1. Introduction
Onychomycosis accounts for 50% of nail diseases, especially more common in toenails than in fingernails, with a mean prevalence in Europe and America of 4.3% in population-based studies [
1]. While non-life-threatening, onychomycosis can lead to serious complications such as cellulitis, sepsis, osteomyelitis, tissue damage, and nail loss [
2]. Overall, 10% of onychomycosis cases are considered moderate-to-severe [
3]. Also, the diagnosis of onychomycosis may become a source of increase concern, with commonly reported esthetic issues and psychosocial factors with embarrassment, low self-esteem, social withdrawal, and reduction of the quality of life, as well as significant economic burden of topical and oral therapies [
4,
5,
6].
Dermatophytes, especially
Trichophyton rubrum, are the most frequent causative agents, but other dermatophytes, including
Trichophyton mentagrophytes and
Epidermophyton floccosum can also cause it. Dermatophytes are identified in 90% of toenail infections and in 50% of fingernail onychomycosis [
7].
Candida albicans accounts for 2% of onychomycosis, particularly affecting the fingernails. Other non-dermatophyte molds include
Aspergillus spp.,
Fusarium spp.,
Acremonium spp.,
Scytalidium, and
Scopulariopsis brevicaulis [
8]. Confirmatory laboratory testing should be routinely performed before prescribing antifungal therapy to avoid treatment failures [
7]. However, in clinical practice, only 40% of patients with onychomycosis underwent diagnostic testing [
9]. In recent years, sensitive tests (fungal culture testing or PCR) are useful to identify the infection cause especially in atypical cases or when a primary saprophytic infection is suspected [
10].
The goal of treatment to eradicate the infecting organism and returning the nail to its normal appearance may be difficult to achieve due to the high recurrence rates, treatment failures, and limited cure rates obtained with topical antifungals, so that the use of oral antifungal treatment is needed in most cases [
2]. The choice of treatment should be individualized according to the clinical characteristics of the disease, the causative organism, the presence of comorbidities and concurrent medications, and the patient’s preferences [
11]. Oral agents including terbinafine, itraconazole and fluconazole are recommended for moderate to severe onychomycosis, with the advantage of high cure rates and short treatment periods, but disadvantages of potential drug interactions and risk of adverse events (AE) (e.g., hepatotoxicity) [
12]. Additionally, many patients have a strong personal preference for a topical approach [
7].
Topical antifungal nail lacquers have been formulated to provide efficient delivery to the nail unit [
13,
14]. Topical ciclopirox 8% hydroxypropyl chitosan (CPX 8% HPCH) has a fungicidal/fungistatic as well as sporicidal activity, with HPCH acting as a film-forming agent, protecting the nail and increasing the penetration of ciclopirox. Hence, CPX 8% HPCH is indicated for the treatment of mild-to-moderate fungal infections of the nails that are caused by ciclopirox-sensitive fungi, without nail matrix involvement, and has been found to be generally well tolerated [
15]. In some cases, when there is extensive involvement of one or several finger and toenails, additional treatment with oral antifungals may be considered, such as combined therapy of oral terbinafine and topical ciclopirox [
16]. However, no clinical evidence has been reported regarding combination of topical ciclopirox with oral itraconazole or fluconazole. Moreover, it could be possible that evidence reported for clinical studies might not reflect the reality in clinical practice.
Therefore, the purpose of this study was to assess the effectiveness and safety of CPX 8% HPCH nail lacquer combined with oral antifungal treatment for treating onychomycosis in the setting of real-world clinical practice in Spain. We used natural language processing (NLP) and machine learning (ML) techniques for extracting real-world data from electronic health records (EHRs). In addition, we described the demographic and clinical characteristics of onychomycosis patients. Studies using this methodology in the assessment of topical and oral antifungals treatment approach in patients with onychomycosis have not been previously reported.
2. Materials and Methods
2.1. Design and Study Population
This was a retrospective multicenter observational real-world evidence study based on secondary use of structured and unstructured data extracted from EHRs from patients diagnosed with onychomycosis attended at three acute-care tertiary hospitals from the public Spanish National Health Care System between January 1, 2014, and March 31, 2023. The participating hospitals were located in Madrid (Hospital Universitario Fundación Alcorcón, Hospital Universitario Infanta Leonor, and Hospital Universitario Puerta de Hierro). Subjects aged 18 years or older, diagnosed with onychomycosis, and treated with CPX 8% HPCH nail lacquer (Ony-Tec®, Almirall S.A., Barcelona, Spain. Other registered (R) brands: Ciclopoli, Fulcare, Kitonail, Myconail, Niogermos, Niogermox, Onytec, Polinail, Privex, Rejuvenail) in combination with oral antifungals were eligible.
Approval of the study from the local Ethics Committee for Clinical Research of the participating centers was obtained. Patient informed consent was not required because aggregated and anonymized data were analyzed from deidentified EHRs.
