Submitted:
10 July 2024
Posted:
24 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Bioactives Compounds
3. Method
4. Results
5. Discussion
6. Conclusions
References
- Petersen, R.C. Mild Cognitive Impairment. Contin. Lifelong Learn. Neurol. 2016, 22, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Eshkoor, S.A.; Mun, C.Y.; Ng, C.K.; Hamid, T.A. Mild cognitive impairment and its management in older people. Clin. Interv. Aging 2015, 10, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Hugo, J.; Ganguli, M. Dementia and Cognitive Impairment. Clin. Geriatr. Med. 2014, 30, 421–442. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Lopez, O.; Armstrong, M.J.; Getchius, T.S.D.; Ganguli, M.; Gloss, D.; Gronseth, G.S.; Marson, D.; Pringsheim, T.; Day, G.S.; et al. Practice guideline update summary: Mild cognitive impairment. Neurology 2018, 90, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Jongsiriyanyong, S.; Limpawattana, P. Mild Cognitive Impairment in Clinical Practice: A Review Article. Am. J. Alzheimer's Dis. Other Dementiasr 2018, 33, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Manly, J.J.; Jones, R.N.; Langa, K.M.; Ryan, L.H.; Levine, D.A.; McCammon, R.; Heeringa, S.G.; Weir, D. Estimating the Prevalence of Dementia and Mild Cognitive Impairment in the US. JAMA Neurol. 2022, 79, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Langa, K.M.; Levine, D.A. The Diagnosis and Management of Mild Cognitive Impairment. JAMA 2014, 312, 2551–2561. [Google Scholar] [CrossRef] [PubMed]
- Hovens, I.B.; Dalenberg, J.R.; Small, D.M. A Brief Neuropsychological Battery for Measuring Cognitive Functions Associated with Obesity. Obesity (Silver Spring, Md) 2019, 27, 1988–96. [Google Scholar] [CrossRef] [PubMed]
- AMERICAN PSYCHIATRIC ASSOCIATION. Diagnostic and statistical manual of mental disorders. 5th-TR ed. [s.l.] American Psychiatric Association, 2022. Available online: https://integrada.minhabiblioteca.com.br/#/books/9786558820949/ (accessed on 6 May 2024).
- Dominguez, L.J.; Veronese, N.; Vernuccio, L.; Catanese, G.; Inzerillo, F.; Salemi, G.; Barbagallo, M. Nutrition, Physical Activity, and Other Lifestyle Factors in the Prevention of Cognitive Decline and Dementia. Nutrients 2021, 13, 4080. [Google Scholar] [CrossRef]
- Grabska-Kobyłecka, I.; Szpakowski, P.; Król, A.; Książek-Winiarek, D.; Kobyłecki, A.; Głąbiński, A.; Nowak, D. Polyphenols and Their Impact on the Prevention of Neurodegenerative Diseases and Development. Nutrients 2023, 15, 3454. [Google Scholar] [CrossRef]
- Calabrò, R.S.; De Cola, M.C.; Gervasi, G.; Portaro, S.; Naro, A.; Accorinti, M.; Manuli, A.; Marra, A.; De Luca, R.; Bramanti, P. The Efficacy of Cocoa Polyphenols in the Treatment of Mild Cognitive Impairment: A Retrospective Study. Medicina 2019, 55, 156. [Google Scholar] [CrossRef] [PubMed]
- Haskell-Ramsay, C.F.; Schmitt, J.; Actis-Goretta, L. The Impact of Epicatechin on Human Cognition: The Role of Cerebral Blood Flow. Nutrients 2018, 10, 986. [Google Scholar] [CrossRef] [PubMed]
- Tejada, S.; Sarubbo, F.; Jiménez-García, M.; Ramis, M.R.; Monserrat-Mesquida, M.; Quetglas-Llabrés, M.M.; Capó, X.; Esteban, S.; Sureda, A.; Moranta, D. Mitigating Age-Related Cognitive Decline and Oxidative Status in Rats Treated with Catechin and Polyphenon-60. Nutrients 2024, 16, 368. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Wang, W.; Li, Q.; Han, X.; Xing, J.; Qi, C.; Lan, X.; Wan, J.; Potts, A.; Guan, F.; et al. Cerebroprotection of flavanol (-)-epicatechin after traumatic brain injury via Nrf2-dependent and -independent pathways. Free. Radic. Biol. Med. 2015, 92, 15–28. [Google Scholar] [CrossRef] [PubMed]
- World Obesity Atlas. Obesity and its consequences. 2024. Available online: https://data.worldobesity.org/publications/WOF-Obesity-Atlas-v7.pdf (accessed on 2 June 2024).
