1. Introduction
Parkinson’s disease (PD) is a multifaceted, heterogeneous neurological disorder for which the deficit in dopaminergic neurotransmission represents the core but not the unique pathophysiological mechanism involved [
1,
2,
3]. Glucocerebrosidase gene (GBA) mutations strongly contribute to the development and progression of the disease [
4,
5]. Compared to non-mutated PD (NM-PD), the GBA-PD phenotype is characterized by earlier onset, a faster disease progression, and a prominent non-motor burden, particularly for psychiatric disorders (especially, psychosis) [
5,
6,
7]. The mechanisms responsible for this PD phenotype are still yet to be disclosed. Some evidence suggests a possible link between neurosteroids (NSs) and PD [
8,
9,
10,
11]. An in vivo positron emission tomography (PET) imaging study detected an increased binding of (R)-[11C]PK11195, a prototypical translocator protein 18 kDa (TSPO) radiotracer, to the TSPO in GBA carriers without PD compared to age-matched healthy controls (HC), suggesting that this change could be an early event in GBA-PD [
12]. TSPO mediates the first step leading to the synthesis of NSs [
13,
14]. These molecules are produced by neurons and glial cells and play an important role in modulating the central nervous system (CNS) functions, by regulating neuronal growth, brain development, synapse formation, neural transmission, myelination, neurogenesis, dendritic growth, neuronal survival, and also neuroinflammation [
8,
13,
14]. Interestingly, dysregulation in NSs is emerging as a factor contributing to psychiatric disorders, including anxiety, depression, and psychosis [
15,
16,
17]. Among NSs, allopregnanolone can potentiate the defective inhibitory response observed in prepulse inhibition (PPI) of the startle reflex, a test that evaluates the failure in information processing and response elaboration in patients with schizophrenia [
18]. Interestingly, dopamine and its agonists reduce the PPI response and this alteration was also observed in PD patients [
19,
20]. Psychosis is found in approximately 50% of GBA-PD, and its prevalence is higher in GBA-PD patients compared to NM-PD patients [
5,
6]. Whether NSs could be related to a higher risk of psychosis in PD, or determine a greater predisposition to develop the disease, has not yet been explored. We present here a pilot study that aimed to address these issues, evaluating both the complete serum NSs’ profile and clinical characteristics of a cohort of consecutive GBA-PD patients compared to a matched cohort of consecutive NM-PD patients and two cohorts of healthy subjects with (GBA-C) and without (HC) GBA mutations.
