1. Introduction
Ischemic heart disease, particularly ST-segment elevation myocardial infarction (STEMI), remains a leading cause of global morbidity and mortality [
1]. Despite advancements in acute management of STEMI patients, there is a persistent need for accurate and individualized prognostic markers to better predict outcomes and tailor therapeutic strategy [
2,
3]. Traditional risk stratification methods might not capture the dynamic nature of patient’s risk, requiring an individualized approach to enhance the prognostic accuracy.
Heart rate variability (HRV), a measure of autonomic nervous system function, has emerged as a promising prognostic tool in cardiovascular disease. Alteration of HRV values is associated with adverse outcomes, including increased mortality and major adverse cardiovascular events (MACE) [
4,
5,
6]. Also, by capturing real-time physiological responses, HRV measurements offer individualized insights into the dynamic autonomic nervous system activity [
7]. Nevertheless, the relationship between short-term and instantaneous HRV parameters and clinical and paraclinical variables in STEMI patients is not yet fully understood.
The clinical application of HRV has been limited by the use of 24-hour electrocardiographic recordings, which could be impractical for real-time monitoring in acute settings. To address this limitation, wearable devices have been validated for their accuracy in measuring HRV, offering a practical alternative to traditional electrocardiographic recordings [
8]. These devices facilitate the collection of HRV data in diverse settings, ranging from everyday activities to acute myocardial infarction (AMI) scenarios, thereby broadening the scope of HRV research and its applications. Additionally, wearables enable continuous monitoring, which is particularly beneficial for observing transient physiological changes in HRV parameters [
8].
In a comprehensive overview,
Shaffer and Ginsberg, identified and established norms for various HRV metrics, including short and ultra-short parameters [
9]. These norms provide valuable benchmarks for interpreting HRV in a range of clinical settings. However, despite the broad applicability of these metrics, there remains a notable gap in the literature concerning the HRV measurements in STEMI patients during the acute phase [
9]. A recent study examined HRV and heart rhythm complexity in 33 patients with inferior STEMI within one year following myocardial infarction [
10]. The authors reported significant changes in this metrics over time, underscoring the potential of HRV in monitoring cardiovascular health [
10].
The alterations in HRV during the initial hours following STEMI onset are still not well described, suggesting a need for further research to elucidate early responses in these patients. Capturing real-time physiological data facilitates a more personalized approach to patient care. Our study represents a key step in integrating wearable technology with clinical practice, aiming to improve prognostic accuracy and ultimately enhance patient outcomes in STEMI management.
The primary objective of this study was to assess the dynamic changes in various HRV parameters (time-domain, frequency-domain and non-linear measurements) during the acute phase of STEMI, and to establish baseline metrics and normative ranges for these parameters. Additionally, we sought to explore the association between real-time HRV measurements and adverse outcomes during hospitalization for STEMI.
2. Materials and Methods
This study was a single-center, observational cohort study conducted at the Institute of Cardiovascular Diseases “Prof. Dr. George I.M. Georgescu” in Iasi, Romania. Ethical approval was obtained from both, the Institute’s Ethics Committee (approval date: 14.01.2022) and the University of Medicine and Pharmacy “Grigore T. Popa” Ethics Committee (approval number: 164/21.03.2022). Also, the protocol of the study was registered in ClinicalTrials.gov database (NCT05098977). All participants were informed about the objectives of the study, potential risks and benefits, and written informed consent was obtained from each participant. Confidentiality and data protection were ensured, as all collected data were anonymized and stored in a secure database.
2.1. Study Population
Consecutive patients presenting with STEMI in sinus rhythm, referred for primary percutaneous coronary intervention (PCI) within 12 hours from symptoms onset were enrolled. Inclusion criteria included: (1) age ≥ 18 years, (2) STEMI diagnosis in the first 12 hours from symptoms onset, and (3) the ability to provide informed consent.
Exclusion criteria encompassed conditions interfering with RR intervals and HRV parameters: (1) atrioventricular block or known sinus node dysfunction, (2) atrial fibrillation, (3) paced ventricular rhythm, (4) patients treated with positive inotropic or chronotropic drugs, (5) history of myocardial infarction or revascularization, (6) and inability to sign the informed consent.
