Submitted:
22 July 2024
Posted:
24 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Site and Stand Description
2.2. Field Sampling and Measurements
2.3. Statistical Analysis
3. Results
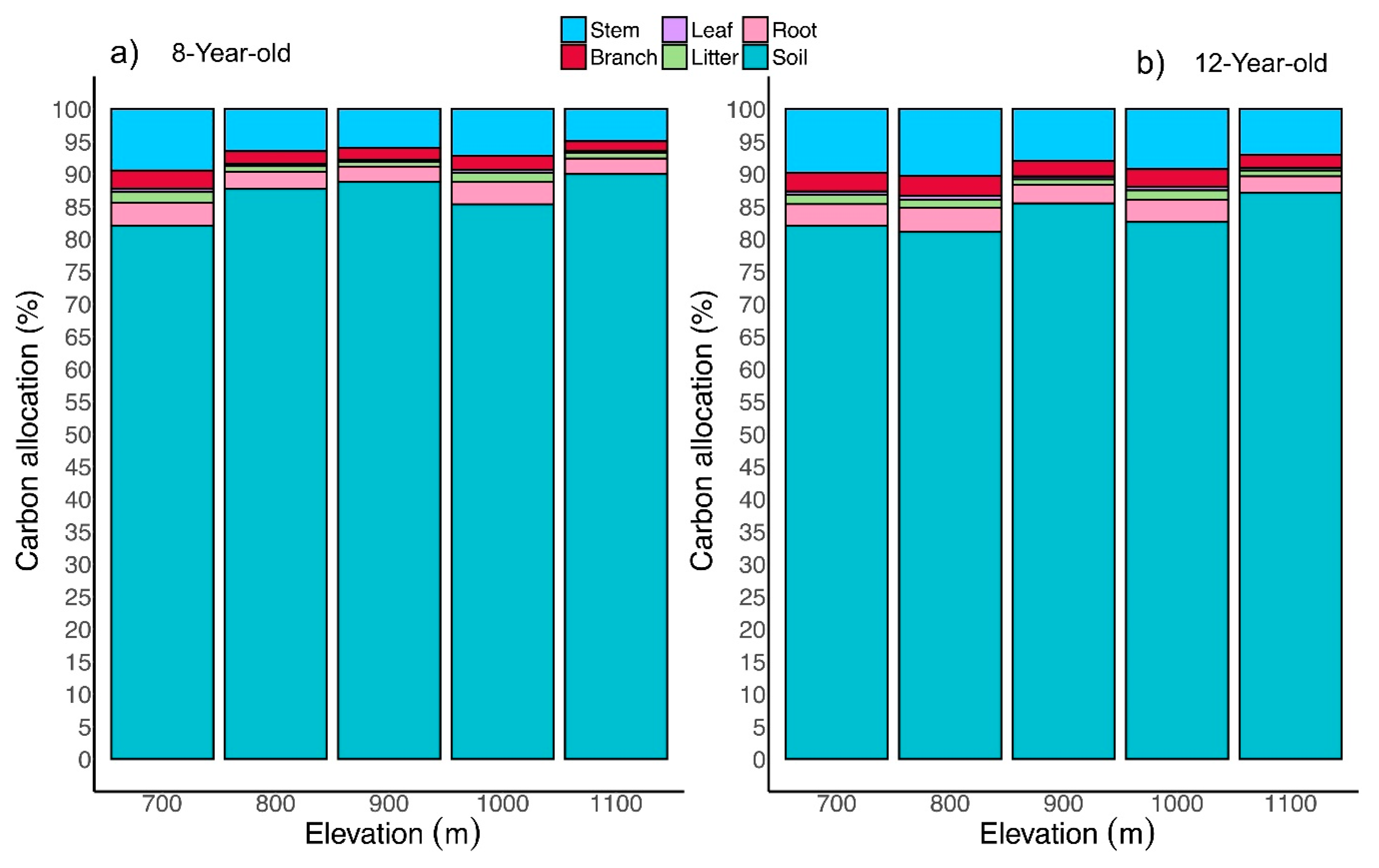
3.1. Tree Biomass Partitioning
3.2. Litter Biomass and C Concentration
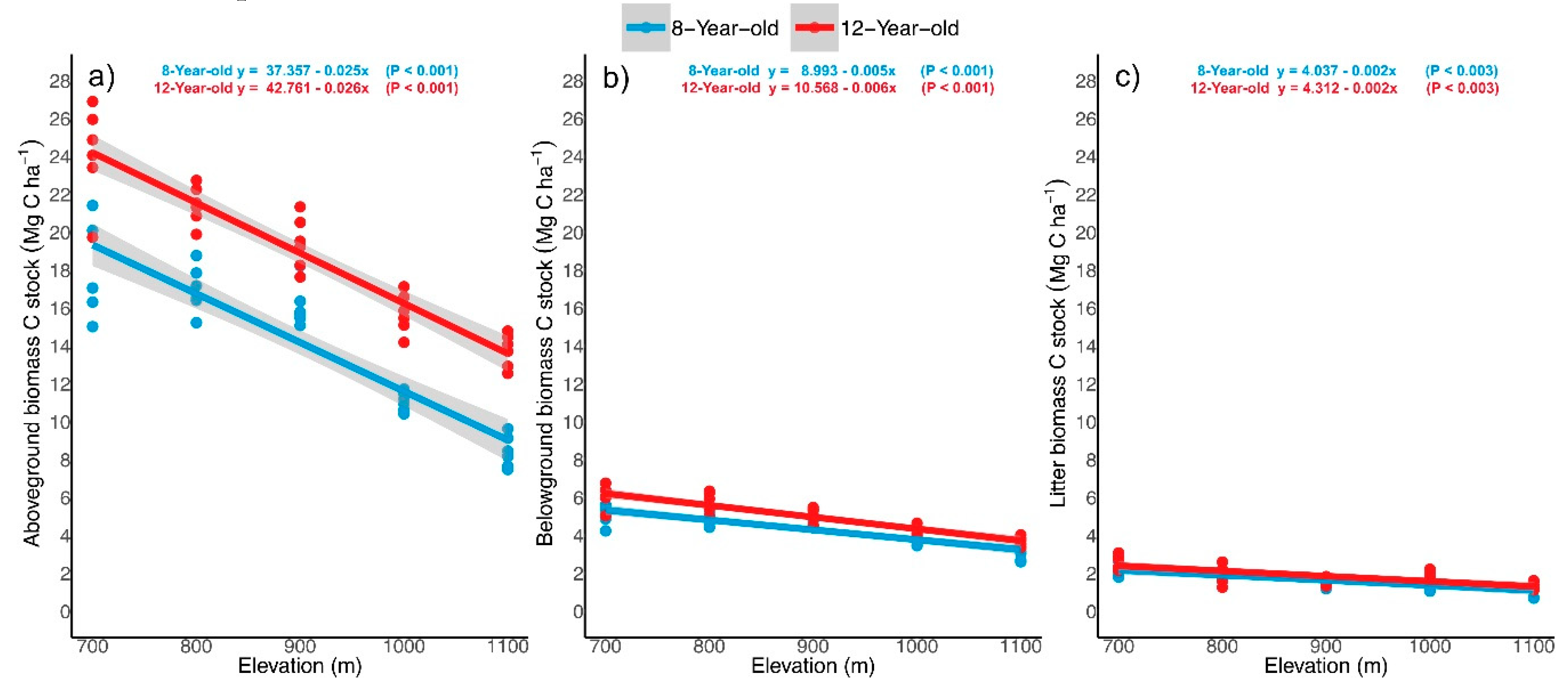
3.3. Tree Components C Stock
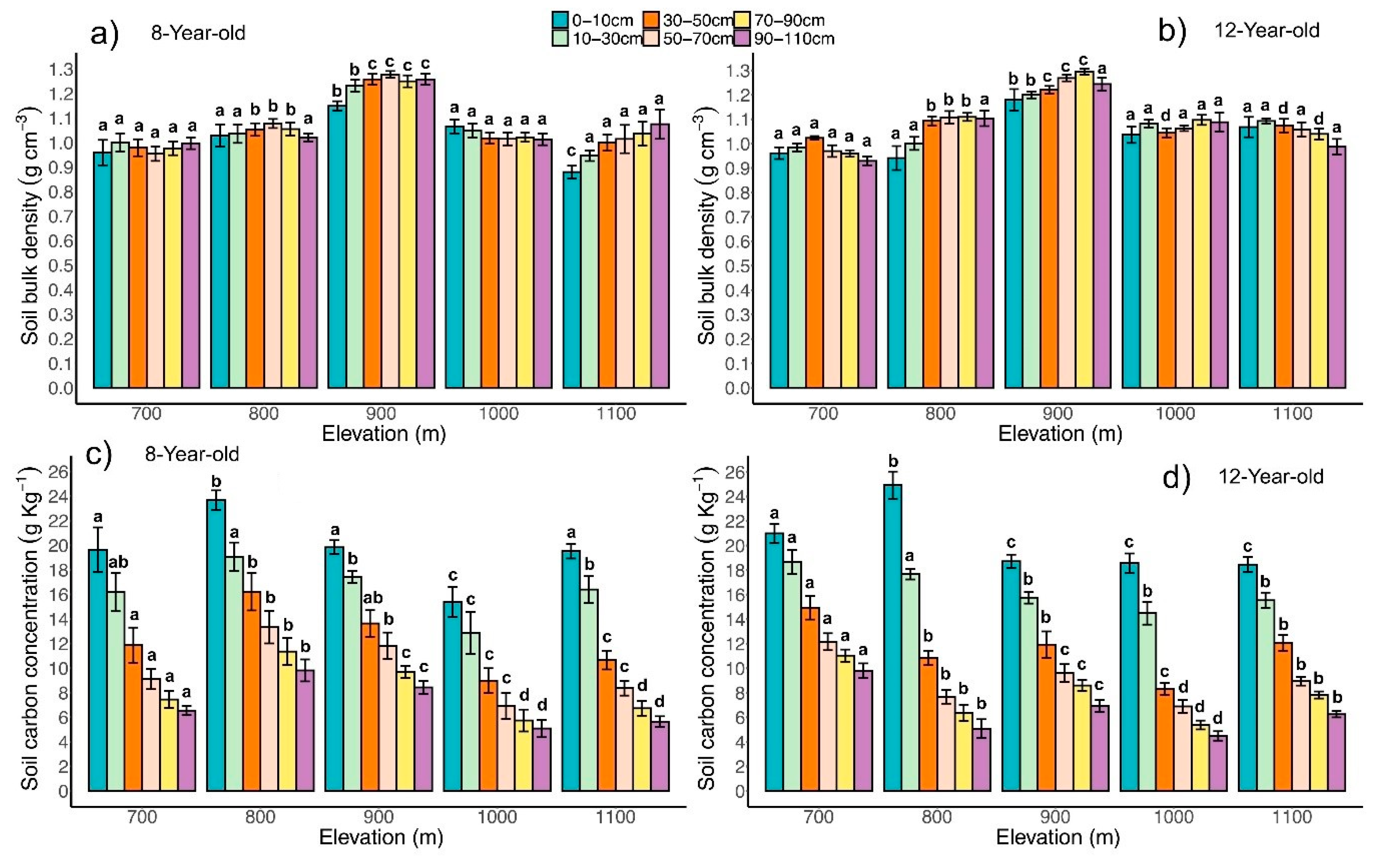
3.4. Soil C Concentration and Stock
3.5. Total Ecosystem C Stock
4. Discussion
4.1. Biomass C Stock
4.2. Soil C Stock
4.3. Total Ecosystem C Stock
4.4. Implications of C Storage on the Elevation Gradient