- Bray, G.A.; Kim, K.K.; Wilding, J.P.H.; on behalf of the World Obesity Federation. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obesity Reviews 2017, 18, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Batista, Â.G.; Soares, E.S.; Mendonça, M.C.; da Silva, J.K.; Dionísio, A.P.; Sartori, C.R.; da Cruz-Höfling, M.A.; Marostica Junior, M.R. Jaboticaba berry peel intake prevents insulin-resistance-induced tau phosphorylation in mice. Mol Nutr Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Dragano, N.R.V.; Marques, A.Y.C.; Cintra, D.E.C.; Solon, C.; Morari, J.; Leite-Legatti, A.V.; Velloso, L.A.; Maróstica-Júnior, M.R. Freeze-dried jaboticaba peel powder improves insulin sensitivity in high-fat-fed mice. Br. J. Nutr. 2013, 110, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Batista, Â.G.; Cazarin, C.B.B.; Sandahl, M.; Turner, C.; Östman, E.; Junior, M.R.M. Characterization of antioxidant polyphenols from Myrciaria jaboticaba peel and their effects on glucose metabolism and antioxidant status: A pilot clinical study. Food Chem. 2016, 211, 185–197. [Google Scholar] [CrossRef]
- Williams, R.J.; Spencer, J.P. Flavonoids, cognition, and dementia: Actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free. Radic. Biol. Med. 2012, 52, 35–45. [Google Scholar] [CrossRef]
- Sugiura, M.; Ogawa, K.; Yano, M. Comparison of bioavailability between β-cryptoxanthin and β-carotene and tissue distribution in its intact form in rats. Biosci. Biotechnol. Biochem. 2014, 78, 307–310. [Google Scholar] [CrossRef]
- Thomas, J.; Garg, M.L.; Smith, D.W. Dietary resveratrol supplementation normalizes gene expression in the hippocampus of streptozotocin-induced diabetic C57Bl/6 mice. J. Nutr. Biochem. 2014, 25, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Jeon, B.T.; Jeong, E.A.; Shin, H.J.; Lee, Y.; Lee, D.H.; Kim, H.J.; Kang, S.S.; Cho, G.J.; Choi, W.S.; Roh, G.S. Resveratrol Attenuates Obesity-Associated Peripheral and Central Inflammation and Improves Memory Deficit in Mice Fed a High-Fat Diet. Diabetes 2012, 61, 1444–1454. [Google Scholar] [CrossRef]
- Dragano, N.R.V.; Marques, A.Y.C.; Cintra, D.E.C.; Solon, C.; Morari, J.; Leite-Legatti, A.V.; Velloso, L.A.; Maróstica-Júnior, M.R. Freeze-dried jaboticaba peel powder improves insulin sensitivity in high-fat-fed mice. Br. J. Nutr. 2013, 110, 447–455. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Kritchevsky, J.; Hargett, K.; Feller, K.; Klobusnik, R.; Song, B.J.; Cooper, B.; Jouni, Z.; Ferruzzi, M.G.; Janle, E.M. Plasma bioavailability and regional brain distribution of polyphenols from apple/grape seed and bilberry extracts in a young swine model. Mol. Nutr. Food Res. 2015, 59, 2432–2447. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Yin, P.; Ma, C.; Liu, Y. Method Development and Validation for Pharmacokinetic and Tissue Distributions of Ellagic Acid Using Ultrahigh Performance Liquid Chromatography-Tandem Mass Spectrometry (UPLC-MS/MS). Molecules 2014, 19, 18923–18935. [Google Scholar] [CrossRef]
