Submitted:
24 July 2024
Posted:
25 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Statistical Analysis
3. Results
3.1. Assessment of the Selected Parameters of Brain Injury in the CSF and the Selected Serum Interleukins in MS Patients
3.1.1. Correlations of the Selected Serum Interleukins with the Selected Parameters of Brain Injury in the CSF in MS Patients
3.1.2. Correlations of the Selected Serum Interleukins with the Selected Parameters of Brain Injury in the CSF in RRMS and PMS Patients
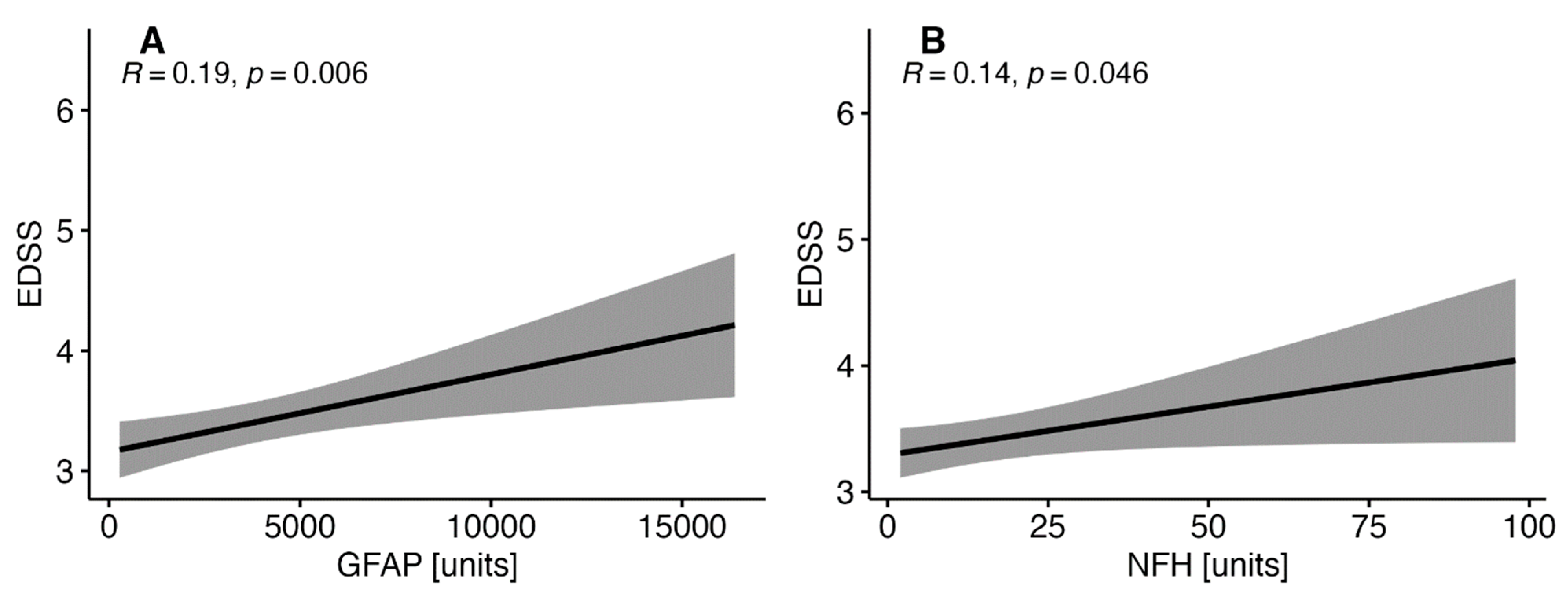
3.2. Correlations between EDSS and the Selected Interleukins in MS Patients
3.3. Correlations between EDSS with the Selected Parameters of Brain Injury in Patients with MS
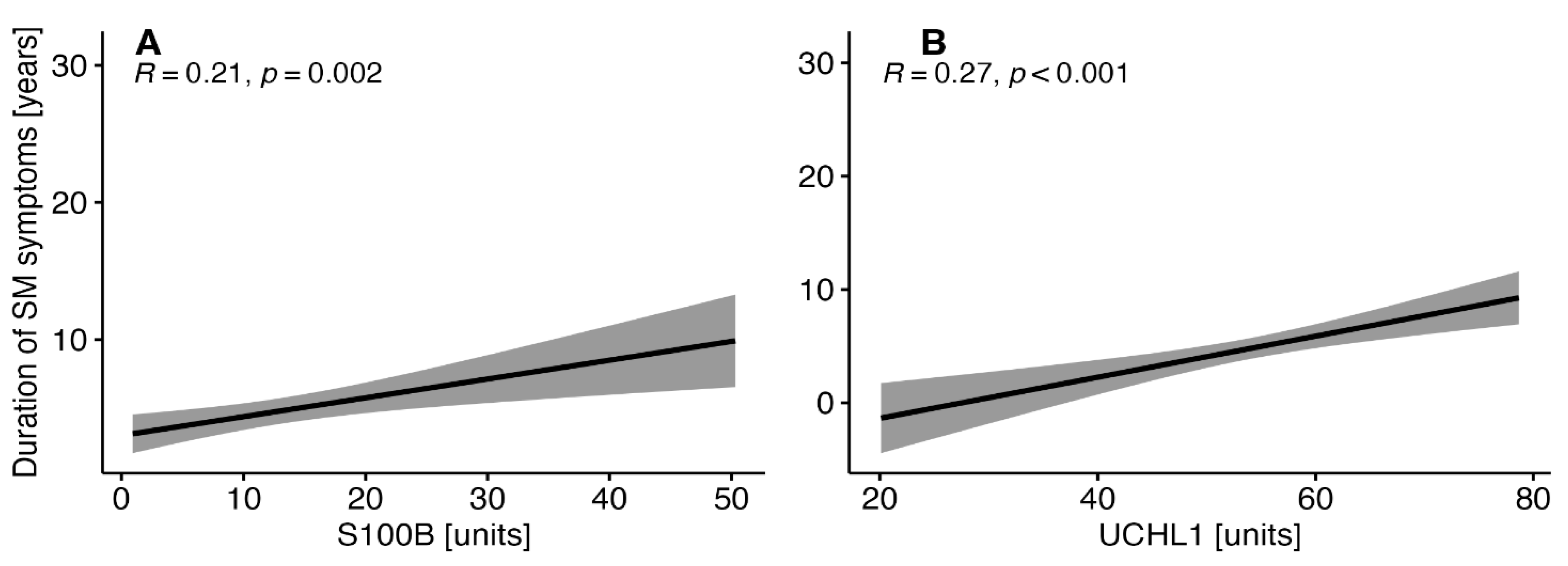
3.3. Correlations between the Duration of MS Symptoms and the Selected Parameters of Brain Injury in MS Patients
4. Discussion
4.1. Limitations
4.2. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murúa, S.R.; Farez, M.F.; Quintana, F.J. The Immune Response in Multiple Sclerosis. Annu. Rev. Pathol. Mech. Dis. 2021, 17, 121–139. [Google Scholar] [CrossRef]
- McGinley, M.P.; Goldschmidt, C.H.; Rae-Grant, A.D. Diagnosis and Treatment of Multiple Sclerosis: A Review. JAMA - J. Am. Med. Assoc. 2021, 325, 765–779. [Google Scholar] [CrossRef]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I.; et al. Rising Prevalence of Multiple Sclerosis Worldwide: Insights from the Atlas of MS, Third Edition. Mult. Scler. 2020, 26, 1816–1821. [Google Scholar] [CrossRef]
- Liu, R.; Du, S.; Zhao, L.; Jain, S.; Sahay, K.; Rizvanov, A.; Lezhnyova, V.; Khaibullin, T.; Martynova, E.; Khaiboullina, S.; et al. Autoreactive Lymphocytes in Multiple Sclerosis: Pathogenesis and Treatment Target. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef]
- Arrambide, G.; Rovira, A.; Sastre-Garriga, J.; Tur, C.; Castilló, J.; Río, J.; Vidal-Jordana, A.; Galán, I.; Rodríguez-Acevedo, B.; Midaglia, L.; et al. Spinal Cord Lesions: A Modest Contributor to Diagnosis in Clinically Isolated Syndromes but a Relevant Prognostic Factor. Mult. Scler. 2018, 24, 301–312. [Google Scholar] [CrossRef]
- Attfield, K.E.; Jensen, L.T.; Kaufmann, M.; Friese, M.A.; Fugger, L. The Immunology of Multiple Sclerosis. Nat. Rev. Immunol. 2022, 22, 734–750. [Google Scholar] [CrossRef]
