Submitted:
22 July 2024
Posted:
24 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Background
1.2. Significance of CNDs in Drug Delivery Systems
1.2. Significance of CNDs in Drug Delivery Systems
2. Phytomedicine in Drug Delivery
2.1. Carbon Nanodots (CNDs): Catalysts of Transformation
2.1.1. Precision Delivery for Optimal Impact
2.1.2. Addressing Bioavailability Challenges
2.1.3. Guardians of Therapeutic Efficacy
2.2. Overview of Phytomedicine
2.2.1. Historical Roots and Evolution
2.2.2. Bioactive Compounds in Phytomedicine
2.2.3. Catalysts of Transformation: Carbon Nanodots (CNDs)
2.2.4. Precision Delivery for Optimal Impact
2.3. Addressing Bioavailability Challenges
2.3.1. Advantages of Phytomedicine
2.3.2. Challenges of Phytomedicine
2.4. Advantages of Carbon Nanodots-Based Drug Delivery for Phytomedicine
2.5. Challenges of Carbon Nanodots-Based Drug Delivery for Phytomedicine
2.6. Role of Drug Delivery Systems in Enhancing Phytomedicine Efficacy
2.6.1. Enhanced Bioavailability
2.6.2. Targeted Drug Delivery
2.6.3. Controlled Release
2.6.4. Synergistic Effects
2.6.5. Overcoming Bioavailability Challenges
2.6.6. Improving Cellular Uptake
2.6.7. Biocompatibility and Reduced Toxicity
2.6.8. Personalized Medicine Approach
3. Carbon Nanodots: Properties and Synthesis
3.1. Sources, Structures and Properties of Carbon Nanodots
3.2. Synthesis and Production of Carbon Nanodots
3.3. Surface Modification and Functionalization of Carbon Nanodots
4. Biomedical Applications of CNDs
| Sources of carbon | Preparation techniques | Size (nm) |
Quantum Yield (%) | Color | Excitation wavelength (nm) | Application | Ref |
|---|---|---|---|---|---|---|---|
| rose-heart radish | Hydrothermal | 1.2- 6.0 | 13.6 | Blue | 330 | Sensing Fe+3 | [208] |
| Prunus persica (peach) | Hydrothermal | 8 | 15 | Blue | 325 | Cellular imaging and oxygen reduction reaction | [209] |
| Trapa bispinosa peel | Hydrothermal | 5-10 | 0.1 | Green | 365 | Cellular imaging | [210] |
| Saccharum officinarum juice | Hydrothermal | 3 | 5.67 | Blue | 365 | Cellular imaging of bacteria and yeast | [211] |
| Unripe fruit extract of Prunus mume | Hydrothermal | 9 | 16 | Blue | 355 | Cellular imaging | [212] |
| Apple juice | Hydrothermal | 4.5 | 4.27 | Blue | 360 | Imaging of mycobacterium and fungal cells | [95] |
| Chionanthus retusus fruit extract | Hydrothermal | 5 | 9 | Blue | 365 | Metal ion sensing and imaging of fungal cells | [213] |
| Pseudo-stem of banana | Hydrothermal | 1-3 | 48 | Green | - | Sensing Fe+3, Imaging of Hela and MCF-7 cells* | [214] |
| Honey | Solvothermal | 2 | 19.18 | Blue | 365 | Sensing Fe+3 and imaging of Hep-2 and Hela cells* | [215] |
| Garlic | Hydrothermal | 11 | 17.5 | Blue | 365 | Cellular imaging and free radical scavenging | [216] |
| Sweet potato | Hydrothermal | 3.39 | 8.64 | Blue | 365 | Fe+3 sensing and cellular imaging | [217] |
| Walnut shell | Hydrothermal | 3.4 | - | Green | 360-460 | Cellular imaging | [218] |
| Glycerine and PEG | Microwave | 3-4 | Blue | 365 | Nitrite sensing | [182] | |
| Bloomed algae | Microwave | 8 | 13 | Blue | 365 | In Vitro imaging | [219] |
| Tissue paper | Microwave | 4.2 | 93 | Blue | - | Determination of Glutathione | [220] |
| Kidney beans | Hydrothermal | 20-30 | 8 | green | 340 | Cellular imaging | [221] |
| Water Chestnut and onion | Hydrothermal | 3.5 | 12 | Green-Blue | 400-600 | Sensing of Cu (II) and Imaging of Coenzyme A | [222] |
| Food waste-derived | Ultrasonic | 4.6 | 2.85 | Blue-Red | 400-470 | In vitro bioimaging | [223] |
| Beer | Gel filtration chromatography | 2.5 | 7.39 | Blue | 360 | Breast cancer cell imaging and drug delivery | [224] |
| Lignin biomass | Ultrasonic and hydrothermal | 2-6 | 21 | Blue Green Red |
310-420-540 | Cellular imaging | [225] |
| Onion waste | Hydrothermal | 15 | 28 | Blue Green Red |
408-488-561- | Sensoring of Fe3+ and cellular imaging | [226] |
| Bee pollens | Hydrothermal | 1-2 | 6-12.8 | Blue-green | 365 | Cellular imaging and catalysis | [227] |
| Coriander leaves | Hydrothermal | 2.4 | 6.48 | green | 320 | Sensoring of Fe3+ and cellular imaging | [228] |
| Grape seed | Microwave | 1-8 | 31.79 | multicolor | 250-550 | Nucleus imaging and pH sensing | [229] |
| Carrot | Hydrothermal | 2.3 | 7.60 | Blue | 365 | Drug delivery | [230] |
| Sugarcane molasses | Hydrothermal | 1.9 | 5.8 | Blue | 365 | Sensoring of Fe3+ and cellular imaging | [231] |
| Mango leaves | Microwave | 2-8 | - | Red | 325 | Cellular imaging and temperature sensors | [232] |
| Papaya juice | Hydrothermal | 3 | 7 | Blue Green Red |
365 488 561 |
Cellular imaging | [233] |
| Latex | Microwave | 2-8 | - | green | 360-520 | Metal sensing and cellular imaging | [234] |
| Mangosteen pulp | Hydrothermal | 5 | - | Blue | 330 | Sensoring of Fe3+ and cellular imaging | [235] |
| Lotus root | Microwave | 9.41 | 19 | Blue | 360 | Heavy metal ion detection and cellular imaging | [236] |
| Date kernel | Hydrothermal | 2.5 | 12.5 | Blue | 365 | Sensing of drugs and cellular imaging | [237] |
| Winter melon | Hydrothermal | 4.5–5.2 | 7.51 | Blue | 360 | Cellular imaging | [238] |
5. Applications of Carbon Nanodots in Drug Delivery
5.1. Overview of Drug Delivery Systems
5.2. Role of Carbon Nanodots in Enhancing Drug Delivery
5.3. Mechanisms of Drug Delivery Using Carbon Nanodots
5.3.1. Increasing Bioavailability and Solubility
5.3.2. Decreased Toxicity and Biocompatibility
5.3.3. Imaging and Tracking in Real Time
5.3.4. Functionalization of Surfaces
5.3.5. Nanoscale Aspects for Improved Cellular Communication
5.3.6. Transport of Different Therapeutic Agents
5.3.7. Mechanisms for Targeted Drug Delivery
6. Phytomedicine-Loaded Carbon Nanodots
Synthesis and Characterization
Improved Bioavailability
Targeted Drug Delivery
Controlled Release Kinetics
Biocompatibility and Safety
Intracellular Delivery and Cellular Uptake
Encapsulation of Diverse Phytochemicals
Application in Multicomponent Phytomedicine Formulations
Future Perspectives and Challenges
6.1. Loading Strategies for Phytomedicine
Physical Adsorption: Recent Developments
Covalent Bonding: Progress in Precision
Encapsulation during Nanodot Synthesis: Tailoring Synthesis Conditions
Emerging Trends and Future Directions
6.2. Stability and Bioavailability Enhancement
Enhanced Stability through Nanoengineering
Smart Nanomaterials for Controlled Release
Advances in Nanotoxicology and Biocompatibility
Precision Bioavailability Enhancement
Emerging Trends and Future Directions
6.3. Controlled Release Mechanisms
Advanced Surface Engineering for Tuneable Release
Microfluidic-Assisted Synthesis for Controlled Encapsulation
Temperature-Responsive Systems for On-Demand Release
Advancements in Biodegradable Nanocarriers
Emerging Trends and Future Prospects
7. Polymeric Carbon Nanodots
7.1. Introduction to Polymer Nanocomposites
7.2. Structural Composition
7.3. Various Synthetic Methods of Polymeric Carbon Nanodots
7.3.1. Role of Polymer Nanocomposites in Drug Delivery
7.3.2. Properties of Polymeric Carbon Nanodots
7.4. Applications of Polymeric Carbon Nanodots in Drug Delivery
- Delivery of Drugs with Specificity:
- Imaging and Diagnosis:
- Theranostics:
- pH-Responsive Drug Release:
- Combination Therapy:
- Intracellular Delivery:
7.5. Polymeric Carbon Nanodots' Mechanisms of Drug Delivery
8. Challenges and Future Perspectives
8.1. Current Challenges in Carbon Nanodots-Based Drug Delivery
8.2. Future Directions and Emerging Trends
8.3. Patents and Publications on CNDs
8.3.1. Publications of CNDs
| No | Title | Findings | Results | Discussions | Ref |
|---|---|---|---|---|---|
| 1 | Effect of carbon nano-dots (CNDs) on structural and optical properties of PMMA polymer composite | Strong intermolecular contacts, an improved amorphous phase, well-dispersed CNDs, increased photoluminescence, a shifted refractive index, and well-defined electron transitions were all displayed by the PMMA/CNDs nanocomposite films that were created using the solution cast process. | Amorphous PMMA/CNDs nanocomposite films with enhanced complexation and optical characteristics were created in this work. Improved photoluminescence and UV-Vis absorption point to the material's applicability for photonic devices, LEDs, and other optoelectronic applications. | The stability, optical qualities, and amorphous phase of PMMA were all enhanced by the addition of CNDs. Their promise in nanotechnology devices is highlighted by their enhanced photoluminescence and UV-Vis absorption, which imply suitable for optoelectronic applications including LEDs and photodetectors. | [323] |
| 2 | One-step synthesis and characterization of N doped carbon nanodots for sensing in organic media | The N-doped CNDs have useful applications in organic media without further functionalization due to their high quantum yield, excitation wavelength-dependent emission, and upconversion characteristics. | N-doped CNDs with 78% QY were created from PMA and showed excellent selectivity when it came to detecting nitroaromatic explosives by the quenching of fluorescence in organic environments. | In spite of their remarkable selectivity and possible commercial uses, such as self-cleaning surfaces, bioimaging of hydrophobic structures, and antiwetting, these N-doped CNDs hold great promise for the detection of nitroaromatic explosives. | [324] |
| 3 | pH-dependent synthesis of novel structure-controllable polymer carbon NanoDots with high acidophilic luminescence and super carbon dots assembly for white-light-emitting diodes | Different nanodot structures are produced by pH-dependent synthesis. White light from SCNDs is appropriate for LEDs. Investigation by PL reveals distinct emission paths. For carbon nanodots, theoretical computations reveal information on their electrical properties. | Changing the pH led to different topologies of carbon nanodots. Super-small carbon nanodots (SCNDs) showed promise for LED technology at pH < 1. They radiated white light. Unique emission channels were found via PL research, which improved knowledge of carbon-based fluorescence. | pH regulation provides customised nanodot morphology, which is essential for a variety of uses. The white emission of SCNDs offers opportunities for affordable LEDs. Understanding of carbon-based fluorescence is advanced by insights into PL processes, which direct future study. | [325] |
| 4 | Freestanding luminescent films of nitrogen-rich carbon nanodots toward large-scale phosphor-based white-light-emitting devices | Under UV light, CNDs produced vivid visible light that was appropriate for phosphor applications. Solid-state quenching was avoided via large-scale freestanding luminous films distributed across a polymer matrix, allowing for flexible, scalable, and thermally robust solid-state lighting systems. | Nitrogen-rich carbon nanodots (CNDs) with a restricted size range and a well-developed graphitic structure were produced by carbonising polyacrylamide using an emulsion template. A high quantum yield of 40% was achieved in the fabrication of large-scale luminous films | CNDs with desired features were produced via synthesis using an emulsion-templated carbonisation process. Because polymer matrix dispersion avoided quenching, flexible lighting systems on a vast scale could be made possible. Under realistic circumstances, white LEDs showed steady emission spectra, demonstrating the promise of CND-based solid-state lighting. | [326] |
| 5 | Photoluminescence of argan-waste-derived carbon nanodots embedded in polymer matrices |
Excitation-dependent emission was demonstrated by blue-emitting CND-polymer nanocomposites, with the blue spectrum exhibiting the highest emission. By placing CNDs within optically transparent matrices, PLQY was increased by two to three times, reaching a 29.6% improvement. |
For use in photonic conversion layers on solar PV cells, luminizing carbon nanodots (CNDs) derived from argan waste were distributed in poly(styrene-co-acrylonitrile) and cyclo-olefin copolymer matrices to generate thin films with a 30% PL conversion efficiency. |
After being distributed in transparent polymers, CNDs made from argan waste maintained their long-term luminescence characteristics and increased radiative efficiency by two to three times. Because thin films are easily processed, they may be used as photonic down-conversion layers to improve the efficiency of solar cells, especially when UV light is used. | [327] |
| 6 | Oxidative synthesis of highly fluorescent boron/nitrogen co-doped carbon nanodots enabling detection of photosensitizer and carcinogenic dye | P-CNDs with PEI passivation showed increased fluorescence after being synthesised in a simple manner. Protoporphyrin (PPD) introduction enabled fluorescence switch-off, allowing dye-doped nanoprobes with a limit of detection (LOD) of 9.9 pM−0.37 nM for Sudan red III (SRIII) and 15 pM for PPD. |
The synthesis of boric acid and N-(4-hydroxyphenyl) glycine by hydrothermal oxidative method resulted in the straightforward production of carbon nanodots (CNDs) co-doped with silicon and nitrogen. polyethyleneimine (PEI) surface passivation improved fluorescence and monodispersity, resulting in polymerized CNDs (P-CNDs) with a 23.71% quantum yield. | Highly fluorescent B/N co-doped CNDs are easily synthesised and have potential uses in a number of fields. The surface passivation of PEI enhances monodispersity and fluorescence. PPD and SRIII may be detected with high sensitivity using dye-doped nanoprobes, indicating the possibility of useful sensing and detection applications. |
[328] |
| 7 | Fluorescent nitrogen-doped carbon nanodots synthesized through a hydrothermal method with different isomers | N-oxide group production was aided by the o-PD precursor, whereas "lattice N" functionalities were inserted by hydrothermal synthesis using the m-PD precursor. While strongly quenched in propylene glycol methyl ether acetate (PGMEA), fluorescence was bright in polar solvents. Radiative emission from N-atom substitutions and N- and O-rich edge groups produced an ultrahigh quantum yield. | Using o-, m-, and p-phenylenediamine (PD) isomers, N-functionalized carbon nanodots (CNDs) were created hydrothermally, allowing for exact control of the N/C atomic ratio (20.2-25.7 at.%). In polar solvents, CNDs showed up to 99% ultrahigh quantum yield of strong fluorescence. |
N-functionalized CNDs can have their characteristics precisely tuned by the hydrothermal process, which has applications in optical, sensing, energy storage/conversion, and biological devices. Strong fluorescence in polar solvents suggests that high-performing nanomaterials may find use in a range of fields. |
[329] |
| 8 | Synthesis of carbon nanodots from sugarcane syrup, and their incorporation into a hydrogel-based composite to fabricate innovative fluorescent microstructured polymer optical fibers | The synthesis of CNDs is scalable, economical, and sustainable. Because of their N and O-rich edge groups, functionalized CNDs showed excellent quantum yields (85–99%), which increased their potential for use in optical and sensing applications. | Using a home microwave oven, CNDs with a 3 nm diameter and low polydispersity were created from sugarcane syrup. They were added to an optical fibre and fluorescent hydrogel composite and displayed fluorescence. |
This work offers a green synthesis approach for CNDs that might potentially replace costly and harmful compounds used in optical fibres. The novel hydrothermal method improves the fluorescence and application of CNDs by precisely controlling N-functionalization. | [330] |
| 9 | Synthesis of highly stable red-emissive carbon polymer dots by modulated polymerization: from the mechanism to application in intracellular pH imaging† |
This study offers a green technique for the synthesis of CNDs that might replace costly, hazardous compounds. R-CPDs' surface state and crosslink enhanced emission (CEE) effect are the sources of their red emission. In optical fibres, they exhibit excellent stability, biocompatibility, and appropriateness for intracellular pH monitoring in HeLa cells. The novel hydrothermal method improves the fluorescence and application of CNDs by precisely controlling N-functionalization. | Red-emissive carbon polymer dots (R-CPDs) were synthesised at 80°C and shown resistance to photobleaching, stability in high salinity, high pH sensitivity (pH 4–6), and adjustable solvent-color effect (λem 528–600 nm). |
This study presents a straightforward, controlled technique for producing highly stable, biocompatible long-wavelength emitting R-CPDs. On the processes of photoluminescence, cellular uptake, and multifunctional uses, more study is required. |
[331] |
| 10 | Synthesis of surface molecularly imprinted poly-o-phenylene diamine/TiO2/ carbon nanodots with a highly enhanced selective photocatalytic degradation of pendimethalin herbicide under visible light | Adsorption and selectivity were enhanced by the imprinted cavities and particular recognition sites on MIP. Photocatalytic activity was increased by the redshifted absorption to visible areas and the lowered band gap energy. The primary species responsible for PM photodegradation were O2% radicals. | Using PM herbicide as a template, a TiO2/CNDs/MIP nanocomposite was created. Under visible light, it demonstrated a high adsorption capacity (86.1 mg/g), good selectivity, and increased photodegradation efficiency (95%). |
The TiO2/CNDs/MIP nanocomposite, with high stability and reusability, effectively adsorbs and degrades PM due to its unique structure. It offers a promising photocatalyst for environmental pollutant removal, leveraging lower energy to produce reactive species. | [332] |
