Submitted:
16 July 2024
Posted:
24 July 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Historical Survey of Omitted Opportunities for Understanding Breast Cancer Behavior
Estrogen Deficiency and ER Resistance Proved to Be Newly Recognized Cancer Risk Factors
Genes Activated by Estrogen Upregulate DNA Stabilization and Silence cell Proliferation in Breast Cancer Cells
In Breast Cancer Cells, Estrogen Upregulates the Genome Stabilizer Circuit Counteracting Pro-Oncogenic Processes
Molecular Classification of Breast Cancer Subtypes Is a Mirror Reflecting the Defect of Estrogen Signaling and Genomic Damage in Patients
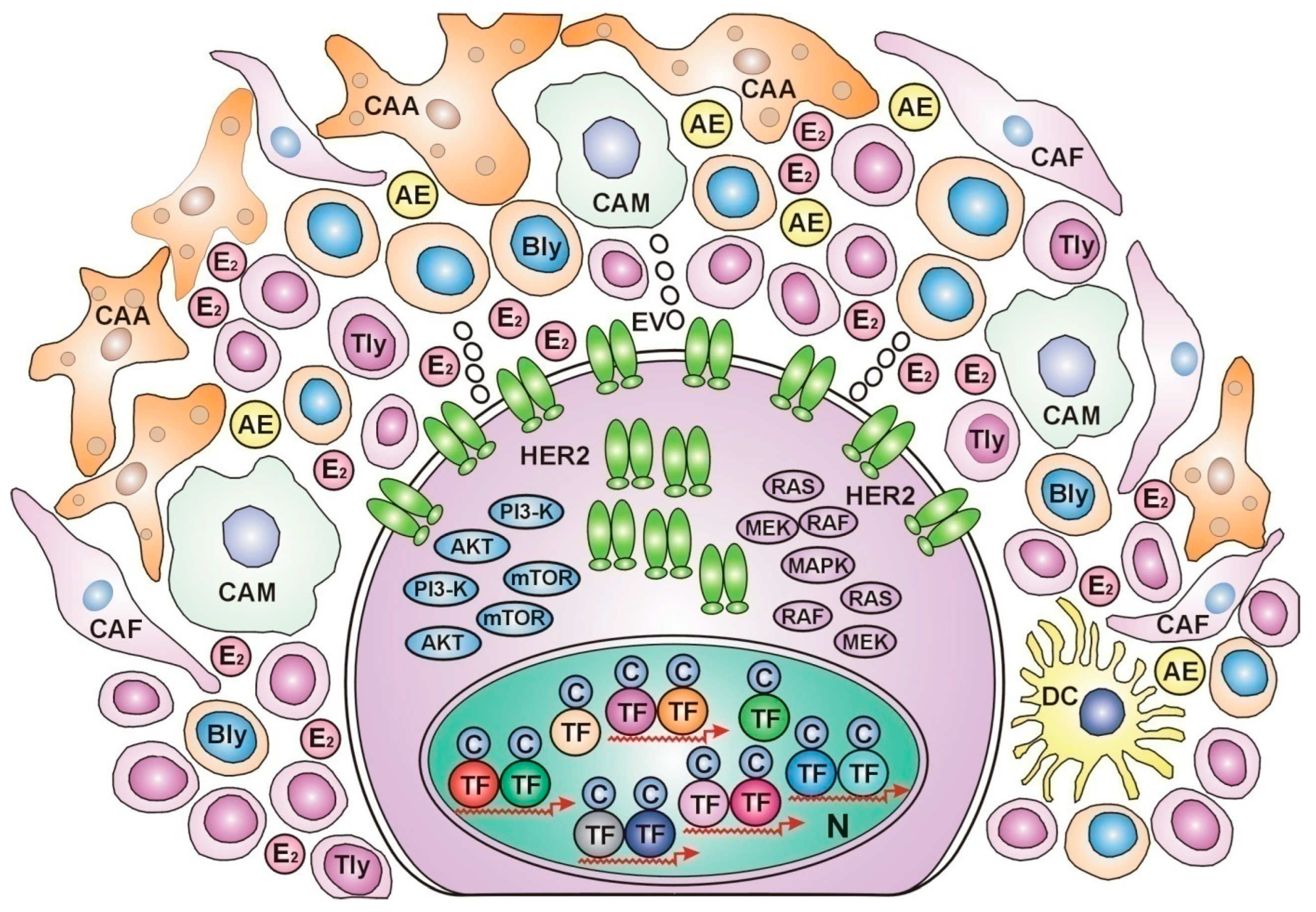
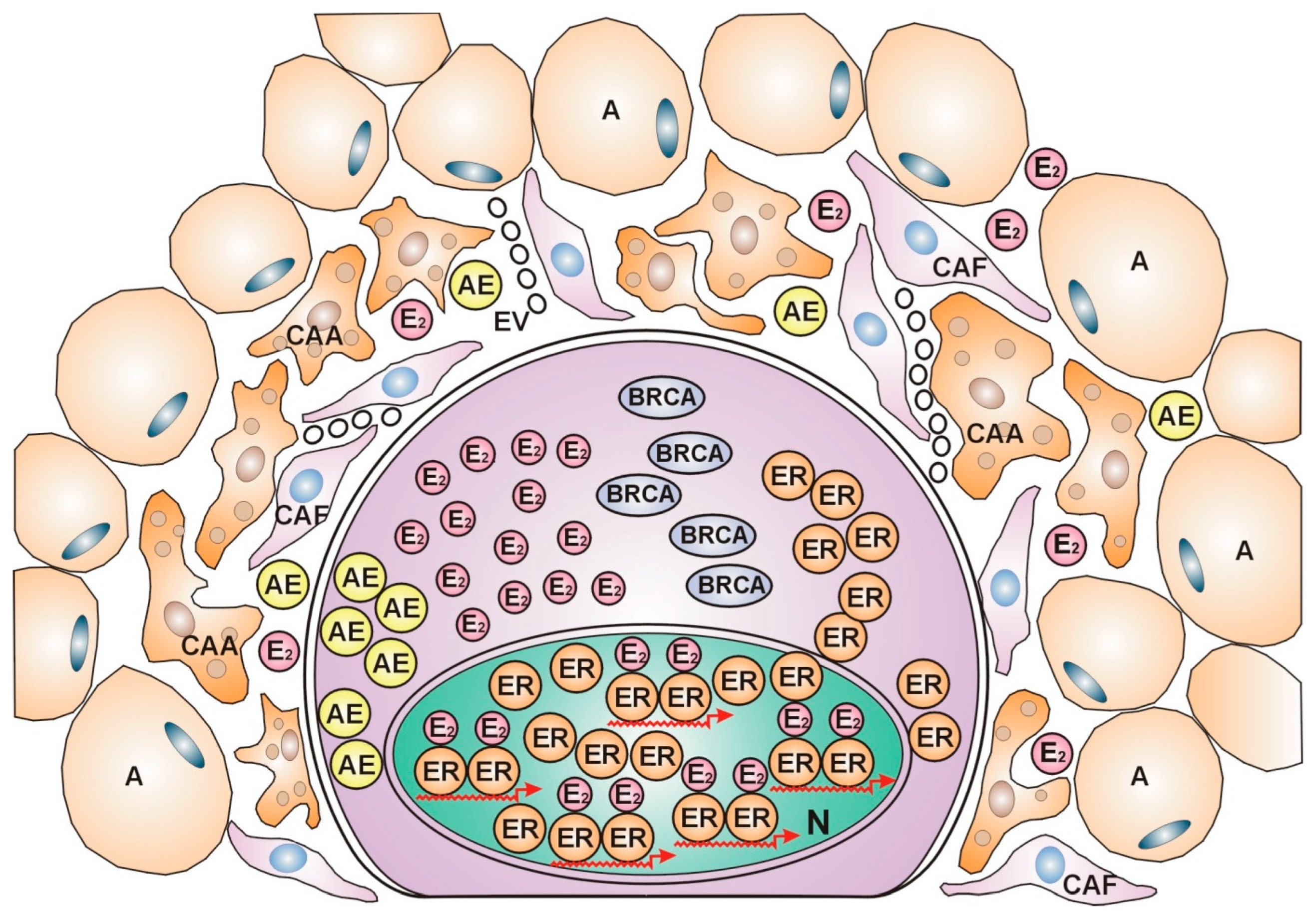
Dynamic Communication between Breast Cancers and Their Microenvironment during Anticancer Fight
Correlations between Breast Cancer Subtypes and the Characteristics of Their Cellular Microenvironment
Estrogen Prevention and Therapy of Breast Cancer
Conclusions
Conflicts of Interest
References
- Wilkinson, L.; Gathani, T. Understanding breast cancer as a global health concern. Br J Radiol. 2022, 95, 20211033. [Google Scholar] [CrossRef]
- Heer, E.; Harper, A.; Escandor, N.; Sung, H.; McCormack, V.; et al. Global burden and trends in premenopausal and postmenopausal breast cancer: A population-based study. The Lancet Global Health 2020, 8, e1027–e1037. [Google Scholar] [CrossRef]
- Huang, J.; Chan, P.S.F.; Lok, V.; Chen, X.; Ding, H.; Jin, Y.; et al. Global incidence and mortality of breast cancer: A trend analysis. Aging 2021, 13, 5748–5803. [Google Scholar] [CrossRef]
- Narod, S.A.; Iqbal, J.; Giannakeas, V.; Sopik, V.; Sun, P. Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol 2015, 1, 888–896. [Google Scholar] [CrossRef]
- Esserman, L.; Yau, C. Rethinking the standard for ductal carcinoma in situ treatment. JAMA Oncol. 2015, 1, 881–883. [Google Scholar] [CrossRef]
- Beatson, G.T. On the treatment of inoperable cases of carcinoma of the mamma: Suggestions for a new method of treatment, with illustrative cases. Lancet 1986, 2, 104–107. [Google Scholar]
- Boyd, S. On oophorectomy in cancer of the breast. BMJ 1900, 2, 1161–1187. [Google Scholar] [CrossRef]
- Jensen, E.V.; Jacobson, H.I. Biological Activities of Steroids in Relation to Cancer; Pincus, G., Vollmer, E.P., Eds.; Academic Press: New York, 1960; p. 161. [Google Scholar]
- Liehr, J.G. Genotoxic effects of estrogens. Mutat Res. 1990, 238, 269–276. [Google Scholar] [CrossRef]
- Yager, D.J. Endogenous estrogens as carcinogens through metabolic activation. Natl Cancer Inst Monogr. 2000, 27, 67–73. [Google Scholar] [CrossRef]
- Cavalieri, E.; Rogan, E. Catechol quinones of estrogens in the initiation of breast, prostate, and other human cancers: Keynote lecture. Ann N Y Acad Sci. 2006, 1089, 286–301. [Google Scholar] [CrossRef]
- Russo, J.; Russo, I.H. The role of estrogen in the initiation of breast cancer. J Steroid Biochem Mol Biol 2006, 102, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Hemsell, D.L.; Grodin, J.M.; Brenner, P.F.; Siiteri, P.K.; MacDonald, P.C. Plasma precursors of estrogen. II. Correlation of the extent of conversion of plasma androstenedione to estrone with age. The Journal of Clinical Endocrinology and Metabolism. 1974, 38, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Mahboobifard, F.; Pourgholami, M.H.; Jorjani, M.; Dargahi, L.; et al. Estrogen as a key regulator of energy homeostasis and metabolic health. Biomed Pharmacother 2022, 156, 113808. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.R.; Mahendroo, M.S.; Nichols, J.E.; Bulun, S.E. Aromatase gene expression in adipose tissue: Relationship to breast cancer. Int J Fertil Menopausal Stud. 1994, 39 (Suppl. S2), 75–83. [Google Scholar] [PubMed]
- Stefanick, M.L. Estrogens and progestins: Background and history, trends in use, and guidelines and regimens approved by the US Food and Drug Administration. Am J Med 2005, 118 (Suppl. S12), 64–73. [Google Scholar] [CrossRef] [PubMed]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar] [PubMed]
