1. Introduction
Eye and vision problems associated with high computer and electronic screen use are increasingly recognized as problems for the community. Symptoms can include ocular and visual symptoms such as itching, burning, dryness, blurred vision, and photophobia; and pain-related conditions such as headaches and neck and shoulder pain. Computer vision syndrome, visual fatigue, and digital eye strain are terms often used to reflect these symptoms [
1]. A commonly posited hypothesis for the relationship between digital screen use and ocular symptoms is that digital screen use changes blinking dynamics, leading to ocular dryness [
2]. Through overexposure to blue light, electronic screen use can also contribute to the excessive production and accumulation of free oxygen radicals in mitochondria and photosensitive molecules [
3,
4].
Lutein and Zeaxanthin (LZ) are fat-soluble antioxidant nutrients in the carotenoid family. Lutein (L) is found in dark green leafy vegetables such as spinach and kale and in corn and egg yolks. Zeaxanthin (Z) is more prominent in orange and yellow foods such as corn, egg yolks, orange capsicums, persimmons, tangerines, mandarins, and oranges. In the body, LZ are found in the tissues of the eye, brain, breast and adipose tissue. Several studies have examined the effects of LZ on eye health, with mostly positive results [
5]. In several reviews and meta-analyses, it was concluded that LZ intake can help with ocular health and reduce the risk of some eye diseases [
6,
7,
8]. The effects of LZ on high-screen users require further investigation; however, its six-month supplementation was associated with improvements in eye strain, eye fatigue, visual performance, sleep, and headache frequency [
9]. Moreover, another study found positive effects on dry eye symptoms after LZ supplementation in adults with dry eye syndrome [
10].
Given the preliminary positive evidence of LZ on eye health, this study aimed to investigate further the effects of LZ supplementation on high electronic screen users. It was hypothesized that LZ supplementation would positively affect ocular symptoms based on outcome measures comprising ophthalmic examinations and self-report questionnaires. Moreover, its effects on sleep and attention were examined as an exploratory investigation.
2. Materials and Methods
2.1. Study Design
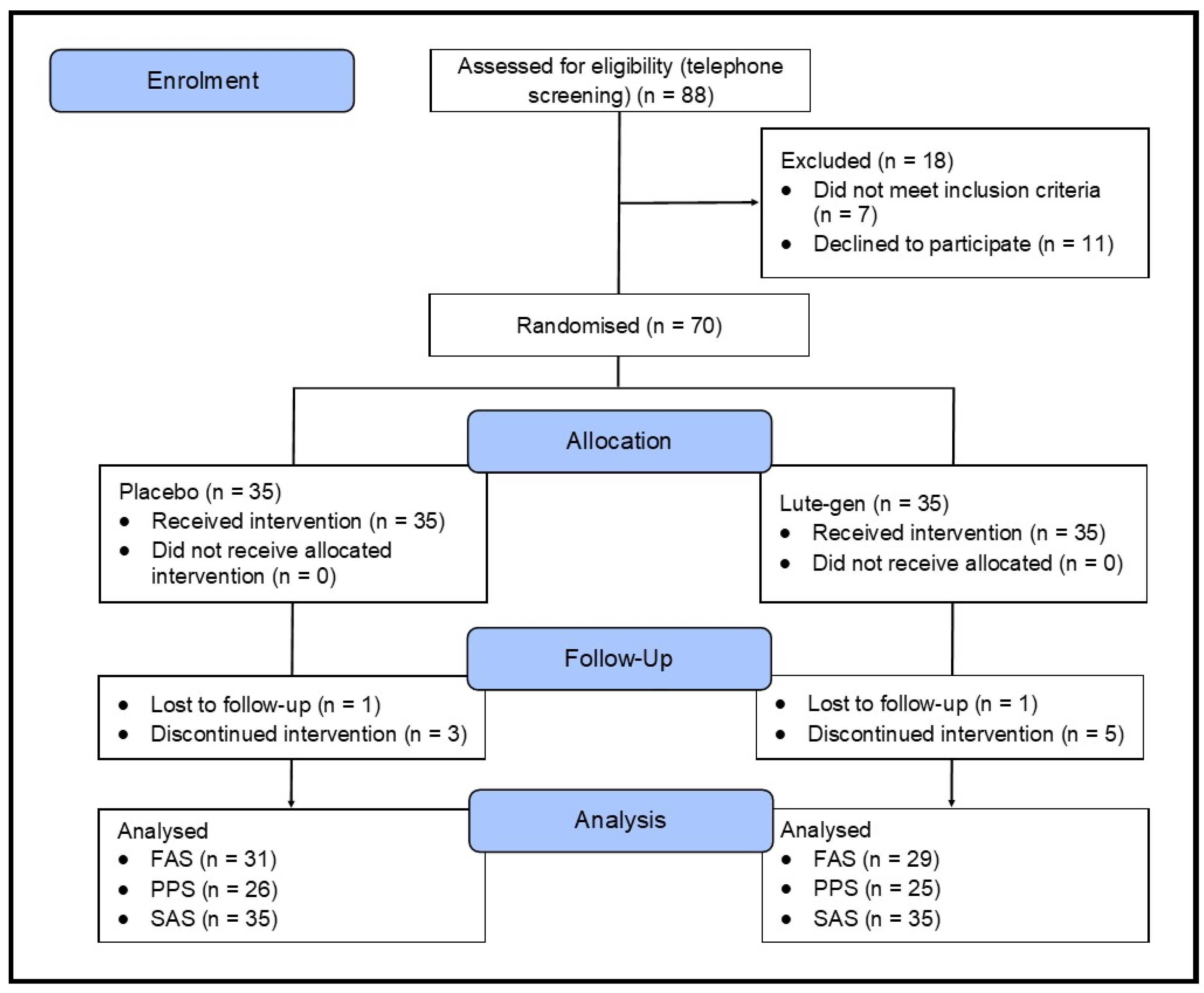
This was a 180-day (6 months), parallel-group, two-arm, randomized, double-blind, placebo-controlled trial (
Figure 1). The study received ethics approval from the National Institute of Integrative Medicine Human Research Ethics Committee (approval number 0122E_2023), and informed consent was acquired from all participants. This trial was registered prospectively with the Australian and New Zealand Clinical Trials Registry (ACTRN12623000427673).