| 11 | Direct solvent-derived polymer-coated nitrogen-doped carbon nanodots with high water solubility for targeted fluorescence imaging of glioma | N-CNDs demonstrated superior dispersibility, shown minimal cytotoxicity, and improved passive targeting to facilitate glioma fluorescence imaging. NMP was used in the synthesis as a source of carbon and nitrogen as well as a solvent. | A direct solvothermal process was used to create pN-CNDs, which produced 5–15 nm particles with a quantum yield of 8.4%, sustained fluorescence, and great water solubility. They facilitated in vivo fluorescence imaging by penetrating glioma cells. | An effective method for producing functional carbon nanomaterials with promise for glioma-targeted imaging is the straightforward solvothermal synthesis of pN-CNDs. Their chemical makeup, growth, and targeting methods require more investigation. | [333] |
| 12 | Evolution and synthesis of carbon dots: from carbon dots to carbonized polymer dots | Different from conventional CDs, CPDs are characterised by partial carbonisation of polymer clusters. The lack of control over structure and performance in current synthesis methods limits their applicability in biolabeling, sensing, LEDs, and other areas. | The unique polymer/carbon hybrid structure of CPDs, a novel type of carbon dots, is apparent. Different bottom-up synthesis techniques show how synthesis circumstances affect the structures and characteristics of CPDs. |
Future studies should concentrate on comprehending the principles of CPD synthesis, reaction mechanisms, and formation processes in order to achieve regulated synthesis and maximise their potential for use in a variety of disciplines. | [334] |
| 13 | Design, synthesis, and functionalization strategies of tailored carbon nanodots | Tuning the emissive, electrochemical, and chiroptical characteristics of the CDs was made possible by regulating the reaction conditions. Their surface chemistry was further modified by post-functionalization, which increased their potential for use in a variety of applications, including energy conversion, sensing, and imaging. | They synthesised nitrogen-doped carbon nanodots (CDs) that generate blue light by a bottom-up, microwave-assisted hydrothermal process. These CDs were effectively utilised in hybrid and composite systems and showed tunable optoelectronic characteristics. |
CDs are appropriate for biomedical and energy applications due to their low cost, low toxicity, and strong photostability. Controlling synthesis for improved structural and performance regulation should be the main goal of future study, since this will increase their usefulness in many other domains. |
[335] |
| 14 | Facile synthesis of multicolor photoluminescent polymer carbon dots with surface-state energy gap-controlled emission |
The multicolor photoluminescence of PCDs was found to be mostly controlled by the surface state, namely the C=N functional groups. As the C=N concentration rose, the band gap shrunk and the emission peak moved. |
Hydroquinone and ethylenediamine were used to develop multicolor emissive PCDs that emitted green, blue, and yellow fluorescence. These PCDs demonstrated outstanding solubility, high stability, and wavelength-independent photoluminescence upon stimulation. | A brand-new, gentle, and simple process was created to create PCDs with outstanding water solubility and brilliant, steady emissions. A thorough characterization revealed how important surface states are in dictating the photoluminescence characteristics of PCDs. | [336] |
| 15 | Synthesis separation, and characterization of small and highly fluorescent nitrogen-doped carbon nanodots | NCNDs demonstrated strong luminescence, excellent fluorescence quantum yields (up to 0.46), and ease of functionalization. The surface states, which are impacted by various emission centres and traps, have a significant impact on the fluorescence. |
Using a microwave-assisted process, nitrogen-doped CNDs (NCNDs) were created, producing particles with a narrow size distribution, adjustable fluorescence emission, and superior water solubility. Their size and surface characteristics were further improved using size-exclusion chromatography. | NCNDs with exceptional optical characteristics were manufactured via a straightforward, programmable microwave-assisted approach that controlled both surface and size. These adaptable NCNDs may find use in biomedicine, bioimaging, and optoelectronics | [337] |
| 16 | Ultrahigh-yield synthesis of N-doped carbon nanodots with down-regulating ROS in zebrafish | With a larger C=C percentage, the synthesis yield of CNDs rose by 3.3 times. By adding nitrogen in the forms of pyridinic-like N (74%) and NH2 (26%), the antioxidative qualities against ROS were strengthened. | A novel approach using carbon-carbon double bonds achieved a record-breaking 85.9% yield in synthesizing nitrogen-doped carbon nanodots (CNDs). These CNDs significantly reduced reactive oxygen species (ROS) by 68% in zebrafish. | A viable method for creating antioxidative nitrogen-doped CNDs is the idea of increasing synthesis yield via carbon-carbon double bonds. These CNDs may be used as nanodrugs to treat illnesses associated with ageing. |
[338] |
8.3.2. Patents on CNDs
| No | Title | Patent number | Findings | Date | Ref |
|---|---|---|---|---|---|
| 1 | Metal enhanced photoluminescence from carbon nanodots | US 10,837,904B2 | The invention enhances the detectable emissions of carbon nanodots through Metal-Enhanced Fluorescence (MEF). By positioning carbon nanodots at an optimal distance from plasmon-supporting materials like silver island films, this technique significantly improves brightness, photostability, and detectability, making it highly effective for biological imaging applications. | Nov. 17, 2020 | [339] |
| 2 | Nanomaterials with enhanced | US 11,478,433B2 | Based on medicinal natural products, supramolecular particles improve bioavailability, stabilise in acidic conditions, and distribute therapeutic agents efficiently, leading to better treatment results for diabetes or tumours. | Oct. 25, 2022 | [340] |
| 3 | Carbon nano-dot, and preparation method and application thereof |
CN102849722B |
The technique solves quenching problems in the production of highly fluorescent carbon nano-dots, which may be used for cryptography, photovoltaics, and biological imaging, among other things. It is straightforward and inexpensive. | 2012-08-29 | [341] |
| 4 | Nanocarbon composite structure having ruthenium oxide trapped therein |
US7572542B2 |
The nanocarbon composite, incorporating ruthenium oxide within graphene via Ketjen black and ultracentrifugal reaction, exhibits enhanced electrochemical activity, making it suitable for high-capacity capacitor applications in electrical energy storage. | 2005-06-10 | [342] |
| 5 | Traditional Chinese medicine bio-based carbon nanodots, preparation method thereof, fluorescent probe, traditional Chinese medicine pharmaceutical preparation and application | CN111778018A |
The innovation proposes a carbon nanodot based on ginsenoside that has a large number of surface functional groups that allow for flexible alterations and good stability. It is biocompatible with other substances and functions as a potential biological fluorescence probe. It also shows selective inhibition on PC12 cells. | 2020-06-08 | [343] |
| 6 | Preparation and regulation method of high-color quality fluorescent carbon nanodots | CN109504375B |
Using this technique, standard white light emission and near spectrum matching with sunlight are achieved, yielding high-quality fluorescent carbon nanodots with good colour attributes. | 2018-12-12 | [344] |
| 7 | Carbon nano-dot compound and preparation method thereof, fluorescent powder and light source material | CN106833631B |
A stable carbon nanodot complex with a silicon dioxide covering is presented in this invention, providing improved fluorescence qualities and resilience to environmental influences. | 2017-02-04 | [345] |
| 8 | Fused carbon dot, preparation method and application thereof | CN113403068B |
This innovation includes fused carbon dots manufactured by a straightforward, economical technique that exhibits improved near-infrared emission and good light-heat conversion. | 2021-06-16 | [346] |
9. Conclusions
Acknowledgments
References
- Khan, S.; Dunphy, A.; Anike, M.S.; Belperain, S.; Patel, K.; Chiu, N.H.; Jia, Z. Recent advances in carbon nanodots: a promising nanomaterial for biomedical applications. Int. J. Mol. Sci. 2021, 22(13):6786.
- Meng-Chih, S. ; Yung, Hui, Y. Introduction to Carbon Structures. Carbon Nanomaterials for Bioimaging, Bioanal., Ther. 2019, 9, 1-4.
- Ðorđević., L.; Arcudi, F.; Prato, M. Ðorđević. L.; Arcudi, F.; Prato, M. Preparation, functionalization and characterization of engineered carbon nanodots. Nat. Protoc. 2019,14(10), 2931-53.
- Sawalha, S.; Assali, M.; Nasasrah, A.; Salman, M.; Nasasrah, M.; Jitan, M.; Hilal, H.S.; Zyuod, A. Optical properties and photoactivity of carbon nanodots synthesized from olive solid wastes at different carbonization temperatures. RSC Adv. 2022, 12(8), 4490–500. [Google Scholar] [CrossRef] [PubMed]
- Manisha, H.; Swetha, P.P.; Shim, Y.B.; Prasad, K.S. Revisiting fluorescent carbon nanodots for environmental, biomedical applications and puzzle about fluorophore impurities. Nano-Struct. Nano-Objects. 2019, 20, 100391.
- Vibhute, A.; Patil, T.; Gambhir, R.; Tiwari, A.P. Fluorescent carbon quantum dots: Synthesis methods, functionalization and biomedical applications. Appl. Surf. Sci. 2022, 11, 100311. [Google Scholar] [CrossRef]
- Singh, A.; Kafle, S.R.; Sharma, M.; Kim, B.S. Comprehensive Review on Multifaceted Carbon Dot Nanocatalysts: Sources and Energy Applications. Catal. 2023, 13(11), 1446. [Google Scholar] [CrossRef]
- Daoudi, W.; Tiwari, A.; Tyagi, M.; Singh, P.; Saxena, A.; Verma, D.K.; Dagdag, O.; Sharma, H.K.; Fuertes, P.O.; El Aatiaoui, A. Carbon dots nanoparticles: A promising breakthrough in biosensing, catalysis, biomedical and authers applications. Nano-Struct. Nano-Objects. 2024, 37, 101074.
- Kumar, Y.R.; Deshmukh, K.; Sadasivuni, K.K.; Pasha, S.K. Graphene quantum dot based materials for sensing, bio-imaging and energy storage applications: a review. RSC Adv. 2020, 10(40), 23861–98. [Google Scholar] [CrossRef] [PubMed]
- Cadranel, A.; Margraf, J. T.; Strauss, V.; Clark, T.; Guldi, D. M. Carbon nanodots for charge-transfer processes. Acc. Chem. Res. 2019, 52(4), 955–63. [Google Scholar] [CrossRef] [PubMed]
- Patra JK, Das G, Fraceto LF, Campos EV, Rodriguez-Torres MD, Acosta-Torres LS, Diaz-Torres LA, Grillo R, Swamy MK, Sharma S, Habtemariam S. Nano based drug delivery systems: recent developments and future prospects. Journal of nanobiotechnology. 2018, 16, 1-33.
- Sahu, T.; Ratre, Y.K.; Chauhan, S.; Bhaskar, L.V.; Nair, M.P.; Verma, H.K. Nanotechnology based drug delivery system: Current strategies and emerging therapeutic potential for medical science. J. Drug Deliv. Sci. Technol. 2021, 63, 102487. [Google Scholar] [CrossRef]
- Stielow, M.; Witczyńska, A.; Kubryń, N.; Fijałkowski, Ł.; Nowaczyk, J.; Nowaczyk, A. The Bioavailability of Drugs—The Current State of Knowledge. Mol. 2023, 28(24), 8038. [Google Scholar] [CrossRef]
- Buya, A.B.; Beloqui, A.; Memvanga, P.B.; Préat, V. Self-nano-emulsifying drug-delivery systems: From the development to the current applications and challenges in oral drug delivery. Pharm. 2020, 12(12), 1194. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Munoz, N.; Urbán-Morlán, Z.; Leyva-Gómez, G.; de la Luz Zambrano-Zaragoza, M.; Quintanar-Guerrero, D. Solid lipid nanoparticles: an approach to improve oral drug delivery. J Pharm. Pharm. Sci. 2021, 24, 509–32. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic nanoparticles and their targeted delivery applications. Mol. 2020, 25(9), 2193. [Google Scholar] [CrossRef]
- Manikkath, J.; Manikkath, A.; Lad, H.; Vora, L.K.; Mudgal, J.; Shenoy, R.R.; Ashili, S.; Radhakrishnan, R. Nanoparticle-mediated active and passive drug targeting in oral squamous cell carcinoma: current trends and advances. Nanomed. 2023, 18(27), 2061–80. [Google Scholar] [CrossRef] [PubMed]
- Vargason, A.M.; Anselmo, A.C.; Mitragotri, S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 2021, 5(9), 951–67. [Google Scholar] [CrossRef] [PubMed]
- Shirvan, A.R.; Bashari, A.; Hemmatinejad, N. New insight into the fabrication of smart mucoadhesive buccal patches as a novel controlled-drug delivery system. Eur. Polym. J. 2019, 119, 541–50. [Google Scholar] [CrossRef]
- Mohammed, J.; Desu, P.K.; Namratha, J.R.; Rao, G.K. Applications of carbon dots (CDs) in drug delivery. Adv. Pharmacol. Pharm. 2023, 11(1), 36–45. [Google Scholar] [CrossRef]
- Wang, B.; Lu, S. The light of carbon dots: From mechanism to applications. Matt. 2022, 5(1), 110–49. [Google Scholar] [CrossRef]
- Bayda, S.; Amadio, E.; Cailotto, S.; Frión-Herrera, Y.; Perosa, A.; Rizzolio, F. Carbon dots for cancer nanomedicine: a bright future. Nanoscale Adv. 2021, 3(18), 5183–221. [Google Scholar] [CrossRef]
- Huang, W.L.; Hsu, C.J.; Wang, J.H.; Hsu, Z.H.; Lu, K.H.; Wang, W.D.; Huang, C.C. Low-Toxicity Antibacterial Carbon Nanodots for Wound Dressings and Food Packaging. ACS Appl. Nano Mater. 2023, 6(20), 19200–9. [Google Scholar] [CrossRef]
- Nair, A.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Natural carbon-based quantum dots and their applications in drug delivery: A review. Biomed. Pharmacotherapy. 2020, 132, 110834. [Google Scholar] [CrossRef]
- Ratre, P.; Nazeer, N.; Kumari, R.; Thareja, S.; Jain, B.; Tiwari, R.; Kamthan, A. ; Srivastava RK, Mishra PK. Carbon-Based Fluorescent Nano-Biosensors for the Detection of Cell-Free Circulating MicroRNAs. Biosensors. 2023, 13(2), 226.
- Hosnedlova, B.; Kepinska, M.; Fernandez, C.; Peng, Q.; Ruttkay-Nedecky, B.; Milnerowicz, H.; Kizek, R. Carbon nanomaterials for targeted cancer therapy drugs: A critical review. Chem. Rec. 2019, 19(2-3), 502-22.
- Guido, C.; Baldari, C.; Maiorano, G.; Mastronuzzi, A.; Carai, A. ; Quintarelli. ; C.; De Angelis, B.; Cortese, B.; Gigli, G.; Palamà, I.E. Nanoparticles for diagnosis and target therapy in pediatric brain cancers. Diagnostics. 2022, 12(1), 173. [Google Scholar]
- Hong, G.; Diao, S.; Antaris, A. L.; Dai, H. Carbon nanomaterials for biological imaging and nanomedicinal therapy. Chem. Rev. 2015, 115(19), 10816–906. [Google Scholar] [CrossRef]
- Panwar, N.; Soehartono, A.M.; Chan, K.K.; Zeng, S.; Xu, G.; Qu, J.; Coquet, P.; Yong, K.T.; Chen, X. Nanocarbons for biology and medicine: sensing, imaging, and drug delivery. Chem. Rev. 2019, 119(16), 9559–656. [Google Scholar] [CrossRef]
- Karami, M.H.; Abdouss, M.; Rahdar, A.; Pandey, S. Graphene quantum dots: Background, synthesis methods, and applications as nanocarrier in drug delivery and cancer treatment: An updated review. Inorg. Chem. Commun. 2024, 11, 112032. [Google Scholar] [CrossRef]
- Dhamodharan, D.; Byun, H.S.; Shree, M.V.; Veeman, D.; Natrayan, L.; Stalin, B. Carbon nanodots: synthesis, mechanisms for bio-electrical applications. J. Ind. Eng. Chem. 2022, 110, 68–83. [Google Scholar] [CrossRef]
- Sivasankarapillai, V.S.; Kirthi, A.V.; Akksadha, M.; Indu, S.; Dharshini, U.D.; Pushpamalar, J.; Karthik, L. Recent advancements in the applications of carbon nanodots: Exploring the rising star of nanotechnology. Nanoscale Adv. 2020, 2(5), 1760–73. [Google Scholar] [CrossRef]
- Ostadhossein, F.; Pan, D. Functional carbon nanodots for multiscale imaging and therapy. Wiley Interdisciplinary Reviews. Nanomed. Nanobiotechnol. 2017, 9(3), e1436. [Google Scholar] [CrossRef]
- Cohen, E.N.; Kondiah, P.P.; Choonara, Y.E.; du Toit, LC.; Pillay, V. Carbon dots as nanotherapeutics for biomedical application. Curr. Pharma. des. 2020, 26(19), 2207–21. [Google Scholar] [CrossRef] [PubMed]
- Paramanantham, P.; Anju, V.T.; Dyavaiah, M.; Siddhardha, B. Applications of carbon-based nanomaterials for antimicrobial photodynamic therapy. Microbial Nanobionics. Basic Res App. 2019, 237–59. [Google Scholar]
- Chiari-Andréo, B.G.; Abuçafy, M.P.; Manaia, E.B.; da Silva, B.L.; Rissi, N.C.; Oshiro-Junior, J.A.; Chiavacci, L.A. Drug delivery using theranostics: an Overview of its use, advantages and safety assessment. Curr. Nanosci. 2020, 16(1), 3–14. [Google Scholar] [CrossRef]
- Ding, S.; Khan, A. I.; Cai, X.; Song, Y.; Lyu, Z.; Du, D.; Dutta, P.; Lin, Y. Overcoming blood–brain barrier transport: Advances in nanoparticle-based drug delivery strategies. Mater. Today. 2020, 37, 112–25. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.N.; Kondiah, P.P.; Choonara, Y.E.; du Toit, L.C.; Pillay, V. Carbon dots as nanotherapeutics for biomedical application. Curr. Pharm. Des. 2020, 26(19), 2207–21. [Google Scholar] [CrossRef]
- Gaur, M.; Misra, C.; Yadav, A.B.; Swaroop, S.; Maolmhuaidh, F.Ó.; Bechelany, M.; Barhoum, A. Biomedical applications of carbon nanomaterials: fullerenes, quantum dots, nanotubes, nanofibers, and graphene. Mater. 2021, 14(20), 5978. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, S.; Mozaffarian, M.; Pazuki, G.; Hadidi, N.; Villate-Beitia, I.; Zárate, J.; Puras, G.; Pedraz, J.L. Carbon-Based Nanostructures as Emerging Materials for Gene Delivery Applications. Pharm. 2024, 16(2), 288. [Google Scholar] [CrossRef]
- Wynn, S.G.; Fougère, B.J. The roots of veterinary botanical medicine. Veterinary. Herbal. Med. 2007, 33–49. [Google Scholar]
- Jamal, A. Embracing nature's therapeutic potential: Herbal medicine. IJMRA. 2023, 2(1), 117–26. [Google Scholar]
- Chawla, S. How to develop more effective policies against crime: some reflections on drugs and crime research in an international context. Eur. J. Crim. Pol Res. 2004, 10(1), 85–98. [Google Scholar] [CrossRef]
- Baishya, R.; Hati, Boruah, J.L; Bordoloi, M.J.; Kumar, D.; Kalita, P. Novel drug delivery system in phytochemicals: modern era of ancient science. Herbal Medicine in India: Indigenous Knowledge, Practice, Innovation and its Value. 2020, 175-89.
