1. Introduction
With the continuous advancement of the new energy sector, lithium-ion batteries, which offer advantages such as long service life and mature technology, have been applied in various aspects of daily life [
1,
2,
3]. Particularly in recent years, following the rapid growth of the new energy sector and the continuous expansion of the electric vehicle market, the consumption of lithium resources has been escalating annually [
4,
5,
6]. In light of the Statistical Multifragmentational Model’s (SMM), the worldwide demand for lithium batteries witnessed a year-on-year growth of 52% in 2022, while the supply of lithium resources reached as high as 840 Kt [
7]. From a global perspective, a notable mismatch in the regional supply and demand of lithium resources is evident. Lithium ore exploitation resources in the upstream of the supply chain are mainly distributed in Australia, Chile, Argentina, and other regions in South America [
8,
9]. Thus, the extraction of lithium resources from secondary resource lithium becomes the focus of researchers. Secondary lithium resources are generated along with the slag from lithium ore [
10], hydrometallurgical leaching the lithium from waste lithium batteries [
11]. With the continuous expansion of the scale of lithium ore production and the recycling of waste lithium-ion batteries, the total amount of lithium-rich solutions shows an increasing trend year by year [
12,
13]. Therefore, the efficient and convenient lithium extraction technology is worthy study in the future.
Currently, a wide array of lithium extraction technologies are employed to recover lithium from lithium-containing solutions, including hydrometallurgy [
14,
15] and electrochemical recovery processes [
16,
17]. The main technology is hydrometallurgy method, through the co-precipitation method to recover the metal elements in the lithium solution, and reproduced as chemical raw materials to achieve the recycling of lithium resources. Electrochemical extraction technology is an efficient lithium extraction technology that has rapidly developed in recent years [
17,
18]. The primary technology used is the hydrometallurgy method, which utilizes co-precipitation to recover metal elements from lithium solutions and reproduce them as chemical raw materials, thereby achieving the recycling of lithium resources [
19,
20,
21]. The electrochemical lithium extraction technology features high efficiency, environmental friendliness, and low energy consumption, with extensive prospects in the future [
22,
23]. This review aims to analyze the properties of various electrochemical lithium extraction methods from lithium-containing solutions, outline the current research status and technical characteristics, and promote the development of more efficient and environmentally friendly electrochemical lithium extraction technologies to meet the increasing demand for lithium extraction.
2. Different Techniques of Electrochemical Lithium Extraction
2.1. Electrochemical Deintercalation Method
LiFePO
4 is a commonly used anode materials of lithium ion battery. LiFePO
4 has a typical olivine-type crystal structure [
23,
24]. The crystal structure of LiFePO
4 is composed of [LiO
6] octahedra and [PO
4] tetrahedra, which facilitate the migration of lithium ions and electronic conduction through the Fe-O-Fe lattice planes. The chemical reactions involved in the removal and insertion of Li
+ in the LiFePO
4 crystal structure are depicted in Equations (1) and (2) [
25] (
Figure 1).
Based on above electrochemical delithiation theory, lithium iron phosphate is widely used for lithium extraction and lithium enrichment. Zhao et al. [
26,
27,
28,
29] have conducted extensive research on lithium extraction using the LiFePO
4 electrode. They developed a “rocking chair” electrode system, which alternates between a lithium-rich LiFePO
4 electrode and a lithium-poor Li(1-x)FePO
4 electrode. This system is divided by an anion-exchange membrane, with the electrolytic cell containing initial liquid on one side and lithium-rich liquid on the other. Under a direct current electric field, the lithiation reaction occurred at the lithium-poor electrode (FePO
4 cathode) where lithium ions were absorbed from initial liquid, while the delithiation reaction occurred at the lithium-rich electrode (LiFePO
4 anode), diffusing lithium ions into the lithium-rich liquid. Anion exchange membranes are used, allowing Cl
- ions to migrate from the cathode to the anode side to maintain charge balance. By switching the positions of the two electrodes and repeating the process, selective enrichment of lithium ions is achieved. Experimental results indicated that the maximum amount of lithium embedded reaches 38.93 mg/g after 10 h, while the maximum amount of embedded Mg
2+ is 5 mg/g in MgCl
2 solution (220 mg/L). The selective enrichment of lithium ions has been confirmed in a simulated brine composed of LiCl and MgCl
2 solutions. The initial concentration of Li
+ was 220 mg/L, and the final Mg/Li ratio decreased from 60 to 45, demonstrating effective lithium ion selectivity in the process.
Figure 1.
Structure of the LiFePO
4/FePO
4 electrolytic cell for lithium extraction [
27].
Figure 1.
Structure of the LiFePO
4/FePO
4 electrolytic cell for lithium extraction [
27].
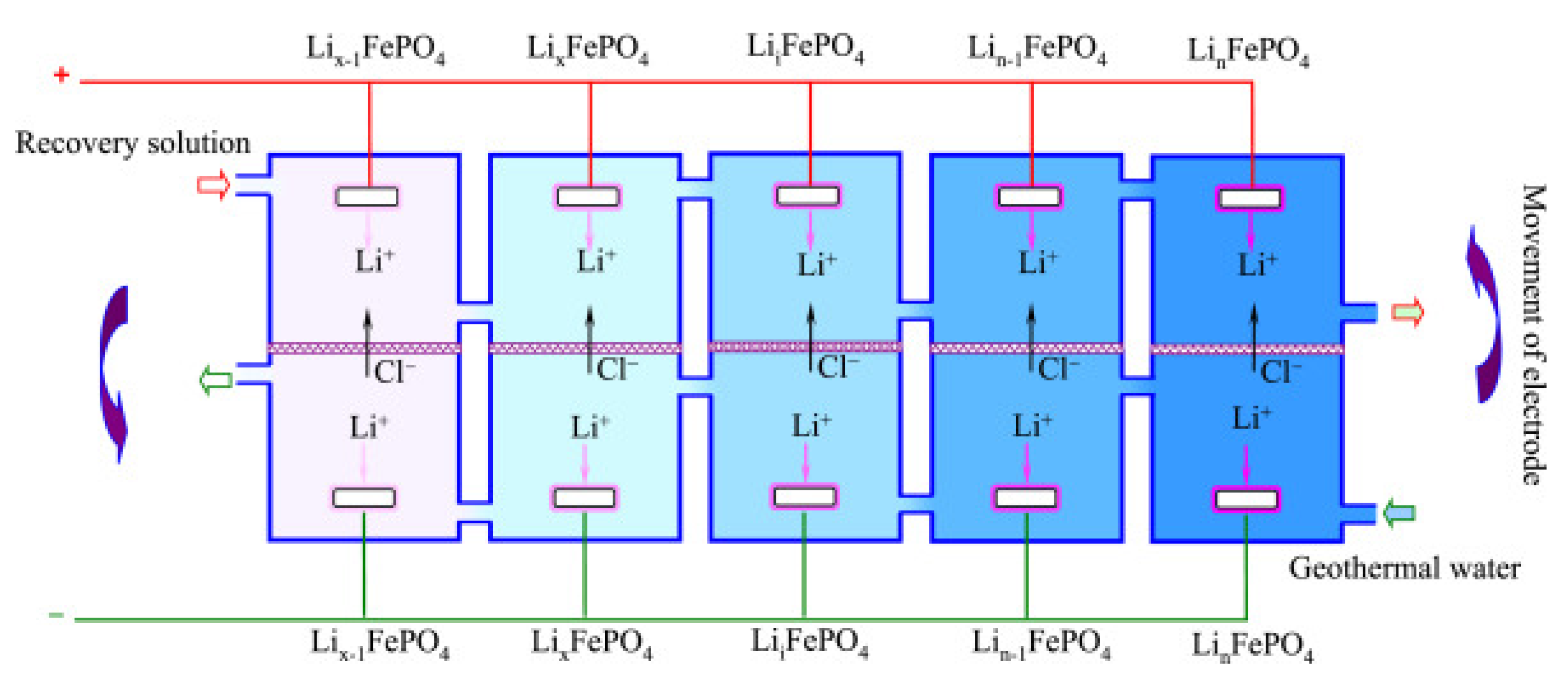
Deng et al. [
30] carried out electrochemical extraction lithium using a LiFePO
4/FePO
4 electrochemical system. The electrode was modified using PEG-6000 as a poregen to obtain abundant pore structures of about 5–20 μm formed on the surface of electrode. This modification increased the adsorption capacity to 17.10 mg g
−1, a 57.02% improvement. In this electrochemical system, delithium occurred at the LiFePO
4 electrode, while the lithiation reaction took place at theFePO
4 electrode. The system was effectively employed to recover lithium resources from geothermal water with low lithium concentration. When the modified LiFePO
4 and FePO
4 electrodes were utilized in an H-type electrolytic cell with operated voltage of 1 V, the retrieval rate of Li
+ reached 90.65% after 8 cycles in the geothermal water, and the electrode structure of both anode and cathode maintained stable (
Figure 2).
