Submitted:
24 July 2024
Posted:
24 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Materials
2.3. Instrumentations
2.4. Extraction and Separation Procedures
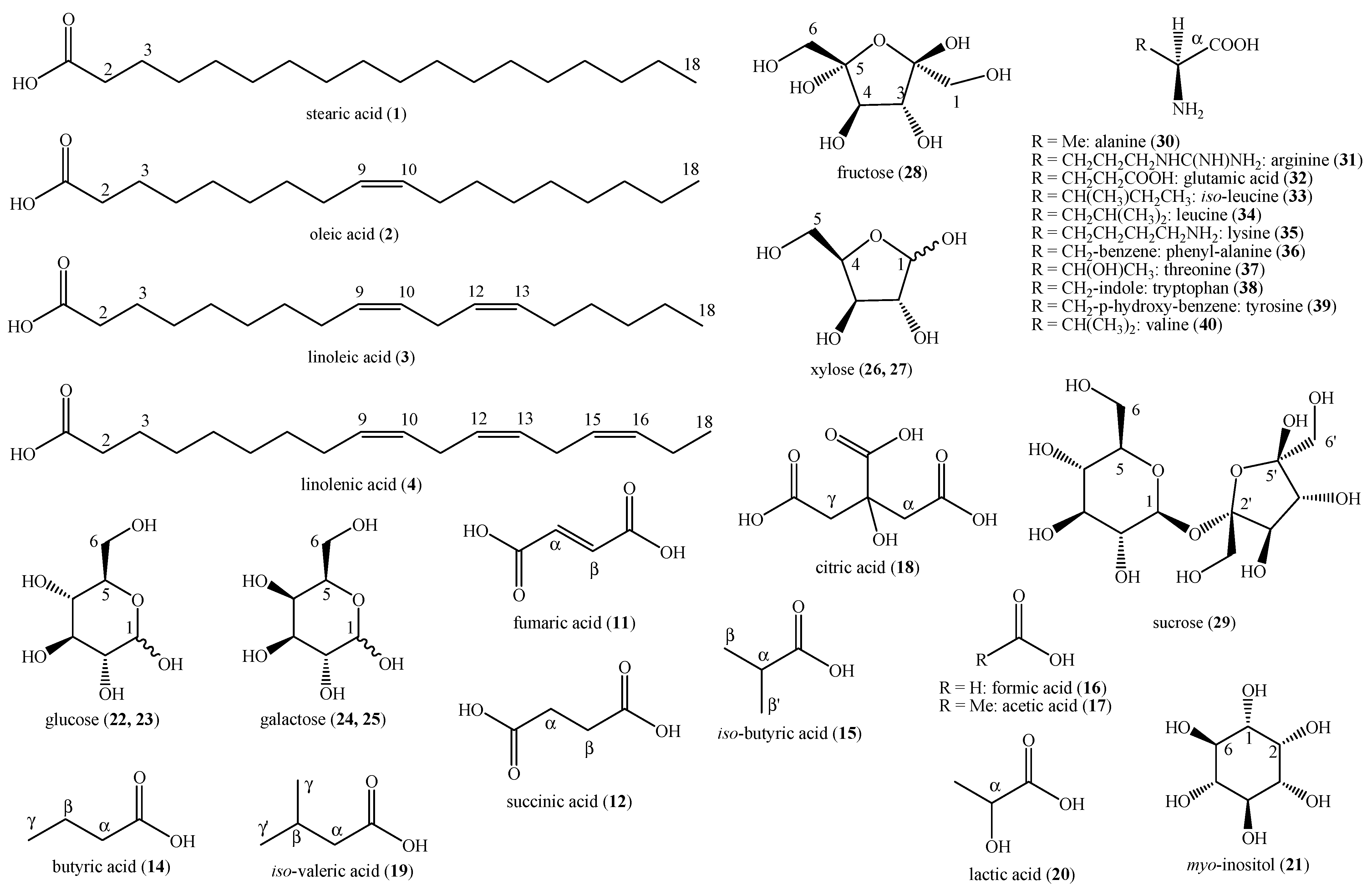
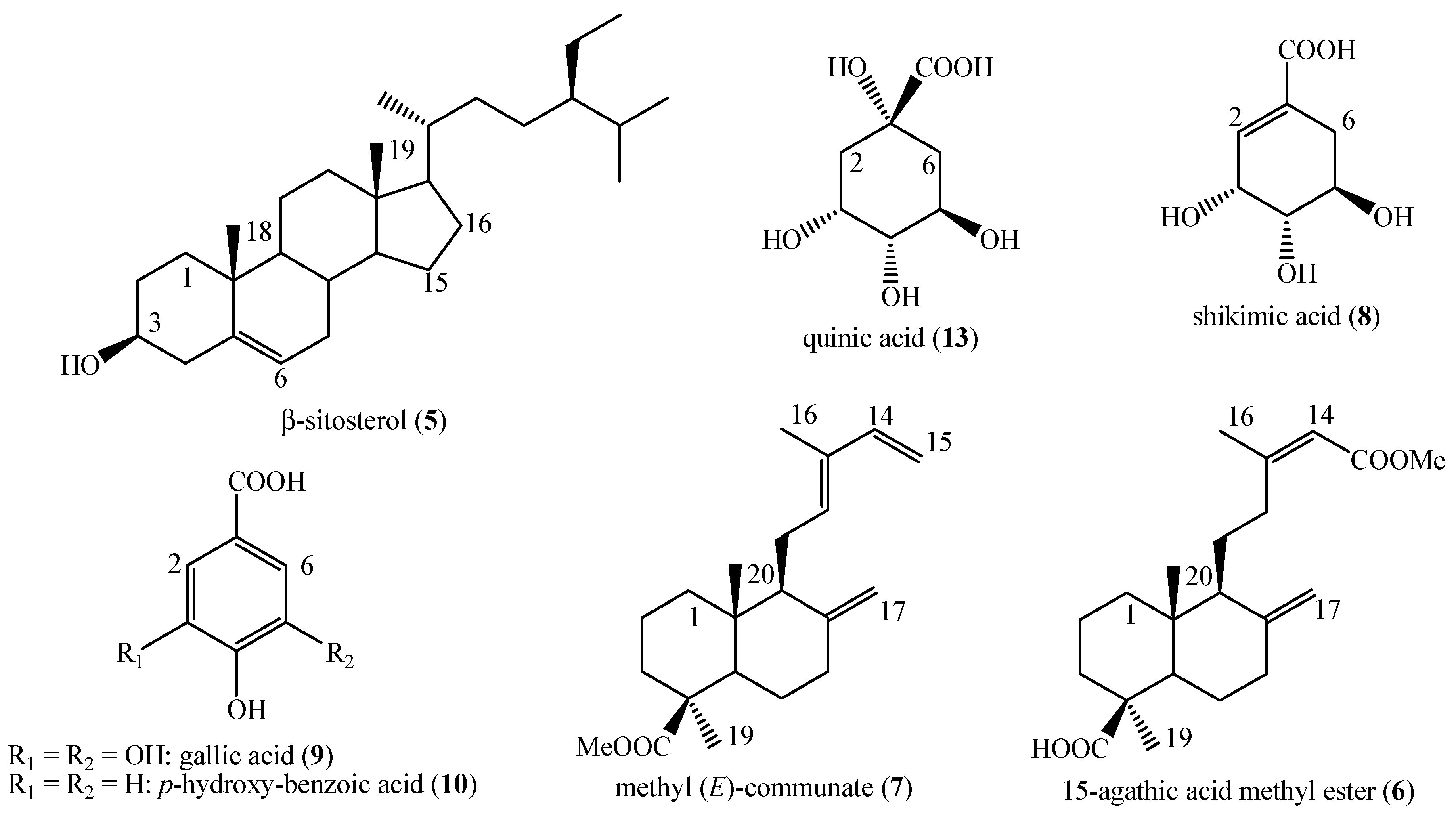
2.5. NMR and MS Data of the Identified Compounds
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Smith, I.A.; Butler, D. The Bunya in Queensland's Forests. Queensland Rev. 2002, 9(2), 31–38. [Google Scholar] [CrossRef]
- Frezza, C.; Venditti, A.; De Vita, D.; Toniolo, C.; Franceschin, M.; Ventrone, A.; Tomassini, L.; Foddai, S.; Guiso, M.; Nicoletti, M.; Bianco, A.; Serafini, M. Phytochemistry, chemotaxonomy, and biological activities of the Araucariaceae family - a review. Plants, 2020, 9, 888. [Google Scholar] [CrossRef] [PubMed]
- Moura Nadolny, J.; Best, O.; Netzel, G.; Shewan, H.M.; Thi Phan, A.D.; Smyth, H.E.; Stokes, J.R. Chemical composition of bunya nuts (Araucaria bidwillii) compared to Araucaria angustifolia and Araucaria araucana species. Food Res. Int. 2023, 163, 112269. [Google Scholar] [CrossRef] [PubMed]
- Huth, J. Introducing the Bunya Pine – A Noble Denizen of the Scrub. Queensland Rev. 2002, 9(2), 7–20. [Google Scholar] [CrossRef]