- Chen, L.H.; Hu, J.N. Development of nano-delivery systems for loaded bioactive compounds: using molecular dynamics simulations. Crit. Rev. Food Sci. Nutr. 2024, 3, 1–22. [Google Scholar] [CrossRef]
- Gaikwad, D.; Sutar, R.; Patil, D. Polysaccharide mediated nanodrug delivery: A review. In6t. J. Biol. Macromol. 2024, 129547. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.K.; Tripathi, A.; Taufeeq, A.; Dar, A.H.; Samrot, A.V.; Rustagi, S.; Malik, S.; Bhattacharya, T.; Kovacs, B.; Shaikh, A.M. Significance and applications of carbon dots in anti cancerous nanodrug conjugate development: A review. Appl. Surf. Sci. 2024, 19, 100550. [Google Scholar] [CrossRef]
- Zeng, M.; Guo, D.; Fernández-Varo, G.; Zhang, X.; Fu, S.; Ju, S.; Casals, E. The Integration of Nanomedicine with Traditional Chinese Medicine: Drug Delivery of Natural Products and Other Opportunities. Mol. Pharmaceutics. 2022, 20(2), 886–904. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, W.; Li, X.; Xu, Y.; Cao, J.; Jiang, W. The anti-obesogenic effects of dietary berry fruits: A review. Food. Res. Int. 2021, 147, 110539. [Google Scholar] [CrossRef]
- Naik, G. G.; Minocha, T.; Verma, A.; Yadav, S. K.; Saha, S.; Agrawal, A. K.; Sahu, A. N. Asparagus racemosus root-derived carbon nanodots as a nano-probe for biomedical applications. J. Mater. Sci. 2022, 57(43), 20380–20401. [Google Scholar] [CrossRef]
- Atran, S. The trouble with memes: Inference versus imitation in cultural creation. Hum. Nat. 2001, 12(4), 351–81. [Google Scholar] [CrossRef] [PubMed]
- Larit, F.; León, F. Therapeutics to Treat Psychiatric and Neurological Disorders: A Promising Perspective from Algerian Traditional Medicine. Plants. 2023, 12(22), 3860. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hu, L. Nanoscale delivery system for nutraceuticals: Preparation, application, characterization, safety, and future trends. Food Eng. Rev. 2020, 12(1), 14–31. [Google Scholar] [CrossRef]
- Hung, P. L.; Chang, W. N.; Huang, L.T. Cardiovascular medicine. Curr. Opin. Pediatr, 2004, 16, 585-613.
- Liu, M.; Anderson, R. C.; Lan, X.; Conti, P. S.; Chen, K. Recent advances in the development of nanoparticles for multimodality imaging and therapy of cancer. Med. Res. Rev. 2020, 40(3), 909–930. [Google Scholar] [CrossRef] [PubMed]
- Sekar, R.; Basavegowda, N.; Jena, S.; Jayakodi, S.; Elumalai, P.; Chaitanyakumar, A.; Somu, P.; Baek, K. H. Recent developments in heteroatom/metal-doped carbon dot-based image-guided photodynamic therapy for cancer. Pharm. 2022, 14(9), 1869. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Patel, R. J.; Saraf, S.; Saraf, S. Recent expansion of pharmaceutical nanotechnologies and targeting strategies in the field of phytopharmaceuticals for the delivery of herbal extracts and bioactives. J. Control. Release. 2016, 241, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.R.; Desu, P.K.; Nakkala, R.K.; Kondi, V.; Devi, S.; Alam, M.S.; Kesharwani, P. Nanotechnology-based approaches applied to nutraceuticals. Drug Deliv. Transl. Res. 2021, 1–15. [Google Scholar]
- Lin, J.; Yin, X.; Zeng, Y.; Hong, X.; Zhang, S.; Cui, B.; Yang, D. Progress and prospect: Biosynthesis of plant natural products based on plant chassis. Biotechnol. Adv. 2023, 108266. [Google Scholar] [CrossRef]
- Dar, R.A.; Shahnawaz, M.; Ahanger, M.A.; ul Majid, I. Exploring the Diverse Bioactive Compounds from Medicinal Plants: A Review. J. Phytopharmacol, 2023, 12, 189-195.
- Abdel-Aziz, S. M.; Aeron, A.; Kahil, T. A. Health benefits and possible risks of herbal medicine. Microbes in food and health. 2016, 97–116. [Google Scholar]
- Cai, R.; Shan, Y.; Du, F.; Miao, Z.; Zhu, L.; Hang, L.; Wang, Z. Injectable hydrogels as promising in situ therapeutic platform for cartilage tissue engineering. International J. Biol. Macromol. 2024, 129537. [Google Scholar] [CrossRef]
- Parveen, B.; Parveen, A.; Parveen, R.; Ahmad, S.; Ahmad, M.; Iqbal, M. Challenges and opportunities for traditional herbal medicine today, with special reference to its status in India. Ann Phytomed. 2020, 9(2), 97–112. [Google Scholar] [CrossRef]
- Pandey, M.; Debnath, M.; Gupta, S.; Chikara, S.K. Phytomedicine: An ancient approach turning into future potential source of therapeutics. J. Pharmacognosy. Phytother. 2011, 3(3), 27–37. [Google Scholar]
- Naik, G.G.; Pratap, R.; Mohapatra, D.; Shreya, S.; Sharma, D.K.; Parmar, A.S.; Sahu, A.N. From phytomedicine to photomedicine: quercetin-derived carbon nanodots—synthesis, characterization and healthcare applications. J. Mater. Sci. 2023, 58(34), 13744–13761. [Google Scholar] [CrossRef]
- Gaikwad, D.; Sutar, R.; Patil, D. Polysaccharide mediated nanodrug delivery: A review. Int. J. Biol. Macromol. 2024, 129547. [Google Scholar] [CrossRef]
- Qiang, R.; Huang, H.; Chen, J.; Shi, X.; Fan, Z.; Xu, G.; Qiu, H. Carbon quantum dots derived from herbal medicine as therapeutic nanoagents for rheumatoid arthritis with ultrahigh lubrication and anti-inflammation. ACS Appl. Mater. Interfaces. 2023, 15(32), 38653–64. [Google Scholar] [CrossRef]
- Viswanath, B.; Kim, S. Influence of nanotoxicity on human health and environment: The alternative strategies. Revi Environ Contam T. 2017, 242, 61–104. [Google Scholar]
- Osazee, F.O.; Mokobia, K.E. , Ifijen, I.H. The Urgent Need for Tungsten-Based Nanoparticles as Antibacterial Agents. Biomed. Mater. Dev. 2023, 1-16.
- Khan, J.; Ikbal, A.M.A.; Debnath, B.; Rajkhowa, A.; Choudhury, P.D.; Sen, S.; Folorunsho, A.A. Management of diabetes mellitus by nano-based drug delivery with special reference to phytosomes. Pharmaceutical and Biosciences Journal. 2021, 11–28. [Google Scholar] [CrossRef]
- Kaurav, H.; Verma, D.; Bansal, A.; Kapoor, D.N.; Sheth, S. Progress in drug delivery and diagnostic applications of carbon dots: a systematic review. Front. Chem, 2023, 11, 1227843.
- Gunasekaran, T.; Haile, T.; Nigusse, T.; Dhanaraju, M.D. Nanotechnology: an effective tool for enhancing bioavailability and bioactivity of phytomedicine. Asian Pac. J. Trop. Biomed. 2014, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Debnath, R.; Jamatia, K.; Choudhury, P.D.; Sen, S.; Saha, S.; Ikbal, A.M.A. Niosome: A Prominent Carrier in Advanced Drug Delivery System. Pharm Biosci J. 2024, 1–19. [Google Scholar]
- Naik, G.G.; Minocha, T.; Verma, A.; Yadav, S.K.; Saha, S.; Agrawal, A.K.; Sahu, A. N. Asparagus racemosus root-derived carbon nanodots as a nano-probe for biomedical applications. Journal of materials science, 2022, 57(43), 20380-20401.
- Truskewycz, A.; Yin, H.; Halberg, N.; Lai, D.T.; Ball, A.S. , Truong, V.K., Cole, I. Carbon dot therapeutic platforms: administration, distribution, metabolism, excretion, toxicity, and therapeutic potential. Small. 2022, 18(16), 2106342.
- Annisa, W.D.; Permatasari, F.A.; Iskandar, F.; Rachmawati, H. Functionalized Phytochemicals-Embedded Carbon Dots Derived from Medicinal Plant for Bioimaging Application. ACS Appl. Bio Mater. 2023, 7(1), 114–23. [Google Scholar] [CrossRef]
- Babu Busi, K.; Palanivel, M.; Kanta Ghosh, K.; Basu Ball, W.; Gulyás, B.; Padmanabhan, P.; Chakrabortty, S. The multifarious applications of copper nanoclusters in biosensing and bioimaging and their translational role in early disease detection. Nanomater. 2022, 12(3), 301. [Google Scholar] [CrossRef] [PubMed]
- Cutshaw, G.; Uthaman, S.; Hassan, N.; Kothadiya, S.; Wen, X.; Bardhan, R. The emerging role of Raman spectroscopy as an omics approach for metabolic profiling and biomarker detection toward precision medicine. Chem. Rev. 2023, 123(13), 8297–346. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L. ; Raker K, Scrivens W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. \J. Phy. Org. Com. 2004, 126(40), 12736-7.
- Baker, S.N.; Baker, G.A. Luminescent carbon nanodots: emergent nanolights. Angewandte Chemie International Edition. 2010, 49(38), 6726–44. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhu, Y.; Yang, X.l.; Li, C. Graphene quantum dots: emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices. Chem Comm. 2012, 48(31), 3686–99. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wang, X.; Meziani, M. J.; Lu, F.; Wang, H.; Luo, P. G.; Lin, Y.; Harruff, B. A.; Veca, L.M.; Murray, D.; Xie, S.Y. Carbon dots for multiphoton bioimaging. J. Am. Chem. Soc. 2007, 129(37), 11318–9. [Google Scholar] [CrossRef]
- Yang, S.T.; Cao, L.; Luo, P.G.; Lu, F.; Wang, X.; Wang, H.; Meziani, M. J.; Liu, Y.; Qi, G.; Sun, Y. P. Carbon dots for optical imaging in vivo. J. Am. Chem. Soc. 2009, 131(32), 11308–9. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, X.; Long, Y.; Wang, X.; Zhang, H.; Zhu, R.; Liang, L.; Teng, P.; Zheng, H. Hollow luminescent carbon dots for drug delivery. Carbon. 2013, 59, 192–9. [Google Scholar] [CrossRef]
- Fang, L.; Zhang, L.; Chen, Z.; Zhu, C.; Liu, J.; Zheng, J. Ammonium citrate derived carbon quantum dot as on-off-on fluorescent sensor for detection of chromium (VI) and sulfites. Mater. Lett. 2017, 191, 1–4. [Google Scholar] [CrossRef]
- Jaiswal, A.; Ghosh, S.S.; Chattopadhyay, A. One step synthesis of C-dots by microwave mediated caramelization of poly (ethylene glycol). Chem Comm. 2012, 48(3), 407–9. [Google Scholar] [CrossRef]
- Schneider, J.; Reckmeier, C.J.; Xiong, Y.; von Seckendorff, M.; Susha, A. S.; Kasák, P.; Rogach, A.L. Molecular fluorescence in citric acid-based carbon dots. J. Phy. Chem C. 2017, 121(3), 2014–22. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, W.; Chen, W.; Wang, J.; Yang, Q.; Zhu, W.; Wang, J. One-pot synthesis of NiFe2O4 integrated with EDTA-derived carbon dots for enhanced removal of tetracycline. J. Chem. Eng. 2017, 310, 187–96. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Cheng, L.; Cao, Z.; Liu, W. Water-soluble and phosphorus-containing carbon dots with strong green fluorescence for cell labeling. J. Mater. Chem B. 2014, 2(1), 46–8. [Google Scholar] [CrossRef]
- Vedamalai, M.; Periasamy, A.P.; Wang, C.W.; Tseng, Y.T. ; Ho, L,C.; Shih, C.C.; Chang, H.T. Carbon nanodots prepared from o-phenylenediamine for sensing of Cu2+ ions in cells. Nanoscale 2014, 6(21), 13119-25.
- Wang, L.; Bi, Y.; Gao, J.; Li, Y.; Ding, H.; Ding, L. Carbon dots based turn-on fluorescent probes for the sensitive determination of glyphosate in environmental water samples. RSC adv. 2016, 6(89), 85820–8. [Google Scholar] [CrossRef]
- Shinde, D.B.; Pillai, V.K. Electrochemical preparation of luminescent graphene quantum dots from multiwalled carbon nanotubes. Chemi. Eur. J. 2012, 18(39), 12522–8. [Google Scholar] [CrossRef]
- Joseph, J.; Anappara, A.A. White-light-emitting carbon dots prepared by the electrochemical exfoliation of graphite. ChemPhysChem. 2017, 18(3), 292–8. [Google Scholar] [CrossRef]
- Tao, H.; Yang, K.; Ma, Z.; Wan, J.; Zhang, Y.; Kang, Z.; Liu, Z. In vivo NIR fluorescence imaging, biodistribution, and toxicology of photoluminescent carbon dots produced from carbon nanotubes and graphite. Small. 2012, 8(2), 281–90. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.N. , et al., One-step hydrothermal approach to fabricate carbon dots from apple juice for imaging of mycobacterium and fungal cells. Sensors and Actuators B: Chemical. 2015, 213, 434-443.
