1. Introduction
Coronaviruses are responsible for multiple novel
diseases in the human population, ranging from acute respiratory infections
such as SARS-CoV, SARS-CoV2, and MERS-CoV to viruses that cause the common cold
like HCoV-229E and HCoV-NL63 [3]. SARS and
SARS-CoV-2 belong to the Nidovirus group of positive-sense single-stranded RNA
viruses. SARS first emerged in 2003, and recent genome sequencing studies revealed
up to 89% sequence similarity to the SARS-CoV-2 genome.
Currently, there are very few approved therapeutic
agents for coronavirus infection: Boosted Protease Inhibitors, which combine a
protease inhibitor with a booster molecule to improve its antiviral activity
and Pharmacokinetic properties, and natural inhibitors. Examples of boosted
protease inhibitors are Nirmatrelvir/ritonavir (Paxlovid) (a combination of 3cl
protease inhibitor and a booster that increases nirmatrelvir's levels in the
body[4]; Molnupiravir (Lagevio) (it introduces
errors into the viral RNA during replication, thus inhibiting the virus's viral
growth and disrupting its function indirectly); Non -boosted Protease
Inhibitors are currently going through clinical trials (GC343, which presently
failed to show significant efficacy in large clinical trials for COVID-19 and
VX-745: Another inhibitor with promising preclinical data, currently in
Phase 2 clinical trials for COVID-19) [5].
Natural Inhibitors include plant extracts, marine
natural products, and naturally accruing peptides: Extracts from various
plants, like licorice root, green tea, and Andrographis paniculata, have shown
potential for inhibiting 3CL protease in laboratory studies. However, their
efficacy in humans remains unclear and requires further investigation[6]. Compounds isolated from marine organisms like
sponges and algae have also exhibited 3CL protease inhibitory activity. Like
plant extracts, more research is needed to assess their potential as antiviral
agents[7]. Naturally occurring peptides
derived from various sources, including food or microorganisms, have shown
promise inhibiting 3CL protease. However, challenges remain in optimizing their
stability and delivery for therapeutic use[8].
While synthetic inhibitors like
Nirmatrelvir/Ritonavir and Molnupiravir have shown clinical efficacy against
COVID-19, continued research is crucial to develop even more potent and broadly
effective drugs. Natural inhibitors hold potential, but challenges often hamper
their development in isolating, purifying, and optimizing them for therapeutic
use. Combination therapies that target different aspects of the viral life
cycle, including the 3CL protease, could be a promising strategy for combating
coronaviruses and other noroviruses. The main target for antiviral therapy for
COVID-19 is the 3CL protease (3CLpro, Main protease, Mpro, Nsp5). The 3CL
protease plays an integral role in viral replication.
One of the most critical viral enzymes produced by
the following two coronaviruses is nsp5 (3CLpro). 3C-like protease
(3CLpro), the main protease (Mpro), refers to the cysteine
protease's critical role in coronavirus gene expression and replicase
processing. The following enzyme is conserved across all nidoviruses and is
considered the “Achilles heel of the SARS-CoV-2 virus”[9].
Among coronaviruses, the 3CL protease within the
same genus exhibits greater than 80% sequence identity. The greatest sequence
conservation exists in and around the enzyme’s active site. Sequence analysis
of the SARS-CoV 3CLpro and SARS-CoV-2 3CLpro revealed
only a difference of 12 amino acid residues, allowing for approximately 96%
sequence identity between the 3CL proteases for the two coronaviruses[10]. The 12 amino acid residues
are: T35V, A46S, S65N, L86V, R88K, S94A, H134F, K180N, L202V, A267S, T285A,
I286L. It is important to note only A46S was revealed to be close to the
protease’s active site but does not affect the enzyme’s proteolytic activity.
The other 11 variant positions are distant from the enzyme’s binding site and
do not affect the enzymatic activity. Furthermore, the superposition of the
active site with and without ligands for the SARS-CoV 3CLpro and
SARS-CoV-2 3CLpro revealed high similarity[11].
Regarding the 3CL protease’s function, the enzyme
mediates the proteolytic cleavage of pp1a and pp1ab by processing at more than ten
distinct cleavage sites with a QS cleavage site. This produces 16
non-structural proteins (nsp1 to nsp16)[12].
The polyprotein pp1a contains non-structural proteins 1-11, while polyprotein
pp1ab contains the translated coding regions of nsp1-16, including the nsp12
RNA-dependent RNA polymerase and helicase. The proteolytic processing of the two
viral polyproteins by 3CL protease is essential for viral replication because
it creates mature and functionally active replication machinery for the virus.
In other words, the proteolytic processing of the two viral polyproteins by 3CL
protease acts as a critical regulatory mechanism in expressing the coronavirus
replicase proteins. Thus, since the SARS-CoV outbreak of 2003-2004, research
has demonstrated 3CL protease as a desirable antiviral medication target
against SARS and other coronaviruse[13]s.
Drugs primarily targeting viral and bacterial
diseases such as SARS and Coronavirus infection are prone to developing
resistance to viral and bacterial mutations[14].