- Giovannoni, G.; Popescu, V.; Wuerfel, J.; Hellwig, K.; Iacobeus, E.; Jensen, M.B.; García-Domínguez, J.M.; Sousa, L.; De Rossi, N.; Hupperts, R.; et al. Smouldering Multiple Sclerosis: The ‘Real MS. ’ Ther. Adv. Neurol. Disord. 2022, 15. [Google Scholar] [CrossRef]
- Lassmann, H.; van Horssen, J.; Mahad, D. Progressive Multiple Sclerosis: Pathology and Pathogenesis. Nat. Rev. Neurol. 2012, 8, 647–656. [Google Scholar] [CrossRef]
- Mey, G.M.; Mahajan, K.R.; DeSilva, T.M. Neurodegeneration in Multiple Sclerosis. WIREs Mech. Dis. 2023, 15. [Google Scholar] [CrossRef]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sørensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the Clinical Course of Multiple Sclerosis: The 2013 Revisions. Neurology 2014, 83, 278–286. [Google Scholar] [CrossRef]
- Vaughn, C.B.; Jakimovski, D.; Kavak, K.S.; Ramanathan, M.; Benedict, R.H.B.; Zivadinov, R.; Weinstock-Guttman, B. Epidemiology and Treatment of Multiple Sclerosis in Elderly Populations. Nat. Rev. Neurol. 2019, 15, 329–342. [Google Scholar] [CrossRef]
- Kappos, L.; Wolinsky, J.S.; Giovannoni, G.; Arnold, D.L.; Wang, Q.; Bernasconi, C.; Model, F.; Koendgen, H.; Manfrini, M.; Belachew, S.; et al. Contribution of Relapse-Independent Progression vs Relapse-Associated Worsening to Overall Confirmed Disability Accumulation in Typical Relapsing Multiple Sclerosis in a Pooled Analysis of 2 Randomized Clinical Trials. JAMA Neurol. 2020, 77, 1132–1140. [Google Scholar] [CrossRef]
- Cree, B.A.C.; Arnold, D.L.; Chataway, J.; Chitnis, T.; Fox, R.J.; Pozo Ramajo, A.; Murphy, N.; Lassmann, H. Secondary Progressive Multiple Sclerosis: New Insights. Neurology 2021, 97, 378–388. [Google Scholar] [CrossRef]
- Slezáková, D.; Kadlic, P.; Jezberová, M.; Boleková, V.; Valkovič, P.; Minár, M. Brain Volume Loss in Multiple Sclerosis Is Independent of Disease Activity and Might Be Prevented by Early Disease-Modifying Therapy. Neurol. Neurochir. Pol. 2023, 57, 282–288. [Google Scholar] [CrossRef]
- Ruiz, F.; Vigne, S.; Pot, C. Resolution of Inflammation during Multiple Sclerosis. Semin. Immunopathol. 2019, 41, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M. Role of GFAP in CNS Injuries. Neurosci. Lett. 2014, 565, 7–13. [Google Scholar] [CrossRef]
- Momtazmanesh, S.; Shobeiri, P.; Saghazadeh, A.; Teunissen, C.E.; Burman, J.; Szalardy, L.; Klivenyi, P.; Bartos, A.; Fernandes, A.; Rezaei, N. Neuronal and Glial CSF Biomarkers in Multiple Sclerosis: A Systematic Review and Meta-Analysis. Rev. Neurosci. 2021, 32, 573–595. [Google Scholar] [CrossRef]