- Humaera, N.A.; Fahri, A.N.; Armynah, B.; Tahir, D. Natural source of carbon dots from part of a plant and its applications: A review. Luminescence. 2021, 36(6), 1354–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sun, W.; Wu, P. Highly photoluminescent carbon dots derived from egg white: facile and green synthesis, photoluminescence properties, and multiple applications. ACS Sustain Chem Eng. 2015, 3(7), 1412–8. [Google Scholar] [CrossRef]
- Feng, J.; Wang, W. J.; Hai, X.; Yu, Y.L.; Wang, J.H. Green preparation of nitrogen-doped carbon dots derived from silkworm chrysalis for cell imaging. J. Mater. Chem B. 2016, 4(3), 387–93. [Google Scholar] [CrossRef]
- Yin, B.; Deng, J.; Peng, X.; Long, Q.; Zhao, J.; Lu, Q.; Chen, Q.; Li, H.; Tang, H.; Zhang, Y.; Yao, S. Green synthesis of carbon dots with down-and up-conversion fluorescent properties for sensitive detection of hypochlorite with a dual-readout assay. Anlst. 2013, 138(21), 6551–7. [Google Scholar] [CrossRef]
- Xu, J.; Lai, T.; Feng, Z.; Weng, X.; Huang, C. Formation of fluorescent carbon nanodots from kitchen wastes and their application for detection of Fe3+. Luminescence. 2015, 30(4), 420–4. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, X.; Sheng, Y.; Shen, J.; Huang, P.; Guo, S.; Pan, J.; Liu, B.; Feng, B. Simple one-step synthesis of water-soluble fluorescent carbon dots from waste paper. New J Chem. 2014, 38(3), 906–9. [Google Scholar] [CrossRef]
- Feng, Y.; Zhong, D.; Miao, H.; Yang, X. Carbon dots derived from rose flowers for tetracycline sensing. Talanta. 2015, 140, 128–33. [Google Scholar] [CrossRef]
- Wang, B.; Lu, S. The light of carbon dots: From mechanism to applications. Matter. 2022, 5(1), 110–49. [Google Scholar] [CrossRef]
- Ozyurt, D.; Al Kobaisi, M.; Hocking, R.K.; Fox, B. Properties, synthesis, and applications of carbon dots: A review. Carbon Trends. 2023, 100276. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, S. Creating high yield water soluble luminescent graphene quantum dots via exfoliating and disintegrating carbon nanotubes and graphite flakes. Chem Comm. 2012, 48(82), 10177–9. [Google Scholar] [CrossRef]
- Nie, H.; Li, M.; Li, Q.; Liang, S.; Tan, Y.; Sheng, L.; Shi, W.; Zhang, S.X. Carbon dots with continuously tunable full-color emission and their application in ratiometric pH sensing. Chem Mater. 2014, 26(10), 3104–12. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): current state and future perspective. Nano Res. 2015, 8, 355–81. [Google Scholar] [CrossRef]
- Bailey, R. E.; Nie, S. Alloyed semiconductor quantum dots: tuning the optical properties without changing the particle size. J. Am. Chem. Soc. 2003, 125(23), 7100–6. [Google Scholar] [CrossRef]
- Ma, P.; Sun, X.; Pan, W.; Yu, G.; Wang, J. Green and orange emissive carbon dots with high quantum yields dispersed in matrices for phosphor-based white LEDs. ACS sustain Chem Eng. 2020, 8(8), 3151–61. [Google Scholar] [CrossRef]
- Han, Z.; Ni, Y.; Ren, J.; Zhang, W.; Wang, Y.; Xie, Z.; Zhou, S.; Yu, S. F. Highly efficient and ultra-narrow bandwidth orange emissive carbon dots for microcavity lasers. Nanoscale. 2019, 11(24), 11577–83. [Google Scholar] [CrossRef]
- Yang, X.; Sui, L.; Wang, B.; Zhang, Y.; Tang, Z.; Yang, B.; Lu, S. Red-emitting, self-oxidizing carbon dots for the preparation of white LEDs with super-high color rendering index. Sci China Chem. 2021, 64(9), 1547–53. [Google Scholar] [CrossRef]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing graphene quantum dots and carbon dots: properties, syntheses, and biological applications. Small. 2015, 11(14), 1620–36. [Google Scholar] [CrossRef]
- Han, M.; Zhu, S.; Lu, S.; Song, Y.; Feng, T.; Tao, S.; Liu, J.; Yang, B. Recent progress on the photocatalysis of carbon dots: Classification, mechanism and applications. Nano Today. 2018, 19, 201–18. [Google Scholar] [CrossRef]
- Ai, L.; Yang, Y.; Wang, B.; Chang, J.; Tang, Z.; Yang, B.; Lu, S. Insights into photoluminescence mechanisms of carbon dots: advances and perspectives. Sci. Bull. 2021, 66(8), 839–56. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kang, Z.; Liu, Y.; Lee, S.T. Carbon nanodots: synthesis, properties and applications. J. Mater. Chem. 2012, 22(46), 24230–53. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem Soc Rev. 2015, 44(1), 362–81. [Google Scholar] [CrossRef]
- Mintz, K.J.; Zhou, Y.; Leblanc, R.M. Recent development of carbon quantum dots regarding their optical properties, photoluminescence mechanism, and core structure. Nanoscale. 2019, 11(11), 4634–52. [Google Scholar] [CrossRef]
- Sheng-Liang H, Pei-Kang B, Shi-Rui C, Jing S. Preparation of fluorescent carbon nanoparticles by pulsed laser. Chem J Chinese U. 2009, 30(8), 1497–500.
- Hu, S.L.; Niu, K.Y.; Sun, J.; Yang, J.; Zhao, N.Q.; Du, X.W. One-step synthesis of fluorescent carbon nanoparticles by laser irradiation. J Mater Chem. 2009, 19(4), 484–8. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Shimizu, Y.; Pyatenko, A.; Kawaguchi, K.; Koshizaki, N. Preparation of carbon quantum dots with tunable photoluminescence by rapid laser passivation in ordinary organic solvents. Chem Comm. 2010, 47(3), 932–4. [Google Scholar] [CrossRef] [PubMed]
- Hu S, Liu J, Yang J, Wang Y, Cao S. Laser synthesis and size tailor of carbon quantum dots. J. Nanoparticle Res. 2011, 13, 7247–52.
- Ming, H.; Ma, Z.; Liu, Y.; Pan, K.; Yu, H.; Wang, F.; Kang, Z. Large scale electrochemical synthesis of high quality carbon nanodots and their photocatalytic property. Dalton Trans. 2012, 41(31), 9526–31. [Google Scholar] [CrossRef]
- Zhou, J.; Booker, C.; Li, R.; Zhou, X.; Sham, T.K.; Sun, X.; Ding, Z. An electrochemical avenue to blue luminescent nanocrystals from multiwalled carbon nanotubes (MWCNTs). J Am Chem Soc. 2007, 129(4), 744–5. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Zhang, Z.L.; Tian, Z.Q.; Zhang, L.; Liu, C.; Lin, Y.; Qi, B.; Pang, D. W. Electrochemical tuning of luminescent carbon nanodots: from preparation to luminescence mechanism. Advanced materials (Deerfield Beach, Fla.). 2011, 23(48), 5801-6.
- Alaghmandfard, A.; Sedighi, O.; Rezaei, N.T.; Abedini, A.A.; Khachatourian, A.M.; Toprak, M.S.; Seifalian, A. Recent advances in the modification of carbon-based quantum dots for biomedical applications. Mater Sci Eng: C. 2021, 120, 111756.
- Wang, N.; Li, L.; Zhou, N.; Chen, S. Cage breaking of C60 into photoluminescent graphene oxide quantum dots: an efficient peroxidase mimic. physica status solidi (b). 2018, 255(4), 1700535. [Google Scholar] [CrossRef]
- Gu, S.; Hsieh, C.T.; Chiang, Y.M.; Tzou, D.Y.; Chen, Y.F.; Gandomi, Y.A. Optimization of graphene quantum dots by chemical exfoliation from graphite powders and carbon nanotubes. Mater Chem Phys. 2018, 215, 104–11. [Google Scholar] [CrossRef]
- Hu, S.; Wei, Z.; Chang, Q.; Trinchi, A.; Yang, J. A facile and green method towards coal-based fluorescent carbon dots with photocatalytic activity. Appl. Surf. Sci. 2016, 378, 402–7. [Google Scholar] [CrossRef]
- Liu, X.; Hao, J.; Liu, J.; Tao, H. Green synthesis of carbon quantum dots from lignite coal and the application in Fe3+ detection. InIOP Conference Series. Earth and Environmental Science 2018, 113, 012063. [Google Scholar] [CrossRef]
- Ventrella, A.; Camisasca, A.; Fontana, A.; Giordani, S. Synthesis of green fluorescent carbon dots from carbon nano-onions and graphene oxide. RSC adv. 2020, 10(60), 36404–12. [Google Scholar] [CrossRef]
- Sousa, H.B.; Martins, C.S.; Prior, J.A. You don’t learn that in school: An updated practical guide to carbon quantum dots. Nanomaterials. 2021, 11(3), 611. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, S.; Sun, Z.; Zhu, H. Study on ultrasonic single-step synthesis and optical properties of nitrogen-doped carbon fluorescent quantum dots. Fuller Nanotub Car Nanostr. 2015, 23(9), 769–76. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, V.B.; Gedanken, A. Sonochemical synthesis of carbon dots, mechanism, effect of parameters, and catalytic, energy, biomedical and tissue engineering applications. Ultrason. sonochem. 2020, 64, 105009. [Google Scholar] [CrossRef]
- Xu, M.; Li, Z.; Zhu, X.; Hu, N.; Wei, H.; Yang, Z.; Zhang, Y. Hydrothermal/Solvothermal Synthesis of Graphene Quantum Dots and Their Biological Applications. Nano Biomed. Eng. 2013, 5(2), 65–71. [Google Scholar] [CrossRef]
- Mohammed, S.J.; Omer, K.M.; Hawaiz, F.E. Deep insights to explain the mechanism of carbon dot formation at various reaction times using the hydrothermal technique: FT-IR, 13 C-NMR, 1 H-NMR, and UV-visible spectroscopic approaches. RSC adv. 2023, 13(21), 14340–9. [Google Scholar] [CrossRef]
- Tyagi, A.; Tripathi, K.M.; Singh, N.; Choudhary, S.; Gupta, R.K. Green synthesis of carbon quantum dots from lemon peel waste: applications in sensing and photocatalysis. RSC adv. 2016, 6(76), 72423–32. [Google Scholar] [CrossRef]
- Bano, D.; Kumar, V.; Singh, V.K.; Hasan, S.H. Green synthesis of fluorescent carbon quantum dots for the detection of mercury (II) and glutathione. New J Chem. 2018, 42(8), 5814–21. [Google Scholar] [CrossRef]
- Tadesse, A.; RamaDevi, D.; Hagos, M.; Battu, G.; Basavaiah, K. Facile green synthesis of fluorescent carbon quantum dots from citrus lemon juice for live cell imaging. Asian J. Nanosci. Mater. 2018, 1(1), 36–46. [Google Scholar]
- Prasannan, A.; Imae, T. One-pot synthesis of fluorescent carbon dots from orange waste peels. Ind. Eng. Chem. 2013, 52(44), 15673–8. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Varma, R.S. Microwave-assisted organic synthesis and transformations using benign reaction media. Acc. Chem. Res. 2008, 41(5), 629–39. [Google Scholar] [CrossRef]
- De Medeiros, T.V.; Manioudakis, J.; Noun, F.; Macairan, J.R.; Victoria, F.; Naccache, R. Microwave-assisted synthesis of carbon dots and their applications. J Mater Chem C. 2019, 7(24), 7175–95. [Google Scholar] [CrossRef]
- Kung, J.C.; Tseng, I.T.; Chien, C.S.; Lin, S.H.; Wang, C.C.; Shih, C.J. Microwave assisted synthesis of negative-charge carbon dots with potential antibacterial activity against multi-drug resistant bacteria. RSC adv. 2020, 10(67), 41202–8. [Google Scholar] [CrossRef] [PubMed]
- Adeola, A.O.; Duarte, M.P.; Naccache, R. Microwave-assisted synthesis of carbon-based nanomaterials from biobased resources for water treatment applications: emerging trends and prospects. Front Carb. 2023, 2, 1220021. [Google Scholar] [CrossRef]
- He, G.; Shu, M.; Yang, Z.; Ma, Y.; Huang, D.; Xu, S.; Wang, Y.; Hu, N.; Zhang, Y.; Xu, L. Microwave formation and photoluminescence mechanisms of multi-states nitrogen doped carbon dots. Appl Surf Sci. 2017, 422, 257–65. [Google Scholar] [CrossRef]
- Ramezani, Z.; Qorbanpour, M.; Rahbar, N. Green synthesis of carbon quantum dots using quince fruit (Cydonia oblonga) powder as carbon precursor: application in cell imaging and As3+ determination. Colloids and Surfaces A: Physicochem Eng Asp. 2018, 549, 58-66.
- Qin, X.; Fu, C.; Zhang, J.; Shao, W.; Qin, X.; Gui, Y.; Wang, L.; Guo, H.; Chen, F. ; Jiang, L, Wu G. Direct preparation of solid carbon dots by pyrolysis of collagen waste and their applications in fluorescent sensing and imaging. Front Chem. 2022, 10, 1006389. [Google Scholar] [PubMed]
- Jiang, H.; Chen, F.; Lagally, M.G.; Denes, F.S. New strategy for synthesis and functionalization of carbon nanoparticles. Langmuir. 2010, 26(3), 1991–5. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Stassinopoulos, A.; Anglos, D.; Zboril, R.; Georgakilas, V.; Giannelis, E.P. Photoluminescent carbogenic dots. Chemistry of Materials. 2008, 20(14), 4539–41. [Google Scholar] [CrossRef]
- Liu, R.; Wu, D.; Liu, S.; Koynov, K.; Knoll, W.; Li, Q. An aqueous route to multicolor photoluminescent carbon dots using silica spheres as carriers. Angew. Chem. Int. Ed. Engl 2009, 48(25), 4598–601. [Google Scholar] [CrossRef]
- Zong, J.; Zhu, Y.; Yang, X.; Shen, J.; Li, C. Synthesis of photoluminescent carbogenic dots using mesoporous silica spheres as nanoreactors. Chem Comm. 2011, 47(2), 764–6. [Google Scholar] [CrossRef]
- Wang, J.; Xin, X.; Lin, Z. Cu 2 ZnSnS 4 nanocrystals and graphene quantum dots for photovoltaics. Nanoscale. 2011, 3(8), 3040–8. [Google Scholar] [CrossRef]
- Hamilton, I. P.; Li, B.; Yan, X.; Li, L.S. Alignment of colloidal graphene quantum dots on polar surfaces. Nano Lett. 2011, 11(4), 1524–9. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.L.; Yan, X.; Dragnea, B.; Li, L.S. Slow hot-carrier relaxation in colloidal graphene quantum dots. Nano Lett. 2011, 11(1), 56–60. [Google Scholar] [CrossRef] [PubMed]
- Liu, R. ; Wu, D; Feng, X. ; Müllen, K. Bottom-up fabrication of photoluminescent graphene quantum dots with uniform morphology. J. Am. Chem. Soc. 2011, 133(39), 15221–3. [Google Scholar]
- Lu, J.; Yeo, P.S.; Gan, C.K.; Wu, P.; Loh, K.P. Transforming C60 molecules into graphene quantum dots. Nat. Nanotechnol. 2011, 6(4), 247–52. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhu, M.; Lee, X.; Zhang, R.; Wang, K.; Wei, J.; Zhong, M.; Wu, D.; Zhu, H. Direct synthesis of graphene quantum dots by chemical vapor deposition. Part. Syst. Charact. 2013, 30(9), 764–9. [Google Scholar] [CrossRef]
- Kurdyukov, D.A.; Eurov, D.A.; Stovpiaga, E.Y.; Kirilenko, D.A.; Konyakhin, S.V.; Shvidchenko, A.V.; Golubev, V.G. Template synthesis of monodisperse carbon nanodots. Solid State Phys. 2016, 58, 2545–9. [Google Scholar] [CrossRef]
- Li, H.; Kang, Z.; Liu, Y.; Lee, S.T. Carbon nanodots: synthesis, properties and applications. J. Mater. Chem. 2012, 22(46), 24230–53. [Google Scholar] [CrossRef]
- Liu, H.; Ye, T.; Mao, C. Fluorescent carbon nanoparticles derived from candle soot. Angew. Chem. 2007, 119(34), 6593–5. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Kang, Z.; Huang, H.; Liu, Y.; Liu, J.; Lian, S.; Tsang, C.H.; Yang, X.; Lee, S.T. Water-soluble fluorescent carbon quantum dots and photocatalyst design. Angew Chem. 2010, 26(122), 4532–6. [Google Scholar] [CrossRef]
- Vinci, J.C.; Ferrer, I.M.; Seedhouse, S.J.; Bourdon, A.K.; Reynard, J.M.; Foster, B.A.; Bright, F.V.; Colón, L.A. Hidden properties of carbon dots revealed after HPLC fractionation. J. Phy. Chem. Lett. 2013, 4(2), 239–43. [Google Scholar] [CrossRef]
- Zheng, X.T.; Than, A.; Ananthanaraya, A.; Kim, D.H.; Chen, P. Graphene quantum dots as universal fluorophores and their use in revealing regulated trafficking of insulin receptors in adipocytes. ACS nano. 2013, 7(7), 6278–86. [Google Scholar] [CrossRef]
- Liu, X.; Hao, J.; Liu, J.; Tao, H. Green synthesis of carbon quantum dots from lignite coal and the application in Fe3+ detection. InIOP Conference Series. Earth Env Sci. 2018, 113, 012063. [Google Scholar] [CrossRef]
- Liu, W.; Li, C.; Ren, Y.; Sun, X.; Pan, W.; Li, Y.; Wang, J.; Wang, W. Carbon dots: surface engineering and applications. J. Mater. Chem B. 2016, 4(35), 5772–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ai, L.; Song, Z.; Nie, M.; Xiao, J.; Li, G.; Lu, S. Surface modification functionalized carbon dots. Chem. Euro. J. 2023, 29(65), e202302383. [Google Scholar] [CrossRef]
- Wang, X.; Cao, L.; Yang, S.T.; Lu, F.; Meziani, M.J.; Tian, L.; Sun, K.W.; Bloodgood, M.A.; Sun, Y.P. Bandgap-like strong fluorescence in functionalized carbon nanoparticles. Angewandte Chemie. 2010, 49(31), 5310. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhou, N.; Lin, X.; Lin, J.; Chi, Y.; Chen, G. Extraction of electrochemiluminescent oxidized carbon quantum dots from activated carbon. Chemistry of Materials. 2010, 22(21), 5895–9. [Google Scholar] [CrossRef]
- Zou, Y.; Yan, F.; Zheng, T.; Shi, D.; Sun, F.; Yang, N.; Chen, L. Highly luminescent organosilane-functionalized carbon dots as a nanosensor for sensitive and selective detection of quercetin in aqueous solution. Talanta 2015, 135, 145–8. [Google Scholar] [CrossRef]
- Hou, J.; Hou, J.; Dong, J.; Zhu, H.; Teng, X.; Ai, S.; Mang, M. A simple and sensitive fluorescent sensor for methyl parathion based on l-tyrosine methyl ester functionalized carbon dots. Biosens and Bioelectron. 2015,15, 68:20-6.