2.2. Data Source and Study Variables
The source of information was free text (i.e., unstructured) and structured information, including outpatient clinic reports, discharge reports, emergency reports, prescriptions, and other medical reports within the EHRs of patients with onychomycosis in the participating hospitals using the EHRead
® technology developed by Medsavana S.L. (Madrid, Spain). EHRead
® technology [
17] is a powerful engine based on NLP and ML techniques for extracting text from EHRs [
18], which is translated into concepts, synonyms, and definitions using specific terminology based on Systematized Nomenclature of Medicine—Clinical Terms (SNOMED-CT) and then, organized and converted into a synthetic study database. To assess the quality of the information gathered from EHRs, the total number of screened records and patients were analyzed per site according to the main data sources and hospital departments or services (dermatology, emergency, and others). These data are later aggregated in the study database. The index date was defined as the earliest time point within the study period at which CPX 8% HPCH appeared in the unstructured text of the patient’s EHRs. The follow-up period was defined as the time elapsed from the index date to the last report available in the EHR. A window of 6 months pre- and post-index date was considered to define clinical manifestations and the concomitant use of oral antifungal treatment. A window of 12 months pre- and 6 months post-index date was considered to assess diagnostic methods and etiological pathogens.
Study variables included demographics and clinical characteristics, diagnostic methods, etiological agents, treatment-related variables, including response type and time to positive response, treatment synchronicity at index, type of oral antifungal agent (terbinafine, itraconazole, fluconazole), treatment persistence, treatment discontinuation, and safety. Response type included positive response (including cure), presumed positive (non-confirmed potential improvement mentioned in the EHRs, referral to a primary care physician due to treatment effectiveness or free-text detection of terms associated to healing), partial response, and negative response. Time to response was calculated (in months) for positive and presumed positive responses. Treatment synchronicity at index defined three study subgroups: i) concomitant start: patients with both earliest mentions of CPX 8% HPCH and oral antifungals are at the same time (i.e., index date); ii) initial oral antifungal: patients with earliest mention of oral antifungal previous to the index date; and iii) initial CPX 8% HPCH: patients with the earliest oral antifungal mention after the index date. Treatment persistence was calculated for all the study population accounting the time from the treatment initiation until its discontinuation. For the combination treatment, treatment persistence was calculated from the date in which the last treatment component was mentioned (either antifungal at index or posterior to index, or CPX 8% HPCH at index) until the earliest discontinuation of any of them. For oral antifungal treatment, it was calculated as the time from the earliest mention of oral antifungal (either previous, at index or posterior to index) until its discontinuation. And, for the CPX 8% HPCH treatment, it was calculated from the index date (i.e., CPX 8% HPCH earliest mention) until its discontinuation. Discontinuation was created using data from the following categories in this order: time to cure, time to switch, and time to severe potential AE, and time to treatment stop. Additional topical treatment during the follow-up period was also registered.
2.3. Statistical Analysis
The sample size was calculated assuming that the total reference population covered by the three included sites is approximately 1 million patients and considering a prevalence of onychomycosis of 4.3% in the general population [
1] and a conservative estimate of all-age frequency of moderate-to-severe disease of 10% [
