Submitted:
18 July 2024
Posted:
24 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
2.1. Experiment Location
2.2. Area History
2.3. Experimental Design and Treatments
2.4. Planting and Cultural Treatments
2.5. Productivity Analysis
2.6. Physical Soil Evaluations
2.7. Statistical Analysis
3. Results and Discussion
| Sources Variations | D.F.1 | Prod | SD | GM | TP | Micro | Macro | RP |
| kg ha-1 | g cm- 3 -----------------------%------------------------- | MPa | ||||||
| BLOCK | 3 | 8,451 | 0,0039 | 13,002 | 17,62 | 13,96 | 4,11 | 0,18 |
| Crops (C) | 3 | 38,01* | 0,0040ns | 4,92* | 81,0* | 133,45* | 11,06ns | 0,142ns |
| Depth (D) | 3 | 0,16ns | 0,0512* | 0,70ns | 1,42ns | 13,39ns | 0,58ns | 7,56ns |
| C x D | 9 | 0,27ns | 0,0056ns | 0,89ns | 10,96ns | 6,47ns | 10,47* | 2,48* |
| Error | 45 | 0,17ns | 0,0052ns | 0,96ns | 9,60ns | 6,33ns | 4,29ns | 3,90ns |
| CV (%) | - | 12,66 | 4,54 | 4,53 | 6,83 | 6,94 | 21,5 | 17,19 |
| Mean | - | 6,45 | 1,59 | 21,61 | 45,35 | 36,26 | 9,83 | 1,71 |
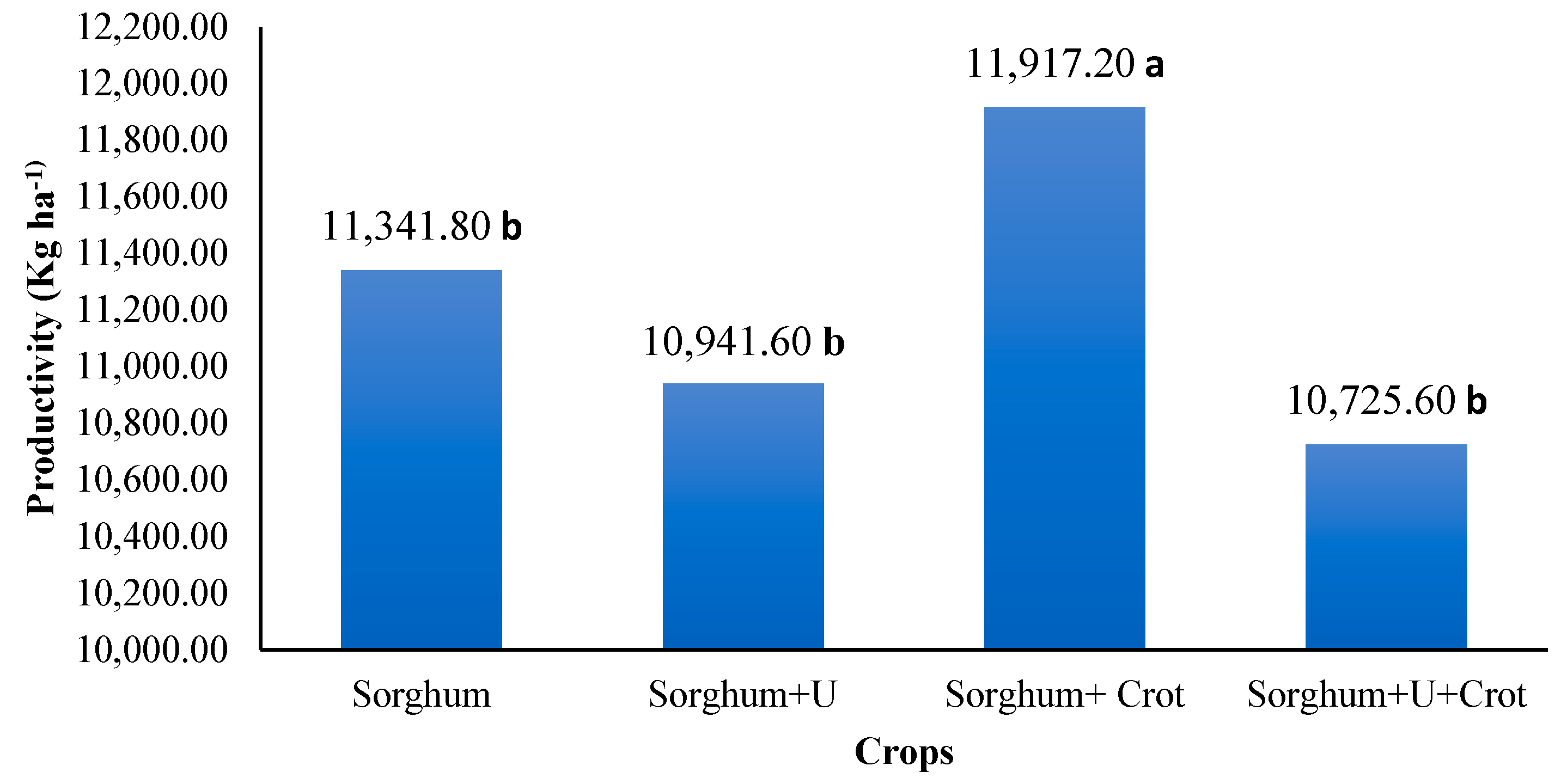
3.1. Productivity
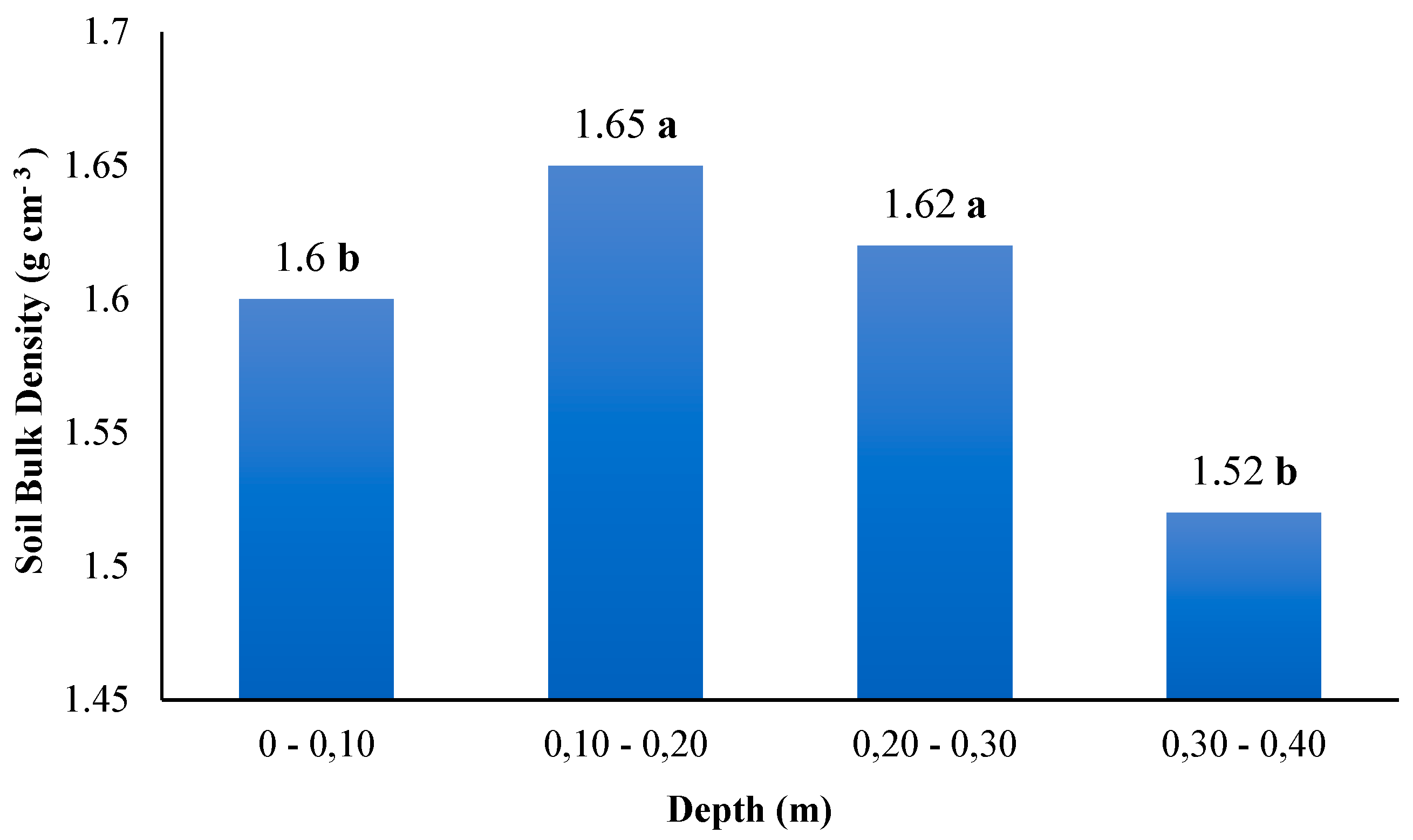
3.2. Soil Bulk Density