- Coelingh-Bennink, H.J.; Verhoeven, C.; Dutman, A.E.; Thijssen, J. The use of high-dose estrogens for the treatment of breast cancer. Maturitas 2017, 95, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Lidegaard, Ø.; Løkkegaard, E.; Jensen, A.; Skovlund, C.W.; Keiding, N.; et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med 2012, 366, 2257–2266. [Google Scholar] [CrossRef] [PubMed]
- Mørch, L.S.; Skovlund, C.W.; Hannaford, P.C.; Iversen, L.; Fielding, S.; Lidegaard, Ø. Contemporary Hormonal Contraception and the Risk of Breast Cancer. N Engl J Med 2017, 377, 2228–2239. [Google Scholar] [CrossRef]
- Cortés, M.E.; Alfaro, A.A. The effects of hormonal contraceptives on glycemic regulation. Linacre Q 2014, 81, 209–218. [Google Scholar] [CrossRef]
- Hilakivi-Clarke, L.; de Assis, S.; Warri, A. Exposures to Synthetic Estrogens at Different Times During the Life, and Their Effect on Breast Cancer Risk. J Mammary Gland Biol Neoplasia. 2013, 18, 25–42. [Google Scholar] [CrossRef]
- Bentrem, D.J.; Jordan, V.C. Role of antiestrogens and aromatase inhibitors in breast cancer treatment. Curr Opin Obstet Gynecol. 2002, 14, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Suba, Z. Amplified crosstalk between estrogen binding and GFR signaling mediated pathways of ER activation drives responses in tumors treated with endocrine disruptors. Recent Pat Anticancer Drug Discov 2018, 13, 428–444. [Google Scholar] [CrossRef]
- Hayes, D.F. Tamoxifen: dr. Jekyll and mr. Hyde? J Natl Cancer Inst 2004, 96, 895–897. [Google Scholar] [CrossRef] [PubMed]
- Osborne, C.K.; Schiff, R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011, 62, 233–247. [Google Scholar] [CrossRef]
- Massarweh, S.; Osborne, C.K.; Creighton, C.J.; Qin, L.; Tsimelzon, A.; Huang, S.; Weiss, H.; Rimawi, M.; Schiff, R. Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer Res. 2008, 68, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Suba, Z. Compensatory estrogen signal is capable of DNA repair in antiestrogen-responsive cancer cells via activating mutations. J Oncol 2020, 2020, 5418365. [Google Scholar] [CrossRef] [PubMed]
- Pujol, P.; Galtier-Dereure, F.; Bringer, J. Obesity and breast cancer risk. Human Reproduction 1997, 12, 116–125. [Google Scholar] [CrossRef]
- García-Estévez, L.; Cortés, J.; Pérez, S.; Calvo, I.; Gallegeos, I.; Moreno-Bueno, G. Obesity and Breast Cancer: A Paradoxical and Controversial Relationship Influenced by Menopausal Status. Front Oncol. 2021, 11, 705911. [Google Scholar] [CrossRef]
- Suba, Z. Circulatory Estrogen Level Protects against Breast Cancer in Obese Women. Recent Patents Anti-Cancer Drug Discov 2013, 8, 154–167. [Google Scholar] [CrossRef]
- Bruning, P.F.; Bonfrèr, J.M.G.; van Noord, P.A.H.; Hart, A.A.M.; et al. Insulin resistance and breast-cancer risk. Int J Cancer 1992, 52, 511–516. [Google Scholar] [CrossRef]
- Rose, D.P.; Vona-Davis, L. The cellular and molecular mechanisms by which insulin influences breast cancer risk and progression. Endocrine-Related Cancer 2012, 19, R225–R241. [Google Scholar] [CrossRef] [PubMed]
- Suba, Z. Interplay between insulin resistance and estrogen deficiency as coactivators in carcinogenesis. Pathol Oncol Res 2012, 18, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Suba, Z. Low estrogen exposure and/or defective estrogen signaling induces disturbances in glucose uptake and energy expenditure. J Diabet Metab 2013, 4, 272–281. [Google Scholar] [CrossRef]
- Amend, K.; Hicks, D.; Ambrosone, C.B. Breast cancer in AfricanAmerican women: Differences in tumor biology from EuropeanAmerican women. Cancer Res 2006, 66, 8327–8330. [Google Scholar] [CrossRef] [PubMed]
- Amirikia, K.C.; Mills, P.; Bush, J.; Newman, L.A. Higher populationbased incidence rates of triple-negative breast cancer among young African-American women: Implications for breast cancer screening recommendations. Cancer 2011, 117, 2747–2753. [Google Scholar] [CrossRef] [PubMed]