The electrochemical deintercalation method is commonly utilized for lithium extraction. Researchers have observed that the diffusion of anions is driven by the concentration differences of cations between the anode and cathode, leading anions to migrate towards the opposite electrode [
31]. As a result, a small number of anions and impurity ions pass through anion-exchange membrane under the driving force of concentration difference, and the separation rate of Li
+ is declined. Specifically, in the treatment of Li2SO4 solutions, the relatively large ionic volume of SO
42- increases migration resistance. This not only necessitates higher voltages for the electrochemical adsorption reaction, prompting numerous side reactions, but also results in substantial electrical resistance and high energy consumption during the process [
32].
While the electrochemical deintercalation method is straightforward and easy to operate, it has several drawbacks that require improvement. Specifically, the ionic conduction ability of the membrane and the stability of the electrode material need significant enhancements to optimize the process.
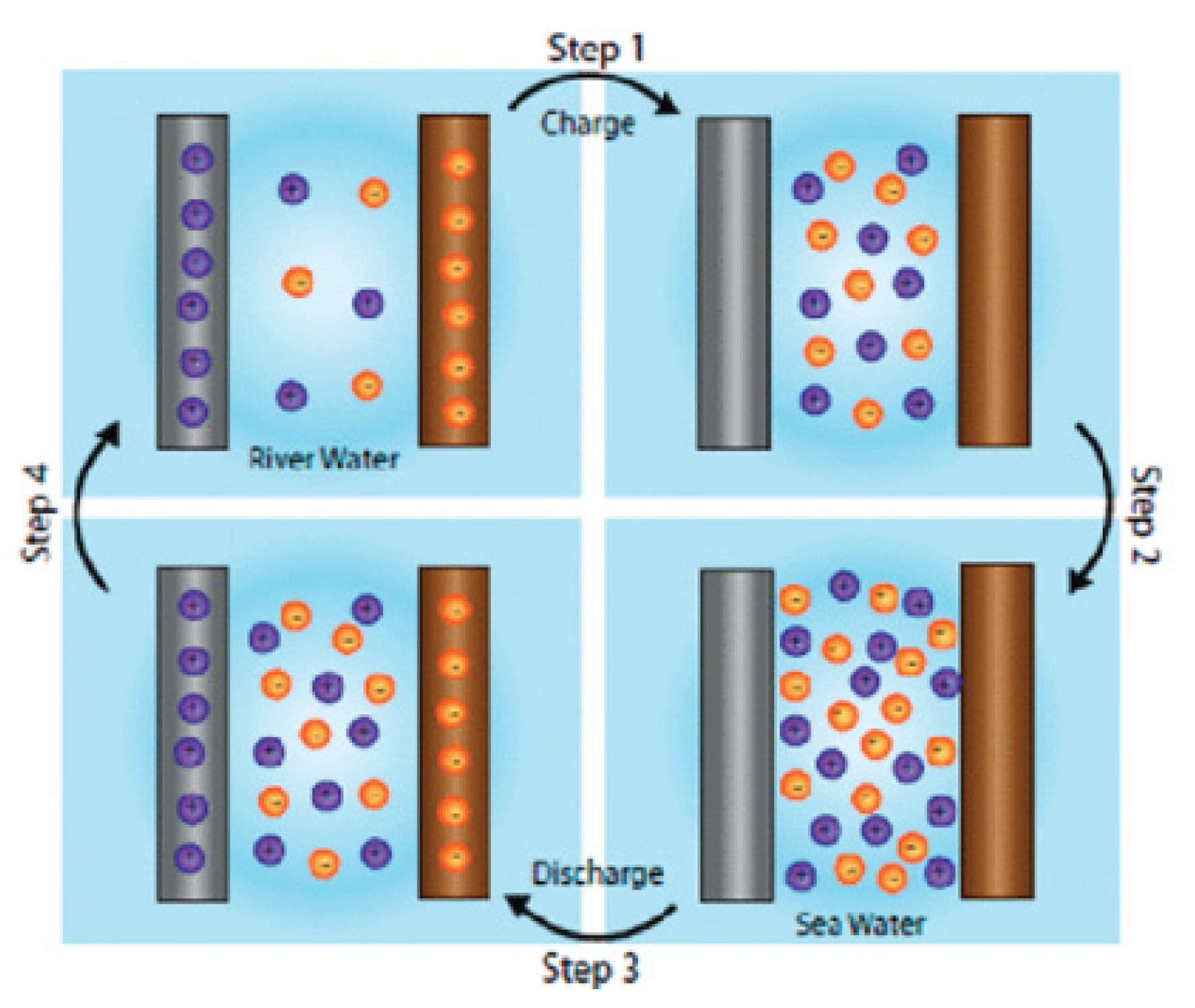
2.2. Electrochemical Ion Pump
The principle of electrochemical ion pump involves extraction lithium under inverse concentration gradient. The Lithium ions are captured selectively by lithium absorption materials, while the counter electrodes (such as Pt and Ag) are adopted to capture anions [
33,
34,
35]. Initially reported in the early 1990s by Kanoh and his team, the electrochemical ion pump method utilized λ-MnO
2 as the working electrode and a Pt wire as the counter electrode to extract lithium ions from a polycationic solution [
36,
37]. Subsequently, La Mantia and Yoon’s group innovated on this by replacing the Pt electrode with an Ag electrode, and its working principle is shown in
Figure 3. The λ-MnO
2 serves as the intercalating electrode, adsorbing and desorbing lithium ions, while the Ag electrode functions as the counter electrode, capturing and releasing chloride ions,, thereby significantly reducing the power consumption during the electrochemical reaction [
38].