- Liu, S.; Zhao, N.; Cheng, Z.; Liu, H. Amino-functionalized green fluorescent carbon dots as surface energy transfer biosensors for hyaluronidase. Nanoscale. 2015, 7(15), 6836–42. [Google Scholar] [CrossRef]
- Shen, P.; Xia, Y. Synthesis-modification integration: one-step fabrication of boronic acid functionalized carbon dots for fluorescent blood sugar sensing. Anal Chem. 2014, 86(11), 5323–9. [Google Scholar] [CrossRef]
- Gonçalves, H.; Jorge, P.A.; Fernandes, J.R.; da Silva, J.C. Hg (II) sensing based on functionalized carbon dots obtained by direct laser ablation. Sensors and Actuators B: Chemical. 2010, 145(2), 702-7.
- Liang, S.; Wang, M.; Gao, W.; Zhao, X. Effects of elemental doping, acid treatment, and passivation on the fluorescence intensity and emission behavior of yellow fluorescence carbon dots. Opt. Mater. 2022, 128, 112471. [Google Scholar] [CrossRef]
- Barman, M.K.; Jana, B.; Bhattacharyya, S.; Patra, A. Photophysical properties of doped carbon dots (N, P, and B) and their influence on electron/hole transfer in carbon dots–nickel (II) phthalocyanine conjugates. J. Phys. Chem C. 2014, 118(34), 20034–41. [Google Scholar] [CrossRef]
- Redondo-Fernandez, G.; Canga, J.C.; Soldado, A.; Encinar, J.R.; Costa-Fernandez, J. M. Functionalized heteroatom-doped carbon dots for biomedical applications: A review. Anal. Chim Acta. 202334 1874. [CrossRef] [PubMed]
- Wang, K.; Gao, Z.; Gao, G.; Wo, Y.; Wang, Y.; Shen, G.; Cui, D. Systematic safety evaluation on photoluminescent carbon dots. Nanoscale Res Lett. 2013, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Ge, S.; Wang, S.; Yan, M.; Zang, D.; Yu, J. Facile and sensitive paper-based chemiluminescence DNA biosensor using carbon dots dotted nanoporous gold signal amplification label. Anal Methods. 2013, 5(5), 1328–36. [Google Scholar] [CrossRef]
- Kumar, A.; Chowdhuri, A.R.; Laha, D.; Mahto, T.K.; Karmakar, P.; Sahu, S.K. Green synthesis of carbon dots from Ocimum sanctum for effective fluorescent sensing of Pb2+ ions and live cell imaging. Sens. Actuators B: Chem. 2017, 242, 679-86.
- Shi, W.; Wang, Q.; Long, Y.; Cheng, Z.; Chen, S.; Zheng, H.; Huang, Y. Carbon nanodots as peroxidase mimetics and their applications to glucose detection. Chem Comm. 2011, 47(23), 6695–7. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Yang, C.; Tong, Y.; Xu, G.; Ma, X.; Lin, Y.; Chen, G. Label-free electrochemiluminescent immunosensor for α-fetoprotein: Performance of Nafion–carbon nanodots nanocomposite films as antibody carriers. Chem Comm. 2012, 48(25), 3055–7. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yan, Y.; Zhu, L.; Li, X.; Li, G. An amperometric biosensor for the detection of hydrogen peroxide released from human breast cancer cells. Biosens Bioelectron. 2013, 41, 815–9. [Google Scholar] [CrossRef] [PubMed]
- Lin, Zhen.; L.Z, Xue.; Wei, X.W.; Chen, Hui, C.H.; Lin JinMing, L.J. Peroxynitrous-acid-induced chemiluminescence of fluorescent carbon dots for nitrite sensing. Anal. Chem. 2011, 83(21), 8245-8251.
- Zhao, H.X.; Liu, L.Q.; De Liu, Z.; Wang, Y.; Zhao, X.J.; Huang, C.Z. Highly selective detection of phosphate in very complicated matrixes with an off–on fluorescent probe of europium-adjusted carbon dots. Chem. Comm. 2011, 47(9), 2604–6. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Lu, W.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Microwave-assisted rapid green synthesis of photoluminescent carbon nanodots from flour and their applications for sensitive and selective detection of mercury (II) ions. Sens. Actuators B: Chem. 2013, 184, 156-62.
- Hou, Y.; Lu, Q.; Deng, J.; Li, H.; Zhang, Y. One-pot electrochemical synthesis of functionalized fluorescent carbon dots and their selective sensing for mercury ion. Anal. Chim. Acta. 2015, 866, 69–74. [Google Scholar] [CrossRef]
- Gogoi, N.; Barooah, M.; Majumdar, G.; Chowdhury, D. Carbon dots rooted agarose hydrogel hybrid platform for optical detection and separation of heavy metal ions. ACS applied materials & interfaces. 2015, 7(5), 3058-67.
- Mohd Yazid, S,N.; Chin, S.F.; Pang, S.C.; Ng, S.M. Detection of Sn (II) ions via quenching of the fluorescence of carbon nanodots. Microchim. Acta. 2013, 180, 137-43.
- Qian, Y.; Deng, Y.; Qiu, X.; Li, H.; Yang, D. Formation of uniform colloidal spheres from lignin, a renewable resource recovered from pulping spent liquor. Green Chem. 2014, 16(4), 2156–63. [Google Scholar] [CrossRef]
- Lan, M. ; Di Y, Zhu, X.; Ng, T. W.; Xia, J.; Liu, W..; Meng, X.; Wang, P.; Lee, C. S.; Zhang, W. A carbon dot-based fluorescence turn-on sensor for hydrogen peroxide with a photo-induced electron transfer mechanism. Chem. Comm. 2015, 51(85), 15574-7.
- Miao, P.; Han, K.; Tang, Y.; Wang, B.; Lin, T.; Cheng, W. Recent advances in carbon nanodots: synthesis, properties and biomedical applications. Nanoscale. 2015, 7(5), 1586–95. [Google Scholar] [CrossRef]
- Ray, S.C.; Saha, A.; Jana, N.R.; Sarkar, R. Fluorescent carbon nanoparticles: synthesis, characterization, and bioimaging application. J, Phys, Chem C. 2009, 113(43), 18546-51.
- Xu, Y.; Wu, M.; Liu, Y.; Feng, X.Z.; Yin, X.B.; He, X.W.; Zhang, Y.K. Nitrogen-doped carbon dots: a facile and general preparation method, photoluminescence investigation, and imaging applications. Chem. Eur. J. 2013, 19(7), 2276–83. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Zhu, C.; Liu, M.; Ji, Y.; Feng, L.; Tao, L.; Wei, Y. Carbon-dots derived from nanodiamond: Photoluminescence tunable nanoparticles for cell imaging. J. Colloid. Interface Sci. 2013, 397, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Pang, H.; Yang, H. B.; Guo, C.; Shao, J.; Chi, Y.; Li, C. M.; Yu, T. Carbon-based dots co-doped with nitrogen and sulfur for high quantum yield and excitation-independent emission. Angews Chem. Int. Ed. 2013, 52(30), 7800–4. [Google Scholar] [CrossRef]
- Wu, Z.L.; Zhang, P.; Gao, M.X.; Liu, C.F.; Wang, W.; Leng, F.; Huang, C.Z. One-pot hydrothermal synthesis of highly luminescent nitrogen-doped amphoteric carbon dots for bioimaging from Bombyx mori silk–natural proteins. J. Mater. Chem B. 2013, 1(22), 2868–73. [Google Scholar] [CrossRef]
- Jiang, C.; Wu, H.; Song, X.; Ma, X.; Wang, J.; Tan, M. Presence of photoluminescent carbon dots in Nescafe® original instant coffee: applications to bioimaging. Talanta. 2014, 127, 68–74. [Google Scholar] [CrossRef]
- Zhu, A.; Luo, Z.; Ding, C.; Li, B.; Zhou, S.; Wang, R.; Tian, Y. A two-photon “turn-on” fluorescent probe based on carbon nanodots for imaging and selective biosensing of hydrogen sulfide in live cells and tissues. Analyst. 2014, 139(8), 1945–52. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Li, F.; Wen, J.; Wang, X.; Sun, R. Gram-scale synthesis of single-crystalline graphene quantum dots derived from lignin biomass. Green Chem. 2018, 20(6), 1383–90. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Dadashzadeh, A.; Moghassemi, S.; Ashrafizadeh, M.; Dehshahri, A.; Pardakhty, A.; Sassan, H.; Sohrevardi, S.M.; Mandegary, A. Shedding light on gene therapy: Carbon dots for the minimally invasive image-guided delivery of plasmids and noncoding RNAs-A review. J. Adv. Res. 2019, 18, 81–93. [Google Scholar] [CrossRef]
- Gogoi, N.; Chowdhury, D. Novel carbon dot coated alginate beads with superior stability, swelling and pH responsive drug delivery. Journal of Materials Chemistry B. 2014, 2(26), 4089–99. [Google Scholar] [CrossRef]
- Karthik, S.; Saha, B.; Ghosh, S. K.; Singh, N.P. Photoresponsive quinoline tethered fluorescent carbon dots for regulated anticancer drug delivery. Chem. Comm. 2013, 49(89), 10471–3. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.U.; Park, S.Y.; Park, E.S.; Son, B.; Lee, S. C.; Lee, J.W.; Lee, Y.C.; Kang, K.S.; Kim, M.I.; Park, H.G.; Choi, S. Photoluminescent carbon nanotags from harmful cyanobacteria for drug delivery and imaging in cancer cells. Sci. Rep. 2014, 4(1), 4665. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Krishnatreya, G.; Gogoi, N.; Thakur, D.; Chowdhury, D. Carbon-dot-coated alginate beads as a smart stimuli-responsive drug delivery system. ACS Appl. Mater. Interfaces. 2016, 8(50), 34179–84. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Liu, C.; Li, X.; Liu, J.; Di, D.; Zhang, Y.; Zhao, Q.; Wang, S. Fluorescent carbon dot modified mesoporous silica nanocarriers for redox-responsive controlled drug delivery and bioimaging. J. Colloid Interface Sci. 2016, 483, 343–52. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Shen, X.; Su, C.; Yang, J.; Piao, M.; Jia, F.; Gao, G.; Zhang, L.; Lin, Q. One-step synthesis of photoluminescent carbon dots with excitation-independent emission for selective bioimaging and gene delivery. J. Colloid Interface Sci. 2017, 492, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, X.; Fang, Z.; Niu, Y.; Lou, J.; Wu, Y.; Zou, S.; Xia, S.; Sun, M.; Du, F. Fabrication of HA/PEI-functionalized carbon dots for tumor targeting, intracellular imaging and gene delivery. RSC Adv. 2017, 7(6), 3369–75. [Google Scholar] [CrossRef]
- Liu, W.; Diao, H.; Chang, H.; Wang, H.; Li, T.; Wei, W. Green synthesis of carbon dots from rose-heart radish and application for Fe3+ detection and cell imaging. Sens Actuators B Chem. 2017, 241, 190–8. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.; Lee, Y.R. Nitrogen-doped carbon dots originating from unripe peach for fluorescent bioimaging and electrocatalytic oxygen reduction reaction. J Colloid Interface Sci. 2016, 482, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Mewada, A.; Pandey, S.; Shinde, S.; Mishra, N.; Oza, G.; Thakur, M.; Sharon, M.; Sharon, M. Green synthesis of biocompatible carbon dots using aqueous extract of Trapa bispinosa peel. Mater. Sci. Eng. C. 2013, 33(5), 2914–7. [Google Scholar] [CrossRef]
- Mehta, V.N.; Jha, S.; Kailasa, S.K. One-pot green synthesis of carbon dots by using Saccharum officinarum juice for fluorescent imaging of bacteria (Escherichia coli) and yeast (Saccharomyces cerevisiae) cells. Mater. Sci. Eng C. 2014, 38, 20–7. [Google Scholar] [CrossRef]
- Vanitha, M.; Aarthy, K.C.; Banupriya, D. GREEN SYNTHESIS OF NANOBIOCONJUGATES FOR BACTERIAL BIOFILM INHIBITION. GJMBT. 2021, 11(1), 1–6. [Google Scholar]
- Atchudan, R.; Edison, T. N.; Chakradhar, D.; Perumal, S.; Shim, J. J.; Lee, Y. R. Facile green synthesis of nitrogen-doped carbon dots using Chionanthus retusus fruit extract and investigation of their suitability for metal ion sensing and biological applications. Sens. Actuators B: CheM. 2017, 246, 497-509.
- Vandarkuzhali, S.A.; Jeyalakshmi, V.; Sivaraman, G.; Singaravadivel, S.; Krishnamurthy, K.R.; Viswanathan, B. Highly fluorescent carbon dots from pseudo-stem of banana plant: applications as nanosensor and bio-imaging agents. Sens. Actuators B: Chemical. 2017, 252, 894-900.
- Yang, X.; Zhuo, Y.; Zhu, S.; Luo, Y.; Feng, Y.; Dou, Y. Novel and green synthesis of high-fluorescent carbon dots originated from honey for sensing and imaging. Biosens Bioelectron. 2014, 60, 292–8. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Lan, M.; Zhu, X.; Xue, H.; Ng, T.W.; Meng, X.; Lee, C.S.; Wang, P.; Zhang, W. Green synthesis of bifunctional fluorescent carbon dots from garlic for cellular imaging and free radical scavenging. ACS appl. Mater. Interfaces. 2015, 7(31), 17054–60. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Shang, S.; Chen, X.; Wang, D.; Cai, Y. Facile synthesis of fluorescence carbon dots from sweet potato for Fe3+ sensing and cell imaging. Mater. Sci. Eng C. 2017, 76, 856–64. [Google Scholar] [CrossRef]
- Cheng, C.; Shi, Y.; Li, M.; Xing, M.; Wu, Q. Carbon quantum dots from carbonized walnut shells: Structural evolution, fluorescence characteristics, and intracellular bioimaging. Mater. Sci. Eng C. 2017, 79, 473–80. [Google Scholar] [CrossRef]
- Ramanan, V.; Thiyagarajan, S.K.; Raji, K.; Suresh, R.; Sekar, R.; Ramamurthy, P. Outright green synthesis of fluorescent carbon dots from eutrophic algal blooms for in vitro imaging. ACS Sustain. Chem. Eng. 2016, 4(9), 4724–31. [Google Scholar] [CrossRef]
- Sivasankaran, U.; Jesny, S.; Jose, A.R.; Kumar, K.G. Fluorescence determination of glutathione using tissue paper-derived carbon dots as fluorophores. Anal. Sci. 2017, 33(3), 281–5. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, K.M.; Tran, T.S.; Tung, T.T.; Losic, D.; Kim, T. Water soluble fluorescent carbon nanodots from biosource for cells imaging. J. Nanomater. 2017, 1–10. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, L.; Li, X.; Liu, R.; Lin, L.; Zhao, S. Green preparation of S and N Co-doped carbon dots from water chestnut and onion as well as their use as an off–on fluorescent probe for the quantification and imaging of coenzyme A. ACS Sustain. Chem. Eng. 2017, 5(6), 4992–5000. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, H.U.; Park, E.S.; Lee, S.C.; Lee, J.W.; Jeong, S.W.; Kim, C.H.; Lee, Y.C.; Huh, Y.S.; Lee, J. Photoluminescent green carbon nanodots from food-waste-derived sources: large-scale synthesis, properties, and biomedical applications. ACS. Appl. Mater. Interfaces. 2014, 6(5), 3365–70. [Google Scholar] [CrossRef]
- Wang, Z.; Liao, H.; Wu, H.; Wang, B.; Zhao, H.; Tan, M. Fluorescent carbon dots from beer for breast cancer cell imaging and drug delivery. Analytical Methods. 2015, 7(20), 8911–8917. [Google Scholar] [CrossRef]
- Ding, Z.; Li, F.; Wen, J.; Wang, X.; Sun, R. Gram-scale synthesis of single-crystalline graphene quantum dots derived from lignin biomass. Green Chem. 2018, 20(6), 1383–90. [Google Scholar] [CrossRef]
- Bandi, R.; Gangapuram, B.R.; Dadigala, R.; Eslavath, R.; Singh, S.S.; Guttena, V. Facile and green synthesis of fluorescent carbon dots from onion waste and their potential applications as sensor and multicolour imaging agents. RSC Adv. 2016, 6(34), 28633–9. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, Y.; Liang, G.; Yu, S.H. Scale-up synthesis of fragrant nitrogen-doped carbon dots from bee pollens for bioimaging and catalysis. Adv. Sci. 2015, 2(4). 1500002.