- Suba, Z. Light deficiency confers breast cancer risk by endocrine disorders. Recent Pat Anticancer Drug Discov. 2012, 7, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Yedjou, C.G.; Sims, J.N.; Miele, L.; Noubissi, F.; Lowe, L.; et al. Health and Racial Disparity in Breast Cancer. Adv Exp Med Biol. 2019, 1152, 31–49. [Google Scholar] [PubMed]
- Phipps, A.I.; Chlebowski, R.T.; Prentice, R.; McTiernan, A.; Stefanick, M.L.; Wactawski-Wende, J.; Kuller, L.H.; Adams-Campbell, L.L.; Lane, D.; Vitolins, M.; et al. Body size, physical activity, and risk of triple-negative and estrogen receptor-positive breast cancer. Cancer Epidemiol Biomarkers Prev 2011, 20, 454–463. [Google Scholar] [CrossRef]
- Suba, Z. Triple-negative breast cancer risk in women is defined by the defect of estrogen signaling: Preventive and therapeutic implications. Onco Targets Ther. 2014, 7, 147–164. [Google Scholar] [CrossRef]
- Miki, Y.; Swensen, J.; Shattuck-Eidens, D.; Futreal, P.A.; Harshman, K.; Tavtigian, S.; et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 1994, 266, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Wooster, R.; Bignell, G.; Lancaster, J.; Swift, S.; Seal, S.; Mangion, J.; et al. Identification of the breast cancer susceptibility gene BRCA2. Nature 1995, 378, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Gorski, J.J.; Kennedy, R.D.; Hosey, A.M.; Harkin, D.P. The complex relationship between BRCA1 and ERalpha in hereditary breast cancer. Clin Cancer Res 2009, 15, 1514–1518. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Di, L.J. BRCA1 and estrogen/estrogen receptor in breast cancer: Where they interact? Int J Biol Sci 2014, 10, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Annab, L.A.; Afshari, C.A.; Lee, W.H.; Boyer, T.G. BRCA1 mediates ligand-independent transcriptional repression of the estrogen receptor. Proc Natl Acad Sci USA. 2001, 98, 9587–9592. [Google Scholar] [CrossRef] [PubMed]
- Chand, A.L.; Simpson, E.R.; Clyne, C.D. Aromatase expression is increased in BRCA1 mutation carriers. BMC Cancer 2009, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, S.R.; Van De Vijver, M.J.; Jacquemier, J.; et al. The pathology of familial breast cancer: Predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2. J Clin Oncol. 2002, 20, 2310–2318. [Google Scholar] [CrossRef] [PubMed]
- Sorlie, T.; Perou, C.M.; Tibshirani, R.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001, 98, 10869–10874. [Google Scholar] [CrossRef]
- Suba, Z. DNA stabilization by the upregulation of estrogen signaling in BRCA gene mutation carriers. Drug Des Devel Ther 2015, 9, 2663–2675. [Google Scholar] [CrossRef]
- Suba, Z. DNA damage responses in tumors are not proliferative stimuli, but rather they are DNA repair actions requiring supportive medical care. Cancers 2024, 16, 1573. [Google Scholar] [CrossRef]
- Björnström, L.; Sjöberg, M. Mechanisms of estrogen receptor signaling: Convergence of genomic and nongenomic actions on target genes. Mol Endocrinol 2005, 19, 833–842. [Google Scholar] [CrossRef]
- Maggi, A. Liganded and unliganded activation of estrogen receptor and hormone replacement therapies. Biochim Biophys Acta 2011, 1812, 1054–1060. [Google Scholar] [CrossRef]
- Pescatori, S.; Berardinelli, F.; Albanesi, J.; Ascenzi, P.; et al. A Tale of Ice and Fire: The Dual Role for 17β-Estradiol in Balancing DNA Damage and Genome Integrity. Cancers 2021, 13, 1583. [Google Scholar] [CrossRef]
- Anderson, G.L.; Limacher, M.; Assaf, A.R.; et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized controlled trial. JAMA 2004, 291, 1701–1712. [Google Scholar]
- Suba, Z. Synthetic Estrogens Deregulate Estrogen Receptors Inducing Thromboembolic Complications and Cancer. In: Topics in Anti-Cancer Research. Vol. 8. Eds: Atta-ur-Rahman and Khurshid Zaman. Bentham Science Publishers 2019, Chapter 2, pp. 44–73. [CrossRef]