2.2. HRV Measurement
HRV parameters were measured during primary PCI using a wearable device approved for medical use (Empatica E4 wristband, CE certified). Measurements were taken throughout the entire duration of the primary PCI, as well as specifically during the initial 5 minutes and the final 5 minutes of the procedure, to capture dynamic short-term physiological responses. The following time-domain HRV parameters were calculated: the standard deviation of all NN intervals (SDNN), the standard deviation of the average NN interval over short time divisions (SDANN), HRV triangular index, the square root of the mean squared differences of consecutive NN intervals (RMSSD), the number of pairs of successive NN (R-R) intervals that differ by more than 50 ms (NN50), and the proportion of NN50 divided by the total number of NN (R-R) intervals (pNN50). Frequency-domain measurements included low-frequency power (LF), very-low-frequency power (VLF), high-frequency power (HF), as well as LF/HF ratio. Additionally, the following non-linear parameters were evaluated: SD1, SD2, SD2/SD1, and approximate entropy.
2.3. Study Outcomes
The primary outcome of this study was to assess the dynamic changes in various short-term HRV parameters (time-domain, frequency-domain, and non-linear measurements) during the acute phase of STEMI, and to establish baseline metrics and normative ranges for these parameters. Additionally, we explored the relationship between real-time HRV measurements and other clinical and paraclinical variables in the first hours from symptoms onset.
Furthermore, we analyzed the association between all HRV parameters and in-hospital adverse outcomes, including all-cause and cardiovascular mortality, as well as MACE. MACE was defined as all-cause and cardiovascular mortality, fatal and non-fatal myocardial infarction, unplanned target vessel revascularization, and ischemic or hemorrhagic stroke.
2.4. Statistical Analysis
Statistical analysis was conducted using SPSS software version 26.0 (IBM SPSS Statistics, NY), and R software (R Foundation for Statistical Computing, Vienna, Austria). Also, Kubios HRV Standard software (Kubios Oy, Kuopio, Finland) was used to analyze extracted interbeat interval data from the wristband and to calculate all HRV parameters. To ensure the accuracy of HRV parameters, any artifacts in the raw data were corrected using a very low threshold algorithm (0.45 s) in Kubios HRV software, minimizing the potential for significant distortions in HRV measurement.
Missing data were addressed using multiple imputation techniques to ensure robustness and reliability of the statistical analysis. Non-parametric statistical tests were used for data that were not normally distributed. The Wilcoxon signed-rank test was employed to analyze dynamic changes in HRV parameters over different time points. Additionally, we performed ROC analysis to predict adverse events during hospitalization. All statistical tests were two-tailed, and a p-value of <0.05 was considered statistically significant. The analyses were conducted in accordance with relevant guidelines and best practices for statistical analysis in medical research
3. Results
Between April 2022 and December 2023, a total of 104 patients with STEMI who underwent primary PCI were included in this study. Among them, 70.2% were male and 29.8% were female, with a mean age of 60.64 ± 13.36 years. Baseline demographics and characteristics of patients were displayed in
Table 1. The median time to PCI was 8 hours with an interquartile range (IQR) of 6.0 to 10.25 hours. Regarding cardiovascular risk factors, 58.65% of the patients were smokers, 55.8% had arterial hypertension, and 21.15% had diabetes mellitus. Additionally, 15.4% had a history of ischemic heart disease, 5.8% had experienced a previous stroke, 2.9% had peripheral artery disease, and 2.9% had chronic obstructive pulmonary disease. Also, 23.1% patients were treated with beta-blockers prior to the index hospitalization.
Most patients presented with inferior STEMI (50%), followed by extensive anterior STEMI (24%), with a median admission left ventricular ejection fraction (LVEF) of 40%. Additionally, the majority of patients were in Killip class I (66.3%) and class II (27.9%). Regarding coronary artery disease severity, 46.2% of patients had single-vessel disease, 31.7% had two-vessel disease, and 22.1% had three-vessel disease. The most frequent culprit artery was the left anterior descending (LAD) artery (48.1%), followed by the right coronary artery (RCA, 35.6%). Final TIMI flow grade 3 was achieved in 88.5% of patients (
Table 2).