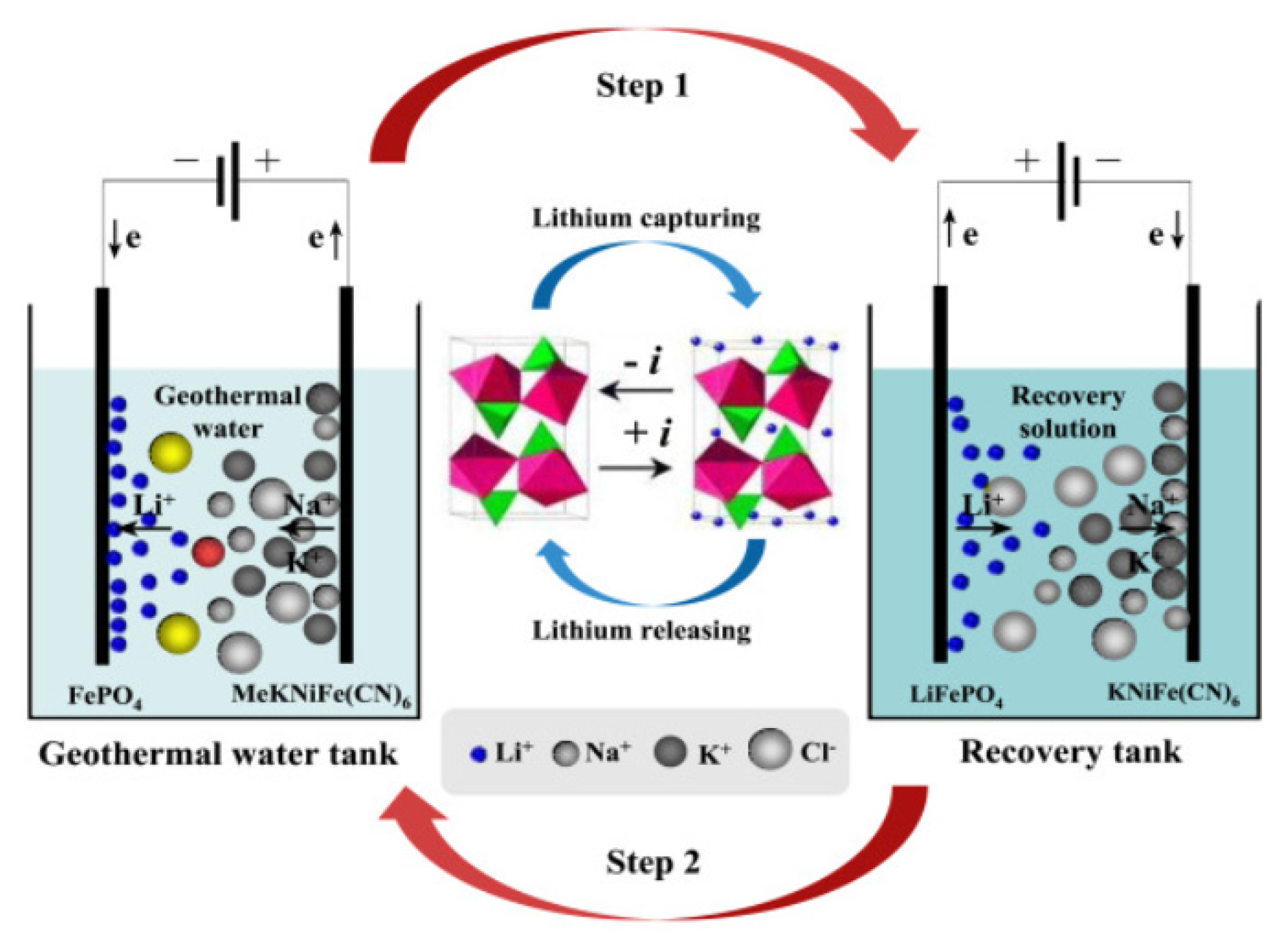
In recent years, with continuous advancements in materials manufacturing technology, the scope of electrochemical ion pumping applications has notably expanded. Deng et.al introduced a green and innovative FePO
4/K
2NiFe(CN)
6 [FPO/KNiFC] electrochemical ion pump technique to recover lithium from low-concentration sources in geothermal water (
Figure 4). The performance of the LiFePO
4 electrode was enhanced by modifying it with multiwalled carbon nanotubes, improving both the insertion capacity and rate. Although the single insertion capacity stands at 14.88 mg/g, the recovery rate can be increased through cycle insertion-release processes [
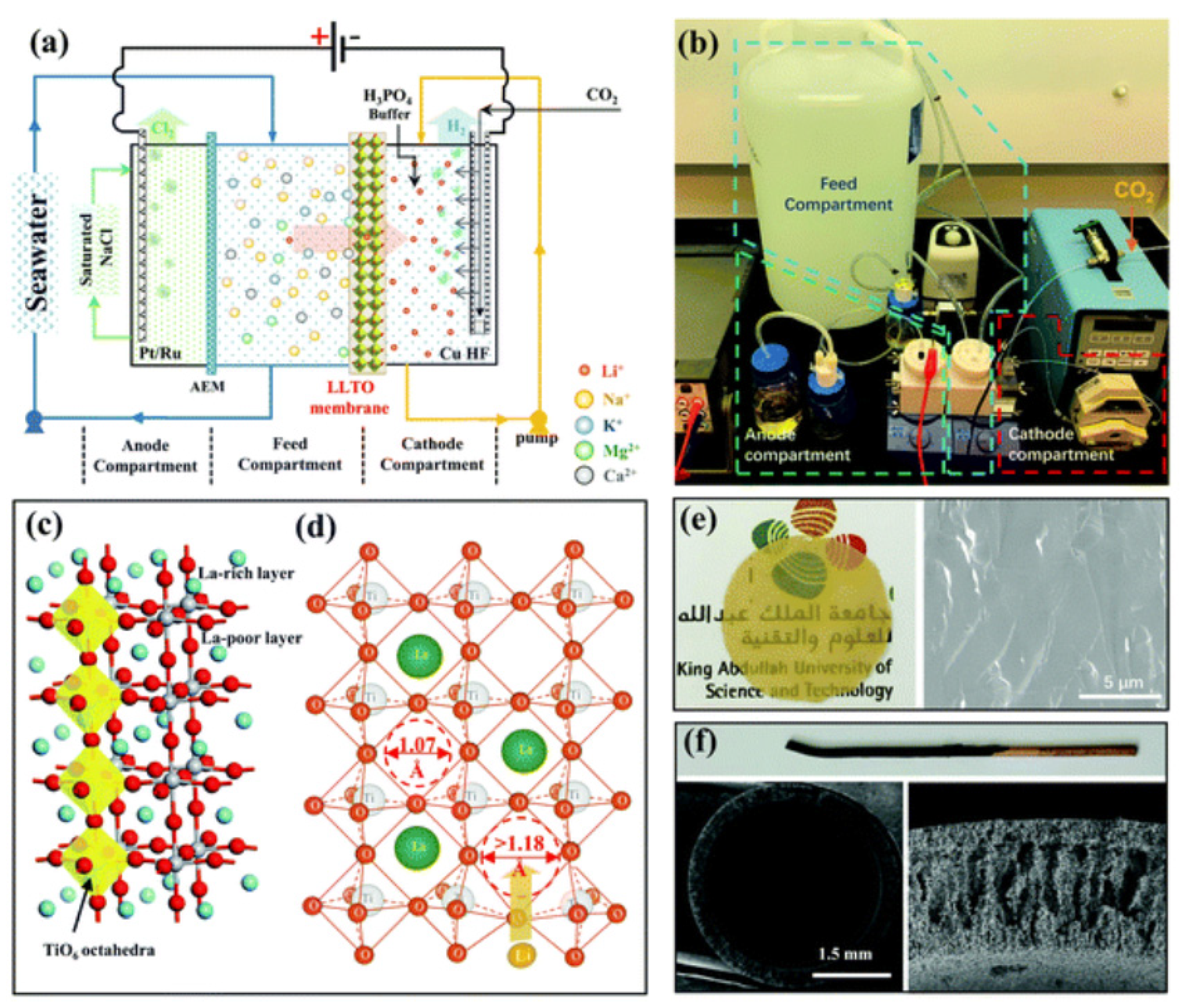
39]. Moreover, Kazuya Sasaki et al. developed a new electrochemical pumping technology for the extraction/recovery of lithium. This system comprises two power supplies, three electrodes, and a La
0.57Li
0.29TiO
3 (LLTO) electrolyte [
40] (
Figure 5). The distinctive structure enables the positive voltage of the third electrodes in the cathode solution to be applied to the conventional battery through a secondary power supply. LLTO serves as an ideal solid electrolyte for high-purity lithium extraction and recovery, effectively blocking the permeation of cations other than lithium ions. Notably, this newly designed system demonstrates flexibility in adapting to a variety of commercial electrolytes and capability of significantly enhancing the lithium collection rate of the mentioned main circuit [
41]. Furthermore, an additional subcircuit provides effective control, accelerating the lithium absorption process by at least 464 times compared to traditional systems.