- Langeh, U.; Singh, S. Targeting S100B Protein as a Surrogate Biomarker and Its Role in Various Neurological Disorders. Curr. Neuropharmacol. 2021, 19, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Górska, E.; Tylicka, M.; Hermanowicz, A.; Matuszczak, E.; Sankiewicz, A.; Gorodkiewicz, E.; Hermanowicz, J.; Karpińska, E.; Socha, K.; Kochanowicz, J.; et al. UCHL1, besides Leptin and Fibronectin, Also Could Be a Sensitive Marker of the Relapsing-Remitting Type of Multiple Sclerosis. 123AD. [CrossRef]
- Kiselev, I.; Bashinskaya, V.; Baulina, N.; Kozin, M.; Popova, E.; Boyko, A.; Favorova, O.; Kulakova, O. Genetic Differences between Primary Progressive and Relapsing-Remitting Multiple Sclerosis: The Impact of Immune-Related Genes Variability. Mult. Scler. Relat. Disord. 2019, 29, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gong, H.; Meng, F. Recent Advances in Btk Inhibitors for the Treatment of Inflammatory and Autoimmune Diseases. Molecules 2021, 26, 4907. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wu, P.F.; Zhang, W.; Liao, X. Circulating Interleukins and Risk of Multiple Sclerosis: A Mendelian Randomization Study. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Lorscheider, J.; Buzzard, K.; Jokubaitis, V.; Spelman, T.; Havrdova, E.; Horakova, D.; Trojano, M.; Izquierdo, G.; Girard, M.; Duquette, P.; et al. Defining Secondary Progressive Multiple Sclerosis. Brain 2016, 139, 2395–2405. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, A.; Cacciaguerra, L.; Pagani, E.; Preziosa, P.; Filippi, M.; Rocca, M.A. Glymphatic System Impairment in Multiple Sclerosis: Relation with Brain Damage and Disability. Brain 2022, 145, 2785–2795. [Google Scholar] [CrossRef] [PubMed]
- Kappos, L.; Wolinsky, J.S.; Giovannoni, G.; Arnold, D.L.; Wang, Q.; Bernasconi, C.; Model, F.; Koendgen, H.; Manfrini, M.; Belachew, S.; et al. Contribution of Relapse-Independent Progression vs Relapse-Associated Worsening to Overall Confirmed Disability Accumulation in Typical Relapsing Multiple Sclerosis in a Pooled Analysis of 2 Randomized Clinical Trials. JAMA Neurol. 2020, 77, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Abdelhak, A.; Foschi, M.; Abu-Rumeileh, S.; Yue, J.K.; D’Anna, L.; Huss, A.; Oeckl, P.; Ludolph, A.C.; Kuhle, J.; Petzold, A.; et al. Blood GFAP as an Emerging Biomarker in Brain and Spinal Cord Disorders. Nat. Rev. Neurol. 