HRV parameters were measured throughout the entire PCI procedure. These measurements were also divided into two 5-minute segments: one at the beginning and one at the end of the procedure, to capture the potential effects of percutaneous myocardial revascularization. All measurements are presented in
Table 3. Notably, all HRV parameters (time-domain, frequency-domain, and non-linear measurements) tended to decrease in the last 5-minute segment compared to the first 5-minute segment, although not all changes achieved statistical significance. Mortality occurred in 5 patients (4.8%), and MACE occurred in 6 cases (5.8%). Cardiac arrest during PCI was observed in 2 patients (1.9%), and ventricular arrhythmias were documented in 12 patients (11.5%). The median ICU stay was 3.0 days (
Supplementary Table S1).
Among time-domain measurements, a significant decrease following PCI was noted for SDNN, RMSSD, and pNN50 (
Table 4). Similarly, in the case of frequency-domain parameters, the decrease in HF reached statistical significance, while the other parameters remained relatively unchanged when comparing the first and last 5 minutes of PCI. Additionally, non-linear parameters also decreased shortly after PCI, with SD1 and the SD2/SD1 ratio showing statistically significant changes (Supplementary
Figures S1-S3).
Compared to patients with RCA as culprit artery, those with LAD as culprit artery tended to have lower HRV parameter values (
Supplementary Table S2). In time-domain measurements, SDNN, RMSSD, pNN50, and the RR triangular index were significantly lower in patients with LAD as the culprit artery. In case of frequency-domain parameters, VLF, LF (ms², log), and HF (ms², log) were significantly higher in RCA-culprit patients, while the LF/HF ratio was similar in both groups. Additionally, SD1, SD2, and the SD2/SD1 ratio were lower in LAD-culprit as compared to RCA-culprit patients.