For example, as Paxlovid continues to be the only known treatment for the SARS-CoV-2
virus, many recent studies have noted potential resistance towards the
3CL-protease inhibitor due to mutations[15].
For instance, a study led by a virologist revealed that after 12 rounds of
Nirmatrelvir treatment, the SARS-CoV-2 virus accumulated three mutations at
amino acid positions 50, 166, and 167 in 3CLpro that reduced the virus’
susceptibility to Nirmatrelvir by 20-fold. Furthermore, another study spotted
potential resistance-conferring mutations at positions 50 and 166 in 3CL pro
that, when combined, the virus was 80 times less susceptible to Nirmatrelvir[16]. In other words, viruses have an extremely high
mutation rate, and these mutations can occur in any region of the viral genome.
More specifically, mutations in the protease region of the COVID-19 genome have
proven to create drug resistance and reduce the protease inhibitory effect of
Paxlovid. Thus, it is essential to develop potent inhibitors that target the
3CL protease’s mechanism to prevent drug resistance in the future. The
QCT technology overcomes the major underlying disadvantage of the
structural drug design method: drug resistance.
1.1. Catalytic Mechanism-Based Drug Design: QCT
Catalytic mechanism-based drug design, also known
as Quantum Core Technology (QCT), stems from a combination of quantum
calculations and physical organic chemistry developed by Dr. Dorit Arad. The
novel methodology for drug design incorporates basic concepts regarding
enzymatic mechanisms, such as the Koshland-induced fit model and the Molecular
Orbital theory[17]. More specifically, the QCT
applies quantum mechanical calculations to protein structure data sets to
systematically ascertain the catalytic transition state of specific enzyme
families to design and synthesize particular enzyme inhibitors. In
other words, the novel technology considers both the conditions required for
the enzymatic reaction and the core of the enzyme’s catalytic site, known as
the reaction center, to create potent and selective viral inhibitors[18]. One of the significant challenges in drug
design is creating inhibitors that can both target and fit perfectly into the
enzyme’s active site. Thus, NLC Pharma utilizes the “Structural Mechanistic
Enzymology” (SME) approach[19] to classify
enzymes based on their enzymatic mechanism and protein structure to create
inhibitors that react and form a covalent bond with the enzyme’s reaction
center, allowing the molecule to bind to the enzyme[20]
tightly. Furthermore, Quantum Core Technology designs molecules that directly
interact with the molecule and the enzyme’s critical catalytic residue in the
active site (the reaction center), activating the enzyme (triggering). In the
enzyme’s resting state, the catalytic residues cannot interact and activate the
enzyme due to a lack of spatial alignment. When the substrate interacts with
the enzyme, it forms the enzyme-substrate complex (ES complex), which consists
of numerous non-covalent interactions between the active site residues and the
substrate, thus increasing the ES binding energy[21].
The formation of the ES complex activates the catalytic machinery of the
enzyme, inducing a change in the spatial conformation of the catalytic residues
and allowing the catalytic residues to react among themselves chemically. As a
result, the catalytic residue reacts with the substrate in a step called
“triggering” and initiates the enzymatic reaction[22].
In application, QCT identifies the triggering reaction of each enzyme using ab
initio quantum mechanical calculations[23].
The QCT drug development process can be broadly broken down into three stages:
analysis, creation of core structures, and optimization. During the analysis
stage, the main task is classifying enzyme families according to their quantum
mechanism. This is followed by designing core structures based on previous
cores of similar enzymes, mechanical analysis, known inhibitors, modeling, and
screening of focused libraries[24]. The
optimization stage can be performed in three forms: modeling and computation
chemistry, virtual libraries, or combinatorial chemistry[25]. The goal of the optimization stage is to gain
the potency and specificity of the molecule. Utilizing the Quantum Core
Technology as a means for mechanism-based drug designs provides the
pharmaceutical industry with a unique and efficient method to obtain novel core
molecules that can serve as the basis for developing new drugs, raising the
overall productivity in the sector.
2. Materials and Methods
2.1. SARS-Cov-2 3CL Protease:
Recombinant SARS-CoV 3CL Protease 50 UG E-718-050
from Doron Scientific, Israel.
2.2. Cloning and Production of SARS-Cov-2 3CL Protease with P132H Mutation:
A G-block of 3CL P132H was ordered from IDT. The
fragment was inserted into the pET14b plasmid as an XbaI-BamHI fragment.