2.2. Recruitment and Randomization
Between May 2023 and September 2023, social media advertisements and e-mail databases were used for volunteer recruitment. Eligible participants were randomly allocated to one of two groups (LZ or placebo; 1:1 ratio) using a randomization calculator with the randomization structure comprising 7 randomly permuted blocks, with 10 participants per block. A participant identification number was assigned based on the order of participant enrollment. The randomization sequence was generated by a researcher not directly involved in volunteer recruitment, and bottle codes were stored by the study sponsor and revealed after all data were analyzed. All softgels were packed in matching bottles. Researchers were blind to the treatment allocation until all outcomes were collected and a blind review was completed.
2.3. Participants
2.3.1. Inclusion Criteria
The inclusion criteria for the study comprised the following: Healthy adults (male and female) 18 to 65 years; devotes at least 6 hours a day viewing a screen at a distance of 1 meter or less; non-smoker; body mass index (BMI) between 18 and 30 kg/m2; has no plan to commence new treatments over the study period; willing to maintain their current diet, exercise, and supplement regimen during the study period; and if wearing spectacles for vision, best corrected visual acuity must be 6/6.
2.3.2. Exclusion Criteria
The exclusion criteria for the study comprised the following: Ocular disorders including but not limited to cataracts, corneal diseases, ocular surface disorders, glaucoma, retinal disease, and myopia (except mild to moderate severity); undergone eye surgery in the past; wears contact lenses more than 3 days a week; suffering from a recently diagnosed or uncontrolled medical condition including but not limited to hyper/hypotension, diabetes, cardiovascular disease, gastrointestinal disease, gallbladder disease, rheumatoid arthritis or another autoimmune disease, endocrine disease, or cancer/malignancy; diagnosed with a psychiatric/neurological condition including but not limited to a severe psychiatric disorder (other than mild-to-moderate depression or anxiety); regular medication intake including but not limited to steroid medications, hormone replacement therapy, eye drops, antihistamines, beta-blockers, or tricyclic antidepressants; change in medication in the last 3 months or an expectation to change during the study duration; taking vitamins or herbal supplements that may affect the study measures; current or 12-month history of illicit drug use; alcohol intake more than 14 standard drinks per week; pregnant, breastfeeding, or an intention to fall pregnant in the next 6 months; any significant surgeries over the last year; and planned major lifestyle change in the next 6 months.
2.4. Interventions
The intervention comprised either a combination of lutein & zeaxanthin isomers (Lute-gen®) or a placebo (sunflower oil). Participants were required to take one capsule daily with a meal, with the active intervention delivering 10mg of lutein and 2mg of zeaxanthin-isomers daily for 180 days. The active and placebo soft gel capsules were identical in appearance, matched for shape, color, size, smell, and taste. The excipients in the soft gels were also identical, comprising sunflower oil. Adherence to intake was assessed by asking participants to estimate capsule intake consistency (0 to 100%) every month and by the return of unused capsules at the day 90 and 180 visits. Treatment blinding was assessed by asking participants to predict group allocation (placebo, lutein/zeaxanthin, or unsure) at the end of the study.
2.5. Outcome Measures
2.5.1. Primary Outcome Measures
Visual Fatigue Scale (VFS): The VFS is a 10-item questionnaire assessing symptoms of eye discomfort experienced after a typical workday. Symptoms are rated from 0 (none) to 6 (severe), with higher scores indicating greater visual fatigue [
11].
Schirmer tear test (STT): The STT assesses tear production, especially in patients with suspected dry eye or tear overproduction. In the test, a special paper strip is placed inside the lower eyelid of each eye and bent at 90 degrees. The eyes are then closed for 5 minutes, after which time the paper is removed, and how much of it has become moist is measured. The Schirmer test score is calculated by the length of the moistened area of the strips (using the scale included on the strips) and the measurement duration in minutes. A score of greater than 10 mm in 5 minutes is considered normal. A score of less than 5 mm in 5 minutes indicates a tear deficiency [
12]. A mean score for tests conducted on the left and right eye at each visit was calculated to evaluate changes over time.
