1. Introduction
In restorative dentistry, the application of dental adhesives has become increasingly common; and as a result, the technique for applying dental adhesive systems has become a critical factor that can impact the bond strength and overall success of the final restoration. As a result, the application of the dental adhesive system can be performed using a variety of different techniques: a) some are applied without removing excess adhesive and immediately light-cured for 20 seconds, b) other dental adhesives can be applied followed by removal of excess with a gentle air stream for 5 seconds, c) some dental adhesives are applied followed by removing excess dental adhesive using a dry micro applicator, d) and finally, some dental adhesives can be applied followed by removal of excess using a dry micro applicator and gentle stream of air. A thin and uniform layer of adhesive is more favorable for good bond strength.[
1]
Throughout the literature, various contaminating factors of the dental adhesive interface have yet to be discovered or analyzed. However, some factors such as the presence of blood and saliva surrounding their contamination potential have already been examined and proven. Regarding bond strength after contamination, the application of water spray for 10 to 30 seconds followed by drying with an air stream has been shown to be effective.[
2,
3]
For the product's effectiveness, According to some manufacturers, active agitation or scrubbing of the dental adhesive (e.g. silane, universal adhesives, self-etch adhesives and desensitizers) is necessary to ensure the product’s effectiveness.. The most commonly used device used to apply dental adhesives is the micro applicator with adhesively-fixed fiber flocks. Among a variety of different types, conventional micro applicators typically feature fiber flocks that are glued on or adhered of the plastic handle in the active area of the micro applicator. New technologies have been developed to optimize the use of micro applicators and reduce the risk of contamination by replacing adhesively-fixed fiber flocks with elastomer bristles in the active area of the micro applicator. Thus, there are two types of micro applicators: Conventional Fiber Flocked Micro applicators (CFFM) and Fiber-Free Elastomer Bristle Micro applicators (FEBM).[
4,
5,
6]
Studies evaluating the efficacy and clinical conditions for using micro applicators as primer carriers or in cases involving fiber posts, have demonstrated clinical effectiveness and the ability to generate a uniform micro-mechanical bonding between the conditioned dentin and the dental adhesive material.[
7,
8] Given the variety of different micro applicators available with different applicator tips, their initial use should be correlated with the specific material used - whether this is desensitizers or dental adhesives. Furthermore, manufacturers advise against the reuse, sterilization or decontamination of the material, since this could lead to the risk of cross-contamination, degradation, or loss of mechanical function of the product.[
4,
5,
6]
The aim of this study was to assess the degree of adhesive absorption and wetting for each micro applicator, and also quantify the amount of lost fiber flocks after three applications of dental adhesive on the inside surface of a veneer restoration in order to reveal a potential contaminating factor at the adhesive interface. The null hypothesis is that the type of micro applicator and composition does not affect dentin adhesion or induce contamination in the substrate.
2. Materials and Methods
2.1. Study Materials
Thirteen different brands of micro applicators, with 5 regular sizes, 4 fine sizes, 4 extra fine sizes listed in Square 1 were used.
Square 1.
Materials used and their Main Component.
Square 1.
Materials used and their Main Component.
| Material |
Manufacturer |
Code |
Main Component |
Type of microapplicator |
| 1 |
Points Regular (SDI Ltd) |
PO-R |
Fiber Flocks |
CFFM |
| 2 |
Points Fine (SDI Ltd) |
PO-F |
Fiber Flocks |
CFFM |
| 3 |
Points Extra fine (SDI Ltd) |
PO-EF |
Fiber Flocks |
CFFM |
| 4 |
Cavibrush Regular (FGM Dental) |
CAV-R |
Fiber Flocks |
CFFM |
| 5 |
Cavibrush Fine (FGM Dental) |
CAV-F |
Fiber Flocks |
CFFM |
| 6 |
Cavibrush Extra-fine (FGM Dental) |
CAV-EF |
Fiber Flocks |
CFFM |
| 7 |
KG Regular (KG Sorensen) |
KG-R |
Fiber Flocks |
CFFM |
| 8 |
KG Fine (KG Sorensen) |
KG-F |
Fiber Flocks |
CFFM |
| 9 |
KG Extra fine (KG Sorensen) |
KG-EF |
Fiber Flocks |
CFFM |
| 10 |
Aplik Regular (Angelus) |
AP-R |
Fiber Flocks |
CFFM |
| 11 |
Aplik Fine (Angelus) |
AP-F |
Fiber Flocks |
CFFM |
| 12 |
Aplik Extra Fine (Angelus) |
AP-EF |
Fiber Flocks |
CFFM |
| 13 |
Zeroflox (MIXPAC Dental) |
ZERO |
Elastomer Bristle |
FEBM |
2.2. Design of Tooth Preparation and Measure Loss of Fiber by Weight
A plastic veneer tooth from a training model was selected (Training models of Marília, MOM, Marília, Brazil), the dental adhesive Scotchbond Universal (3M ESPE, Minnesota, USA) and analytical balance with four scale decimals (Mitutoyo, Tokyo, Japan) was used to weigh all micro applicators. Prior to their use, each micro applicator was shortened to facilitate application of dental adhesive inside the plastic veneer tooth. Before contact with the dental adhesive, the initial weight of the micro applicator was measured which included a total number of 65 specimens (n=5).