pET14b-XopC was then utilized as a template vector. The plasmids were
then purified from bacteria using the MiniPrep kit, NEB. The G-Block (total lug
DNA) was re-suspended in 20ul TE, 50ng/µl
pET14b-XopC, and then digested using restriction enzymes XbaI and BamHI (HF) in
CutSmart buffer (10X) for 1hr at 37°C. We then ran samples in preparative agarose
gel (1-2%) and excised the bands according to the specific size (G-block:
~1000bp, digested pET14b: ~4500bp). Extracted DNA from gel slices (Gel
extraction kit, NEB). Ligation- A ‘self’ reaction was prepared with 13ul
nuclease-free water instead of inserting DNA. The reaction was incubated
overnight at RT. To create competent E. coli bacteria (XL-1 strain, prepared
in-house, Benhar lab), a starter was made with a colony from the transformation
plate that was inoculated into 4ml LB-amp (100ug/ml) and incubated ON at 37°C,
250RPM shaking. We then purified the plasmids from the bacteria (MiniPrep kit,
NEB) using 3ml of culture. 1ml of the culture was stored at 4C. pET14b-3CL mut
P132H: 80ng/µl. The plasmid was then sent
for sequencing. In addition, a glycerol stock was prepared using 1ml of the
culture mixed with 250ul of 80% glycerol and stored at -80°C. The plasmid was
transformed into E. coli BL-21 strain (Rosetta, prepared in-house, Benhar lab):
We then made a starter with a colony from the transformation plate, which we
inoculated into 10ml LB-amp (100ug/ml) and incubated ON at 37°C, 250RPM
shaking. We added Ampicillin to the LB medium (100ug/ml), and the medium was
inoculated with 5mL of the overnight culture of E. coli BL-21 with pET14b-3CL
mut P132H. The culture was grown at 37°C 250rpm, shaking until OD600
= 0.6. At this point, IPTG was added to a final concentration of 1mM (by
directly adding the powder to the culture). The culture was incubated at 18°C
overnight for induction of protein expression. In addition, we centrifuged
bacterial cells (Sorvall, 500mL cups) at 4000rpm for 20min. The sup was
discarded, and the pellet was stored at -20°C ON. Protein was separated in the
FF HisTrap HP column (1mL, GE). Protein size: 35.6Kda. Protein Ext. Co: 0.966.
All eluted fractions (including FT and washes) were quantified by nano-drop,
and protein concentration was calculated (Ext. co: 0.966). SDS-PAGE analyzed
all relevant fractions to verify the purity. Fractions with sufficiently high
concentration (>1.2mg/mL) were mixed and loaded on the Zeba™ Spin Desalting
column (Thermo Fisher, Cat# 89892) for buffer exchange. Storage buffer without
maltose, DTT, and glycerol was prepared x2 (meaning 80 Trism 220mM NaCL, 4.4mM
KCl) and used to wash and elute the protein from the Zeba column (according to
the manufacturer’s instructions). Following buffer exchange, the protein was
quantified by a BCA protein assay kit (Thermo Fisher) according to the
manufacturer’s instructions. Storage buffer x2 was used as blank.
2.3. Bioactivity Assay for 3CL Protease
The following assay was utilized to determine the
raw materials required to produce Tollovid—the assay tested for the inhibitory
activity of various plant extracts against recombinant 3cl protease. Inhibitory
activity (IC50) of plant extracts was performed using 488 (CBR2, by Cambridge
UK) and 670 (Covidyte™ TF670 ) substrates. NLC provided all plant-based
extracts and diagnostics. Thus, specific inhibitory activity cannot be
attributed to particular aspects or impurities of the extract but rather to the
entire plant. Any reference to Shikonin (active ingredient) in the following
report originates from the names of the materials designated by NLC.
“Shikonin”
refers to
5,8-dihydroxy-2-(1-hydroxy-4-methylpent-3-en-1-yl)naphthalene-1,4-dione and
more particularly to the (R)-enantiomer thereof, which is found in the roots
of Lithospermum erythrorhizon (purple gromwell), a plant used in
Chinese herbal medicine (in which it is referred to as “Zicao”) for treating a
variety of inflammatory and infectious diseases. The (S)-enantiomer is known as
“alkannin,” a plant that has been used in folk medicine to treat abscesses and
inflammations, and racemic mixtures are also known as “Shikalkin.”
Shikonin has been reported to exhibit activities
related to the treatment of cancer, inflammation, and wound healing,
as well as antiviral activity against HIV type I, adenovirus 3
(AdV3), and hepatitis C virus (HCV) and distinct anti-inflammation mechanisms,
such as inhibition of leukotriene B4 synthesis, suppression of mast cell degranulation,
inhibition of neutrophil respiratory burst, alteration of
phosphatidylinositol-mediated signaling, and blockade of chemokine binding to
CCR-1 [Andujar et al., Planta Med 2013, 79:1685;] and reduction of
interleukin-6, interleukin-8 and chemokine C—C motif ligand
(CCL)20 production[26]
The controls for the following bioactivity assay
were samples with no assay and samples with DMSO. A stock solution of 20mM was
added to DMSO to prepare the sample based on the percentage of Shikonin
provided by NLC. The solution then underwent serial dilutions in DMSO, all
while light-protected. To prepare a buffer, enzyme solution of 150 ng final
concentration, substrate solution of 1 M final concentration, and reagent
buffer with DTT (RB+) were added. Extract dilutions were incubated with enzyme
for 30 minutes in the dark. Percentage interference was calculated by 1 – [FU
measurement of free fluorophore in RB in RB at the specific extract
concentration divided by FU of no extract]. Normalized slope calculations were
determined by slope x (1 + % interference).
2.4. Preparation of NLC-V
NLC-V is a liposomal formulation of Shikonin, 95% extracted
from Lithospermum erythrorhizon gromwell root and Arnebia euchromatin extract.