2. Results
Twenty-two GBA-PD (males: 11; age: 63.68 years; MDS-UPDRS III: 36.00; MoCa: 22.73), 22 matched NM-PD (age: 63.05 years; MDS-UPDRS III: 29.68; MoCa: 21.82), 14 GBA-C (males: 8; age: 49.36 years; MDS-UPDRS III: .00; MoCa: 29.79) and 15 HC (males: 4; age: 60.60 years; MDS-UPDRS III: .33; MoCa: 29.40) were included in the study (
Table 1).
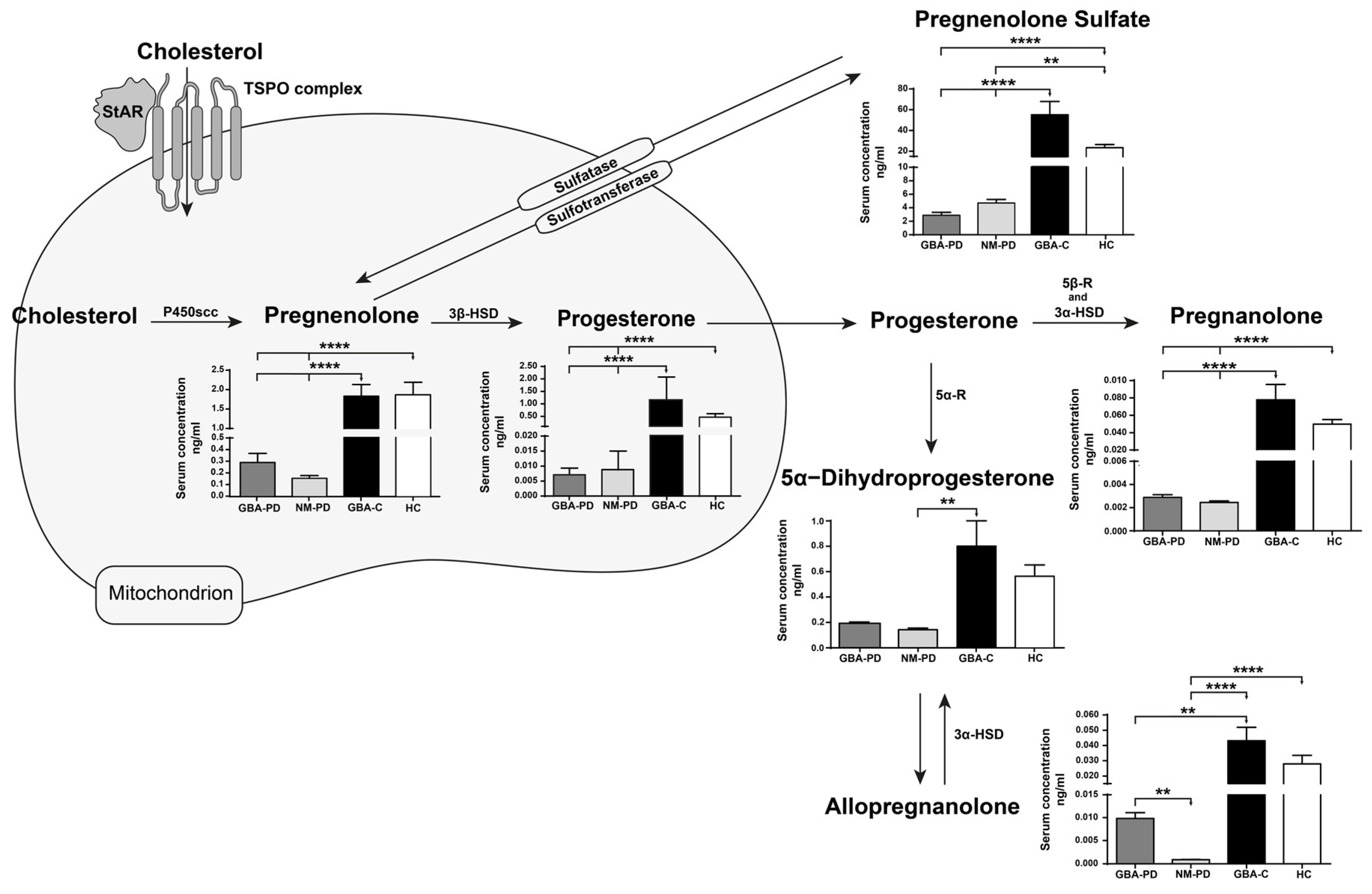
Compared to GBA-PD, NM-PD, and HC, GBA-C were younger (p<0.005, Tukey’s test), while no differences were found in gender distribution between the four cohorts. As shown in
Figure 1, compared to both GBA-PD and NM-PD patients, GBA-C presented significantly higher levels (****p<0.0001, Dunn’s test) of pregnenolone, progesterone, pregnenolone sulfate, pregnanolone, and allopregnanolone (** p<0.01 vs GBA-PD, **** p<0.0001 vs NM-PD). At variance, 5α-DHP was statistically different only in NM-PD compared to GBA-C (**p<0.01), who anyway were younger. The HC cohort presented higher levels for almost all the evaluated NSs when compared to GBA-PD and NM-PD patients (pregnenolone, progesterone, and pregnanolone: ****p<0.0001 vs both cohorts of patients; pregnenolone sulfate: ****p<0.0001 vs GBA-PD, **p<0.001 vs NM-PD), with the only exception of 5α-DHP. Notably, allopregnanolone was significantly reduced only in NM-PD (****p<0.001) when compared to HC, and non-significantly reduced in GBA-PD vs HC. It is interesting to note that the only significant difference between GBA-PD and NM-PD cohorts was for allopregnanolone (
**p<0.01), which was remarkably reduced in NM-PD patients (-88% vs GBA-PD). Lastly, no differences were found between GBA-C and HC cohorts.
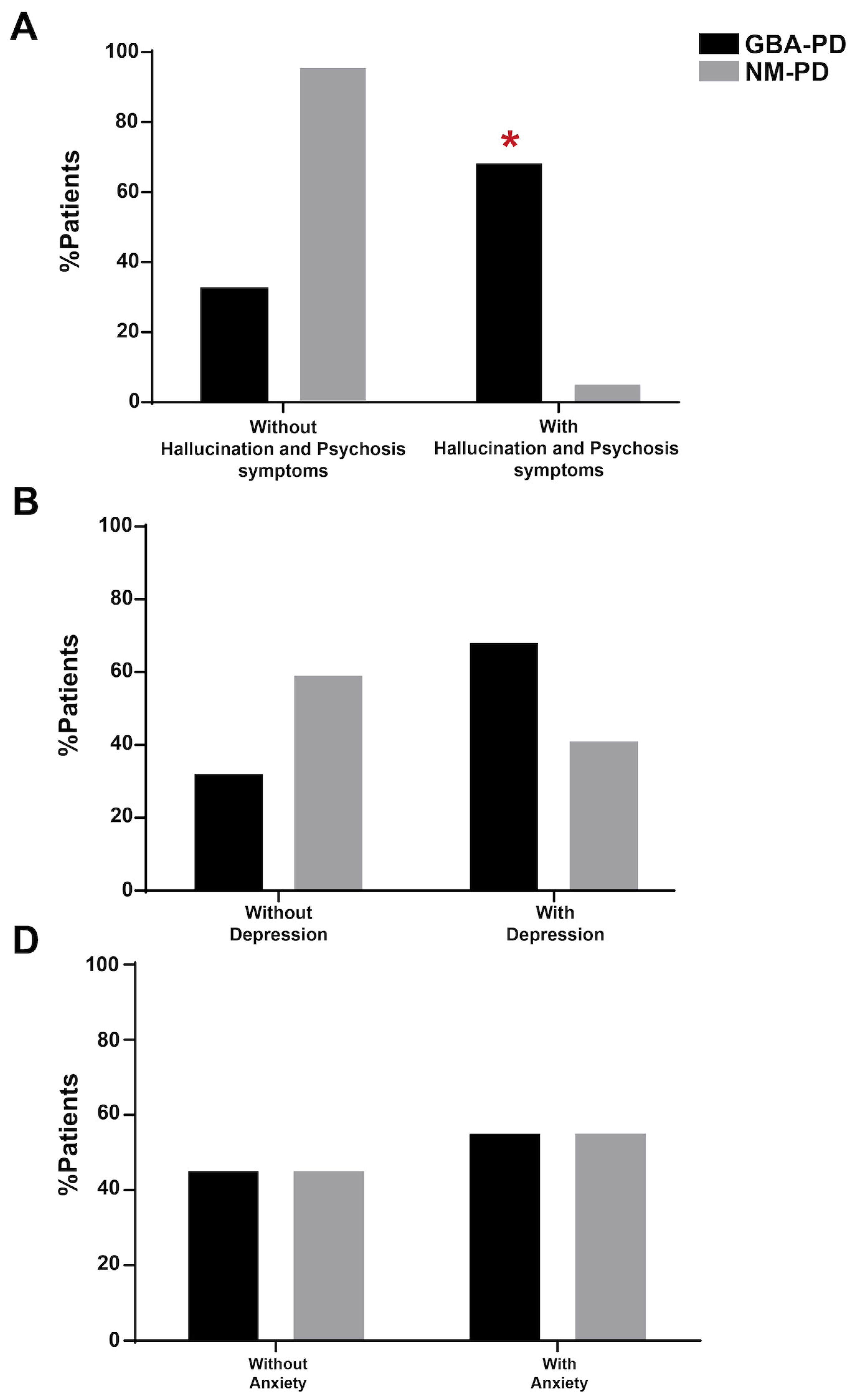
In terms of clinical features, the GBA cohort showed more hallucinations and psychosis symptoms quantified with the MDS-UPDRS item 1.2 (p<0.05, Fisher Exact Test;
Figure 2A) and a trend for depression (p=0.069,
Figure 2B). No differences emerged in anxiety disorders (
Figure 2C).
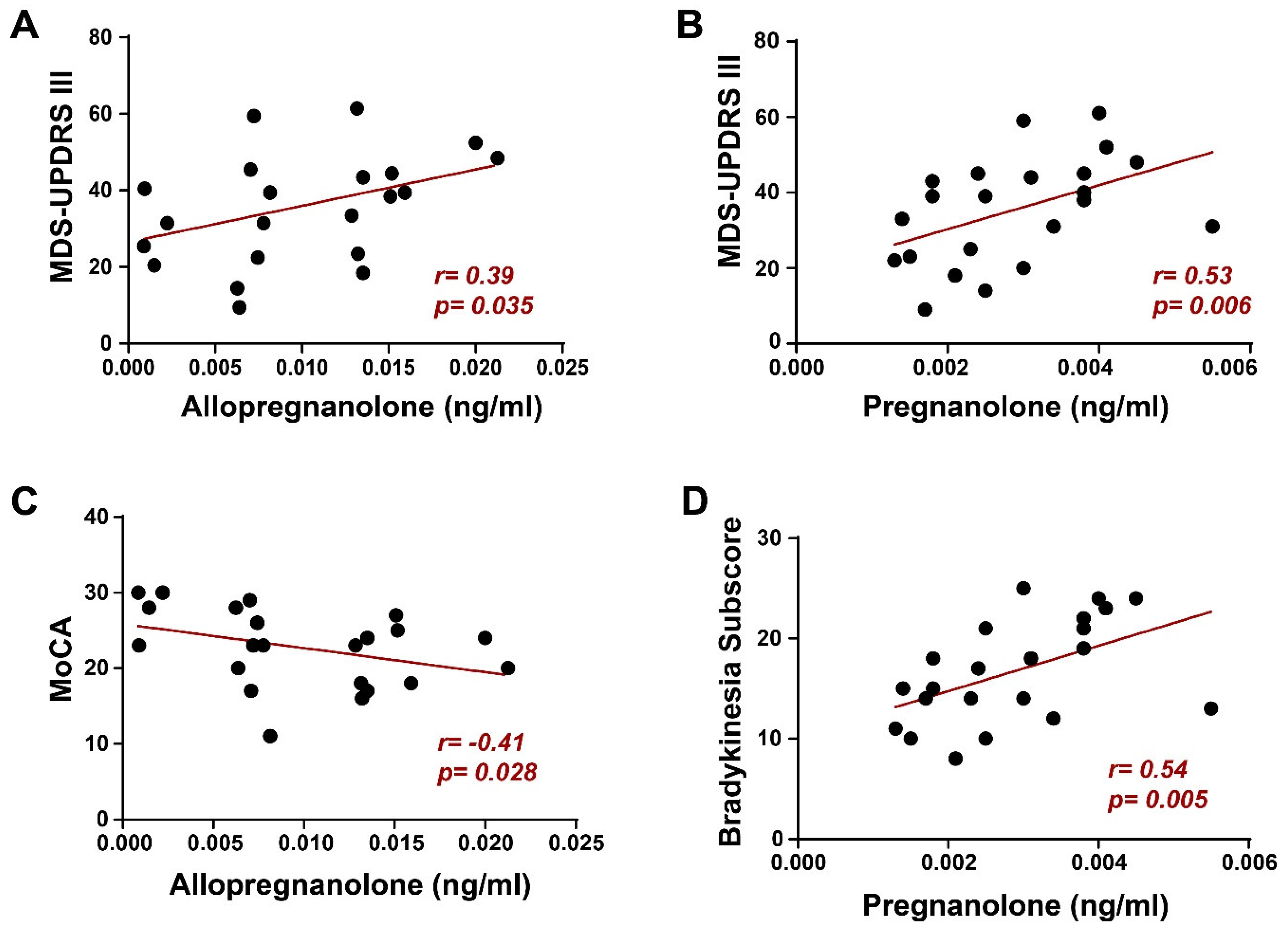
Also, motor aspects of experiences of daily living (MDS-UPDRS part II) were more compromised in GBA-PD patients (p<0.05), with a trend towards significance for axial manifestations such as balance, walking, freezing, swallowing, and speech (quantified through the PIGD subscore, p=0.06). Expectedly, both GBA-C and HC presented significantly lower MDS-UPDRS and, respectively, higher MoCA scores (p<0.05) if compared to GBA-PD and NM-PD.We found a positive correlation between allopregnanolone serum levels and the MDS-UPDRS III score in GBA-PD patients (Spearman’s r = 0.39, p = 0.035,
