Submitted:
23 July 2024
Posted:
24 July 2024
You are already at the latest version
Abstract
Keywords:
Epigraph
1. Introduction
- Emil von Behring, who received the first Nobel Prize for Physiology and Medicine in 1901 for introducing serum therapy as passive vaccination against diphtheria and tetanus [15]
- Robert Koch, famed for his studies on Tuberculosis and tuberculin-induced delayed hypersensitivity [16]
- Ramon Y. Cajal [17], who adapted Golgi’s silver staining method to map the intricate distribution of neurons in exquisite detail;
2. The Reticulo-Endothelial System (RES)
3.1. The Mononuclear Phagocyte System (MPS)
3.2. Enter the Lymphocytes
3.3. Enter Dendritic Cells (DCs)
3.4. Enter Monoclonal Antibodies
3.5. Enter Monoclonal Antibodies
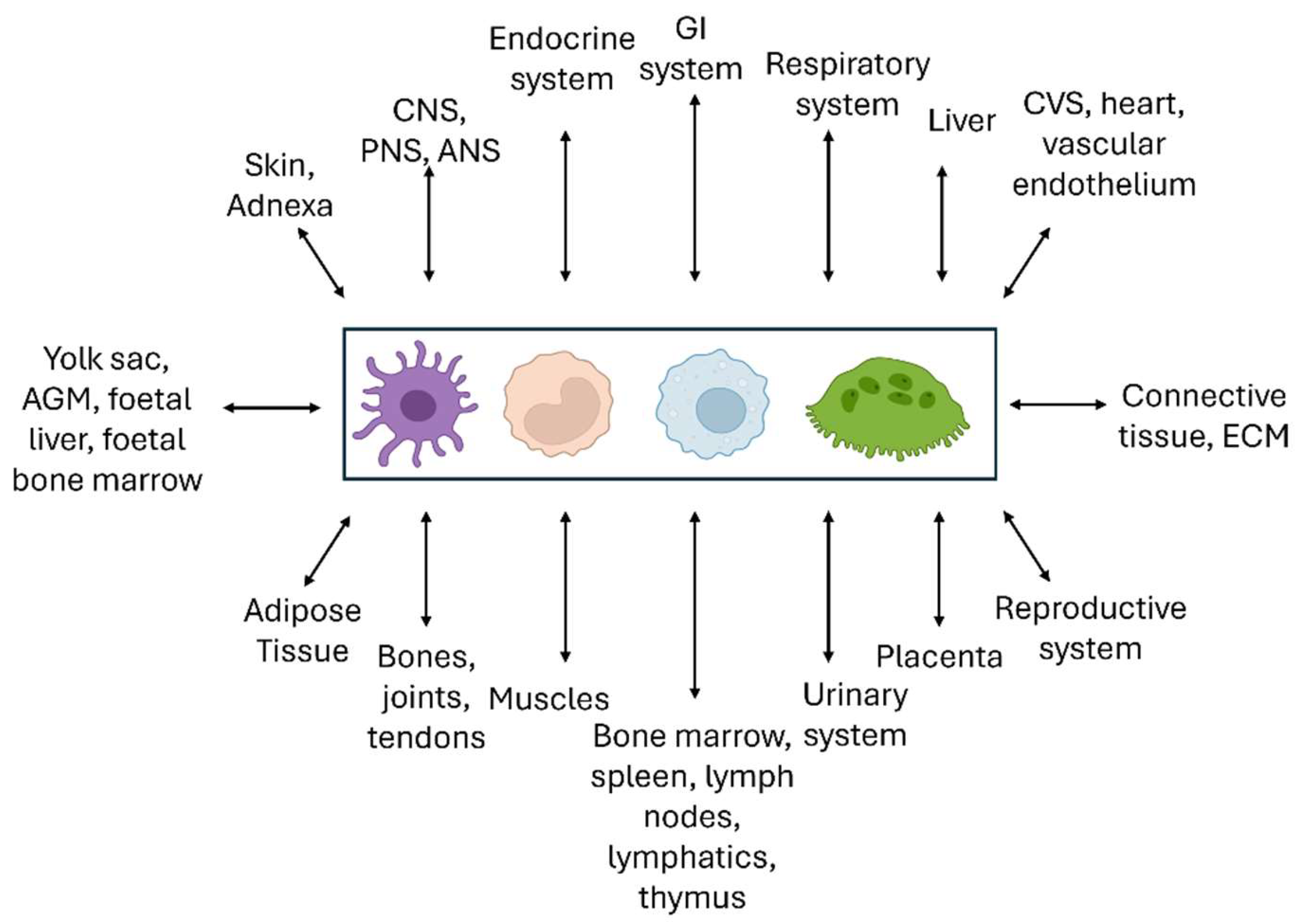
3.6. Development and Distribution
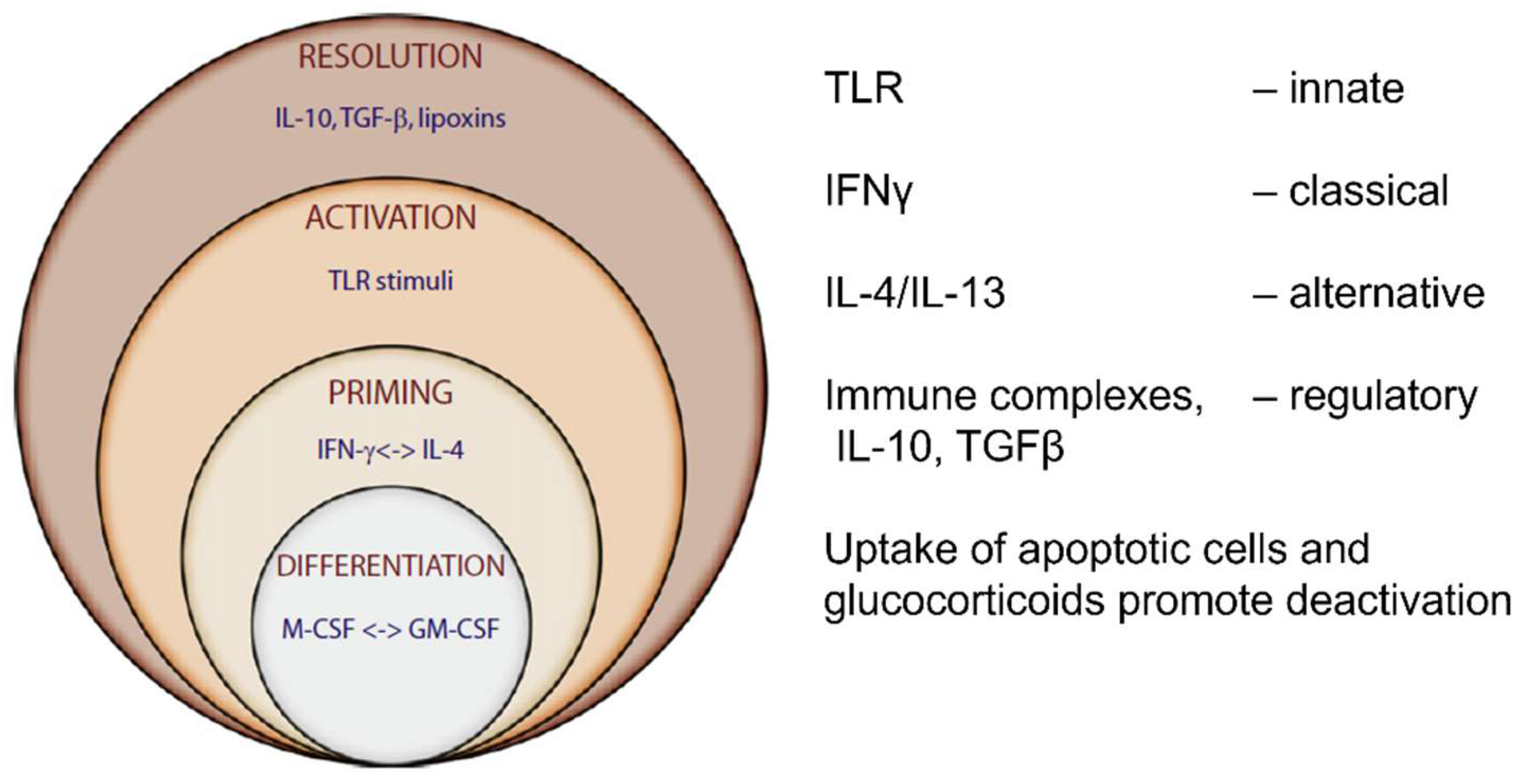
3.7. Distinct Properties of Elicited Monocyte-Derived Mononuclear Phagocytes
3.8. Re-Enter Complement
4. Cellular Functions of Mononuclear Phagocytes
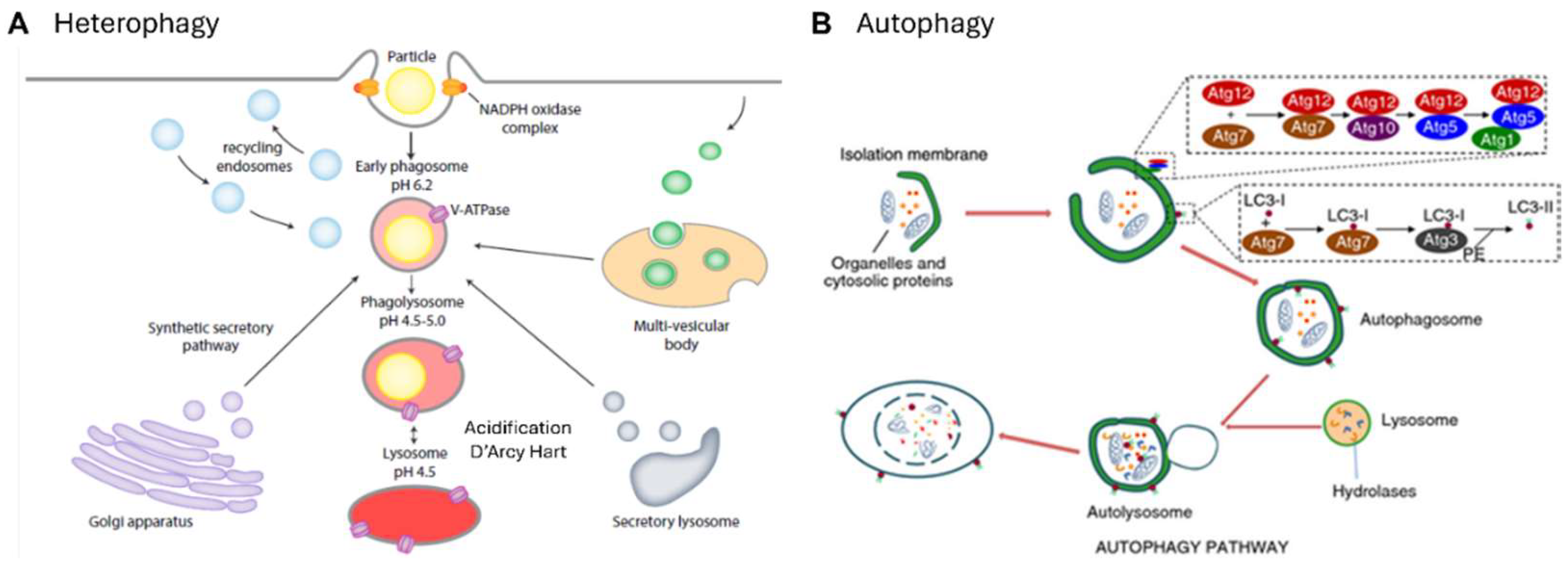
4.1. Recognition, Uptake and Degradation
4.2. Biosynthesis, Gene Expression, Metabolism, Cell Activation and Secretion
4.3. Cellular Immunity and Granuloma Formation
5. Discussion
6. Conclusion
Funding
Acknowledgements
Conflicts of Interest
Abbreviations
References
- Darwin, C. and G. Beer, On the Origin of Species. 2008: OUP Oxford.