- Sachdev, A.; Gopinath, P. Green synthesis of multifunctional carbon dots from coriander leaves and their potential application as antioxidants, sensors and bioimaging agents. Analyst. 2015, 140(12), 4260–9. [Google Scholar] [CrossRef]
- Kumawat, M.K.; Thakur, M.; Gurung, R.B.; Srivastava, R. Graphene quantum dots for cell proliferation, nucleus imaging, and photoluminescent sensing applications. Sci. Rep. 2017, 7(1), 15858. [Google Scholar] [CrossRef] [PubMed]
- D’souza, S.L.; Chettiar, S.S.; Koduru, J.R.; Kailasa, S.K. Synthesis of fluorescent carbon dots using Daucus carota subsp. sativus roots for mitomycin drug delivery. Optik. 2018, 158, 893–900. [Google Scholar]
- Huang, G.; Chen, X.; Wang, C.; Zheng, H.; Huang, Z.; Chen, D.; Xie, H. Photoluminescent carbon dots derived from sugarcane molasses: synthesis, properties, and applications. RSC Adv. 2017, 7(75), 47840–7. [Google Scholar] [CrossRef]
- Kumawat, M.K.; Thakur, M.; Gurung, R.B.; Srivastava, R. Graphene quantum dots from mangifera indica: application in near-infrared bioimaging and intracellular nanothermometry. ACS Sustain. Chem. Eng. 2017, 5(2), 1382–91. [Google Scholar] [CrossRef]
- Kasibabu, B.S.; D’souza, S.L.; Jha, S.; Kailasa, S.K. Imaging of bacterial and fungal cells using fluorescent carbon dots prepared from carica papaya juice. J. Fluoresc. 2015, 25, 803–10. [Google Scholar] [CrossRef]
- Murugesan, B.; Sonamuthu, J.; Pandiyan, N.; Pandi, B.; Samayanan, S.; Mahalingam, S. Photoluminescent reduced graphene oxide quantum dots from latex of Calotropis gigantea for metal sensing, radical scavenging, cytotoxicity, and bioimaging in Artemia salina: A greener route. J. Photochem. Photobiol. B. 2018, 178, 371–9. [Google Scholar] [CrossRef]
- Yang, R.; Guo, X.; Jia, L.; Zhang, Y.; Zhao, Z.; Lonshakov, F. Green preparation of carbon dots with mangosteen pulp for the selective detection of Fe3+ ions and cell imaging. Appl. Surf. Sci. 2017, 423, 426–32. [Google Scholar] [CrossRef]
- Gu, D.; Shang, S.; Yu, Q.; Shen, J. Green synthesis of nitrogen-doped carbon dots from lotus root for Hg (II) ions detection and cell imaging. Appl. Surf. Sci. 2016, 390, 38–42. [Google Scholar] [CrossRef]
- Amin, N.; Afkhami, A.; Hosseinzadeh, L.; Madrakian, T. Green and cost-effective synthesis of carbon dots from date kernel and their application as a novel switchable fluorescence probe for sensitive assay of Zoledronic acid drug in human serum and cellular imaging. Anal. Chim. Acta. 2018, 1030, 183–93. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Jiang, Y.; Zhao, J.; Miao, M.; Cao, S.; Fang, J.; Shi, L. Easy synthesis of photoluminescent N-doped carbon dots from winter melon for bio-imaging. Rsc Adv. 2015, 5(40), 31250–4. [Google Scholar] [CrossRef]
- Naik, G.G.; Pratap, R.; Mohapatra, D.; Shreya, S.; Sharma, D.K.; Parmar, A.S.; Sahu, A.N. From phytomedicine to photomedicine: quercetin-derived carbon nanodots—synthesis, characterization and healthcare applications. J. Mater. Sci. 2023, 58(34), 13744–13761. [Google Scholar] [CrossRef]
- Yadav, T.C.; Bachhuka, A. Tuning foreign body response with tailor-engineered nanoscale surface modifications: fundamentals to clinical applications. J. Mater. Chem. B, 2023, 11(33), 7834-7854.
- Leppert, W.; Malec–Milewska, M.; Zajaczkowska, R.; Wordliczek, J. Transdermal and topical drug administration in the treatment of pain. Mol. 2018, 23(3), 681. [Google Scholar] [CrossRef] [PubMed]
- Sahana, S.; Gautam, A.; Singh, R.; Chandel, S. A recent update on development, synthesis methods, properties and application of natural products derived carbon dots. Nat. Prod. Bioprospect. 2023, 13(1), 51. [Google Scholar] [CrossRef] [PubMed]
- Adepu, S.; Ramakrishna, S. Controlled drug delivery systems: current status and future directions. Mol. 2021, 26(19), 5905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Molecularly imprinted nanoparticles for biomedical applications. Adv Mater. 2020, 32(3), 1806328. [Google Scholar] [CrossRef]
- Das, A.; Adhikari, S.; Deka, D.; Baildya, N.; Sahare, P.; Banerjee, A.; Pathak, S. An updated review on the role of nanoformulated phytochemicals in colorectal cancer. Medicina. 2023, 59(4), 685. [Google Scholar] [CrossRef]
- Walia, S.; Sharma, C.; Acharya, A. Biocompatible Fluorescent nanomaterials for molecular imaging applications. nanomaterial-based biomedical applications in molecular imaging, diagnosis. Ther. 2023, 27-53.
- Javed, M.N.; Dahiya, E.S.; Ibrahim, A.M.; Alam, M.S.; Khan, F.A.; Pottoo, F.H. Recent advancement in clinical application of nanotechnological approached targeted delivery of herbal drugs. Nanophytomedicine: Concept to Clinic. 2020, 151-172.
- Soni, V.; Raizada, P.; Singh, P.; Cuong, H.N.; Rangabhashiyam, S.; Saini, A.; Nguyen, V.H. Sustainable and green trends in using plant extracts for the synthesis of biogenic metal nanoparticles toward environmental and pharmaceutical advances: A review. Environ. Res. 2021, 202, 111622. [Google Scholar] [CrossRef] [PubMed]
- John, V.L.; Nair, Y.; Vinod, T.P. Doping and surface modification of carbon quantum dots for enhanced functionalities and related applications. Part. Syst. Charact. 2021, 38(11), 2100170. [Google Scholar] [CrossRef]
- Arsene, M.L.; Răut, I.; Călin, M.; Jecu, M.L.; Doni, M.; Gurban, A.M. Versatility of reverse micelles: From biomimetic models to nano (bio) sensor design. Processes, 2021, 9(2), 345.
- Valizadeh, A.; Asghari, S.; Abbaspoor, S.; Jafari, A. , Raeisi, M., & Pilehvar, Y. Implantable smart hyperthermia nanofibers for cancer therapy: Challenges and opportunities. Nanomed. Nanobiot. 2023, 15(6), 1909.
- Vallejo, F.A.; Sigdel, G.; Veliz, E.A.; Leblanc, R.M.; Vanni, S.; Graham, R.M. Carbon Dots in Treatment of Pediatric Brain Tumors: Past, Present, and Future Directions. Int. J. Mol. Sci. 2023, 24(11), 9562. [Google Scholar] [CrossRef]
- Bhattacharya, T.; Shin, G. H.; Kim, J. T. Carbon dots: Opportunities and challenges in cancer therapy. Pharm. 2023, 15(3), 1019. [Google Scholar] [CrossRef] [PubMed]
- Yun, W.S.; Cho, H.; Jeon, S.I.; Lim, D.K.; Kim, K. Fluorescence-Based Mono-and Multimodal Imaging for In Vivo Tracking of Mesenchymal Stem Cells. Biomol. 2023, 13(12), 1787. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Fadeel, B. Understanding the bidirectional interactions between two-dimensional materials, microorganisms, and the immune system. Adv. Drug Deliv. Rev. 2022, 114422. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.P.; Gaharwar, A.K. Light-responsive inorganic biomaterials for biomedical applications. Adv. Sci. 2020, 7(17), 2000863. [Google Scholar] [CrossRef] [PubMed]
- Bakhle, S. S.; Avari, J.G. Development and characterization of solid self-emulsifying drug delivery system of cilnidipine. Chem. Pharm. Bull. 2015, 63(6), 408–17. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Dong, Y.; Wang, F. ; Zhang. Nanoformulations to enhance the bioavailability and physiological functions of polyphenols. Mol. 2020, 25(20), 4613. [Google Scholar]
- Mauro, N.; Utzeri, M.A.; Varvarà, P.; Cavallaro, G. Functionalization of metal and carbon nanoparticles with potential in cancer theranostics. Mol, 2021, 26(11), 3085.
- Naik, G.G.; Pratap, R.; Mohapatra, D.; Shreya, S.; Sharma, D.K.; Parmar, A. S.; Sahu, A.N. From phytomedicine to photomedicine: quercetin-derived carbon nanodots—synthesis, characterization and healthcare applications. J. Mater. Sci. 2023, 58(34), 13744–13761. [Google Scholar] [CrossRef]
- Ji, Z.; Yin, Z.; Jia, Z.; Wei, J. Carbon nanodots derived from urea and citric acid in living cells: Cellular uptake and antioxidation effect. Langmuir. 2020, 36(29), 8632–8640. [Google Scholar] [CrossRef]
- Ji, D. K.; Dali, H.; Guo, S.; Malaganahally, S.; Vollaire, J.; Josserand, V.; Bianco, A. Multifunctional Carbon Nanodots: Enhanced Near-Infrared Photosensitizing, Photothermal Activity, and Body Clearance. Small Science. 2022, 2(2), 2100082. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Saravanan, A.; Margel, S.; Gedanken, A.; Srinivasan, S.; Rajabzadeh, A. R. Naturally derived carbon dots in situ confined self-healing and breathable hydrogel monolith for anomalous diffusion-driven phytomedicine release. ACS Appl. Bio Mater. 2022, 5(12), 5617–5633. [Google Scholar] [CrossRef]
- Zahin, N.; Anwar, R.; Tewari, D.; Kabir, M.T.; Sajid, A.; Mathew, B.; Abdel-Daim, M.M. Nanoparticles and its biomedical applications in health and diseases: special focus on drug delivery. Environ. Sci. Pollut. Res. 2020, 27, 19151–19168. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. Current status and future prospects of drug delivery systems. Drug Deliv. Sys. 2014, 1–56. [Google Scholar]
- Chatterjee, P.; Dhibar, S. Nanomaterial marvels: Pioneering applications and cutting-edge advancements in drug delivery. Nano. Med. Mater. 2023, 3(2):220.
- Ranganathan, R.; Madanmohan, S.; Kesavan, A.; Baskar, G.; Krishnamoorthy, Y.R.; Santosham, R.; Ponraju, D.; Rayala, S. K.; Venkatraman, G. Nanomedicine: towards development of patient-friendly drug-delivery systems for oncological applications. Int J Nanomedicine. 2012, 1043–60. [Google Scholar]
- Thakuria, A.; Kataria, B.; Gupta, D. Nanoparticle-based methodologies for targeted drug delivery—an insight. J. Nanoparticle Res. 2021, 1–30. [Google Scholar] [CrossRef]
- Ho, J.R.; Chien, J. Trends in translational medicine and drug targeting and delivery: new insights on an old concept—targeted drug delivery with antibody–drug conjugates for cancers. J. Pharm. Sci. 2014, 103(1), 71–7. [Google Scholar]
- Manzari, M.T.; Shamay, Y.; Kiguchi, H.; Rosen, N.; Scaltriti, M.; Heller, D.A. Targeted drug delivery strategies for precision medicines. Nature Reviews Materials. 2021, 6(4), 351–70. [Google Scholar] [CrossRef]
- Khan, F.A.; Narasimhan, K.; Swathi, C.S.; Mustak, S.; Mustafa, G.; Ahmad, M. Z.; Akhter, S. 3D printing technology in customized drug delivery system: current state of the art, prospective and the challenges. Curr. Pharm. Des. 2018, 24(42), 5049–61. [Google Scholar] [CrossRef]
- Zaidi, Z.; Maiti, N.; Ali, MI.; Sharma, G.; Moin, S.; Padhy, H.; Balaji, G.L.; Sundramurthy, V. P. Fabrication, characteristics, and therapeutic applications of carbon-based nanodots. J. Nanomater. 2022. [Google Scholar] [CrossRef]
- Luong, A.D.; Buzid, A.; Luong, J.H. Important roles and potential uses of natural and synthetic antimicrobial peptides (AMPs) in oral diseases: Cavity, periodontal disease, and thrush. J. Func. Biomater. 2022, 13(4), 175. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.; Rodriguez-Torres, M.D.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; Habtemariam, S. Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnology. 2018, 16, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, B.K.; Singh, V.V.; Solanki, M.K.; Kumar, A.; Ruokolainen, J.; Kesari, K.K. Smart nanomaterials in cancer theranostics: challenges and opportunities. ACS omega. 2023, 8(16), 14290–320. [Google Scholar] [CrossRef] [PubMed]
- Sameer, M.; Arif, Y.; Aqil, A.; Nadaf, A.; Rafiya, K.; Hasan, N.; Kesharwani, P.; Ahmad, F. J. Carbon nanodots as a remedial nanovesicles for drug delivery. Eur. Polym. J. 2023, 14, 112515. [Google Scholar] [CrossRef]
- Merino, S.; Martín, C.; Kostarelos, K.; Prato, M.; Vázquez, E. Nanocomposite hydrogels: 3D polymer–nanoparticle synergies for on-demand drug delivery. ACS nano. 2015, 9(5), 4686–97. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, S.; Mozaffarian, M.; Pazuki, G.; Hadidi, N.; Villate-Beitia, I.; Zárate, J.; Puras, G.; Pedraz, J.L. Carbon-Based Nanostructures as Emerging Materials for Gene Delivery Applications. Pharm. 2024, 16(2), 288. [Google Scholar] [CrossRef]
- Priyam, J.; Saxena, U. Therapeutic applications of carbon nanomaterials in renal cancer. Biotechnol. Lett. 2023, 45(11):1395-416.
- Fernandes, N.B.; Nayak, Y.; Garg, S.; Nayak, U.Y. Multifunctional engineered mesoporous silica/inorganic material hybrid nanoparticles: Theranostic perspectives. Coord. Chem. Rev. 2023, 478, 214977. [Google Scholar] [CrossRef]
- Iqbal, B.; Ali, J.; Baboota, S. Recent advances and development in epidermal and dermal drug deposition enhancement technology. Int. J. Dermatol. 2018, 57(6), 646–60. [Google Scholar] [CrossRef]
- Li, D.; Han, D.; Qu, S. N.; Liu, L.; Jing, P. T.; Zhou, D.; Ji, W.Y.; Wang, X.Y.; Zhang, T.F.; Shen, D.Z. Supra-(carbon nanodots) with a strong visible to near-infrared absorption band and efficient photothermal conversion. Light. Sci. Appl. 2016, 5(7), pp.e16120-e16120.
- Mao, Q.X.; Han, L.; Shu, Y.; Chen, X.W.; Wang, J.H. Improving the biocompatibility of carbon nanodots for cell imaging. Talanta. 2016, 161, 54–61. [Google Scholar] [CrossRef]
- Loukanov, A.; Mladenova, P.; Toshev, S.; Karailiev, A.; Ustinovich, E.; Nakabayashi, S. Real time monitoring and quantification of uptake carbon nanodots in eukaryotic cells. Microsc Res and Tech. 2018, 81(12), 1541–7. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Yoo, J.; Lim, B.; Kwon, W.; Rhee, S.W. Improving the functionality of carbon nanodots: doping and surface functionalization. J. Mater. Chem A. 2016, 4(30), 11582–603. [Google Scholar] [CrossRef]
- Henna, T.K.; Raphey, V.R.; Sankar, R.; Shirin, V.A.; Gangadharappa, H.V.; Pramod, K. Carbon nanostructures: The drug and the delivery system for brain disorders. Int. J. Pharm. 2020, 587, 119701. [Google Scholar] [CrossRef]
- Saleemi, M.A.; Kong, Y.L.; Yong, P.V.; Wong, E.H. An overview of recent development in therapeutic drug carrier system using carbon nanotubes. J. Drug Deliv. Sci. Technol. 2020, 59, 101855. [Google Scholar] [CrossRef]
- Akharame, M.O.; Fatoki, O.S.; Opeolu, B.O.; Olorunfemi, D.I.; Oputu, O.U. Polymeric nanocomposites (PNCs) for wastewater remediation: an overview. Polym-Plast. Technol. Eng. 2018, 57(17), 1801-27.
- Fu, S.; Sun, Z.; Huang, P.; Li, Y.; Hu, N. Some basic aspects of polymer nanocomposites: A critical review. Nano Mater Sci. 2019, 1(1), 2–30. [Google Scholar] [CrossRef]
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and synthesis of carbon dots: from carbon dots to carbonized polymer dots. Adv Sci. 2019, 6(23), 1901316. [Google Scholar] [CrossRef]
- De, Oliveira, A.D.; Beatrice, C.A. Polymer nanocomposites with different types of nanofiller. Nanocomposites-recent evolutions. 2018, 103-4.
