1. Introduction
Protein-based therapeutics have revolutionized medicine over the past few decades, offering highly specific and potent treatments for a wide range of diseases [
1,
2]. As of 2023, over 350 protein-based drugs have been approved for clinical use, with many more in development [
3]. The success of protein therapeutics can be attributed to their ability to perform complex biological functions with high specificity and low toxicity compared to small molecule drugs [
2,
3]. However, natural proteins often lack optimal pharmaceutical properties such as stability, half-life, and manufacturability [
1,
4]. Protein design and engineering approaches have emerged as powerful tools to overcome these limitations and create improved biotherapeutics with enhanced efficacy, safety, and developability [
5].
The field of therapeutic protein engineering has expanded rapidly, driven by advances in computational modeling, high-throughput screening techniques, and our deepening understanding of protein structure-function relationships [
3,
5,
6]. These developments have enabled researchers to modify existing proteins and even create entirely novel protein structures tailored for specific therapeutic applications. Key areas of focus include antibody engineering, enzyme replacement therapies, and the development of cytokine-based drugs [
3,
5].
Computational protein design has played an increasingly important role in this field [
7]. Tools such as Rosetta, RoseTTAFold, and RF Diffusion have dramatically improved our ability to predict protein structures, design stable proteins, and engineer proteins for specific molecular interactions [
8]. These computational approaches, when combined with experimental validation, have led to breakthroughs such as the de novo design of protein binders, enzymes with novel catalytic activities, and protein-based vaccines [
3].
Experimental protein engineering techniques have also seen significant advancements [
9,
10]. Directed evolution methods, including phage display and yeast surface display, have been refined to rapidly evolve proteins with desired properties [
3,
11,
12]. Additionally, the integration of non-canonical amino acids and chemical modifications has expanded the toolkit available for protein engineering, enabling the creation of biotherapeutics with enhanced stability, pharmacokinetics, and novel functionalities [
11,
13,
14,
15,
16].
One of the most exciting areas of therapeutic protein engineering is the development of bispecific and multispecific antibodies [
17]. These engineered proteins can simultaneously bind to multiple targets, opening up new possibilities for cancer immunotherapy and the treatment of complex diseases [
18]. Other emerging applications include the design of intracellular protein therapeutics, conditionally activated proteins, and protein-based nanocarriers for drug delivery [
19,
20,
21].
Despite these advances, significant challenges remain in the field of therapeutic protein engineering [
3]. These include improving the accuracy of computational design methods, enhancing the efficiency of experimental screening techniques, and addressing issues related to immunogenicity and manufacturing scalability [
22]. Additionally, the development of strategies for targeted delivery of protein therapeutics to specific tissues or cellular compartments remains an active area of research [
23].
This review will examine recent advances in computational and experimental protein engineering methods and their applications in developing next-generation protein therapeutics. We will discuss strategies for optimizing protein stability, pharmacokinetics, targeting, and functionality, as well as emerging approaches for creating novel protein-based drugs with unique capabilities. For each application, we will explore the specific challenges addressed by protein engineering and highlight notable successes and ongoing clinical trials. We will also discuss the integration of protein engineering with other emerging technologies, such as cell and gene therapies. Finally, we will explore future directions and challenges in the field of therapeutic protein engineering, including emerging computational tools, novel experimental techniques, and potential new therapeutic modalities. We will also consider the broader implications of advances in protein engineering for personalized medicine and the development of treatments for currently intractable diseases.
By providing a comprehensive overview of the current state of the art in therapeutic protein engineering, this review aims to serve as a valuable resource for researchers, clinicians, and biotechnology professionals working at the forefront of this rapidly evolving field.
2. Computational Protein Design
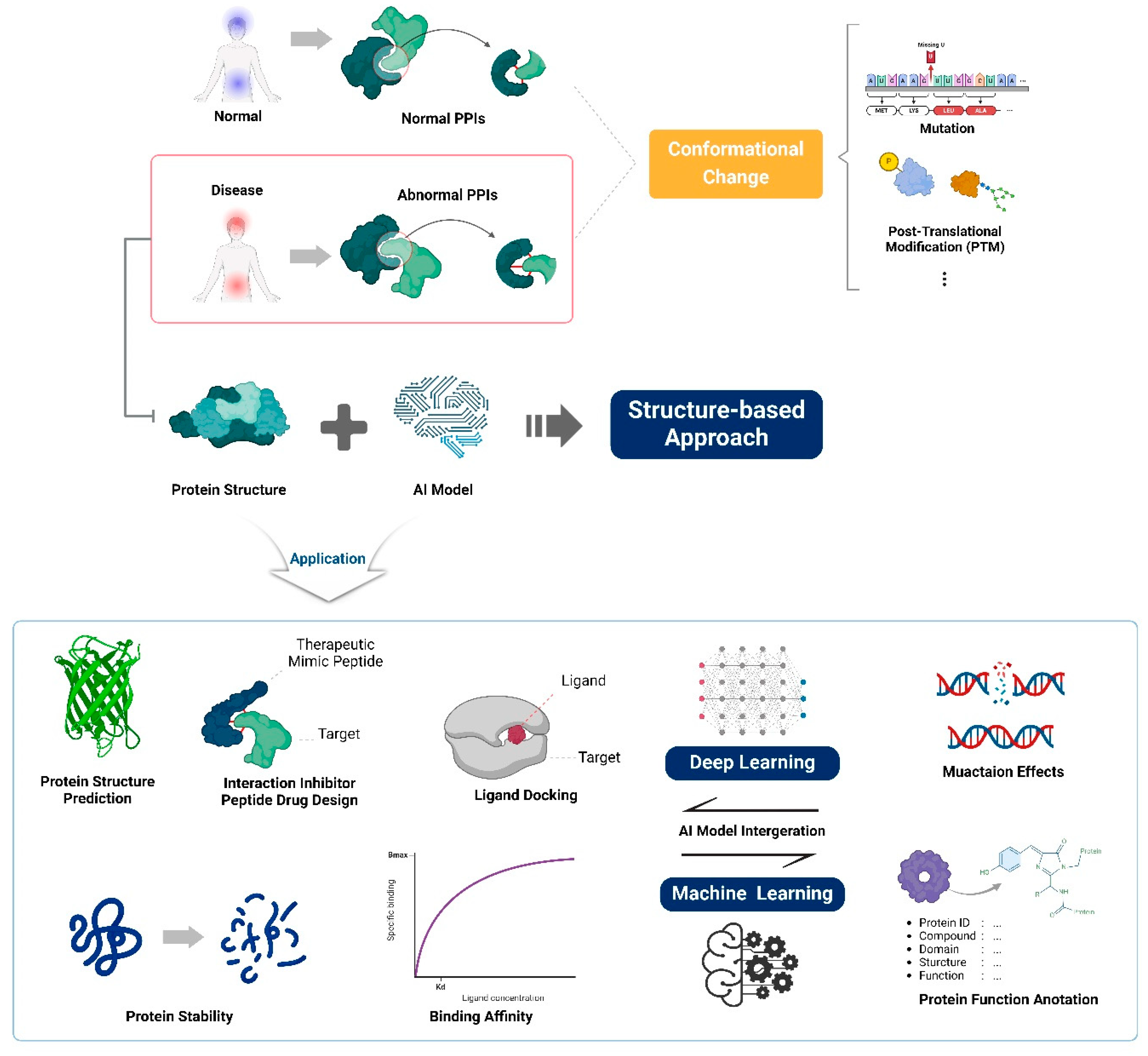
Figure 1.
Overview and applications of structural proteome-based integrated artificial intelligence. Structure-based computational design has advanced dramatically through the integration of existing machine learning and deep learning algorithms. It can now be used in various fields, including protein structure and enzyme design, ligand docking, structure prediction of biological macromolecules and complexes, and protein function annotation. The integration of these algorithms and the advancement of structure-based computational techniques contribute to the optimization and advancement of structural biology for therapeutic protein engineering applications.
Figure 1.
Overview and applications of structural proteome-based integrated artificial intelligence. Structure-based computational design has advanced dramatically through the integration of existing machine learning and deep learning algorithms. It can now be used in various fields, including protein structure and enzyme design, ligand docking, structure prediction of biological macromolecules and complexes, and protein function annotation. The integration of these algorithms and the advancement of structure-based computational techniques contribute to the optimization and advancement of structural biology for therapeutic protein engineering applications.
2.1. Structure-Based Design
Structure-based computational design has become an invaluable tool for engineering therapeutic proteins with improved properties [
7]. This approach leverages available protein structural data and physics-based modeling to predict the effects of amino acid mutations on protein stability, binding affinity, and function [
24].
2.1.1. Machine Learning Integration
The integration of machine learning, particularly deep learning models, has revolutionized computational protein engineering by dramatically improving protein structure prediction and design capabilities [
25,
26]. AlphaFold, developed by DeepMind, has achieved unprecedented accuracy in predicting protein structures from amino acid sequences, with many predictions reaching atomic-level precision. This breakthrough has accelerated research across structural biology and enabled new approaches to protein design and engineering. The success of AlphaFold has inspired the development of other AI-powered tools for protein structure prediction and design, such as RoseTTAFold and ESMFold, further expanding the toolkit available to researchers [
27,
28]. Integration of these deep learning models with traditional physics-based algorithms is enhancing both the accuracy and scope of computational protein engineering [
29]. For example, researchers have developed methods to incorporate physics-based force fields as differentiable modules within deep learning frameworks, allowing for more physically realistic predictions and designs. This synergy between data-driven machine learning approaches and physics-based modeling is enabling more robust and reliable computational protein engineering pipelines. The impact of these advancements extends beyond structure prediction to areas such as protein-protein interaction prediction, enzyme design, and drug discovery, opening up new possibilities for creating novel proteins with tailored functions [
26]. As the field continues to evolve, the integration of machine learning with experimental techniques and high-throughput screening methods promises to further accelerate the discovery and optimization of engineered proteins for therapeutic and biotechnological applications.
2.1.2. Rosetta Software Suite
The Rosetta software suite is a comprehensive platform for macromolecular modeling, docking, and design that has been extensively developed over two decades by a global community of researchers [
30]. It includes algorithms for computational modeling and analysis of protein structures, enabling notable scientific advances in areas such as de novo protein design, enzyme design, ligand docking, and structure prediction of biological macromolecules and complexes. Originally developed in the laboratory of David Baker at the University of Washington for protein structure prediction, Rosetta has since expanded to address a wide range of computational challenges in structural biology. Recent applications of Rosetta include the design of miniprotein binders against targets like SARS-CoV-2 and influenza hemagglutinin [
31]. The software has been continuously refined and extended, with recent developments incorporating deep learning techniques such as RoseTTAFold for rapid protein structure prediction and RFdiffusion for generative protein design [
8]. Rosetta is freely available to academic and non-profit users, while commercial entities can obtain licenses through the University of Washington [
30]. The collaborative nature of Rosetta's development, involving researchers from over 60 institutions, has contributed to its widespread use and ongoing innovation in the field of computational structural biology.