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alam, A., A. Kilpeläinen, and S. Kellomäki, Impacts of initial stand density and thinning regimes on energy wood production and management-related CO2 emissions in boreal ecosystems. European Journal of Forest Research, 2012. 131(3): p. 655-667. [CrossRef]
- Zhang, Y. and S. Liang, Changes in forest biomass and linkage to climate and forest disturbances over Northeastern China. Global Change Biology, 2014. 20(8): p. 2596-2606. [CrossRef]
- Houghton, R.A., et al., Carbon emissions from land use and land-cover change. Biogeosciences, 2012. 9(12): p. 5125-5142. [CrossRef]
- Grace, J., E. Mitchard, and E. Gloor, Perturbations in the carbon budget of the tropics. Global Change Biology, 2014. 20(10): p. 3238-3255. [CrossRef]
- Green, R.E., et al., Farming and the fate of wild nature. science, 2005. 307(5709): p. 550-555. [CrossRef]
- Fitzherbert, E.B., et al., How will oil palm expansion affect biodiversity? Trends in ecology & evolution, 2008. 23(10): p. 538-545. [CrossRef]
- Goetz, S.J., et al., Measurement and monitoring needs, capabilities and potential for addressing reduced emissions from deforestation and forest degradation under REDD+. Environmental Research Letters, 2015. 10(12): p. 123001. [CrossRef]
- Fox, J., et al., Rubber plantations expand in mountainous Southeast Asia: what are the consequences for the environment? 2014.