- Rendeiro, C.; Rhodes, J.S.; Spencer, J.P. The mechanisms of action of flavonoids in the brain: Direct versus indirect effects. Neurochem. Int. 2015, 89, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Gautam, D.; Surjo, D.; Ueki, K.; Baudler, S.; Schubert, D.; Kondo, T.; Alber, J.; Galldiks, N.; Küstermann, E.; et al. Role for neuronal insulin resistance in neurodegenerative diseases. Proc. Natl. Acad. Sci. USA 2004, 101, 3100–3105. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, J.; Zhang, N.; Fu, P.; Jing, Z.; Yu, C.; Zhao, D.; Hao, W.; Zhou, C. Body mass index and mild cognitive impairment among rural older adults in China: the moderating roles of gender and age. BMC Psychiatry 2021, 21, 1–11. [Google Scholar] [CrossRef]
- Hughes, T.; Borenstein, A.; Schofield, E.; Wu, Y.; Larson, E. Association between late-life body mass index and dementia: The Kame Project. Neurology 2009, 72, 1741–1746. [Google Scholar] [CrossRef]
- Atti, A.R.; Palmer, K.; Volpato, S.; Winblad, B.; De Ronchi, D.; Fratiglioni, L. Late-life body mass index and dementia incidence: Nine-year follow-up data from the Kungsholmen Project. J. Am. Geriatr. Soc. 2008, 56, 111–116. [Google Scholar] [CrossRef]
- Blancas-Flores, G.; César Almanza-Pérez, J.; Ivette López-Roa, R.; Javier Alarcón-Aguilar, F.; García-Macedo, R.; Cruz, M. Obesity as an inflammatory process. Bol. Med. Hosp. Infant. Mix. 2010, 67, 88–97. [Google Scholar]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int J Mol Sci 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.S.; Alvarez-Leite, J.I. Low-Grade Inflammation, Obesity, and Diabetes. Curr. Obes. Rep. 2014, 3, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Cook, K.S.; Min, H.Y.; Johnson, D.; Chaplinsky, R.J.; Flier, J.S.; Hunt, C.R.; Spiegelman, B.M. Adipsin: a circulating serine protease homolog secreted by adipose tissue and sciatic nerve. Science 1987, 237, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Flier, J.S.; Cook, K.S.; Usher, P.; Spiegelman, B.M. Severely impaired adiposin expression in genetics and acquired obesity. Science 1987, 237, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.C.; et al. Adipsin is an adipokine that improves beta cell function in diabetes. Cell 2014, 158, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Sánchez, A.; Martínez-Ortega, A.J.; Remón-Ruiz, P.J.; Piñar-Gutiérrez, A.; Pereira-Cunill, J.L.; García-Luna, P.P. Therapeutic Properties and Use of Extra Virgin Olive Oil in Clinical Nutrition: A Narrative Review and Literature Update. Nutrients 2022, 14, 1440. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Morató, J.; Xicota, L.; Fitó, M.; Farré, M.; Dierssen, M.; De La Torre, R. Potential Role of Olive Oil Phenolic Compounds in the Prevention of Neurodegenerative Diseases. Molecules 2015, 20, 4655–4680. [Google Scholar] [CrossRef]
- Daccache, A.; Lion, C.; Sibille, N.; Gerard, M.; Slomianny, C.; Lippens, G.; Cotelle, P. Oleuropein and derivatives from olives as Tau aggregation inhibitors. Neurochem. Int. 2011, 58, 700–707. [Google Scholar] [CrossRef]