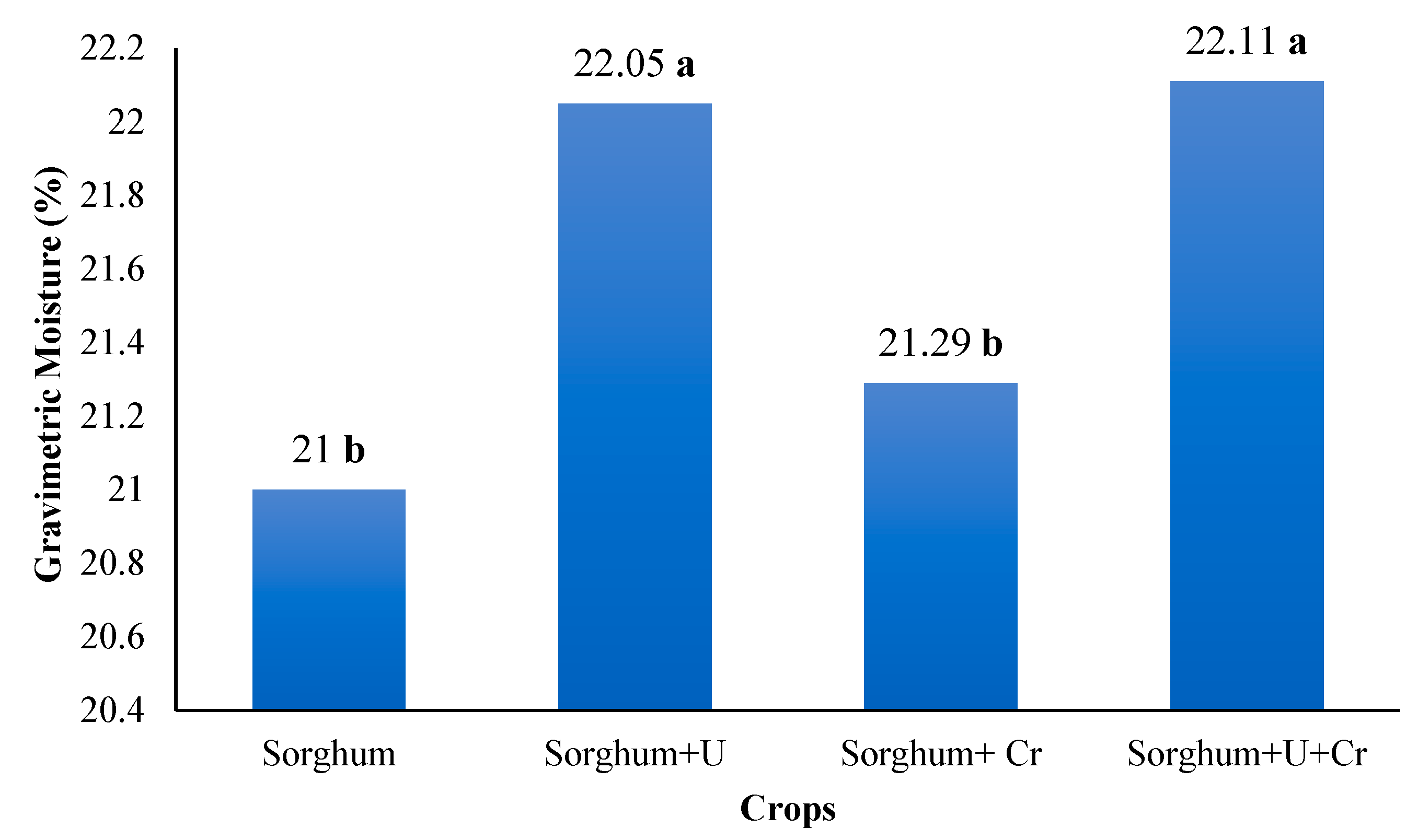
3.3. Gravimetric Moisture
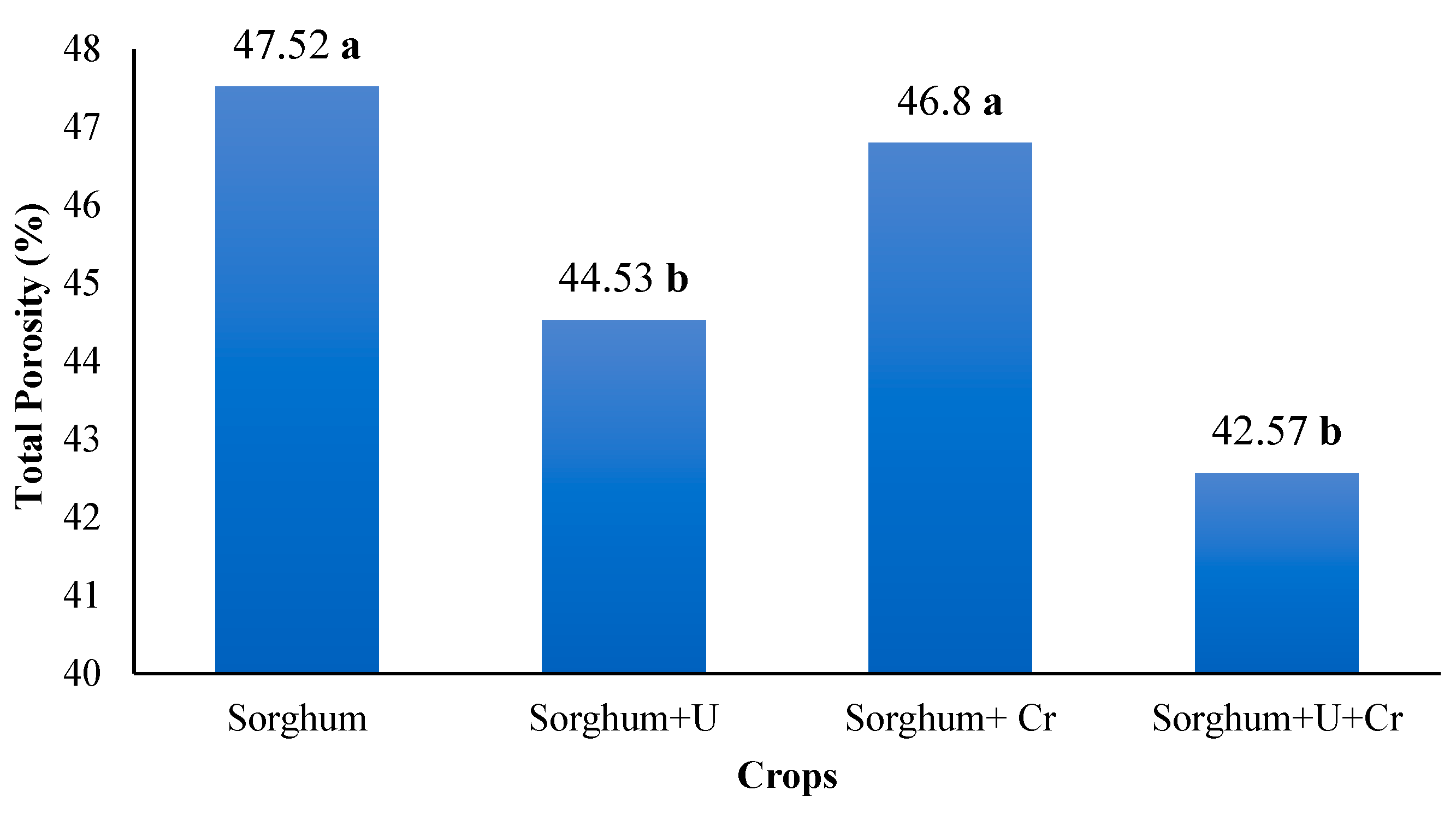
3.4. Total Porosity
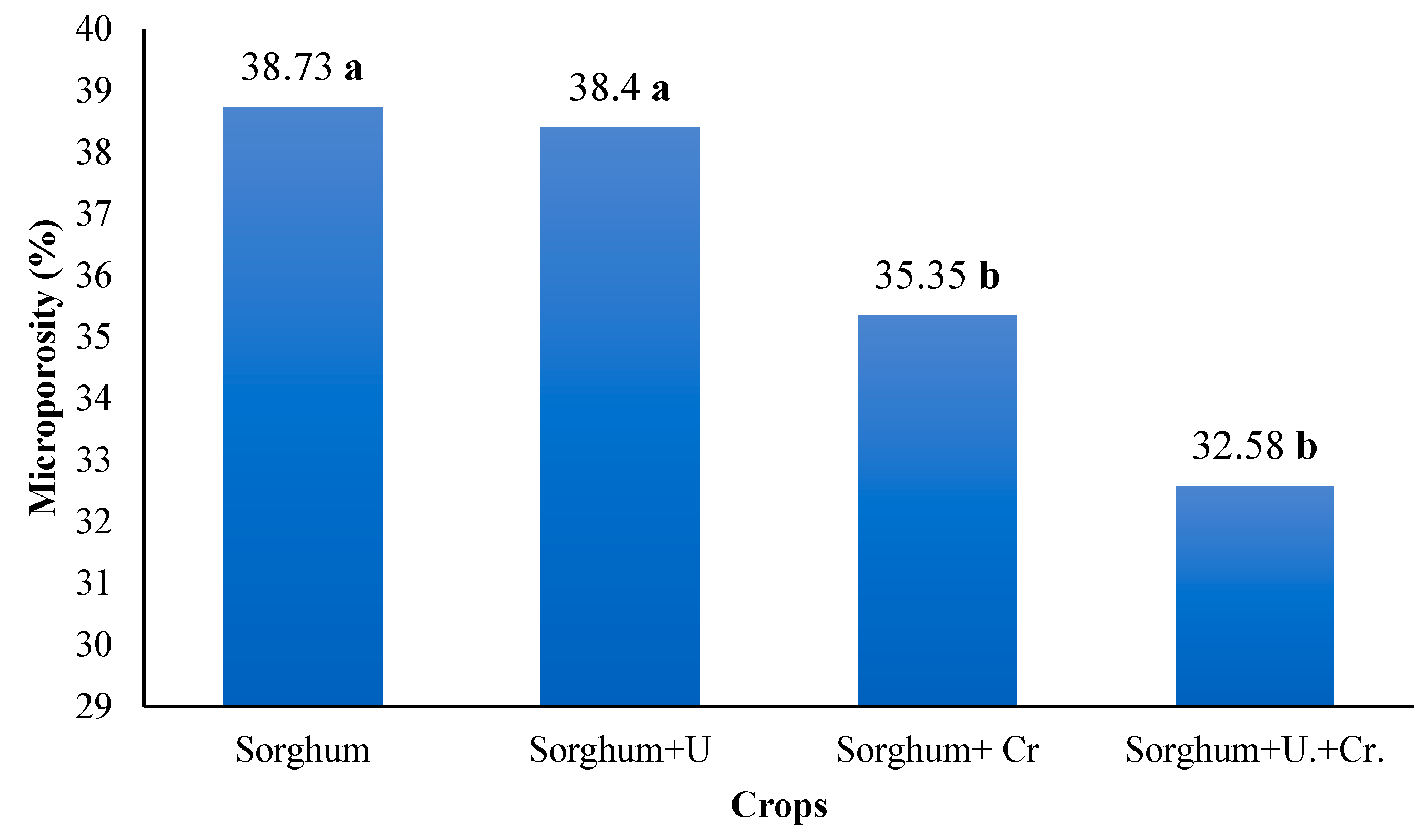
3.5. Microporosity
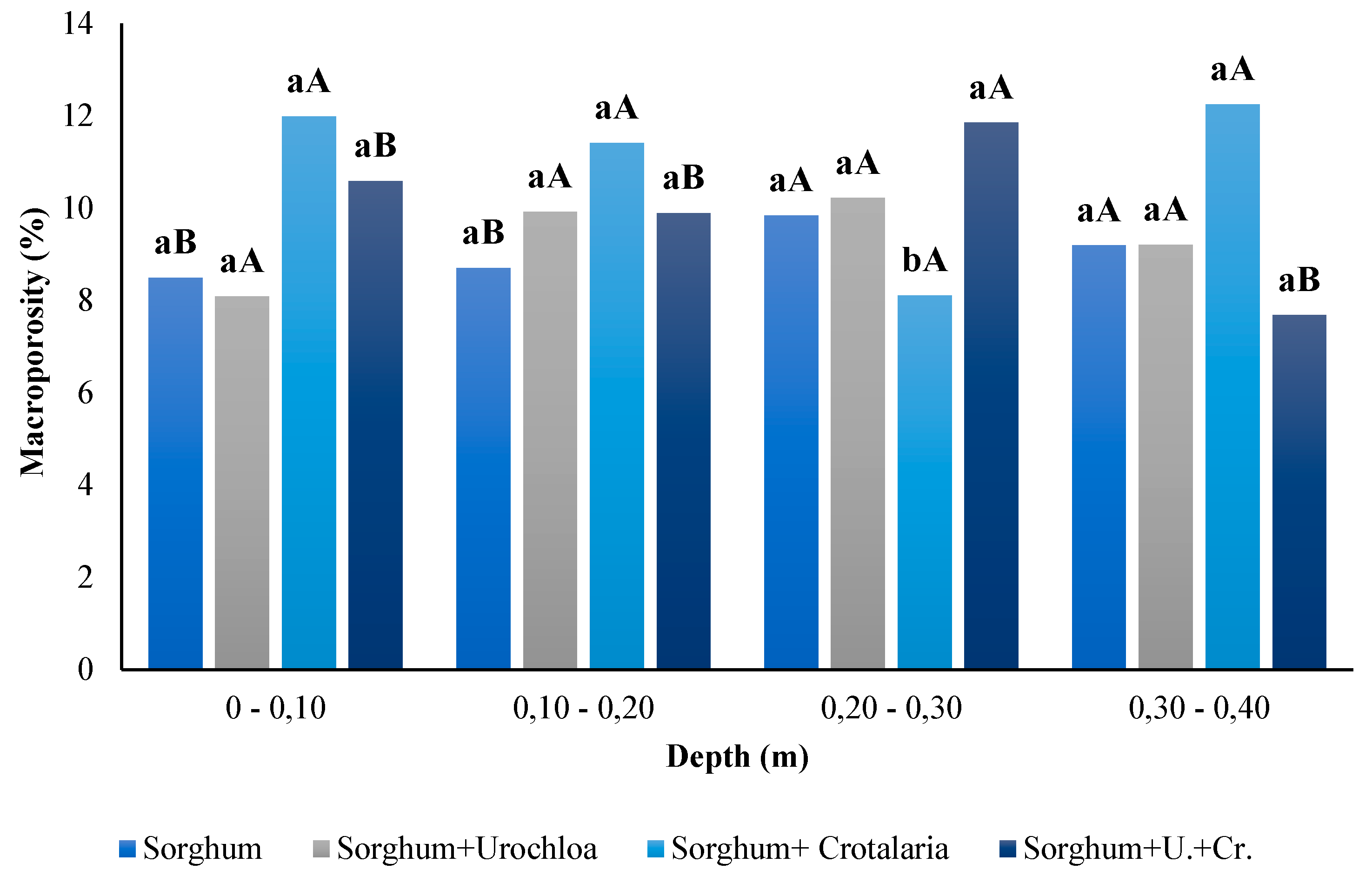
3.6. Macroporosity
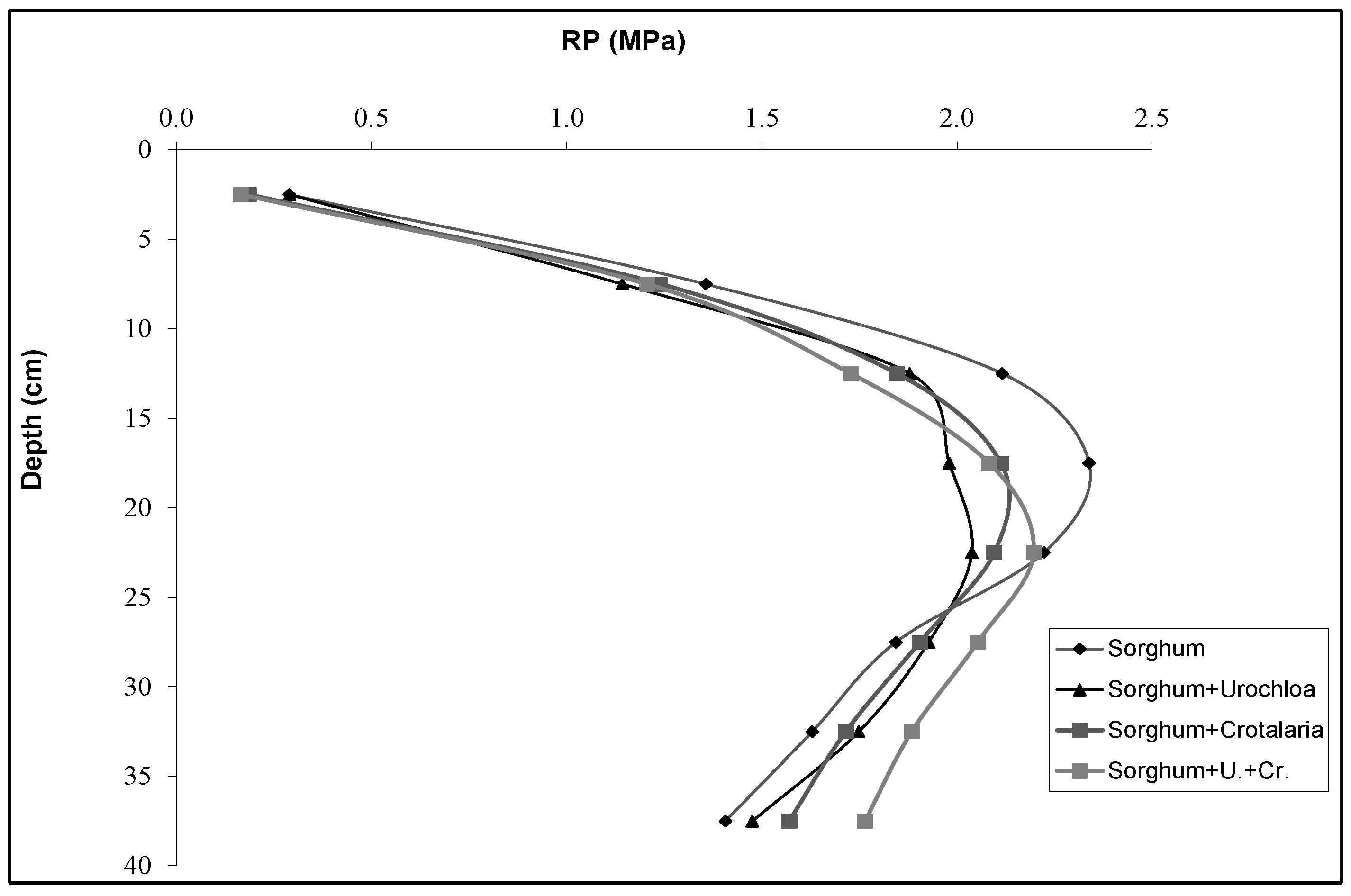
3.7. Resistance to Root Penetration
4. Conclusions
Acknowledgments
References
- Alakukku, L.; Weisskopf, P.; Chamen, W.C.T.; Tijink, F.G.J.; van der Linden, J.P.; Pires, S.; et al. Prevention strategies for soil compaction in modern agriculture: A review. Soil Tillage Res. 2023, 230, 105293. [Google Scholar]