Concerning in-hospital mortality, the SD2/SD1 ratio was significantly lower in deceased patients compared to survivors throughout the entire PCI procedure, including measurements taken in 5-minute segments (p = 0.008). Additionally, the approximate entropy measure for the entire PCI duration was significantly lower in deceased patients (p = 0.019), although the 5-minute segments did not reach statistical significance (
Table 5). In the correlation analysis using the Spearman test, LF (log), SD2/SD1, and approximate entropy were significantly correlated with in-hospital mortality (p = 0.048, p = 0.007, and p = 0.017, respectively) (
Supplementary Table S3).
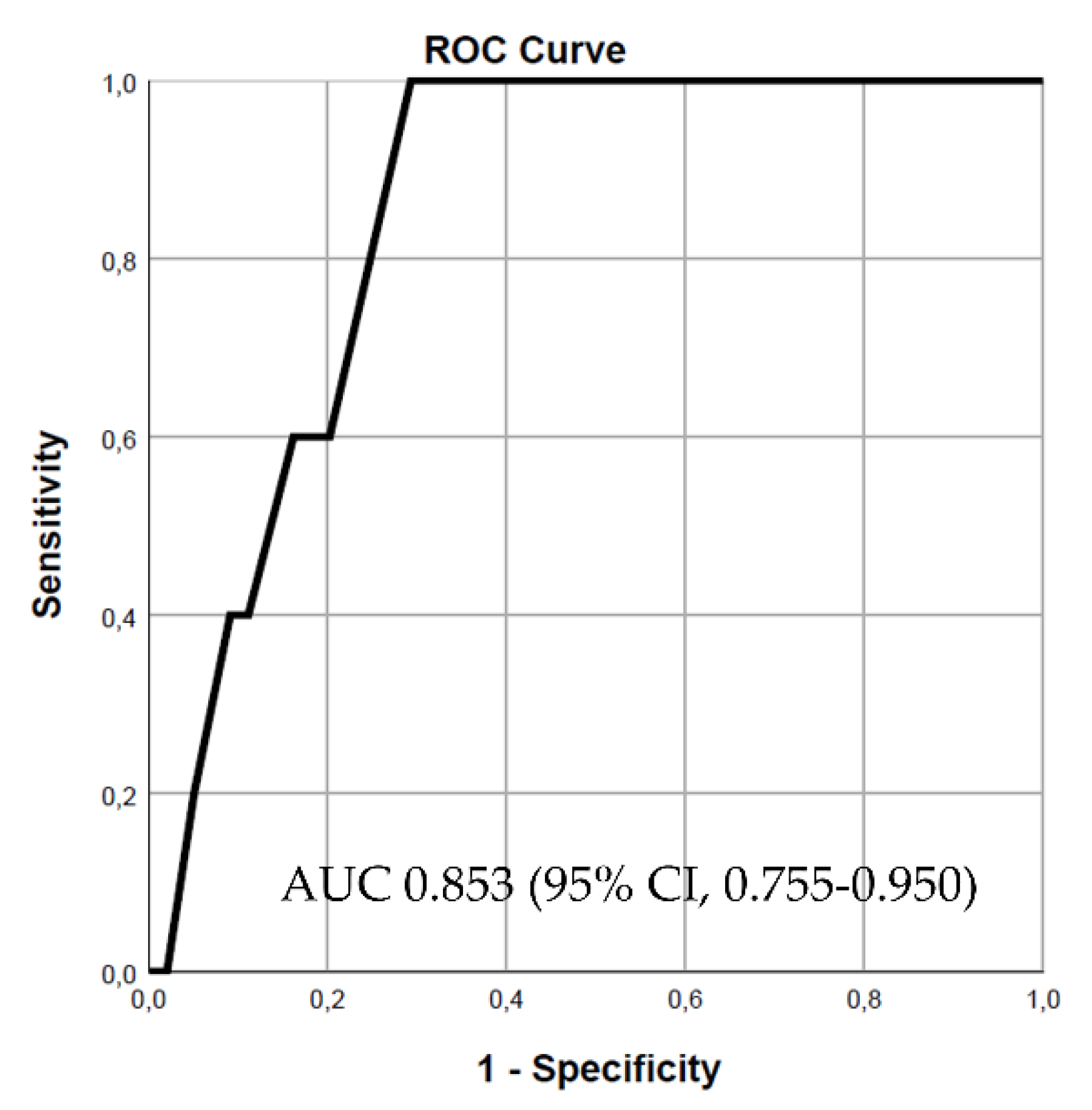
Both variables, the SD2/SD1 ratio and approximate entropy, were incorporated into various models to predict in-hospital mortality. In the first model, the SD2/SD1 ratio demonstrated good predictive power, with an area under the curve (AUC) of 0.853 (95% CI, 0.755-0.950, p = 0.008) (
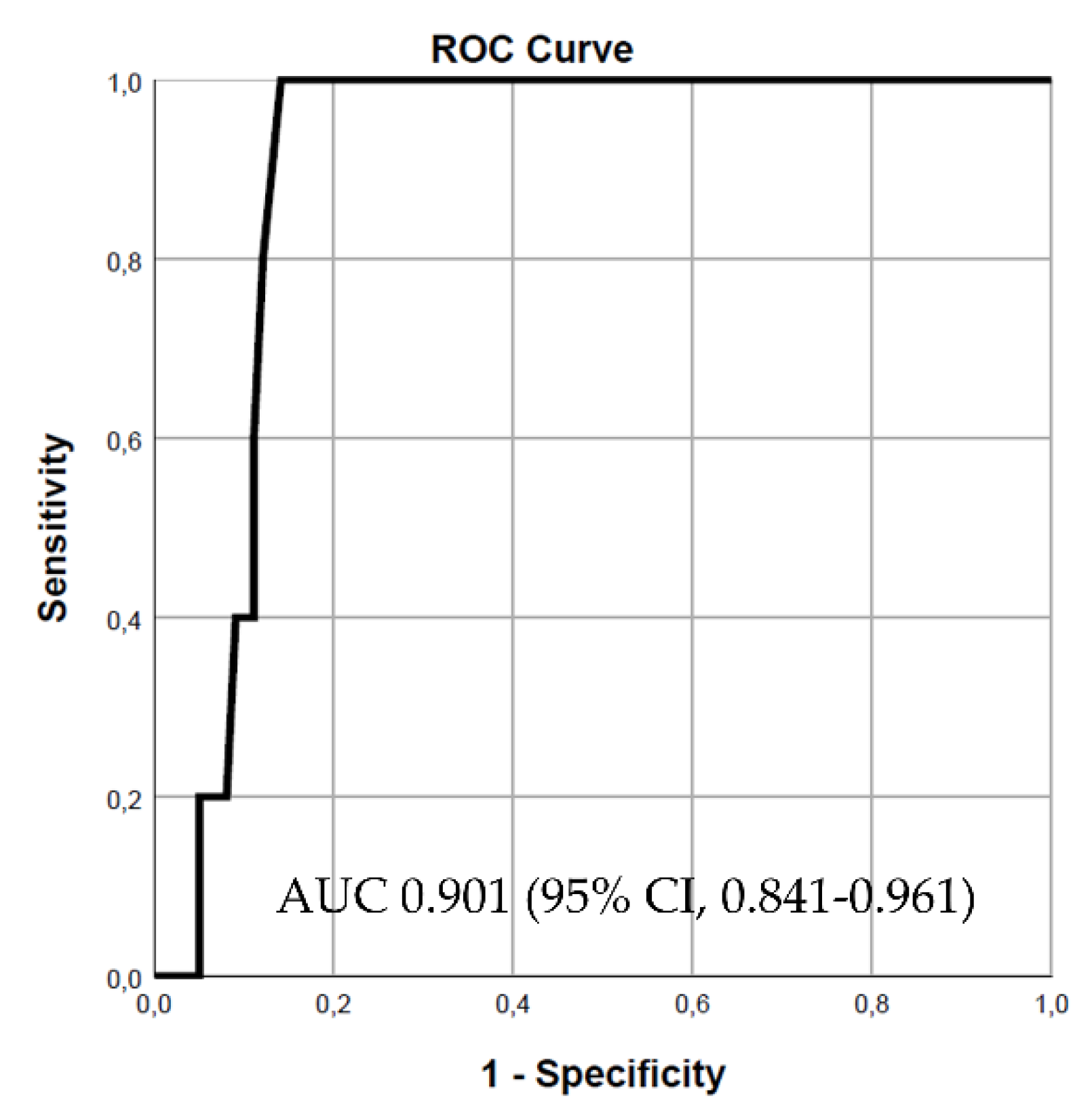
Figure 1). Adding approximate entropy to the model increased the predictive power, resulting in an AUC of 0.901 (95% CI, 0.841-0.961, p = 0.003) (
Figure 2). However, including LVEF in the final model to assess its impact on predictive power did not lead to a statistically significant change (chi-square test, p = 0.234). Notably, for patients with severe systolic dysfunction (LVEF ≤ 30%), the predictive power of the combined model using the SD2/SD1 ratio and approximate entropy remained strong, with an AUC of 0.816 (95% CI, 0.641-0.990) (