2.5.2. Secondary Outcome Measures
Computer Vision Syndrome Questionnaire (CVS-Q): The CVS-Q is a 16-item self-report measure assessing computer-related visual and ocular symptoms associated with computer/ screen use. Symptoms are rated based on frequency (never, occasional, often/always) and intensity (moderate and intense), with higher scores indicating greater symptom severity [
13].
Photo-stress recovery time (PSRT): PSRT is the time (in seconds) taken for visual acuity to return to normal after the retina has been bleached by a bright light source. The test involves exposing the eye to the light from the ophthalmoscope for 30 seconds and measuring the time taken for acuity to return to within one line of pre-bleach acuity. The PSRT can be used to differentiate between retinal (macular) and post-retinal (e.g., optic nerve) diseases.
Contrast sensitivity (CS): Contrast sensitivity test measures a patient’s ability to differentiate between finer and finer increments of light versus dark (contrast). CS was assessed using the Melbourne Edge Test. This test presents 20 circular patches containing edges with reducing contrast. Accurate identification of the orientation of the edges on the patches provides a measure of contrast sensitivity in decibel units, where dB=-10log10 contrast [
14].
Visual Acuity Test (VAT): VAT measures the eye’s ability to see and read a letter or a symbol from a distance. During a VAT using a Snellen chart, random letters and numbers of varying sizes are displayed on a chart 6 meters away from the patient. The patient was required to cover one eye as they read the letters or numbers from top to bottom. Results are presented as a fraction ranging from 6/150 to 6/6 (reflecting normal vision) [
15]. Decimal notations can be calculated by dividing 6 meters by the participant’s corresponding score on the VAT. A mean score for tests conducted on the left and right eye (unaided) at each visit was calculated to evaluate changes over time.
Tear film break-up time (TBUT): TBUT is the time taken for the first dry spot to appear on the cornea after a full blink. TBUT is a method for assessing tear film stability and evaporative dry eye, and is a standard diagnostic procedure used in dry eye clinics. In TBUT test, sodium fluorescein dye is added to the eye, and the tear film is examined under the slit lamp while the patient avoids blinking until tiny dry spots develop. Generally, greater than 10 seconds is considered normal, 5 to 10 seconds marginal, and less than 5 seconds low. Short tear break-up time is a sign of a poor tear film, and the longer it takes, the more stable the tear film [
16]. A mean score for tests conducted on the left and right eye at each visit was calculated to evaluate changes over time.
2.5.3. Exploratory Outcome Measures
PROMIS Sleep Disturbance and Sleep-Related Impairment Scale (PROMIS Sleep): The PROMIS Sleep is a validated—self-report questionnaire that assesses sleep quality and sleep-related impairment over the last seven days. The measure comprises 16 items, creating 2 component scores (sleep disturbance and sleep-related impairment) [
17].
Everyday Life Attention Scale (ELAS): The ELAS was developed as a self-report questionnaire for the evaluation of attention in everyday life that takes into account different situational contexts. The ELAS contains questions about several attentional capacities in a variety of situations (reading a book, watching a movie, performing an indoor activity, attending a lecture/ open evening, having a conversation, doing an assignment/ administration, preparing a meal, cleaning up, driving a car) which are rated on a scale based on how long the respondent can engage in the task without a break, how well he/she can focus on the task, and his/her level of motivation to do the task well [
18].
2.5.4. Safety Outcome Measures
The tolerability of capsule intake was assessed monthly through an online question about the experience of any adverse events. Researchers also asked about adverse events at visits 2 and 3, and participants were requested to contact researchers if they experienced any adverse reactions. Several safety blood measures were also collected comprising a full blood examination (hemoglobin, red blood cell count, hematocrit, mean corpuscular hemoglobin, mean corpuscular volume, mean corpuscular hemoglobin concentration, red blood cell distribution width, white cell count, neutrophils, lymphocytes, monocytes, eosinophils, basophils, platelets, mean platelet volume), liver function test (aspartate transaminase, alanine transaminase, alkaline phosphatase, gamma-glutamyl transferase, bilirubin (total), total protein, globulin, and albumin), renal function test (urea, creatinine, estimated glomerular filtration rate, sodium, potassium, chloride, bicarbonate, anion Gap), and blood lipid profile (cholesterol, triglycerides, high-density lipoprotein, and high-density lipoprotein).
2.6. Sample Size Calculations
An a priori power analysis was completed to estimate the required sample size. In a study on the effects of LZ supplementation on high-screen users, effect sizes on various outcome measures associated with eye health ranged from 0.25 to 1.7 [
9]. Based on this study, an effect size of 0.7 was anticipated. Assuming a power of 80% and a type one error rate of 5%, the number of participants per group required to find an effect based on a single outcome measure was estimated as 52. Assuming a 20% dropout rate, it was planned to recruit 35 participants per group (70 participants in total), which was hypothesized to give enough power to find an effect compared to the placebo, even after dropouts.