2.2. Sequence-Based Design
Complementing structure-based methods, sequence-based computational approaches leverage the wealth of genomic and protein sequence data to guide protein engineering efforts:
2.2.1. Machine Learning on Sequence Data
Deep learning models trained on large protein sequence databases have emerged as powerful tools for predicting the effects of mutations and guiding directed evolution experiments in protein engineering. Convolutional neural networks built with amino acid property descriptors have demonstrated strong performance in predicting protein redesign outcomes across diverse datasets [
32]. These models can efficiently screen large numbers of novel sequences
in silico, accelerating the protein engineering process. Notable examples of generative models for protein sequences include Protein-GAN, which uses generative adversarial networks to expand functional protein sequence spaces, and ProteinMPNN, a graph neural network approach for designing stable and functional de novo proteins [
8]. ProteinMPNN in particular has shown outstanding performance in both computational and experimental tests, with higher native sequence recovery (52.4%) compared to traditional methods like Rosetta (32.9%) when redesigning protein backbones [
33]. It can generate sequences for complex protein structures including monomers, cyclic homo-oligomers, and binding proteins [
34]. The model incorporates noise during training to improve robustness when designing sequences for predicted protein structures [
35]. ProteinMPNN has been successfully applied to rescue previously failed designs of various protein architectures, demonstrating its broad utility [
36]. By combining sequence-based machine learning with structure-based methods and experimental validation, researchers are developing increasingly accurate computational pipelines for engineering proteins with enhanced stability, binding affinity, and catalytic activity [
37].
2.2.2. Language Models for Proteins
Large language models trained on protein sequences have emerged as powerful tools for various protein engineering tasks. ESM-1b, developed by Meta AI (formerly Facebook AI Research), is a prominent example of such models, trained on 250 million protein sequences using masked language modeling [
38]. This model has demonstrated impressive capabilities in predicting protein properties and functions directly from individual sequences ESM-1b can be used for tasks such as predicting the effects of mutations, inferring protein structure, and annotating protein function [
38]. The model's learned representations have been shown to contain information about protein secondary and tertiary structure, which can be extracted through linear projections. ESM-1b has achieved state-of-the-art performance in zero-shot prediction of mutational effects and secondary structure prediction. The model's success has led to the development of more advanced versions, such as ESM-2 and ESMFold, which enable even more accurate structure prediction. These language models have also been applied to tasks like protein design, with tools like ESM-IF1 for inverse folding. Recent work has explored fine-tuning these models on small amounts of experimental data to enhance their predictive capabilities for specific proteins or properties [
39]. The integration of these language models with other computational and experimental techniques is advancing the field of protein engineering, enabling more efficient exploration of protein sequence space and the design of proteins with desired properties [
40].
3. Experimental Protein Engineering
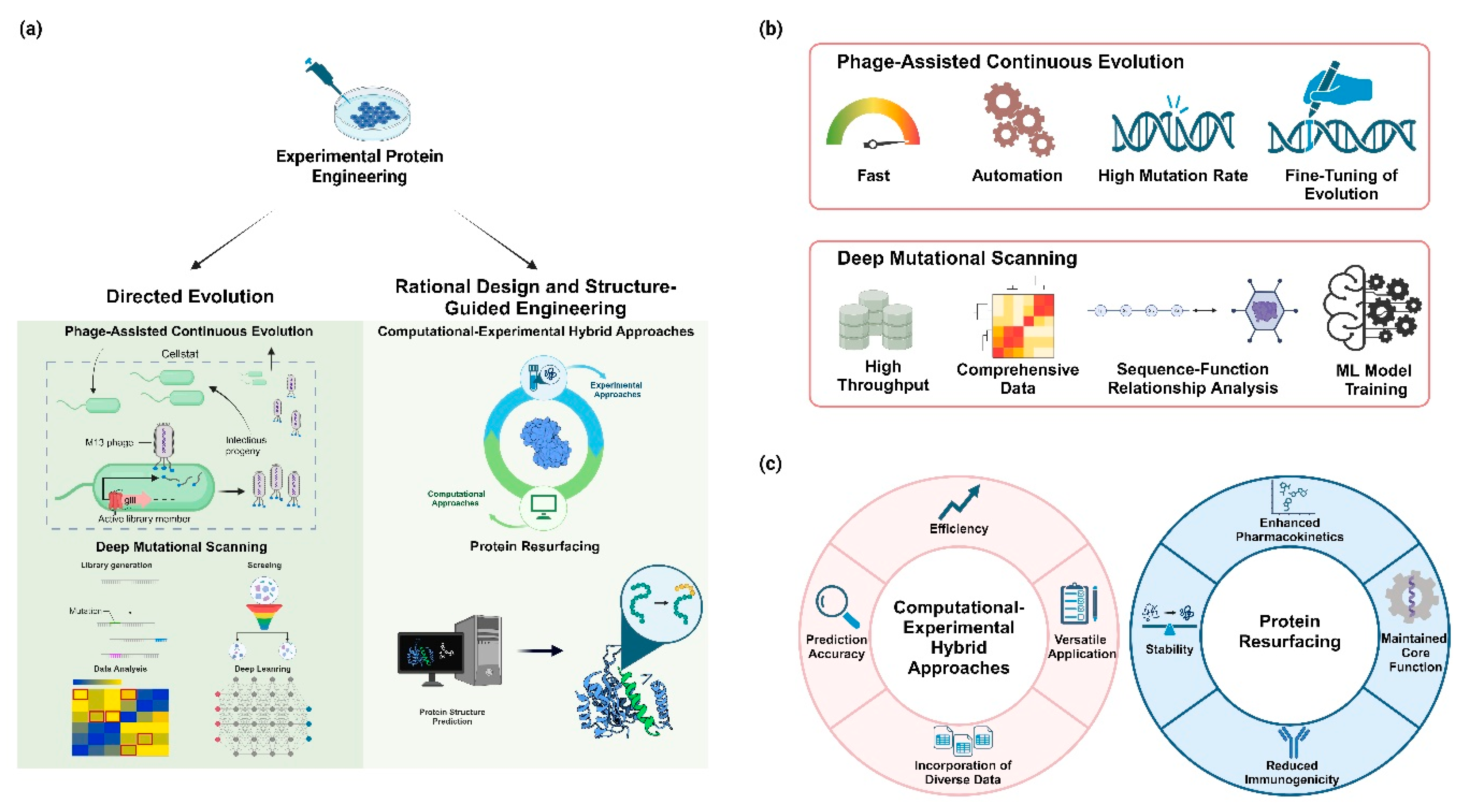
Figure 2.
Experimental protein engineering has achieved significant advancements through directed evolution as well as rational design and structure-guided engineering. (a) A schematic diagram explaining Phage-Assisted Continuous Evolution and Deep Mutational Scanning within Directed Evolution, and Computational-Experimental Hybrid Approaches and Protein Resurfacing within Rational Design and Structure-Guided Engineering. (b) A comparison highlighting the advantages of Phage-Assisted Continuous Evolution and Deep Mutational Scanning compared to traditional methods. (c) A detailed description of the advantages of Computational-Experimental Hybrid Approaches and Protein Resurfacing compared to traditional methods.
Figure 2.
Experimental protein engineering has achieved significant advancements through directed evolution as well as rational design and structure-guided engineering. (a) A schematic diagram explaining Phage-Assisted Continuous Evolution and Deep Mutational Scanning within Directed Evolution, and Computational-Experimental Hybrid Approaches and Protein Resurfacing within Rational Design and Structure-Guided Engineering. (b) A comparison highlighting the advantages of Phage-Assisted Continuous Evolution and Deep Mutational Scanning compared to traditional methods. (c) A detailed description of the advantages of Computational-Experimental Hybrid Approaches and Protein Resurfacing compared to traditional methods.
3.1. Directed Evolution
Directed evolution remains a cornerstone of protein engineering, allowing the creation of proteins with dramatically enhanced or novel functions through iterative rounds of mutation and selection.
3.1.1. Phage-Assisted Continuous Evolution
Phage-assisted continuous evolution (PACE) is a powerful system that enables rapid directed evolution of proteins and other biomolecules. PACE exploits the life cycle of M13 bacteriophage to continuously evolve gene-encoded molecules that can be linked to phage production in E. coli host cells [
41,
42]. The system allows for continuous mutagenesis, selection, and replication without researcher intervention, enabling hundreds of rounds of evolution to occur in days or weeks rather than months. PACE achieves this acceleration by coupling the desired activity to the production of phage infectivity protein pIII, which is essential for phage propagation. Mutagenesis is driven by an error-prone DNA polymerase expressed in the host cells, generating diversity continuously during phage replication [
43]. The use of a "lagoon" vessel with continuous influx of fresh host cells and outflow of depleted cells allows evolution to proceed indefinitely [
44]. PACE has been successfully applied to evolve a wide range of proteins, including polymerases, proteases, genome editing tools, and antibody fragments, often yielding variants with dramatically improved properties after just days of evolution [
41]. The system can be modulated through strategies like negative selection or modifying export levels to the periplasm, allowing fine-tuning of selection stringency. Overall, PACE represents a significant advance in directed evolution technology, enabling the rapid generation of biomolecules with novel and enhanced functions.
3.1.2. Deep Mutational Scanning
Deep mutational scanning (DMS) is a high-throughput approach that systematically assesses the effects of all possible single amino acid substitutions on protein function, providing comprehensive mutational landscapes to guide engineering efforts [
45,
46,
47]. DMS involves creating large libraries of protein variants containing thousands to millions of mutations, followed by selection or screening and high-throughput sequencing to quantify the functional effects of each mutation [
45,
46]. This technique enables the exploration of protein sequence-function relationships at an unprecedented scale and can reveal intrinsic protein properties, protein behavior within cells, and the consequences of genetic variations. DMS has been applied to study protein stability, binding affinity, enzymatic activity, and other functional properties across diverse proteins including enzymes, antibodies, and viral proteins [
48]. The comprehensive mutational data generated by DMS can guide rational protein engineering by identifying key functional residues, revealing permissive sites for modification, and uncovering non-obvious beneficial mutations. Additionally, DMS data can be used to train machine learning models for predicting the effects of mutations, further enhancing protein engineering capabilities [
45,
46]. Recent advances in DMS methodology, including the use of CRISPR-based genome editing for in situ mutagenesis and the development of more sophisticated selection schemes, have expanded the applicability of this approach to studying proteins in their native genomic context [
48].
3.2. Rational Design and Structure-Guided Engineering
Rational design approaches leverage structural and mechanistic insights to make targeted modifications to proteins.
3.2.1. Computational-Experimental Hybrid Approaches
Computational-experimental hybrid approaches, involving iterative cycles of computational prediction and experimental validation, have emerged as powerful strategies for efficiently optimizing protein properties. These approaches leverage the strengths of both computational modeling and experimental testing to accelerate the protein engineering process [
49,
50]. Typically, the workflow begins with computational predictions of promising protein variants, which are then experimentally tested [
51]. The experimental results are used to refine the computational models, creating an iterative feedback loop that improves prediction accuracy over multiple rounds [
49]. This approach has been successfully applied to various protein engineering challenges, including enhancing enzyme activity, stability, and specificity. For example, Voigt et al. demonstrated the power of this method by rapidly optimizing β-lactamase activity using a combination of structure-guided computational design and high-throughput screening [
52]. The integration of machine learning techniques with experimental data has further enhanced the efficiency of these hybrid approaches, enabling more accurate predictions of protein properties from limited experimental data [
53]. Additionally, these methods can incorporate diverse types of experimental data, including structural information from X-ray crystallography, NMR, and cryo-EM, as well as functional data from high-throughput assays [
54]. By combining computational and experimental techniques, researchers can explore larger sequence spaces more efficiently than through experimental methods alone, while also overcoming limitations of purely computational approaches [
50].