2022, 18, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Storoni, M.; Verbeek, M.M.; Illes, Z.; Marignier, R.; Teunissen, C.E.; Grabowska, M.; Confavreux, C.; Plant, G.T.; Petzold, A. Serum GFAP Levels in Optic Neuropathies. J. Neurol. Sci. 2012, 317, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.J.; Liu, N.; Xie, Q.F.; Li, X.; Sun, J.; Wang, H.; Wang, M.X. A Candidate Biomarker of Glial Fibrillary Acidic Protein in CSF and Blood in Differentiating Multiple Sclerosis and Its Subtypes: A Systematic Review and Meta-Analysis. Mult. Scler. Relat. Disord. 2021, 51. [Google Scholar] [CrossRef] [PubMed]
- Kassubek, R.; Gorges, M.; Schocke, M.; Hagenston, V.A.M.; Huss, A.; Ludolph, A.C.; Kassubek, J.; Tumani, H. GFAP in Early Multiple Sclerosis: A Biomarker for Inflammation. Neurosci. Lett. 2017, 657, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Abdelhak, A.; Huss, A.; Kassubek, J.; Tumani, H.; Otto, M. Serum GFAP as a Biomarker for Disease Severity in Multiple Sclerosis. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Adamczyk, B.; Morawiec, N.; Mamak, G.; Boczek, S.; Brzęk, D.; Trędota, N.; Walocha, P.; Czuba, Z.P.; Błachut, M.; Bartman, W.; et al. The Comparison of the Selected Parameters of Brain Injury and Interleukins in the CSF in Patients Diagnosed De Novo with RRMS Compared to the Control Group. Diagnostics (Basel, Switzerland) 2023, 13. [Google Scholar] [CrossRef]
- Högel, H.; Rissanen, E.; Barro, C.; Matilainen, M.; Nylund, M.; Kuhle, J.; Airas, L. Serum Glial Fibrillary Acidic Protein Correlates with Multiple Sclerosis Disease Severity. Mult. Scler. 2020, 26, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.A.M.; Olsson, B.; Bau, L.; Matas, E.; Calvo, Á.C.; Andreasson, U.; Blennow, K.; Romero-Pinel, L.; Martínez-Yélamos, S.; Zetterberg, H. Glial and Neuronal Markers in Cerebrospinal Fluid Predict Progression in Multiple Sclerosis. Mult. Scler. 2015, 21, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Norgren, N.; Sundström, P.; Svenningsson, A.; Rosengren, L.; Stigbrand, T.; Gunnarsson, M. Neurofilament and Glial Fibrillary Acidic Protein in Multiple Sclerosis. Neurology 2004, 63, 1586–1590. [Google Scholar] [CrossRef] [PubMed]