- da Silva Lemos, A.B.; da Fonseca, A.R.; de Souza, A.E.S.; Vivan, L.B.; Lomonaco, L.; Dias, S.T.; Salles, B.C.C.; Cerdeira, C.D.; Barros, G.B.S. Effects of the hydroalcoholic extract from the leaves and flour of the fruits of Guabiju (Myrcianthes pungens) on laboratory parameters of diabetic rats. RSD [Internet] 2021, 10, e39910817442. [Google Scholar]
- Geraldi, M.V.; Betim Cazarin, C.B.; Cristianini, M.; Vasques, A.C.J.; Geloneze, B.; Maróstica Júnior, M.R. Jabuticaba juice improves postprandial glucagon-like peptide-1 and antioxidant status in healthy adults: a randomized crossover trial. British Journal of Nutrition 2022, 128, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Gutierres, J.M.; Carvalho, F.B.; Schetinger, M.R.; Marisco, P.; Agostinho, P.; Rodrigues, M.; Rubin, M.A.; Schmatz, R.; da Silva, C.R.; de PCognato, G.; Farias, J.G.; Signor, C.; Morsch, V.M.; Mazzanti, C.M.; Bogo, M.; Bonan, C.D.; Spanevello, R. Anthocyanins restore behavioral and biochemical changes caused by streptozotocin-induced sporadic dementia of Alzheimer’s type. Life Sci. 2014, 96, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Devore, E.E.; Kang, J.H.; Breteler, M.M.; Grodstein, F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann. Neurol. 2012, 72, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Krikorian, R.; Shidler, M.D.; Nash, T.A.; Kalt, W.; Vinqvist-Tymchuk, M.R.; Shukitt-Hale, B.; Joseph, J.A. Blueberry Supplementation Improves Memory in Older Adults. J. Agric. Food Chem. 2010, 58, 3996–4000. [Google Scholar] [CrossRef] [PubMed]
- Small, G.W.; Siddarth, P.; Li, Z.; Miller, K.J.; Ercoli, L.; Emerson, N.D.; Martinez, J.; Wong, K.-P.; Liu, J.; Merrill, D.A.; et al. Memory and Brain Amyloid and Tau Effects of a Bioavailable Form of Curcumin in Non-Demented Adults: A Double-Blind, Placebo-Controlled 18-Month Trial. Am. J. Geriatr. Psychiatry 2018, 26, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Moura, M.H.; Donado-Pestana, C.M.; Rodrigues, L.; Pessoa, E.V.; e Silva, R.R.; Festuccia, W.T.; Genovese, M.I. Long-term supplementation with phenolic compounds from jaboticaba (Plinia jaboticaba (Vell.) Berg) reduces adiposophaty and improves glucose, lipid, and energy metabolism. Food Res. Int. 2021, 143, 110302. [Google Scholar] [CrossRef]
- Ho, L.; Ferruzzi, M.G.; Janle, E.M.; Wang, J.; Gong, B.; Chen, T.-Y.; Lobo, J.; Cooper, B.; Wu, Q.L.; Talcott, S.T.; Percival, S.S.; Simon, J.E.; Pasinetti, G.M. Identification of brain-targeted bioactive dietary quercetin-3-O-glucuronide as a novel intervention for Alzheimer’s disease. The FASEB Journal 2013, 27, 769–781. [Google Scholar] [CrossRef]
- Stull, A.J.; Cash, K.C.; Champagne, C.M.; Gupta, A.K.; Boston, R.; Beyl, R.A.; Johnson, W.D.; Cefalu, W.T. Blueberries Improve Endothelial Function, but Not Blood Pressure, in Adults with Metabolic Syndrome: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2015, 7, 4107–4123. [Google Scholar] [CrossRef]