Figure 3A), suggesting that the differences found in this NS levels between GBA-PD and NM-PD. However, surprisingly we also found a similar result for pregnanolone (Spearman’s r = 0.53, p = 0.006,
Figure 3B), in which peripheral levels did not differ in the two cohorts of patients. To further investigate the role of these NSs, we tested their correlation with more specific indexes of motor impairment and found that pregnanolone significantly correlated with the bradykinesia subscore (Spearman’s r = 0.54, p = 0.005,
Figure 3D). Conversely, focusing on non-motor symptoms we found that allopregnanolone correlated negatively (Spearman’s r = -0.41 p = 0.028,
Figure 3C) with the MoCA score, suggesting a role of this NS in the cognitive impairment of GBA-PD. Moreover, we did not find any correlation between allopregnanolone and pregnanolone and clinical features in NM-PD.
3. Discussion
GBA gene mutations represent the most frequent genetic determinant of PD, accounting for 5-15% of PD patients worldwide [
2,
4,
6]. Numerous mechanisms have been proposed to explain the GBA-associated neurodegenerative phenotype. However, most related studies have focused on the impact of mutated GCase, especially the loss of enzymatic activity, in neurons, thus neglecting the involvement of other cells composing the CNS [
3,
32]. In this study, we showed an unprecedented observation of NSs peripheral dysregulation in GBA-PD patients, reporting a significant difference in allopregnanolone levels in the serum of GBA-PD, with higher values in comparison to NM-PD.
The differences in allopregnanolone serum levels between GBA-C and GBA-PD, and between GBA-C and NM-PD for 5α-DHP, could be affected and probably explained by the younger age of subjects composing the GBA-C cohort [
33]. These because these differences were not confirmed by the comparison with HC. GBA-PD patients have an increased risk of developing more severe motor and non-motor symptoms, including axial impairment, and cognitive and behavioral problems
5. Considering the link established between behavioral disorders and NSs, we included in our analysis also the evaluation of psychiatric disorders, highlighting their preponderance in GBA-PD. In our study, the GBA-PD cohort showed more hallucinations and psychotic symptoms, a higher impairment in the motor aspects of experiences of daily living, and a trend towards significance for more severe axial PD manifestations compared to NM-PD. These findings were associated with remarkable differences in allopregnanolone serum levels. Thus, it is tentatively suggested that allopregnanolone could be not simply associated, but might be possibly involved in the GBA-PD phenotype, even if further studies will be crucial to confirm this preliminary hypothesis.