- Ayrignac, X.; Le Bars, emmanuelle; Duflos, C.; Hirtz, christophe; Maleska Maceski, A.; carra-Dallière, clarisse; charif, M.; pinna, frédéric; prin, pauline; Menjot de Champfleur, N.; et al. Serum GfAp in Multiple Sclerosis: Correlation with Disease Type and MRi Markers of Disease Severity. 2020, 10, 10923. [CrossRef]
- Camara-Lemarroy, C.; Silva, C.; Gohill, J.; Yong, V.W.; Koch, M. Serum Neurofilament-Light and Glial Fibrillary Acidic Protein Levels in Hydroxychloroquine-Treated Primary Progressive Multiple Sclerosis. Eur. J. Neurol. 2023, 30, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Cadavid, D.; Cohen, J.A.; Freedman, M.S.; Goldman, M.D.; Hartung, H.P.; Havrdova, E.; Jeffery, D.; Kapoor, R.; Miller, A.; Sellebjerg, F.; et al. The EDSS-Plus, an Improved Endpoint for Disability Progression in Secondary Progressive Multiple Sclerosis. Mult. Scler. 2017, 23, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Bjornevik, K.; Munger, K.L.; Cortese, M.; Barro, C.; Healy, B.C.; Niebuhr, D.W.; Scher, A.I.; Kuhle, J.; Ascherio, A. Serum Neurofilament Light Chain Levels in Patients With Presymptomatic Multiple Sclerosis. JAMA Neurol. 2020, 77, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.I.; Kölliker-Frers, R.A.; Otero-Losada, M.; Perez Lloret, S.; Filippo, M.; Tau, J.; Capani, F.; Villa, A.M. A Pilot Cross-Sectional Study to Investigate the Biomarker Potential of Phosphorylated Neurofilament-H and Immune Mediators of Disability in Patients With 5 Year Relapsing-Remitting Multiple Sclerosis. Front. Neurol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.J.; McGlasson, S.; Hunt, D.; Overell, J. Cerebrospinal Fluid Neurofilament Light Chain in Multiple Sclerosis and Its Subtypes: A Meta-Analysis of Case-Control Studies. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1059–1067. [Google Scholar] [CrossRef]
- Sellebjerg, F.; Börnsen, L.; Ammitzbøll, C.; Nielsen, J.E.; Vinther-Jensen, T.; Hjermind, L.E.; von Essen, M.; Ratzer, R.L.; Soelberg Sørensen, P.; Romme Christensen, J. Defining Active Progressive Multiple Sclerosis. Mult. Scler. 2017, 23, 1727–1735. [Google Scholar] [CrossRef]
- Benkert, P.; Meier, S.; Schaedelin, S.; Manouchehrinia, A.; Yaldizli, Ö.; Maceski, A.; Oechtering, J.; Achtnichts, L.; Conen, D.; Derfuss, T.; et al. Serum Neurofilament Light Chain for Individual Prognostication of Disease Activity in People with Multiple Sclerosis: A Retrospective Modelling and Validation Study. 2022.