3]. Considering 100% of these patients with moderate-to-severe onychomycosis attending a specialist for treatment in the hospital setting, a total of 4,300 patients (95% confidence interval [CI] 1,900 to 6,800) were estimated to be included in the study. Therefore, we expected to include around 500 patients treated with CPX 8% HPCH plus an oral antifungal agent if the rate of use of the combination therapy exceeds 12% of the hospital population with moderate-to-severe onychomycosis. Categorical variables are expressed as frequencies and percentages, and continuous variables as mean and standard deviation (SD) or median and quartiles (Q1, Q3). The frequency of available or missing values are also reported. Outcomes were analyzed as time-to-event analysis using the Kaplan-Meier method. Data analysis was carried out using “R” software (v4.0.2) (The R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Overall and Clinical Data of the Study Population
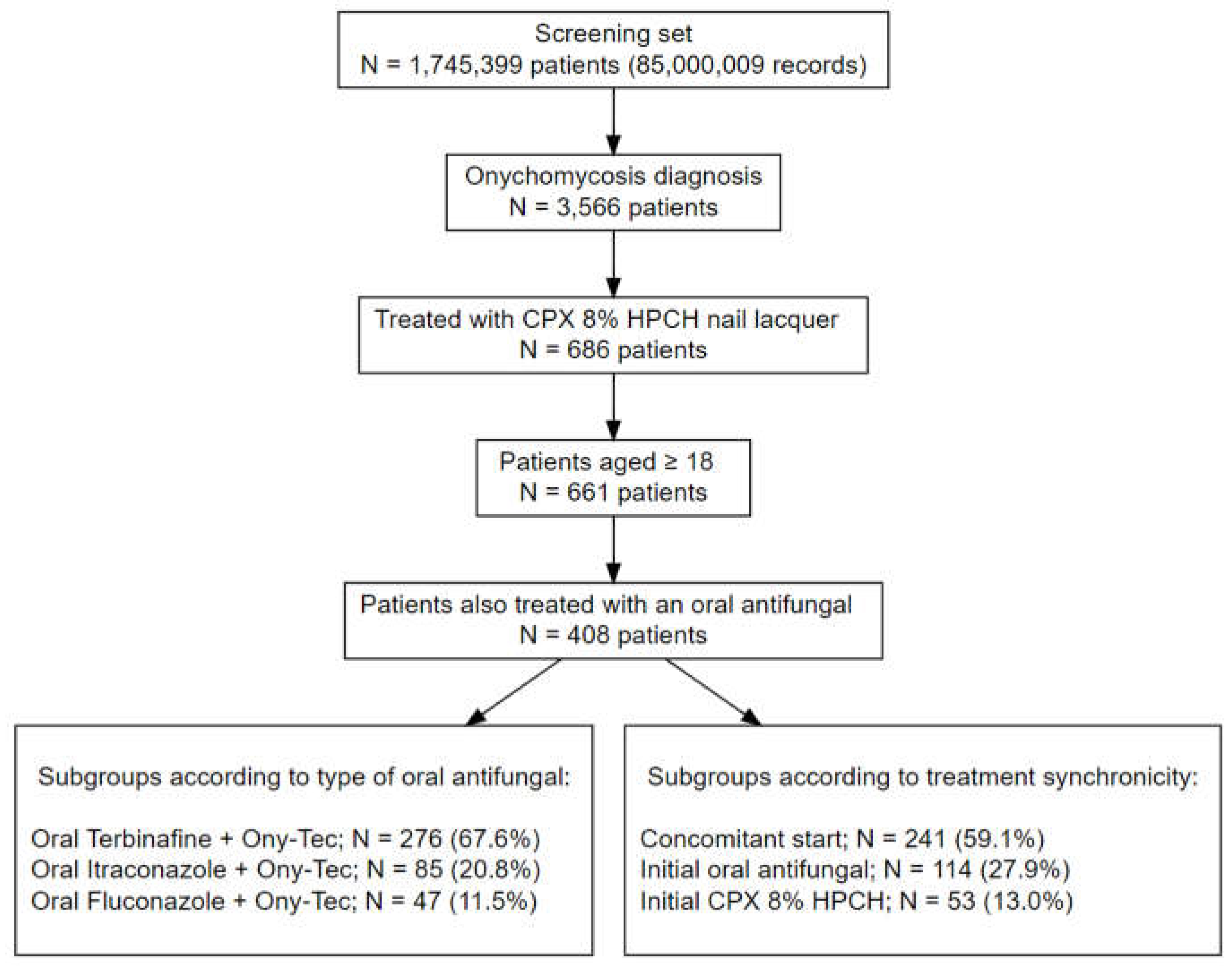
The EHRs from 1,745,399 patients (85,000,009 EHRs in total) were processed from the three participating hospitals. A total of 234,473 patients belonged to the dermatology departments, 3,566 of which had a registered onychomycosis diagnosis. After applying the eligibility criteria and filters of antifungal treatment, there were 661 patients aged ≥ 18 years treated with CPX 8% HPCH, and 61.7% (n = 408) were concomitantly treated with an oral antifungal agent. Therefore, a study population of 408 patients treated with CPX 8% HPCH and oral antifungals were selected for the study. The most common antifungal agent was terbinafine in 67.7% of patients (n = 276) followed by itraconazole in 20.8% (n = 85) and fluconazole in 11.5% (n = 47).
Figure 1 shows the flow chart of the study population and details of the subgroups according to the type of oral antifungal agent and treatment synchronicity.
There were 186 men and 222 women (45.6%, and 54.4%, respectively), with a mean (SD) age of 51.1 (14.8) years. The mean body mass index (BMI) was 27.9 (5.3) kg/m
2. Dermatological comorbidities included tinea pedis interdigitalis in 12.3% of patients, psoriasis in 5.4%, nail psoriasis in 0.7%, and vitiligo in 0.5%. Hypertension and dyslipidemia were also frequent comorbid conditions, which were recorded in 17.4% and 14.5% of patients, respectively. In more than half of patients (51.7%), no comorbidities were found in the EHRs and risk factors for onychomycosis were not registered in 89.7% of patients. Repeated nail trauma and chemotherapy as risk factors for onychomycosis were registered in 7.1% and 2.0% of patients, respectively. Salient features at the index date are summarized in
Table 1.
In relation to clinical symptoms, subungual hyperkeratosis was the most frequent (26.2%) followed by onycholysis (18.6%). The most common diagnostic methods were fungal culture (56.6%) and dermatophyte test strip (13.2%). Among the etiological agents, dermatophytes were the most common, especially
Trichophyton rubrum (33.8%). Clinical, diagnostic, and etiological data are shown in
Table 2.
3.2. Response Type and Time to Response
The response type and time to response according to treatment synchronicity is shown in
Table 3. The median time to positive response was 4.38 months in the overall study population, with the shortest median time (1.84 months) in the subgroup of patients who started oral antifungal therapy before topical use of CPX 8% HPCH nail lacquer (initial oral antifungal subgroup). This subgroup also showed a higher rate of presumed positive and positive response (84.2%) as compared with the subgroups of concomitant start of combined therapy (70.9%) and initial CPX 8% HPCH (77.4%).
In relation to time to response according to the use of different antifungals in the combination therapy with CPX 8% HPCH including terbinafine, itraconazole and fluconazole, the median time was superior to 4 months in the three subgroups (
Table 4). The percentage of patients with positive and presumed positive responses were 85.1%, 81.2%, and 72.1% in those treated with fluconazole, itraconazole, and terbinafine, respectively (
Table 4).