3.2.2. Protein Resurfacing
Protein resurfacing is a powerful protein engineering approach that involves modifying surface residues to improve stability, reduce immunogenicity, and alter pharmacokinetics without disrupting core protein function [
55,
56]. This technique allows for extensive modification of protein surfaces, with some studies achieving up to 58 substitutions resulting in direct modification of 35% of surface residues [
19]. Resurfacing can significantly reduce binding to pre-existing anti-drug antibodies, with greater reductions observed as mutational distance from the native protein increases [
15]. In addition to reducing immunogenicity, resurfacing can enhance protein stability by optimizing surface charge distribution and introducing favorable electrostatic interactions [
57,
58]. The approach has been successfully applied to various therapeutic proteins, including enzymes like L-asparaginase, to generate variants with improved properties while maintaining catalytic function [
1]. Computational design methods play a crucial role in resurfacing efforts, allowing researchers to explore large sequence spaces and predict mutations that maintain protein folding and function [
59,
60]. When combined with experimental validation, these computational approaches enable the efficient development of resurfaced proteins with enhanced pharmaceutical properties. As the field advances, machine learning techniques and large-scale design-test-learn cycles are likely to further improve our ability to optimize the trade-offs between immunogenicity, function, and expression in resurfaced proteins [
19,
37].
4. Applications in Therapeutic Protein Engineering
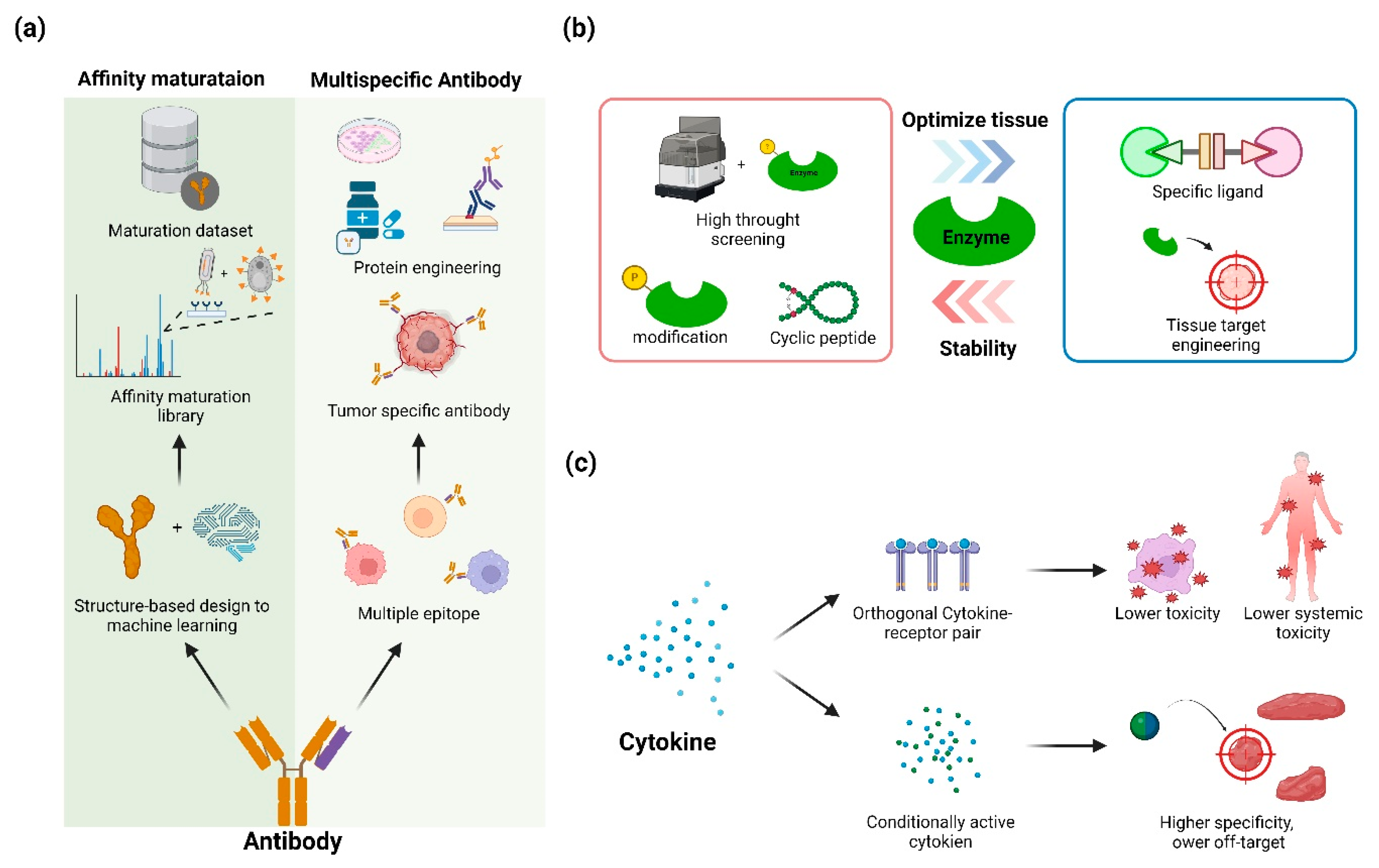
Figure 3.
An overview and applications of therapeutic protein engineering using advanced biological molecules. (a) The workflows of protein engineering using antibodies, which can be applied through computational and experimental methods. (b) An overview of the development direction of protein engineering for enzyme stability and tissue optimization. (c) The workflow of developing lower toxicity and off-target effects using cytokine engineering.
Figure 3.
An overview and applications of therapeutic protein engineering using advanced biological molecules. (a) The workflows of protein engineering using antibodies, which can be applied through computational and experimental methods. (b) An overview of the development direction of protein engineering for enzyme stability and tissue optimization. (c) The workflow of developing lower toxicity and off-target effects using cytokine engineering.
4.1. Antibody Engineering
Monoclonal antibodies represent the largest class of protein therapeutics. Engineering efforts have focused on:
4.1.1. Affinity Maturation
Affinity maturation is a critical process in antibody engineering that aims to enhance the binding affinity and specificity of antibodies to their target antigens [
61,
62]. This process typically involves iterative cycles of introducing mutations into the antibody sequence, particularly in the complementarity-determining regions (CDRs), followed by screening and selection of variants with improved binding properties. Computational approaches have become increasingly important in guiding affinity maturation efforts, with methods ranging from structure-based design to machine learning models [
63,
64]. Structure-guided computational design can identify promising mutations by analyzing the antibody-antigen interface and predicting energetically favorable substitutions [
65]. Machine learning models, trained on experimental data and structural features, can predict the effects of mutations on binding affinity and guide the selection of candidates for experimental validation [
66]. Deep learning approaches, such as those based on language models trained on antibody sequences, have shown promise in generating diverse sets of potentially affinity-enhancing mutations [
67]. Experimental methods for affinity maturation include display technologies like phage and yeast display, which allow for high-throughput screening of large antibody libraries [
68,
69]. Advanced techniques like deep mutational scanning provide comprehensive mutational landscapes that can inform both computational and experimental approaches [
46,
70]. The integration of computational predictions with experimental validation in iterative cycles has emerged as a powerful strategy for efficient affinity maturation, allowing researchers to explore larger sequence spaces and identify non-obvious beneficial mutations [
71,
72].
4.1.2. Bispecific and Multispecific Antibodies
Bispecific and multispecific antibodies represent a significant advancement in antibody engineering, enabling the creation of molecules that can simultaneously bind two or more distinct epitopes, thus facilitating novel therapeutic mechanisms [
73]. These engineered antibodies can be designed to engage multiple targets on the same or different cells, offering potential advantages over traditional monoclonal antibodies in terms of efficacy and specificity [
74]. Bispecific antibodies, in particular, have gained considerable attention, with over 100 candidates in clinical development and several FDA approvals since 2014. Various formats have been developed, including IgG-like structures and smaller fragments, each with distinct properties affecting pharmacokinetics, tissue penetration, and effector functions [
75]. Common applications include T-cell redirection in cancer immunotherapy, where one arm binds a tumor antigen and the other engages CD3 on T cells, as exemplified by the FDA-approved blinatumomab for acute lymphoblastic leukemia [
76]. Beyond oncology, bispecific and multispecific antibodies are being explored for autoimmune diseases, infectious diseases, and neurodegenerative disorders [
77]. The development of these complex molecules presents unique challenges in design, production, and characterization, requiring innovative approaches in protein engineering, cell line development, and manufacturing processes [
78,
79]. As the field advances, computational tools, including machine learning approaches, are increasingly being employed to optimize antibody design and predict properties such as stability and aggregation propensity [
80].
4.2. Enzyme Replacement Therapies
For genetic disorders caused by enzyme deficiencies, protein engineering has been used to:
4.2.1. Enhance Enzyme Stability
Enhancing enzyme stability, particularly thermostability and resistance to proteolysis, is crucial for increasing circulatory half-life and improving the efficacy of therapeutic proteins. Thermostability can be improved through various strategies, including loop scanning and site-saturation mutagenesis to identify stabilizing mutations in flexible regions [
81]. Computational approaches like structure-guided design and machine learning models can predict stabilizing mutations and guide protein engineering efforts [
82]. Increasing the rigidity of enzymes, particularly in the active site region, has been shown to enhance both thermostability and proteolytic resistance [
83,
84]. Specific techniques to improve protease resistance include terminal modifications like N-terminal acetylation and C-terminal amidation, as well as incorporation of non-canonical amino acids [
85,
86]. Cyclization of peptides and proteins can also significantly enhance stability against proteolytic degradation [
87]. For larger proteins, engineering disulfide bonds or introducing covalent "staples" using non-canonical amino acids can rigidify the structure and improve thermostability [
88]. Directed evolution approaches, coupled with high-throughput screening, remain powerful tools for identifying stabilizing mutations that may not be rationally predicted. By combining multiple stabilization strategies, dramatic improvements in enzyme half-life can be achieved, with some engineered variants showing 40-fold longer half-lives at elevated temperatures [
84].