- Sciubba, F.; Di Cocco, M.E.; Gianferri, R.; Capuani, G.; De Salvador, F.R.; Fontanari, M.; Gorietti, D.; Delfini, M. Nuclear magnetic resonance-based metabolic comparative analysis of two apple varieties with different resistances to apple scab attacks. J. Agric. Food Chem. 2015, 63, 8339–8347. [Google Scholar] [CrossRef] [PubMed]
- Sciubba, F.; Capuani, G.; Di Cocco, M.E.; Avanzato, D.; Delfini, M. Nuclear magnetic resonance analysis of water soluble metabolites allows the geographic discrimination of pistachios (Pistacia vera). Food Res. Int. 2014, 62, 66–73. [Google Scholar] [CrossRef]
- Frezza, C.; Sciubba, F.; De Vita, D.; Toniolo, C.; Foddai, S.; Tomassini, L. , Petrucci, R.; Bianco, A.; Serafini, M. Non-volatile compounds from Araucaria columnaris (G.Forst.) Hook leaves. Biochem. Syst. Ecol. 2022, 103, 104430. [Google Scholar] [CrossRef]
- Venditti, A.; Frezza, C.; Sciubba, F.; Foddai, S.; Serafini, M.; Bianco, A. Terpenoids and more polar compounds from the male cones of Wollemia nobilis. Chem. Biodiversity 2017, 14, e1600332. [Google Scholar] [CrossRef] [PubMed]
- Rehan, M. Biosynthesis of diverse class flavonoids via shikimate and phenylpropanoid pathway. In Bioactive compounds - biosynthesis, characterization and applications; Queiroz Zepka, L., Casagrande do Nascimento, T., Jacob-Lopes, T., Eds. IntechOpen, London, UK, 2021; pp. 75392.