2.7. Statistical Analysis
For baseline data, an independent samples t-test was used to compare group data for continuous variables, and a Pearson’s Chi-square test was used to compare categorical data. Outcome analyses were conducted on the full analysis set (FAS) and per protocol set (PPS), with all participants retained in originally allocated groups. Generalized Linear Mixed Models (GLMM) assessed differences between intervention groups on primary and secondary outcomes over time, with intervention effects assessed via entry of the intervention group (placebo and LZ) x time interaction. The time points considered for each eye assessment were days 0, 90, and 180; and for self-report questionnaires, days 0 through 180. Random intercepts were utilized in each model, and covariates of age, sex, and BMI were included. Where appropriate, gamma (with log link function) and normal (with identity link function) target distributions were used. Applicable covariance structures were used to model correlation related with repeated time measurements in gamma models. Robust estimations were used to control for any violations of model assumptions. Moreover, GLMM was used to examine the change in the intervention group (placebo and LZ) from baseline to day 180. Covariates age, sex, BMI, and corresponding baseline values were included. All data were analyzed using SPSS (version 29; IBM, Armonk, NY), and the critical p-value was set at p ≤ 0.05 for all analyses.
3. Results
3.1. Study Population
A total of 153 people completed the online screening questionnaire, 88 people underwent a telephone screening, and 70 people attended an in-person screening assessment. Of the 88 people who participated in the telephone screening, the most common reasons for exclusion were failure to attend their in-person assessment appointment (n=11) and withdrawing consent (n=4).
3.2. Baseline Questionnaire and Demographic Information
Baseline demographic and clinical characteristics are detailed in
Table 1. Analyses revealed that the groups were similarly matched with no statistically between-group differences, except for the physical activity category, where participants in the LZ group were less physically active (p=.003).
3.3. Outcome Measures
3.3.1. Primary Outcome Measures
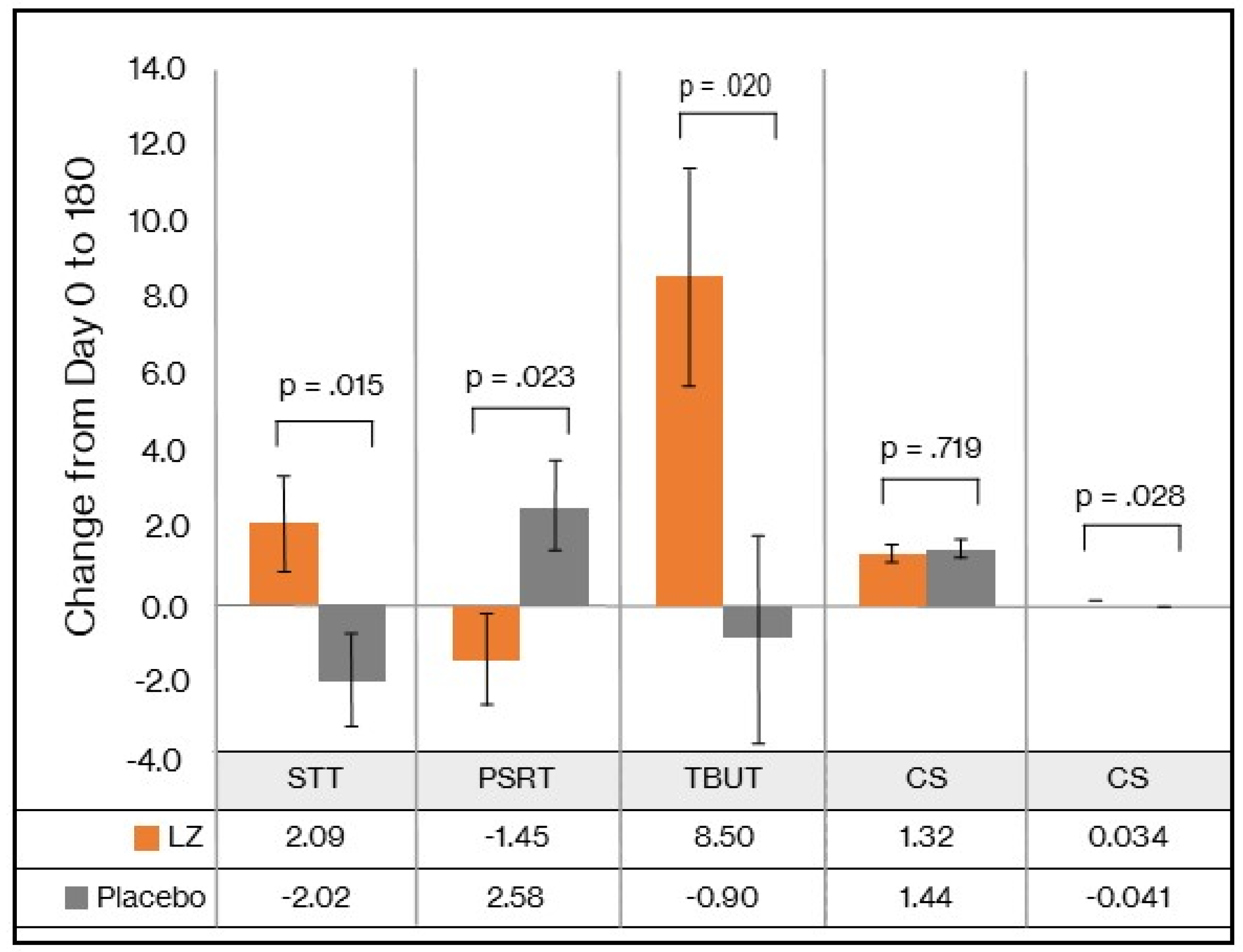
STT: As demonstrated in
Table 2, the GLMM revealed a statistically significant time x group interaction in STT scores (p = 0.024). As detailed in
Table 3 and
Figure 2, from baseline to day 180, there was a statistically significant group difference in change in STT (p = 0.015), represented by a mean increase of 2.09 mm in the LZ group and a 2.02mm decrease in the placebo group (Cohen’s D effect size of 0.66). An analysis of the PPS revealed similar findings (see
Supplementary Tables S1 and S2), as demonstrated by statistically significant group differences and an effect size of 0.66.