- Harris, H. , The Birth of the Cell. 2000: Yale University Press.
- Ackerknecht, E.H. , Rudolf Virchow: doctor, statesman, anthropologist. 1953.
- Brock, T.D., G. L. Geison, and R.J. Dubos, Pasteur and Modern Science. 2014: Springer Berlin Heidelberg.
- Bernard, C. and L.L.S. University College, An Introduction to the Study of Experimental Medicine. 1865: Schuman.
- Cannon, W.B. , The Wisdom of the Body. 1939: W.W. Norton, Incorporated.
- Metchnikoff, E. , Lectures on the Comparative Pathology of Inflammation: Delivered at the Pasteur Institute in 1891. 1968: Dover Publications.
- Kaufmann, S.H. , Immunology’s foundation: the 100-year anniversary of the Nobel Prize to Paul Ehrlich and Elie Metchnikoff. Nat Immunol, 2008. 9(7): p. 705-12.
- Ehrlich, P. Paul Ehrlich - Nobel Lecture. Partial Cell Functions 1908 [cited 2024 17.06.]; Available from: https://www.nobelprize.org/prizes/medicine/1908/ehrlich/lecture/.
- Himmelweit, F. , The Collected Papers of Paul Ehrlich: in Four Volumes Including a Complete Bibliography. 2017: Elsevier Science.
- Ehrlich, P. , et al., The Collected Papers of Paul Ehrlich: Immunology and cancer research. 1957: Pergamon P.
- Ehrlich, P., T. Himmelweit, and M. Marquardt, The Collected Papers of Paul Ehrlich: Chemotherapy. 1960: Pergamon Press.
- Metchnikoff, E. Ilya Mechnikov - Nobel Lecture. On the Present State of the Question of Immunity in Infectious Diseases 1908 [cited 2024 17.06.]; Available from: https://www.nobelprize.org/prizes/medicine/1908/mechnikov/lecture/.
- Metchnikoff, O. , Life of Elie Metchnikoff 1845-1916. 1921: Houghton Mifflin, New York.
- Behring, E.v. Emil von Behring - Nobel Lecture. Serum Therapy in Therapeutics and Medical Science 1901 [cited 2024 17.06.2024]; Available from: https://www.nobelprize.org/prizes/medicine/1901/behring/lecture/.
- Brock, T.D. , Robert Koch: A Life in Medicine and Bacteriology. 1999: ASM Press.
- Ehrlich, B. , The Brain in Search of Itself: Santiago Ramón Y Cajal and the Story of the Neuron. 2022: Farrar, Straus and Giroux.
- Dormandy, T. , The White Death: A History of Tuberculosis. 1999: Hambledon Press.
- Paolicelli, R.C. , et al., Microglia states and nomenclature: A field at its crossroads. Neuron, 2022. 110(21): p. 3458-3483.
- Vikhanski, L. , Immunity: How Elie Metchnikoff Changed the Course of Modern Medicine. 2016: Chicago Review Press Incorporated.
- Cavaillon, J.M. , The historical milestones in the understanding of leukocyte biology initiated by Elie Metchnikoff. J Leukoc Biol, 2011. 90(3): p. 413-24.
- Clarke, D.J.B. , et al., Gene set predictor for post-treatment Lyme disease. Cell Reports Medicine, 2022. 3(11).
- Rasmussen, N. , René Dubos, the Autochthonous Flora, and the Discovery of the Microbiome. J Hist Biol, 2022. 55(3): p. 537-558.
- Stambler, I.S. , Elie Metchnikoff- The founder of longevity science and a founder of modern medicine: In honor of the 170th anniversary Adv Gerontol, 2015. 28(2): p. 207-17.
- Maisonneuve, P., E. Metchnikoff, and P.P.E. Roux, The Experimental Prophylaxis Of Syphilis. 2015: Creative Media Partners, LLC.
- Tolstoy, L. , The Death of Ivan Ilyich. 2020: Open Road Media.
- Rietschel, E.T. and J.-M. Cavaillon, Richard Pfeiffer and Alexandre Besredka: creators of the concept of endotoxin and anti-endotoxin. Microbes and Infection, 2003. 5(15): p. 1407-1414.
- Schama, S. , Foreign Bodies: Pandemics, Vaccines and the Health of Nations. 2023: Simon & Schuster UK.
- Bordet, J. The Nobel Prize in Physiology or Medicine 1919. 1919 [cited 2024 18.06.]; Available from: https://www.nobelprize.org/prizes/medicine/1919/summary/.
- Hansson, N. and D. Angetter-Pfeiffer, Remembering innovative paediatrician Clemens von Pirquet on the 150th anniversary of his birth. Acta Paediatrica. n/a(n/a).
- NobelPrize.org. Charles Richet - Nobel Lecture. [cited 2024 25.06.]; Available from: https://www.nobelprize.org/prizes/medicine/1913/richet/lecture/.
- Nijweide, P.J., E. H. Burger, and J.H. Feyen, Cells of bone: proliferation, differentiation, and hormonal regulation. Physiological Reviews, 1986. 66(4): p. 855-886.
- NobelPrize.org. The Nobel Prize in Physiology or Medicine 1945. [cited 2024 15.07.]; Available from: https://www.nobelprize.org/prizes/medicine/1945/summary/.
- NobelPrize.org. The Nobel Prize in Physiology or Medicine 1999. [cited 2024 15.07.]; Available from: https://www.nobelprize.org/prizes/medicine/1999/summary/.
- Hamann, J. and A.G. Petrenko, Introduction: History of the Adhesion GPCR Field. Handb Exp Pharmacol, 2016. 234: p. 1-11.
- Brown, G.D. and S. Gordon, Immune recognition. A new receptor for beta-glucans. Nature, 2001. 413(6851): p. 36-7.
- NobelPrize.org. The Nobel Prize in Physiology or Medicine 2011. [cited 2024 15.07.]; Available from: https://www.nobelprize.org/prizes/medicine/2011/summary/.
- NobelPrize.org. The Nobel Prize in Physiology or Medicine 1980. [cited 2024 15.07.]; Available from: https://www.nobelprize.org/prizes/medicine/1980/summary/.
- Dzierzak, E. and E. de Pater, Regulation of Blood Stem Cell Development. Curr Top Dev Biol, 2016. 118: p. 1-20.
- NobelPrize.org. The Nobel Prize in Physiology or Medicine 2019. [cited 2024 15.07.]; Available from: https://www.nobelprize.org/prizes/medicine/2019/summary/.
- Wake, K. , Karl Wilhelm Kupffer And His Contributions To Modern Hepatology. Comp Hepatol, 2004. 3 Suppl 1(Suppl 1): p. S2.
- Kodama, T. , et al., Type I macrophage scavenger receptor contains α-helical and collagen-like coiled coils. Nature, 1990. 343(6258): p. 531-535.
- Stanley, E.R. and V. Chitu, CSF-1 receptor signaling in myeloid cells. Cold Spring Harb Perspect Biol, 2014. 6(6).
- Pappenheim, A. and A. Ferrata, Über die verschiedenen lymphoiden Zellformen des normalen und pathologischen Blutes. Vol. 10. 1911: Klinkhardt.