- LaCroix, A.Z.; Chlebowski, R.T.; Manson, J.E.; Aragaki, A.K.; Johnson, K.C.; Martin, L.; et al. Health Outcomes after Stopping Conjugated Equine Estrogens Among Postmenopausal Women with Prior Hysterectomy: A Randomized Controlled Trial. JAMA 2011, 305, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Chlebowski, R.T.; Stefanick, M.L.; Aragaki, A.K.; Rossouw, J.E.; Prentice, R.L.; et al. Menopausal Hormone Therapy and Health Outcomes during the Intervention and Extended Poststopping Phases of the Women’s Health Initiative Randomized Trials. JAMA 2013, 310, 1353–1368. [Google Scholar] [CrossRef] [PubMed]
- Chlebowski, R.T.; Anderson, G.L.; Aragaki, A.K.; Manson, J.E.; Stefanick, M.L.; Pan, K.; et al. Association of Menopausal Hormone Therapy with Breast Cancer Incidence and Mortality during Long-Term Follow-Up of the Women’s Health Initiative Randomized Clinical Trials. JAMA 2020, 324, 369–380. [Google Scholar] [CrossRef]
- Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: Individual participant meta-analysis of the worldwide epidemiological evidence. Lancet 2019, 394, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Al-Shami, K.; Awadi, S.; Khamees, A.; Alsheikh, A.A.; Sumaiya Al-Sharif, S.; et al. Estrogens and the risk of breast cancer: A narrative review of literature. Heliyon 2023, 9, e20224. [Google Scholar] [CrossRef]
- Suba, Z. Gender-related hormonal risk factors for oral cancer. Pathol Oncol Res 2007, 13, 195–202. [Google Scholar] [CrossRef]
- Suba, Z. Common soil of smoking-associated and hormone-related cancers: Estrogen deficiency. Oncol Rev 2010, 4, 73–87. [Google Scholar] [CrossRef]
- McGuire, A.; Brown, J.A.L.; Malone, C.; McLaughlin, R.; Kerin, M.J. Effects of Age on the Detection and Management of Breast Cancer. Cancers 2015, 7, 908–929. [Google Scholar] [CrossRef]
- Kaaks, R.; Berrino, F.; Key, T.; Rinaldi, S.; Dossus, L.; Biessy, C.; Secreto, G.; et al. Serum Sex Steroids in Premenopausal Women and Breast Cancer Risk Within the European Prospective Investigation into Cancer and Nutrition (EPIC). J Natl Cancer Inst 2005, 97, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Baglietto, L.; Severi, G.; English, D.R.; Krishnan, K.; Hopper, J.L.; McLean, C.; Morris, H.A.; Tilley, W.D.; Giles, G.G. Circulating steroid hormone levels and risk of breast cancer for postmenopausal women. Cancer Epidemiol Biomarkers Prev 2010, 19, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Hartley, M.C.; McKinley, B.P.; Rogers, E.A.; Kalbaugh, C.A.; Messich, H.S.; Blackhurst, D.W.; Lokey, J.S.; Trocha, S.D. Differential expression of prognostic factors and effect on survival in young (< or =40) breast cancer patients: A case-control study. Am Surg 2006, 72, 1189–1194. [Google Scholar]
- Britt, K.; Ashworth, A.; Smalley, M. Pregnancy and the risk of breast cancer. Endocrine-Related Cancer 2007, 14, 907–933. [Google Scholar] [CrossRef]
- Papaioannou, S.; Tzafettas, J. Anovulation with or without PCO, hyperandrogenaemia and hyperinsulinaemia as promoters of endometrial and breast cancer. Best Practice Res Clin Obstet Gynaecol 2010, 24, 19–27. [Google Scholar] [CrossRef]
- Gleicher, N. Why are reproductive cancers more common in nulliparous women? Reprod Biomed Online 2013, 26, 416–419. [Google Scholar] [CrossRef]
- Talley, L.I.; Grizzle, W.E.; Waterbor, J.W.; Brown, D.; Weiss, H.; Frost, A.R. Hormone receptors and proliferation in breast carcinomas of equivalent histologic grades in pre- and postmenopausal women. Int J Cancer 2002, 98, 118–127. [Google Scholar] [CrossRef]
- Dolle, J.M.; Daling, J.R.; White, E.; Brinton, L.A.; Doody, D.R.; Porter, P.L.; Malone, K.E. Risk factors for triple-negative breast cancer in women under the age of 45 years. Cancer Epidemiol Biomarkers Prev 2009, 18, 1157–1166. [Google Scholar] [CrossRef]