Patients were administered NLC-V through an oral capsule containing 10mg of
Shikonin.
3. Results
3.1. In-Vitro Assay Results:
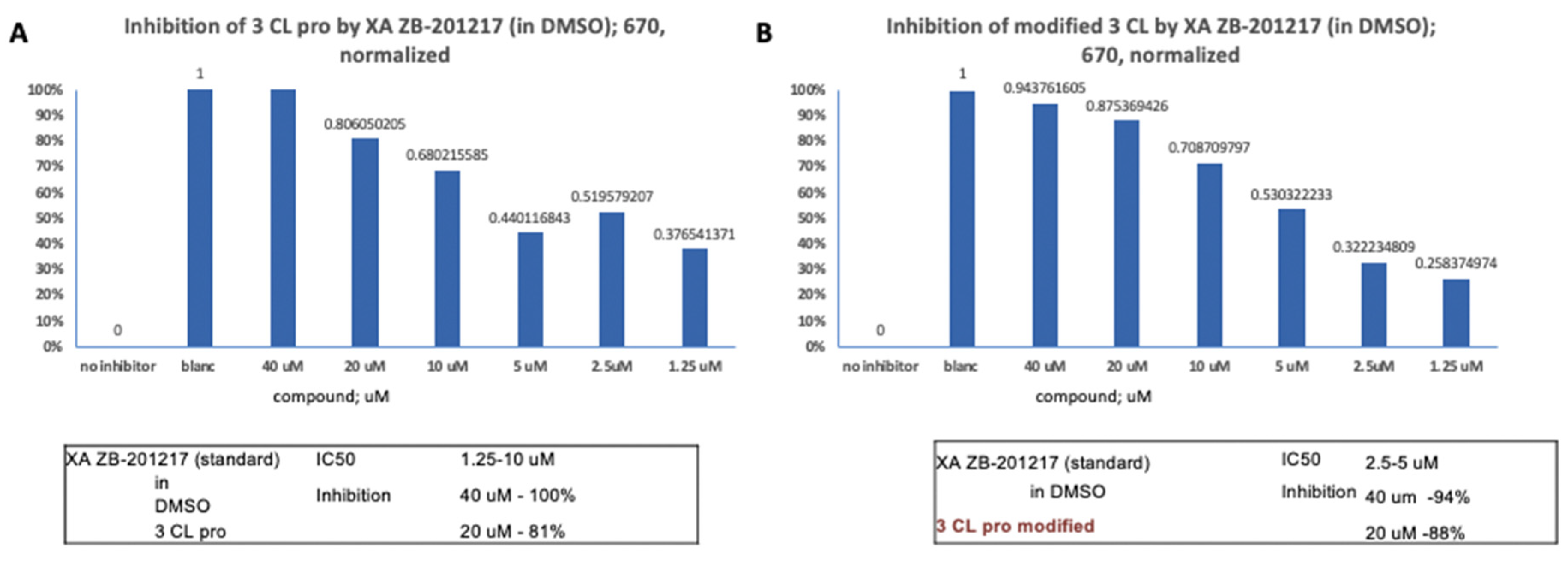
40uM of XA ZB-201217 (in DMSO) displayed 100%
inhibitory activity against 3CL pro (B), and 40uM of XA ZB-201217 (in DMSO)
showed 94% inhibitory activity against modified 3CL (
Figure 1).
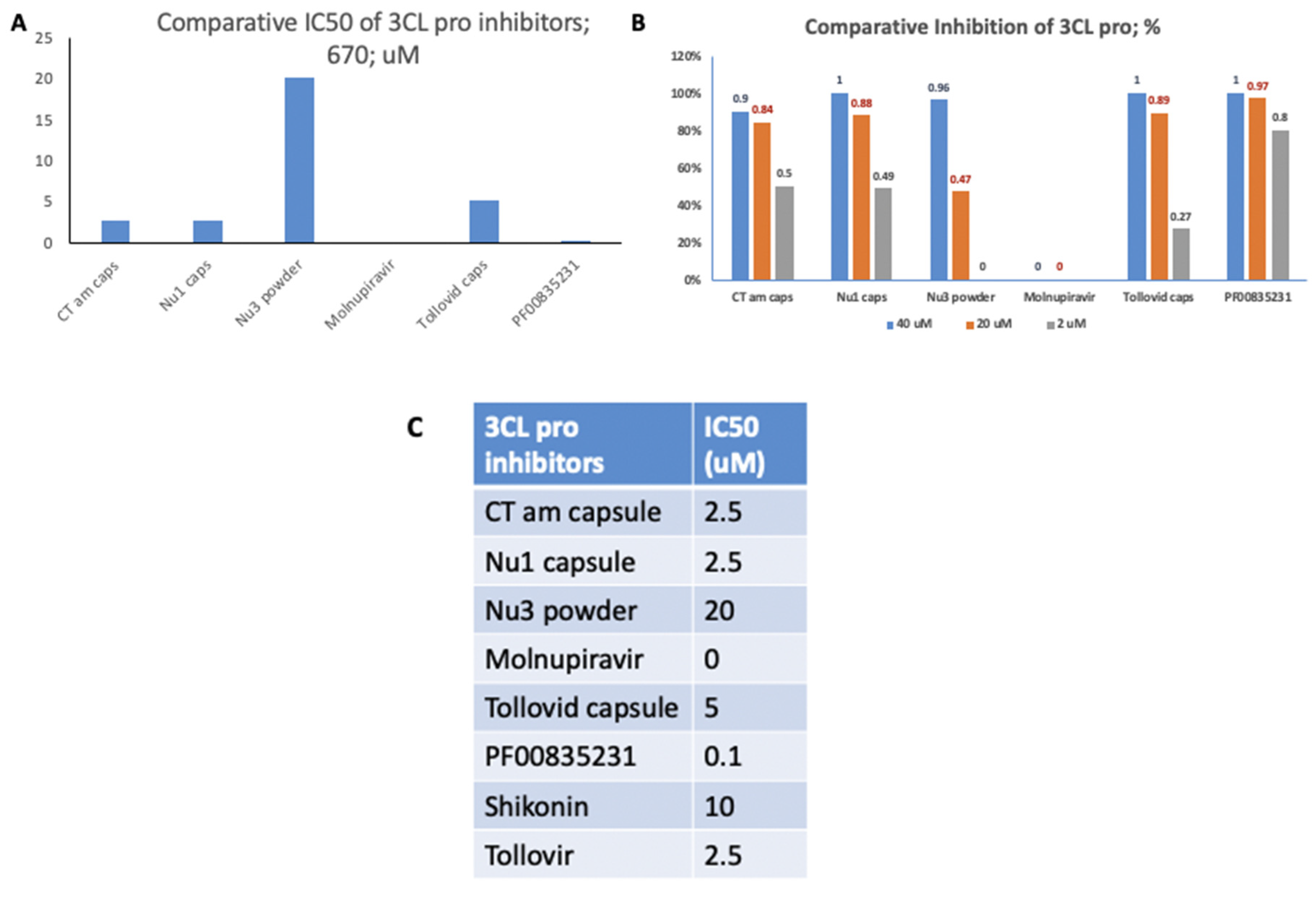
In the assessment of 3CL pro inhibitors, Nu3 powder