Ion pump lithium-extraction technology surpasses other electrochemical methods in selectivity, cycling stability, and energy efficiency, offering significant advantages for practical applications. The success of this technology hinges on electrode materials that exhibit high selectivity and a robust ion exchange capacity. Currently, the use of ion pump technology for lithium recovery remains predominantly within the realm of laboratory research. For it to transition to industrial applications, extensive further research is essential to address practical implementation challenges and scale-up processes.
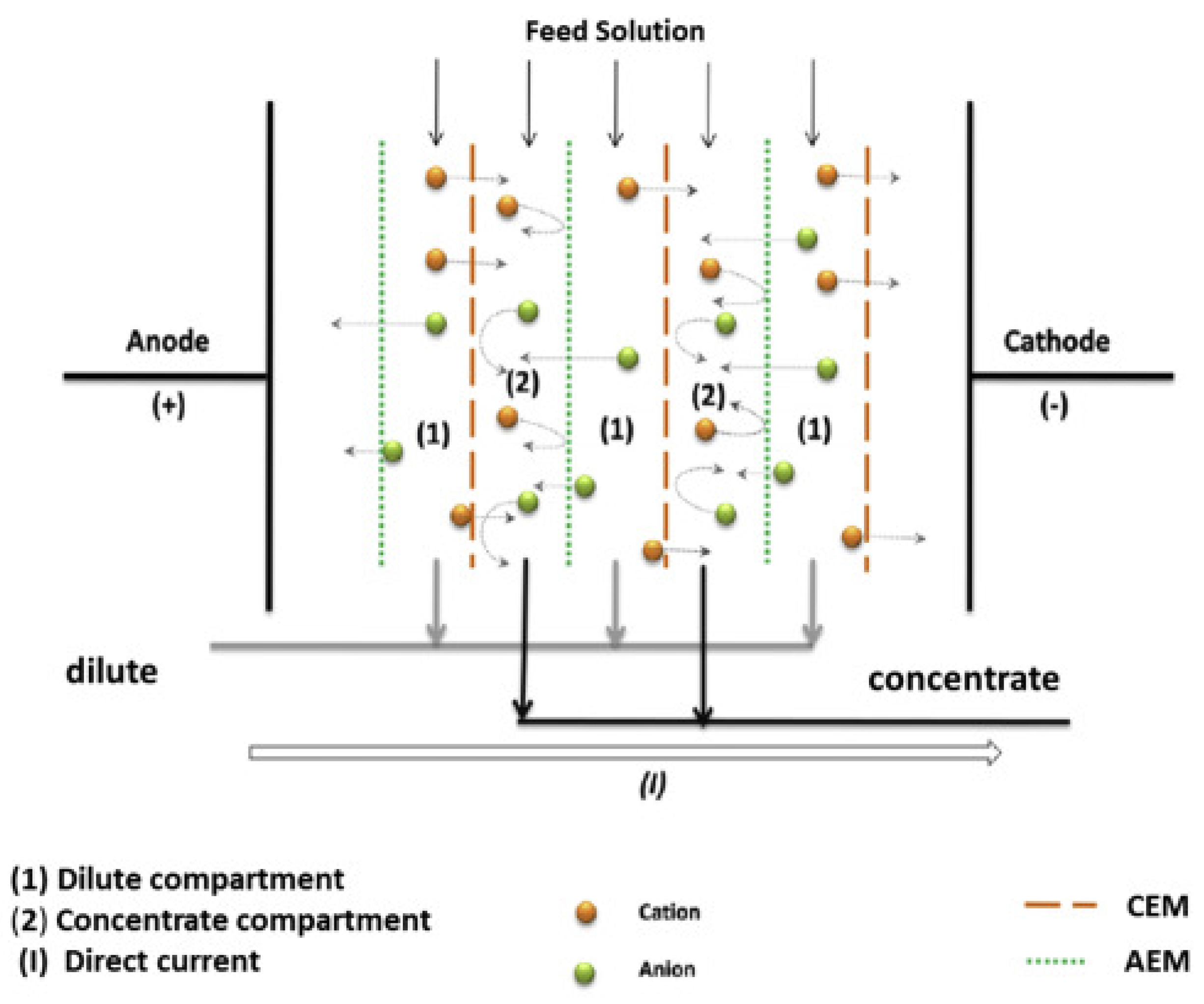
2.3. Electrodialysis Method
Electrodialysis is an electrochemistry separation technology based on selectivity membrane, which was originally developed in the field of seawater desalination [
42,
43,
44]. And with the development of membrane materials, electrodialysis began to be applied for lithium recovery from saline and other lithium solution. The principle of electrodialysis technology is that a voltage is applied between the two electrodes, driving ions migration across the membranes to achieve the enrichment of target ions [
45,
46]. The working principle of the electrodialysis system is schemed in
Figure 6. The electrodialysis system consists of repetitive units including cation-exchange membrane (CEM) and an anion-exchange membrane (AEM), which separate concentration and dilution chambers alternately. The monovalent ions (e.g., Li
+, Na
+, K
+, and Cl
-) could permeated through these chambers and pass through the CEM and the AEM into the concentration chamber, while the divalent ions (e.g., Mg
2+, Ca
2+, SO
42-) are blocked by the ion exchange membrane and retained in the desalination chamber.