- Beutler, B. and A. Cerami, The biology of cachectin/TNF--a primary mediator of the host response. Annu Rev Immunol, 1989. 7: p. 625-55.
- Henson, P.M. and D.L. Bratton, Recognition and removal of apoptotic cells, in Phagocyte-pathogen interactions: macrophages and the host response to infection, D.G. Russell and S. Gordon, Editors. 2009, ASM Press: Washington, D.C. p. 341-365.
- Crocker, P.R. and S. Gordon, Isolation and characterization of resident stromal macrophages and hematopoietic cell clusters from mouse bone marrow. J Exp Med, 1985. 162(3): p. 993-1014.
- Nagata, S. , Apoptosis and Clearance of Apoptotic Cells. Annu Rev Immunol, 2018. 36: p. 489-517.
- Rossi, F. , The O2- -forming NADPH oxidase of the phagocytes: nature, mechanisms of activation and function. Biochim Biophys Acta, 1986. 853(1): p. 65-89.
- Waddington, C.H. Epigenetics and evolution. in Symp Soc Exp Biol. 1953.
- Blackburn, D.G. and J.R. Stewart, Morphological research on amniote eggs and embryos: An introduction and historical retrospective. Journal of Morphology, 2021. 282(7): p. 1024-1046.
- Bessis, M. , L’ilot erythroblastique. Unite functionelle de la moelle osseuse. Rev Hematol, 1958. 13(1): p. 8-11.
- Kaufmann, S.H.E. , Immunology’s Coming of Age. Front Immunol, 2019. 10: p. 684.
- Silverstein, A.M. , Paul Ehrlich’s Receptor Immunology: The Magnificent Obsession. 2001: Elsevier Science.
- Barry Kay, A. , Paul Ehrlich and the Early History of Granulocytes, in Myeloid Cells in Health and Disease. 2017. p. 1-15.
- Aschoff, L. , Das reticulo-endotheliale system. Ergeb Inn Med Kinderheilk, 1924. 26: p. 1-118.
- Cannon, W.B. , The Way of an Investigator: A Scientist’s Experiences in Medical Research. 1984: W.W. Norton.
- Tan, S.Y. and A. Yip, Hans Selye (1907-1982): Founder of the stress theory. Singapore Med J, 2018. 59(4): p. 170-171.
- Dunnill, M.S. , The Plato of Praed Street: The Life and Times of Almroth Wright. 2000: Royal Society of Medicine Press.
- NobelPrize.org. The Nobel Prize in Physiology or Medicine 1972. 1972 [cited 2024 18.06.]; Available from: https://www.nobelprize.org/prizes/medicine/1972/summary/.
- Mastellos, D.C., G. Hajishengallis, and J.D. Lambris, A guide to complement biology, pathology and therapeutic opportunity. Nature Reviews Immunology, 2024. 24(2): p. 118-141.
- Janeway, C. , et al., Immunobiology: The Immune System in Health and Disease. 2005: Garland Science.
- MacPhail, T. , Allergic: How Our Immune System Reacts to a Changing World. 2023: Penguin Books Limited.
- Shaw, B. and D. Laurence, The Doctor’s Dilemma. 1987: Penguin Adult.
- Berry, A. and J. Browne, Mendel and Darwin. Proc Natl Acad Sci U S A, 2022. 119(30): p. e2122144119.
- Bearn, A.G. , Archibald Garrod and the Individuality of Man. 1993: Clarendon Press.
- Roos, D. and M. de Boer, Molecular diagnosis of chronic granulomatous disease. Clin Exp Immunol, 2014. 175(2): p. 139-49.
- Bastard, P. , et al., Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science, 2020. 370(6515).
- Savic, S., J. Coe, and P. Laws, Autoinflammation: Interferonopathies and Other Autoinflammatory Diseases. Journal of Investigative Dermatology, 2022. 142(3, Part B): p. 781-792.
- Grabowski, G.A. and D. Hughes, Emerging Therapies, in Lysosomal Storage Disorders. 2022. p. 287-294.
- Rous, P. and J.W. Beard, Selection with the magnet and cultivation of reticulo-endothelial cells (Kupffer cells). J Exp Med, 1934. 59(5): p. 577-91.
- Sabin, F.R. , Cunningham, Robert S., Doan, Charles A., Studies of the Blood in Experimental Tuberculosis: The Monocyte-Lymphocyte Ratio; The Anemia-Leucopenia Phase. National Tuberculosis Association, 1926(Transactions of the 22nd Annual Meeting of the National Tuberculosis Association).
- Dannenberg, A.M. , Cellular hypersensitivity and cellular immunity in the pathogensis of tuberculosis: specificity, systemic and local nature, and associated macrophage enzymes. Bacteriological reviews, 1968. 32(2): p. 85-102.
- NobelPrize.org. Karl Landsteiner - Nobel Lecture. On Individual Differences in Human Blood [cited 2024 25.06.]; Available from: https://www.nobelprize.org/prizes/medicine/1930/landsteiner/lecture/.
- Landsteiner, K. and M.W. Chase, Experiments on Transfer of Cutaneous Sensitivity to Simple Compounds. Proceedings of the Society for Experimental Biology and Medicine, 1942. 49(4): p. 688-690.
- Fleming, A. and A.E. Wright, On a remarkable bacteriolytic element found in tissues and secretions. Proceedings of the Royal Society of London. Series B, Containing Papers of a Biological Character, 1922. 93(653): p. 306-317.
- Leonard, C. , Alexander Fleming, 1881-1955. Biogr. Mems Fell. R. Soc.2117–127, 1956.
- Fleming, A. , On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzae. 1929. Bull World Health Organ, 2001. 79(8): p. 780-90.
- NobelPrize.org. The Nobel Prize in Physiology or Medicine 1945. [cited 2024 25.06.]; Available from: https://www.nobelprize.org/prizes/medicine/1945/summary/.
- Florey, H. , General Pathology. 1970: W. B. Saunders Company.
- Mackaness, G.B. , The action of drugs on intracellular tubercle bacilli. The Journal of Pathology and Bacteriology, 1952. 64(3): p. 429-446.
- Gowans, J.L., E. J. Knight, and H.W. Florey, The route of re-circulation of lymphocytes in the rat. Proceedings of the Royal Society of London. Series B. Biological Sciences, 1964. 159(975): p. 257-282.
- Harris, H. , Role of chemotaxis in inflammation. Physiol Rev, 1954. 34(3): p. 529-62.
- Billingham, R.E., L. Brent, and P.B. Medawar, ‘Actively Acquired Tolerance’ of Foreign Cells. Nature, 1953. 172(4379): p. 603-606.
- Medawar, P.B. , Immunological tolerance. Science, 1961. 133(3449): p. 303-6.
- Porterfield, J.S. , The National Institute for Medical Research, Mill Hill. Archives of Virology, 1995. 140(7): p. 1329-1336.
- Isaacs, A., J. Lindenmann, and C.H. Andrewes, Virus interference. I. The interferon. Proceedings of the Royal Society of London. Series B - Biological Sciences, 1957. 147(927): p. 258-267.
- Armstrong, J.A. and P.D. Hart, Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med, 1971. 134(3 Pt 1): p. 713-40.
- Humphrey, J.H. and D. Grennan, Different macrophage populations distinguished by means of fluorescent polysaccharides. Recognition and properties of marginal-zone macrophages. Eur J Immunol, 1981. 11(3): p. 221-8.
- Mitchison, T.J. and Beverly, C.L., Nicholas Avrion Mitchison. 5 May 1928 — 28 December 2022. Biogr. Mems Fell. R. Soc., 2024.
- Moberg, C.L. and R.M. Steinman, James Gerald Hirsch. Biogr Mem Natl Acad Sci, 2004. 84: p. 182-203.
- Steinman, R.M. and C.L. Moberg, Zanvil Alexander Cohn 1926-1993. J Exp Med, 1994. 179(1): p. 1-30.
- Moberg, C.L. , An appreciation of Ralph Marvin Steinman (1943-2011). J Exp Med, 2011. 208(12): p. 2337-42.
- Moberg, C.L. , René Dubos, Friend of the Good Earth: Microbiologist, Medical Scientist, Environmentalist. 2005: ASM Press.
- Woodruff, H.B. , Selman A. Waksman, winner of the 1952 Nobel Prize for physiology or medicine. Appl Environ Microbiol, 2014. 80(1): p. 2-8.
- Pringle, P. , Experiment Eleven: Dark Secrets Behind the Discovery of a Wonder Drug. 2012: Bloomsbury Publishing.
- Hirsch, J.G. , Bactericidal action of histone Journal of Experimental Medicine, 1958. 108(6): p. 925-944.
- Horwitz, M.A. , The Legionnaires’ disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med, 1983. 158(6): p. 2108-26.
- Jones, T.C. and J.G. Hirsch, The interaction between Toxoplasma gondii and mammalian cells. II. The absence of lysosomal fusion with phagocytic vacuoles containing living parasites. J Exp Med, 1972. 136(5): p. 1173-94.
- Johnson, W.D., Jr., B. Mei, and Z.A. Cohn, The separation, long-term cultivation, and maturation of the human monocyte. J Exp Med, 1977. 146(6): p. 1613-26.
- NobelPrize.org. George E. Palade - Facts. [cited 2024 20.06.]; Available from: https://www.nobelprize.org/prizes/medicine/1974/palade/facts/.
- De Duve, C. , Vital Dust: The Origin and Evolution of Life on Earth. 1995: Basic Books.
- Rabinovitch, M. , Professional and non-professional phagocytes: an introduction. Trends in Cell Biology, 1995. 5(3): p. 85-87.