exhibited the highest IC50 value compared to the other inhibitors tested.
Moreover, 40uM of Tollovid capsules and 40uM of Nu1 capsules demonstrated
complete inhibitory activity against 3CL pro. The IC50 values of all 3CL pro
inhibitors were also examined (
Figure 2).
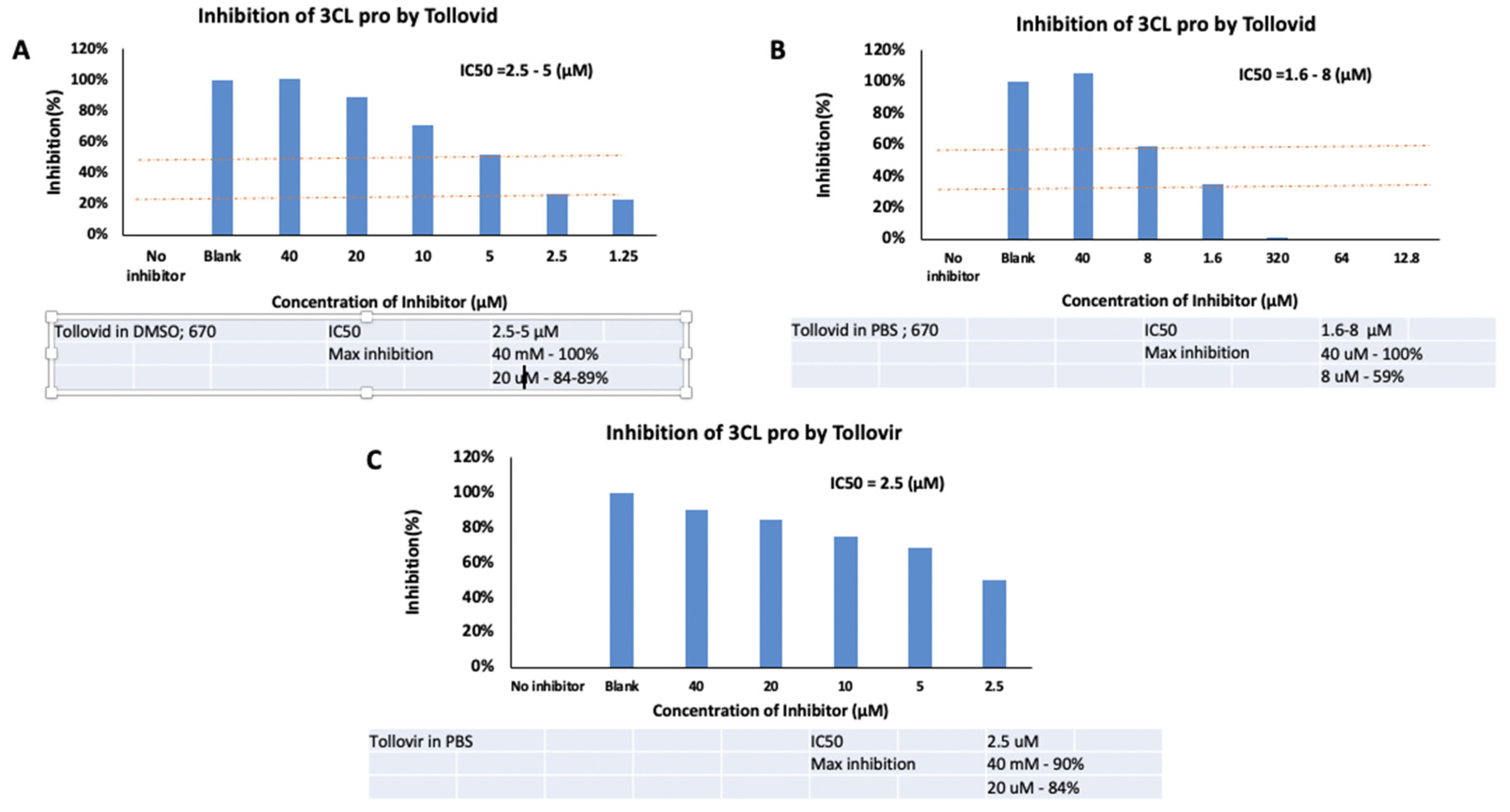
At a concentration of 40μM, Tollovid in DMSO showed
100% maximum inhibition of 3CL pro. Similarly, at the same concentration,
