12, 169–174. [Google Scholar] [CrossRef]
- Abdul Majid, N.A.; Edzuan Abdullah, M.F.; Diana, A.A. Review of Quality Coffee Roasting Degree Evaluation. J Appl Sci Agric. 2015, 10, 18–23. [Google Scholar]
- Münchow, M.; Alstrup, J.; Steen, I.; Giacalone, D. Roasting conditions and coffee flavor: A multi-study empirical investigation. Beverages. 2020, 6, 1–14. [Google Scholar] [CrossRef]
- Schenker, S.; Heinemann, C.; Huber, M.; Pompizzi, R.; Perren, R.; Escher, F. Impact of roasting conditions on the formation of aroma compounds in coffee beans. J Food Sci. 2002, 67, 60–66. [Google Scholar] [CrossRef]
- Bekedam, E.K.; Loots, M.J.; Schols, H.A.; Van Boekel, M.A.J.S.; Smit, G. Roasting effects on formation mechanisms of coffee brew melanoidins. J Agric Food Chem. 2008, 56, 7138–7145. [Google Scholar] [CrossRef]
- Böger, B.R.; Acre, L.B.; Viegas, M.C.; Kurozawa, L.E.; Benassi, M.T. Roasted coffee oil microencapsulation by spray drying and complex coacervation techniques: Characteristics of the particles and sensory effect. Innov Food Sci Emerg Technol. 2021, 72, 102739. [Google Scholar] [CrossRef]
- Budryn, G.; Nebesny, E.; Zyzelewicz, D.; Oracz, J.; Miśkiewicz, K.; Rosicka-Kaczmarek, J. Influence of roasting conditions on fatty acids and oxidative changes of Robusta coffee oil. Eur J Lipid Sci Technol. 2012, 114, 1052–1061. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Rocha, F.; Santos, L.; Alves, A. Microencapsulation with chitosan by spray drying for industry applications - A review. Trends Food Sci Technol. 2013, 31, 138–155. [Google Scholar] [CrossRef]
- Dong, W.; Hu, R.; Chu, Z.; Zhao, J.; Tan, L. Effect of different drying techniques on bioactive components, fatty acid composition, and volatile profile of robusta coffee beans. Food Chem. 2017, 234, 121–130. [Google Scholar] [CrossRef]
- Silva, R.S.V.; Brand, A.L.M.; Tinoco, N.; Freitas, S.P.; Rezende, C.M. Bioactive Diterpenes and Serotonin Amides in Cold-Pressed Green Coffee Oil (Coffea arabica L.). J Braz Chem Soc 2024, 35, 1–8. [Google Scholar] [CrossRef]
- Venkitasamy, C.; The, H.E.; Atungulu, G.G.; McHugh, T.H.; Pan, Z. Optimization of mechanical extraction conditions for producing grape seed oil. Trans ASABE. 2014, 57, 1699–1705. [Google Scholar]
- Ramalho, H.F.; Suarez, P. The chemistry of oils and fats and their extraction and refining processes. Rev Virtual Quim. 2013, 5, 2–15. [Google Scholar] [CrossRef]
- Hussein, A.A. Cold Pressed Green Coffee Oil; Elsevier Inc., 2020. [Google Scholar]
- Patel, T.; Sheth, S.M.; Chauhan, P.; Vishvakarma, B. Design and Development of Hydraulic Press with Die. 5th Natl Conf “Recent Adv Manuf (RAM-2015).” 2015.
- Ferreira, J.A.; Sun, P.; Grácio, J.J. Close loop control of a hydraulic press for springback analysis. J Mater Process Technol. 2006, 177, 377–381. [Google Scholar] [CrossRef]
- Efthymiopoulos, I.; Hellier, P.; Ladommatos, N.; Kay, A.; Mills-Lamptey, B. Integrated strategies for water removal and lipid extraction from coffee industry residues. Sustain Energy Technol Assessments. 2018, 29, 26–35. [Google Scholar] [CrossRef]
- Ibrahim, A.; Onwualu, A.P. Technologies for Extraction of Oil From Oil-Bearing Agricultural Products: a Review. J Agric Eng Technol. 2005, 13, 58–89. [Google Scholar]
- Savoire, R.; Lanoisellé, J.L.; Vorobiev, E. Mechanical Continuous Oil Expression from Oilseeds: A Review. Food Bioprocess Technol. 2013, 6, 1–16. [Google Scholar] [CrossRef]
- Oliveira, L.S.; Franca, A.S.; Mendonça, J.C.F.; Barros-Júnior, M.C. Proximate composition and fatty acids profile of green and roasted defective coffee beans. LWT. 2006, 39, 235–239. [Google Scholar] [CrossRef]
- Vidal, O.L.; Tsukui, A.; Garrett, .; Rocha-Leão, M.H.M.; Carvalho, C.W.P.; Freitas, S.P.; Rezende, C.M.; Ferreira, M.S.L. Production of bioactive films of carboxymethyl cellulose enriched with green coffee oil and its residues. Int J Biol Macromol. 2020, 146, 730–738. [CrossRef]
- Thiex, N. Evaluation of analytical methods for the determination of moisture, crude protein, crude fat, and crude fiber in distillers dried grains with solubles. J AOAC Int. 2009, 92, 61–73. [Google Scholar] [CrossRef]
- German Society for Lipid Sciences (DGF). In: Einheitsmethoden 1950-1975, Wissenschaftliche Verlagsgesellschaft MbH. ; Stuttgart, 1952.