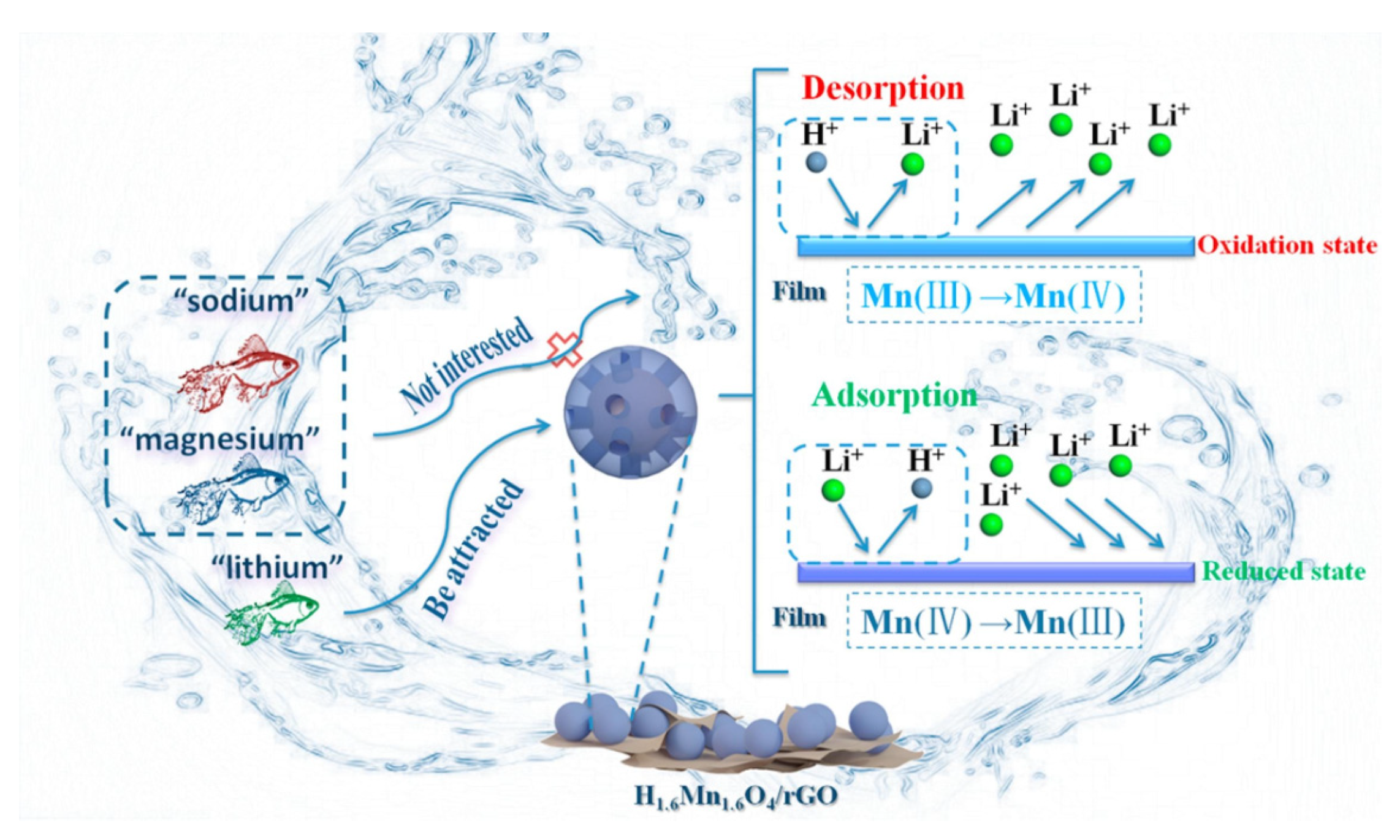
Abuliti Abudula et. al. developed a novel lithium-ion separation composite membrane composed of H
1.6Mn
1.6O
4 nanoparticles and reduced graphene oxide (rGO) by vacuum filtration technology [
47]. The membrane’s effectiveness in selectively extracting low-concentration lithium ions from aqueous solutions is attributed to the selective adsorption properties of H
1.6Mn
1.6O
4 and the excellent conductivity of rGO (
Figure 7). The results showed that the lithium ion adsorption capacity of H
1.6Mn
1.6O
4/rGO composite membranes reached an equilibrium of 38.78 mg/g after 5 h. Impressively, the adsorption capacity of these composite membranes was maintained at 99% even after 5 cycles. Specifically, during selective adsorption experiments with initial molar ratios of Li
+/Na
+ and Li
+/Mg
2+ set at 1:1, the adsorption ratios achieved were 10.39 for Li
+/Na
+ and 10.23 for Li
+/Mg
2+, respectively, demonstrating significant selectivity in ion exchange.
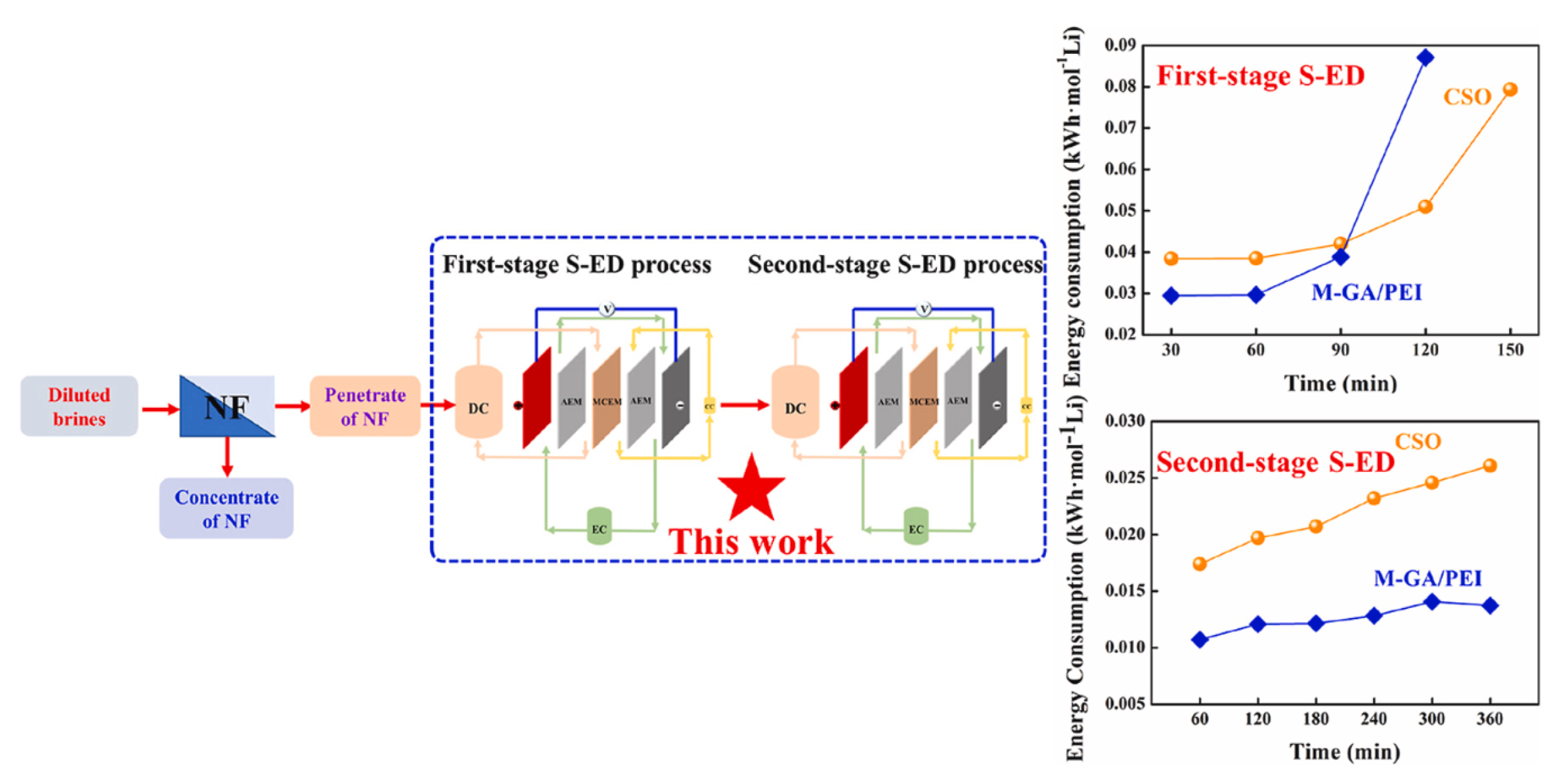
Shao et al. [
48] developed a multistage selective electrodialysis (S-ED) process utilizing high-performance monovalent cation exchange membranes (MCEMs) as shown in
Figure 8. These MCEMs were tailored to treat simulated osmotic water produced by a one-stage nanofiltration (NF) process. The mussel-inspired gallic acid/polyethyleneimine assembled membrane (M-GA/PEI) its unique specific architecture and charge properties demonstrated better separation performance than CSO (a commercial MCEM) during the multi-stage S-ED process. Leveraging its specific architectural and charge characteristics, the M-GA/PEI achieved a Li
+-rich solution with a high concentration (approximately 8.33 g/L) and purity over 96.4% simultaneously, under a volume ratio of 10:1 (under DC:CC converter) during both the first and second-stage S-ED processes.