- Keledi, G.; Hári, J.; Pukánszky, B. Polymer nanocomposites: structure, interaction, and functionality. Nanoscale. 2012, 4(6), 1919–38. [Google Scholar] [CrossRef]
- Singh, R.K.; Kumar, R.; Singh, D.P.; Savu, R.; Moshkalev, S.A. Progress in microwave-assisted synthesis of quantum dots (graphene/carbon/semiconducting) for bioapplications: a review. Mater. Today Chem. 2019, 12, 282–314. [Google Scholar] [CrossRef]
- Lan, G.; Yang, J.; Ye, R.P.; Boyjoo, Y.; Liang, J.; Liu, X.; Li, Y.; Liu, J.; Qian, K. Sustainable carbon materials toward emerging applications. Small Methods. 2021, 5(5), 2001250. [Google Scholar] [CrossRef]
- Rawat, P.; Nain, P.; Sharma, S.; Sharma, P.K.; Malik, V.; Majumder, S.; Verma, V. P.; Rawat, V.; Rhyee, J. S. An overview of synthetic methods and applications of photoluminescence properties of carbon quantum dots. Luminescence. 2023, 38(7), 845–66. [Google Scholar] [CrossRef]
- Purohit, A.; Panda, P.K. Thread of hope: Weaving a comprehensive review on electrospun nanofibers for cancer therapy. J. Drug Deliv. Sci. Technol. 2023, 27, 105100. [Google Scholar] [CrossRef]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64(5), 1020–37. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Peng, Z.; Seven, E.S.; Leblanc, R.M. Crossing the blood-brain barrier with nanoparticles. J Control Release. 2018, 270, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Shariati, L.; Esmaeili, Y.; Rahimmanesh, I.; Babolmorad, S.; Ziaei, G.; Hasan, A.; Boshtam, M.; Makvandi, P. Nanobased platform advances in cardiovascular diseases: Early diagnosis, imaging, treatment, and tissue engineering. Environ. Res. 2023, 116933. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V. P, Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discov. 2014, 13(11), 813–27. [Google Scholar] [CrossRef]
- Coelho, J.F.; Ferreira, P.C.; Alves, P.; Cordeiro, R.; Fonseca, A.C.; Góis, J.R.; Gil, M. H. Drug delivery systems: Advanced technologies potentially applicable in personalized treatments. EPMA J. 2010, 1, 164–209. [Google Scholar] [CrossRef] [PubMed]
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G. Z. Stimuli-responsive polymeric nanocarriers for drug delivery, imaging, and theragnosis. Polym. 2020, 12(6), 1397. [Google Scholar] [CrossRef]
- Benko, A.; Medina-Cruz, D.; Vernet-Crua, A.; O’Connell, C. P.; Świętek, M.; Barabadi, H.; Saravanan, M.; Webster, T.J. Nanocarrier drug resistant tumor interactions: Novel approaches to fight drug resistance in cancer. Cancer Drug Resist. 2021, 4(2), 264. [Google Scholar] [CrossRef] [PubMed]
- Vithalani, R.; Patel, D.; Modi, C.K.; Suthar, D.H. Glowing photoluminescene in carbon-based nanodots: current state and future perspectives. J. Mater. Sci. 2020, 55, 8769–92. [Google Scholar] [CrossRef]
- Ding, H.; Zhou, X.X.; Wei, J. S.; Li, X.B.; Qin, B.T.; Chen, X.B.; Xiong, H.M. Carbon dots with red/near-infrared emissions and their intrinsic merits for biomedical applications. Carbon. 2020, 167, 322–44. [Google Scholar] [CrossRef]
- Bagyalakshmi, S.; Sivakami, A.; Pal, K.; Sarankumar, R.; Mahendran, C. Manufacturing of electrochemical sensors via carbon nanomaterials novel applications: a systematic review. J. Nanoparticle. Res. 2022, 24(10), 201. [Google Scholar] [CrossRef]
- MP, A.; Pardhiya, S.; Rajamani, P. Carbon dots: an excellent fluorescent probe for contaminant sensing and remediation. Small. 2022, 18(15), 2105579. [Google Scholar]
- Xiao. L.; Sun, H. Novel properties and applications of carbon nanodots. Nanoscale Horiz. 2018, 3(6), 565–97.
- Barhoum, A.; Meftahi, A. ; Kashef. Sabery, M.S., Momeni, Heravi, M.E., Eds.; A review on carbon dots as innovative materials for advancing biomedical applications: synthesis, opportunities, and challenges. J. Mater. Sci. 2023, 58(34), 13531-79.
- Aseri, V.; Kumari, P.; Nagar, V.; Godara, V. ; Kumar, Verma, R.; Awasthi, G.; Awasthi, K.K.; Sankhla, M.S. Synthetization and Functionalization of Natural, Polymer, and Quantum-Based Carbon Nanodots and Their Application in Biomedicine. Macromol. Symp. 2024, 413, 2300052.
- Gawrońska, M.; Kowalik, M.; Makowski, M. Recent advances in medicinal chemistry of ampicillin: Derivatives, metal complexes, and sensing approaches. Trac. 2022, 155, 116691. [Google Scholar]
- Chen, B.B.; Liu, M.L.; Huang, C.Z. Recent advances of carbon dots in imaging-guided theranostics. TrAC Trends. Anal. Chem. 2021, 134, 116116. [Google Scholar] [CrossRef]
- Deb, A.; Konwar, A.; Chowdhury, D. pH-responsive hybrid jute carbon dot-cotton patch. ACS Sustain. Chem. Eng. 2020, 8(19), 7394–402. [Google Scholar] [CrossRef]
- Bankoti, K.; Rameshbabu, A.P.; Datta, S.; Roy, M.; Goswami, P.; Roy, S.; Das, A.K.; Ghosh, S.K.; Dhara, S. Carbon nanodot decorated acellular dermal matrix hydrogel augments chronic wound closure. J. Mater Chem B. 2020; 8(40), 9277-94.
- Pardo, J.; Peng, Z.; Leblanc, R.M. Cancer targeting and drug delivery using carbon-based quantum dots and nanotubes. Mol. 2018, 23(2), 378. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, X. The utilization of carbon-based nanomaterials in bone tissue regeneration and engineering: respective featured applications and future prospects. Med. Nove Tech. Dev. 2022, 16, 100168. [Google Scholar] [CrossRef]
- Sajjadi, M.; Nasrollahzadeh, M.; Jaleh, B.; Soufi, G.J.; Iravani, S. Carbon-based nanomaterials for targeted cancer nanotherapy: Recent trends and future prospects. J. Drug Target. 2021, 9(7), 716–41. [Google Scholar] [CrossRef]
- Shi, Y.; Xu, H.; Yuan, T.; Meng, T.; Wu, H.; Chang, J.; Wang, H.; Song, X.; Li, Y.; Li, X.; Zhang, Y. Carbon dots: An innovative luminescent nanomaterial: Photovoltaics: Special Issue Dedicated to Professor Yongfang Li. Aggregate. 2022, 3(3), 108. [Google Scholar] [CrossRef]
- Shakeri, A.; Jarad, N.A.; Khan, S.; Didar, T.F. Bio-functionalization of microfluidic platforms made of thermoplastic materials: A review. Anal. Chim. Acta. 2022, 209, 339283. [Google Scholar] [CrossRef] [PubMed]
- Reina, G.; González-Domínguez, J.M.; Criado, A.; Vázquez, E.; Bianco, A.; Prato, M. Promises, facts and challenges for graphene in biomedical applications. Chem. Soc. Rev. 2017, 46(15), 4400–16. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharipov, M.; Turaev, A.; Azizov, S.; Azizov, I.; Makhado, E.; Rahdar, A.; Kumar, D.; Pandey, S. Polymer-based hybrid nanoarchitectures for cancer therapy applications. Polym. 2022, 14(15), 3027. [Google Scholar] [CrossRef] [PubMed]
- Kasouni, A.; Chatzimitakos, T.; Stalikas, C. Bioimaging applications of carbon nanodots: A review. C. 2019, 5(2), 19. [Google Scholar] [CrossRef]
- Chan, M.H.; Chen, B. G. ; Ngo, L, T.; Huang, W. T., Li, C.H., Liu, R.S., Eds.; Hsiao, M. Natural carbon nanodots: Toxicity assessment and theranostic biological application. Pharm. 2021, 13(11), 1874. [Google Scholar]
- Chan, M.H.; Chen, B. G.; Ngo, L, T.; Huang, W. T.; Li, C.H.; Liu, R.S.; Hsiao, M. Natural carbon nanodots: Toxicity assessment and theranostic biological application. Pharm. 2021, 13(11), 1874. [CrossRef]
- Cayuela, A.; Carrillo-Carrión, C.; Soriano, M.L.; Parak, W.J.; Valcárcel, M. One-step synthesis and characterization of N-doped carbon nanodots for sensing in organic media. Anal Chem. 2016, 88(6), 3178–85. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Cong, R.; Zhu, S.; Zhao, X.; Liu, J.S.; Tse, J.; Meng, S.; Yang, B. pH-dependent synthesis of novel structure-controllable polymer-carbon nanodots with high acidophilic luminescence and super carbon dots assembly for white light-emitting diodes. ACS Appl. Mater Interfaces. 2016, 8(6), 4062–8. [Google Scholar] [CrossRef] [PubMed]
- Kwon, W.; Do, S.; Lee, J.; Hwang, S.; Kim, J.K.; Rhee, S.W. Freestanding luminescent films of nitrogen-rich carbon nanodots toward large-scale phosphor-based white-light-emitting devices. Chem Mater. 2013, 25(9), 1893–9. [Google Scholar] [CrossRef]
- Stan, C.S.; Elouakassi, N.; Albu, C.; Ania, C.O.; Coroaba, A.; Ursu, L.E.; Popa, M.; Kaddami, H.; Almaggoussi, A. Photoluminescence of Argan-Waste-Derived Carbon Nanodots Embedded in Polymer Matrices. Nanomater. 2023, 14(1), 83. [Google Scholar]
- Jahan, S.; Mansoor, F.; Naz, S.; Lei, J.; Kanwal, S. Oxidative synthesis of highly fluorescent boron/nitrogen co-doped carbon nanodots enabling detection of photosensitizer and carcinogenic dye. Anal. Chem. 2013, 85(21), 10232–9. [Google Scholar]
- Yang, P.C.; Ting, Y.X.; Gu, S.; Gandomi, Y.A.; Hsieh, C.T. Fluorescent nitrogen-doped carbon nanodots synthesized through a hydrothermal method with different isomers. J. Taiwan Inst. Chem. Eng. 2021, 123, 302–9. [Google Scholar] [CrossRef]
- Perli, G.; Soares, M.C.; Cabral, T.D.; Bertuzzi, D.L.; Bartoli, J.R.; Livi, S.; Duchet-Rumeau, J.; Cordeiro, C.M.; Fujiwara, E.; Ornelas, C. Synthesis of carbon nanodots from sugarcane syrup, and their incorporation into a hydrogel-based composite to fabricate innovative fluorescent microstructured polymer optical fibers. Gels. 2022, 8(9), 553. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Chen, S.; Zou, G.Y.; Yu, Y.L.; Wang, J.H. Synthesis of highly stable red-emissive carbon polymer dots by modulated polymerization: from the mechanism to application in intracellular pH imaging. Nanoscale. 2018, 10(47), 22484–92. [Google Scholar] [CrossRef]
- Shahnazi, A.; Nabid, M.R.; Sedghi, R. Synthesis of surface molecularly imprinted poly-o-phenylenediamine/TiO2/carbon nanodots with a highly enhanced selective photocatalytic degradation of pendimethalin herbicide under visible light. React. Funct. Polym. 2020, 151, 104580. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, Y.; Wang, S.; Li, C.; Shi, W.; Chen, J.; Wang, J.; Huang, R. Direct Solvent-Derived Polymer-Coated Nitrogen-Doped Carbon Nanodots with High Water Solubility for Targeted Fluorescence Imaging of Glioma. Small. 2015, 11(29), 3575–81. [Google Scholar] [CrossRef]
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and synthesis of carbon dots: from carbon dots to carbonized polymer dots. Adv. Sci. 2019, 6(23), 1901316. [Google Scholar] [CrossRef]
- Arcudi, F.; Đorđević, L.; Prato, M. Design, synthesis, and functionalization strategies of tailored carbon nanodots. Acc. Chem. Res. 2019, 52(8), 2070–9. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Liu, S.G.; Dong, J.X.; Liang, J.Y.; Li, L.J.; Li, N.B.; Luo, H.Q. Facile synthesis of multicolor photoluminescent polymer carbon dots with surface-state energy gap-controlled emission. J. Mater. Chem C. 2017, 5(41), 10785–93. [Google Scholar] [CrossRef]
- Arcudi, F.; Đorđević, L.; Prato, M. Synthesis, separation, and characterization of small and highly fluorescent nitrogen-doped carbon nanodots. Angew. Chem. Int. Ed. 2016, 55(6), 2107–12. [Google Scholar] [CrossRef]
- Wang, L.; Li, B.; Li, L.; Xu, F.; Xu, Z.; Wei, D.; Feng, Y.; Wang, Y.; Jia, D.; Zhou, Y. Ultrahigh-yield synthesis of N-doped carbon nanodots that down-regulate ROS in zebrafish. J. Mater. Chem. B. 2017, 5(38), 7848–60. [Google Scholar] [CrossRef]
- https://patentimages.storage.googleapis.com/62/e4/c2/0addaa60e6dfef/US10837904.pdf.
- https://patentimages.storage.googleapis.com/03/96/a9/28ce82d2c74544/US11478433.pdf.
- https://patentimages.storage.googleapis.com/82/4d/1a/d6b3bc5dd09ffb/CN102849722B.pdf.
- https://patentimage zs.storage.googleapis.com/b6/28/0b/4149a74fffe4a8/US7572542.pdf.
- https://patentimages.storage.googleapis.com/a9/f9/8a/f57f618a5f3384/CN111778018A.pdf.
- https://patentimages.storage.googleapis.com/85/e0/b8/e07c9f2159c602/CN109504375B.pdf.
- https://patents.google.com/patent/CN106833631B/en.
- https://patents.google.com/patent/CN113403068B/en.