- Li, Z. and J.M. Fox, Mapping rubber tree growth in mainland Southeast Asia using time-series MODIS 250 m NDVI and statistical data. Applied Geography, 2012. 32(2): p. 420-432. [CrossRef]
- Yi, Z.F., et al., Developing indicators of economic value and biodiversity loss for rubber plantations in Xishuangbanna, southwest China: A case study from Menglun township. 2013.
- Fox, J., J.C. Castella, and A.D. Ziegler, Swidden, rubber and carbon: Can REDD+ work for people and the environment in Montane Mainland Southeast Asia? Global Environmental Change, 2014. 29: p. 318-326. [CrossRef]
- Sahner, J., et al., Degradation of root community traits as indicator for transformation of tropical lowland rain forests into oil palm and rubber plantations. PloS one, 2015. 10(9): p. e0138077. [CrossRef]
- Ziegler, A.D., J.M. Fox, and J. Xu, The rubber juggernaut. Science, 2009. 324(5930): p. 1024-1025. [CrossRef]
- Warren-Thomas, E., P.M. Dolman, and D.P. Edwards, Increasing demand for natural rubber necessitates a robust sustainability initiative to mitigate impacts on tropical biodiversity. Conservation Letters, 2015. 8(4): p. 230-241. [CrossRef]
- Fox, J. and J.C. Castella, Expansion of rubber (Hevea brasiliensis) in Mainland Southeast Asia: what are the prospects for smallholders? The Journal of Peasant Studies, 2013. 40(1): p. 155-170. [CrossRef]
- Zomer, R.J., et al., Environmental stratification to model climate change impacts on biodiversity and rubber production in Xishuangbanna, Yunnan, China. Biological Conservation, 2014. 170: p. 264-273. [CrossRef]
- Li, H., et al., Demand for rubber is causing the loss of high diversity rain forest in SW China. Biodivers Conserv, 2007. 16: p. 1731-1745. [CrossRef]
- Li, M. and F. Yuan, Rubber plantations–green desert. Chinese National Geography, 2008. 4: p. 60-78.
- Xiao, G. and S. Zhong, Mountain climate with high elevation and rubber suitable region in Xishuangbanna. Trop Agric Sci Technol, 2007. 30(2): p. 1-6.
- Yi, Z.F., et al., Developing indicators of economic value and biodiversity loss for rubber plantations in Xishuangbanna, southwest China: A case study from Menglun township. Ecological Indicators, 2014. 36: p. 788-797. [CrossRef]
- Dar, J.A. and S. Sundarapandian, Variation of biomass and carbon pools with forest type in temperate forests of Kashmir Himalaya, India. Environmental monitoring and assessment, 2015. 187(2): p. 55. [CrossRef]
- Ensslin, A., et al., Effects of elevation and land use on the biomass of trees, shrubs and herbs at Mount Kilimanjaro. Ecosphere, 2015. 6(3): p. 1-15. [CrossRef]
- Van Do, T., et al., Aboveground biomass and tree species diversity along altitudinal gradient in Central Highland, Vietnam. Tropical Ecology, 2017. 58(1).
- Thompson, I., et al., V., Kurz, WA, Spalding, M. and van Vliet, N., 2012. Forest biodiversity, carbon and other ecosystem services: Relationships and impacts of deforestation and forest degradation. Understanding Relationships Between Biodiversity, Carbon, Forests and People: The Key to Achieving REDD+ Objectives, 2012.