Initially, one drop of dental adhesive was dispensed into a glass dental mixing well. Each micro applicator was coated with the dental adhesive simulating clinical conditions; and a chronometer was activated for 30s. During this time, active application inside the surface of the veneer was done; and after 30s, the micro applicator was weighed again. For each micro applicators, this sequence was repeated three times and measured by the same operator.
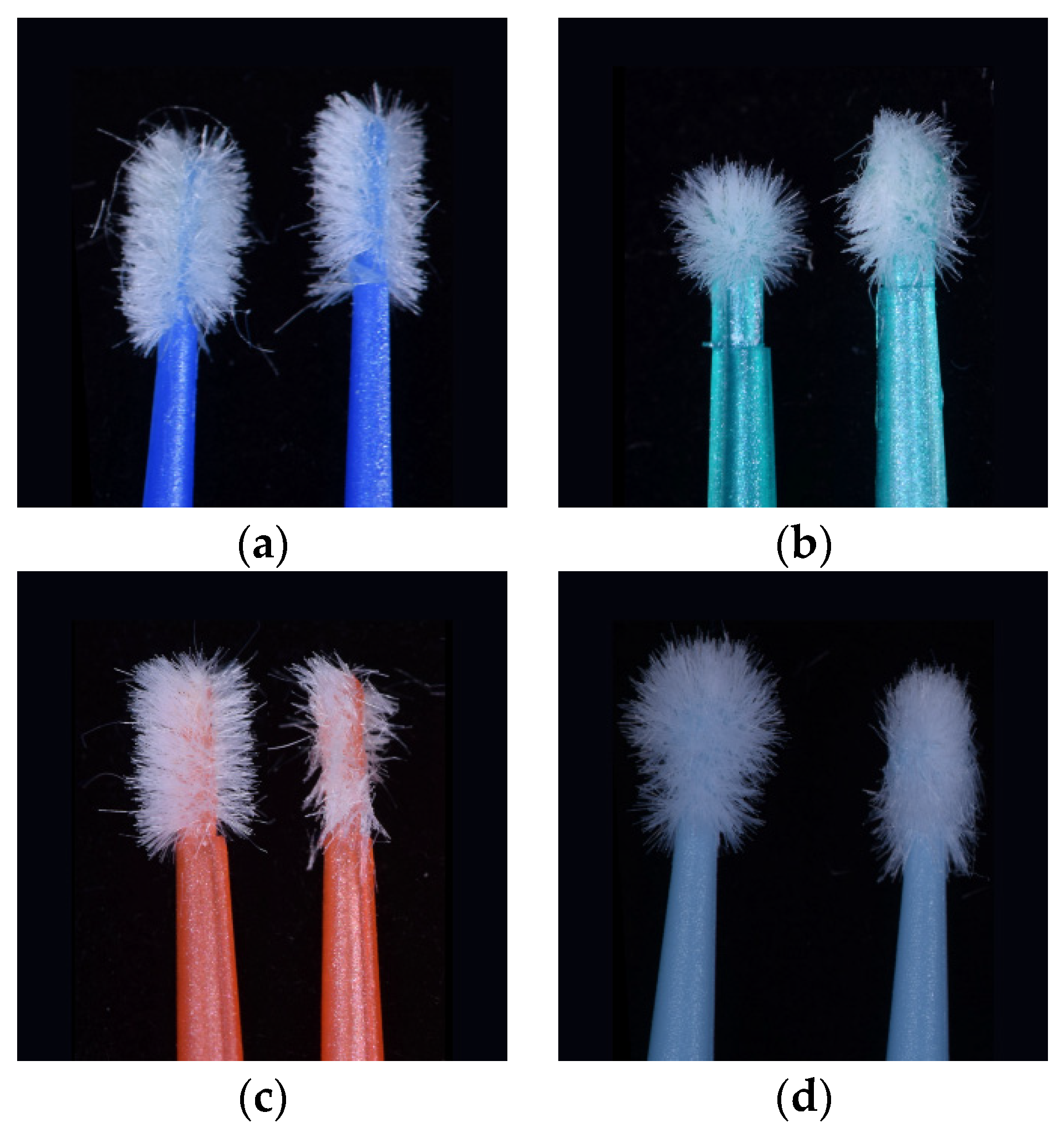
In between all measurements, the plastic tooth veneer was cleaned with a lint wipe and 70% alcohol. The same tooth and application area was used to quantify the amount of fiber flocks lost for each brand of micro applicator.. After three applications, which represented a total application time of 90s per micro applicator, the difference between the first, second and third measured