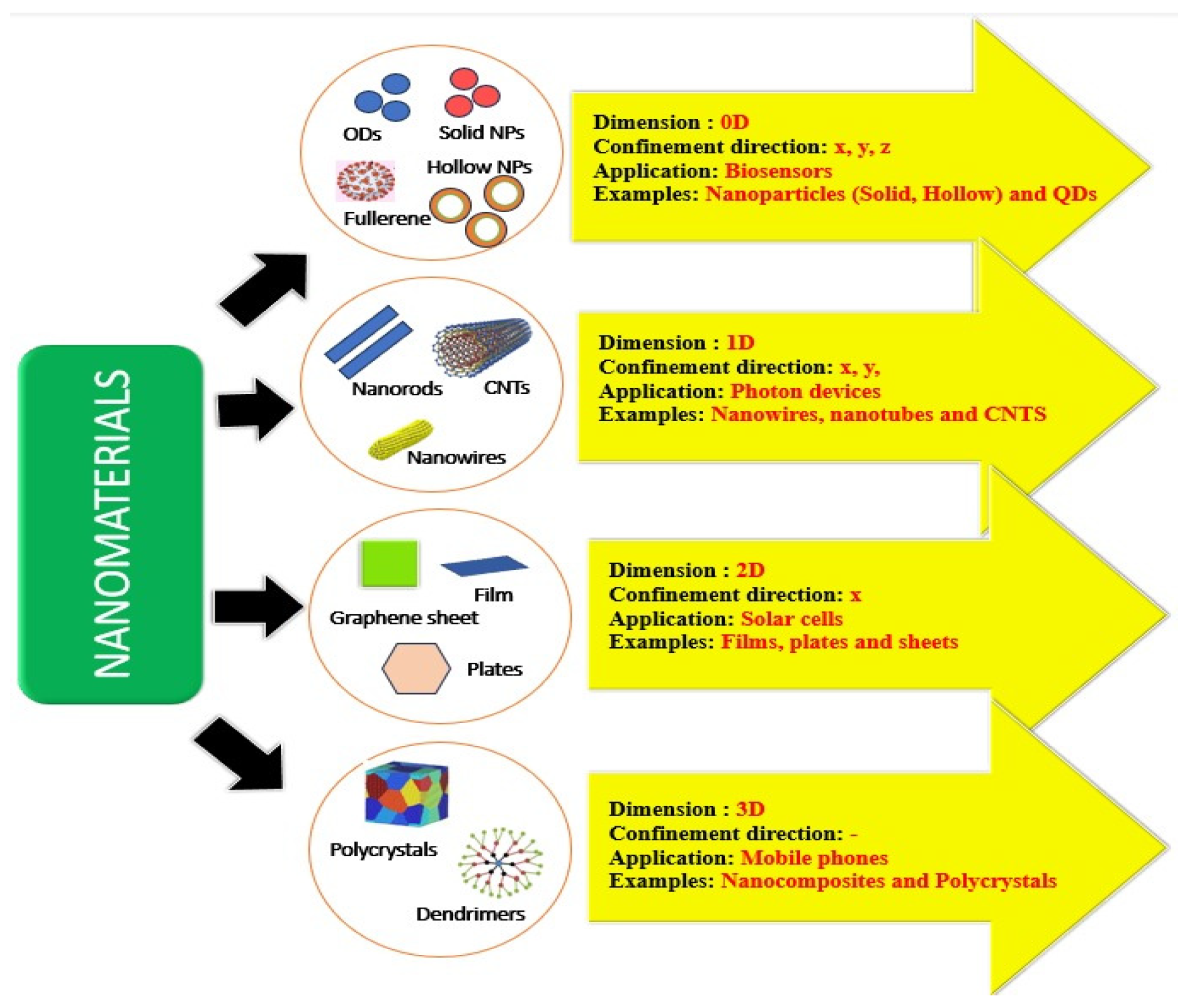
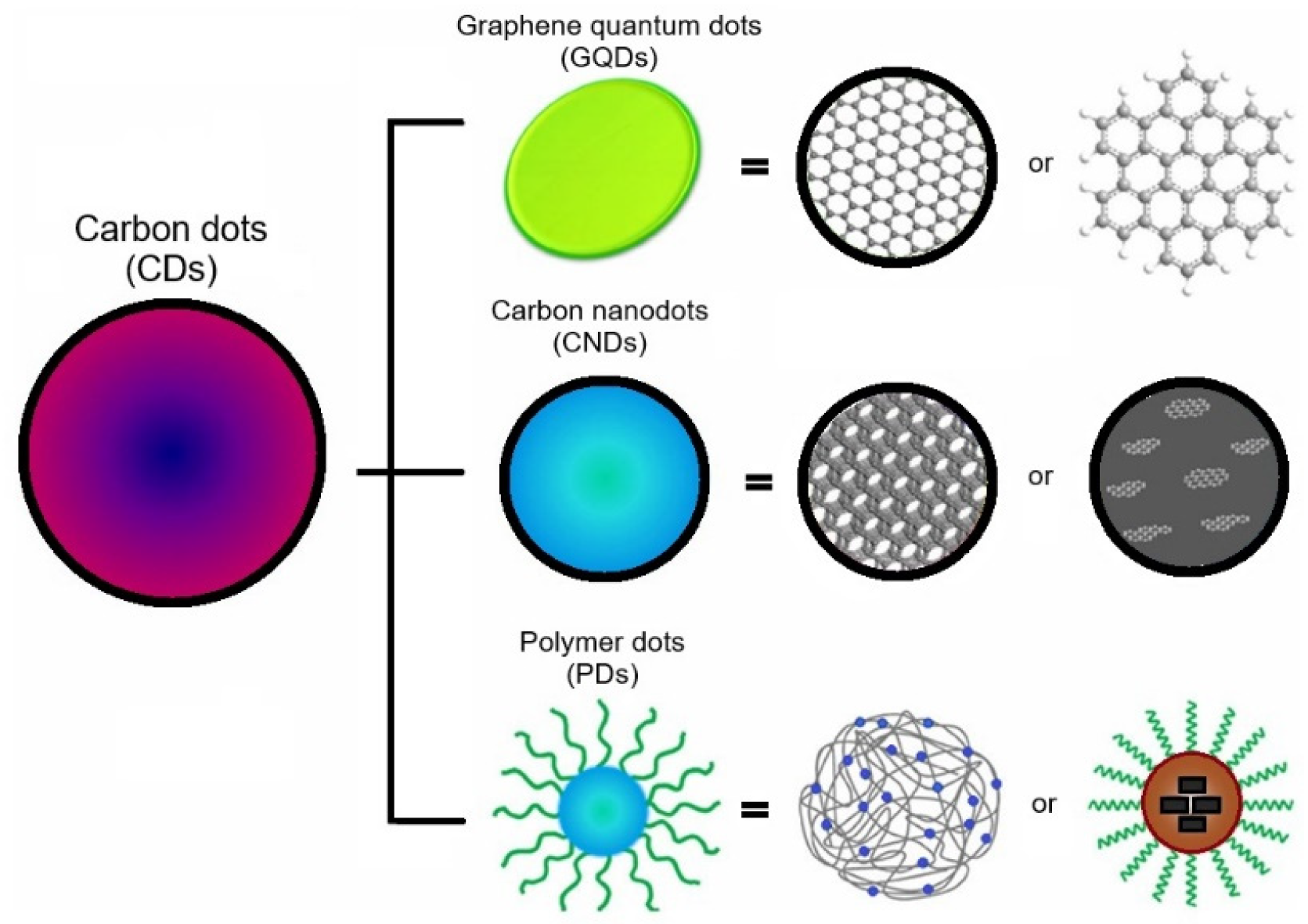
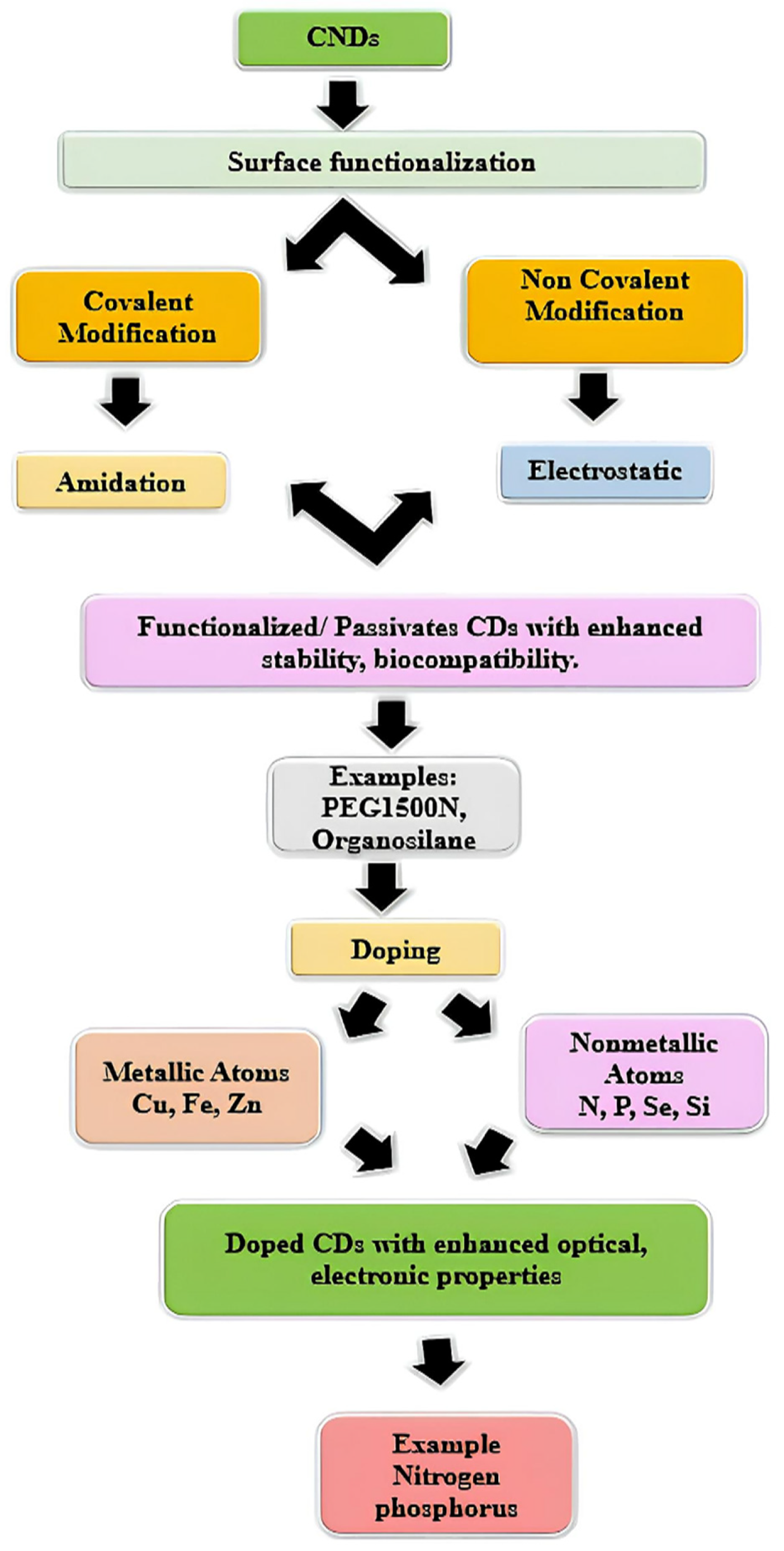
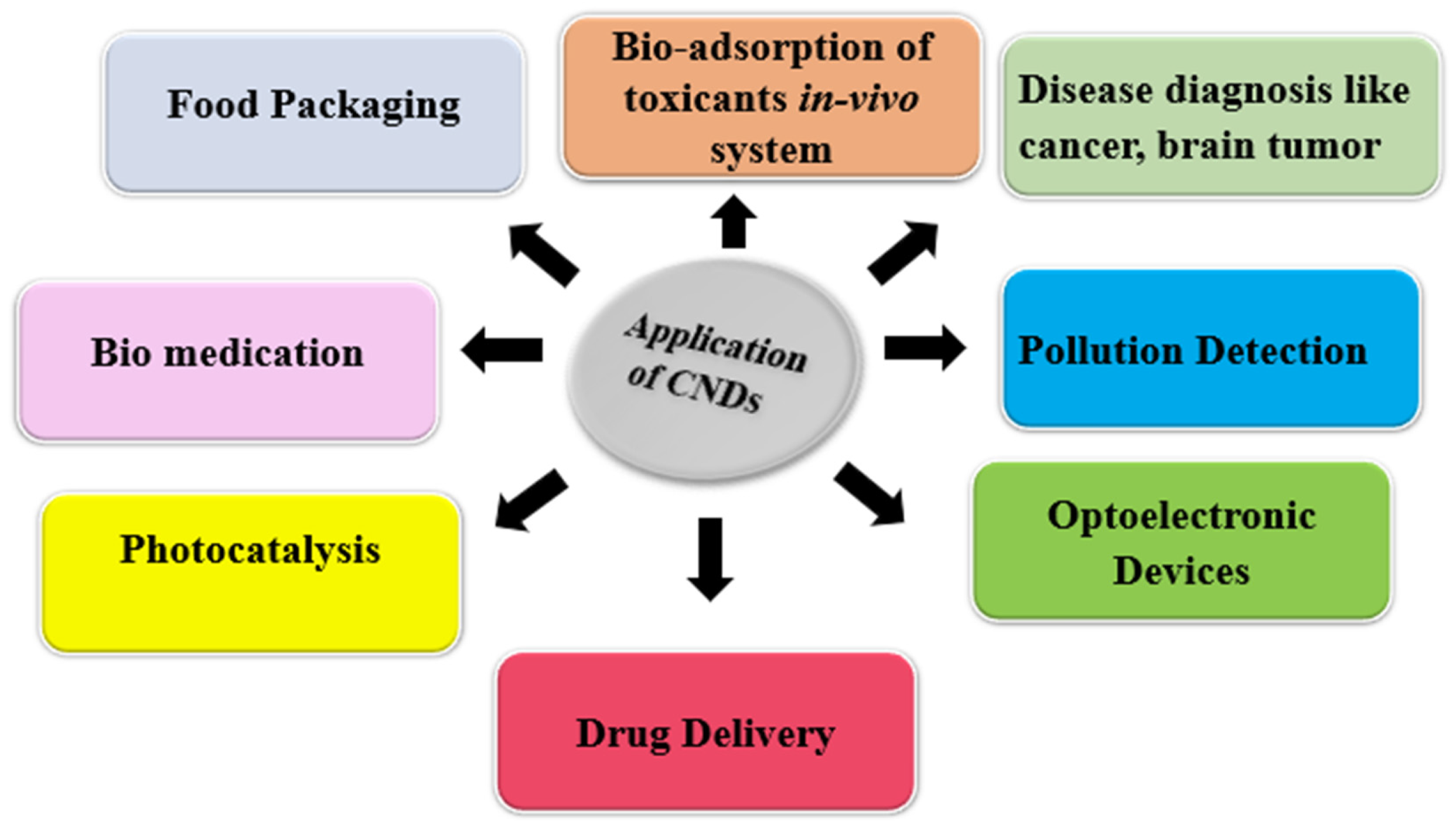
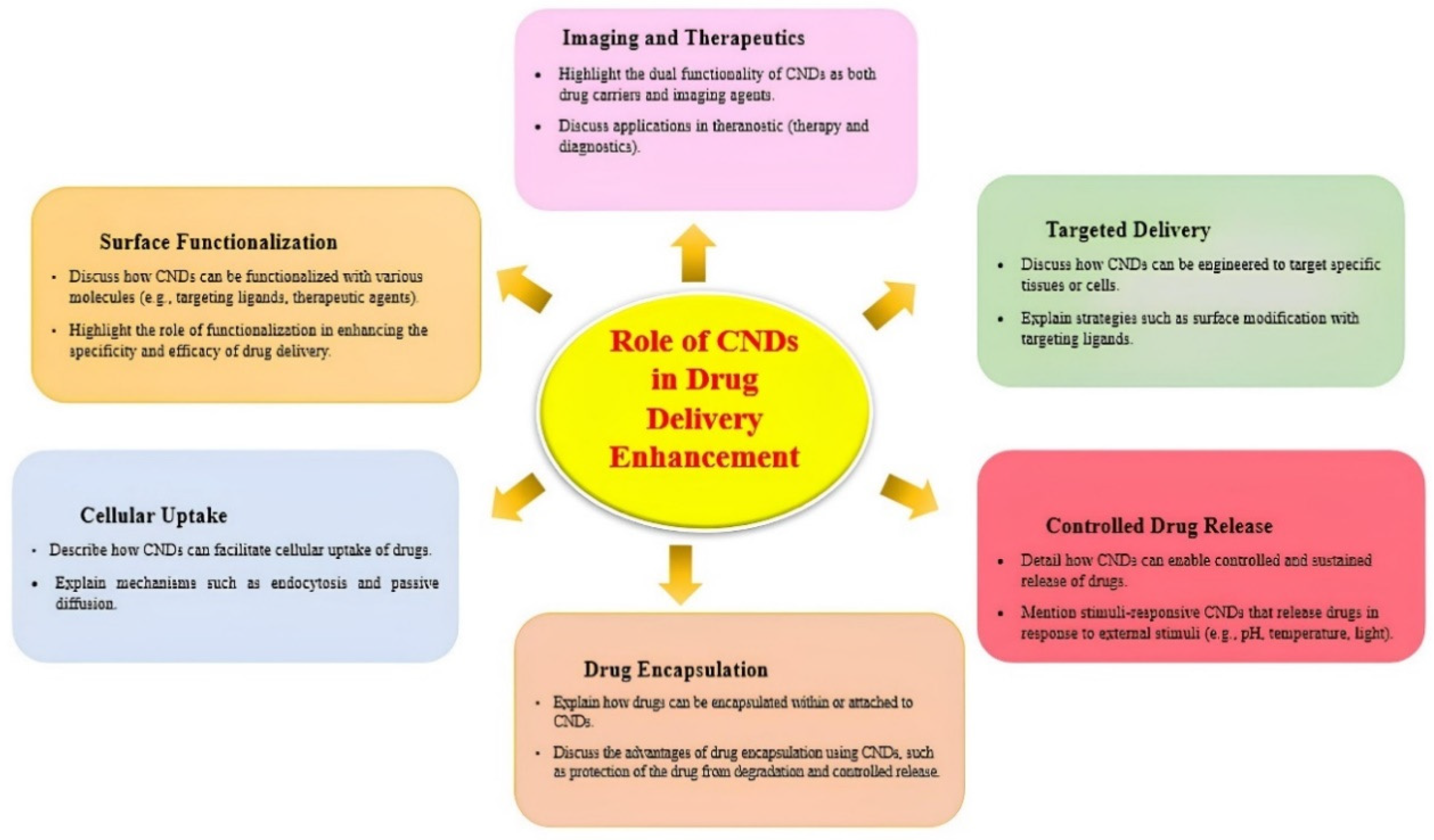
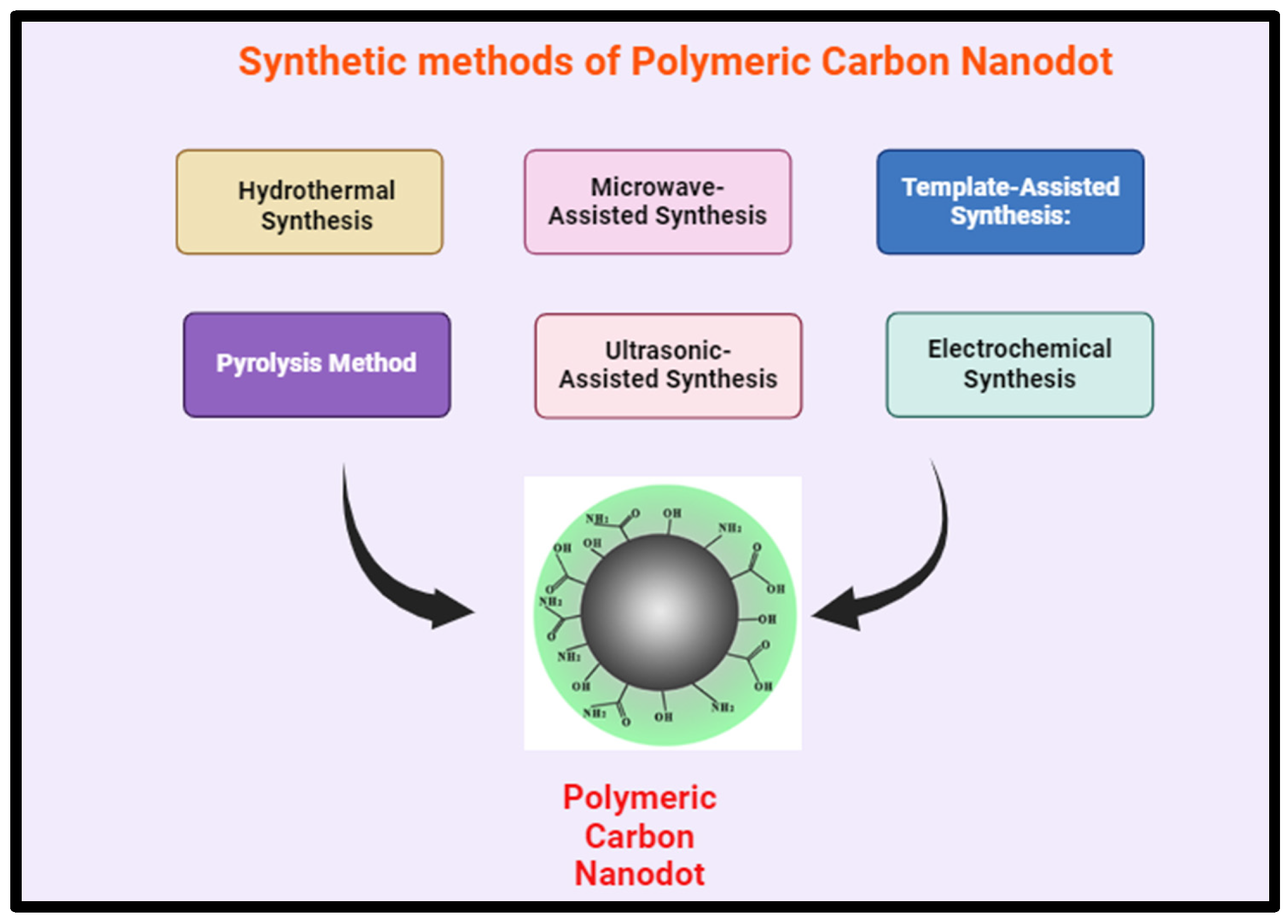
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