- Junior, A.A.B.; Dos Santos, J.C.F.; Debiasi, H.; Yokoyama, A.H. Contribution of roots and shoots of Brachiaria species to soybean performance in succession. Pesqui. Agropecu. Bras. 2017, 52, 592–598. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Ruis, S.J. Cover crops and soil health. Soil Tillage Res. 2020, 198, 104558. [Google Scholar]
- Blanco-Canqui, H.; Lal, R.; Post, W.M.; Izaurralde, R.C.; Owens, L.B. Soil mechanical properties and organic carbon dynamics as influenced by cropping systems. Soil Tillage Res 2022, 221, 105360. [Google Scholar]
- Borges, J.A.; Pires, L.F.; Cássaro, F.A.; Roque, W.L.; Heck, R.J.; Rosa, J.A.; Wolf, F.G. X-ray microtomography analysis of representative elementary volume (REV) of soil morphological and geometrical properties. Soil Tillage Res. 2018, 182, 112–122. [Google Scholar] [CrossRef]
- Braida, J.A.; Reichert, J.M.; Veiga, M.D.; Reinert, D.J. Resíduos vegetais na superfície e carbono orgânico e suas relações com a densidade máxima obtida no ensaio proctor. Revista Brasileira de Ciência do Solo 2006, 30, 605–614. [Google Scholar] [CrossRef]
- Cambaúva, V.; et al. Crescimento, produtividade e palhada de milho exclusivo e consorciado com crotalárias em diferentes espaçamentos. Revista Brasileira de Milho e Sorgo 2019, 18, 99–111. [Google Scholar] [CrossRef]
- Carof, M.; Barbillon, P.; Soudais, J.; Doré, T. Cover cropping with legume living mulches in conventional and no-tillage organic farming: Effects on the growth and yield of winter wheat. European Journal of Agronomy 2007, 26, 65–72. [Google Scholar]
- Ciampitti, I.A; Carcedo. A. P. (2023). Sorghum Checkoff. KSU Agronomy eUpdates.