4.2.2. Optimize Tissue Targeting
Optimizing tissue targeting by adding or modifying targeting motifs is a crucial strategy to improve enzyme delivery to affected tissues in enzyme replacement therapies. The addition of specific targeting ligands can enhance cellular uptake and tissue distribution of therapeutic enzymes [
23,
89]. For example, glycosylation with mannose-6-phosphate (M6P) residues enables targeting to M6P receptors that are highly expressed in many cell types, facilitating lysosomal enzyme delivery [
90]. Modifying enzymes with cell-penetrating peptides like TAT or polyarginine can improve cellular uptake and tissue penetration [
91]. Antibody-enzyme fusion proteins have been developed to target specific cell surface receptors and enhance tissue-specific delivery [
92,
93]. Nanocarrier systems functionalized with targeting ligands such as transferrin, folate, or RGD peptides can improve enzyme biodistribution to target tissues [
94]. Glycoengineering approaches to modify enzyme glycosylation patterns can alter tissue tropism and pharmacokinetics [
95]. Site-specific PEGylation or polymer conjugation can be used to modulate enzyme circulation time and tissue accumulation [
94]. Biomimetic strategies like erythrocyte membrane coating have shown promise for prolonging circulation and enhancing tissue-specific targeting [
96]. Recent advances in protein engineering and synthetic biology are enabling the design of enzymes with intrinsic tissue-targeting properties [
97,
98].
4.3. Cytokine Engineering
Engineered cytokines offer improved therapeutic windows and targeted activity:
4.3.1. Orthogonal Cytokine-Receptor Pairs
Orthogonal cytokine-receptor pairs represent an innovative approach to creating targeted therapies with reduced off-target effects by engineering both the cytokine and its receptor to interact exclusively with each other [
99]. This strategy was pioneered with the development of an orthogonal IL-2/IL-2R pair (ortho2 and ortho2R) that can stimulate only engineered T cells expressing the modified receptor while remaining inert to endogenous immune cells. Similar approaches have been applied to other cytokines, such as the split, conditionally active mimetic of IL-2/IL-15 (Neo-2/15) that requires colocalization of two components for activity, enabling selective activation in specific tissue microenvironments [
100]. Orthogonal engineering has also been used to create synthetic T cell states that escape canonical exhaustion, as demonstrated by T cells engineered to secrete an IL-2 variant binding the IL-2Rβγ receptor and the alarmin IL-33 [
101]. These approaches can be extended to other cytokines and receptors, potentially allowing precise control of engineered therapeutic cells like CAR-T cells [
102]. The development of non-natural "synthekines" that assemble non-natural receptor heterodimers represents another frontier in orthogonal cytokine engineering [
103]. As the field advances, combining orthogonal cytokine-receptor pairs with engineered cellular delivery systems may offer powerful ways to autonomously target and modulate local disease environments while minimizing systemic toxicity.
4.3.2. Conditionally Active Cytokines
Conditionally active cytokines are engineered variants designed to be selectively activated in specific tissue microenvironments, such as tumors, to improve their therapeutic index and reduce systemic toxicity [
100]. One approach involves splitting cytokines into two inactive components that require colocalization for activity, as demonstrated with a split IL-2/IL-15 mimetic that showed enhanced antitumor efficacy and reduced toxicity when the components were independently targeted [
100]. Another strategy utilizes protease-activated cytokine prodrugs that leverage the dysregulated protease activity in tumors, exemplified by WTX-124, a conditionally activated IL-2 prodrug that is preferentially cleaved and activated in the tumor microenvironment [
104]. pH-dependent cytokines have also been developed to exploit the acidic tumor microenvironment, with engineered variants showing selective activation under low pH conditions [
105]. Some approaches focus on modifying cytokine-receptor interactions, such as engineering IL-2 variants with reduced affinity for CD25 to avoid preferential activation of regulatory T cells [
106]. Targeted delivery systems, including antibody-cytokine fusions and nanoparticle formulations, can also achieve conditional activation by concentrating cytokines in desired tissues [
107]. More complex designs involve multi-component systems, such as the cytokine-PACE platform, which combines split cytokines with antibody-driven chain exchange for targeted reconstitution of active cytokines on tumor cells [
108]. These diverse engineering strategies aim to overcome the limitations of conventional cytokine therapies by enhancing tumor specificity and minimizing off-target effects, potentially expanding the therapeutic window for cytokine-based cancer immunotherapies [
106,
109,
110].
5. Emerging Approaches and Future Directions
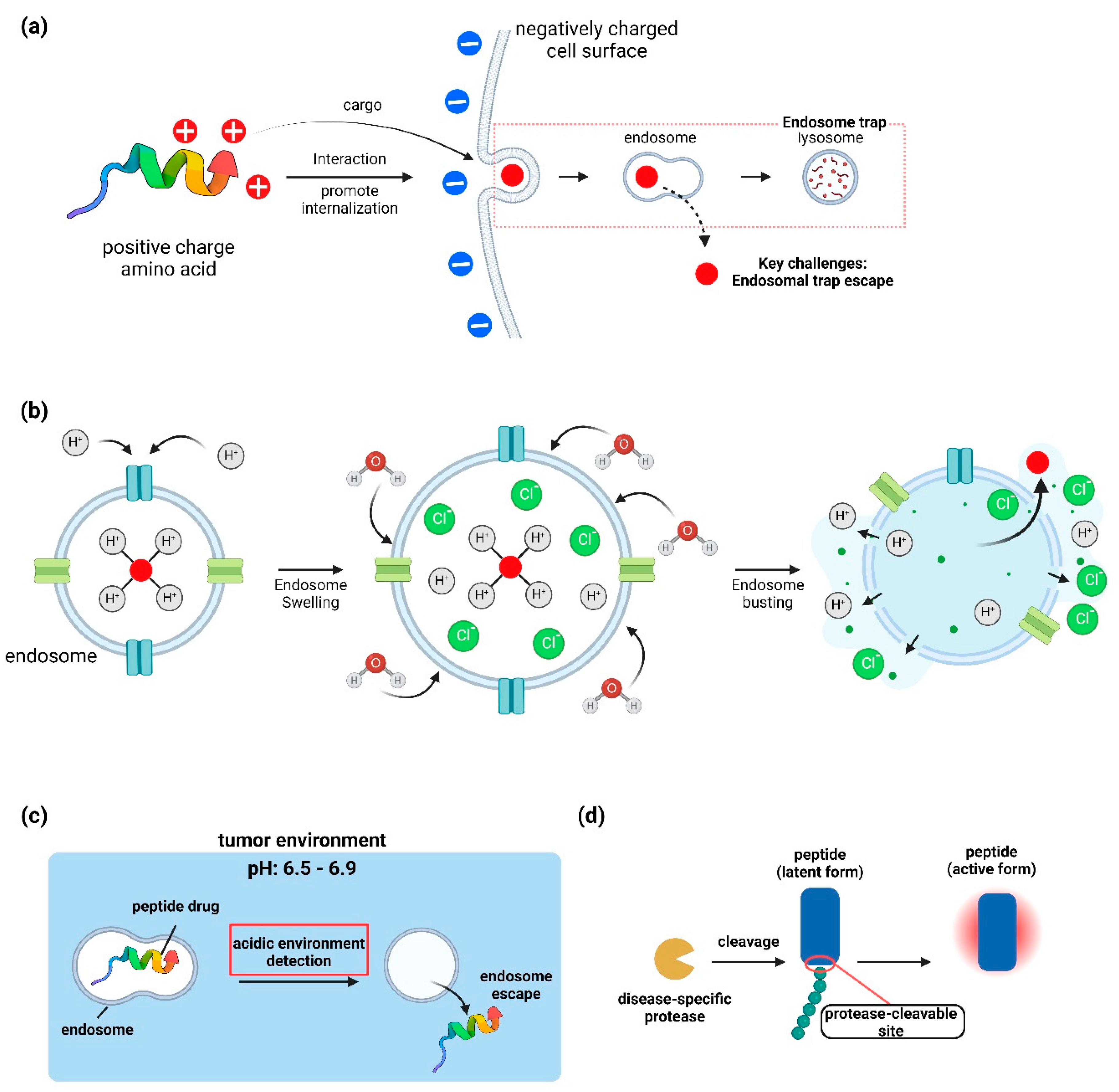
Figure 4.
Mechanisms of cell-penetrating peptide (CPP) delivery and endosomal escape. (a) Mechanism of cellular membrane penetration by a Cell-Penetrating Peptide (CPP) containing positively charged amino acids, and subsequent evasion of the endosome trap. (b) Schematic of the proton sponge mechanism, one of the mechanisms for the escape of peptides from endosomal membranes. The diagram illustrates the proton sponge mechanism, where the peptide captures protons, leveraging osmotic pressure to facilitate the influx of water molecules into the endosome. This influx induces endosomal swelling, culminating in the rupture of the endosome and the subsequent release of the peptide. (c) Escape of peptide drugs from the endosome upon detection of the acidic environment of a tumor. (d) Diagram depicting the activation of a latent form peptide by a disease-specific protease via cleavage.
Figure 4.
Mechanisms of cell-penetrating peptide (CPP) delivery and endosomal escape. (a) Mechanism of cellular membrane penetration by a Cell-Penetrating Peptide (CPP) containing positively charged amino acids, and subsequent evasion of the endosome trap. (b) Schematic of the proton sponge mechanism, one of the mechanisms for the escape of peptides from endosomal membranes. The diagram illustrates the proton sponge mechanism, where the peptide captures protons, leveraging osmotic pressure to facilitate the influx of water molecules into the endosome. This influx induces endosomal swelling, culminating in the rupture of the endosome and the subsequent release of the peptide. (c) Escape of peptide drugs from the endosome upon detection of the acidic environment of a tumor. (d) Diagram depicting the activation of a latent form peptide by a disease-specific protease via cleavage.
5.1. Intracellular Protein Delivery
Achieving efficient delivery of therapeutic proteins to intracellular targets remains a major challenge. Promising approaches include:
5.1.1. Cell-Penetrating Peptides
Cell-penetrating peptides (CPPs) are short peptide sequences, typically 5-30 amino acids long, that can facilitate the cellular uptake of attached cargo proteins across biological membranes [
111]. CPPs are often rich in positively charged amino acids like arginine and lysine, which interact with negatively charged cell surface molecules to promote internalization [
112]. Common CPP sequences include HIV-1 Tat, penetratin, and polyarginine peptides. The mechanisms of CPP-mediated cellular entry are still debated but likely involve both direct membrane translocation and endocytic uptake [
113]. A key challenge for CPP-protein delivery is escaping endosomal entrapment after internalization [
114]. Some CPPs can disrupt endosomal membranes through mechanisms like the proton sponge effect or pore formation. Engineering approaches to enhance endosomal escape include incorporating pH-sensitive domains or fusogenic peptides [
57]. CPPs have been successfully used to deliver a wide range of protein cargoes including enzymes, antibodies, and transcription factors both in vitro and in vivo [
115]. Recent advances include the development of activatable CPPs that are triggered by tumor microenvironment conditions and cell-type specific CPPs for targeted delivery. While promising, challenges remain in optimizing the efficiency of cytosolic delivery and reducing potential toxicity of CPPs for therapeutic applications [
116].