weights were observed. Here the amount of lost fiber flocks was associated with the remains of adhesive incorporation in the fiber flocks. The balance of weight for each application was established by measuring the difference with the initial weight under these three conditions. In
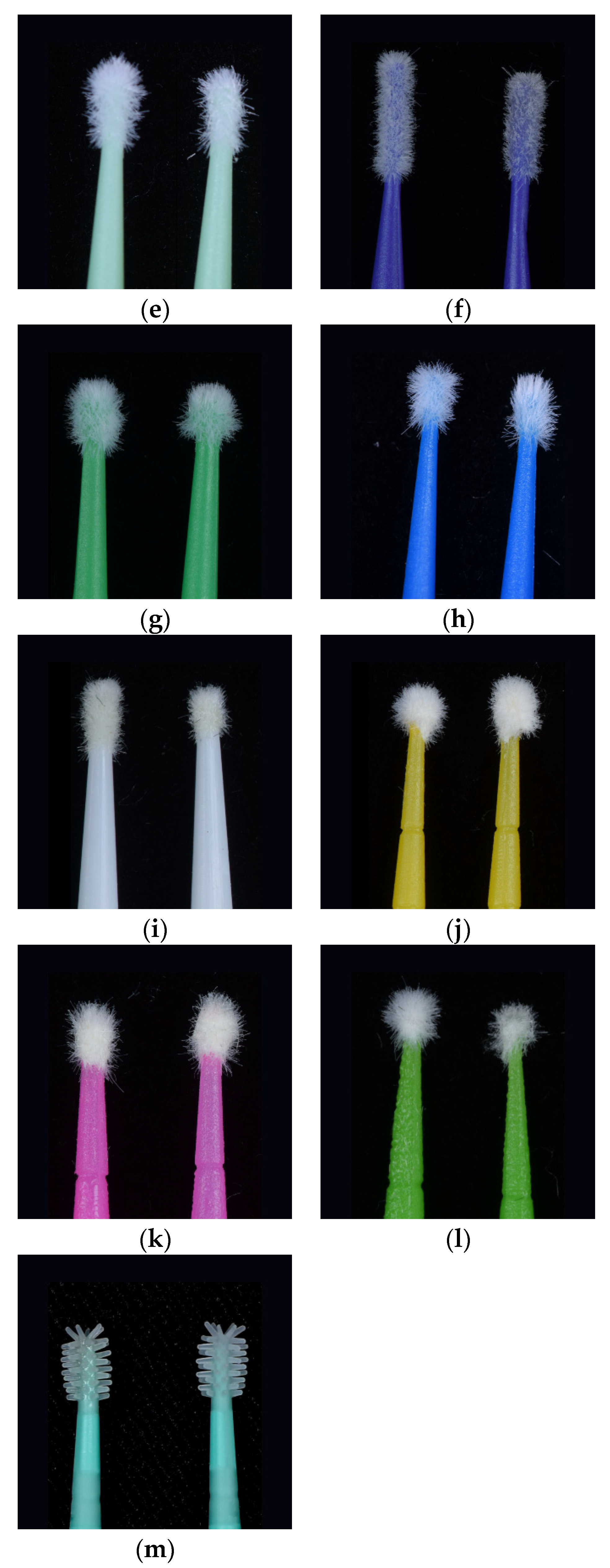
Figure 1, a difference in appearance some of the micro applicators can be observed the before and after micro applicator was used.
2.3. Scanning Electron Microscopy (SEM) Observation
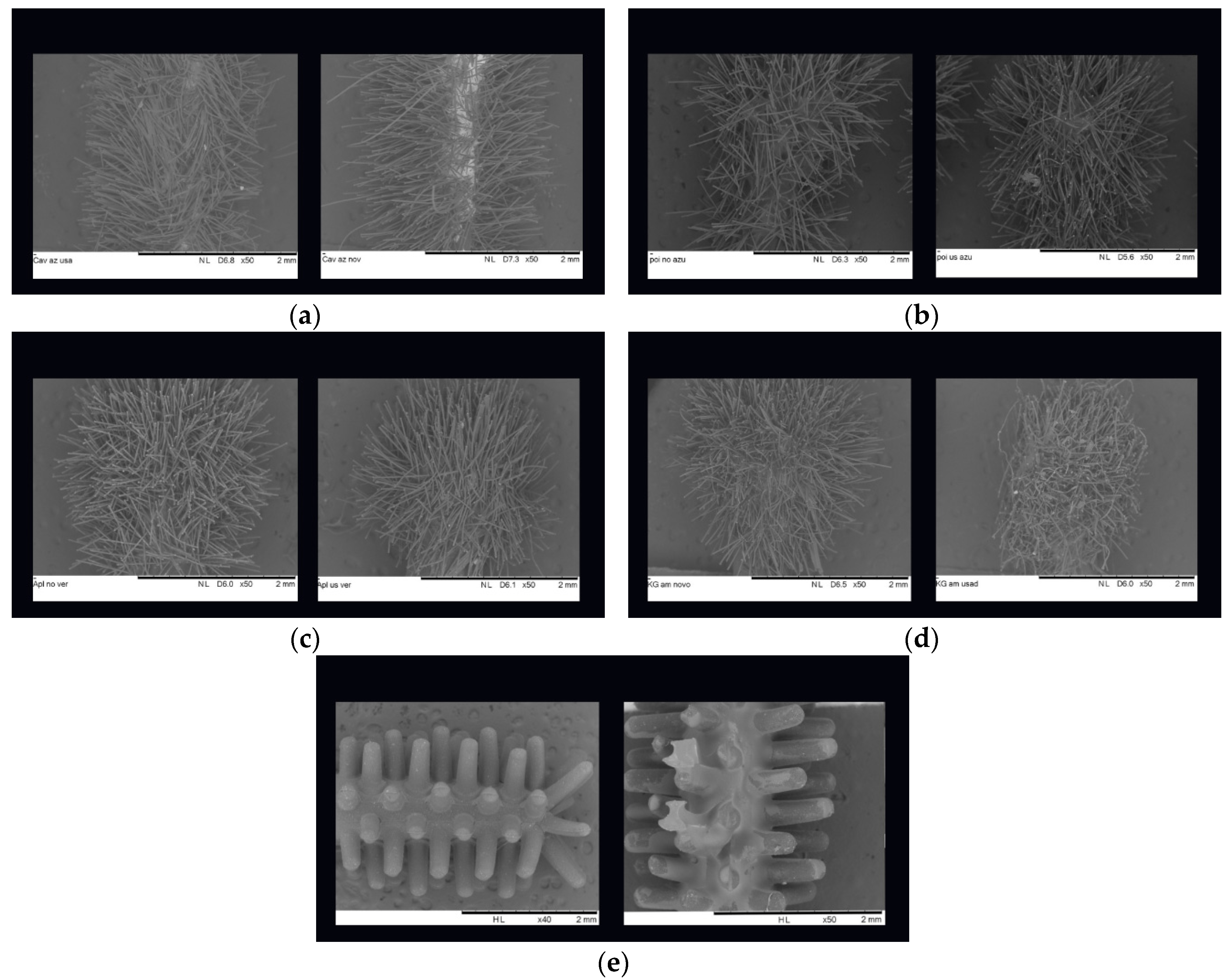
Some regular micro applicators were chosen, aleatory and obtained images of SEM (Zeiss, Swiss) (
Figure 2). These SEM images also demonstrate the critical importance of the clinical situation related to the amount of fiber flocks and quality of glue that comes into to contact with the active part of the micro applicator.