- Meier, S.; Willemse, E.A.J.; Schaedelin, S.; Oechtering, J.; Lorscheider, J.; Melie-Garcia, L.; Cagol, A.; Barakovic, M.; Galbusera, R.; Subramaniam, S.; et al. Serum Glial Fibrillary Acidic Protein Compared With Neurofilament Light Chain as a Biomarker for Disease Progression in Multiple Sclerosis. JAMA Neurol. 2023, 80, 287–297. [Google Scholar] [CrossRef]
- Gafson, A.R.; Jiang, X.; Shen, C.; Kapoor, R.; Zetterberg, H.; Fox, R.J.; Belachew, S. Serum Neurofilament Light and Multiple Sclerosis Progression Independent of Acute Inflammation. JAMA Netw. Open 2022, 5. [Google Scholar] [CrossRef]
- Donato, R.; Sorci, G.; Bianchi, R.; Riuzzi, F.; Tubaro, C.; Arcuri, C.; Giambanco, I. S100B Protein, A Damage-Associated Molecular Pattern Protein in the Brain and Heart, and Beyond. Cardiovasc. Psychiatry Neurol. 2010, 2010. [Google Scholar] [CrossRef]
- Riuzzi, F.; Sorci, G.; Donato, R. S100B Protein Regulates Myoblast Proliferation and Differentiation by Activating FGFR1 in a BFGF-Dependent Manner. J. Cell Sci. 2011, 124, 2389–2400. [Google Scholar] [CrossRef] [PubMed]
- Barateiro, A.; Afonso, V.; Santos, G.; Cerqueira, J.J.; Brites, D.; van Horssen, J.; Fernandes, A. S100B as a Potential Biomarker and Therapeutic Target in Multiple Sclerosis. Mol. Neurobiol. 2016, 53, 3976–3991. [Google Scholar] [CrossRef] [PubMed]
- Petzold, A.; Eikelenboom, M.J.; Gveric, D.; Keir, G.; Chapman, M.; Lazeron, R.H.C.; Cuzner, M.L.; Polman, C.H.; Uitdehaag, B.M.J.; Thompson, E.J.; et al. Markers for Different Glial Cell Responses in Multiple Sclerosis: Clinical and Pathological Correlations. Brain 2002, 125, 1462–1473. [Google Scholar] [CrossRef] [PubMed]
- Bartosik-Psujek, H.; Psujek, M.; Jaworski, J.; Stelmasiak, Z. Total Tau and S100b Proteins in Different Types of Multiple Sclerosis and during Immunosuppressive Treatment with Mitoxantrone. Acta Neurol. Scand. 2011, 123, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Mañé-Martínez, M.A.; Olsson, B.; Bau, L.; Matas, E.; Cobo-Calvo, Á.; Andreasson, U.; Blennow, K.; Romero-Pinel, L.; Martínez-Yélamos, S.; Zetterberg, H. Glial and Neuronal Markers in Cerebrospinal Fluid in Different Types of Multiple Sclerosis. J. Neuroimmunol. 2016, 299, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.H.; Gelfand, J.M.; Thebault, S.; Bennett, J.L.; Von Büdingen, H.C.; Cameron, B.; Carruthers, R.; Edwards, K.; Fallis, R.; Gerstein, R.; et al. Emerging Cerebrospinal Fluid Biomarkers of Disease Activity and Progression in Multiple Sclerosis. JAMA Neurol. 2024, 81, 373–383. [Google Scholar] [CrossRef]
- Matuszczak, E.; Tylicka, M.; Komarowska, M.D.; Debek, W.; Hermanowicz, A. Ubiquitin Carboxy-Terminal Hydrolase L1 - Physiology and Pathology. Cell Biochem. Funct. 2020, 38, 533–540. [Google Scholar] [CrossRef]
- Mi, Z.; Graham, S.H. Role of UCHL1 in the Pathogenesis of Neurodegenerative Diseases and Brain Injury. Ageing Res. Rev. 2023, 86. [Google Scholar] [CrossRef]
- Papa, L.; Akinyi, L.; Liu, M.C.; Pineda, J.A.; Tepas, J.J.; Oli, M.W.; Zheng, W.; Robinson, G.; Robicsek, S.A.; Gabrielli, A.; et al. Ubiquitin C-Terminal Hydrolase Is a Novel Biomarker in Humans for Severe Traumatic Brain Injury. Crit. Care Med. 2010, 38, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Brocker, C.; Thompson, D.; Matsumoto, A.; Nebert, D.W.; Vasiliou, V. Evolutionary Divergence and Functions of the Human Interleukin (IL) Gene Family.