- Kaplan, G. and Z.A. Cohn, The immunobiology of leprosy. Int Rev Exp Pathol, 1986. 28: p. 45-78.
- Kaplan, G. and Z.A. Cohn, Leprosy and cell-mediated immunity. Curr Opin Immunol, 1991. 3(1): p. 91-6.
- McElrath, M.J., R. M. Steinman, and Z.A. Cohn, Latent HIV-1 infection in enriched populations of blood monocytes and T cells from seropositive patients. J Clin Invest, 1991. 87(1): p. 27-30.
- Van Furth, R. and L. Conference on Mononuclear Phagocytes, Mononuclear Phagocytes. Edited by Ralph Van Furth. 1970: Blackwell Scientific Publications (C1970).
- Van Furth, R. , Mononuclear Phagocytes in Immunity, Infection and Pathology. 1975: Mosby, Incorporated.
- van Furth, R. , Mononuclear Phagocytes: Functional Aspects. 1980: Springer Netherlands.
- van Furth, R. , Mononuclear Phagocytes: Characteristics, Physiology and Function. 1985: Springer Netherlands.
- van Furth, R. , et al., The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ, 1972. 46(6): p. 845-52.
- Tauber, A.I. , The birth of immunology: III. The fate of the phagocytosis theory. Cellular Immunology, 1992. 139(2): p. 505-530.
- Gowans, J.L. , First Medawar prize lecture. The recirculating small lymphocyte. Transplant Proc, 1991. 23(1 Pt 1): p. 7-8.
- Volkman, A. and J.L. Gowans, The origin of macrophages from bone marrow in the rat. Br J Exp Pathol, 1965. 46(1): p. 62-70.
- Marchesi, V.T. and J.L. Gowans, The migration of lymphocytes through the endothelium of venules in lymph nodes: an electron microscope study. Proceedings of the Royal Society of London. Series B. Biological Sciences, 1964. 159: p. 283 - 290.
- Miller, J.F.A.P. , Immunological function of the thymus The Lancet, 1961. 278(7205): p. 748-749.
- Miller, J.F. , The discovery of thymus function and of thymus-derived lymphocytes. Immunol Rev, 2002. 185: p. 7-14.
- Nature Milestones: T cells. 2022 [cited 2024 20.06.]; Available from: https://www.nature.com/immersive/d42859-022-00032-7/index.html.
- Gitlin, A.D. and M.C. Nussenzweig, Immunology: Fifty years of B lymphocytes. Nature, 2015. 517(7533): p. 139-141.
- Zoltan Fehervari, Y.B. , Cosma Dellisanti, Nicholas Bernard & Jamie D. K. Wilson. Nature Milestones in Antibodies. 2016 [cited 2024 20.06.]; Available from: https://www.nature.com/articles/d42859-021-00068-1.
- Burnet, M. , Self and Not-Self: Cellular Immunology Book One. 1969: Cambridge University Press.
- Burnet, F.M. , Immunological recognition of self. Science, 1961. 133(3449): p. 307-11.
- NobelPrize.org. The Nobel Prize in Physiology or Medicine 1996. [cited 2024 20.6.]; Available from: https://www.nobelprize.org/prizes/medicine/1996/summary/.
- Metcalf, D. , Growth and Differentiation Factors. Microbiol Spectr, 2016. 4(4).
- Pixley, F.J. and E.R. Stanley, CSF-1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol, 2004. 14(11): p. 628-38.
- Mackaness, G.B. , The Immunological Basis of Acquired Cellular Resistance. J Exp Med, 1964. 120: p. 105-20.
- Sorg, C. and B.R. Bloom Products of activated lymphocytes: I. The use of radiolabeling techniques in the characterization and partial purification of the migration inhibitory factor of the guinea pig Journal of Experimental Medicine, 1973. 137(1): p. 148-170.
- Dinarello, C.A. , Historical insights into cytokines. Eur J Immunol, 2007. 37 Suppl 1(Suppl 1): p. S34-45.
- Mosmann, T.R. and R.L. Coffman, TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol, 1989. 7: p. 145-73.
- Nathan, C.F. , et al., Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. Journal of Experimental Medicine, 1983. 158(3): p. 670-689.
- Gray, P.W. and D.V. Goeddel, Structure of the human immune interferon gene. Nature, 1982. 298(5877): p. 859-863.
- Moberg, C.L. , The discovery of dendritic cells. Journal of Experimental Medicine, 2021. 218(6).
- Steinman, R. , Dendritic cells: linking innate to different forms of adaptive immunity in The Biomedical & Biomedical Life Sciences Collection. 2009, Henry Stewart Talks.
- Unanue, E.R. and B.A. Askonas, Persistence of immunogenicity of antigen after uptake by macrophages. J Exp Med, 1968. 127(5): p. 915-26.
- Silverstein, S.C., R. M. Steinman, and Z.A. Cohn, Endocytosis. Annu Rev Biochem, 1977. 46: p. 669-722.
- Elliott, T. , et al., Structural requirements for the peptide-induced conformational change of free major histocompatibility complex class I heavy chains. European Journal of Immunology, 1992. 22(8): p. 2085-2091.
- Bjorkman, P.J. , et al., Structure of the human class I histocompatibility antigen, HLA-A2. Nature, 1987. 329(6139): p. 506-512.
- Steinman, R.M. and Z.A. Cohn, Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med, 1973. 137(5): p. 1142-62.
- Nussenzweig, M.C. and R.M. Steinman, Contribution of dendritic cells to stimulation of the murine syngeneic mixed leukocyte reaction. J Exp Med, 1980. 151(5): p. 1196-212.
- Paul, W.E. , Dendritic cells bask in the limelight. Cell, 2007. 130(6): p. 967-70.
- Inaba, K. , et al., Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med, 1992. 176(6): p. 1693-702.
- Merad, M. , et al., The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol, 2013. 31: p. 563-604.
- Bálint, Š. , et al., Supramolecular attack particles are autonomous killing entities released from cytotoxic T cells. Science, 2020. 368(6493): p. 897-901.
- Horton, C., K. Shanmugarajah, and P.J. Fairchild, Harnessing the properties of dendritic cells in the pursuit of immunological tolerance. Biomedical Journal, 2017. 40(2): p. 80-93.
- Qin, S. , et al., “Infectious” Transplantation Tolerance. Science, 1993. 259(5097): p. 974-977.
- Mellman, I. , et al., The cancer-immunity cycle: Indication, genotype, and immunotype. Immunity, 2023. 56(10): p. 2188-2205.
- Bearn, A.G., F. J. Dixon, and B. Benacerraf, Henry G. Kunkel 1916-1983. An appreciation of the man and his scientific contributions & a bibliography of his research papers. J Exp Med, 1985. 161(5): p. 869-95.
- Harris, H. , et al., Artificial heterokaryons of animal cells from different species. J Cell Sci, 1966. 1(1): p. 1-30.
- Klein, G., S. Friberg, Jr., and H. Harris, Two kinds of antigen suppression in tumor cells revealed by cell fusion. J Exp Med, 1972. 135(4): p. 839-49.
- Köhler, G. and C. Milstein, Continuous cultures of fused cells secreting antibody of predefined specificity. Nature, 1975. 256(5517): p. 495-7.
- Crumpton, M.J., Alan Frederick Williams 25 May 1945 - 9 April 1992. Biographical Memoirs of Fellows of the Royal Society, 2004. 50: p. 351-366.
- Springer, T. , et al., Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol, 1979. 9(4): p. 301-6.
- Diamond, M.S. , et al., Binding of the integrin Mac-1 (CD11b/CD18) to the third immunoglobulin-like domain of ICAM-1 (CD54) and its regulation by glycosylation. Cell, 1991. 65(6): p. 961-971.
- Feldman, M. , et al., Anti-TNF alpha therapy is useful in rheumatoid arthritis and Crohn’s disease: analysis of the mechanism of action predicts utility in other diseases. Transplant Proc, 1998. 30(8): p. 4126-7.
- Austyn, J.M. and S. Gordon, F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol, 1981. 11(10): p. 805-15.
- Gordon, S. and A. Pluddemann, Tissue macrophages: heterogeneity and functions. BMC Biol, 2017. 15(1): p. 53.
- Taylor, P.R. , et al., Macrophage receptors and immune recognition. Annu Rev Immunol, 2005. 23: p. 901-44.
- Lin, H.H. , et al., Ligands and Beyond: Mechanosensitive Adhesion GPCRs. Pharmaceuticals (Basel), 2022. 15(2).
- I, K.Y. , et al., Activation of Adhesion GPCR EMR2/ADGRE2 Induces Macrophage Differentiation and Inflammatory Responses via Gα(16)/Akt/MAPK/NF-κB Signaling Pathways. Front Immunol, 2017. 8: p. 373.
- Lin, H.H. , et al., The macrophage F4/80 receptor is required for the induction of antigen-specific efferent regulatory T cells in peripheral tolerance. J Exp Med, 2005. 201(10): p. 1615-25.
- Gordon, S., A. Plüddemann, and S. Mukhopadhyay, Plasma membrane receptors of tissue macrophages: functions and role in pathology. J Pathol, 2020. 250(5): p. 656-666.
- Zulu, M.Z. , et al., The Elusive Role of Placental Macrophages: The Hofbauer Cell. J Innate Immun, 2019: p. 1-10.
- Takahashi, K. and S. Yamanaka, Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 2006. 126(4): p. 663-76.