Supplementary Figure S4).
Patients who experienced MACE and ventricular arrhythmias exhibited altered non-linear HRV parameters compared to those without adverse events. Specifically, patients with MACE during hospitalization had significantly lower SD2/SD1 ratio and approximate entropy values (p = 0.006 and p = 0.005, respectively). Similarly, ventricular arrhythmias were associated with a significantly reduced SD2/SD1 ratio (p = 0.002) (
Supplementary Table S4).
4. Discussion
Our study is the first prospective investigation to evaluate dynamic changes in HRV parameters during the acute phase of STEMI using real-time measurements from a CE-marked wearable device (Empatica E4 wristband). This innovative approach enabled continuous monitoring of patients during the primary PCI procedure, offering valuable insights into autonomic nervous system activity during this critical period.
The primary novelty of our study lies in the use of real-time HRV monitoring with a wearable device, which allowed continuous data collection throughout the entire PCI procedure. Previous studies have typically relied on 24-hour electrocardiographic recordings, which are impractical for real-time monitoring in acute settings [
11,
12]. The wearable device used in our study is validated for medical use, ensuring the accuracy and reliability of the HRV measurements obtained [
8]. By capturing short-term physiological responses, one could assess transient physiological changes, providing data regarding autonomic responses to acute myocardial infarction and subsequent revascularization.
The present study demonstrates several key strengths.