- Wood, E.; Hein, S.; Mesnage, R.; Fernandes, F.; Abhayaratne, N.; Xu, Y.; Zhang, Z.; Bell, L.; Williams, C.; Rodriguez-Mateos, A. Wild blueberry (poly)phenols can improve vascular function and cognitive performance in healthy older individuals: a double-blind randomized controlled trial. Am. J. Clin. Nutr. 2023, 117, 1306–1319. [Google Scholar] [CrossRef]
- Huang, F.; Marungruang, N.; Martinsson, I.; Ferrer, L.C.; Nguyen, T.D.; Gondo, T.F.; Karlsson, E.N.; Deierborg, T.; Öste, R.; Heyman-Lindén, L. A mixture of Nordic berries improves cognitive function, metabolic function and alters the gut microbiota in C57Bl/6J male mice. Front. Nutr. 2023, 10, 1257472. [Google Scholar] [CrossRef]
- Truzzi, F.; Tibaldi, C.; Zhang, Y.; Dinelli, G.; D′Amen, E. An Overview on Dietary Polyphenols and Their Biopharmaceutical Classification System (BCS). Int. J. Mol. Sci. 2021, 22, 5514. [Google Scholar] [CrossRef] [PubMed]
- Kuszewski, J.C.; Howe, P.R.C.; Wong, R.H.X. Evaluation of Cognitive Performance following Fish-Oil and Curcumin Supplementation in Middle-Aged and Older Adults with Overweight or Obesity. J. Nutr. 2020, 150, 3190–3199. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.M.; Gamage, E.; Du, S.; Ashtree, D.N.; McGuinness, A.J.; Gauci, S.; et al. Ultra-processed food exposure and adverse health outcomes: umbrella review of epidemiological meta-analyses. BMJ 2024, 384, e077310. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-M.; Yoon, Y.; Yoon, H.; Park, H.-M.; Song, S.; Yeum, K.-J. Dietary Anthocyanins against Obesity and Inflammation. Nutrients 2017, 9, 1089. [Google Scholar] [CrossRef]
- de Vries, K.; Medawar, E.; Korosi, A.; Witte, A.V. The Effect of Polyphenols on Working and Episodic Memory in Non-pathological and Pathological Aging: A Systematic Review and Meta-Analysis. Front. Nutr. 2022, 8, 720756. [Google Scholar] [CrossRef]
- Lenquiste, S.A.; Lamas, C.d.A.; Marineli, R.d.S.; Moraes, A.; Borck, P.C.; Camargo, R.L.; Quitete, V.H.A.C.; Carneiro, E.M.; Junior, M.R.M. Jaboticaba peel powder and jaboticaba peel aqueous extract reduces obesity, insulin resistance and hepatic fat accumulation in rats. Food Res. Int. 2018, 120, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Lamas, C.; Lenquiste, S.; Baseggio, A.; Cuquetto-Leite, L.; Kido, L.; Aguiar, A.; Erbelin, M.; Collares-Buzato, C.; Maróstica, M.; Cagnon, V. Jaboticaba extract prevents prediabetes and liver steatosis in high-fat-fed aging mice. J. Funct. Foods 2018, 47, 434–446. [Google Scholar] [CrossRef]
- Ngamsamer, C.; Sirivarasai, J.; Sutjarit, N. The Benefits of Anthocyanins against Obesity-Induced Inflammation. Biomolecules 2022, 12, 852. [Google Scholar] [CrossRef]
- Lee, J.-S.; Cha, Y.-J.; Lee, K.-H.; Yim, J.-E. Onion peel extract reduces the percentage of body fat in overweight and obese subjects: a 12-week, randomized, double-blind, placebo-controlled study. Nutrition Research Practice 2016, 10, 175–181. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F. Flavonols (kaempeferol, quercetin, myricetin) contents of selected fruits, vegetables and medicinal plants. Food Chem. 2008, 108, 879–884. [Google Scholar] [CrossRef]