- Fejer, G. , et al., Nontransformed, GM-CSF-dependent macrophage lines are a unique model to study tissue macrophage functions. Proc Natl Acad Sci U S A, 2013. 110(24): p. E2191-8.
- Mass, E. , et al., Specification of tissue-resident macrophages during organogenesis. Science, 2016. 353(6304).
- Guilliams, M. and C.L. Scott, Liver macrophages in health and disease. Immunity, 2022. 55(9): p. 1515-1529.
- Amit, I., D. R. Winter, and S. Jung, The role of the local environment and epigenetics in shaping macrophage identity and their effect on tissue homeostasis. Nat Immunol, 2016. 17(1): p. 18-25.
- Ginhoux, F. , et al., Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science, 2010. 330(6005): p. 841-5.
- Hume, D.A., V. H. Perry, and S. Gordon, The mononuclear phagocyte system of the mouse defined by immunohistochemical localisation of antigen F4/80: macrophages associated with epithelia. Anat Rec, 1984. 210(3): p. 503-12.
- Lawson, L.J. , et al., Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience, 1990. 39(1): p. 151-70.
- Silverstein, S.C., J. Michl, and J.D. Loike, Studies of the Mechanism of Phagocytosis, in International Cell Biology 1980–1981: Papers Presented at the Second International Congress on Cell Biology Berlin (West), August 31 – September 5, 1980, H.G. Schweiger, Editor. 1981, Springer Berlin Heidelberg: Berlin, Heidelberg. p. 604-612.
- Yona, S. , et al., Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity, 2013. 38(1): p. 79-91.
- Mariani, S.A. , et al., Pro-inflammatory Aorta-Associated Macrophages Are Involved in Embryonic Development of Hematopoietic Stem Cells. Immunity, 2019. 50(6): p. 1439-1452.e5.
- Schulz, C. , et al., A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science, 2012. 336(6077): p. 86-90.
- Ginhoux, F. and M. Guilliams, Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity, 2016. 44(3): p. 439-449.
- Orsenigo, F. , et al., Unifying considerations and evidence of macrophage activation mosaicism through human CSF1R and M1/M2 genes. Cell Reports, 2024. 43(6).
- Ziegler-Heitbrock, L. , et al., Nomenclature of monocytes and dendritic cells in blood. Blood, 2010. 116(16): p. e74-80.
- Carlin, L. , et al., Functions and molecular mechanisms of patrolling monocytes. Vascular Pharmacology, 2012. 56: p. 328.
- Janssen, H. , et al., Monocytes re-enter the bone marrow during fasting and alter the host response to infection. Immunity, 2023. 56(4): p. 783-796.e7.
- Hossain, M. and P. Kubes, Innate immune cells orchestrate the repair of sterile injury in the liver and beyond. European Journal of Immunology, 2019. 49(6): p. 831-841.
- Bellingan, G.J. , et al., In vivo fate of the inflammatory macrophage during the resolution of inflammation: inflammatory macrophages do not die locally, but emigrate to the draining lymph nodes. J Immunol, 1996. 157(6): p. 2577-85.
- Bonnardel, J. , et al., Stellate Cells, Hepatocytes, and Endothelial Cells Imprint the Kupffer Cell Identity on Monocytes Colonizing the Liver Macrophage Niche. Immunity, 2019. 51(4): p. 638-654.e9.
- Cugurra, A. , et al., Skull and vertebral bone marrow are myeloid cell reservoirs for the meninges and CNS parenchyma. Science, 2021. 373(6553): p. eabf7844.
- Jacome-Galarza, C.E. , et al., Developmental origin, functional maintenance and genetic rescue of osteoclasts. Nature, 2019. 568(7753): p. 541-545.
- Alvarez, D., E. H. Vollmann, and U.H. von Andrian, Mechanisms and consequences of dendritic cell migration. Immunity, 2008. 29(3): p. 325-42.
- Towiwat, P., A. Chhana, and N. Dalbeth, The anatomical pathology of gout: a systematic literature review. BMC Musculoskeletal Disorders, 2019. 20(1): p. 140.
- Turner, M.W. , The role of mannose-binding lectin in health and disease. Mol Immunol, 2003. 40(7): p. 423-9.
- Sawaya, A.P. , et al., Calreticulin: a multifunctional protein with potential therapeutic applications for chronic wounds. Front Med (Lausanne), 2023. 10: p. 1207538.
- Mantovani, A. , et al., The long pentraxin PTX3: a paradigm for humoral pattern recognition molecules. Annals of the New York Academy of Sciences, 2013. 1285(1): p. 1-14.
- Colten, H.R. , Ontogeny of the human complement system: in vitro biosynthesis of individual complement components by fetal tissues. J Clin Invest, 1972. 51(4): p. 725-30.
- Wu, X. , et al., Complement C1q drives microglia-dependent synaptic loss and cognitive impairments in a mouse model of lipopolysaccharide-induced neuroinflammation. Neuropharmacology, 2023. 237: p. 109646.
- West, E.E. and C. Kemper, Complosome - the intracellular complement system. Nat Rev Nephrol, 2023. 19(7): p. 426-439.
- Matzinger, P. , Tolerance, Danger, and the Extended Family. Annual Review of Immunology, 1994. 12(Volume 12, 1994): p. 991-1045.
- Medzhitov, R. and C. Janeway, Jr., Innate immune recognition: mechanisms and pathways. Immunol Rev, 2000. 173: p. 89-97.
- Kerr, J.F., A. H. Wyllie, and A.R. Currie, Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer, 1972. 26(4): p. 239-57.
- Willingham, S.B. , et al., The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proceedings of the National Academy of Sciences, 2012. 109(17): p. 6662-6667.
- Griffin, F.M., Jr. Bianco, and S.C. Silverstein, Characterization of the macrophage receptro for complement and demonstration of its functional independence from the receptor for the Fc portion of immunoglobulin G. J Exp Med, 1975. 141(6): p. 1269-77.
- Botelho, R.J. , et al., Localized Biphasic Changes in Phosphatidylinositol-4,5-Bisphosphate at Sites of Phagocytosis. Journal of Cell Biology, 2000. 151(7): p. 1353-1368.
- Goodridge, H.S. , et al., Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’. Nature, 2011. 472(7344): p. 471-5.
- Davis, S.J. and P.A. Van Der Merwe, The kinetic-segregation model: TCR triggering and beyond. Nature immunology, 2006. 7(8): p. 803-809.
- Halstead, S.B. , Antibodies determine virulence in dengue. Ann N Y Acad Sci, 2009. 1171 Suppl 1: p. E48-56.
- Peiris, J.S. , et al., Monoclonal anti-Fc receptor IgG blocks antibody enhancement of viral replication in macrophages. Nature, 1981. 289(5794): p. 189-91.
- Cardosa, B.M.J., J. S. Porterfield, and S. Gordon, Complement receptor mediates enhanced flavivirus replication in macrophages. The Journal of Experimental Medicine, 1983. 158: p. 258 - 263.
- He, Y. , et al., In vitro enhancement of Zika virus infection by preexisting West Nile virus antibodies in human plasma-derived immunoglobulins revealed after P2 binding site-specific enrichment. Microbiology Spectrum, 2024. 12(6): p. e00758-24.
- Schafer, D.P. , et al., Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron, 2012. 74(4): p. 691-705.
- Menon, V. and S. Ghaffari, Erythroid enucleation: a gateway into a “bloody” world. Exp Hematol, 2021. 95: p. 13-22.
- Nicolás-Ávila, J.A. , et al., A Network of Macrophages Supports Mitochondrial Homeostasis in the Heart. Cell, 2020. 183(1): p. 94-109.e23.
- Brestoff, J.R. , et al., Intercellular Mitochondria Transfer to Macrophages Regulates White Adipose Tissue Homeostasis and Is Impaired in Obesity. Cell Metab, 2021. 33(2): p. 270-282.e8.
- Steinman, R.M. , et al., Endocytosis and the recycling of plasma membrane. J Cell Biol, 1983. 96(1): p. 1-27.
- Hilligan, K.L. and F. Ronchese, Antigen presentation by dendritic cells and their instruction of CD4+ T helper cell responses. Cellular & Molecular Immunology, 2020. 17(6): p. 587-599.
- Randolph, G.J., C. Jakubzick, and C. Qu, Antigen presentation by monocytes and monocyte-derived cells. Curr Opin Immunol, 2008. 20(1): p. 52-60.
- Puleston, D.J. and A.K. Simon, Autophagy in the immune system. Immunology, 2014. 141(1): p. 1-8.
- Russell, D.G. , Mycobacterium tuberculosis and the intimate discourse of a chronic infection. Immunological reviews, 2011. 240(1): p. 252-268.
- Gordon, S. , Phagocytosis: An Immunobiologic Process. Immunity, 2016. 44(3): p. 463-75.
- NobelPrize.org. The Nobel Prize in Chemistry 2004. [cited 2024 27.06.]; Available from: https://www.nobelprize.org/prizes/chemistry/2004/summary/.
- Hernández-Morán, B.A. , et al., Degron tagging for rapid protein degradation in mice. Disease Models & Mechanisms, 2024. 17(4).
- NobelPrize.org. The Nobel Prize in Physiology or Medicine 1985. [cited 2024 27.06.]; Available from: https://www.nobelprize.org/prizes/medicine/1985/summary/.
- Nairz, M. , et al., “Pumping iron”-how macrophages handle iron at the systemic, microenvironmental, and cellular levels. Pflugers Arch, 2017. 469(3-4): p. 397-418.
- Nathan, C. , Secretory products of macrophages: twenty-five years on. J Clin Invest, 2012. 122(4): p. 1189-90.