5.1.2. Nanocarrier-Based Delivery
Nanocarrier-based delivery systems, including nanoparticles and liposomes, have emerged as promising approaches for protein encapsulation and targeted delivery. These nanocarriers can protect proteins from premature degradation, enhance their stability and circulation time, enable controlled release, and facilitate cellular uptake and intracellular delivery [
117]. Liposomes, composed of phospholipid bilayers, can encapsulate hydrophilic proteins in their aqueous core or incorporate hydrophobic proteins in the lipid bilayer. Polymeric nanoparticles, such as those made from PLGA or chitosan, offer tunable degradation and release properties for sustained protein delivery [
118]. Inorganic nanoparticles like mesoporous silica can achieve high protein loading capacity and enable stimuli-responsive release [
119]. Surface modification of nanocarriers with targeting ligands or cell-penetrating peptides can enhance tissue- or cell-specific delivery [
120]. Key challenges include maintaining protein stability during encapsulation, achieving efficient intracellular delivery and endosomal escape, and optimizing the nanocarrier degradation rate for controlled protein release [
121]. Recent advances such as biomimetic nanoparticles and exosome-based delivery systems show promise for improving the in vivo performance of protein therapeutics. While several nanocarrier-based protein delivery systems have reached clinical trials, continued research is needed to overcome biological barriers and enhance the therapeutic efficacy of this approach.
5.2. Stimulus-Responsive Proteins
Creating proteins that can be activated or deactivated in response to specific stimuli offers precise spatiotemporal control of therapeutic activity:
5.2.1. pH-Sensitive Proteins
The acidic tumor microenvironment, characterized by extracellular pH values typically ranging from 6.5 to 6.9 compared to 7.2-7.4 in normal tissues, provides a unique opportunity for developing targeted cancer therapies using pH-sensitive proteins [
122]. Designing such proteins involves incorporating pH-responsive elements that undergo conformational changes or altered interactions in response to acidic conditions [
123]. Common strategies include introducing histidine residues, which become protonated at slightly acidic pH, into key functional regions of proteins [
124]. For example, antibodies have been engineered with pH-dependent binding, allowing them to release their cargo specifically in acidic endosomes [
125]. Another approach involves designing pH-sensitive protein switches using computational methods, as demonstrated by the de novo design of proteins that assemble into micron-scale fibers at neutral pH but rapidly disassemble when exposed to acidic conditions [
126]. pH-responsive protein nanocarriers have also been developed, utilizing materials that change conformation or disassemble in acidic environments to release encapsulated drugs. These pH-sensitive designs can be further enhanced by combining them with other stimuli-responsive elements or targeting moieties to improve specificity and efficacy [
127]. The development of conditionally active biologics (CABs) that are preferentially activated in the acidic tumor microenvironment represents another promising approach, as exemplified by protease-activated cytokine prodrugs [
126]. While these strategies show great promise, challenges remain in optimizing the pH transition point, maintaining protein stability, and achieving efficient intracellular delivery for therapeutic applications.
5.2.2. Protease-Activated Proteins
Protease-activated proteins are engineered latent proteins that remain inactive until cleaved by disease-specific proteases, enabling targeted therapeutic activity. A key strategy involves designing proteins with inhibitory pro-domains that are removed by proteolytic cleavage, as demonstrated by engineered pro-forms of antibodies and cytokines that are activated in the tumor microenvironment [
128,
129]. Computational approaches like structure-guided design and machine learning models can predict optimal cleavage sites and pro-domain sequences to achieve desired activation profiles. Directed evolution techniques have also been applied to engineer highly specific protease recognition sequences [
130]. Some designs incorporate multiple protease cleavage sites to require coincident protease activity for full activation, improving specificity [
131]. Protein cages that disassemble upon protease cleavage to release encapsulated cargo offer another promising approach. Beyond oncology, protease-activated proteins are being explored for applications in infectious diseases, cardiovascular disorders, and other conditions with dysregulated protease activity [
128]. Key challenges include optimizing the dynamic range between latent and active states, minimizing premature activation, and achieving efficient intracellular delivery for some applications. As the field advances, combining protease-activation with other targeting strategies may further enhance the therapeutic window of engineered proteins [
129].
5.3. De Novo Designed Therapeutic Proteins
Advances in de novo protein design are enabling the creation of entirely novel therapeutic modalities:
5.3.1. Protein Switches
Protein switches are engineered proteins that undergo programmable conformational changes in response to specific molecular cues, enabling sensing and actuation functions in synthetic biology applications. A key strategy involves designing proteins with multiple stable conformational states separated by energy barriers that can be modulated by stimuli like small molecules, pH changes, or protein-protein interactions [
132]. Computational approaches have become increasingly important for switch design, with methods like structure-guided design and machine learning models enabling the prediction of conformational changes and optimization of switching behavior [
133,
134]. Common design principles include incorporating ligand-binding domains, using mutually exclusive folding states, and engineering allosteric coupling between distant protein regions [
134,
135]. Directed evolution and high-throughput screening approaches have also been successful in developing switches with desired properties. Recent advances include the de novo design of modular protein switches and sensors using approaches like deep learning. Key challenges in the field include improving the dynamic range between states, minimizing unwanted activation, and achieving rapid and reversible switching [
136]. As computational and experimental methods continue to advance, protein switches are likely to play an increasingly important role in synthetic biology applications like cell-based therapeutics and biosensors [
137,
138].
5.3.2. Artificial Enzymes
Artificial enzymes are designed catalytic proteins that aim to mimic or improve upon the functions of natural enzymes for therapeutic applications. A key strategy involves computational design of protein scaffolds with precisely positioned catalytic residues to facilitate desired reactions, as demonstrated by early work on de novo enzyme design [
139]. Machine learning approaches are increasingly being used to optimize artificial enzyme designs and predict beneficial mutations. Directed evolution remains a powerful complementary method for enhancing the activity and specificity of designed enzymes [
140]. For prodrug activation, artificial enzymes have been engineered to catalyze bond-forming reactions that construct a drug's pharmacophore, enabling highly selective activation [
141]. Incorporation of non-canonical amino acids or metal cofactors can introduce novel catalytic functionalities not found in natural enzymes [
142,
143]. Artificial metalloenzymes combining protein scaffolds with synthetic metal catalysts show promise for expanding the repertoire of biocompatible reactions [
144]. Computational design of enzyme switches that are selectively activated by disease-specific triggers offers another avenue for targeted therapeutics [
145]. While significant progress has been made, challenges remain in achieving the catalytic efficiency and substrate specificity of natural enzymes [
146,
147]. Key hurdles include improving in vivo half-life, enhancing targeted action, and controlling immune responses to enzyme therapeutics [
144]. Continued advances in computational methods, high-throughput screening, and our understanding of enzyme mechanisms will be key to realizing the full potential of artificial enzymes as therapeutics [
138].
6. Challenges and Future Outlook
While protein engineering has made tremendous strides, several challenges remain:
Improving our ability to predict protein behavior in complex physiological environments remains a major challenge for engineered protein therapeutics [
148]. Developing scalable manufacturing processes for increasingly complex engineered proteins will be crucial as more sophisticated designs enter clinical development [
149]. Enhancing immunogenicity prediction and mitigation strategies is critical to reduce adverse immune responses that can limit efficacy and safety [
150]. Improving targeted delivery methods to specific tissues and cell types could dramatically enhance therapeutic index and enable new applications. Designing protein therapeutics that synergize effectively with other treatment modalities like small molecules or cell therapies offers exciting potential for combination approaches. Addressing these challenges will require continued advances in computational modeling, high-throughput screening methods, protein engineering techniques, drug delivery technologies, and systems biology approaches to understand complex in vivo environments.
Looking ahead, the integration of advanced computational methods, high-throughput experimental techniques, and a deepening understanding of protein structure-function relationships will continue to drive innovations in therapeutic protein engineering. Emerging technologies like mRNA therapeutics and in vivo protein evolution may further expand the possibilities for creating and delivering engineered proteins. As these approaches mature, we can expect to see a new generation of highly tailored protein therapeutics with improved efficacy, safety, and applicability across a wide range of diseases.
7. Conclusion
Protein design and engineering have become indispensable tools in the development of advanced biotherapeutics. By harnessing computational and experimental approaches, researchers are creating protein drugs with enhanced stability, efficacy, and safety profiles. As our ability to manipulate protein structure and function continues to improve, we can anticipate a future where highly customized protein therapeutics provide precise and powerful treatments for a wide range of human diseases.