Our hypothesis can be supported by preclinical evidence showing the involvement of allopregnanolone in the development of psychosis triggered by psychosocial stress [
34]. In particular, by reducing the synthesis of allopregnanolone with the 5α-reductase irreversible inhibitor finasteride the impairment in PPI was alleviated in rats modeling schizophrenia [
35,
36]. The negative impact of allopregnanolone on PPI was related to the modulation of D1 receptor activity by dopamine [
18]. This may explain at least in part the increased risk of developing psychosis and impulse control disorders in GBA patients under dopaminergic chronic oral treatment (particularly with dopaminoagonists). Based on these premises we may hypothesize that the significant increase in the content of allopregnanolone may possibly influence the GBA-PD neuropsychiatric profile. Of course, this hypothesis requires confirmation and that could be further challenged by further studies considering GBA-PD patients possibly treated with 5α-reductase inhibitors.
In addition, we also found an inverse correlation between allopregnanolone serum levels and cognitive status in GBA-PD. In particular, GBA-PD patients with higher levels of allopregnanolone showed lower MoCA scores. This is not surprisingly if we keep in mind the negative effects of chronic benzodiazepines use on cognition [
37,
38,
39,
40] and that both benzodiazepines and allopregnanolone modulate positively the γ-aminobutyric acid type A (GABA-A) receptor complex at the postsynaptic membrane of nerve cells [
10]. Indeed, allopregnanolone possesses benzodiazepine-like properties, since it’s able to positively modulate GABA-A receptors. Allopregnanolone administration was associated with the appearance of memory and learning impairment in rats, and, along with other GABA-A positive modulators, could potentially cause a cognitive impairment [
41]. Recently, GABA-A chronic stimulation by benzodiazepines has been associated with cognitive decline during aging in healthy subjects [
42]. Although we cannot firmly propose that allopregnanolone might have contributed to the cognitive decline in GBA-PD, the positive relationship we found between allopregnanolone levels and the worsening in the MoCA scores is another piece of evidence supporting the involvement of GABA-A receptors in this phenomenon.