- Liu, C., et al., Carbon stocks across a fifty year chronosequence of rubber plantations in tropical China. Forests, 2017. 8(6): p. 209. [CrossRef]
- Ziegler, A.D., et al., Carbon outcomes of major land-cover transitions in SE Asia: great uncertainties and REDD+ policy implications. Global change biology, 2012. 18(10): p. 3087-3099. [CrossRef]
- Le Quéré, C., et al., Global carbon budget 2016. Earth System Science Data (Online), 2016. 8(2). [CrossRef]
- Körner, C., The use of ‘altitude’in ecological research. Trends in ecology & evolution, 2007. 22(11): p. 569-574. [CrossRef]
- Malhi, Y., et al., Introduction: elevation gradients in the tropics: laboratories for ecosystem ecology and global change research. Global Change Biology, 2010. 16(12): p. 3171-3175. [CrossRef]
- Gavazov, K., et al., Biotic and abiotic constraints on the decomposition of Fagus sylvatica leaf litter along an altitudinal gradient in contrasting land-use types. Ecosystems, 2014. 17(8): p. 1326-1337. [CrossRef]
- Nations, A.O.f.t.U., World reference base for soil resources. Vol. 3. 1998: Food & Agriculture Org.
- Yang, X., et al., Land-use change impact on time-averaged carbon balances: Rubber expansion and reforestation in a biosphere reserve, South-West China. Forest Ecology and Management, 2016. 372: p. 149-163. [CrossRef]
- Yang, X., et al., Rubber tree allometry, biomass partitioning and carbon stocks in mountainous landscapes of sub-tropical China. Forest ecology and management, 2017. 404: p. 84-99. [CrossRef]
- Grieco, E., Land use change and carbon stock dynamics in Sub-Saharan Africa-Case study of Western Africa–Ghana. 2011.
- Choudhary, B.K., K. Majumdar, and B.K. Datta, Carbon sequestration potential and edaphic properties along the plantation age of rubber in Tripura, Northeastern India. Current World Environment, 2016. 11(3): p. 756. [CrossRef]
- Selma Regina, M., et al., Potential carbon sequestration in rubber tree plantations in the northwestern region of the Paraná State, Brazil. Acta Scientiarum. Agronomy, 2014. 36(2). [CrossRef]
- Diniz, A.R., et al., Contrasts in areas of rubber tree clones in regard to soil and biomass carbon stocks. Revista Brasileira de Ciência do Solo, 2015. 39(5): p. 1378-1385. [CrossRef]
- Fernandes, T.J.G., et al., Quantificação do carbono estocado na parte aérea e raízes de Hevea sp., aos 12 anos de idade, na Zona da Mata Mineira. Revista Arvore, 2007. 31: p. 657-665. [CrossRef]
- Yanci, S., et al., Temporal changes of ecosystem carbon stocks in rubber plantations in Xishuangbanna, Southwest China. Pedosphere, 2017. 27(4): p. 737-746.
- Li, H., et al., Past, present and future land-use in Xishuangbanna, China and the implications for carbon dynamics. Forest Ecology and Management, 2008. 255(1): p. 16-24. [CrossRef]
- Lü, X.T., et al., Ecosystem carbon storage and partitioning in a tropical seasonal forest in Southwestern China. Forest Ecology and Management, 2010. 260(10): p. 1798-1803. [CrossRef]
- N’Dri, J.K., et al., Can litter production and litter decomposition improve soil properties in the rubber plantations of different ages in Côte d’Ivoire? Nutrient cycling in agroecosystems, 2018. 111: p. 203-215.
- de Blécourt, M., et al., Soil carbon stocks decrease following conversion of secondary forests to rubber (Hevea brasiliensis) plantations. PloS one, 2013. 8(7): p. e69357. [CrossRef]
- Wauters, J.B., et al., Carbon stock in rubber tree plantations in Western Ghana and Mato Grosso (Brazil). Forest Ecology and Management, 2008. 255(7): p. 2347-2361. [CrossRef]
- Zheng, Z., et al., Carbon storage and distribution law of the rubber forest ecological system in Danzhou of Hainan. Trop. Agric. Eng, 2010. 34: p. 45-50.
- Zhou, Y.R., Z.L. Yu, and S.D. Zhao, Carbon storage and budget of major Chinese forest types. Chinese Journal of Plant Ecology, 2000. 24(5): p. 518.
- Jianwei, T., et al., A Preliminary Study on the Biomass of Secondary Tropical Forest in Xishuangbana. Chinese Journal of Plant Ecology, 1998. 22(6): p. 489.
- FAO, Food and Agriculture Organization of the United Nations. 2021.