Author Contributions
Conceptualization, investigation, writing, and original draft preparation, A.S. (Ahrum Son); H.K. (Hyunsoo Kim) – Visualization, and proofreading, J.P. (Jongham Park); W.K. (Woojin Kim); W.L. (Wonseok Lee); Y.Y. (Yoonki Yoon) – Supervision, Project Administration, Funding Acquisition, Review and Editing, H.K. (Hyunsoo Kim). All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Korea Basic Science Institute (National research Facilities and Equipment Center) grant funded by the Korea government (MSIT) (RS-2024-00402298). This work was supported by BK21 FOUR Program by Chungnam National University Research Grant, 2023. This work was partly supported by Institute of Information & communications Technology Planning & Evaluation (IITP) grant funded by the Korea government (MSIT) (No.RS-2022-00155857, Artificial Intelligence Convergence Innovation Human Resources Development (Chungnam National University)) and supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (RS-2023-00209456).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lagasse, H.A.; Alexaki, A.; Simhadri, V.L.; Katagiri, N.H.; Jankowski, W.; Sauna, Z.E.; Kimchi-Sarfaty, C. Recent advances in (therapeutic protein) drug development. F1000Res 2017, 6, 113. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, D.S. Therapeutic proteins. Methods Mol Biol 2012, 899, 1–26. [Google Scholar] [CrossRef]
- Ebrahimi, S.B.; Samanta, D. Engineering protein-based therapeutics through structural and chemical design. Nat Commun 2023, 14, 2411. [Google Scholar] [CrossRef]
- Akbarian, M.; Chen, S.H. Instability Challenges and Stabilization Strategies of Pharmaceutical Proteins. Pharmaceutics 2022, 14. [Google Scholar] [CrossRef]
- Tobin, P.H.; Richards, D.H.; Callender, R.A.; Wilson, C.J. Protein engineering: a new frontier for biological therapeutics. Curr Drug Metab 2014, 15, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Derat, E.; Kamerlin, S.C.L. Computational Advances in Protein Engineering and Enzyme Design. J Phys Chem B 2022, 126, 2449–2451. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, X.; Chen, X.; Huang, J.; Wang, C.; Wang, J.; Wang, Z. Accelerating therapeutic protein design with computational approaches toward the clinical stage. Comput Struct Biotechnol J 2023, 21, 2909–2926. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.L.; Juergens, D.; Bennett, N.R.; Trippe, B.L.; Yim, J.; Eisenach, H.E.; Ahern, W.; Borst, A.J.; Ragotte, R.J.; Milles, L.F.; et al. De novo design of protein structure and function with RFdiffusion. Nature 2023, 620, 1089–1100. [Google Scholar] [CrossRef]
- Selles Vidal, L.; Isalan, M.; Heap, J.T.; Ledesma-Amaro, R. A primer to directed evolution: current methodologies and future directions. RSC Chem Biol 2023, 4, 271–291. [Google Scholar] [CrossRef]
- Cherf, G.M.; Cochran, J.R. Applications of Yeast Surface Display for Protein Engineering. Methods Mol Biol 2015, 1319, 155–175. [Google Scholar] [CrossRef]
- Link, A.J.; Mock, M.L.; Tirrell, D.A. Non-canonical amino acids in protein engineering. Curr Opin Biotechnol 2003, 14, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Boutureira, O.; Bernardes, G.J. Advances in chemical protein modification. Chem Rev 2015, 115, 2174–2195. [Google Scholar] [CrossRef]
- Li, C.; Zhang, R.; Wang, J.; Wilson, L.M.; Yan, Y. Protein Engineering for Improving and Diversifying Natural Product Biosynthesis. Trends Biotechnol 2020, 38, 729–744. [Google Scholar] [CrossRef]
- Naowarojna, N.; Cheng, R.; Lopez, J.; Wong, C.; Qiao, L.; Liu, P. Chemical modifications of proteins and their applications in metalloenzyme studies. Synth Syst Biotechnol 2021, 6, 32–49. [Google Scholar] [CrossRef] [PubMed]
- Rahban, M.; Ahmad, F.; Piatyszek, M.A.; Haertle, T.; Saso, L.; Saboury, A.A. Stabilization challenges and aggregation in protein-based therapeutics in the pharmaceutical industry. RSC Adv 2023, 13, 35947–35963. [Google Scholar] [CrossRef] [PubMed]
- Castro, T.G.; Melle-Franco, M.; Sousa, C.E.A.; Cavaco-Paulo, A.; Marcos, J.C. Non-Canonical Amino Acids as Building Blocks for Peptidomimetics: Structure, Function, and Applications. Biomolecules 2023, 13. [Google Scholar] [CrossRef]
- Brinkmann, U.; Kontermann, R.E. The making of bispecific antibodies. MAbs 2017, 9, 182–212. [Google Scholar] [CrossRef]
- Thoreau, F.; Chudasama, V. Enabling the next steps in cancer immunotherapy: from antibody-based bispecifics to multispecifics, with an evolving role for bioconjugation chemistry. RSC Chem Biol 2022, 3, 140–169. [Google Scholar] [CrossRef] [PubMed]
- Porello, I.; Cellesi, F. Intracellular delivery of therapeutic proteins. New advancements and future directions. Front Bioeng Biotechnol 2023, 11, 1211798. [Google Scholar] [CrossRef]
- Li, Y.; Yang, G.; Gerstweiler, L.; Thang, S.H.; Zhao, C.X. Design of Stimuli-Responsive Peptides and Proteins. Advanced Functional Materials 2022, 33. [Google Scholar] [CrossRef]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G.; Lee, W.; Park, H.H. Protein-Based Nanoparticles as Drug Delivery Systems. Pharmaceutics 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, M.; Telzerow, A.; Quax, W.J.; Boersma, Y.L. High-Throughput Screening in Protein Engineering: Recent Advances and Future Perspectives. Int J Mol Sci 2015, 16, 24918–24945. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ukidve, A.; Kim, J.; Mitragotri, S. Targeting Strategies for Tissue-Specific Drug Delivery. Cell 2020, 181, 151–167. [Google Scholar] [CrossRef]
- Hwang, I.; Park, S. Computational design of protein therapeutics. Drug Discov Today Technol 2008, 5, e43–48. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Bertoline, L.M.F.; Lima, A.N.; Krieger, J.E.; Teixeira, S.K. Before and after AlphaFold2: An overview of protein structure prediction. Front Bioinform 2023, 3, 1120370. [Google Scholar] [CrossRef]
- Baek, M.; DiMaio, F.; Anishchenko, I.; Dauparas, J.; Ovchinnikov, S.; Lee, G.R.; Wang, J.; Cong, Q.; Kinch, L.N.; Schaeffer, R.D.; et al. Accurate prediction of protein structures and interactions using a three-track neural network. Science 2021, 373, 871–876. [Google Scholar] [CrossRef]
- Lin, Z.; Akin, H.; Rao, R.; Hie, B.; Zhu, Z.; Lu, W.; Smetanin, N.; Verkuil, R.; Kabeli, O.; Shmueli, Y.; et al. Evolutionary-scale prediction of atomic-level protein structure with a language model. Science 2023, 379, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Orlando, G.; Serrano, L.; Schymkowitz, J.; Rousseau, F. Integrating physics in deep learning algorithms: a force field as a PyTorch module. Bioinformatics 2024, 40. [Google Scholar] [CrossRef]
- Leman, J.K.; Weitzner, B.D.; Lewis, S.M.; Adolf-Bryfogle, J.; Alam, N.; Alford, R.F.; Aprahamian, M.; Baker, D.; Barlow, K.A.; Barth, P.; et al. Macromolecular modeling and design in Rosetta: recent methods and frameworks. Nat Methods 2020, 17, 665–680. [Google Scholar] [CrossRef]
- Cao, L.; Goreshnik, I.; Coventry, B.; Case, J.B.; Miller, L.; Kozodoy, L.; Chen, R.E.; Carter, L.; Walls, A.C.; Park, Y.J.; et al. De novo design of picomolar SARS-CoV-2 miniprotein inhibitors. Science 2020, 370, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K.E.; Fannjiang, C.; Wittmann, B.J.; Hie, B.L.; Yang, K.K.; Wu, Z. Machine Learning for Protein Engineering. ArXiv 2023. [Google Scholar]
- Sumida, K.H.; Nunez-Franco, R.; Kalvet, I.; Pellock, S.J.; Wicky, B.I.M.; Milles, L.F.; Dauparas, J.; Wang, J.; Kipnis, Y.; Jameson, N.; et al. Improving Protein Expression, Stability, and Function with ProteinMPNN. J Am Chem Soc 2024, 146, 2054–2061. [Google Scholar] [CrossRef] [PubMed]
- Mardikoraem, M.; Wang, Z.; Pascual, N.; Woldring, D. Generative models for protein sequence modeling: recent advances and future directions. Brief Bioinform 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Dolorfino, M.; Samanta, R.; Vorobieva, A. ProteinMPNN Recovers Complex Sequence Properties of Transmembrane beta-barrels. bioRxiv 2024. [Google Scholar] [CrossRef]
- Dauparas, J.; Anishchenko, I.; Bennett, N.; Bai, H.; Ragotte, R.J.; Milles, L.F.; Wicky, B.I.M.; Courbet, A.; de Haas, R.J.; Bethel, N.; et al. Robust deep learning-based protein sequence design using ProteinMPNN. Science 2022, 378, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Repecka, D.; Jauniskis, V.; Karpus, L.; Rembeza, E.; Rokaitis, I.; Zrimec, J.; Poviloniene, S.; Laurynenas, A.; Viknander, S.; Abuajwa, W.; et al. Expanding functional protein sequence spaces using generative adversarial networks. Nature Machine Intelligence 2021, 3, 324–333. [Google Scholar] [CrossRef]
- Rives, A.; Meier, J.; Sercu, T.; Goyal, S.; Lin, Z.; Liu, J.; Guo, D.; Ott, M.; Zitnick, C.L.; Ma, J.; et al. Biological structure and function emerge from scaling unsupervised learning to 250 million protein sequences. Proc Natl Acad Sci U S A 2021, 118. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, L.; Yu, Y.; Wu, B.; Li, M.; Hong, L.; Tan, P. Enhancing efficiency of protein language models with minimal wet-lab data through few-shot learning. Nat Commun 2024, 15, 5566. [Google Scholar] [CrossRef]
- Frisby, T.S.; Langmead, C.J. Identifying promising sequences for protein engineering using a deep transformer protein language model. Proteins 2023, 91, 1471–1486. [Google Scholar] [CrossRef]
- Popa, S.C.; Inamoto, I.; Thuronyi, B.W.; Shin, J.A. Phage-Assisted Continuous Evolution (PACE): A Guide Focused on Evolving Protein-DNA Interactions. ACS Omega 2020, 5, 26957–26966. [Google Scholar] [CrossRef] [PubMed]
- Esvelt, K.M.; Carlson, J.C.; Liu, D.R. A system for the continuous directed evolution of biomolecules. Nature 2011, 472, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.M.; Wang, T.; Liu, D.R. Phage-assisted continuous and non-continuous evolution. Nat Protoc 2020, 15, 4101–4127. [Google Scholar] [CrossRef] [PubMed]
- Morrison, M.S.; Wang, T.; Raguram, A.; Hemez, C.; Liu, D.R. Disulfide-compatible phage-assisted continuous evolution in the periplasmic space. Nat Commun 2021, 12, 5959. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Li, X. Deep mutational scanning: A versatile tool in systematically mapping genotypes to phenotypes. Front Genet 2023, 14, 1087267. [Google Scholar] [CrossRef] [PubMed]
- Fowler, D.M.; Fields, S. Deep mutational scanning: a new style of protein science. Nat Methods 2014, 11, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Burton, T.D.; Eyre, N.S. Applications of Deep Mutational Scanning in Virology. Viruses 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Arcos, S.; Sabsay, K.R.; Te Velthuis, A.J.W.; Lauring, A.S. Deep mutational scanning reveals the functional constraints and evolutionary potential of the influenza A virus PB1 protein. J Virol 2023, 97, e0132923. [Google Scholar] [CrossRef] [PubMed]