2.4. Statistical Analysis
To determine the appropriate sample size for the amount loss of fibers, a statistical analysis was performed. After gathering the data, post hoc power tests were performed using StatPlus build 8.0.3/core v7.8.11(x86_64) (Brandon, Florida, USA). One-way analysis of variance and Tukey post hoc tests were used with a significance level of 0.05
3. Results
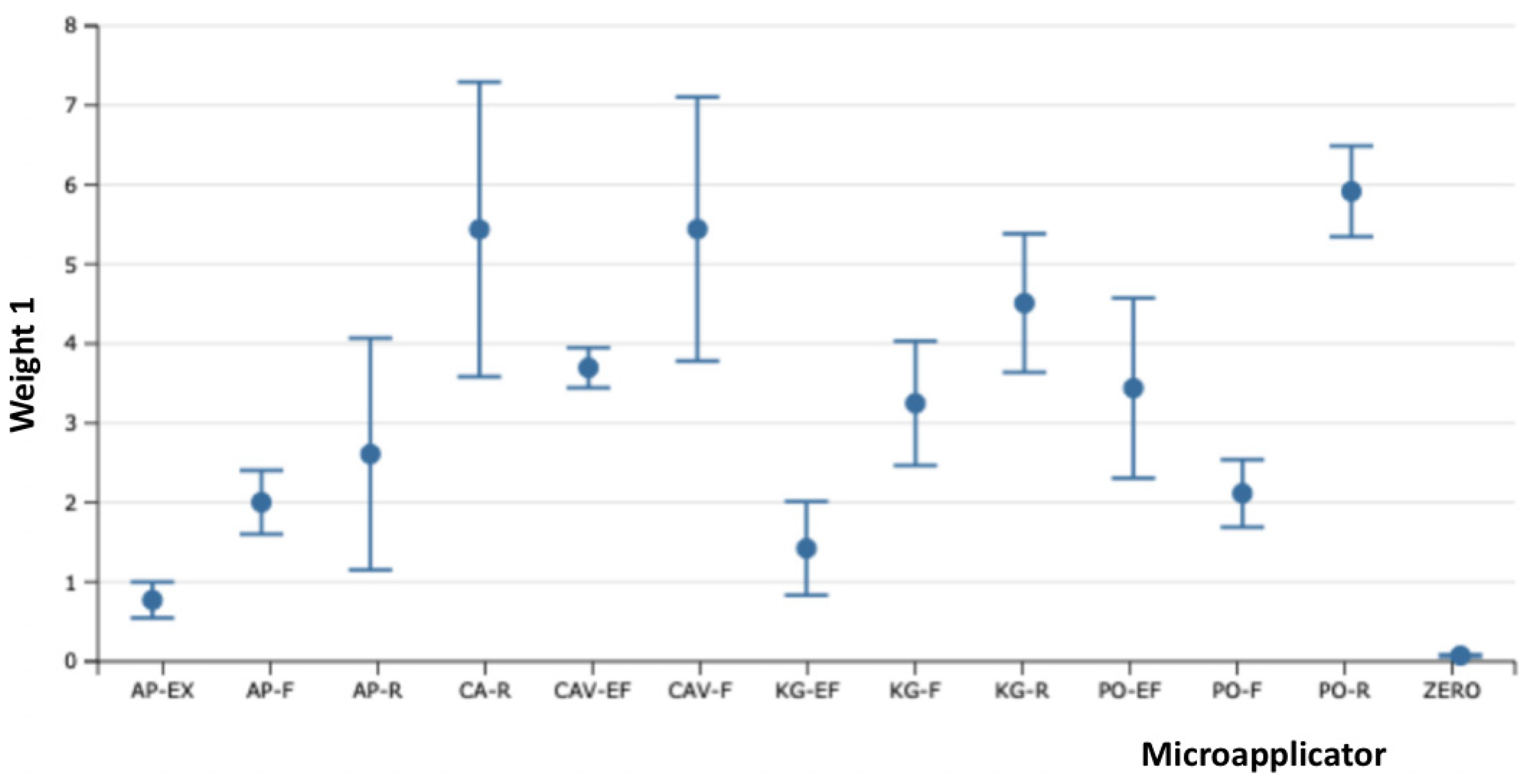
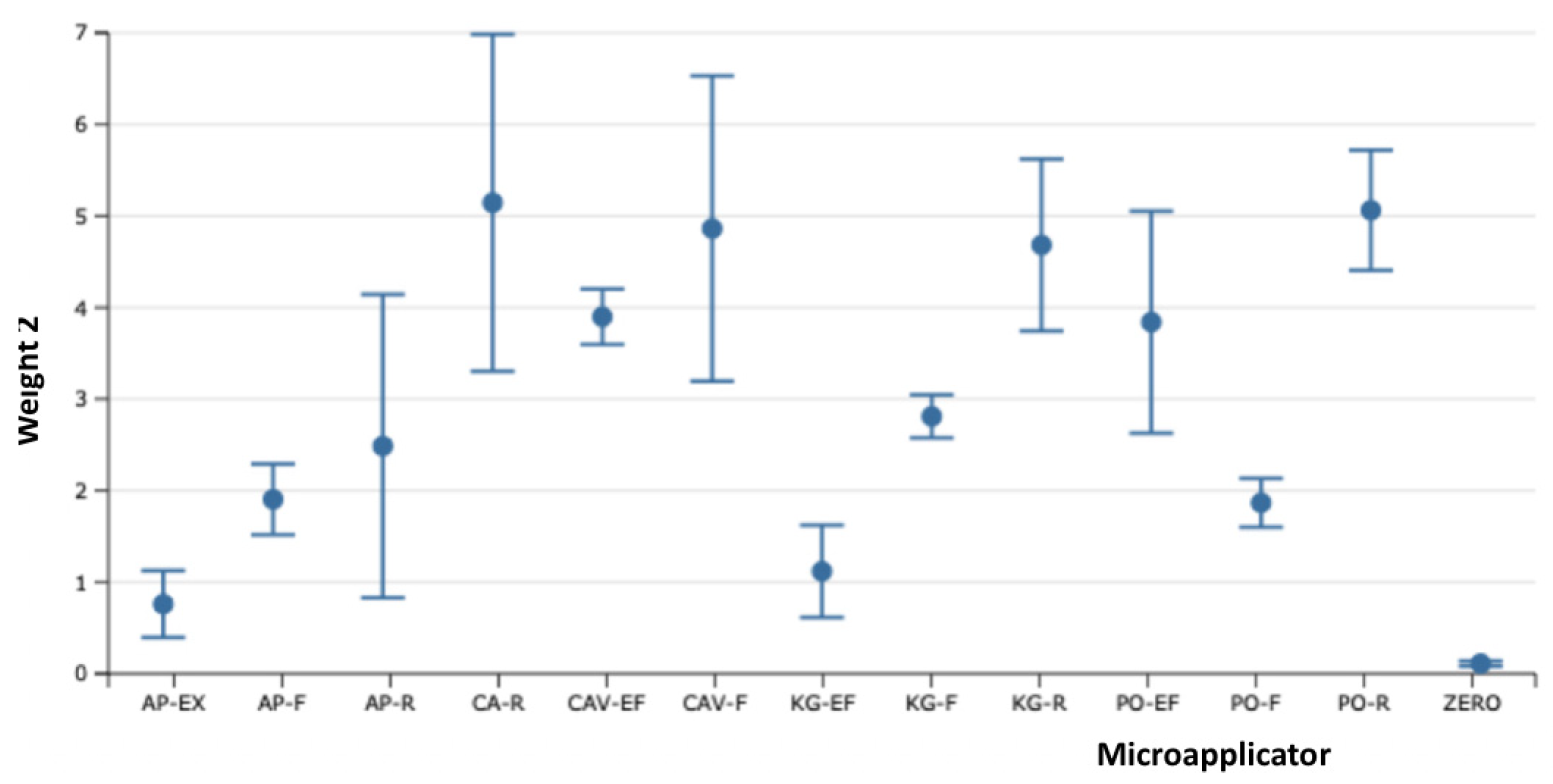
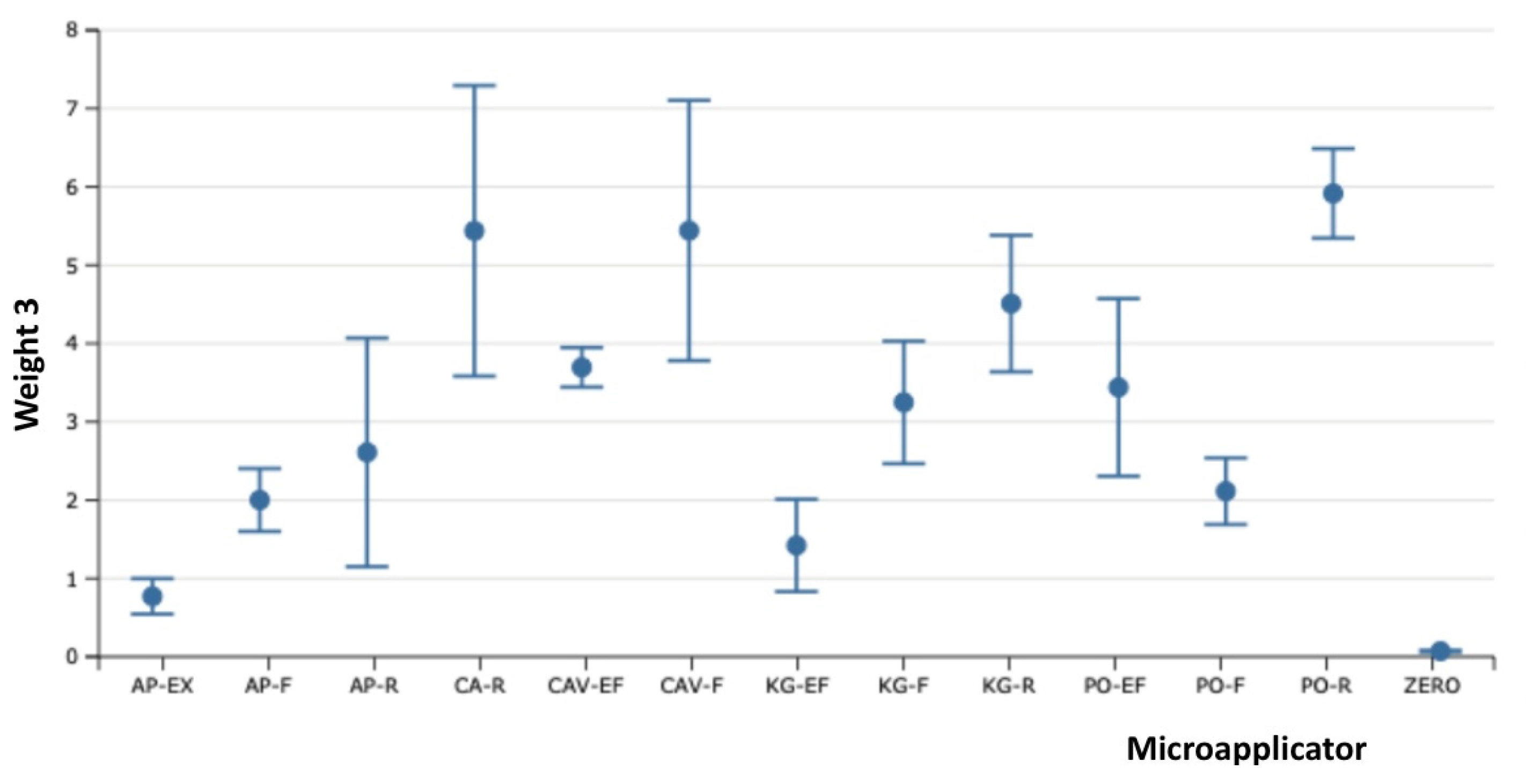
The influence of each adhesive application was analyzed separately to show the values independently and compare them to the initial weight. The tables and graphics below show the influence of adhesive application compared with the initial weight of micro applicator.
Table 1 and graphics 1 show the difference between the first and initial application of dental adhesive applied.