Our findings revealed a significant decrease in the majority of HRV parameters shortly after myocardial revascularization, including SDNN, RMSSD, pNN50, HF, SD1, and the SD2/SD1 ratio, underscoring the impact of PCI on autonomic function. Moreover, our analysis highlighted different HRV values in patients with LAD as the culprit artery compared to those with RCA as the culprit artery. LAD-culprit patients showed significantly lower values in parameters such as SDNN, RMSSD, and pNN50.
Furthermore, we evaluated adverse in-hospital outcomes such as cardiovascular mortality, MACE, and ventricular arrhythmias, establishing correlations with HRV metrics. Notably, reduced values of SD2/SD1 and approximate entropy were significantly associated with increased in-hospital mortality. Similarly, a lower SD2/SD1 ratio was linked to a higher incidence of ventricular arrhythmias. In case of patients who experienced MACE, both SD2/SD1 and approximate entropy were significantly decreased.
Chakko et al. [
12], reported similar findings regarding HRV parameter alterations following myocardial reperfusion. They observed a significant decrease in HRV parameters, measured over 24 hours, following myocardial reperfusion primarily achieved through thrombolysis. This decrease was attributed to acute autonomic imbalance induced by myocardial reperfusion, underscoring the immediate impact of revascularization on autonomic function [
12].
In an earlier study,
Huikuri et al. [
13], found that HRV metrics were linked to an increased risk of mortality and arrhythmic events. However, PCI was performed in only 25% of patients presenting with AMI, contrasting with contemporary practice where PCI is the standard of care [
13]. Therefore, the data on HRV monitoring in these patients needed updating, particularly with real-time derived parameters, to better stratify the risk of adverse events.
A recent study examined the impact of immediate versus delayed stenting strategies (high thrombus burden) on short-term HRV in STEMI patients undergoing primary PCI [
14]. The delayed stenting approach was linked to improved postoperative cardiac electrical stability, indicated by fewer premature ventricular contractions and higher HRV parameters such as SDNN and HF. This study reinforces the utility of HRV assessment in STEMI patients, aiding in the identification of those at higher risk for adverse events [
14].
While our study provides novel insights into the real-time measurement of HRV parameters during PCI, it also has several limitations.
Firstly, the single-center design may limit the generalizability of our findings to other settings or populations. Additionally, the relatively small sample size may reduce the statistical power to detect more subtle differences or associations. Furthermore, the observational nature of the study precludes any conclusions about causality between HRV changes and clinical outcomes. Lastly, other potential confounding factors may impact autonomic function and influence HRV measurements. Future multicenter studies with larger sample sizes are required to validate our findings and to incorporate HRV metrics into future risk scores for STEMI patients.
Our study highlights the significant advantages of real-time HRV monitoring during PCI in STEMI patients, demonstrating its utility in improving risk stratification and patient outcomes. These promising results support the integration of HRV metrics into routine clinical practice, fostering better management and prognosis for STEMI patients. While our study constitutes a foundational background for the utility of HRV analysis in STEMI patients, future multicenter studies with larger sample sizes are required to validate our findings.
5. Conclusions
The present study demonstrates the significant potential of real-time HRV monitoring using wearable technology during the acute phase of STEMI. This approach could offer a new perspective for enhancing patient care through individualized and dynamic risk stratification. The primary findings illustrate that PCI significantly impacts HRV parameters, with a significant decrease in key metrics such as SDNN, RMSSD, and pNN50, highlighting the acute autonomic responses to myocardial revascularization. The association between reduced HRV parameters, particularly the SD2/SD1 ratio and approximate entropy, and adverse in-hospital outcomes, underscores the prognostic utility of HRV monitoring. The integration of HRV metrics into routine clinical practice, facilitated by wearable devices, could improve the risk stratification in STEMI patients.
Supplementary Materials
The following supporting information can be downloaded at:
www.mdpi.com/xxx/s1, Figure S1: Time-domain parameters during PCI; Figure S2: Frequency-domain parameters during PCI; Figure S3: Non-linear parameters during PCI; Figure S4: SD2SD1 and ApEn mortality low FE; Table S1: In-hospital outcomes; Table S2: HRV in LAD vs RCA; Table S3: Mortality correlation analysis; Table S4: HRV and MACE and VA.