- Seffernick, J.T.; Lindert, S. Hybrid methods for combined experimental and computational determination of protein structure. J Chem Phys 2020, 153, 240901. [Google Scholar] [CrossRef]
- Chi, X.; Hou, J. An iterative approach of protein function prediction. BMC Bioinformatics 2011, 12, 437. [Google Scholar] [CrossRef]
- Hayes, R.J.; Bentzien, J.; Ary, M.L.; Hwang, M.Y.; Jacinto, J.M.; Vielmetter, J.; Kundu, A.; Dahiyat, B.I. Combining computational and experimental screening for rapid optimization of protein properties. Proc Natl Acad Sci U S A 2002, 99, 15926–15931. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, V.K.; Workman, S.; Sun, T.; Rettie, S.; Li, X.; Worrall, L.J.; Craven, T.W.; King, D.T.; Hosseinzadeh, P.; Watkins, A.M.; et al. Computationally designed peptide macrocycle inhibitors of New Delhi metallo-beta-lactamase 1. Proc Natl Acad Sci U S A 2021, 118. [Google Scholar] [CrossRef]
- Yang, K.K.; Wu, Z.; Arnold, F.H. Machine-learning-guided directed evolution for protein engineering. Nat Methods 2019, 16, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Lenz, S.; MacCallum, J.L.; Perez, A. Hybrid computational methods combining experimental information with molecular dynamics. Curr Opin Struct Biol 2023, 81, 102609. [Google Scholar] [CrossRef]
- Turner, M.R.; Balu-Iyer, S.V. Challenges and Opportunities for the Subcutaneous Delivery of Therapeutic Proteins. J Pharm Sci 2018, 107, 1247–1260. [Google Scholar] [CrossRef]
- Teufl, M.; Zajc, C.U.; Traxlmayr, M.W. Engineering Strategies to Overcome the Stability-Function Trade-Off in Proteins. ACS Synth Biol 2022, 11, 1030–1039. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: current applications and future directions. Signal Transduct Target Ther 2022, 7, 48. [Google Scholar] [CrossRef]
- Bootwala, A.; An, H.H.; Franklin, M.W.; Manning, B.J.; Xu, L.Y.; Panchal, S.; Garlick, J.D.; Baral, R.; Hudson, M.E.; Grigoryan, G.; et al. Protein re-surfacing of E. coli L-Asparaginase to evade pre-existing anti-drug antibodies and hypersensitivity responses. Front Immunol 2022, 13, 1016179. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Cholkar, K.; Mitra, A.K. Recent developments in protein and peptide parenteral delivery approaches. Ther Deliv 2014, 5, 337–365. [Google Scholar] [CrossRef]
- Jarvi, N.L.; Balu-Iyer, S.V. Immunogenicity Challenges Associated with Subcutaneous Delivery of Therapeutic Proteins. BioDrugs 2021, 35, 125–146. [Google Scholar] [CrossRef]
- Cannon, D.A.; Shan, L.; Du, Q.; Shirinian, L.; Rickert, K.W.; Rosenthal, K.L.; Korade, M., 3rd; van Vlerken-Ysla, L.E.; Buchanan, A.; Vaughan, T.J.; et al. Experimentally guided computational antibody affinity maturation with de novo docking, modelling and rational design. PLoS Comput Biol 2019, 15, e1006980. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.; Subramanian, V.; Jayaraman, A.; Fitzpatrick, E.; Gopal, R.; Pentakota, N.; Rurak, T.; Anand, S.; Viglione, A.; Raman, R.; et al. Enhancing antibody affinity through experimental sampling of non-deleterious CDR mutations predicted by machine learning. Commun Chem 2023, 6, 244. [Google Scholar] [CrossRef]
- Kim, J.; McFee, M.; Fang, Q.; Abdin, O.; Kim, P.M. Computational and artificial intelligence-based methods for antibody development. Trends Pharmacol Sci 2023, 44, 175–189. [Google Scholar] [CrossRef]
- Bostrom, J.; Lee, C.V.; Haber, L.; Fuh, G. Improving antibody binding affinity and specificity for therapeutic development. Methods Mol Biol 2009, 525, 353–376. [Google Scholar] [CrossRef]
- Parkinson, J.; Hard, R.; Wang, W. The RESP AI model accelerates the identification of tight-binding antibodies. Nat Commun 2023, 14, 454. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, Z.; Zhou, Y. AbAgIntPre: A deep learning method for predicting antibody-antigen interactions based on sequence information. Front Immunol 2022, 13, 1053617. [Google Scholar] [CrossRef]
- Hie, B.L.; Shanker, V.R.; Xu, D.; Bruun, T.U.J.; Weidenbacher, P.A.; Tang, S.; Wu, W.; Pak, J.E.; Kim, P.S. Efficient evolution of human antibodies from general protein language models. Nat Biotechnol 2024, 42, 275–283. [Google Scholar] [CrossRef]
- Boder, E.T.; Wittrup, K.D. Yeast surface display for screening combinatorial polypeptide libraries. Nat Biotechnol 1997, 15, 553–557. [Google Scholar] [CrossRef] [PubMed]
- McCafferty, J.; Griffiths, A.D.; Winter, G.; Chiswell, D.J. Phage antibodies: filamentous phage displaying antibody variable domains. Nature 1990, 348, 552–554. [Google Scholar] [CrossRef]
- Adams, R.M.; Mora, T.; Walczak, A.M.; Kinney, J.B. Measuring the sequence-affinity landscape of antibodies with massively parallel titration curves. Elife 2016, 5. [Google Scholar] [CrossRef]
- Wu, N.C.; Dai, L.; Olson, C.A.; Lloyd-Smith, J.O.; Sun, R. Adaptation in protein fitness landscapes is facilitated by indirect paths. Elife 2016, 5. [Google Scholar] [CrossRef]
- Kuroda, D.; Tsumoto, K. Antibody Affinity Maturation by Computational Design. Methods Mol Biol 2018, 1827, 15–34. [Google Scholar] [CrossRef]
- Wei, J.; Yang, Y.; Wang, G.; Liu, M. Current landscape and future directions of bispecific antibodies in cancer immunotherapy. Front Immunol 2022, 13, 1035276. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Mo, Y.; Tang, M.; Shen, J.; Qi, Y.; Zhao, W.; Huang, Y.; Xu, Y.; Qian, C. Bispecific Antibodies: From Research to Clinical Application. Front Immunol 2021, 12, 626616. [Google Scholar] [CrossRef] [PubMed]
- Keri, D.; Walker, M.; Singh, I.; Nishikawa, K.; Garces, F. Next generation of multispecific antibody engineering. Antib Ther 2024, 7, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.; Stein, A.; Gokbuget, N.; Fielding, A.K.; Schuh, A.C.; Ribera, J.M.; Wei, A.; Dombret, H.; Foa, R.; Bassan, R.; et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N Engl J Med 2017, 376, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies (Basel) 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Hatano, R.; Itoh, T.; Otsuka, H.; Okamoto, S.; Komiya, E.; Iwata, S.; Aune, T.M.; Dang, N.H.; Kuwahara-Arai, K.; Ohnuma, K.; et al. Characterization of novel anti-IL-26 neutralizing monoclonal antibodies for the treatment of inflammatory diseases including psoriasis. MAbs 2019, 11, 1428–1442. [Google Scholar] [CrossRef]
- Spiess, C.; Zhai, Q.; Carter, P.J. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol Immunol 2015, 67, 95–106. [Google Scholar] [CrossRef]
- Mason, D.M.; Friedensohn, S.; Weber, C.R.; Jordi, C.; Wagner, B.; Meng, S.M.; Ehling, R.A.; Bonati, L.; Dahinden, J.; Gainza, P.; et al. Optimization of therapeutic antibodies by predicting antigen specificity from antibody sequence via deep learning. Nat Biomed Eng 2021, 5, 600–612. [Google Scholar] [CrossRef]
- Ahmad, S.; Kumar, V.; Ramanand, K.B.; Rao, N.M. Probing protein stability and proteolytic resistance by loop scanning: a comprehensive mutational analysis. Protein Sci 2012, 21, 433–446. [Google Scholar] [CrossRef]
- Stromstedt, A.A.; Pasupuleti, M.; Schmidtchen, A.; Malmsten, M. Evaluation of strategies for improving proteolytic resistance of antimicrobial peptides by using variants of EFK17, an internal segment of LL-37. Antimicrob Agents Chemother 2009, 53, 593–602. [Google Scholar] [CrossRef]
- Che Hussian, C.H.A.; Leong, W.Y. Thermostable enzyme research advances: a bibliometric analysis. J Genet Eng Biotechnol 2023, 21, 37. [Google Scholar] [CrossRef]
- Zhang, X.F.; Yang, G.Y.; Zhang, Y.; Xie, Y.; Withers, S.G.; Feng, Y. A general and efficient strategy for generating the stable enzymes. Sci Rep 2016, 6, 33797. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Tsai, C.J.; Nussinov, R. Factors enhancing protein thermostability. Protein Eng 2000, 13, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Iannuzzelli, J.A.; Bacik, J.P.; Moore, E.J.; Shen, Z.; Irving, E.M.; Vargas, D.A.; Khare, S.D.; Ando, N.; Fasan, R. Tuning Enzyme Thermostability via Computationally Guided Covalent Stapling and Structural Basis of Enhanced Stabilization. Biochemistry 2022, 61, 1041–1054. [Google Scholar] [CrossRef]
- Lucana, M.C.; Arruga, Y.; Petrachi, E.; Roig, A.; Lucchi, R.; Oller-Salvia, B. Protease-Resistant Peptides for Targeting and Intracellular Delivery of Therapeutics. Pharmaceutics 2021, 13. [Google Scholar] [CrossRef]
- Turner, P.; Mamo, G.; Karlsson, E.N. Potential and utilization of thermophiles and thermostable enzymes in biorefining. Microb Cell Fact 2007, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Persano, S.; Shen, H.; Zhao, Y.; Blanco, E.; Ferrari, M.; Wolfram, J. Strategies for improving drug delivery: nanocarriers and microenvironmental priming. Expert Opin Drug Deliv 2017, 14, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Do, M.A.; Levy, D.; Brown, A.; Marriott, G.; Lu, B. Targeted delivery of lysosomal enzymes to the endocytic compartment in human cells using engineered extracellular vesicles. Sci Rep 2019, 9, 17274. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, M.; Zabit, S.; Hauser, N.; Farouz, S.; Melloul, O.; Hirbawi, J.; Lorberboum-Galski, H. TAT for Enzyme/Protein Delivery to Restore or Destroy Cell Activity in Human Diseases. Life (Basel) 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Na, J.; Liu, X.; Wu, P. Different Targeting Ligands-Mediated Drug Delivery Systems for Tumor Therapy. Pharmaceutics 2024, 16. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.B. Glyco-engineering strategies for the development of therapeutic enzymes with improved efficacy for the treatment of lysosomal storage diseases. BMB Rep 2015, 48, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Dozier, J.K.; Distefano, M.D. Site-Specific PEGylation of Therapeutic Proteins. Int J Mol Sci 2015, 16, 25831–25864. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Guo, S.; Ren, X.; Wu, Z.; Liu, S.; Yao, X. Current Strategies for Exosome Cargo Loading and Targeting Delivery. Cells 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Modery-Pawlowski, C.L.; Menegatti, S.; Kumar, S.; Vogus, D.R.; Tian, L.L.; Chen, M.; Squires, T.M.; Sen Gupta, A.; Mitragotri, S. Platelet-like nanoparticles: mimicking shape, flexibility, and surface biology of platelets to target vascular injuries. ACS Nano 2014, 8, 11243–11253. [Google Scholar] [CrossRef] [PubMed]