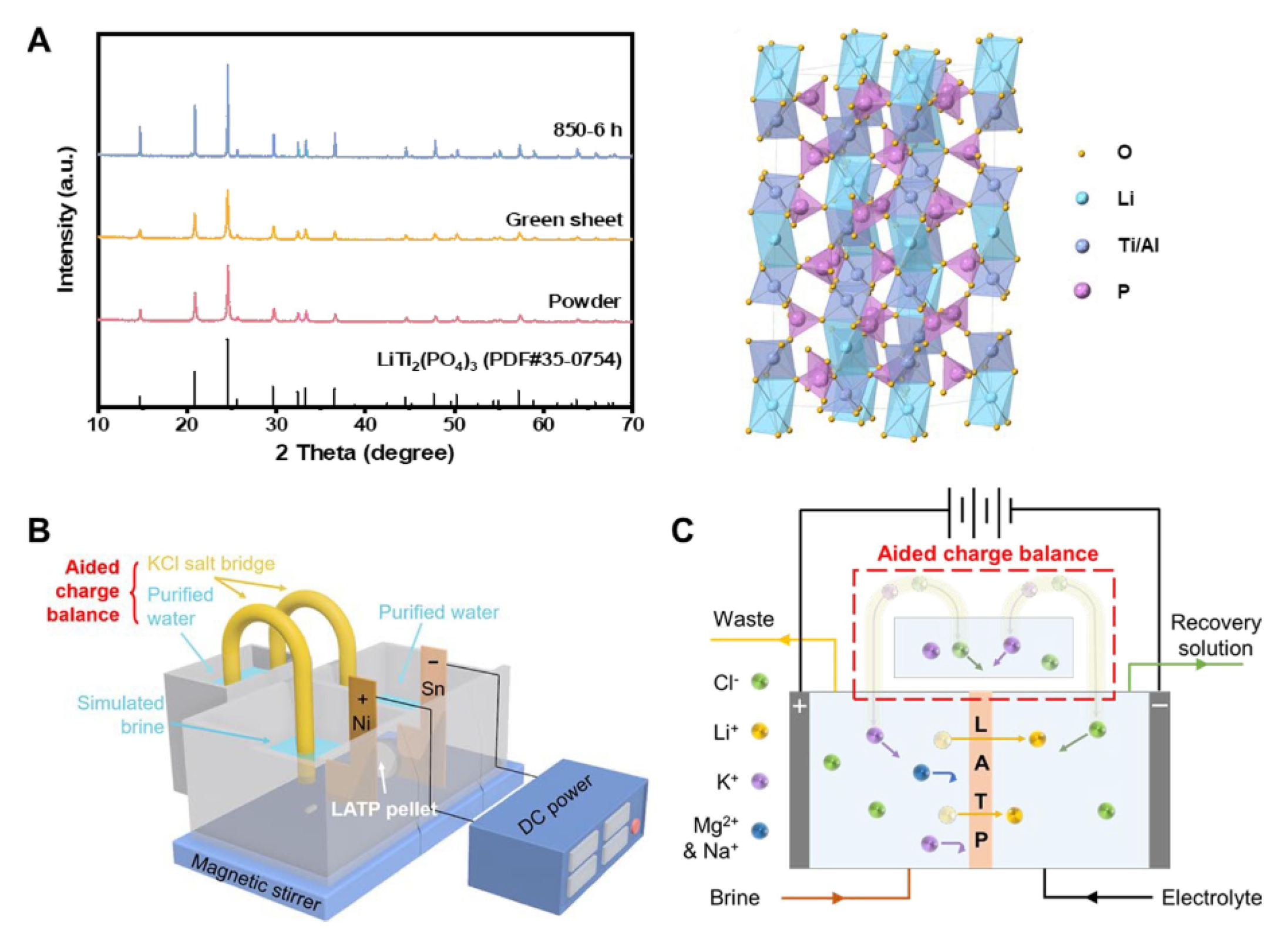
Liu et al. introduced an innovative electrodialysis technology which utilized Li
1.3Al
0.3Ti
1.7(PO
4)
3 (LATP), a lithium superionic conductors, and incorporates an assisted charge balance (ACB) system [
49]. The electrodialysis technology was operated all under low voltage and ultra-low current conditions (
Figure 9). The excellent ion selectivity of LATP, coupled with the improved recovery of ACB, enable this technology to achieve exceptionally high separation efficiencies and significantly reduced energy consumption. By implementing a two-stage extraction process on aged brine, the application of directly producing battery-grade Li
2CO
3 with a purity of 99.93%. Remarkably, when applied to pristine brine, the technology achieves an impressive Li/Mg separation coefficient as high as 5924. This simplified production process is expected to significantly reduce the cost of Li
2CO
3 production.
The electrodialysis method offers significant advantages in terms of cost-efficiency, environmental sustainability, and effective separation of lithium ions from bivalent cations. However, its effectiveness in separating lithium from sodium ions is limited. Additionally, the presence of specific anions in the solution, particularly high concentrations of SO
42-, can dramatically reduce the lifespan of the electrodialysis membranes [
50,
51]. Another challenge with this method is the generation of substantial amounts of wastewater that require further treatment. Moreover, the migration rate of lithium ions during electrodialysis is relatively slow, leading to lengthy processing times. Consequently, while electrodialysis presents potential benefits, its application in lithium extraction requires more in-depth study to overcome these operational challenges.
3. Conclusions
Electrochemical technology holds promising prospects in lithium extraction and adsorption, encompassing methods such as electrochemical deintercalation, ion pumps, and electrodialysis. These techniques offer advantages such as low cost, environmental sustainability, and reusability. Despite their potential, many of these technologies are still under development, facing significant barriers to commercialization. To advance the field of electrochemical lithium extraction, further research should focus on the following aspects:
(1) Electrochemical materials play a crucial role in determining the performance of lithium extraction systems. The effectiveness of both the anode and cathode materials in the electrochemical system, the ion exchange active materials in electrically controlled setups, and the membrane materials in electrodialysis processes directly influences the efficiency of lithium extraction. Therefore, there is a pressing need to enhance basic research into the development of new materials. Challenges such as low ionic mobility and suboptimal cycling stability persist in current materials. Addressing these issues could involve doping and coating materials, as well as engineering electrodes with perforations to modify their microstructures and chemical compositions, thereby improving their functional properties. In electrodialysis, the choice of membrane materials is critical for effectively separating monovalent from polyvalent ions. Advancements in this area should focus on increasing the ion conductivity, minimizing electrochemical impedance, and enhancing the chemical stability of these membranes. Such improvements will not only boost the performance of lithium extraction but also enhance the overall sustainability and efficiency of the electrochemical methods employed.