- Suba, Z. Diverse pathomechanisms leading to the breakdown of cellular estrogen surveillance and breast cancer development: New therapeutic strategies. Drug Design Devel Ther 2014, 8, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Suba, Z. Rosetta Stone for Cancer Cure: Comparison of the Anticancer Capacity of Endogenous Estrogens, Synthetic Estrogens and Antiestrogens. Oncol Rev 2023, 17, 10708. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ruan, X.; Schultz, S.; Neubauer, H.; Fehm, T.; Seeger, H.; et al. Oestetrol stimulates proliferation and oestrogen receptor expression in breast cancer cell lines: Comparison of four oestrogens. Eur J Contracept Reprod Health Care 2015, 20, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Stoica, G.E.; Franke, T.F.; Moroni, M.; Mueller, S.; Morgan, E.; Iann, M.C.; et al. Effect of estradiol on estrogen receptor-alpha gene expression and activity can be modulated by the ErbB2/PI 3-K/Akt pathway. Oncogene 2003, 22, 7998–8011. [Google Scholar] [CrossRef] [PubMed]
- Holst, F.; Stahl, P.R.; Ruiz, C.; Hellwinkel, O.; Jehan, Z.; Wendland, M.; et al. Estrogen receptor alpha (ESR1) gene amplification is frequent in breast cancer. Nat Genet 2007, 39, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Tomita, S.; Abdalla, M.O.; Fujiwara, S.; Matsumori, H.; Maehara, K.; Ohkawa, Y.; et al. A cluster of noncoding RNAs activates the ESR1 locus during breast cancer adaptation. Nat Commun 2015, 6, 6966. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, P.; Wang, L.; Piao, H.L.; Ma, L. Long non-coding RNA HOTAIR in carcinogenesis and metastasis. Acta Biochim Biophys Sin (Shanghai) 2014, 46, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Bhan, A.; Mandal, S.S. Estradiol-Induced Transcriptional Regulation of Long Non-Coding RNA, HOTAIR. Methods Mol Biol 2016, 1366, 395–412. [Google Scholar] [PubMed]
- Suba, Z. Activating mutations of ESR1, BRCA1 and CYP19 aromatase genes confer tumor response in breast cancers treated with antiestrogens. Recent Pat Anticancer Drug Discov 2017, 12, 136–147. [Google Scholar] [CrossRef]
- Lu, L.; Zhu, G.; Zhang, C.; Deng, Q.; Katsaros, D.; Mayne, S.T.; et al. Association of large noncoding RNA HOTAIR expression and its downstream intergenic CpG island methylation with survival in breast cancer. Breast Cancer Res Treat 2012, 136, 875–883. [Google Scholar] [CrossRef]
- Kinoshita, Y.; Chen, S. Induction of aromatase (CYP19) expression in breast cancer cells through a nongenomic action of estrogen receptor alpha. Cancer Res 2003, 63, 3546–3555. [Google Scholar] [PubMed]
- Catalano, S.; Giordano, C.; Panza, S.; Chemi, F.; Bonofiglio, D.; Lanzino, M.; et al. Tamoxifen through GPER upregulates aromatase expression: A novel mechanism sustaining tamoxifen-resistant breast cancer cell growth. Breast Cancer Res Treat 2014, 146, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Sasano, H.; Miki, Y.; Nagasaki, S.; Suzuki, T. In situ estrogen production and its regulation in human breast carcinoma: From endocrinology to intracrinology. Pathology International 2009, 59, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.R.J.; Rowlands, M.G.; Silva, M.C.; Law, M.; Coombes, R.C. Prognostic significance of aromatase and estrone sulfatase enzymes in human breast cancer. J Steroid Biochem Mol Biol 1993, 44, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Bollet, M.A.; Savignoni, A.; De Koning, L.; Tran-Perennou, C.; Barbaroux, C.; Degeorges, A.; et al. Tumor aromatase expression as a prognostic factor for local control in young breast cancer patients after breast-conserving treatment. Breast Cancer Res 2009, 11, R54. [Google Scholar] [CrossRef]
- Kininis, M.; Chen, B.S.; Diehl, A.G.; Isaacs, G.D.; Zhang, T.; Siepel, A.C.; et al. Genomic analyses of transcription factor binding, histone acetylation, and gene expression. Mol Cell Biol 2007, 27, 5090–5104. [Google Scholar] [CrossRef]
- Hosey, A.M.; Gorski, J.J.; Murray, M.M.; Quinn, J.E.; Chung, W.Y.; Stewart, G.E.; et al. Molecular basis for estrogen receptor alpha deficiency in BRCA1-linked breast cancer. J Natl Cancer Inst 2007, 99, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Scherbakov, A.M.; Shestakova, E.A.; Galeeva, K.E.; et al. BRCA1 and Estrogen Receptor α Expression Regulation in Breast Cancer Cells. Mol Biol 2019, 53, 442–451. [Google Scholar] [CrossRef]