- AOAC Official Method 945.16 (2005). Official Methods of Analysis of AOAC INTERNATIONAL, 18th Ed.; AOAC INTERNATIONAL: Gaithersburg, MD, 2005.
- Picard, H.; Guyot, B.; Vincent, J.C. Etudes des composés stéroliques de l’huile de café Coffea canephora. Café, Cacao, Thé. 1984, 28, 47–62. [Google Scholar]
- Dibert, K.; Cros, E.; Andrieu, J. Solvent extraction of oil and chlorogenic acid from green coffee. Part II: Kinetic data. J Food Eng 1989, 10, 199–214. [Google Scholar]
- Carrera, F.; León-Camacho, M.; Pablos, F.; González, A.G. Authentication of green coffee varieties according to their sterolic profile. Anal Chim Acta. 1998, 370, 131–139. [Google Scholar] [CrossRef]
- Araújo, J.M.A.; Sandi, D. Extraction of coffee diterpenes and coffee oil using supercritical carbon dioxide. Food Chem. 2007, 101, 1087–1094. [Google Scholar] [CrossRef]
- Oliveira, L.S.; Franca, A.S.; Camargos, R.R.S.; Ferraz, V.P. Coffee oil as a potential feedstock for biodiesel production. Bioresour Technol. 2008, 99, 3244–3250. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.C.E.; De Faria, A.F.; Mercadante, A.Z.; Bragagnolo, N.; De Benassi, M.T. Comparison of extraction methods for kahweol and cafestol analysis in roasted coffee. J Braz Chem Soc. 2013, 24, 492–499. [Google Scholar] [CrossRef]
- Tsukui, A.; Santos Júnior, H.M.; Oigman, S.S.; De Souza, R.O.M.A.; Bizzo, H.R.; Rezende, C.M. Microwave-assisted extraction of green coffee oil and quantification of diterpenes by HPLC. Food Chem. 2014, 164, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Sparr Eskilsson, C.; Björklund, E. Analytical-scale microwave-assisted extraction. J Chromatogr A. 2000, 902, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Topala, C.M.; Tataru, L.D. Infrared spectra of green arabica coffee extraction using supercritical carbon dioxide and soxhlet technique. Rev Chim. 2015, 66, 1128–1131. [Google Scholar]
- Oliveira, É.R.; Silva, R.F.; Santos, P.R.; Queiroz, F. Potential of alternative solvents to extract biologically active compounds from green coffee beans and its residue from the oil industry. Food Bioprod Process. 2019, 115, 47–58. [Google Scholar] [CrossRef]
- Ribeiro, R.C.; Teixeira, R.S.S.; Rezende, C.M. Extrusion pretreatment of green Arabica coffee for lipid extraction. Ind. Crops. submitted.
- Luque de Castro, M.D.; García-Ayuso, L.E. Soxhlet extraction of solid materials: An outdated technique with a promising innovative future. Anal Chim Acta. 1998, 369, 1–10. [Google Scholar] [CrossRef]
- Tsukui, A. Extração assistida por micro-ondas de óleo de café verde ( Coffea arabica L .) e quantificação de diterpenos verde ( Coffea arabica L .). PhD. Federal University of Rio de Janeiro, Rio de Janeiro, Brazil, 2013.