Table 2 and graphics 2 show the difference between the second and initial application of dental adhesive applied.
Table 3 and graphics 3 show difference between the third and initial application of dental adhesive applied.
Table 1.
One-way variance analysis – difference first and initial adhesive application.
Table 1.
One-way variance analysis – difference first and initial adhesive application.
| Microapplicators |
Samples |
M |
SD |
| AP-EX |
5 |
0,77200 |
0,18226 |
| AP-F |
5 |
2,00200 |
0,32337 |
| AP-R |
5 |
2,61000 |
1,17539 |
| CA-R |
5 |
5,43800 |
1,49398 |
| CAV-EF |
5 |
3,69600 |
0,20379 |
| CAV-F |
5 |
5,44200 |
1,33790 |
| KG-EF |
5 |
1,42200 |
0,47484 |
| KG-F |
5 |
3,24800 |
0,63085 |
| KG-R |
5 |
4,51000 |
0,70189 |
| PO-EF |
5 |
3,44000 |
0,91269 |
| PO-F |
5 |
2,11400 |
0,34290 |
| PO-R |
5 |
5,91600 |
0,46003 |
| ZERO |
5 |
0,07200 |
0,00837 |
| Total |
65 |
3,12938 |
1,92560 |
Graphic 1.
Median graphic.