Tollovid in PBS demonstrated 100% maximum inhibition of 3CL pro. However,
Tollovir in PBS, at a concentration of 40μM, presented 90% maximum inhibition
of 3CL pro (
Figure 3).
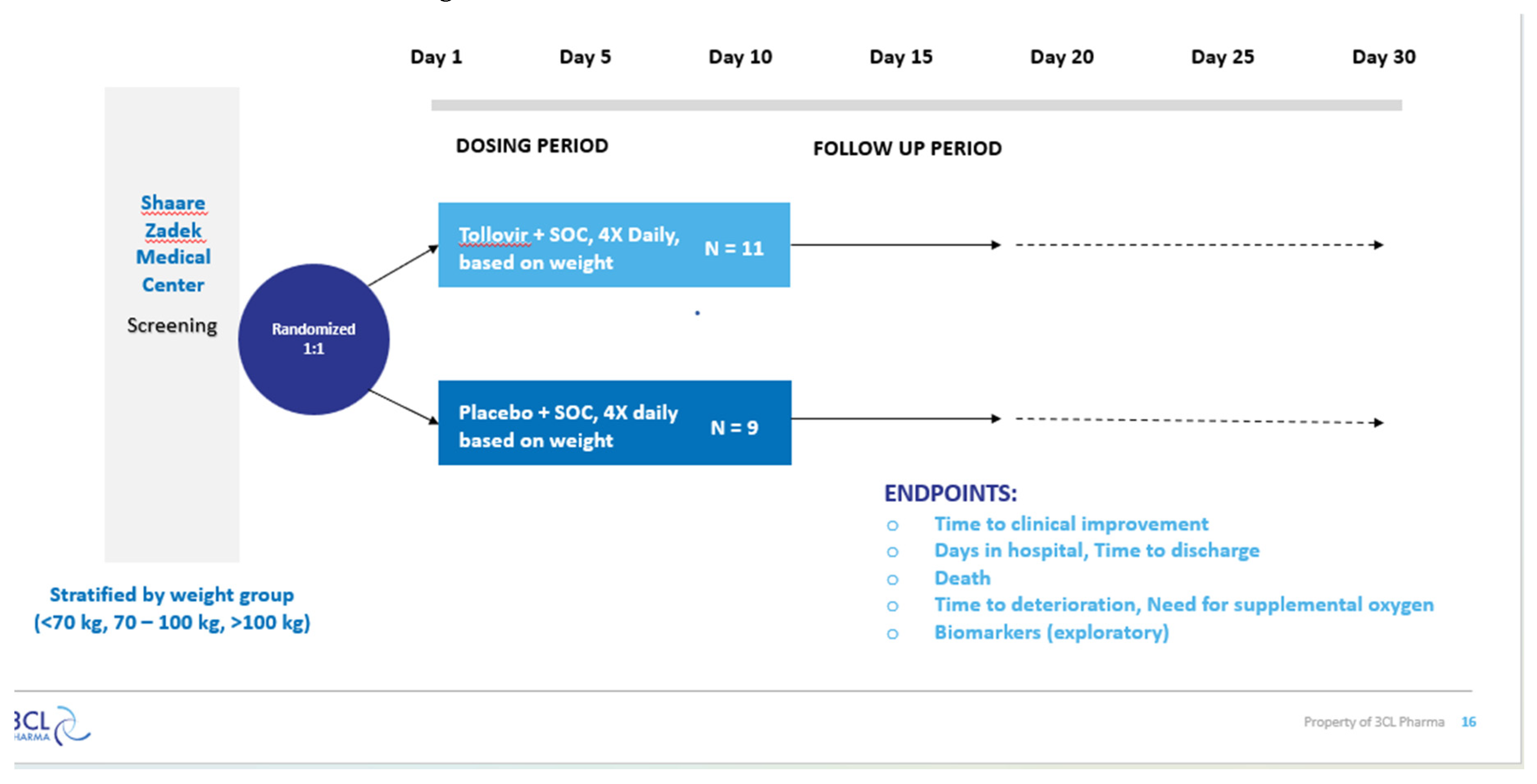
3.2. Clinical Trial Results:
Tollovir is a highly potent dual mechanism 3CL
protease inhibitor that can be utilized as a potential antiviral therapy for
COVID-19. The essential compounds in the antiviral are NLC-EXT-1 and NLC-EXT-2.