- Liebold, I. , et al., Apoptotic cell identity induces distinct functional responses to IL-4 in efferocytic macrophages. Science, 2024. 384(6691): p. eabo7027.
- Martinez, F.O. and S. Gordon, The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep, 2014. 6: p. 13.
- Gordon, S. and F.O. Martinez, Alternative activation of macrophages: mechanism and functions. Immunity, 2010. 32(5): p. 593-604.
- Gordon, S., J. Todd, and Z.A. Cohn In vitro synthesis and secretion of lysozyme by mononuclear phagocytes Journal of Experimental Medicine, 1974. 139(5): p. 1228-1248.
- Gordon, S. and Z. Werb, Secretion of macrophage neutral proteinase is enhanced by colchicine. Proceedings of the National Academy of Sciences of the United States of America, 1976. 73(3): p. 872-876.
- Amersfoort, J., G. Eelen, and P. Carmeliet, Immunomodulation by endothelial cells — partnering up with the immune system? Nature Reviews Immunology, 2022. 22(9): p. 576-588.
- Winkler, J. , et al., Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nature Communications, 2020. 11(1): p. 5120.
- NobelPrize.org. The Nobel Prize in Physiology or Medicine 1931. [cited 2024 27.06.]; Available from: https://www.nobelprize.org/prizes/medicine/1931/summary/.
- NobelPrize.org. [cited 2024 27.06.]; Available from: https://www.nobelprize.org/prizes/medicine/1953/summary/.
- Murphy, M.P. and L.A.J. O’Neill, A break in mitochondrial endosymbiosis as a basis for inflammatory diseases. Nature, 2024. 626(7998): p. 271-279.
- Mantovani, et al., Macrophage Polarization Comes of Age. Immunity, 2005. 23(4): p. 344-346.
- Gordon, S. , et al., Macrophage Class A Scavenger Receptors – A Functional Perspective. 2022.
- Pick, E. , In Memoriam: Filippo Rossi (1926–2022). Journal of Leukocyte Biology, 2023. 113.
- Nathan, C. , Role of iNOS in human host defense. Science, 2006. 312(5782): p. 1874-5; author reply 1874-5.
- Stein, M. , et al., Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med, 1992. 176(1): p. 287-92.
- Mantovani, A. , et al., The chemokine system in diverse forms of macrophage activation and polarization. Trends in Immunology, 2004. 25(12): p. 677-686.
- Park, M.D. , et al., On the Biology and Therapeutic Modulation of Macrophages and Dendritic Cells in Cancer. Annual Review of Cancer Biology, 2023. 7(Volume 7, 2023): p. 291-311.
- Cassetta, L. and J.W. Pollard, Tumor-associated macrophages. Curr Biol, 2020. 30(6): p. R246-r248.
- Balkwill, F.R. and A. Mantovani, Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol, 2012. 22(1): p. 33-40.
- Blériot, C. , et al., A temporal perspective for tumor-associated macrophage identities and functions. Cancer Cell, 2024. 42(5): p. 747-758.
- Mantovani, A. , et al., Interleukin-1 and Related Cytokines in the Regulation of Inflammation and Immunity. Immunity, 2019. 50(4): p. 778-795.
- Martinon, F., K. Burns, and J. Tschopp, The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell, 2002. 10(2): p. 417-26.
- Barnett, K.C. , et al., A 360° view of the inflammasome: Mechanisms of activation, cell death, and diseases. Cell, 2023. 186(11): p. 2288-2312.
- Hertzog, J. and J. Rehwinkel, Regulation and inhibition of the DNA sensor cGAS. EMBO Rep, 2020. 21(12): p. e51345.
- Lawn, S.D. and G. Meintjes, Pathogenesis and prevention of immune reconstitution disease during antiretroviral therapy. Expert Rev Anti Infect Ther, 2011. 9(4): p. 415-30.
- Webb, B.J. , Defining COVID-19-associated hyperinflammatory syndrome in specific populations. The Lancet Rheumatology, 2021. 3(9): p. e609-e611.
- Dexamethasone in Hospitalized Patients with Covid-19. New England Journal of Medicine, 2021. 384(8): p. 693-704.
- Roberti, A., L. E. Chaffey, and D.R. Greaves, NF-κB Signaling and Inflammation-Drug Repurposing to Treat Inflammatory Disorders? Biology (Basel), 2022. 11(3).
- Altmann, D.M. , et al., The immunology of long COVID. Nature Reviews Immunology, 2023.
- Mariano, M. and W.G. Spector, The formation and properties of macrophage polykaryons (inflammatory giant cells). J Pathol, 1974. 113(1): p. 1-19.
- Hughes, E.J. and D.M. Tobin, Decoding the tuberculous granuloma. Immunity, 2022. 55(5): p. 819-821.
- Henderson, S.R. , et al., Proteinase 3 promotes formation of multinucleated giant cells and granuloma-like structures in patients with granulomatosis with polyangiitis. Annals of the Rheumatic Diseases, 2023. 82(6): p. 848-856.
- Spector, W.G. , Immunologic components of granuloma formation. Epithelioid cells, giant cells, and sarcoidosis. Ann N Y Acad Sci, 1976. 278: p. 3-6.
- Schwartz, C. and P.G. Fallon, Schistosoma “Eggs-Iting” the Host: Granuloma Formation and Egg Excretion. Frontiers in Immunology, 2018. 9.
- Ahmadzadeh, K. , et al., Multinucleation resets human macrophages for specialized functions at the expense of their identity. EMBO Rep, 2023: p. e56310.
- Brooks, P.J., M. Glogauer, and C.A. McCulloch, An Overview of the Derivation and Function of Multinucleated Giant Cells and Their Role in Pathologic Processes. The American Journal of Pathology, 2019. 189(6): p. 1145-1158.
- Pereira, M. , et al., Common signalling pathways in macrophage and osteoclast multinucleation. J Cell Sci, 2018. 131(11).
- Chung, K.F., D. Togbe, and B. Ryffel, Editorial: Ozone as a Driver of Lung Inflammation and Innate Immunity and as a Model for Lung Disease. Frontiers in Immunology, 2021. 12.
- Dobzhansky, T. , Nothing in Biology Makes Sense except in the Light of Evolution. The American Biology Teacher, 1973. 35(3): p. 125-129.
- Cooper, S.J. , From Claude Bernard to Walter Cannon. Emergence of the concept of homeostasis. Appetite, 2008. 51(3): p. 419-427.
- Pirzgalska, R.M. , et al., Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat Med, 2017. 23(11): p. 1309-1318.
- Hulsmans, M. , et al., Macrophages Facilitate Electrical Conduction in the Heart. Cell, 2017. 169(3): p. 510-522 e20.
- Schulthess, J. , et al., The Short Chain Fatty Acid Butyrate Imprints an Antimicrobial Program in Macrophages. Immunity, 2019. 50(2): p. 432-445.e7.
- Tabas, I. , 2016 Russell Ross Memorial Lecture in Vascular Biology. Arteriosclerosis, Thrombosis, and Vascular Biology, 2017. 37(2): p. 183-189.
- Huby, T. and E.L. Gautier, Immune cell-mediated features of non-alcoholic steatohepatitis. Nature Reviews Immunology, 2022. 22(7): p. 429-443.
- Mildner, A. , et al., Distinct and non-redundant roles of microglia and myeloid subsets in mouse models of Alzheimer’s disease. J Neurosci, 2011. 31(31): p. 11159-71.
- Cooper, M. , Which came first, T cells or B cells? Nature Reviews Immunology, 2021. 21(10): p. 616-617.
- NobelPrize.org. Svante Pääbo - Nobel Lecture. [cited 2024 01.07.]; Available from: https://www.nobelprize.org/prizes/medicine/2022/paabo/lecture/.
- Quintana-Murci, L. and A.G. Clark, Population genetic tools for dissecting innate immunity in humans. Nature Reviews Immunology, 2013. 13(4): p. 280-293.
- Barreiro, L.B. and L. Quintana-Murci, From evolutionary genetics to human immunology: how selection shapes host defence genes. Nature Reviews Genetics, 2010. 11(1): p. 17-30.
- Margulis, L. and U.M.A.M.L. Margulis, Symbiosis in Cell Evolution: Life and Its Environment on the Early Earth. 1981: W. H. Freeman.
- Margulis, L. and D. Sagan, Lynn Margulis: The Life and Legacy of a Scientific Rebel. 2012: Chelsea Green Publishing.
- Dopkins, N. and D.F. Nixon, Activation of human endogenous retroviruses and its physiological consequences. Nat Rev Mol Cell Biol, 2024. 25(3): p. 212-222.
- Xu, C. , et al., Living material assembly of bacteriogenic protocells. Nature, 2022. 609(7929): p. 1029-1037.