- Fu, Y., et al., Smallholder rubber plantation expansion and its impact on local livelihoods, land use and agrobiodiversity, a case study from Daka, Xishuangbanna, southwestern China. International Journal of Sustainable Development & World Ecology, 2009. 16(1): p. 22-29. [CrossRef]
- Jianchu, X., et al., Land-use and land-cover change and farmer vulnerability in Xishuangbanna prefecture in southwestern China. Environmental Management, 2005. 36: p. 404-413. [CrossRef]
- Min, S., et al., Adoption of intercropping among smallholder rubber farmers in Xishuangbanna, China. International Journal of Agricultural Sustainability, 2017. 15(3): p. 223-237. [CrossRef]
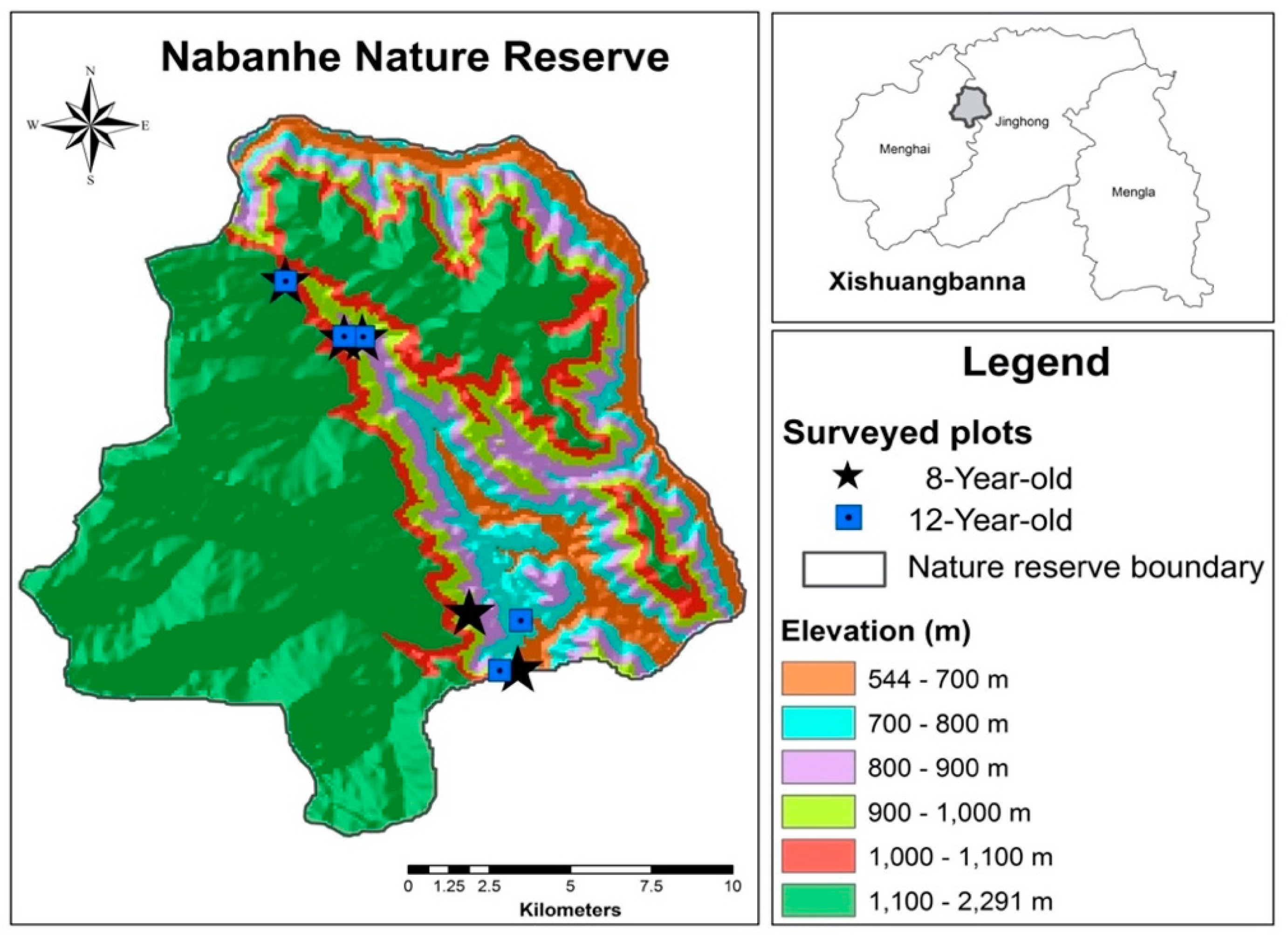

| Age | Elevation (m) | Longitude | Latitude | Aspect (°) | Slope (°) | Planting spacing (m) |
|---|---|---|---|---|---|---|
| 8-Year-old | 700 | 100°40′33.58″ | 22°07′53.10″ | Southeast (20) | 25 | 6 × 2.5 |
| 800 | 100°39′53.21″ | 22°08′51.55″ | Southeast (10) | 30 | 6 × 2.5 | |
| 900 | 100°37′47.52″ | 22°13′26.74″ | Southwest (20) | 30 | 6 × 2.5 | |
| 1000 | 100°37′44.91″ | 22°13′26.89″ | Southeast (20) | 25 | 6 × 2.5 | |
| 1100 | 100°36′46.93″ | 22°14′25.33″ | Southeast (30) | 25 | 6 × 2.5 | |
| 12-Year-old | 700 | 100°40′03.48″ | 22°07′49.81″ | Southeast (15) | 30 | 6 × 2.5 |
| 800 | 100°40′38.58″ | 22°08′36.33″ | Southeast (20) | 25 | 6 × 2.5 | |
| 900 | 100°37′47.62″ | 22°13′26.79″ | Southwest (24) | 30 | 6 × 2.5 | |
| 1000 | 100°37′44.69″ | 22°13′28.65″ | Southeast (20) | 25 | 6 × 2.5 | |
| 1100 | 100°36′47.46″ | 22°14′24.47″ | Southeast (60) | 30 | 6 × 2.5 |
| Age | Elevation (m) | Stem | Branch | Foliage | Root | Total |
|---|---|---|---|---|---|---|