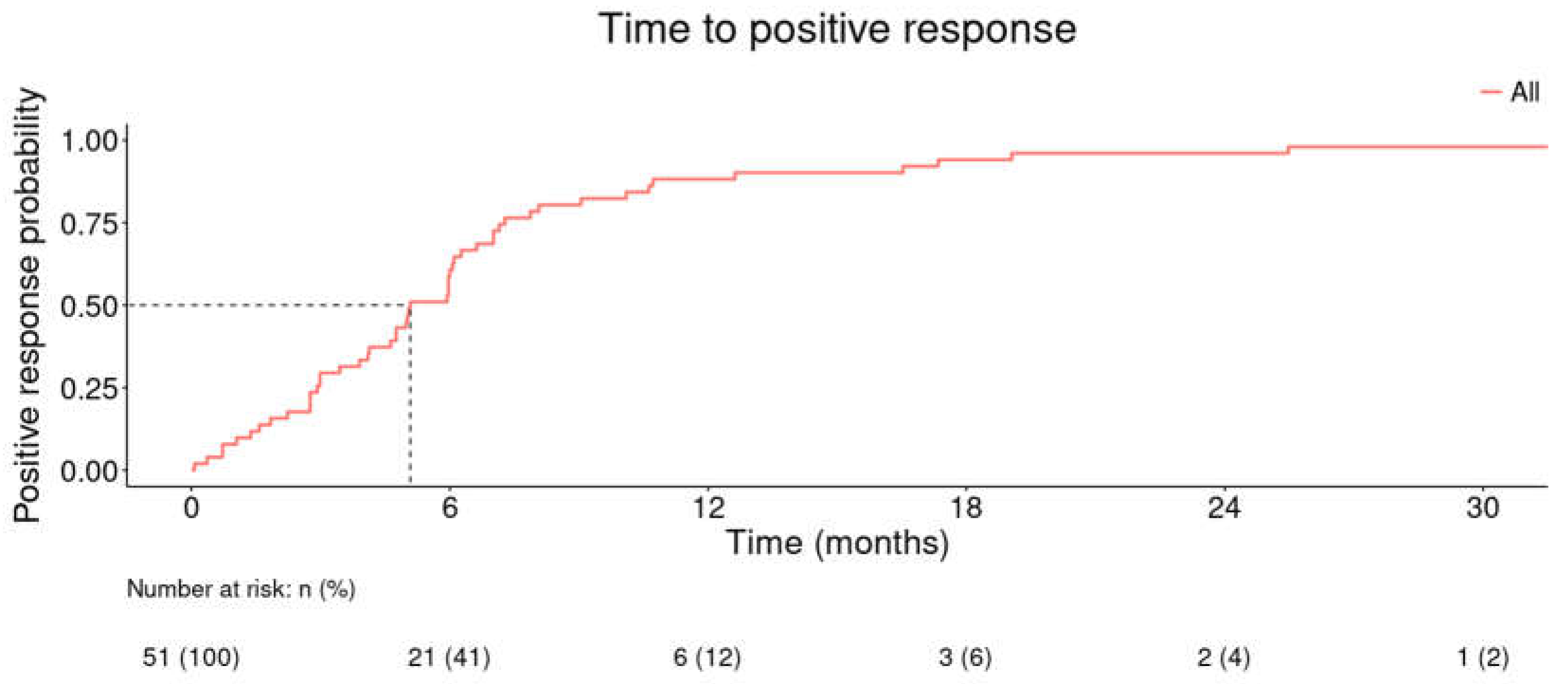
The median time to positive response was 5.08 months for the combination of CPX 8% HPCH and oral antifungals in patients who registered a positive response (including cure) within the study period (
Figure 2).
3.3. Treatment Persistence
Treatment persistence for CPX 8% HPCH, oral antifungal agents, and combined treatment are shown in
Table 5. The median (Q1, Q3) treatment persistence for the treatment combination, for oral antifungal agents, and for CPX 8% HPCH was 2.72 (0.95, 4.72), 2.93 (1.38, 6.07), and 4.98 (1.48, 6.77) months, respectively.