- Mauro, L.; Salerno, M.; Panno, M.L.; Bellizzi, D.; Sisci, D.; Miglietta, A.; et al. Estradiol increases IRS-1 gene expression and insulin signaling in breast cancer cells. Biochem Biophys Res Commun. 2001, 288, 685–689. [Google Scholar] [CrossRef]
- Garrido, P.; Morán, J.; Alonso, A.; González, S.; González, C. 17β-estradiol activates glucose uptake via GLUT4 translocation and PI3K/Akt signaling pathway in MCF-7 cells. Endocrinology 2013, 154, 1979–8. [Google Scholar] [CrossRef]
- Clusan, L.; Ferrière, F.; Flouriot, G.; Pakdel, F. A Basic Review on Estrogen Receptor Signaling Pathways in Breast Cancer. Int J Mol Sci 2023, 24, 6834. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular Portraits of Human Breast Tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Mandapati, A.; Lukong, K.E. Triple negative breast cancer: Approved treatment options and their mechanisms of action. J Cancer Res Clin Oncol 2023, 149, 3701–3719. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.T.; Gou, X.; Seker, S.; Ellis, M.J. ESR1 alterations and metastasis in estrogen receptor positive breast cancer. J Cancer Metastasis Treat. 2019, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Singer, C.F.; Holst, F.; Steurer, S.; Eike, C.; Burandt, E.C.; Lax, S.F.; et al. Estrogen Receptor Alpha Gene Amplification Is an Independent Predictor of Long-Term Outcome in Postmenopausal Patients with Endocrine-Responsive Early Breast Cancer. Clin Cancer Res. 2022, 28, 4112–4120. [Google Scholar] [CrossRef] [PubMed]
- Toy, W.; Weir, H.; Razavi, P.; Lawson, M.; Goeppert, A.U.; Mazzola, A.M.; Smith, A.; Wilson, J.; et al. Activating ESR1 Mutations Differentially Affect the Efficacy of ER Antagonists. Cancer Discov 2017, 7, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Munzone, E.; Colleoni, M. Optimal management of luminal breast cancer: How much endocrine therapy is long enough? Ther Adv Med Oncol. 2018, 10, 1758835918777437. [Google Scholar] [CrossRef] [PubMed]
- Arpino, G.; Weiss, H.; Lee, A.V.; Schiff, R.; De Placido, S.; Osborne, C.K.; et al. Estrogen receptor-positive, progesterone receptor-negative breast cancer: Association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst 2005, 97, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.S.; Mullins, M.; Cheang, M.C.U.; Leung, V.D.; Vickery, T.; Davies, S.; Fauron, C.; He, X.; Hu, Z.; et al. Supervised Risk Predictor of Breast Cancer Based on Intrinsic Subtypes. J Clin Oncol Off J Am Soc Clin Oncol 2009, 27, 1160–1167. [Google Scholar] [CrossRef]
- Rouanet, P.; Roger, P.; Rousseau, E.; Thibault, S.; Romieu, G.; et al. HER2 overexpression a major risk factor for recurrence in pT1a-bN0M0 breast cancer: Results from a French regional cohort. Cancer Med. 2014, 3, 134–142. [Google Scholar] [CrossRef]
- Chlebowski, R.T.; Aragaki, A.K.; Pan, K. Breast Cancer Prevention: Time for Change. JCO Oncol Pract 2021, 17, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Pegram, M.; Jackisch, C.; Johnston, S.R.D. Estrogen/HER2 receptor crosstalk in breast cancer: Combination therapies to improve outcomes for patients with hormone receptor-positive/HER2-positive breast cancer. npj Breast Cancer 2023, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Du, F.; Gao, S.L.; Si, Y.R.; Hu, N.L.; Liu, D.X. Combined analysis of receptor expression reflects inter-and intra-tumor heterogeneity in HR+/HER2+ breast cancer. Breast Cancer Research and Treatment 2022, 194, 221–230. [Google Scholar] [CrossRef]
- Deligiannis, N.G.; Sosa, S.; Danilowicz, K.; Rizzo, L.F. Endocrine dysfunction induced by immune checkpoint inhibitors. Medicina (B Aires) 2021, 81, 269–278. [Google Scholar] [PubMed]