| 8-Year-old | 700 | 31.64 ± 1.81a | 8.82 ± 0.50a | 1.58 ± 0.07a | 11.62 ± 0.46a | 53.66 ± 2.85a |
| 800 | 29.27 ± 0.87a | 8.18 ± 0.24a | 1.51 ± 0.05a | 11.36 ± 0.36a | 50.32 ± 1.52a | |
| 900 | 27.75 ± 0.79a | 7.75 ± 0.22a | 1.42 ± 0.04a | 10.65 ± 0.25a | 47.57 ± 1.29a | |
| 1000 | 18.93 ± 0.35b | 5.34 ± 0.10b | 1.09 ± 0.02b | 8.86 ± 0.19b | 34.21 ± 0.66b | |
| 1100 | 14.39 ± 0.59c | 4.07 ± 0.16c | 0.84 ± 0.03c | 6.87 ± 0.26c | 26.17 ± 1.05c | |
| 12-Year-old | 700 | 41.84 ± 1.78a | 11.60 ± 0.49a | 1.97 ± 0.08a | 13.90 ± 0.54a | 69.31 ± 2.89a |
| 800 | 37.04 ± 0.71b | 10.30 ± 0.20b | 1.81 ± 0.05ab | 13.18 ± 0.49ab | 62.33 ± 1.45ab | |
| 900 | 33.58 ± 0.98b | 9.33 ± 0.27b | 1.63 ± 0.04b | 11.75 ± 0.33b | 56.29 ± 1.62b | |
| 1000 | 27.14 ± 0.75c | 7.56 ± 0.21c | 1.35 ± 0.03c | 9.88 ± 0.22c | 45.93 ± 1.21c | |
| 1100 | 23.95 ± 0.72c | 6.67 ± 0.20c | 1.19 ± 0.03c | 8.66 ± 0.24c | 40.46 ± 1.19c |
| Age | Elevation (m) | Leaf | Twig | Flowers/fruits | Miscellaneous | BDB | Total |
| 8-Year-old | 700 | 4.17 ± 0.25a | 0.50 ± 0.10a | 0.04 ± 0.02a | 0.08 ± 0.00a | 0.17 ± 0.05ab | 4.95 ± 0.43a |
| 800 | 2.48 ± 0.25b | 0.66 ± 0.09a | 0.09 ± 0.08a | 0.12 ± 0.02ab | 0.33 ± 0.10a | 3.67 ± 0.55b | |
| 900 | 2.39 ± 0.08b | 0.37 ± 0.06a | 0.01 ± 0.00a | 0.06 ± 0.00b | 0.09 ± 0.05ab | 2.90 ± 0.19bc | |
| 1000 | 2.55 ± 0.33b | 0.75 ± 0.08a | ND | 0.06 ± 0.01b | 0.12 ± 0.04ab | 3.47 ± 0.46b | |
| 1100 | 1.63 ± 0.21b | 0.49 ± 0.22a | ND | 0.05 ± 0.01b | 0.06 ± 0.03b | 2.23 ± 0.47c | |
| 12-Year-old | 700 | 4.80 ± 0.24a | 0.23 ± 0.03a | 0.30 ± 0.07a | 0.09 ± 0.01a | 0.21 ± 0.06a | 5.62 ± 0.40a |
| 800 | 3.23 ± 0.19b | 0.30 ± 0.07a | 0.02 ± 0.01b | 0.06 ± 0.01b | 0.43 ± 0.20a | 4.04 ± 0.48b | |