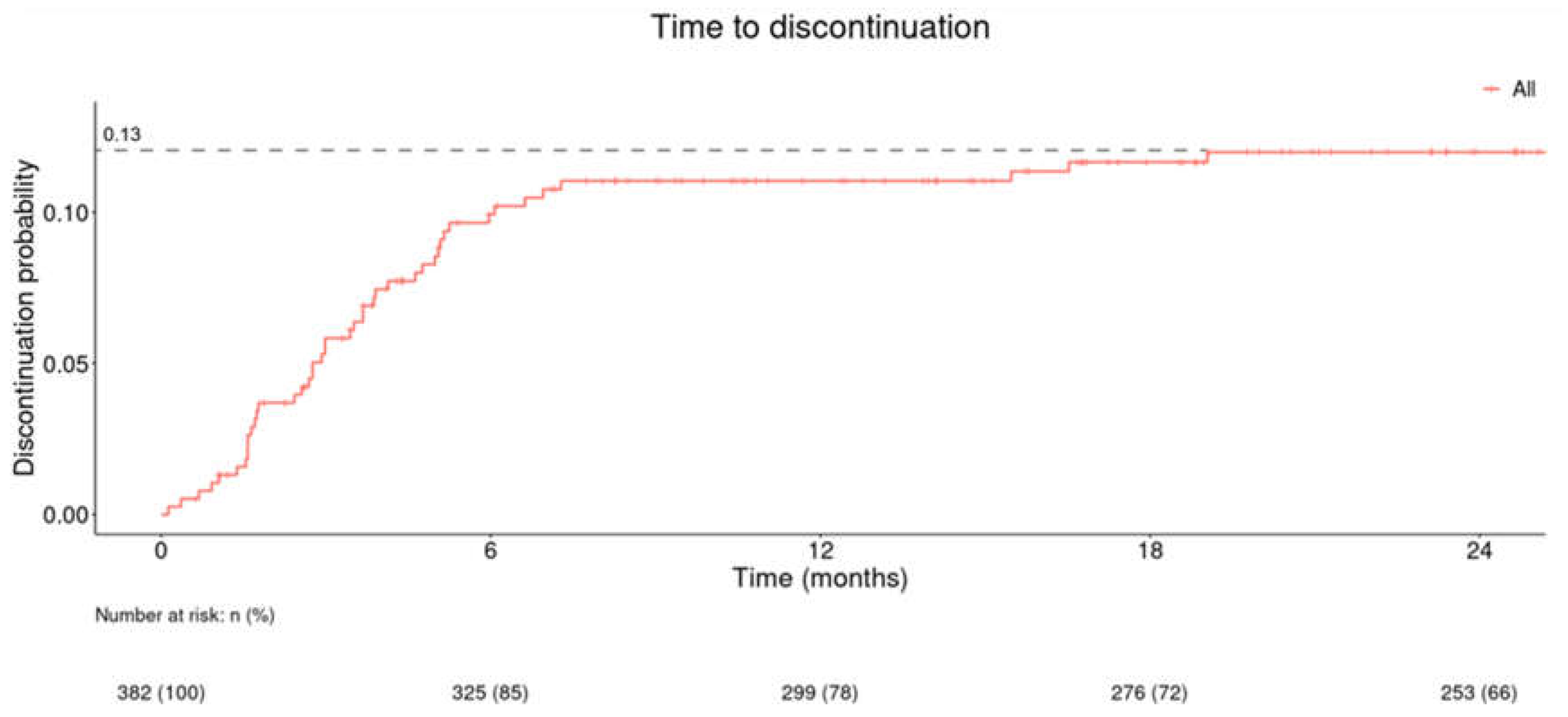
Time to discontinuation (in months) of onychomycosis combination treatment (CPX 8% HPCH and oral antifungal) in all study population is shown in
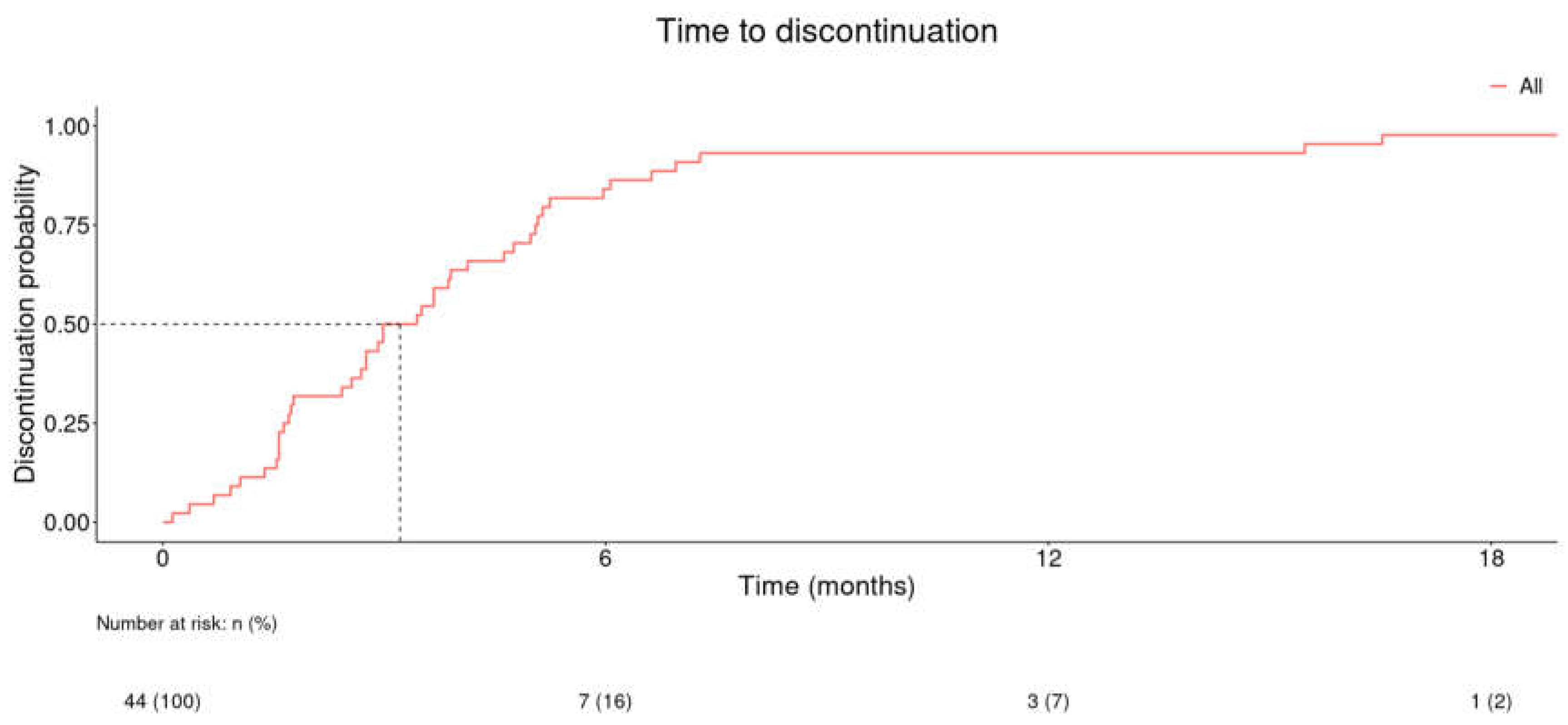
Figure 3 and in patients with registered positive response in
Figure 4. The probability of discontinuation reached 0.13 at the 24-month point for the study population (
Figure 3). The median time to discontinuation in patients with registered positive response was 3.21 months (
Figure 4).