NLC-EXT-1 inhibits the 3CL protease and, as a result, prevents viral
replication. On the other hand, NLC-EXT-2 inhibits cytokine storm, normalizing
the immune function. The following study evaluates the safety and efficacy of
NLC-V in patients diagnosed with COVID-19. NLC-V was administered to patients
according to their weight. Patients under the weight of 70kg received two
capsules four times a day, a total of 80mg NLC-V daily. On the other hand,
patients above 100 kilograms received four capsules four times a day for a
total of 160mg NLC-V daily. Patients weighing between 70 and 100kg received three
capsules four times a day for 120mg NLC-V daily. The patients completed the
treatment for ten days. The phase 2b/3 clinical trial was conducted at the
Shaare Zadek Medical Center with hospitalized patients who confirmed SARS-CoV-2
infection between the ages of 40 – 90. The primary endpoint is the time to
clinical improvement, defined as the time from randomization to either an
improvement of two points on a seven-category original scale or discharge from
the hospital, whichever comes first. The consenting subjects were randomly
assigned to receive a standard of care with a placebo or a standard of care
with Tollovir. The hospitalized patients received four capsules every day. The
results of the phase 2b/3 clinical trial can be seen in
Table 1 below.
4. Discussion
The Phase 2b/3 clinical trial revealed that 100% of hospitalized patients who received standard of care and Tollovir significantly improved health according to the NEWS2 scale. In comparison, only 66% of hospitalized patients who received standard of care and placebo showed improvement in their health. Furthermore, it is essential to note that the mortality rate was 0% for hospitalized patients who received Tollovir but a 22% mortality rate for hospitalized patients who received the placebo. In addition, the number of hospitalized patients who required oxygen support decreased after receiving Tollovir, and the time spent on mechanical ventilation also reduced significantly compared to hospitalized patients who received the placebo. The clinical trial results revealed a decrease in IL-6, CRP, and D-dimer levels for hospitalized patients treated with Tollovir compared to patients treated with the placebo. More importantly, neutralizing antibody levels increased for patients who received Tollovir compared to patients who received a placebo. Lastly, unlike Paxlovid (21), the renal and haptic biomarkers revealed no safety signals. Thus, based on the research and clinical results, Tollovir is safe and significantly decreases mortality rates for hospitalized COVID-19 patients. However, additional research is required to understand further the anti-viral and anti-cytokine therapeutic benefits of the botanical drug Tollovir.
Figure 6.
Tollovir Phase 2a Clinical Trial Design, Part 2.
Figure 6.
Tollovir Phase 2a Clinical Trial Design, Part 2.
5. Conclusions
Acute COVID-19 and Long-Haul Syndrome continue to threaten millions of lives nationally and globally. While much research focuses on developing an effective antiviral drug to target the viral 3CL protease, the study is focused mainly on creating therapeutic agents for COVID-19 using structure-based drug design. The major flaw with the following method is that it is prone to drug resistance and is not a long-term solution. Thus, 3CL Pharma has used QCT, a mechanism-based drug design, to create an oral antiviral to treat COVID-19. Tollovir has proven to be safe and decrease mortality rates in hospitalized patients.
6. Patents
Patent number 11857517: Described herein are compounds of Formula I, wherein R1-R6 are as described herein, for use in the treatment of a coronavirus infection; a method of inhibiting a coronavirus 3CL protease by contacting the 3CL protease with a compound of Formula I; as well as methods pharmaceutical composition comprising a compound of Formula I and at least one phospholipid, wherein a weight ratio of the phospholipid(s) to the compound in the composition is in a range of from 10:1 to 1:10. Further described herein is a method of treating a coronavirus infection in a subject in need thereof, by administering to the subject at least one compound that exhibits at least two of inhibition of activity of a 3CL protease of the coronavirus; inhibition of inflammation in the subject; and inhibition of autophagy in the subject.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Funding
This research received no external funding.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Conflicts of Interest
Dorit Arad is employed by 3CL Pharma LTD, an affiliate of Todos Medical USA.
References
- Kaeuffer; et al. Clinical characteristics and risk factors associated with severe COVID-19: Perspective analysis of 1,045 hospitalized cases in North-Eastern France, March 2020. NIH, 25(48). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7716399/.