- Crusciol, C.A.C.; Nascente, A.S.; Mateus, G.P.; Borghi, E.; Leles, E.P.; Santos, N.C.B. Effect of Intercropping on Yields of Corn with Different Relative Maturities and Palisadegrass. Agron. J. 2013, 105, 599–606. [Google Scholar] [CrossRef]
- Crusciol, C.A.C.; Lima, E.V.; Andreotti, M.; Moro, E.; Venâncio, R.S. Persistência de palhada e liberação de nutrientes do sorgo granífero, milheto e Urochloa. Pesquisa Agropecuária Brasileira 2010, 45, 516–520. [Google Scholar]
- Crusciol, C.A.; Momesso, L.; Portugal, J.R.; Costa, C.H.; Bossolani, J.W.; Costa, N.R.; Pariz, C.M.; Castilhos, A.M.; Rodrigues, V.A.; Costa, C.; et al. Upland rice intercropped with forage grasses in an integrated crop-livestock system: Optimizing nitrogen management and food production. Field Crop. Res. 2021, 261. [Google Scholar] [CrossRef]
- Crusciol CA, C.; Soratto, R.P.; Mateus, G.P.; Costa CH, M.; Pariz, C.M. Improving soil fertility and crop yield in a tropical region with palisadegrass cover crops. Agronomy Journal 2013, 105, 1437–1445. [Google Scholar] [CrossRef]
- da Silva, R.F.; Severiano, E.d.C.; de Oliveira, G.C.; Barbosa, S.M.; Peixoto, D.S.; Tassinari, D.; Silva, B.M.; Silva, S.H.G.; Júnior, M.d.S.D.; Figueiredo, T.D.F.R. Changes in soil profile hydraulic properties and porosity as affected by deep tillage soil preparation and Brachiaria grass intercropping in a recent coffee plantation on a naturally dense Inceptisol. Soil Tillage Res. 2021, 213, 105127. [Google Scholar] [CrossRef]
- Desalegn, T.; Mekonnen, T.; Getachew, S.; Lemma, T. Impacts of Cover Crops on Soil Health and Crop Productivity: A Review. Soil Tillage Res. 2023, 223, 105345. [Google Scholar]
- Drescher, M.S. Estratégias para descompactação do solo por escarificação e hastes sulcadoras em sistema plantio direto. Tese (Doutorado em Ciência do Solo), Centro de Ciências Rurais, Universidade Federal de Santa Maria, Santa Maria, 2015. [Google Scholar]
- Ferreira, A.C.; Costa, A.R.; Silva, M.A.; Gomes, L.C. Benefits of Crotalaria juncea as a cover crop in tropical soils. Soil Tillage Res. 2021, 213, 105122. [Google Scholar]
- Ferreira, A.C.; Costa, A.R.; Silva, M.A.; Gomes, L.C. Benefits of Crotalaria juncea as a cover crop in tropical soils. Soil Tillage Res. 2021, 213, 105122. [Google Scholar]
- Ferreira, L.L.; de Souza, B.R.; Pereira, A.I.A.; Curvêlo, C.R.D.S.; Fernandes, C.D.S.; Dias, N.D.S.; Nascimento, E.K. .D. BIOESTIMULANTE E NITROGÊNIO DE LIBERAÇÃO GRADUAL NO DESEMPENHO DO SORGO. Nativa 2019, 7, 330–335. [Google Scholar] [CrossRef]
- Gentsch, N.; et al. Increased root biomass through cover crop mixtures enhances soil biogeochemical cycling. Agricultural Systems 2020, 182, 102839. [Google Scholar]
- Gilbert, R.A.; Morse, S.; Roberts, C.A.; Zartman, R.E. Evaluation of leguminous cover crops for weed suppression and soil fertility improvement. Journal of Agronomy and Crop Science 2008, 194, 183–190. [Google Scholar]
- Hamza, M.A.; Anderson, W.K. Soil compaction in cropping systems: A review of the nature, causes and possible solutions. Soil Tillage Res. 2005, 82, 121–145. [Google Scholar] [CrossRef]
- Haskel, M.K. Atributos físicos do solo conduzido sob escarificação mecânica, biológica e plantio direto: influência na produtividade biológica das culturas. Dissertação (Mestrado em Agronomia), Departamento Acadêmico de Ciências Agrárias, Universidade Técnológica Federal do Paraná, Pato Branco, 2020. [Google Scholar]
- Hellner, Q.; Koestel, J.; Ulén, B.; Larsbo, M. Effects of tillage and liming on macropore networks derived from X-ray tomography images of a silty clay soil. Soil Use Manag. 2018, 34, 197–205. [Google Scholar] [CrossRef]
- Hudek, C.; et al. Root traits of cover crops for soil health improvement. Soil Biology and Biochemistry 2022, 157, 108265. [Google Scholar]
- Keller, T.; Sandin, M.; Colombi, T.; Horn, R.; Or, D. Historical and future perspectives of soil compaction research: From foot marks to modern agriculture. Soil Tillage Res. 2022, 218, 105234. [Google Scholar]
- Lal, R. Enhancing soil health to mitigate climate change. Geoderma 2020, 364, 114182. [Google Scholar]
- Li, Y.; Li, Z.; Cui, S.; Jagadamma, S.; Zhang, Q.P. Residue retention and minimum tillage improve physical environment of the soil in croplands: A global meta-analysis. Soil Tillage Res. 2019, 194, 104292. [Google Scholar] [CrossRef]
- Liu, S.; Qin, W.; Oenema, O. Soil compaction and crop production: A meta-analysis. Soil Use and Management 2022, 38, 276–288. [Google Scholar]
- Martin-Guay, M.-O.; Paquette, A.; Dupras, J.; Rivest, D. The new Green Revolution: Sustainable intensification of agriculture by intercropping. Sci. Total. Environ. 2018, 615, 767–772. [Google Scholar] [CrossRef]