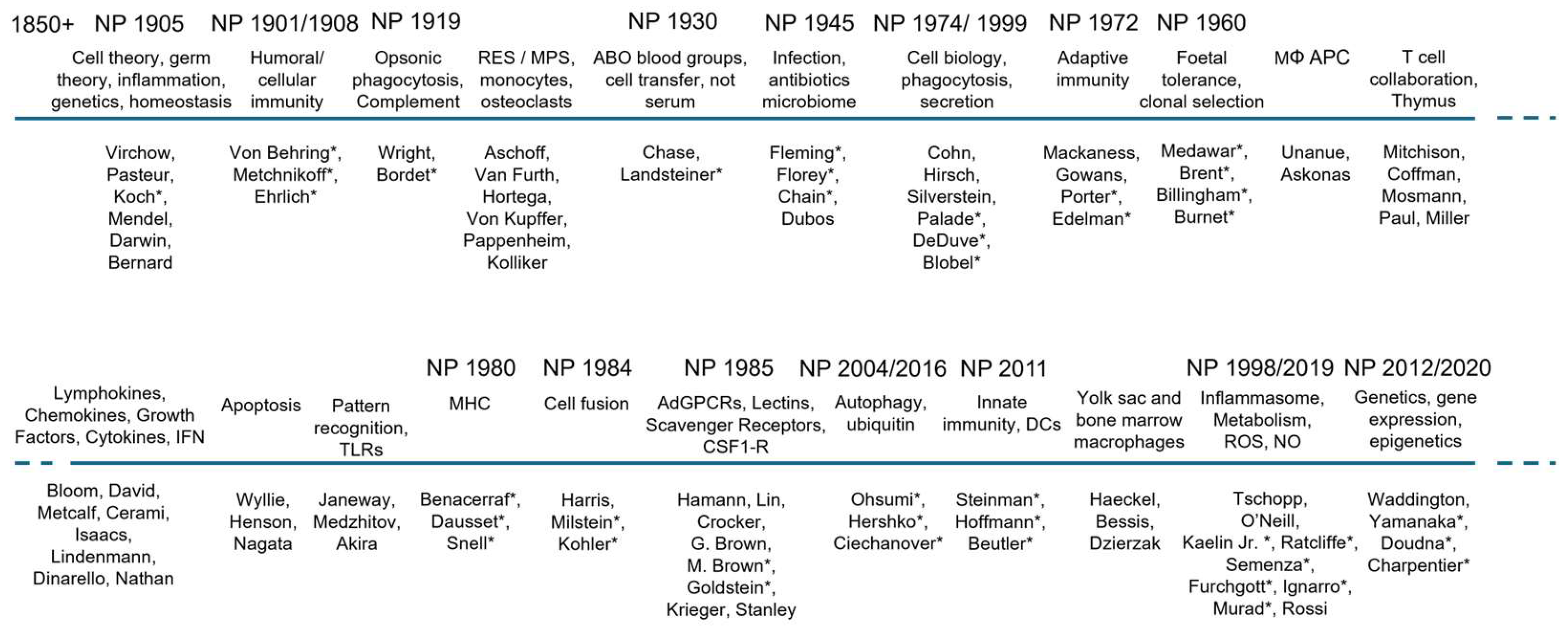
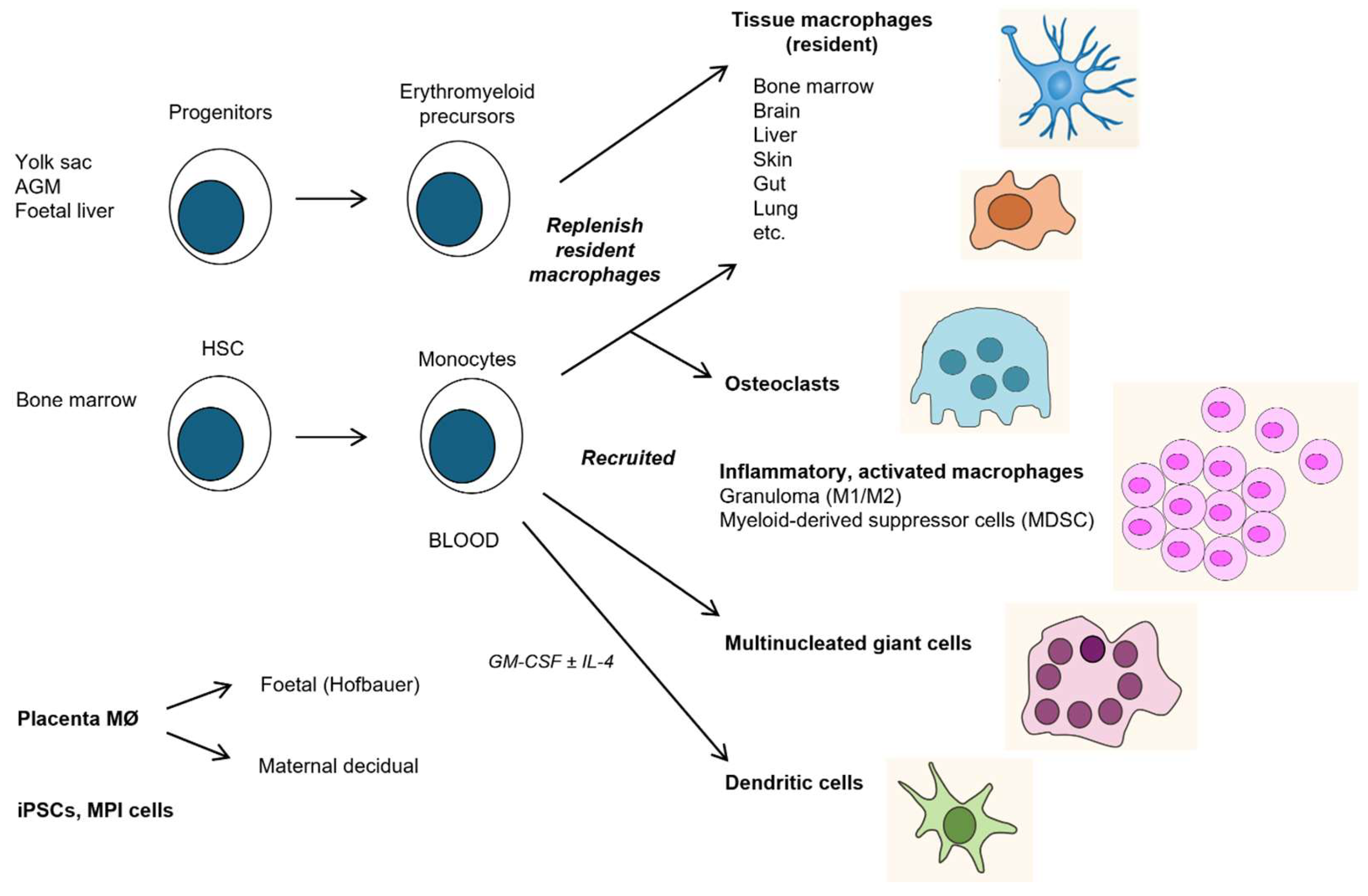
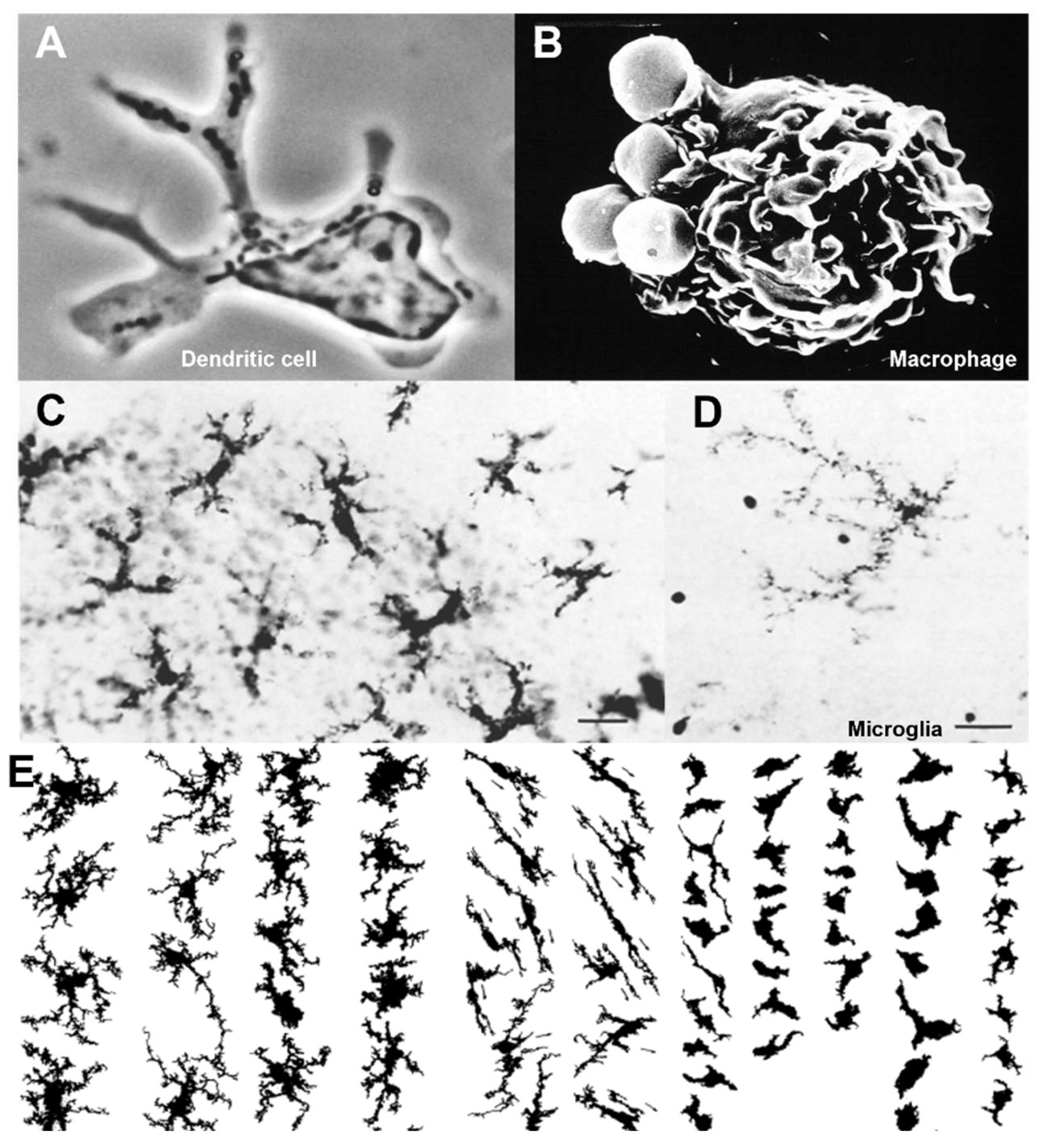
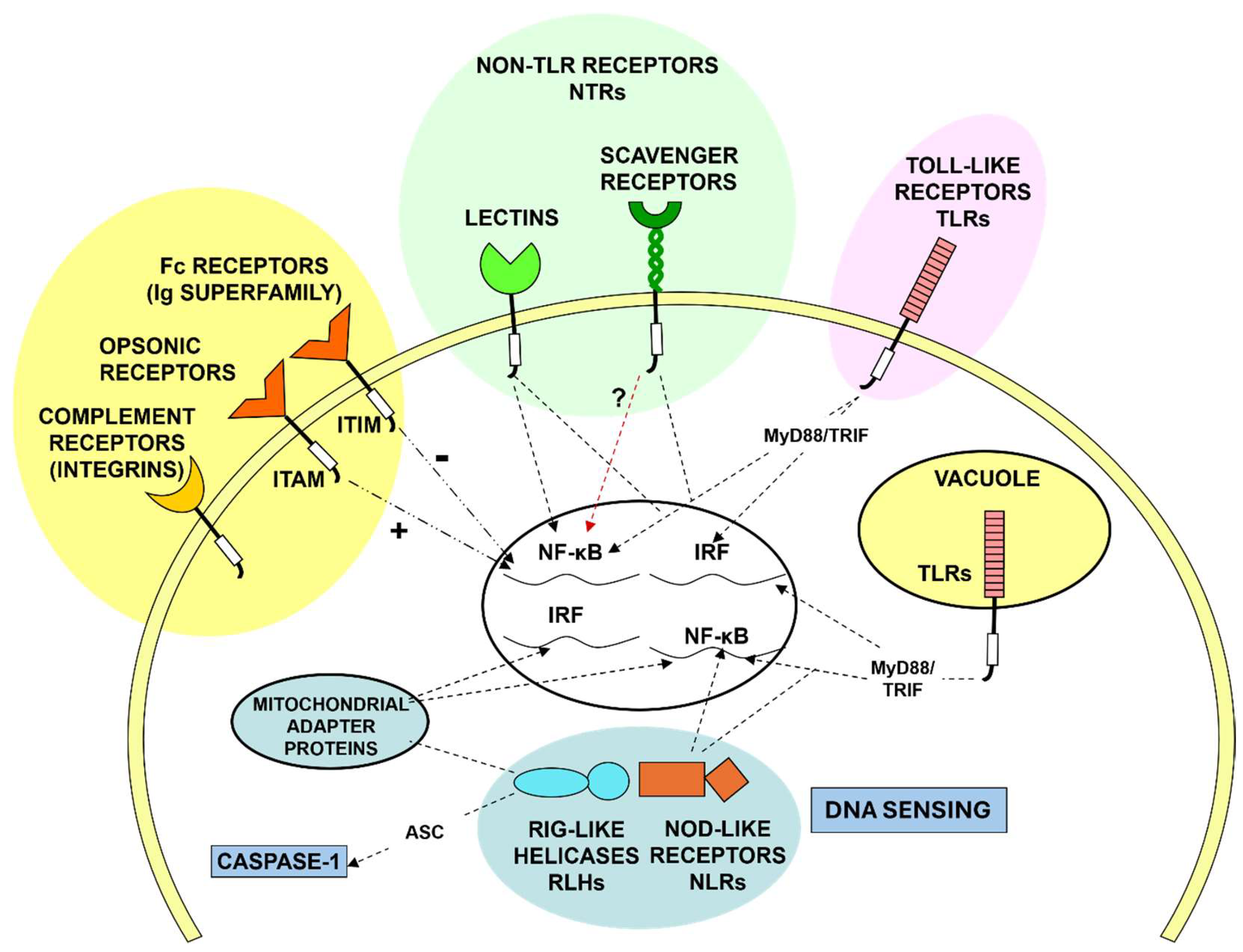
| Year | Names | Topic | Comment |
| 1901 | Von Behring | Diphtheria | Antibodies |
| 1905 | Koch | TB | Cell-mediated |
| 1908 | Metchnikoff, Ehrlich | Phagocytosis, Receptors | Cell-mediated and antibodies |
| 1912 | Carrel | Work on vascular suture and the transplantation of blood vessels and organs | Cell culture |
| 1913 | Richet | Anaphylaxis | Cell-mediated and antibodies |
| 1919 | Bordet | Complement | Humoral |
| 1930 | Landsteiner | Blood groups | Antibodies |
| 1931 | Warburg | Respiration | Metabolism |
| 1945 | Fleming, Chain, Florey | Penicillin | Antibiotics |
| 1950 | Kendall, Reichstein, Hench | Corticosteroids | Anti-inflammatory |
| 1951 | Theiler | Yellow fever vaccine | Adaptive |
| 1952 | Waksman | Streptomycin | Antibiotic TB |
| 1953 | Krebs, Lipmann | Citric acid cycle, coenzyme A | Metabolism |
| 1954 | Enders, Weller, Robbins | Polio virus | Isolation |
| 1960 | Burnet, Medawar | Self and non-self, tolerance | Cell-mediated, transplantation |
| 1966 | Rous, Huggins | Viral malignancy | Magnetic beads macrophage isolation |
| 1972 | Edelman, Porter | Antibodies | Structure |
| 1974 | Claude, de Duve, Palade | Cell structure | Cell biology, transmission EM |
| 1975 | Baltimore, Dulbecco, Temin | Viral replication | NF-κB pathway |
| 1976 | Blumberg, Gajdusek | Hepatitis, Prions | Infection |
| 1980 | Benacerraf, Dausset, Snell | Histocompatibility antigens | Genetics |
| 1982 | Bergstrom, Samuelsson, Vane | Prostaglandins | Regulatory |
| 1984 | Jerne, Kohler, Milstein | Monoclonal antibodies | Specific targeting |
| 1985 | Brown, Goldstein | Cholesterol | Scavenger receptor |
| 1987 | Tonegawa | Genetic control of antibodies | Lymphocytes |
| 1990 | Murray, Thomas | Transplantation | Cell-mediated |
| 1994 | Gilman, Rodbell | G-proteins | Signalling |
| 1996 | Doherty, Zinkernagel | MHC peptide recognition | Cell-mediated and lymphocytes |
| 1998 | Furchgott, Ignarro, Murad | NO | Effector metabolite |
| 1999 | Blobel | Protein signal peptide | Membrane transport |