In our cohorts, PD led to a reduction of several NSs, as already reported in previous studies [
9,
10]. In particular, the reduction of allopregnanolone may be related to the neurodegenerative process involving the nigral area [
9]. Indeed, the substantia nigra of the human brain expresses high concentrations of allopregnanolone [
10]. In this setting it has been assumed that the decrease of allopregnanolone may represent a biochemical marker of dopaminergic cell loss [
9]. This is particularly relevant if we bear in mind the correlation found between the MDS-UPDRS part III and both allopregnanolone and pregnenolone levels in GBA-PD patients. However, the significant reduction of allopregnanolone in our cohort was only found by comparing GBA-PD with GBA-C, but with a significant difference in age which could alternatively explain the statistical finding, and not by comparing age-and gender- matched HC. This finding highlights the need for future studies with age- and gender- matched cohorts of GBA-PD, GBA-C and HC in order to better understand the possible role of GBA heterozygous mutations in allopregnanolone expression. This is particularly relevant if considering that GBA is only a risk factor for developing PD and only 10-30% of GBA mutation carriers develop PD eventually [
2,
6]. Therefore, it is possible that NSs dysregulation could be present in GBA carriers that will never develop PD, hallucinations or psychosis. It was also unexpected the evidence of a correlation between pregnanolone levels and motor impairment in GBA-PD, especially because no significant difference was found in pregnanolone levels measures in NM-PD and GBA-PD. This suggests that NSs able to similarly modulate the GABA-A receptors may display a different specificity in their effects, probably related to other different characteristics that are currently unknown.
In summary, the findings reported here represent the first assessment of NSs in GBA-PD patients. We acknowledge the limitations of the study, in particular the small sample size and the assessment of psychosis through the MDS-UPDRS part I and the significant difference in age between GBA-C and the other three cohorts. Moreover, the significantly higher values for all NS in both control groups compared to both PD groups and the weak differences in all but 1/6 examined NS between NM-PD and GBA-PD raise the question if peripheral NS dysregulation is rather a feature of PD pathophysiology or neurodegeneration in general rather than being associated with GBA-PD. In this setting future studies with larger and age-matched samples and specific scales for the evaluation of neuropsychiatric symptoms will be needed to better assess these issues. Considering that only a minority of GBA gene mutation carriers develop PD, the identification of cofactors capable of impacting the development and progression of the disease such as NSs is of undoubtable relevance. From this perspective, understanding the role of NSs in the GBA-PD phenotype may represent a fundamental step to finding new therapeutic targets for this disabling disease.
4. Materials and Methods
4.1. Patients
Two consecutive cohorts of GBA-PD and NM-PD patients, one GBA-C, and an HC cohort were included. The genetic profile was obtained by testing PD patients for 11 pathogenic or likely pathogenic LRKK2 variants and GBA sequences. If negative, a next-generation sequencing panel targeting 68 genes involved in PD was performed [21). Each consecutive GBA-PD patient has been matched with a 1:1 pairing method with a consecutive NM-PD subject. In particular, the variables considered for a 1:1 match were: sex, age (tolerance of ± 2 years), age at disease onset (with a tolerance of ± 2 years), disease duration, and the Charlson Comorbidity score (CCI) (tolerance of ± 2 points). The consecutive GBA-PD patients were selected from an original pull of 60 GBA-PD patients followed at our center, while, for each NM-PD patient, a 1:1 match was performed searching from a pull of 400 consecutive NM-PD subjects [
22,
23]. In particular, the first anonymized patient in a list in random order with matched sex, age, age at disease onset (with a tolerance interval of 2 years), and comorbidity index (CCI) has been selected. During the pairing method, no other clinical or instrumental data has been considered except the one already mentioned. GBA-C and NM-HC have been previously screened for the GBA gene through a next-generation sequencing gene panel. The clinical evaluation of GBA-C showed no premotor PD symptoms (rapid eye movement [REM] sleep behavior disorder [RBD], hyposmia, severe constipation, or mood disorders). These clinical characteristics have been evaluated by retrospectively reviewing medical records and also by interviewing the patients specifically about these premotor symptoms during the clinical assessment. This study was approved by the Ethical Committee of the Area Vasta Emilia Nord (n= 2022/0139218) and written informed consent was obtained from each participant included according to the Declaration of Helsinki [
24].
4.2. Clinical Assessment
PD patients were evaluated through the following clinical scales: the four parts of the Movement Disorder Society revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) [
25] and the Hoehn and Yahr scale [
26] while the GBA-C and the HC only with the MDS-UPDRS. The following subscores were also extrapolated from the MDS-UPDRS: tremor (items 2.10, 3.15, 3.16, 3.17, 3.18); bradykinesia (items 3.4, 3.5, 3.6, 3.7, 3.8, 3.14); rigidity (items 3.3); postural instability and gait difficulties (PIGD) (items 2.12, 2.13, 3.10, 3.11, 3.12); gait (items 3.10, 3.11); dyskinesia (items 4.1, 4.2), fluctuations (items 4.3, 4.4, 4.5, 4.6), hallucinations and psychosis (item 1.2), depressed mood (item 1.3) and anxious mood (item 1.4). In addition, cognitive function was tested in all subjects through the Montreal Cognitive Assessment (MoCA) [
27]. The total amount of the dopaminergic treatment was converted into the L-dopa equivalent daily dose (LEDD) using the updated conversion formulae [
28]. In addition, the following variables were also collected for NM-PD and GBA-PD cohorts: age at evaluation, age at disease onset, disease duration, and the motor phenotype (akineto-rigid, tremor-dominant).