(2) Optimization of electrochemical processes. In the past, the industrial production of lithium primarily focused on generating industrial-grade Li2CO3. However, advances in electrochemical production technology are now paving the way for the direct one-step production of battery-grade lithium compounds such as Li2CO3, LiOH, and LiCl. This shift enables not only a transition from single to multiple electrochemical processes but also a transformation of the industry towards refining chemicals and achieving battery-grade production through process optimization. Electrochemical lithium extraction optimally utilizes lithium-containing solutions and recovers lithium from low-concentration and economically unviable brines efficiently. As global efforts to reduce carbon emissions continue to evolve, industrial practices must adapt to minimize environmental impact, carbon emissions, energy, and freshwater consumption. Upgrades in electrochemical lithium extraction technology are essential for significantly improving these parameters, which in turn will accelerate the rapid industrialization and commercialization of lithium extraction processes. This evolution is critical for supporting the growing demand for lithium in various high-tech applications, including electric vehicles and renewable energy storage solutions.
Author Contributions
Qingyuan Dong: Methodology, Funding acquisition, Data curation, Writing-original draft, Investigation. Haiyin Gang: Methodology, Review, Conceptualization. Jinxiao Xu: Data curation, Review, Methodology. Zuxiang Li: Investigation, Review, Methodology. Zhongxiang Wang: Conceptualization, Project administration, Writing-review & editing.
Acknowledgments
This research was supported a grant from Mianyang Municipal Social Science Research Planning Project (MY2024YB226).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; Li, B. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. Journal of Energy Chemistry. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Liu, B.; Jia, Y.; Yuan, C.; Wang, L.; Gao, X.; Yin, S.; Xu, J. Safety issues and mechanisms of lithium-ion battery cell upon mechanical abusive loading: A review. Energy Storage Materials. 2020, 24, 85–112. [Google Scholar] [CrossRef]
- Shang, Z.; Yu, W.; Zhou, J.; Zhou, X.; Zeng, Z.; Tursun, R.; Liu, X.; Xu, S. Recycling of spent lithium-ion batteries in view of graphite recovery: A review. eTransportation. 2024, 20, 100320. [Google Scholar] [CrossRef]
- Yang, L.; Gao, Z.; Liu, T.; Huang, M.; Liu, G.; Feng, Y.; Shao, P.; Luo, X. Direct Electrochemical Leaching Method for High-Purity Lithium Recovery from Spent Lithium Batteries. Environmental Science & Technology. 2023, 57, 4591–4597. [Google Scholar]
- Swain, B. Recovery and recycling of lithium: A review. Separation and Purification Technology. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Wang, J.; Koenig Jr, G. M. Direct Lithium Extraction Using Intercalation Materials. Chemistry—A European Journal. 2024, 30, e202302776. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, S.; Gao, Z.; Wang, G.; Zong, L.; Liu, J.; Zhu, F.; Ming, H.; Zheng, Y.; Chen, F.; Cao, N.; Yang, S. A statistical distribution-based pack-integrated model towards state estimation for lithium-ion batteries. eTransportation. 2024, 19, 100302. [Google Scholar] [CrossRef]
- Jin, P.; Wang, S.; Meng, Z.; Chen, B. China’s lithium supply chains: Network evolution and resilience assessment. Resources Policy. 2023, 87, 104339. [Google Scholar] [CrossRef]
- Shao, L.; Jin, S. Resilience assessment of the lithium supply chain in China under impact of new energy vehicles and supply interruption. Journal of Cleaner Production. 2020, 252, 119624. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B. D.; Mankhand, T. R. Extraction of lithium from primary and secondary sources by pre-treatment, leaching and separation: A comprehensive review. Hydrometallurgy. 2014, 150, 192–208. [Google Scholar] [CrossRef]
- Talens Peiró, L.; Villalba Méndez, G.; Ayres, R. U. Lithium: Sources, Production, Uses, and Recovery Outlook. JOM. 2013, 65, 986–996. [Google Scholar] [CrossRef]
- Yelatontsev, D.; Mukhachev, A. Processing of lithium ores: Industrial technologies and case studies—A review. Hydrometallurgy. 2021, 201, 105578. [Google Scholar] [CrossRef]
- Gu, G.; Gao, T. Sustainable production of lithium salts extraction from ores in China: Cleaner production assessment. Resources Policy. 2021, 74, 102261. [Google Scholar] [CrossRef]
- Vieceli, N.; Casasola, R.; Lombardo, G.; Ebin, B.; Petranikova, M. Hydrometallurgical recycling of EV lithium-ion batteries: Effects of incineration on the leaching efficiency of metals using sulfuric acid. Waste Management. 2021, 125, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; He, Y.; Li, J.; Qu, L.; Yang, Y.; Guo, X.; Xie, W. Improved hydrometallurgical extraction of valuable metals from spent lithium-ion batteries via a closed-loop process. Journal of Alloys and Compounds. 2020, 847, 156489. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, F.; Ding, H.; He, P.; Zhou, H. Lithium Metal Extraction from Seawater. Joule. 2018, 2, 1648–1651. [Google Scholar] [CrossRef]
- Wang, J.; Yue, X.; Wang, P.; Yu, T.; Du, X.; Hao, X.; Abudula, A.; Guan, G. Electrochemical technologies for lithium recovery from liquid resources: A review. Renewable and Sustainable Energy Reviews. 2022, 154, 111813. [Google Scholar] [CrossRef]
- Farahbakhsh, J.; Arshadi, F.; Mofidi, Z.; Mohseni-Dargah, M.; Kök, C.; Assefi, M.; Soozanipour, A.; Zargar, M.; Asadnia, M.; Boroumand, Y.; Presser, V.; Razmjou, A. Direct lithium extraction: A new paradigm for lithium production and resource utilization. Desalination. 2024, 575, 117249. [Google Scholar] [CrossRef]
- Delmas, C.; Maccario, M.; Croguennec, L.; Le Cras, F.; Weill, F. Lithium deintercalation in LiFePO4 nanoparticles via a domino-cascade model. Nature Materials. 2008, 7, 665–671. [Google Scholar] [CrossRef]
- Odetallah, M.; Kuss, C. A Review of Chemically Induced Intercalation and Deintercalation in Battery Materials. Energy Technology. 2023, 11, 2201060. [Google Scholar] [CrossRef]
- Allen, J. L.; Jow, T. R.; Wolfenstine, J. Kinetic Study of the Electrochemical FePO4 to LiFePO4 Phase Transition. Chemistry of Materials. 2007, 19, 2108–2111. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, H.; Wang, Y.; Sha, Z. Review on the electrochemical extraction of lithium from seawater/brine. Journal of Electroanalytical Chemistry. 2019, 850, 113389. [Google Scholar] [CrossRef]
- Battistel, A.; Palagonia, M. S.; Brogioli, D.; La Mantia, F.; Trócoli, R. Electrochemical Methods for Lithium Recovery: A Comprehensive and Critical Review. 2020, 32, e1905440. [Google Scholar] [CrossRef]