| 2001 | Hartwell, Hunt, Nurse | Regulators of cell cycle | Cell cycle |
| 2002 | Brenner, Horvitz, Sulston | Genetics of organ development, programmed cell death | Evolution, C. elegans |
| 2005 | Marshall, Warren | Helicobacter pylori infection | Peptic ulcers |
| 2008 | Zur Hausen, Barre-Sinoussi, Montagnier | HPV and HIV | CD4 depletion, opportunistic infection |
| 2011 | Hoffmann, Beutler, Steinman | Insect, TNF, TLR, DCs | Innate immunity and antigen presentation |
| 2012 | Gurdon, Yamanaka | Reprogramming differentiation | iPSC |
| 2013 | Rothman, Schekman, Sudhof | Vesicular trafficking | Cell biology |
| 2016 | Ohsumi | Autophagy | Macrophages |
| 2018 | Allison, Honjo | Cancer immunotherapy | Checkpoint inhibitors |
| 2019 | Ratcliffe, Kaelin, Semenza | Oxygen sensing | Respiratory burst |
| 2020 | Houghton, Alter, Rice | Hepatitis C virus | Virus |
| 2021 | Patapoutian, Julius | Receptors for temperature and touch | Receptor biology |
| 2022 | Paabo | Genomes of extinct hominins and human evolution | Evolution and genetics |
| Chemistry and Physics | |||
| Year | Names | Topic | Comment |
| 1902 | Fischer | Sugar and purine synthesis | Biochemistry |
| 1948 | Tiselius | Electrophoresis | Method |
| Pauling | Protein bonds | Structural Biology/Chemistry | |
| 1958 | Sanger | Proteins | Sequence |
| 1962 | Perutz, Kendrew | Protein Structure | Analytic |
| 1972 | Anfinsen | Ribonuclease | Protein strucure |
| 1980 | Berg, Gilbert, Sanger | DNA | Sequencing |
| 1982 | Klug | Crystallography | Protein structure |
| 1993 | Mullis, Smith | PCR and mutagenesis | Nucleic acid |
| 2004 | Ciechanover, Hershko, Rose | Protein ubiquitination | Degradation |
| 2009 | Ramakrishnan, Steitz, Yonath | Ribosome | Structural biology |
| 2008 | Shimomura, Chalfie, Tsien | Green fluorescent protein | Detection, microscopy |
| 2012 | Lefkowitz, Kobilka | GPCRs | Signalling |
| 2018 | Arnold, Smith, Winter | Enzymes, phage display of peptides and antibodies | Directed evolution |
| 2020 | Charpentier, Doudna | Genome editing | CRISPR |
| 2022 | Bertozzi, Meldal, Sharpless | Click chemistry and biorthogonal chemistry | Protein and therapeutics |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