4.3. Quantitative Analysis of NSs in Serum by Liquid Chromatography-Electrospray Tandem Mass Spectrometry (LC-MS/MS)
4.3.1. Chemicals and Reagents
All standards were purchased from Sigma (St. Louis, MO, U.S.A.). Internal standards with isotope labeling were the following: 5a-pregnan-3a-ol-20-one-17a,21,21,21-d4 (ALLO-D4) and sodium pregnenolone-17a,21,21,21-d4 sulfate (PS-D4), purchased from Sigma Aldrich. All solvents for high-performance liquid chromatography/electrospray ionization tandem mass spectrometry (HPLC/ESI-MS/MS) were liquid chromatography-mass spectrometry (LC-MS) purity grade, whereas other solvents used for sample preparation were analytical grade (Sigma-Fluka, St. Louis, MO, U.S.).
4.3.2. Standard Solutions
A stock solution including pregnenolone, pregnenolone sulfate, progesterone, 5α-dihydroprogesterone (5α-DHP), allopregnanolone, and pregnanolone, was serially diluted with methanol to obtain calibration solutions at ten concentrations. The internal standard solution (IS) was prepared apart. Samples preparations were performed as previously described [
29,
30,
31].
4.3.3. Sample Preparation
Briefly, serum samples obtained from the recruited patients were spiked with IS solution, vortexed, and added with acetonitrile/methanol (70/30; +1.0% formic acid). The samples were then sonicated, centrifuged, and purified on Phree-SPE cartridges to remove endogenous phospholipids. Eluates were concentrated, derivatized with Amplifex Keto Reagent, and transferred in autosampler vials for LC-MS/MS analysis.
4.3.4. LC-MS/MS Analysis
The LC-MS/MS analysis was performed at Centro Interdipartimentale Grandi Strumenti (CIGS), University of Modena and Reggio Emilia. The chromatographic separation was performed on an Agilent 1200 Series Binary Pump (Agilent, Waldbronn, Germany). Mass spectrometric detection was performed using an Agilent QQQ-MS/MS (6410B) triple quadrupole mass analyzer equipped with an ESI ion source (Agilent), operating in the positive mode, as described previously [
29].
4.4. Statistical Analyses
Continuous variables were expressed as mean (±SD) and median (range), while frequencies and percentages were calculated for categorical variables. First, we tried to compare NS serum levels using a two-way analysis of variance (ANOVA), considering as main factors GBA mutation and PD, but since Shapiro-Wilk normality test failed, and variances in groups were unequal, we secondly used the Kruskal-Wallis test, followed by the Dunn’s test for multiple comparison. Outliers were identified using the Grubbs test and removed before statistical analysis. Clinical continuous variables were compared by using one-way ANOVA followed by Tukey’s post hoc test. Differences in categorical variables between the two groups (hallucinations and psychosis symptoms; depression; anxiety) were assessed by applying Fisher’s exact test. The correlation analyses were performed by calculating the respective Spearman’s r values and their significance level. Data are presented as mean ± standard error of the mean (SEM) or as percentages, and they were regarded as significantly different at p<0.05. Statistical analyses were performed with Sigmaplot 11; Systat Software, San Jose, CA, U.S.A and IBM SPSS Statistics for Windows version 20.0 (IBM, Armonk, NY, USA).
5. Conclusions
This pilot study provides the first observation of changes in neurosteroid peripheral levels in GBA-PD. The involvement of the observed changes in the development of neuro-psychological and motor symptoms of GBA-PD deserves further attention.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, F.C., C.L., G.B., F.V.; methodology and statistical analyses, F.C., C.L., S.G., E.M., A.D.F., G.B., F.V.; investigation, F.C., C.L., S.G., E.M., V.F., G.T., G.D.R, J.R., A.D.F., G.B., F.V.; writing—original draft preparation, F.C., C.L., G.B., F.V.; writing—review and editing, F.C., C.L., S.G., E.M., A.D.F., G.B., F.V.; investigation, F.C., C.L., S.G., E.M., V.F., G.T., G.D.R, J.R., A.D.F., G.B., F.V.; supervision, A.D.F., G.B., F.V. All authors have read and agreed to the published version of the manuscript.
Informed Consent Statement
Written informed consent has been obtained from the patients.
Acknowledgments
This study was partially supported by Italian Ministry of Health – Ricerca Corrente Annual Program 2024.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kalia LV, Lang AE. Parkinson’s disease. Lancet Lond Engl. 2015;386:896–912.
- Riboldi, G.M.; Di Fonzo, A.B. GBA, Gaucher Disease, and Parkinson’s Disease: From Genetic to Clinic to New Therapeutic Approaches. Cells 2019, 8, 364. [CrossRef]
- Cavallieri F, Cury RG, Guimarães T, et al. Recent Advances in the Treatment of Genetic Forms of Parkinson’s Disease: Hype or Hope? Cells. 2023;12:764.