3.4. Adverse Events
Potential AEs reported in the EHRs during the study period in the overall study population and in the subgroups of patients according to the oral antifungal agent combined with CPX 8% HPCH is shown in
Table 6. The most frequent AEs were erythema (5.6%), diarrhea (4.9%), and fever (4.2%), although the occurrence of AEs was similar in the different subgroups of oral antifungal agents.
3.5. Additional Topical Treatments
A small percentage of patients received additional topical treatments during the study period, including topical amorolfine in 9.1% (n = 37), laser therapy in 4.9% (n = 20), topical miconazole in 4.4% (n = 18), topical ketoconazole in 1% (n = 4), topical clotrimazole in 0.7% (n = 3), and photodynamic therapy in 0.2% (n = 1).
4. Discussion
The present study provides real-world evidence of the management of patients diagnosed with onychomycosis in daily practice conditions. A key finding of the study was that treatment combinations of CPX 8% HPCH with terbinafine, itraconazole, and fluconazole were commonly used in our clinical practice setting, which is consistent with data of other studies indicating that the association of antifungal agents to topical therapy may be useful to accelerate the clinical and microbiological healing of superficial dermatophytes infections [
19].
When contextualizing our results within the framework of previously described literature findings, it is crucial to emphasize the distinctions between the research product utilized in the current study (CPX 8% HPCH nail lacquer) and the ciclopirox 8% employed in other studies. In this regard, the addition of HPCH, a water-soluble biopolymer, has shown to improve the efficacy of ciclopirox nail lacquer as shown in a multicenter, randomized, three-arm, placebo-controlled study of 467 patients [
19]. While brief evidence exists regarding combination of ciclopirox 8% with terbinafine [
16,
20,
21], no previous studies have evaluated the combinations of ciclopirox 8% with other oral antifungals. In a clinical series of 8 patients randomly assigned to oral terbinafine 250 mg/day for 16 weeks or a combination of oral terbinafine 250 mg/day for 16 weeks and topical ciclopirox nail lacquer once daily for 9 months, the mycological cure rates were 64.7% for terbinafine monotherapy vs. 88.2% for the combined therapy [
16]. In a multicenter randomized pilot study of 73 patients with moderate to severe toenail onychomycosis, mycological cure was observed in 70.4% of patients treated with the combination of ciclopirox 8% nail lacquer topical solution and terbinafine 250 mg/day and in 56% of patients treated with oral terbinafine alone [
20]. However, in an open randomized comparative study of 96 patients, a small difference in mycological cure rates was found between oral terbinafine pulse therapy in combination with topical ciclopirox olamine 8% (cure rate 83.3%) and terbinafine pulse therapy as monotherapy (cure rate 82.6%) [
21]. Although all these results showed higher mycological cure rates in patients treated with the combination therapy, results are conflicting due to methodological differences among the three studies [
22].
As far as we are aware, this is the first study describing the use of ciclopirox 8% HPCH combined with three different oral antifungals in a large study population of 408 patients with onychomycosis. The present results show that the response rates as reported by clinicians in EHRs were similar across the combinations of CPX 8% HPCH with terbinafine, itraconazole and fluconazole, with an overall positive response of 15.7%, although we also found that 59.8% of patients had a presumed positive response non-confirmed but registered in their clinical records. When positive and presumed positive responses were considered, the overall response rate was 75.5%. This percentage is in line with the high variability of response rates reported by Falotico et al. in a recently published systematic review [
10]. The authors reported that cure percentages for the combination of oral terbinafine plus ciclopirox (non-HPCH) range from 33% to 83.3% depending on the treatment protocol, time to event and type of endpoints assessed, such as mycological cure, clinical cure or clinical improvement, among others. In our study, the median time to positive response was 4.4 months. Interestingly, a similar trend of response was observed when stratified by treatment synchronicity (concomitant start, initial oral antifungal, and initial CPX 8% HPCH), and by type of antifungal agent (terbinafine, itraconazole, and fluconazole). This observation facilitates flexibility in the use of combined treatment with CPX 8% HPCH and systemic antifungals in daily practice.
In this study, treatment persistence was calculated for the combination therapy and for each treatment component, reporting median times of 2.72, 2.93, and 4.98 months for the combination therapy, oral antifungal, and CPX 8% HPCH, respectively. In parallel, analysis of time to CPX 8% HPCH + oral antifungal combination discontinuation in patients with a registered positive response showed that the median time to discontinuation was 3.21 months and that almost all patients discontinued the treatment at the time point of 18 months.
Another interesting finding was the very small percentage of patients in which additional topical treatment was recorded during the follow-up period, with laser therapy and photodynamic therapy being used by only 4.9% and 0.2% of the patients, which may indirectly support the effectiveness of CPX 8% HPCH nail lacquer associated with oral antifungal treatment for the management of onychomycosis.