- Pick, N.; Rawat, M.; Arad, D.; Lan, J.; Fan, J.; Kende, A.S.; Av-Gay, Y. In vitro properties of antimicrobial bromotyrosine alkaloids. J. Med Microbiol. 2006, 55, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.H.; Tomlinson, A.C.; Zhou, D.; Satkunarajah, M.; Chen, K.; Sharon, C.; Desforges, M.; Talbot, P.J.; Rini, J.M. Receptor-binding loops in alphacoronavirus adaptation and evolution. Nature communications 2017, 8, 1735. [Google Scholar] [CrossRef] [PubMed]
- Teli, D.; Balar, P.; Patel, K.; Sharma, A.; Chavda, V.; Vora, L. Molnupiravir: A Versatile Prodrug against SARS-CoV-2 Variants. Metabolites 2023, 13, 309. [Google Scholar] [CrossRef] [PubMed]
- Teli, D.; Balar, P.; Patel, K.; Sharma, A.; Chavda, V.; Vora, L. Molnupiravir: A Versatile Prodrug against SARS-CoV-2 Variants. Metabolites 2023, 13, 309. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Bose, S.; Srivastava, A.K.; Chaudhary, A.A.; Lall, R.; Prasad, S. Jeopardy of COVID-19: Rechecking the Perks of Phytotherapeutic Interventions. Molecules 2021, 26, 6783. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.; Ali, A.; Wang, Q.; Irfan, M.; Khan, A.; Zeb, M.T.; Zhang, Y.-J.; Chinnasamy, S.; Wei, D.-Q. Marine natural compounds as potents inhibitors against the main protease of SARS-CoV-2—A molecular dynamic study. J. Biomol. Struct. Dyn. 2020, 39, 3627–3637. [Google Scholar] [CrossRef] [PubMed]
- Mohamed NM, Ali EM, AboulMagd AM. Ligand-based design, molecular dynamics, and ADMET studies of suggested SARS-CoV-2 M pro inhibitors. RSC advances. 2021;11(8):4523-38. [CrossRef]
- Gyebi, G.A.; Ogunro, O.B.; Adegunloye, A.P.; Ogunyemi, O.M.; Afolabi, S.O. Potential inhibitors of coronavirus 3-chymotrypsin-like protease (3CLpro): An in silico screening of alkaloids and terpenoids from African medicinal plants. J. Biomol. Struct. Dyn. 2021, 39, 3396–3408. [Google Scholar] [CrossRef] [PubMed]
- Roe, M.K.; Junod, N.A.; Young, A.R.; Beachboard, D.C.; Stobart, C.C. Targeting novel structural and functional features of coronavirus protease nsp5 (3CLpro, Mpro) in the age of COVID-19. J. Gen. Virol. 2021, 102, 001558. [Google Scholar] [CrossRef] [PubMed]
- Anand, K.; Ziebuhr, J.; Wadhwani, P.; Mesters, J.R.; Hilgenfeld, R. Coronavirus main proteinase (3CLpro) structure: Basis for design of anti-SARS drugs. Science 2003, 300, 1763–1767. [Google Scholar] [CrossRef] [PubMed]
- Service, R.F. Bad news for Paxlovid? Resistance may be coming. Science 2022, 377, 138–139. [Google Scholar] [CrossRef] [PubMed]
- Koshland, D.E., Jr. The key–lock theory and the induced fit theory. Angewandte Chemie International Edition in English. 1995, 33, 2375–3278. [Google Scholar] [CrossRef]
- Pople, J.A.; Beveridge, D.L. Molecular orbital theory. C0., NY. 1970. [CrossRef]
- Arad, D.; Kreisberg, R.; Shokhen, M. Structural and mechanical aspects of 3C proteases from the picornavirus family. Journal of chemical information and computer sciences. 1993, 33, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Bugg, T.D.H. The development of mechanistic enzymology in the 20th century. Nat. Prod. Rep. 2001, 18, 465–493. [Google Scholar] [CrossRef] [PubMed]
- Shokhen, M.; Arad, D. The Source for the Difference Between Sulfhydryl and Hydroxyl Anions in Their Nucleophilic Addition Reaction to a Carbonyl Group: A DFT Approach. J. Mol. Model. 1996, 2, 399–409. [Google Scholar] [CrossRef]
- Arad, D. The QCT technology for the design of novel protease inhibitors. Drug Design SMI Conf, 2002.
- Halford, B. The Path to Paxlovid. ACS Central Sci. 2022, 8, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Arad D, inventor; Nlc Pharma Ltd, assignee. Compounds for treating coronavirus infection. United States patent US 11,857,517. 2024 Jan 2.
- Shindo, S.; Hosokawa, Y.; Hosokawa, I.; Ozaki, K.; Matsuo, T. Shikonin Inhibits Inflammatory Cytokine Production in Human Periodontal Ligament Cells. Inflammation 2016, 39, 1124–1129. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; He, J.; Song, X.; Tan, L.; Wang, M.; Jiang, P.; Li, Y.; Cao, Z.; Peng, C. Pharmacological properties and derivatives of shikonin—A review in recent years. Pharmacol. Res. 2019, 149, 104463. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).