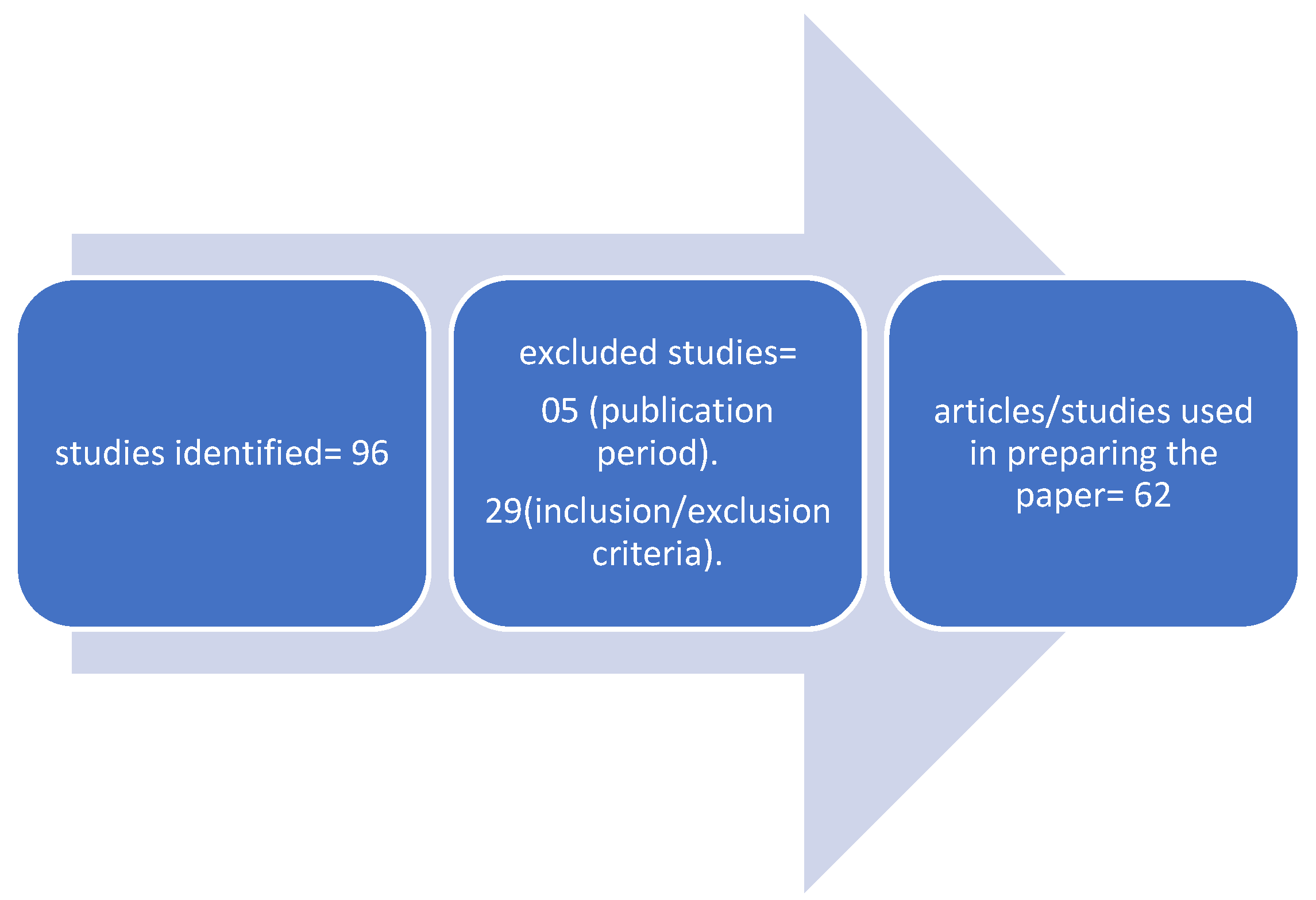
| Compound evaluated | Dose, time | Model used (humans, age, sex, conditions, inclusion criteria | Main results (biomarkers used) |
Reference |
|---|---|---|---|---|
| Guabiju (myrcianthes pungens) | hydroethanolic extract of Guabiju leaves at a concentration of 300 mg/Kg. The treatment was carried out for 90 days. | Wistar rats | Treatment with Guabiju leaf extract prevented the increase in liver damage, and liver function results showed significant beneficial changes in animals treated with Guabiju leaf extract, with a decrease in AST (aspartate aminotransferase) levels. This action in preventing typical complications of DM, such as liver damage, at low doses, demonstrates that this plant may have possible therapeutic activity, which must be refined with new tests and clinical trials that prove its efficacy and safety. | 42 |
| Plinia Jabuticaba | 250 ml Jabuticaba solution; 16 participants | Humans; 11 women, 5 men; healthy men and women, aged between 18 and 40, and with weight (BMI 18–25 kg/m2 ). Exclusion criteria included: age <18 years; allergy, hypersensitivity or intolerance to any food/ food ingredients; smoke; a vegetarian or vegan diet; taking a food supplement or receive any medication treatment; pregnancy or breastfeeding; diagnosis of diabetes mellitus, hypertension or reported medical history of CVD, cancer, liver, kidney, or bowel illness. |
250 ml of jaboticaba juice before a carbohydrate meal was able to improve antioxidant status and GLP-1 concentrations in healthy individuals. Compared to placebo, jaboticaba juice ingestion resulted in a GLP-1 response greater than the AUC and peaking at 60 min. Jaboticaba juice also resulted in greater antioxidant capacity. | 43 |
| Anthocyanins (extracted and purified from grape skins) | Anthocyanin10mg/KG | Male Wistar rats (3 months old) weighing 350-400 g were used in the study |
Biomarker evaluated: Acetylcholinesterase. The results demonstrated that Anthocyanin is capable of regulating ion pump activity and cholinergic neurotransmission, in addition to being capable of improving memory and acting as an anxiolytic compound in animals. | 44 |
| Blueberry, Strawberry | 145,4 a 684,1 mg/dia | 16010 participants over 70 years of age | A higher intake of blueberries and strawberries has been linked to slower rates of cognitive decline. These effect estimates were equivalent to those found in previous cohort studies, indicating that berry intake appears to delay cognitive aging by up to 2.5 years. Furthermore, in further supporting evidence, higher intakes of anthocyanidins and total flavonoids have been associated with slower rates of cognitive decline. | 45 |
| Blueberry | 734 mg/ml (phenolics); Anthocyanins 877 mg | 444 to 621 ml per day; 12 weeks 5 men, 4 women |