VFS: As demonstrated in
Table 4, based on the GLMM, there was a non-significant time x group interaction in VFS scores (p = 0.312). In the LZ and placebo groups, there were statistically significant within-group changes over time in VFS scores (p < 0.001). PPS data revealed similar non-significant group differences in changes in VFS scores (see
Supplementary Table S3).
3.3.2. Secondary Outcome Measures
TBUT: As demonstrated in
Table 2, the GLMM revealed a statistically significant time x group interaction in TBUT scores (p = 0.031). As detailed in
Table 3 and
Figure 2, from baseline to day 180, there was a statistically significant group difference in change in TBUT scores (p = 0.020), represented by a mean increase of 8.50 secs in the LZ group and a 0.90 sec decrease in the placebo group (Cohen’s D effect size of 0.63). An analysis of the PPS revealed similar findings (see
Supplementary Tables S1 and S2), as demonstrated by statistically significant group differences and an effect size of 0.59.
PSRT: As demonstrated in
Table 2, based on the GLMM, there was a near statistically significant time x group interaction in PSRT scores (p = 0.063). As detailed in
Table 3 and
Figure 2, from baseline to day 180, there was a statistically significant group difference in change in PSRT (p = 0.023), represented by a mean decrease of 1.45 secs in the LZ group and a 2.58 sec increase in the placebo group (Cohen’s D effect size of 0.62). When data from the PPS were analyzed (see
Supplementary Tables S1 and S2), group differences in changes in PSRT no longer remained statistically significant. However, strong trends remained with a moderate effect size of 0.44.
CS: As demonstrated in
Table 2, based on the GLMM, there was no significant time x group interaction in CS scores (p = 0.694). As detailed in
Table 3 and
Figure 2, from baseline to day 180, there was no significant group difference in change in CS (p = 0.719). PPS data revealed similar non-significant group differences in changes in CS scores (see
Supplementary Tables S1 and S2).
VAT: As demonstrated in
Table 2, based on the GLMM, there was no significant time x group interaction in VAT scores (p = 0.258). However, as detailed in
Table 3 and
Figure 2, from baseline to day 180, there was a statistically significant group difference in change in VAT scores (p = 0.028), with a Cohen’s D effect size of 0.59. PPS data revealed similar findings as there was a non-significant time x group interaction (p = 0.157), but a near statistically significant difference when examining changes in VAT scores from baseline to day 180 (p = 0.053) (see
Supplementary Tables S1 and S2).
CVS-Q
: As demonstrated in
Table 4, based on the GLMM, there was a non-significant time x group interaction in CVS-Q scores (p = 0.575). In the LZ and placebo groups, there were statistically significant within-group changes over time in VFS scores (p < 0.001 and p = 0.005, respectively). PPS data revealed similar non-significant group differences in changes in CVS-Q scores (see
Supplementary Table S3).
3.3.3. Exploratory Outcome Measures
PROMIS Sleep: As demonstrated in
Table 4, based on the GLMM, there was a non-significant time x group interaction in PROMIS Sleep Disturbance (p = 0.963) and Sleep-Related Impairment (p = 0.730) scores. PPS data revealed similar non-significant group differences in changes in PROMIS Sleep scores (see
Supplementary Table S3).
ELAS: As demonstrated in
Table 4, based on the GLMM, there was a non-significant time x group interaction in ELAS scores (p = 0.206). In the LZ and placebo groups, there were no statistically significant within-group changes over time in ELAS scores (p = 0.292 and p = 0.610, respectively). PPS data revealed similar non-significant group differences in changes in ELAS scores (see
Supplementary Table S3).
3.4. Intake of Supplements
IP bottles with remaining capsules were returned on visits 2 and 3. Based on these details, 98% of participants who completed the study took over 80% of their capsules.
3.5. Efficacy of Participant Blinding
To assess the effectiveness of condition concealment during the trial, participants predicted their condition allocation (i.e., placebo, LZ, or unsure) at the end of the study. Overall group concealment was high, as 67% of participants were unsure or incorrectly guessed treatment allocation.
3.6. Adverse Reactions and Treatment Discontinuation
Participants reported no serious adverse events, and there was a tendency for a greater frequency of adverse events in the placebo group.