Graphic 1.
Median graphic.
Table 2.
One-way variance analysis – difference between the second and initial adhesive application.
Table 2.
One-way variance analysis – difference between the second and initial adhesive application.
| Microapplicators |
Samples |
M |
SD |
| AP-EX |
5 |
0,76000 |
0,29385 |
| AP-F |
5 |
1,90400 |
0,31118 |
| AP-R |
5 |
2,48600 |
1,33478 |
| CA-R |
5 |
5,14400 |
1,48172 |
| CAV-EF |
5 |
3,89800 |
0,24386 |
| CAV-F |
5 |
4,86200 |
1,34358 |
| KG-EF |
5 |
1,11800 |
0,40586 |
| KG-F |
5 |
2,81000 |
0,18868 |
| KG-R |
5 |
4,68200 |
0,75642 |
| PO-EF |
5 |
3,84000 |
0,97622 |
| PO-F |
5 |
1,86600 |
0,21455 |
| PO-R |
5 |
5,06200 |
0,52893 |
| ZERO |
5 |
0,11000 |
0,02236 |
| Total |
65 |
2,96477 |
1,82862 |
Graphic 2.
Median graphic.
Graphic 2.
Median graphic.
Table 3.
One-way variance analysis – difference between the third and initial adhesive application.
Table 3.