| 900 | 2.63 ± 0.13bc | 0.36 ± 0.08a | ND | 0.05 ± 0.01b | 0.20 ± 0.07a | 3.23 ± 0.28bc | |
| 1000 | 3.20 ± 0.18b | 0.43 ± 0.08a | ND | 0.04 ± 0.00b | 0.15 ± 0.07a | 3.82 ± 0.34bc | |
| 1100 | 2.31 ± 0.16dc | 0.32 ± 0.06a | 0.02 ± 0.02b | 0.03 ± 0.00b | 0.10 ± 0.04a | 2.78 ± 0.29c |
| Age | Depth (cm) | 0–10 | 10–30 | 30–50 | 50–70 | 70–90 | 90–110 | Total |
| 8-Year-old | 700m | 18.39 ± 0.80ba | 31.87 ± 2.12bc | 22.91 ± 2.37b | 17.31 ± 1.56b | 14.44 ± 1.18b | 13.00 ± 0.67b | 117.91 ± 1.45b |
| 800m | 24.17 ± 0.48a | 39.13 ± 1.38ab | 33.83 ± 2.66a | 28.61 ± 2.68a | 23.79 ± 2.07a | 20.11 ± 2.05a | 169.64 ± 1.89a | |
| 900m | 22.80 ± 0.83b | 42.92 ± 1.50a | 34.36 ± 2.96a | 30.19 ± 2.82a | 24.25 ± 1.41a | 21.25 ± 1.54a | 175.77 ± 11.06a | |
| 1000m | 16.47 ± 1.55b | 26.86 ± 3.58c | 18.40 ± 2.27b | 14.11 ± 2.29b | 11.61 ± 1.72b | 9.03 ± 1.30b | 96.49 ± 12.72b | |
| 1100m | 17.19 ± 0.81b | 30.92 ± 1.87bc | 21.25 ± 1.5b | 16.93 ± 1.42b | 13.89 ± 1.28b | 12.11 ± 1.16b | 112.29 ± 8.08b | |
| 12-Year-old | 700m | 19.12 ± 0.5a | 26.25 ± 3.69ab | 34.83 ± 2.10a | 27.05 ± 2.31b | 22.88 ± 1.97b | 20.14 ± 1.66c | 150.27 ± 2.04a |
| 800m | 23.26 ± 0.95a | 35.31 ± 0.92ab | 23.79 ± 1.58ab | 17.03 ± 1.35a | 14.17 ± 1.59a | 11.16 ± 1.71a | 124.71 ± 1.35ab | |
| 900m | 21.67 ± 1.20a | 33.26 ± 3.58a | 31.71 ± 3.14a | 25.24 ± 2.26b | 22.85 ± 1.47b | 18.78 ± 2.07bc | 153.51 ± 13.71a | |
| 1000m | 19.24 ± 0.99a | 31.26 ± 1.74b | 17.33 ± 0.94b | 14.61 ± 1.09a | 11.81 ± 0.89a | 9.65 ± 0.63a | 103.90 ± 6.28b | |
| 1100m | 19.66 ± 0.85a | 33.99 ± 1.55ab | 25.78 ± 1.00a | 18.94 ± 1.00ab | 16.26 ± 0.62a | 12.39 ± 0.80ab | 127.01 ± 5.82ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