Table 5 details the adverse events classified as possibly or probably related to the investigational products. In the placebo group, 14.3% of participants experienced a treatment-related adverse event, and in the LZ group, 8.6% of participants experienced a treatment-related adverse event.
No participants experienced clinically significant changes in blood markers over time (complete blood count, liver function test, blood lipids and renal function test), with concentrations remaining within or close to established reference ranges and none reaching clinically significant levels. Based on the FAS, there were statistically significant group differences in changes in concentrations of neutrophils (p = 0.046), albumin (p = 0.049), anion gap (p = 0.045), and eGFR (p = 0.024). However, these differences between groups must be viewed tentatively as the number of analyses conducted increases the likelihood of type 1 error. Moreover, changes in these markers were small and not clinically meaningful. There were also no group differences in weight, BMI, blood pressure, and pulse rate changes over the 6 month study period.
A total of 10 people discontinued the study. Six people in the LZ group withdrew from the study, three due to adverse effects believed to be associated with capsule intake (mild severity), one due to personal stressors, one due to inconsistent capsule intake, and one where no reason was given. Reasons for study withdrawal in the three people in the LZ group comprised worsening eye symptoms (n=1), stomach bloating (n=1), and pain above the right eye (n=1). In the placebo group, four people withdrew from the study, two due to adverse events believed to be associated with capsule intake (mild severity), one due to unexpected travel, and one where no reason was given. Reasons for study withdrawal in the two people in the placebo group comprised swollen lips (n=1) and stomach pain/ bloating (n=1).
4. Discussion
In this randomized, double-blind, placebo-controlled trial, the effects of 6 months of LZ supplementation (Lute-gen®) on visual fatigue, CVS, dry eyes, visual performance, sleep quality, and attention were examined in high users of electronic screens. Outcome measures comprised a combination of self-report questionnaires and ophthalmic examinations, with the VFS, a self-report measure of visual fatigue, and the STT, a measure of dry eyes/ tear production, comprising the primary outcome measures. Compared to the placebo, LZ supplementation did not have a differential effect on the VFS score but resulted in larger improvements in the STT. Moreover, LZ supplementation was associated with improvements in several other ophthalmic examinations, including the TBUT, PSRT, and VAT. However, these positive changes in eye health and function were not corroborated by group differences in changes in self-report measures.
Dry eye disease is a multifactorial disease characterized by discomfort, visual disturbance, and tear film instability with potential damage to the ocular surface. Estimates of the prevalence of dry eye disease vary based on the population examined, the definition used, and the assessment methods utilized, with global prevalence rates ranging from 5 to 34% [
19]. Risk factors for dry eyes include ageing, female gender, contact lens use, history of ocular or laser refractive surgery, and systemic diseases such as Sjogren’s syndrome, rheumatoid arthritis, gout, thyroid disease, and autoimmune disorders. In addition, environmental factors such as low room humidity, high temperature, air pollution, and certain lighting conditions can exacerbate dry eyes. Moreover, excessive digital screen use can contribute to dry eyes [
19]. A commonly accepted hypothesis for the relationship between digital screen use and dry eye disease is that digital screen use alters blinking dynamics, leading to ocular dryness [
2]. Through overexposure to blue light, electronic screen use can also contribute to the excessive production and accumulation of free oxygen radicals in mitochondria and photosensitive molecules [
3,
4].
Based on the results from two ophthalmic examinations comprising the STT and TBUT, this study demonstrated that LZ supplementation for 6 months can increase tear production. The STT is a commonly used measure of total tear secretion [
20], and the TBUT provides a measure of tear film instability [
21,
22]. Lutein and zeaxanthin are the main carotenoids in the human macula, often referred to as macular pigments. Even though further investigation is required to understand the photo-protective actions associated with LZ supplementation, it may be via their antioxidant and anti-inflammatory actions. Several studies have demonstrated that lutein inhibits the pro-inflammatory cytokine cascade and the transcription factor nuclear factor-kB. There is also evidence that lutein and zeaxanthin reduce reactive oxygen species production and the expression of inducible nitric oxide synthase [
2,
5]. Another protective effect of L may be through its ability to filter blue light, thereby reducing phototoxic damage to photoreceptor cells [
23]. In a study on adults with high screen use, LZ supplementation for 6 months was associated with improvements in macular pigment optical density (MPOD) and several visual performance measures [
9]. In addition to changes in tear production, improvements in VAT and PSRT were also demonstrated in this study. Visual acuity is a measure of the capacity of the eye to distinguish shapes and the details of objects at a specified distance. In this study, it was measured using the Snellen chart. PSRT is an objective quantitative measure of macular function, and several diseases influencing central vision, including age-related macular degeneration, central serous retinopathy, retinal detachments, and retinitis pigmentosa, can affect recovery time [
24,
25]. In meta-analyses examining the association between LZ supplementation, MPOD, and visual function, it was concluded that LZ supplementation increased MPOD, and LZ intake/ supplementation and MPOD are associated with reduced photostress recovery and improved visual acuity [
26,
27,
28,
29]. Therefore, the positive changes in VAT and PSRT identified in this study are consistent with previous trials conducted in the area. However, despite several improvements in ophthalmic examinations being identified, this did not translate into between-group differences in changes in self-report questionnaires assessing dry eyes, visual fatigue, eye soreness, and other eye-related symptoms associated with high electronic screen use. Placebo responses are common in clinical trials, with subjective evaluations particularly susceptible to placebo responses [
30]. However, such placebo responses did not occur when investigator-administered ophthalmic examinations were undertaken. It is important to note that baseline scores on the self-report questionnaires were low, indicating that despite recruiting high electronic screen users, complaints associated with dry eyes and computer vision syndrome were not highly prevalent in the recruited population. This suggests that the recruited population did not experience dry eye and visual symptoms of sufficient severity to result in noticeable problems for participants, and/or visual-related symptoms were accepted as part of participants’ everyday experiences. Even though there is no consensus on the diagnostic criteria of dry eye in STT, a reading of less than 5 mm indicates dry eyes and less than 10 mm marginally dry eyes [
20]. At baseline, the mean STT score of participants in this study was approximately 20mm, indicating normal tear production in most participants. Moreover, despite research demonstrating excessive electronic screen time can contribute to sleep and attention problems [
31,
32], such difficulties were not present in the population examined.