The treatment schedule of CPX 8% HPCH combined with oral antifungals was well tolerated and safe, with erythema (5.6%), diarrhea (4.9%) and fever (4.2%) as the most frequently registered potential AEs. The interpretation of AEs is limited by the fact that symptoms caused by CPX 8% HPCH from those attributable to treatment with oral antifungals cannot be differentiated. Retrospective analysis of AEs with topical onychomycosis medications reported to the United States Food and Drug Administration showed that drug ineffectiveness was the most common AE associated with ciclopirox 8% solution [
23], whereas taste disturbance (terbinafine) and drug interactions (itraconazole and fluconazole) were the most frequent AEs associated with systemic onychomycosis medications [
24].
The use of artificial intelligence (AI) related tools for extracting data from EHRs is a novel approach for the assessment of the clinical management of medical conditions in the real-world setting. The extent to which clinicians accurately described the patients’ conditions and clinical status in their medical records and the extension of missing reports is a limitation for this type of studies. In addition, the study population included only patients attended at hospital level, limiting the generalization of the results to other settings. However, the multicenter approach and the EHRead® technical resource optimizes the analysis of a representative characterization of patients with onychomycosis and their medical care in daily practice.
5. Conclusions
This study offers a comprehensive overview of onychomycosis patients in a real-world hospital setting, describing effectiveness and safety profile of a product containing ciclopirox 8% formulated with HPCH in combination with oral terbinafine, itraconazole and fluconazole. Our results corroborate that CPX 8% HPCH combination with terbinafine is the most common association prescribed, being either itraconazole or fluconazole combinations similarly used. Singularly, we do not observe differences in response rates or in time to response according to oral antifungal or treatment synchronicity, being frequency of response rates analogous to those previously published. In addition, no threatening AEs were detected, highlighting the safety profile of the combinations studied. The large study population and the robustness of data extracted from EHRs and analyzed using AI-related tools provides evidence of the benefit of combined treatment, which is clinically relevant for the management of patients with onychomycosis in daily practice.
Author Contributions
Conceptualization, G. Roustan, J. López-Estebaranz, P. de la Cueva, Savana Research, and J. Galván; methodology, Savana Research; software, Savana Research; validation, G. Roustan, J. López-Estebaranz, P. de la Cueva, Savana Research, and J. Galván; formal analysis, Savana Research; investigation, G. Roustan, J. López-Estebaranz, P. de la Cueva, Savana Research, F. Pajuelo, M. Tamarit, A. Valmaseda, and J. Galván; resources, F. Pajuelo, M. Tamarit, A. Valmaseda, and J. Galván; data curation, Savana Research; writing—original draft preparation, Savana Research; writing—review and editing, G. Roustan, J. López-Estebaranz, P. de la Cueva, Savana Research, and J. Galván. All authors have read and agreed to the published version of the manuscript.
Funding
This research was founded by Polichem S.A.
Institutional Review Board Statement
Approval by the Ethics Committee of the participating hospitals was obtained.
Informed Consent Statement
Not applicable.
Data Availability Statement
The study data are available from the corresponding author upon request.
Acknowledgments
The authors thank Dr. Alba Martínez-Alcocer Martínez, Dr. Ángel Rosell Díaz, Dr. Ángela García Miñarro, Dr. Elena Garcia Zamora, Dr. Joseph Griffiths, and Dr. María Gamo Guerrero for performing the external evaluation of clinical variables extracted with NLP. The authors thank Dr. Marta Pulido for editing the manuscript and editorial assistance. Savana Research Group members in alphabetical order: Lucía Cabal-Hierro, David Casadevall, Judith Marín-Corral, Luisa Martínez, Claudia Maté, Sebastian Menke, Natalia Polo, Margarita Posso, Ignacio Salcedo, Daniel Salvador and Miren Taberna.
Conflicts of Interest
P. de la Cueva served as a consultant, speaker and investigator for Abbvie, Almirall, BMS, Boehringer, Celgene, Janssen, LEO Pharma, Lilly, MSD, Novartis, Pfizer, Roche, Sanofi, UCB. J. López-Estebaranz served as a consultant, participated in clinical trials and/or received speaking fees from Almirall, Janssen, Leo-Pharma, Lilly, Abbvie, Bioderma, Galderma, UCB, Novartis, Pierre-Fabre, Invasix, Isdin and Incyte. G. Roustan has been involved in training and consulting activities and conferences sponsored by Almirall. A. Valmaseda, J. Galván, F. Pajuelo, and M. Tamarit are full-time employees of Almirall S.A. The study was conducted by Medsavana S.L and Savana Research S.L, of which Savana Research Group members are employees.