- Billingsley, K.J.; Ding, J.; Jerez, P.A.; Illarionova, A.; Levine, K.; Grenn, F.P.; Makarious, M.B.; Moore, A.; Vitale, D.; Reed, X.; et al. Genome-Wide Analysis of Structural Variants in Parkinson Disease. Ann. Neurol. 2023, 93, 1012–1022. [CrossRef]
- Petrucci S, Ginevrino M, Trezzi I, et al. GBA-Related Parkinson’s Disease: Dissection of Genotype-Phenotype Correlates in a Large Italian Cohort. Mov Disord Off J Mov Disord Soc. 2020;35:2106–2111.
- Menozzi E, Schapira AHV. Exploring the Genotype-Phenotype Correlation in GBA-Parkinson Disease: Clinical Aspects, Biomarkers, and Potential Modifiers. Front Neurol. 2021;12:694764.
- Cilia R, Tunesi S, Marotta G, et al. Survival and dementia in GBA-associated Parkinson’s disease: The mutation matters. Ann Neurol. 2016;80:662–673.
- Yılmaz, C.; Karali, K.; Fodelianaki, G.; Gravanis, A.; Chavakis, T.; Charalampopoulos, I.; Alexaki, V.I. Neurosteroids as regulators of neuroinflammation. Front. Neuroendocrinol. 2019, 55, 100788. [CrossRef]
- di Michele, F.; Longone, P.; Romeo, E.; Lucchetti, S.; Brusa, L.; Pierantozzi, M.; Bassi, A.; Bernardi, G.; Stanzione, P. Decreased plasma and cerebrospinal fluid content of neuroactive steroids in Parkinson’s disease. Neurol. Sci. 2003, 24, 172–173. [CrossRef]
- di Michele, F.; Luchetti, S.; Bernardi, G.; Romeo, E.; Longone, P. Neurosteroid and neurotransmitter alterations in Parkinson’s disease. Front. Neuroendocr. 2013, 34, 132–142. [CrossRef]
- Luchetti, S.; Liere, P.; Pianos, A.; Verwer, R.W.; Sluiter, A.; Huitinga, I.; Schumacher, M.; Swaab, D.F.; Mason, M.R. Disease stage-dependent changes in brain levels and neuroprotective effects of neuroactive steroids in Parkinson's disease. Neurobiol. Dis. 2023, 183, 106169. [CrossRef]
- Mullin, S.; Stokholm, M.G.; Hughes, D.; Mehta, A.; Parbo, P.; Hinz, R.; Pavese, N.; Brooks, D.J.; Schapira, A.H. Brain Microglial Activation Increased in Glucocerebrosidase (GBA) Mutation Carriers without Parkinson's disease. Mov. Disord. 2021, 36, 774–779. [CrossRef]
- Compagnone, N.A.; Mellon, S.H. Neurosteroids: Biosynthesis and Function of These Novel Neuromodulators. Front. Neuroendocr. 2000, 21, 1–56. [CrossRef]
- Mellon, S.H.; Griffin, L.D. Neurosteroids: biochemistry and clinical significance. Trends Endocrinol. Metab. 2002, 13, 35–43. [CrossRef]
- Frau, R.; Abbiati, F.; Bini, V.; Casti, A.; Caruso, D.; Devoto, P.; Bortolato, M. Targeting neurosteroid synthesis as a therapy for schizophrenia-related alterations induced by early psychosocial stress. Schizophr. Res. 2015, 168, 640–648. [CrossRef]
- Rupprecht R, Wetzel CH, Dorostkar M, et al. Translocator protein (18kDa) TSPO: a new diagnostic or therapeutic target for stress-related disorders? Mol Psychiatry. 2022;27:2918–2926.
- Walton, N.L.; Antonoudiou, P.; Maguire, J.L. Neurosteroid influence on affective tone. Neurosci. Biobehav. Rev. 2023, 152, 105327. [CrossRef]
- Frau, R.; Traccis, F.; Concas, L.; Cadeddu, R.; Mosher, L.J.; Nordkild, P.; Gaikwad, N.W.; Bortolato, M. Prefrontal allopregnanolone synergizes with D1 receptor activation to disrupt sensorimotor gating in male Sprague-Dawley rats. Psychopharmacology 2023, 240, 1359–1372. [CrossRef]
- Zoetmulder, M.; Biernat, H.B.; Nikolic, M.; Korbo, L.; Friberg, L.; Jennum, P.J. Prepulse Inhibition is Associated with Attention, Processing Speed, and 123I-FP-CIT SPECT in Parkinson's Disease. J. Park. Dis. 2014, 4, 77–87. [CrossRef]
- Lipari, N.; Centner, A.; Glinski, J.; Cohen, S.; Manfredsson, F.P.; Bishop, C. Characterizing the relationship between L-DOPA-induced-dyskinesia and psychosis-like behaviors in a bilateral rat model of Parkinson's disease. Neurobiol. Dis. 2023, 176, 105965. [CrossRef]
- Skrahina, V.; Gaber, H.; Vollstedt, E.; Förster, T.M.; Usnich, T.; Curado, F.; Brüggemann, N.; Paul, J.; Bogdanovic, X.; Zülbahar, S.; et al. The Rostock International Parkinson's Disease (ROPAD) Study: Protocol and Initial Findings. Mov. Disord. 2021, 36, 1005–1010. [CrossRef]
- Grisanti, S.; Fraternali, A.; Cavallieri, F.; Fioravanti, V.; Casali, M.; Toschi, G.; Ferri, L.; Sabadini, R.; Zedde, M.; Salomone, G.; et al. Author response for "Quantitative dopamine transporter imaging assessment in Parkinson's disease patients carrying GBA gene mutations compared with idiopathic PD patients: A case-control study". 2023. [CrossRef]
- Grisanti, S.; Ferri, L.; Cavallieri, F.; Fioravanti, V.; Vincenzi, C.; Toschi, G.; Grisendi, I.; Sabadini, R.; Paul, J.J.; Bauer, P.; et al. Increased Stroke Risk in Patients with Parkinson's Disease with LRRK2 Mutations. Mov. Disord. 2022, 37, 1117–1118. [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194.