- Abbas, M.; Ahmed, D.; Qamar, M.T.; Ihsan, S.; Noor, Z.I. Optimization of ultrasound-assisted, microwave-assisted and Soxhlet extraction of bioactive compounds from Lagenaria siceraria: A comparative analysis. Bioresour Technol Reports. 2021, 15, 100746. [Google Scholar] [CrossRef]
- Dutta, R.; Sarkar, U. Design modifications and scale-up of a novel soxhlet apparatus: Optimization of batch extraction of a biofuel oil from Crotalaria juncea seeds. Sustain Energy Technol Assessments. 2023, 60, 103472. [Google Scholar] [CrossRef]
- Okpo, S.O.; Otaraku, I.J. Modelling of Soxhlet Extraction of Lemongrass Oil. Int J Chem Eng Res. 2020, 7, 24–29. [Google Scholar] [CrossRef]
- Sihvonen, M.; Järvenpää, E.; Hietaniemi, V.; Huopalahti, R. Advances in supercritical carbon dioxide technologies. Trends Food Sci Technol. 1999, 10, 217–222. [Google Scholar] [CrossRef]
- Sandi, D. Extração do óleo e diterpenos do café com CO2 supercrítico. PhD. Federal University of Viçosa, Viçosa, Brazil, 2003.
- Vinitha, U.G.; Sathasivam, R.; Muthuraman, M.S.; Park, S.U. Intensification of supercritical fluid in the extraction of flavonoids: A comprehensive review. Physiol Mol Plant Pathol. 2022, 118, 101815. [Google Scholar] [CrossRef]
- Rodríguez-España, M.; Mendoza-Sánchez, L.G.; Magallón-Servín, P.; Salgado-Cervantes, M.A.; Acosta-Osorio, A.A.; García, H.S. Supercritical fluid extraction of lipids rich in DHA from Schizochytrium sp. J Supercrit Fluids. 2021, 179, 105391. [Google Scholar] [CrossRef]
- Colucci, C.R.; Garella, I.; Gallo, M.; Nigro, R. Effect of moisture content on the extraction rate of coffee oil from spent coffee grounds using Norflurane as solvent. Chem Eng Res Des. 2021, 165, 172–179. [Google Scholar] [CrossRef]
- Khaw, K.Y.; Parat, M.O.; Shaw, P.N.; Falconer, J.R. Solvent supercritical fluid technologies to extract bioactive compounds from natural sources: A review. Molecules. 2017, 22, 1186. [Google Scholar] [CrossRef] [PubMed]
- De Azevedo, A.B.A.; Mazzafera, P.; Mohamed, R.S.; Vieira De Melo, S.A.B.; Kieckbusch, T.G. Extraction of caffeine, chlorogenic acids and lipids from green coffee beans using supercritical carbon dioxide and co-solvents. Brazil. J. Chem. Eng. 2008, 25, 543–552. [Google Scholar] [CrossRef]
- De Oliveira, P.M.A.; De Almeida, R.H.; De Oliveira, N.A.; Bostyn, S.; Gonçalves, C.B.; De Oliveira, A.L. Enrichment of diterpenes in green coffee oil using supercritical fluid extraction - Characterization and comparison with green coffee oil from pressing. J Supercrit Fluids. 2014, 95, 137–145. [Google Scholar] [CrossRef]
- Hurtado-Benavides, A.; Dorado, D.A.; Sánchez-Camargo, A.D.P. Study of the fatty acid profile and the aroma composition of oil obtained from roasted Colombian coffee beans by supercritical fluid extraction. J Supercrit Fluids. 2016, 113, 44–52. [Google Scholar] [CrossRef]
- Cornelio-Santiago, H.P.; Gonçalves, C.B.; de Oliveira, N.A.; de Oliveira, A.L. Supercritical CO2 extraction of oil from green coffee beans: Solubility, triacylglycerol composition, thermophysical properties and thermodynamic modelling. J Supercrit Fluids. 2017, 128, 386–394. [Google Scholar] [CrossRef]
- Bitencourt, R.G.; Ferreira, N.J.; Oliveira, A.L.; Cabral, F.A.; Meirelles, A.J.A. High pressure phase equilibrium of the crude green coffee oil – CO2 – ethanol system and the oil bioactive compounds. J Supercrit Fluids. 2018, 133, 49–57. [Google Scholar] [CrossRef]
- Barajas-Álvarez, P.; Castillo-Herrera, G.A.; Guatemala-Morales, G.M.; Corona-González, R.I.; Arriola-Guevara, E.; Espinosa-Andrews, H. Supercritical CO2-ethanol extraction of oil from green coffee beans: optimization conditions and bioactive compound identification. J Food Sci Technol. 2021, 58, 4514–4523. [Google Scholar] [CrossRef]
- Dong, W.; Chen, Q.; Wei, C.; Hu, R.; Long, Y.; Zong, Y.; Chu, Z. Comparison of the effect of extraction methods on the quality of green coffee oil from Arabica coffee beans: Lipid yield, fatty acid composition, bioactive components, and antioxidant activity. Ultrason Sonochem. 2021, 74, 105578. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