- Chew, V.; Toh, H.; Abastado, J. Immune Microenvironment in Tumor Progression: Characteristics and Challenges for Therapy. Journal of Oncology 2012, 2012, 608406. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, X.; Zhang, Y.; Zhu, M.; Wang, F.X.C.; et al. Role of tumor microenvironment in cancer progression and therapeutic strategy. Cancer Med. 2023, 12, 11149–11165. [Google Scholar] [CrossRef] [PubMed]
- Linares, J.; Marín-Jiménez, J.A.; Badia-Ramentol, J.; Calon, A. Determinants and Functions of CAFs Secretome during Cancer progression and Therapy. Front Cell Dev Biol 2021, 8, 621070. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as tools and targets in cancer therapy. Nat Rev Drug Discov 2022, 21, 799–820. [Google Scholar] [CrossRef]
- Chen, Y.; McAndrews, K.M.; Kalluri, R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat Rev Clin Oncol 2021, 18, 792–804. [Google Scholar] [CrossRef]
- Pejerrey, S.M.; Dustin, D.; Kim, J.A.; Gu, G.; Rechoum, Y.; et al. The impact of ESR1 mutations on the treatment of metastatic breast cancer. Horm Cancer 2018, 9, 215–228. [Google Scholar] [CrossRef]
- Caizzi, L.; Ferrero, G.; Cutrupi, S.; Cordero, F.; Ballaré, C.; Miano, V.; et al. Genome-wide activity of unliganded estrogen receptor-α in breast cancer cells. Proc Natl Acad Sci USA 2014, 111, 4892–4897. [Google Scholar] [CrossRef] [PubMed]
- Stellato, C.; Porreca, I.; Cuomo, D.; Tarallo, R.; Nassa, G.; Ambrosino, C. The “busy life” of unliganded estrogen receptors. Proteomics 2016, 16, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Jordan, V.C. New insights into acquired endocrine resistance of breast cancer. Cancer Drug Resist 2019, 2, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wu, K.; Wang, X.; Zhang, J.; Wang, L.; Jiang, Y.; et al. Periostin secreted by cancer-associated fibroblasts promotes cancer stemness in head and neck cancer by activating protein tyrosine kinase 7. Cell Death Dis 2018, 9, 1082. [Google Scholar] [CrossRef] [PubMed]
- Rothenberger, N.J.; Somasundaram, A.; Stabile, L.P. The Role of the Estrogen Pathway in the Tumor Microenvironment. Int J Mol Sci 2018, 19, 611. [Google Scholar] [CrossRef] [PubMed]
- Purohit, A.; Newman, S.P.; Reed, M.J. The role of cytokines in regulating estrogen synthesis: Implications for the etiology of breast cancer. Breast Cancer Research 2002, 4, 65. [Google Scholar] [CrossRef] [PubMed]
- Suba, Z. Crossroad between obesity and cancer: A defective signaling function of heavily lipid laden adipocytes (Online First). In Crosstalk in Biological Processes; El-Esawi, M.A., Ed.; InTechOpen: London, 2019. [Google Scholar] [CrossRef]
- Dannenfelser, R.; Nome, M.; Tahiri, A.; Ursini-Siegel, J.; Vollan, H.K.M.; Haakensen, V.D.; Helland, A.; Naume, B.; Caldas, C.; Borresen-Dale, A.L.; et al. Data-driven analysis of immune infiltrate in a large cohort of breast cancer and its association with disease progression, er activity, and genomic complexity. Oncotarget 2017, 8, 57121–57133. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Miki, Y.; Akahira, J.I.; Moriya, T.; Ohuchi, N.; Sasano, H. Review: Aromatase in human breast carcinoma as a key regulator of intratumoral sex steroid concentrations. Endocrine J 2008, 55, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Dong, S.; Huang, R.; Chen, X. Cancer-Associated Adipocytes and Breast Cancer: Intertwining in the Tumor Microenvironment and Challenges for Cancer Therapy. Cancers 2023, 15, 726. [Google Scholar] [CrossRef]
- Ali, H.R.; Provenzano, E.; Dawson, S.J.; Blows, F.M.; Liu, B.; Shah, M.; et al. Association between cd8+ t-cell infiltration and breast cancer survival in 12,439 patients. Ann. Oncol. 2014, 25, 1536–1543. [Google Scholar] [CrossRef]
- Li, J.; Wu, J.; Han, J. Analysis of Tumor Microenvironment Heterogeneity among Breast Cancer Subtypes to Identify Subtype-Specific Signatures. Genes 2022, 14, 44. [Google Scholar] [CrossRef]
- Sinha, D.K.; Pazik, J.E.; Dao, T.L. Prevention of mammary carcinogenesis in rats by pregnancy: Effect of full-term and interrupted pregnancy. Br J Cancer 1988, 57, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Medina, D.; Smith, G.H. Chemical carcinogen-induced tumorigenesis in parous, involuted mouse mammary glands. J Natl Cancer Inst. 1999, 91, 967–969. [Google Scholar] [CrossRef]