One-way variance analysis – difference between the third and initial adhesive application.
| Microapplicators |
Samples |
M |
SD |
| AP-EX |
5 |
0,72000 |
0,32195 |
| AP-F |
5 |
1,68600 |
0,30803 |
| AP-R |
5 |
2,13000 |
0,81228 |
| CA-R |
5 |
3,91400 |
1,00256 |
| CAV-EF |
5 |
2,92200 |
1,14755 |
| CAV-F |
5 |
3,82200 |
1,01050 |
| KG-EF |
5 |
1,33200 |
0,51066 |
| KG-F |
5 |
2,67000 |
0,25826 |
| KG-R |
5 |
4,15200 |
0,30475 |
| PO-EF |
5 |
3,64000 |
1,04547 |
| PO-F |
5 |
1,89000 |
0,15248 |
| PO-R |
5 |
4,81600 |
0,33931 |
| ZERO |
5 |
0,13000 |
0,03162 |
| Total |
65 |
2,60185 |
1,51080 |
Graphic 3.
Median graphic.
Graphic 3.
Median graphic.
The samples of Fiber-Free Elastomer Bristle Micro Applicators (FEBM) demonstrated the best performance with a good degree of absorption and wetting of the dental adhesive without the lost fiber flocks and fiber release-induced weight loss. The other samples of Conventional Fiber Flocked Micro Applicators (CFFM) demonstrated good initial absorption and wetting of the dental adhesive, but visually shed several fiber flocks which resulted in significant weight loss. Furthermore, this also be considered a contaminating factor at the adhesive interface during clinical procedures due to the observed loss of fiber flocks. Among the fine micro applicators, similar behavior was observed, except for CAV-F, which despite higher absorption capacity, it shed the most fibers over the three applications.
4. Discussion
During the 1970s and early 1980s, dental adhesive systems were generally inefficient. With technological advancements leading to the emergence of new dental adhesive materials such as bonding agents for enamel and dentin, the so-called "cosmetic revolution" was initiated. This advancement enabled improvements in the cementation of ceramic laminates and the longevity of direct restorations. [
9]
Adhesion to dentin is crucial in contemporary restorative dentistry, replacing traditional mechanical methods. Dentin bonding agents have been developed to strengthen the bond of restorative materials and reduce micro leakage. [
10,
11,
12] Thus, there is an interaction between the dental adhesive, primer, desensitizer, and dental substrate. The bond strength of dental enamel to universal dental adhesives is enhanced by the application of phosphoric acid, while such application is generally not necessary on dentin. In current practice, aiming to streamline the number of steps in the adhesive protocol, manufacturers have made the dental adhesives more acidic and hydrophilic [
13,
14] using micro applicators as carrier and application device for such products.
However, there is a risk of contamination and decreased bond strength to dentin and micro-tensile strength depending on how the micro applicators are used by dentists. Therefore, it is not recommended to reuse micro applicators in more than one cavity. [
15] Loss fiber flocks from conventional flocked type micro applicators may also be present in the adhesive interface after applications, potentially compromising sealing effectiveness. [
16]
The results of this study demonstrate that conventional micro applicators release more contaminating agents during use compared to "fiber flock-free" micro applicators, regardless of the brand used. This study confirms that the FEBM types are more effective, safer, and do not present contamination factors during the application of universal dental adhesives after active application on different surfaces when compared to CFM types. It was observed that conventional micro applicators with a "glue-layer" showed the highest amount of contamination due to the dissolution of the bonding agent between the fiber flocks and the active tip of the micro applicator. Samples from the "glue-free" conventional micro applicator groups released more fibers due to friction with the surface after application simulations. FEBM samples exhibited higher friction resistance and did not release bristles during tests due to their composition and methods of connection with the active tip of the micro applicator.
In performing dental restorative protocols, the use of absolute isolation is relevant to assist with intervention-free clinical procedures. However, the use of absolute isolation does not always guarantee the absence of contaminating influences, which may include such factors related to: marginal gaps, deep or gingival margin-proximal restorations; and operate areas, among others, which can contribute to contamination from saliva, gloves, crevicular fluids, water, and blood. This can compromise the bond strength of the dental adhesive system used to dentin. [
17,
18] Additionally, a new factor to consider regarding contamination is the type of micro applicator, since loss of fiber flocks may pose a risk towards the overall success of the adhesive protocol.
When analyzing other contamination caused by saliva and blood, it is observed that when the dental adhesive material is contaminated before light-curing, its conversion rate is affected due to hydrophilic molecules such as HEMA, which retain water during the development of the limiting chain in the polymerization process, resulting in oxidation of carbon-carbon double bonds (C=C) and a plasticizing effect on the polymer. The presence of hydrolytic enzymes from contaminants can degrade Bis-GMA, thereby hindering adhesion. Additionally, the higher viscosity of blood reduces light permeation and adhesive polymer formation. Furthermore, when discussing contamination of already polymerized adhesive, glycoproteins can adhere to the material surface inhibited by air; thus creating a physical barrier that prevents co-polymerization between dental adhesive and restorative material. [
19] Throughout this application process, micro applicators are commonly used in the adhesive procedure, most of which release fiber flocks, potentially becoming another factor in contamination processes, thereby exacerbating negative effects on bond strength.
Therefore, it is believed that a new contaminant factor such as lost fiber flocks is likely to negatively impact adhesion.The authors of this investigation encourage further clinical studies to substantiate this hypothesis. Additionally, more research is needed to understand the outcomes and contamination process in restorative dental procedures to help guide the selection of materials with lower contamination potential.