- Lorent, J.H.; Levental, K.R.; Ganesan, L.; Rivera-Longsworth, G.; Sezgin, E.; Doktorova, M.; Lyman, E.; Levental, I. Plasma membranes are asymmetric in lipid unsaturation, packing and protein shape. Nat Chem Biol 2020, 16, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Muro, S. Challenges in design and characterization of ligand-targeted drug delivery systems. J Control Release 2012, 164, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Sockolosky, J.T.; Trotta, E.; Parisi, G.; Picton, L.; Su, L.L.; Le, A.C.; Chhabra, A.; Silveria, S.L.; George, B.M.; King, I.C.; et al. Selective targeting of engineered T cells using orthogonal IL-2 cytokine-receptor complexes. Science 2018, 359, 1037–1042. [Google Scholar] [CrossRef]
- Quijano-Rubio, A.; Bhuiyan, A.M.; Yang, H.; Leung, I.; Bello, E.; Ali, L.R.; Zhangxu, K.; Perkins, J.; Chun, J.H.; Wang, W.; et al. A split, conditionally active mimetic of IL-2 reduces the toxicity of systemic cytokine therapy. Nat Biotechnol 2023, 41, 532–540. [Google Scholar] [CrossRef]
- Corria-Osorio, J.; Carmona, S.J.; Stefanidis, E.; Andreatta, M.; Ortiz-Miranda, Y.; Muller, T.; Rota, I.A.; Crespo, I.; Seijo, B.; Castro, W.; et al. Orthogonal cytokine engineering enables novel synthetic effector states escaping canonical exhaustion in tumor-rejecting CD8(+) T cells. Nat Immunol 2023, 24, 869–883. [Google Scholar] [CrossRef] [PubMed]
- Li, A.W.; Lim, W.A. Engineering cytokines and cytokine circuits. Science 2020, 370, 1034–1035. [Google Scholar] [CrossRef] [PubMed]
- Moraga, I.; Spangler, J.B.; Mendoza, J.L.; Gakovic, M.; Wehrman, T.S.; Krutzik, P.; Garcia, K.C. Synthekines are surrogate cytokine and growth factor agonists that compel signaling through non-natural receptor dimers. Elife 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res 2014, 2014, 149185. [Google Scholar] [CrossRef] [PubMed]
- Rybchenko, V.S.; Aliev, T.K.; Panina, A.A.; Kirpichnikov, M.P.; Dolgikh, D.A. Targeted Cytokine Delivery for Cancer Treatment: Engineering and Biological Effects. Pharmaceutics 2023, 15. [Google Scholar] [CrossRef]
- Fu, Y.; Tang, R.; Zhao, X. Engineering cytokines for cancer immunotherapy: a systematic review. Front Immunol 2023, 14, 1218082. [Google Scholar] [CrossRef] [PubMed]
- Nirschl, C.J.; Brodkin, H.R.; Hicklin, D.J.; Ismail, N.; Morris, K.; Seidel-Dugan, C.; Steiner, P.; Steuert, Z.; Sullivan, J.M.; Tyagi, E.; et al. Discovery of a Conditionally Activated IL-2 that Promotes Antitumor Immunity and Induces Tumor Regression. Cancer Immunol Res 2022, 10, 581–596. [Google Scholar] [CrossRef]
- Vasic, V.; Buldun, C.; Ritz, M.; Dickopf, S.; Georges, G.J.; Spick, C.; Peuker, A.; Meier, T.; Mayer, K.; Brinkmann, U. Targeted chain-exchange-mediated reconstitution of a split type-I cytokine for conditional immunotherapy. MAbs 2023, 15, 2245111. [Google Scholar] [CrossRef] [PubMed]
- Deckers, J.; Anbergen, T.; Hokke, A.M.; de Dreu, A.; Schrijver, D.P.; de Bruin, K.; Toner, Y.C.; Beldman, T.J.; Spangler, J.B.; de Greef, T.F.A.; et al. Engineering cytokine therapeutics. Nat Rev Bioeng 2023, 1, 286–303. [Google Scholar] [CrossRef]
- Pires, I.S.; Hammond, P.T.; Irvine, D.J. Engineering Strategies for Immunomodulatory Cytokine Therapies - Challenges and Clinical Progress. Adv Ther (Weinh) 2021, 4. [Google Scholar] [CrossRef]
- Bottens, R.A.; Yamada, T. Cell-Penetrating Peptides (CPPs) as Therapeutic and Diagnostic Agents for Cancer. Cancers (Basel) 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Trabulo, S.; Cardoso, A.L.; Mano, M.; De Lima, M.C. Cell-Penetrating Peptides-Mechanisms of Cellular Uptake and Generation of Delivery Systems. Pharmaceuticals (Basel) 2010, 3, 961–993. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Bi, Y.; Zhang, H.; Dong, S.; Teng, L.; Lee, R.J.; Yang, Z. Cell-Penetrating Peptides in Diagnosis and Treatment of Human Diseases: From Preclinical Research to Clinical Application. Front Pharmacol 2020, 11, 697. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.G.; Sayers, E.J.; He, L.; Narayan, R.; Williams, T.L.; Mills, E.M.; Allemann, R.K.; Luk, L.Y.P.; Jones, A.T.; Tsai, Y.H. Cell-penetrating peptide sequence and modification dependent uptake and subcellular distribution of green florescent protein in different cell lines. Sci Rep 2019, 9, 6298. [Google Scholar] [CrossRef] [PubMed]
- Khairkhah, N.; Namvar, A.; Bolhassani, A. Application of Cell Penetrating Peptides as a Promising Drug Carrier to Combat Viral Infections. Mol Biotechnol 2023, 65, 1387–1402. [Google Scholar] [CrossRef]
- Ouyang, J.; Sheng, Y.; Wang, W. Recent Advances of Studies on Cell-Penetrating Peptides Based on Molecular Dynamics Simulations. Cells 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wu, J.; Shi, J.; Farokhzad, O.C. Nanotechnology for protein delivery: Overview and perspectives. J Control Release 2016, 240, 24–37. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Habibi, N.; Mauser, A.; Ko, Y.; Lahann, J. Protein Nanoparticles: Uniting the Power of Proteins with Engineering Design Approaches. Adv Sci (Weinh) 2022, 9, e2104012. [Google Scholar] [CrossRef]
- Yau, A.; Lee, J.; Chen, Y. Nanomaterials for Protein Delivery in Anticancer Applications. Pharmaceutics 2021, 13. [Google Scholar] [CrossRef]
- Begarani, F.; Cassano, D.; Margheritis, E.; Marotta, R.; Cardarelli, F.; Voliani, V. Silica-Based Nanoparticles for Protein Encapsulation and Delivery. Nanomaterials (Basel) 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res 2013, 73, 1524–1535. [Google Scholar] [CrossRef] [PubMed]
- AlSawaftah, N.M.; Awad, N.S.; Pitt, W.G.; Husseini, G.A. pH-Responsive Nanocarriers in Cancer Therapy. Polymers (Basel) 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Shi, X.; Tian, Y.; Gao, F. pH-Responsive Polymer Nanomaterials for Tumor Therapy. Front Oncol 2022, 12, 855019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, Y.; Gillies, R.J. Tumor pH and its measurement. J Nucl Med 2010, 51, 1167–1170. [Google Scholar] [CrossRef] [PubMed]
- Karanth, H.; Murthy, R.S. pH-sensitive liposomes--principle and application in cancer therapy. J Pharm Pharmacol 2007, 59, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yue, R.; Guan, G.; Zhang, C.; Zhou, Y.; Song, G. Recent development of pH-responsive theranostic nanoplatforms for magnetic resonance imaging-guided cancer therapy. Exploration (Beijing) 2023, 3, 20220002. [Google Scholar] [CrossRef] [PubMed]
- Dyer, R.P.; Weiss, G.A. Making the cut with protease engineering. Cell Chem Biol 2022, 29, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Bleuez, C.; Koch, W.F.; Urbach, C.; Hollfelder, F.; Jermutus, L. Exploiting protease activation for therapy. Drug Discov Today 2022, 27, 1743–1754. [Google Scholar] [CrossRef]
- Song, P.; Zhang, X.; Wang, S.; Xu, W.; Wang, F.; Fu, R.; Wei, F. Microbial proteases and their applications. Front Microbiol 2023, 14, 1236368. [Google Scholar] [CrossRef]
- Pan, L.; Sun, J.; Qi, H.; Han, M.; Dai, Q.; Xu, J.; Yao, S.; Li, Q.; Wei, L.; Zhao, T. Dead-zone-compensated design as general method of flow field optimization for redox flow batteries. Proc Natl Acad Sci U S A 2023, 120, e2305572120. [Google Scholar] [CrossRef] [PubMed]
- Stein, V.; Alexandrov, K. Synthetic protein switches: design principles and applications. Trends Biotechnol 2015, 33, 101–110. [Google Scholar] [CrossRef]
- Liang, Y.; Qie, Y.; Yang, J.; Wu, R.; Cui, S.; Zhao, Y.; Anderson, G.J.; Nie, G.; Li, S.; Zhang, C. Programming conformational cooperativity to regulate allosteric protein-oligonucleotide signal transduction. Nat Commun 2023, 14, 4898. [Google Scholar] [CrossRef] [PubMed]
- Alberstein, R.G.; Guo, A.B.; Kortemme, T. Design principles of protein switches. Curr Opin Struct Biol 2022, 72, 71–78. [Google Scholar] [CrossRef]
- Sekhon, H.; Ha, J.H.; Loh, S.N. Enhancing response of a protein conformational switch by using two disordered ligand binding domains. Front Mol Biosci 2023, 10, 1114756. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, L.B.; Stratton, M.R. Mutational signatures: the patterns of somatic mutations hidden in cancer genomes. Curr Opin Genet Dev 2014, 24, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.; Tillegreen, C.B.; Petranovic, D. Innovation trends in industrial biotechnology. Trends Biotechnol 2022, 40, 1160–1172. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Penet, M.F.; Chen, Z.; Li, C.; Winnard, P.T., Jr.; Bhujwalla, Z.M. Prodrug enzymes and their applications in image-guided therapy of cancer: tracking prodrug enzymes to minimize collateral damage. Drug Deliv Transl Res 2012, 2, 22–30. [Google Scholar] [CrossRef]
- Mann, D.L. Synthetic Biology, Directed Evolution, and the Rational Design of New Cardiovascular Therapeutics: Are We There Yet? JACC Basic Transl Sci 2023, 8, 905–906. [Google Scholar] [CrossRef]
- de la Fuente, M.; Lombardero, L.; Gomez-Gonzalez, A.; Solari, C.; Angulo-Barturen, I.; Acera, A.; Vecino, E.; Astigarraga, E.; Barreda-Gomez, G. Enzyme Therapy: Current Challenges and Future Perspectives. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Hennigan, J.N.; Lynch, M.D. The past, present, and future of enzyme-based therapies. Drug Discov Today 2022, 27, 117–133. [Google Scholar] [CrossRef] [PubMed]
- Nasibullin, I.; Smirnov, I.; Ahmadi, P.; Vong, K.; Kurbangalieva, A.; Tanaka, K. Synthetic prodrug design enables biocatalytic activation in mice to elicit tumor growth suppression. Nat Commun 2022, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Michailidou, F. Engineering of Therapeutic and Detoxifying Enzymes. Angew Chem Int Ed Engl 2023, 62, e202308814. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, F.Z.; Arnold, F.H. Opportunities and Challenges for Machine Learning-Assisted Enzyme Engineering. ACS Cent Sci 2024, 10, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.S.; Boyken, S.E.; Baker, D. The coming of age of de novo protein design. Nature 2016, 537, 320–327. [Google Scholar] [CrossRef]
- Korendovych, I.V.; DeGrado, W.F. De novo protein design, a retrospective. Q Rev Biophys 2020, 53, e3. [Google Scholar] [CrossRef] [PubMed]
- Grassmann, G.; Miotto, M.; Desantis, F.; Di Rienzo, L.; Tartaglia, G.G.; Pastore, A.; Ruocco, G.; Monti, M.; Milanetti, E. Computational Approaches to Predict Protein-Protein Interactions in Crowded Cellular Environments. Chem Rev 2024, 124, 3932–3977. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, F.; Markgren, J.; Hedenqvist, M.; Johansson, E. Modeling to Understand Plant Protein Structure-Function Relationships-Implications for Seed Storage Proteins. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Avery, C.; Patterson, J.; Grear, T.; Frater, T.; Jacobs, D.J. Protein Function Analysis through Machine Learning. Biomolecules 2022, 12. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).