- Haase, S.; Linker, R.A. Inflammation in Multiple Sclerosis. Ther. Adv. Neurol. Disord. 2021, 14. [Google Scholar] [CrossRef] [PubMed]
- Papiri, G.; D’Andreamatteo, G.; Cacchiò, G.; Alia, S.; Silvestrini, M.; Paci, C.; Luzzi, S.; Vignini, A. Multiple Sclerosis: Inflammatory and Neuroglial Aspects. Curr. Issues Mol. Biol. 2023, 45, 1443–1470. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Kiczmer, M.; Niedziela, N.; Czuba, Z.P.; Sowa, P.; Wierzbicki, K.; Lubczyński, M.; Adamczyk-Sowa, M. A Comparison of Serum Inflammatory Parameters in Progressive Forms of Multiple Sclerosis. Mult. Scler. Relat. Disord. 2023, 79. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, H.; Won, S.J.; Basu, J.; Kapfhamer, D.; Swanson, R.A. Microglial Activation Induced by the Alarmin S100B Is Regulated by Poly(ADP-Ribose) Polymerase-1. Glia 2016, 64, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Matsushige, T.; Ichiyama, T.; Anlar, B.; Tohyama, J.; Nomura, K.; Yamashita, Y.; Furukawa, S. CSF Neurofilament and Soluble TNF Receptor 1 Levels in Subacute Sclerosing Panencephalitis. J. Neuroimmunol. 2008, 205, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Mindur, J.E.; Valenzuela, R.M.; Yadav, S.K.; Boppana, S.; Dhib-Jalbut, S.; Ito, K. IL-27: A Potential Biomarker for Responders to Glatiramer Acetate Therapy. J. Neuroimmunol. 2017, 304, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Zhang, C.X.; Li, R.F.; Man, Q.W. IL-8 Is Upregulated in the Tissue-Derived EVs of Odontogenic Keratocysts. Biomed Res. Int. 2022, 2022. [Google Scholar] [CrossRef]
- Daoud, H.; Alharfi, I.; Alhelali, I.; Charyk Stewart, T.; Qasem, H.; Fraser, D.D. Brain Injury Biomarkers as Outcome Predictors in Pediatric Severe Traumatic Brain Injury. Neurocrit. Care 2014, 20, 427–435. [Google Scholar] [CrossRef]
- Zhang, X.; Putoczki, T.; Markovic-Plese, S. IL-11 in Multiple Sclerosis. Oncotarget 6.