5. Conclusions
Within the limitations of this study, it can be confirmed that the type of micro applicator can influence contamination during clinical procedures. FEBM samples showed superior results in terms of durability and the degree of absorption, and wetting compared to conventional fiber flocked micro applicators.
Funding
This research receive no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- De Menezes, F.C.H.; Da Silva, S.B.; Valentino, T.A.; Oliveira, M.A.H.D.M.; Rastelli, A.N.D.S.; Conçalves, L.D.S. Evaluation of bond strength and thickness of adhesive layer according to the techniques of applying adhesives in composite resin restorations. Quintessence Int. 2013, 44, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.M.-C.; Ekambaram, M.; Li, K.C.; Cooper, P.R.; Mei, M.L. A scoping review of the influence of clinical contaminants on bond strength in direct adhesive restorative procedures. J. Dent. 2024, 145, 104985. [Google Scholar] [CrossRef] [PubMed]
- Bourgi, R.; Cuevas-Suarez, C.E.; Devoto, W.; Monjarás-Ávila, A.J.; Monteiro, P.; Kharma, K.; Lukomska-Szymanska, M.; Hardan, L. Effect of contamination and decontamination methods on the bond strength of adhesive systems to dentin: A systematic review. J. Esthet. Restor. Dent. 2023, 35, 1218–1238. [Google Scholar] [CrossRef] [PubMed]
- Microbrush International. Instruction for Use (IFU) Microbrush Applicators. Young Innovations, USA. 2020. Link available: http://microbrush.younginnovations.com/wp-content/uploads/sites/6/2020/12/MB_Applicators_IFU.pdf.
- Points. Disposable brush applicators. SDI, AU. Points. Disposable brush applicators. SDI, AU. Link available: https://www.sdi.com.au/pdfs/brochures/au/points_sdi_brochures_au.pdf.
- Cowen M, Powers JM. In Vitro Evaluation of ZerofloX an Innovative Applicator Brush. DENTAL ADVISOR Biomaterials Research Center, USA. 2023. Link available: https://www.medmix.swiss/-/media/Files/Products/zeroflox/Dental-Advisor-ZerofloX-In-Vitro-Evaluation.pdf.
- Ferrari, M.; Grandini, S.; Simonetti, M.; Monticelli, F.; Goracci, C. Influence of a microbrush on bonding fiber post into root canals under clinical conditions. Oral Surgery, Oral Med. Oral Pathol. Oral Radiol. Endodontology 2002, 94, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Vichi, A.; Grandini, S.; Ferrari, M. Comparison Between Two Clinical Procedures for Bonding Fiber Posts into a Root Canal: A Microscopic Investigation. J. Endod. 2002, 28, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Alex, G. Universal adhesives: the next evolution in adhesive dentistry? Compend Contin Educ Dent. 2015 Jan;36(1):15-26; quiz 28, 40. [PubMed]
- Swift, E.J., Jr.; Perdigãao, J.; Heymann, H.O. Bonding to enamel and dentin: a brief history and state of the art, 1995. Quintessence Int. (Berlin, Ger. : 1985) 1995, 26, 95–110. [Google Scholar]
- Pashley, D.; Carvalho, R. Dentine permeability and dentine adhesion. J. Dent. 1997, 25, 355–372. [Google Scholar] [CrossRef] [PubMed]
- Van Meerbeek, B.; Perdigão, J.; Lambrechts, P.; Vanherle, G. The clinical performance of adhesives. J. Dent. 1998, 26, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.V.; de Almeida Neves, A.; Mine, A.; Coutinho, E.; Van Landuyt, K.; De Munck, J.; Van Meerbeek, B. Current aspects on bonding effectiveness and stability in adhesive dentistry. Aust. Dent. J. 2011, 56, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Rosa, W.L.d.O.d.; Piva, E.; da Silva, A.F. Bond strength of universal adhesives: A systematic review and meta-analysis. J. Dent. 2015, 43, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Kazemi AD, Davari A, Nasab SMM, Geravand E. Effect of re-application of microbrush on micro tensile bond strength of an adhesive to dentin. Journal of Dental Medicine-Tehran University of Medical Sciences. 2013; 25 (4): 266-272.
- Berton, F.; Rapani, A.; Zotti, M.; Stacchi, C.; Berton, T.; Porrelli, D. Presence of microbrush remnants on the adhesion surface: A microscopical analysis. J. Dent. 2022, 127, 104320. [Google Scholar] [CrossRef] [PubMed]
- Bourgi, R.; Cuevas-Suarez, C.E.; Devoto, W.; Monjarás-Ávila, A.J.; Monteiro, P.; Kharma, K.; Lukomska-Szymanska, M.; Hardan, L. Effect of contamination and decontamination methods on the bond strength of adhesive systems to dentin: A systematic review. J. Esthet. Restor. Dent. 2023, 35, 1218–1238. [Google Scholar] [CrossRef] [PubMed]
- Elkassas, D.; Arafa, A. Assessment of post-contamination treatments affecting different bonding stages to dentin. Eur. J. Dent. 2016, 10, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.M.-C.; Ekambaram, M.; Li, K.C.; Cooper, P.R.; Mei, M.L. A scoping review of the influence of clinical contaminants on bond strength in direct adhesive restorative procedures. J. Dent. 2024, 145, 104985. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).