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [CrossRef]
- Goetz CG, Poewe W, Rascol O, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord Off J Mov Disord Soc. 2004;19:1020–1028.
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [CrossRef]
- Jost, S.T.; Kaldenbach, M.; Antonini, A.; Martinez-Martin, P.; Timmermann, L.; Odin, P.; Katzenschlager, R.; Borgohain, R.; Fasano, A.; Stocchi, F.; et al. Levodopa Dose Equivalency in Parkinson's Disease: Updated Systematic Review and Proposals. Mov. Disord. 2023, 38, 1236–1252. [CrossRef]
- Trivisano, M.; Lucchi, C.; Rustichelli, C.; Terracciano, A.; Cusmai, R.; Ubertini, G.M.; Giannone, G.; Bertini, E.S.; Vigevano, F.; Gecz, J.; et al. Reduced steroidogenesis in patients with PCDH19-female limited epilepsy. Epilepsia 2017, 58, e91–e95. [CrossRef]
- Meletti, S.; Lucchi, C.; Monti, G.; Giovannini, G.; Bedin, R.; Trenti, T.; Rustichelli, C.; Biagini, G. Decreased allopregnanolone levels in cerebrospinal fluid obtained during status epilepticus. Epilepsia 2017, 58, e16–e20. [CrossRef]
- Meletti, S.; Lucchi, C.; Monti, G.; Giovannini, G.; Bedin, R.; Trenti, T.; Rustichelli, C.; Biagini, G. Low levels of progesterone and derivatives in cerebrospinal fluid of patients affected by status epilepticus. J. Neurochem. 2018, 147, 275–284. [CrossRef]
- Brunialti E, Villa A, Mekhaeil M, et al. Inhibition of microglial β-glucocerebrosidase hampers the microglia-mediated antioxidant and protective response in neurons. J Neuroinflammation. 2021;18:220.
- Genazzani AR, Petraglia F, Bernardi F, et al. Circulating Levels of Allopregnanolone in Humans: Gender, Age, and Endocrine Influences. J Clin Endocrinol Metab. 1998;83:2099–2103.
- Bali, A.; Jaggi, A.S. Multifunctional aspects of allopregnanolone in stress and related disorders. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2014, 48, 64–78. [CrossRef]
- Mosher, L.J.; Cadeddu, R.; Yen, S.; Staudinger, J.L.; Traccis, F.; Fowler, S.C.; Maguire, J.L.; Bortolato, M. Allopregnanolone is required for prepulse inhibition deficits induced by D1 dopamine receptor activation. Psychoneuroendocrinology 2019, 108, 53–61. [CrossRef]
- Darbra, S.; Mòdol, L.; Pallarès, M. Allopregnanolone infused into the dorsal (CA1) hippocampus increases prepulse inhibition of startle response in Wistar rats. Psychoneuroendocrinology 2012, 37, 581–585. [CrossRef]
- Zetsen, S.P.; Schellekens, A.F.; Paling, E.P.; Kan, C.C.; Kessels, R.P. Cognitive Functioning in Long-Term Benzodiazepine Users. Eur. Addict. Res. 2022, 28, 377–381. [CrossRef]
- Penninx, B.W.J.H.; Pine, D.S.; A Holmes, E.; Reif, A. Benzodiazepines for the long-term treatment of anxiety disorders? – Authors' reply. Lancet 2021, 398, 120–120. [CrossRef]
- de Gage, S.B.; Moride, Y.; Ducruet, T.; Kurth, T.; Verdoux, H.; Tournier, M.; Pariente, A.; Bégaud, B. Benzodiazepine use and risk of Alzheimer's disease: case-control study. BMJ 2014, 349, g5205–g5205. [CrossRef]
- Leng, Y.; Stone, K.L.; Yaffe, K. Race Differences in the Association Between Sleep Medication Use and Risk of Dementia. J. Alzheimer's Dis. 2023, 91, 1133–1139. [CrossRef]
- Bäckström T, Turkmen S, Das R, Doverskog M, Blackburn TP. The GABA system, a new target for medications against cognitive impairment—Associated with neuroactive steroids. J Intern Med. 2023;294:281–294.
- del Ser, T.; Zea, M.-A.; Valentí, M.; Olazarán, J.; López-Álvarez, J.; Rebollo-Vázquez, A.; Ávila-Villanueva, M.; Frades, B.; Medina, M.; A Fernández-Blázquez, M. Effects of commonly prescribed drugs on cognition and mild cognitive impairment in healthy elderly people. J. Psychopharmacol. 2019, 33, 965–974. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).