- Patil, A.; Patil, V.; Wook Shin, D.; Choi, J.-W.; Paik, D.-S.; Yoon, S.-J. Issue and challenges facing rechargeable thin film lithium batteries. Materials Research Bulletin. 2008, 43, 1913–1942. [Google Scholar] [CrossRef]
- Tarascon, J. M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature. 2001, 414, 359–67. [Google Scholar] [CrossRef]
- He, L.; Xu, W.; Song, Y.; Luo, Y.; Liu, X.; Zhao, Z. New Insights into the Application of Lithium-Ion Battery Materials: Selective Extraction of Lithium from Brines via a Rocking-Chair Lithium-Ion Battery System. Global Challenges. 2018, 2, 1700079. [Google Scholar] [CrossRef]
- Zhao, Z.; Si, X.; Liu, X.; He, L.; Liang, X. Li extraction from high Mg/Li ratio brine with LiFePO4/FePO4 as electrode materials. Hydrometallurgy. 2013, 133, 75–83. [Google Scholar] [CrossRef]
- Zhao, Z.-w.; Si, X.-f.; Liang, X.-x.; Liu, X.-h.; He, L.-h. Electrochemical behavior of Li+, Mg2+, Na+ and K+ in LiFePO4/ FePO4 structures. Transactions of Nonferrous Metals Society of China. 2013, 23, 1157–1164. [Google Scholar] [CrossRef]
- Li, Z.; Liu, D.; Xiong, J.; He, L.; Zhao, Z.; Wang, D. Selective recovery of lithium and iron phosphate/carbon from spent lithium iron phosphate cathode material by anionic membrane slurry electrolysis. Waste Management. 2020, 107, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Yu, X.; Li, M.; Duo, J.; Guo, Y.; Deng, T. Green recovery of lithium from geothermal water based on a novel lithium iron phosphate electrochemical technique. Journal of Cleaner Production. 2020, 247, 119178. [Google Scholar] [CrossRef]
- Sata, T.; Mine, K.; Higa, M. Change in permselectivity between sulfate and chloride ions through anion exchange membrane with hydrophilicity of the membrane. Journal of Membrane Science. 1998, 141, 137–144. [Google Scholar] [CrossRef]
- Mehanna, M.; Saito, T.; Yan, J.; Hickner, M.; Cao, X.; Huang, X.; Logan, B. E. Using microbial desalination cells to reduce water salinity prior to reverse osmosis. Energy & Environmental Science. 2010, 3, 1114–1120. [Google Scholar]
- Luo, G.; Li, X.; Chen, L.; Chao, Y.; Zhu, W. Electrochemical lithium ion pumps for lithium recovery: A systematic review and influencing factors analysis. Desalination. 2023, 548, 116228. [Google Scholar] [CrossRef]
- Zhou, G.; Chen, L.; Chao, Y.; Li, X.; Luo, G.; Zhu, W. Progress in electrochemical lithium ion pumping for lithium recovery. Journal of Energy Chemistry. 2021, 59, 431–445. [Google Scholar] [CrossRef]
- Luo, G.; Zhu, L.; Li, X.; Zhou, G.; Sun, J.; Chen, L.; Chao, Y.; Jiang, L.; Zhu, W. Electrochemical lithium ions pump for lithium recovery from brine by using a surface stability Al2O3–ZrO2 coated LiMn2O4 electrode. Journal of Energy Chemistry. 2022, 69, 244–252. [Google Scholar] [CrossRef]
- Battistel, A.; Palagonia, M. S.; Brogioli, D.; La Mantia, F.; Trócoli, R. Electrochemical Methods for Lithium Recovery: A Comprehensive and Critical Review. Advanced materials. 2020, 32, 1905440. [Google Scholar] [CrossRef] [PubMed]
- Calvo, E. J. Electrochemical methods for sustainable recovery of lithium from natural brines and battery recycling. Current Opinion in Electrochemistry. 2019, 15, 102–108. [Google Scholar] [CrossRef]
- Pasta, M.; Wessells, C. D.; Cui, Y.; La Mantia, F. A Desalination Battery. Nano letters. 2012, 12, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Yu, X.; Guo, Y.; Li, M.; Duo, J.; Deng, T. Green recovery of low concentration of lithium from geothermal water by a novel FPO/KNiFC ion pump technique. Electrochimica Acta. 2020, 350, 136385. [Google Scholar] [CrossRef]
- Sasaki, K.; Shin-mura, K.; Honda, S.; Tazoe, H.; Niwa, E. A three-electrode dual-power-supply electrochemical pumping system for fast and energy efficient lithium extraction and recovery from solutions. Communications Engineering. 2024, 3, 29. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.; Liu, X.; Cao, L.; Li, P.; Wei, R.; Li, X.; Guo, D.; Huang, K.-W.; Lai, Z. Continuous electrical pumping membrane process for seawater lithium mining. Energy & Environmental Science. 2021, 14, 3152–3159. [Google Scholar]
- Al-Amshawee, S.; Yunus, M. Y. B. M.; Azoddein, A. A. M.; Hassell, D. G.; Dakhil, I. H.; Hasan, H. A. Electrodialysis desalination for water and wastewater: A review. Chemical Engineering Journal. 2020, 380, 122231. [Google Scholar] [CrossRef]
- Gonçalves, F.; Fernandes, C.; Cameira dos Santos, P.; de Pinho, M. N. Wine tartaric stabilization by electrodialysis and its assessment by the saturation temperature. Journal of Food Engineering. 2003, 59, 229–235. [Google Scholar] [CrossRef]
- Zhao, D.; Lee, L. Y.; Ong, S. L.; Chowdhury, P.; Siah, K. B.; Ng, H. Y. Electrodialysis reversal for industrial reverse osmosis brine treatment. Separation and Purification Technology. 2019, 213, 339–347. [Google Scholar] [CrossRef]
- Gmar, S.; Chagnes, A. Recent advances on electrodialysis for the recovery of lithium from primary and secondary resources. Hydrometallurgy. 2019, 189, 105124. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, Z. Recovery of lithium from spent lithium-ion batteries using precipitation and electrodialysis techniques. Separation and Purification Technology. 2018, 206, 335–342. [Google Scholar] [CrossRef]
- Wang, Q.; Du, X.; Gao, F.; Liu, F.; Liu, M.; Hao, X.; Tang, K.; Guan, G.; Abudula, A. A novel H1.6Mn1.6O4/reduced graphene oxide composite film for selective electrochemical capturing lithium ions with low concentration. Separation and Purification Technology. 2019, 226, 59–67. [Google Scholar] [CrossRef]
- Wang, W.; Hong, G.; Zhang, Y.; Yang, X.; Hu, N.; Zhang, J.; Sorokin, P.; Shao, L. Designing an energy-efficient multi-stage selective electrodialysis process based on high-performance materials for lithium extraction. Journal of Membrane Science. 2023, 675, 121534. [Google Scholar] [CrossRef]
- Li, B.; Jiang, L.; Xiao, N.; Liu, S.; Zhang, Z.; Liu, F.; Free, M. L. Enhanced lithium separation with Li1.3Al0.3Ti1.7(PO4)3 lithium superionic conductor and aided charge balance. Separation and Purification Technology. 2024, 351, 128058. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Pang, B.; Liu, C.; Liu, Z. Study on the optimal conductivity titration parameters for SO42- in cement-based materials. Measurement. 2024, 115277. [Google Scholar] [CrossRef]
- Wenten, I. G.; Bazant, M. Z.; Khoiruddin, K. Mitigating electrodialysis membrane fouling in seawater desalination. Separation and Purification Technology. 2024, 345, 127228. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).