4.2. Limitations and Directions for Future Research
Although the ophthalmic examinations completed in the study are considered acceptable measures, they have several limitations. For example, the TBUT use of fluorescein dye does not allow for observation of the physiological state of the ocular surface. Moreover, the breakup time depends on the amount of fluorescein dye used, and it is sometimes difficult to determine when the tear film begins to breakup. This can affect the reproducibility of results [
21,
22]. In several studies, the STT did not reliably detect the efficacy of drugs in patients undergoing treatment for dry eye, and its weaknesses include poor repeatability, low sensitivity and specificity, and sharp patient discomfort. Changes in light, room humidity, temperature and patient’s anxiety can also influence the reproducibility of results [
20]. Therefore, additional examinations will be important to validate the results of this trial further. This includes MPOD assessments to examine the effects of LZ supplementation on MPOD and visual changes. In a study by Stringham and colleagues [
9], LZ supplementation improved MPOD, and MPOD was correlated with improvements in visual performance. Assessing for and controlling for changes in diet quality and the intake of carotenoid-rich foods will be helpful to ensure visual changes are not the result of changes in dietary patterns during the study. The recruitment of participants with identified dry eye syndrome, CVS, poor sleep and attentional problems will also help to understand the effects of LZ supplementation in people presenting with such difficulties.
5. Conclusions
In summary, the results from this study provide support for the beneficial effects of 6 months of LZ supplementation on regular users of electronic screens. Compared to the placebo, there were improvements in several ophthalmic examinations for dry eyes and visual health; however, these findings were not corroborated by group differences in the administered self-report questionnaires. LZ was well tolerated, with no serious adverse effects or significant changes in blood safety measures of liver function, renal function, and blood lipids.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Supplementary tables.
Author Contributions
Conceptualization and methodology, A.L.L. and S.J.S; formal analysis, A.L.L.; investigation, A.L.L. and S.J.S.; writing—original draft preparation, A.L.L.; writing—review and editing, A.L.L. and S.J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study received funding from Bio-gen Extracts Pvt. Ltd. Bio-gen Extracts Pvt. Ltd. also provided the investigational products used in this study.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee at the National Institute of Integrative Medicine (approval number: 0122E_2023; date of approval: 21 February 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data collected for this study can be requested by contacting the corresponding author.
Acknowledgments
The authors gratefully acknowledge Bio-gen Extracts Pvt. Ltd. for funding the project and supplying the investigational products used for this study; Lilly Wall for conducting the ophthalmic assessments; and Dr Mohsina Mehkri, MBBS, MS (Ophthalmology) for assistance in the study design.
Conflicts of Interest
A.L.L. is the managing director of Clinical Research Australia, a contract research organization that has received research funding from nutraceutical companies. A.L.L. has also received presentation honoraria from nutraceutical companies. S.J.S. is an employee of Clinical Research Australia and declares no other conflicts of interest. The funder was not involved in data collection, interpretation of data, or the decision to submit it for publication.