- Gupta, E. B-Sitosterol: predominant phytosterol of therapeutic potential. In Innovations in Food Technology; Mishra, P., Mishra, R.R., Adetunji, C.O. (eds). Springer, Singapore. pp. 465-477.
- Candeias, N.R.; Assoah, B.; Simeonov, S.P. Production and synthetic modifications of shikimic acid. Chem. Rev. 2018, 118, 10458–10550. [Google Scholar] [CrossRef] [PubMed]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: a versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Manuja, R.; Sachdeva, S.; Jain, A.; Chaudhary, J. A Comprehensive review on biological activities of p-hydroxy benzoic acid and its derivatives. Int. J. Pharm. Sci. Rev. Res. 2013, 22(2), 109–115. [Google Scholar]
- Benali, T.; Bakrim, S. , Ghchime, R., Benkhaira, N.; El Omari, N.; Balahbib, A.; Taha, D.; Zengin, G.; Hasan, M.M.; Bibi, S.; Bouyahya, A. Pharmacological insights into the multifaceted biological properties of quinic acid. Biotechnol. Gen. Engineer. Rev. 1–30. [CrossRef]
- Carman, R.M.; Marty, R.A. Diterpenoids* IX. Agathis microstachya oleoresin. Aust. J. Chem. 1966, 19, 2403–2406. [Google Scholar] [CrossRef]
- Poinar Jr., G. O. Resinites, with examples from New Zealand and Australia. Fuel Process. Technol. 1991, 28(2), 135–148. [Google Scholar] [CrossRef]
- Sahu, B.; Bhardwaj, N.; Chatterjee, E.; Dey, B.; Tripathi, N.; Goel, B.; Kushwaha, M. , Kumar, B. ; Singh, B.; Guru, S.K.; Jain, S.K. LCMS-DNP based dereplication of Araucaria cunninghamii Mudie gum-resin: identification of new cytotoxic labdane diterpene, Nat. Prod. Res. 2022, 36(24), 6207–6214. [Google Scholar] [PubMed]
- Frezza, C.; De Vita, D.; Fonti, L.; Giampaoli, O.; Dal Bosco, C.; Sciubba, F.; Venditti, A.; Scintu, C.; Attorre, F. Secondary metabolites of Araucaria cunninghamii Mudie from central Italy. Plant Biosyst. 2024, In press. [CrossRef]
- Patial, P.K.; Cannoo, D.S. Phytochemical profile, antioxidant potential and DFT study of Araucaria columnaris (G. Forst.) Hook. Branch extracts. Nat. Prod. Res. 2021, 35(22), 4611-4615.
- Frezza, C.; Venditti, A.; Scandurra, C.; Ciccòla, A.; Serafini, I.; Sciubba, F.; Foddai, S.; Franceschin, M.; Bianco, A.; Serafini, M. Phytochemical profile of Wollemia nobilis half-matured female cones and their potential ethnopharmacological and nutraceutical activities. J. Agric. Sci. Technol. A 2018, 8, 162–170. [Google Scholar]
- Venditti, A.; Frezza, C.; Vincenti, F.; Brodella, A.; Sciubba, F.; Montesano, C.; Franceschin, M.; Sergi, M.; Foddai, S.; Di Cocco, M.E.; Curini, R.; Delfini, M.; Bianco, A.; Serafini, M. A syn-ent-labdadiene derivative with a rare spiro-β-lactone function from the male cones of Wollemia nobilis. Phytochemistry 2019, 158, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Venditti, A.; Frezza, C.; Rossi, G.; Sciubba, F.; Ornano, L.; De Vita, D.; Toniolo, C.; Tomassini, L.; Foddai, S.; Nicoletti, M.; Di Cocco, M.E.; Bianco, A.; Serafini, M. A new diterpene and other compounds from the unripe female cones of Wollemia nobilis. Nat. Prod. Res. 2021, 35(21), 3839–3849. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