- Yang, J.; Yoshizawa, K.; Nandi, S.; Tsubura, A. Protective effects of pregnancy and lactation against N-methyl-N-nitrosourea-induced mammary carcinomas in female Lewis rats. Carcinogenesis 1999, 20, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Guzman, R.C.; Yang, J.; Rajkumar, L.; Thordarson, G.; Chen, X.; Nandi, S. Hormonal prevention of breast cancer: Mimicking the protective effect of pregnancy. Proc Natl Acad Sci USA. 1999, 96, 2520–2525. [Google Scholar] [CrossRef]
- Rajkumar, L.; Guzman, R.C.; Yang, J.; Thordarson, G.; Talamantes, F.; Nandi, S. Short-term exposure to pregnancy levels of estrogen prevents mammary carcinogenesis. Proc Natl Acad Sci USA 2001, 98, 11755–11759. [Google Scholar] [CrossRef]
- Rajkumar, L.; Guzman, R.C.; Yang, J.; Thordarson, G.; Talamantes, F.; Nandi, S. Prevention of mammary carcinogenesis by short-term estrogen and progestin treatments. Breast Cancer Res 2004, 6, R31–R37. [Google Scholar] [CrossRef]
- Rajkumar, L.; Kittrell, F.S.; Guzman, R.C.; Brown, P.H.; Nandi, S.; Medina, D. Hormone-induced protection of mammary tumorigenesis in genetically engineered mouse models. Breast Cancer Res. 2007, 9, R12. [Google Scholar] [CrossRef]
- Jerry, J. Roles for estrogen and progesterone in breast cancer prevention. Breast Cancer Res 2007, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, A.C.; Shenton, A.; Maher, E.R.; Watson, E.; Woodward, E.; et al. Parity and breast cancer risk among BRCA1 and BRCA2 mutation carriers. Breast Cancer Res 2006, 8, R72. [Google Scholar] [CrossRef] [PubMed]
- Quaynor, S.D.; Stradtman, E.W.; Kim, H.G.; Shen, Y.; Chorich, L.P.; Schreihofer, D.A.; Layman, L.C. Delayed puberty and estrogen resistance in a woman with estrogen receptor α variant. N Engl J Med 2013, 369, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Manna, S.; Holz, M.K. Tamoxifen action in ER-negative breast cancer. Sign Transduct Insight 2016, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- van Barele, M.; Heemskerk-Gerritsen, B.A.M.; Louwers, Y.V.; Vastbinder, M.B.; et al. Estrogens and Progestogens in Triple Negative Breast Cancer: Do They Harm? Cancers 2021, 13, 2506. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Qarmali, M.; Siegal, G.P.; Wei, S. Receptor conversion in metastatic breast cancer: Analysis of 390 cases from a single institution. Mod Pathol. 2020, 33, 2499–2506. [Google Scholar] [CrossRef] [PubMed]
- Jordan, V.C. Linking estrogen induced apoptosis with decreases in mortality following long term adjuvant tamoxifen therapy. Journal of the National Cancer Institute 2014, 106, dju296. [Google Scholar] [CrossRef]
- Jordan, V.C. The new biology of estrogen induced apoptosis applied to treat and prevent breast cancer. Endocr. Relat. Cancer 2015, 22, R1–R31. [Google Scholar] [CrossRef] [PubMed]
- Abderrahman, B.; Jordan, V.C.C. Estrogen for the Treatment and Prevention of Breast Cancer: A Tale of 2 Karnofsky Lectures. Cancer, J. 2022, 28, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Derocq, D.; Freiss, G.; Rochefort, H. Activation of estrogen receptor transfected into a receptor-negative breast cancer cell line decreases the metastatic and invasive potential of the cells. Proc Natl Acad Sci USA 1992, 89, 11538–11542. [Google Scholar] [CrossRef]
- Malone, R.W.; Felgner, P.L.; Verma, I.M. Cationic liposome-mediated RNA transfection. Proceedings of the National Academy of Sciences 1989, 86, 6077–6081. [Google Scholar] [CrossRef]
- Wolff, J.A.; Malone, R.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; et al. Direct gene transfer into mouse muscle in vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef]
- Dolgin, E. The tangled history of mRNA vaccines. Nature News Feature. 14 September 2021. Correction 22 October 2021.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