References
- Sheppard, A.L.; Wolffsohn, J.S. Digital eye strain: prevalence, measurement and amelioration. BMJ Open Ophthalmol 2018, 3, e000146. [Google Scholar] [CrossRef] [PubMed]
- Al-Mohtaseb, Z.; et al. The Relationship Between Dry Eye Disease and Digital Screen Use. Clin Ophthalmol 2021, 15, 3811–3820. [Google Scholar] [CrossRef] [PubMed]
- Fietz, A.; et al. Blue Light Damage and p53: Unravelling the Role of p53 in Oxidative-Stress-Induced Retinal Apoptosis. Antioxidants (Basel) 2023, 12. [Google Scholar] [CrossRef]
- Cougnard-Gregoire, A.; et al. Blue Light Exposure: Ocular Hazards and Prevention-A Narrative Review. Ophthalmol Ther 2023, 12, 755–788. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; et al. The Effect of Lutein on Eye and Extra-Eye Health. Nutrients 2018, 10. [Google Scholar] [CrossRef]
- Ma, L.; Lin, X.M. Effects of lutein and zeaxanthin on aspects of eye health. J Sci Food Agric 2010, 90, 2–12. [Google Scholar] [CrossRef]
- Wilson, L.M.; et al. The Effect of Lutein/Zeaxanthin Intake on Human Macular Pigment Optical Density: A Systematic Review and Meta-Analysis. Adv Nutr 2021, 12, 2244–2254. [Google Scholar] [CrossRef]
- Mares, J. Lutein and Zeaxanthin Isomers in Eye Health and Disease. Annu Rev Nutr 2016, 36, 571–602. [Google Scholar] [CrossRef]
- Stringham, J.M.; Stringham, N.T.; O’Brien, K.J. Macular Carotenoid Supplementation Improves Visual Performance, Sleep Quality, and Adverse Physical Symptoms in Those with High Screen Time Exposure. Foods 2017, 6. [Google Scholar] [CrossRef]
- Radkar, P.; et al. A Novel Multi-Ingredient Supplement Reduces Inflammation of the Eye and Improves Production and Quality of Tears in Humans. Ophthalmol Ther 2021, 10, 581–599. [Google Scholar] [CrossRef]
- Hayes, J.R.; et al. Computer use, symptoms, and quality of life. Optom Vis Sci 2007, 84, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Brott, N.R.; Ronquillo, Y. Schirmer Test. 2022.
- Segui Mdel, M.; et al. A reliable and valid questionnaire was developed to measure computer vision syndrome at the workplace. J Clin Epidemiol 2015, 68, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Haymes, S.A.; Chen, J. Reliability and validity of the Melbourne Edge Test and High/Low Contrast Visual Acuity chart. Optom Vis Sci 2004, 81, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Marsden, J.; Stevens, S.; Ebri, A. How to measure distance visual acuity. Community Eye Health 2014, 27, 16. [Google Scholar] [PubMed]
- Tsubota, K. Short Tear Film Breakup Time-Type Dry Eye. Invest Ophthalmol Vis Sci 2018, 59, DES64–DES70. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; et al. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep 2010, 33, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Groen, Y.; et al. A situation-specific approach to measure attention in adults with ADHD: The everyday life attention scale (ELAS). Appl Neuropsychol Adult 2019, 26, 411–440. [Google Scholar] [CrossRef] [PubMed]
- Messmer, E.M. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int 2015, 112, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; et al. Advances in Dry Eye Disease Examination Techniques. Front Med (Lausanne) 2021, 8, 826530. [Google Scholar] [CrossRef]
- Nichols, K.K.; Mitchell, G.L.; Zadnik, K. The repeatability of clinical measurements of dry eye. Cornea 2004, 23, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Norn, M.S. Desiccation of the precorneal film. I. Corneal wetting-time. Acta Ophthalmol (Copenh) 1969, 47, 865–880. [Google Scholar] [CrossRef]
- Junghans, A.; Sies, H.; Stahl, W. Macular pigments lutein and zeaxanthin as blue light filters studied in liposomes. Arch Biochem Biophys 2001, 391, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Karampatakis, V.; et al. Design of a novel smartphone-based photostress recovery time test for detecting abnormalities in the macula. A cross-sectional study. Ann Med Surg (Lond) 2022, 77, 103699. [Google Scholar]
- Brandl, C.; et al. Photostress Recovery Time as a Potential Predictive Biomarker for Age-Related Macular Degeneration. Transl Vis Sci Technol 2023, 12, 15. [Google Scholar] [CrossRef]
- Hu, W.; et al. Effect of Antioxidant Supplementation on Macular Pigment Optical Density and Visual Functions: A systematic review and network meta-analysis of randomized controlled trials. Adv Nutr, 2024; 100216. [Google Scholar]
- Liu, R.; et al. Lutein and zeaxanthin supplementation and association with visual function in age-related macular degeneration. Invest Ophthalmol Vis Sci 2014, 56, 252–258. [Google Scholar] [CrossRef]
- Hu, W.; et al. Effect of xanthophyll-rich food and supplement intake on visual outcomes in healthy adults and those with eye disease: a systematic review, meta-analysis, and meta-regression of randomized controlled trials. Nutr Rev 2023, 82, 34–46. [Google Scholar] [CrossRef]
- Johnson, E.J.; et al. The association between macular pigment optical density and visual function outcomes: a systematic review and meta-analysis. Eye (Lond) 2021, 35, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Vera, J.; et al. Manipulating expectancies in optometry practice: Ocular accommodation and stereoacuity are sensitive to placebo and nocebo effects. Ophthalmic Physiol Opt 2022, 42, 1390–1398. [Google Scholar] [CrossRef]
- Wallace, J.; et al. Screen time, impulsivity, neuropsychological functions and their relationship to growth in adolescent attention-deficit/hyperactivity disorder symptoms. Sci Rep 2023, 13, 18108. [Google Scholar] [CrossRef]
- Gringras, P.; et al. Bigger, Brighter, Bluer-Better? Current Light-Emitting Devices—Adverse Sleep Properties and Preventative Strategies. Front Public Health 2015, 3, 233. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).