References
- Sigurgeirsson, B.; Baran, R. The prevalence of onychomycosis in the global population—A literature study. J. Eur. Acad. Dermatol. Venereol. 2013, 28, 1480–1491. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, N.M.; Rodríguez-Tamez, G.; Perez, S.; Tosti, A. Onychomycosis: Old and New. J. Fungi 2023, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Jain, H.C.; Lynde, C.W.; MacDonald, P.; Cooper, E.A.; Summerbell, R.C. Prevalence and epidemiology of onychomycosis in patients visiting physicians’ offices: A multicenter Canadian survey of 15,000 patients. J. Am. Acad. Dermatol. 2000, 43, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Chacon, A.; Franca, K.; Fernandez, A.; Nouri, K. Psychosocial impact of onychomycosis: a review. Int. J. Dermatol. 2013, 52, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Mays, R.R. The Impact of Onychomycosis on Quality of Life: A Systematic Review of the Available Literature. Ski. Appendage Disord. 2018, 4, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K. Pharmacoeconomic Analysis of Oral Antifungal Therapies Used to Treat Dermatophyte Onychomycosis of the Toenails. PharmacoEconomics 1998, 13, 243–256. [Google Scholar] [CrossRef]
- Leung, A.K.; Lam, J.M.; Leong, K.F.; Hon, K.L.; Barankin, B.; Leung, A.A.; Wong, A.H. Onychomycosis: An Updated Review. Recent Patents Inflamm. Allergy Drug Discov. 2020, 14, 32–45. [Google Scholar] [CrossRef]
- Valentín-Martín, A.; Hernández-Pérez, N.; Romero-Noreña, A.; Molina-Moreno, J.M. Onychomycosis of rare etiology. Enferm Infecc Microbiol Clin 2022, 40, 330–331. [Google Scholar] [CrossRef]
- Geizhals, S.; Cooley, V.; Lipner, S.R. Diagnostic testing for onychomycosis: A retrospective study over 17 years. J. Am. Acad. Dermatol. 2020, 83, 239–241. [Google Scholar] [CrossRef]
- Falotico, J.M.; Lipner, S.R. Updated Perspectives on the Diagnosis and Management of Onychomycosis. Clin. Cosmet. Investig. Dermatol. 2022, ume 15, 1933–1957. [Google Scholar] [CrossRef]
- Lipner, S.R.; Scher, R.K. Onychomycosis: Treatment and prevention of recurrence. J. Am. Acad. Dermatol. 2018, 80, 853–867. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Targhotra, M.; Kumar, B.; Sahoo, P.; Chauhan, M. Treatment and management strategies of onychomycosis. J. Med Mycol. 2020, 30, 100949. [Google Scholar] [CrossRef] [PubMed]
- Baran, R.; Kaoukhov, A. Topical antifungal drugs for the treatment of onychomycosis: an overview of current strategies for monotherapy and combination therapy. J. Eur. Acad. Dermatol. Venereol. 2004, 19, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Xiong, X.; Ran, Y. Efficacy and tolerability of amorolfine 5% nail lacquer in combination with systemic antifungal agents for onychomycosis: A meta-analysis and systematic review. Dermatol. Ther. 2017, 30, e12457. [Google Scholar] [CrossRef] [PubMed]
- Piraccini, B.M.; Iorizzo, M.; Lencastre, A.; Nenoff, P.; Rigopoulos, D. Ciclopirox Hydroxypropyl Chitosan (HPCH) Nail Lacquer: A Review of Its Use in Onychomycosis. Dermatol. Ther. 2020, 10, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Avner, S.; Nir, N.; Henri, T. Combination of oral terbinafine and topical ciclopirox compared to oral terbinafine for the treatment of onychomycosis. J. Dermatol. Treat. 2005, 16, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Medrano, I.H.; Guijarro, J.T.; Belda, C.; Ureña, A.; Salcedo, I.; Espinosa-Anke, L.; Saggion, H. Savana: Re-using Electronic Health Records with Artificial Intelligence. Int. J. Interact. Multimedia Artif. Intell. 2018, 4, 8–12. [Google Scholar] [CrossRef]
- Espinosa-Anke, L.; Tello, J.; Pardo, A.; Medrano, I.; Ureña, A.; Salcedo, I.; Saggion, H. Savana: A Global information extraction and terminology expansion framework in the medical domain. Procesamiento del Lenguaje Natural 2016, 57, 23–30. [Google Scholar]
- Baran, R.; Tosti, A.; Hartmane, I.; Altmeyer, P.; Hercogova, J.; Koudelkova, V.; Ruzicka, T.; Combemale, P.; Mikazans, I. An innovative water-soluble biopolymer improves efficacy of ciclopirox nail lacquer in the management of onychomycosis. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 773–781. [Google Scholar] [CrossRef]
- Gupta, A.K.; Onychomycosis Combination Therapy Study Group. Ciclopirox topical solution, 8% combined with oral terbinafine to treat onychomycosis: a randomized, evaluator-blinded study. J. Drugs Dermatol. 2005, 4, 481–485. [Google Scholar]
- Sharma, R.; Garg, A.; Jaiswal, A. An open randomized comparative study to test the efficacy and safety of oral terbinafine pulse as a monotherapy and in combination with topical ciclopirox olamine 8% or topical amorolfine hydrochloride 5% in the treatment of onychomycosis. Indian J. Dermatol. Venereol. Leprol. 2007, 73, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Falotico, J.M.; Lapides, R.; Lipner, S.R. Combination Therapy Should Be Reserved as Second-Line Treatment of Onychomycosis: A Systematic Review of Onychomycosis Clinical Trials. J. Fungi 2022, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lipner, S.R. Retrospective analysis of adverse events with topical onychomycosis medications reported to the United States Food and Drug Administration. Arch. Dermatol. Res. 2020, 312, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lipner, S.R. Retrospective analysis of adverse events with systemic onychomycosis medications reported to the United States Food and Drug Administration. J. Dermatol. Treat. 2021, 32, 783–787. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).