- Autieri, M. V. IL-19 and Other IL-20 Family Member Cytokines in Vascular Inflammatory Diseases. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Gilliet, M.; Modlin, R.L. Immunobiology of IL-26. J. Invest. Dermatol. 2024, 144, 1217–1222. [Google Scholar] [CrossRef] [PubMed]

| Parameter | RRMS (N=123) |
PMS (N=88) |
p-value |
| Age (years) | 36 (29 - 41) | 52 (47 - 54) | <0.001 |
| Sex (females) | 87 / 123 (71%) | 48 / 88 (55%) | 0.016 |
| MS relapse | 30 / 123 (24%) | 0 / 88 (0%) | <0.001 |
| MS duration (years) | 0 (0 - 1) | 1 (0 - 12) | <0.001 |
| MS symptoms duration (years) | 1 (0 - 3) | 5 (1 - 13) | <0.001 |
| EDSS (score) | 2.50 (2.00 - 3.00) | 4.50 (4.00 - 5.00) | <0.001 |
| DMT | 18 / 123 (15%) | 8 / 88 (9.1%) | 0.227 |
| Gd+ lesions (brain MRI) |
48 / 123 (39%) | 8 / 88 (9.1%) | <0.001 |
| Gd+ lesions (cervical and thoracic MRI) |
12 / 123 (9.8%) | 8 / 88 (9.1%) | 0.871 |
| Elevated IgG in CSF | 75 / 105 (71%) | 64 / 88 (73%) | 0.841 |
| OCBs | 78 / 117 (67%) | 48 / 88 (55%) | 0.078 |
| Parameter | RRMS (N=123) |
PMS (N=88) |
p-value |
| GFAP (pg/ml) | 1,878 (1,260 - 3,055) | 3,626 (1,755 - 8,495) | <0.001 |
| NF-H (pg/ml) | 4 (3 - 4) | 4 (3 - 25) | 0.070 |
| S100B (pg/ml) | 10 (3 - 16) | 13 (10 - 17) | 0.018 |
| UCHL1 (pg/ml) | 54 (48 - 58) | 59 (51 - 62) | <0.001 |
| IL-8 (pg/ml) | 47 (42 - 59) | 49 (40 - 59) | 0.236 |
| IL-10 (pg/ml) | 12.9 (9.8 - 14.7) | 12.3 (9.5 - 16.8) | 0.238 |
| IL-11 (pg/ml) | 3.51 (2.74 - 4.77) | 4.52 (2.87 - 7.02) | 0.005 |
| IL12p70 (pg/ml) | 1.40 (0.80 - 1.96) | 1.40 (0.80 - 1.40) | 0.172 |
| IL-19 (pg/ml) | 33 (21 - 44) | 33 (15 - 44) | 0.868 |
| IL-20 (pg/ml) | 11.58 (8.46 - 11.58) | 11.58 (10.03 - 13.13) | <0.001 |
| IL-22 (pg/ml) | 27 (22 - 34) | 26 (21 - 34) | 0.440 |
| IL-26 (pg/ml) | 348 (150 - 528) | 197 (95 - 582) | 0.225 |
| IL-27p28 (pg/ml) | 69 (45 - 87) | 50 (10 - 64) | <0.001 |
| Parameter (pg/ml) |
RRMS | PMS | ||||||||||
| IL-10 | IL-27p28 | IL-8 | IL-11 | IL-20 | IL-26 | IL-10 | IL-27p28 | IL-8 | IL-11 | IL-20 | IL-26 | |
| GFAP | - | R= - 0.31 p <0.001 |
- | R=0.82 p <0.001 |
R=0.4 p <0.001 |
R=0.33 p<0.001 |
- | - | R= 0.32 p= 0.002 |
R= 0.65 P < 0.001 |
- | R= 0.25 p= 0.0017 |
| NF-H | R= - 0.2 p= 0.024 |
R= -0.2 p= 0.024 |
R= 0.34 p, 0.001 |
R= 0.18 p= 0.047 |
- | R= -0.22, p= 0.014 | R= - 0.62 p< 0.001 |
- | R=0.42 p<0.001 |
- | - | - |
| S100B | - | - | R= 0.18 p= 0.042 |
R= 0.34 p<0.001 |
- | - | R= - 0.55 p< 0.001 |
- | - | - | - | - |
| UCHL1 | - | - | - | - | - | - | R= - 0.59 p < 0.001 |
- | - | - | R=0.34 p= 0.001 |
- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