- Melo, W.J.; Pereira, M.G.; Pavinato, P.S. The role of Crotalaria juncea in soil nitrogen fixation and organic matter enhancement. Agricultural Research 2017, 6, 126–134. [Google Scholar]
- Melo, W.J.; Pereira, M.G.; Pavinato, P.S. The role of Crotalaria juncea in soil nitrogen fixation and organic matter enhancement. Agricultural Research 2017, 6, 126–134. [Google Scholar]
- Mingotte, F.L.C.; Jardim, C.A.; Amaral, C.B.D.; Coelho, A.P.; Morello, O.F.; Leal, F.T.; Lemos, L.B.; Filho, D.F. Maize yield under Urochloa ruziziensis intercropping and previous crop nitrogen fertilization. Agron. J. 2020, 113, 1681–1690. [Google Scholar] [CrossRef]
- Molin, J.P.; Dias, C.T.d.S.; Carbonera, L. Estudos com penetrometria: novos equipamentos e amostragem correta. Rev. Bras. De Eng. Agricola E Ambient. 2012, 16, 584–590. [Google Scholar] [CrossRef]
- Moraes, M.T.; Debiasi, H.; Franchini, J.C.; Silva, V.R. Benefícios das plantas de cobertura sobre as propriedades físicas do solo. In Manejo e conservação do solo e da água em pequenas propriedades rurais no sul do Brasil: práticas alternativas de manejo visando a conservação do solo e da água; Tiecher, T., Ed.; UFRGS: Porto Alegre, 2016; pp. 34–48. [Google Scholar]
- Pacheco, L.P.; Monteiro MM, S.; Silva, A.F.; Petter, F.A.; Carvalho, M.C.S. Desempenho de plantas de cobertura em função do manejo do solo e da adubação nitrogenada no cerrado piauiense. Revista Brasileira de Ciência do Solo 2011, 35, 2031–2039. [Google Scholar]
- Pires, L.F.; Borges JA, R.; Rosa, J.A.; Mooney, S.J. Soil compaction in Brazil: Current status and future approaches for mitigation. Soil and Tillage Research 2023, 234, 105411. [Google Scholar]
- Pitol, C. Crotalaria juncea: uma leguminosa promissora para o Brasil; IAPAR: Londrina, 2008. [Google Scholar]
- Possamai, E.J.; Conceição, P.C.; Amadori, C.; Bartz, M.L.C.; Ralisch, R.; Vicensi, M.; Marx, E.F. Adoption of the no-tillage system in Paraná State: A (re)view. Rev. Bras. De Cienc. Do Solo 2022, 46. [Google Scholar] [CrossRef]
- Reichert, J.M.; Suzuki, L.E.A.S.; Reinert, D.J. Compactação do solo em sistemas agropecuários e florestais: identificação, efeitos, limites críticos e mitigação. In Tópicos ciência do solo; Ceretta, C.A., Silva, L.S., Reichert, J.M., Eds.; Editora Sociedade Brasileira de Ciência do Solo: Viçosa, 2007; pp. 49–134. [Google Scholar]
- Ruffato, G.G.; Secco, D.; Junior, L.A.Z.; Tokura, L.K.; de Marins, A.C.; de Villa, B.; Miranda, A.G.G.; Chang, P.; Roehrs, S.A.; Savioli, M.R.; et al. Structuring of a Haplortox by Cover Crops and Their Effects on the Yield of Soybean Grains. J. Agric. Sci. 2019, 11, 309. [Google Scholar] [CrossRef]
- Santos, G.G.; da Silveira, P.M.; Marchão, R.L.; Becquer, T.; Balbino, L.C. Macrofauna edáfica associada a plantas de cobertura em plantio direto em um Latossolo Vermelho do Cerrado. Pesqui. Agropecu. Bras. 2008, 43, 115–122. [Google Scholar] [CrossRef]
- Shaheb, M.R. A study on the effect of tyre inflation pressure on soil properties, growth and yield of maize and soybean in Central Illinois. Ph.D. (Thesis), Harper Adams University, Newport, United Kingdom, 2020. [Google Scholar]
- Silva, R.F.; Muraoka, T.; Buzetti, S.; Trivelin, P.C.O.; Oliveira, R. Nitrogen fertilization and cover crops affecting soil attributes and productivity of an upland rice-cowpea rotation. Scientia Agricola 2011, 68, 478–484. [Google Scholar]
- Soares de Faria, R.; Quintão Lana, R.M. Sorghum–Grass Intercropping Systems under Varying Planting Densities in a Semi-Arid Region: Focusing on Soil Carbon and Grain Yield in the Conservation Systems. Agriculture 2022, 12, 1762. [Google Scholar] [CrossRef]
- Sousa, D. M. G. De; Lobato, E. Cerrado Correção e Adubação. Embrapa. 2004.
- Stegarescu, R.; et al. Root activity and aggregate stability in cover crop systems. Plant and Soil 2021, 463, 123–138. [Google Scholar]
- Sulzbach, L.G.; Secco, D.; Tokura, L.K.; De Villa, B.; Ruffato, G.G. Implicações de espécies de cobertura em parâmetros físico-hídricos de um Latossolo argiloso e no rendimento de grãos de soja. Acta Iguazu 2017, 6, 280–286. [Google Scholar]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de métodos de análise de solo - 3. ed. rev. e ampl.; Embrapa: Brasília, DF, 2017; 573p. [Google Scholar]
- Valicheski, R.R.; Grossklaus, F.; Stürmer, S.L.K.; Tramontin, A.L.; Baade, E.S.A.S. Desenvolvimento de plantas de cobertura e produtividade da soja conforme atributos físicos em solo compactado. Rev. Bras. De Eng. Agricola E Ambient. 2012, 16, 969–977. [Google Scholar] [CrossRef]
- Steponavičienė, V.; Žiūraitis, G.; Rudinskienė, A.; Jackevičienė, K.; Bogužas, V. Long-Term Effects of Different Tillage Systems and Their Impact on Soil Properties and Crop Yields. Agronomy 2024, 14, 870. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