Author Contributions
Conceptualization, A.B., M.F.; methodology, A.B., C.B.; software, C.B., A.B.; formal analysis, A.B., M.F., C.B.; A.B., M.F., C.B.; resources, A.M.C., A.C., D.V.S.; data curation, A.B., M.F., C.B.; writing—original draft preparation, A.B., M.F., C.B., D.VS.; writing—review and editing, A.B., M.F., C.B.; visualization, A.B., M.F., C.B., A.M.C., A.C., D.V.S.; supervision, A.B., M.F.; project administration, A.B., M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Institute of Cardiovascular Diseases “Prof. Dr. George I.M. Georgescu” (approval date: 14.01.2022) and the University of Medicine and Pharmacy “Grigore T. Popa” Ethics Committee (approval number: 164/21.03.2022). Protocol approved by clinicaltrials.gov, NCT05098977.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data available upon request.
Acknowledgments
No acknowledgments to declare.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics—2019 Update: A Report From the American Heart Association. Circulation. 2019;139(10):e56-e528.
- Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth Universal Definition of Myocardial Infarction (2018). Circulation. 2018;138(20):e618-e51. [CrossRef]
- Byrne RA, Rossello X, Coughlan JJ, Barbato E, Berry C, Chieffo A, et al. 2023 ESC Guidelines for the management of acute coronary syndromes: Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). European Heart Journal. 2023;44(38):3720-826. [CrossRef]
- Bošković A, Belada N, Knežević B. Prognostic value of heart rate variability in post-infarction patients. Vojnosanitetski pregled. 2014;71(10):925-30.
- Coviello I, Pinnacchio G, Laurito M, Stazi A, Battipaglia I, Barone L, et al. Prognostic role of heart rate variability in patients with ST-segment elevation acute myocardial infarction treated by primary angioplasty. Cardiology. 2013;124(1):63-70. [CrossRef]
- Ablonskytė-Dūdonienė R, Bakšytė G, Čeponienė I, Kriščiukaitis A, Drėgūnas K, Ereminienė E. Impedance cardiography and heart rate variability for long-term cardiovascular outcome prediction after myocardial infarction. Medicina (Kaunas, Lithuania). 2012;48(7):350-8.
- Balanescu S, Corlan AD, Dorobantu M, Gherasim L. Prognostic value of heart rate variability after acute myocardial infarction. Medical science monitor : international medical journal of experimental and clinical research. 2004;10(7):Cr307-15. [CrossRef]
- Schuurmans AAT, de Looff P, Nijhof KS, Rosada C, Scholte RHJ, Popma A, et al. Validity of the Empatica E4 Wristband to Measure Heart Rate Variability (HRV) Parameters: a Comparison to Electrocardiography (ECG). Journal of medical systems. 2020;44(11):190.
- Shaffer F, Ginsberg JP. An Overview of Heart Rate Variability Metrics and Norms. Frontiers in public health. 2017;5:258.
- Tang S-Y, Ma H-P, Lin C, Lo M-T, Lin L-Y, Chen T-Y, et al. Heart rhythm complexity analysis in patients with inferior ST-elevation myocardial infarction. Scientific Reports. 2023;13(1):20861.
- Kida N, Tsubakihara Y, Kida H, Ageta S, Arai M, Hamada Y, et al. Usefulness of measurement of heart rate variability by holter ECG in hemodialysis patients. BMC nephrology. 2017;18(1):8.
- Chakko S, Fernandez A, Sequeira R, Kessler KM, Myerburg RJ. Heart rate variability during the first 24 hours of successfully reperfused acute myocardial infarction: Paradoxic decrease after reperfusion. American Heart Journal. 1996;132(3):586-92.
- Huikuri HV, Tapanainen JM, Lindgren K, Raatikainen P, Mäkikallio TH, Juhani Airaksinen KE, et al. Prediction of sudden cardiac death after myocardial infarction in the beta-blocking era. Journal of the American College of Cardiology. 2003;42(4):652-8.
- Lin S, Yang X, Guo X, Ye J, Hu X, Dong H, et al. Impact of Short-Term Heart Rate Variability in Patients with STEMI Treated by Delayed versus Immediate Stent in Primary Percutaneous Coronary Intervention: A Prospective Cohort Study. Computational and Mathematical Methods in Medicine. 2022;2022(1):2533664.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).