This preliminary study suggests, through its findings, that moderate-term blueberry supplementation may provide neurocognitive benefits with preventive potential related to neuronal mechanisms. | 46 |
| Curcumin | 40 participants without dementia, aged 51 to 84; 90 mg of Curcumin twice a day |
Human beings | Daily oral use of curcumin can improve memory and attention in adults without dementia, that is, without a neurodegenerative process. The results show that the benefits of symptoms are associated with a decrease in the accumulation of amyloid and tau proteins in brain regions that modulate mood and memory. | 47 |
| Plinia Jaboticaba | 2 groups “Food” group: 20 Control group: 46 Preparation of Phenolic Extract |
Mices | Several therapeutic properties against diet-induced obesity were demonstrated in this study. Polyphenols derived from Jabuticaba (PEJ) prevented weight gain in mice with already established obesity. Animals treated with PEJ showed a decrease in adipocyte hyperplasia and inflammation caused by adiposopathy. PEJ improved glucose metabolism by reducing FBG, glucose intolerance, insulinemia and insulin resistance, similarly, PEJ also improved lipid metabolism, decreasing plasma levels of total cholesterol and LDL-cholesterol. The study concludes that long-term PEJ supplementation can be used as an adjuvant against obesity and associated metabolic changes. | 48 |
| Quercetina-3-0-Glucoronide | Polyphenolic compounds | Cell cultures | We demonstrated that quercetin-3-O-glucuronide significantly reduced the generation of β-amyloid (Aβ) peptides by primary neuron cultures; It has also been demonstrated that quercetin-3-O-glucuronide is also capable of interfering with the initial protein-protein interaction of Aβ1–40 and Aβ1–42 that is necessary for the formation of neurotoxic oligomeric Aβ species | 49 |
| Strawberry, Blueberry | 44 men and women 45 g powder (Strawberry and Blueberry) daily for 6 weeks |
human beings | The study concludes that daily consumption of blueberries improved (increased) endothelial function over six weeks in individuals with metabolic syndrome. |
50 |
| Wild blueberry | 61 healthy older individuals aged 65-80 – 12 weeks | human beings | There is improvement in vascular and cognitive function and reduction in 24-hour ambulatory systolic blood pressure in healthy elderly people.; polyphenols may improve episodic memory processes and executive functioning in older adults at risk for cognitive decline | 51 |
| Nordic berries | 4,5 months | male mice | Supplementing with the berry mixture may have beneficial effects on spatial memory, as seen by better performance in the T-maze and Barnes maze compared to rats given the high-fat diet without fruit. It has also been observed that berry intake may help counteract high-fat diet-induced weight gain and may influence neuroinflammatory status, as suggested by increased levels of the inflammation-modifying cytokine IL-10 in mouse hippocampal extracts. supplemented with red fruits. | 52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




