1. Introduction
The main objective of anesthesia is to guarantee patient safety and comfort while improving surgical conditions. When general anesthesia is used for this purpose, it is crucial to have three critical components converge: analgesia, anesthesia, and, if necessary, neuromuscular blockade. This convergence is necessary to achieve the overall goal [
1,
2]
As a result, diverse methodologies and systems have been developed for the real-time and continuous monitoring of these components. Nevertheless, existing techniques and systems are currently optimized solely for monitoring depth of anesthesia (DOA) and neuromuscular blockade, as outlined in current guidelines. These include the Bispectral Index (BIS) for assessing DOA and neuromuscular monitoring (NM) for monitoring muscle function [
1,
2].
However, adequate direct monitoring of the third crucial factor of anesthesia has not been reliably established yet: the analgesia or the nociception. For a long time, direct and validated monitoring of perioperative nociception was unavailable, and the anesthetist had to rely on indirect indicators such as blood pressure, heart rate, and sweating to estimate sufficient analgesia [
3].
However, these surrogate parameters are not independent and can be susceptible to influences from various other factors, such as blood loss, volume shifts, environmental temperature or individual medications [
4].
These associations can carry significant ramifications. Inadequate pain management may result in various complications, including elevated stress responses and postoperative pain due to suboptimal dosage. On the other hand, excessive medication can result in more hemodynamic events and postoperative nausea and vomiting [
5].
In recent years, several monitoring systems have been developed to assess various parameters of nociception [
6,
7]. These techniques allow for the optimization of perioperative analgesic therapy and help to avoid overmedication.
The PMD-200-Nociception Monitor (NOL
®; Medasense, Ramat Gan, Israel) is an approach that non-invasively analyzes four different parameters (photoplethysmography, galvanic skin response, peripheral temperature, accelerometery) related to the activation of the sympathetic nervous system. These parameters are then consolidated into an index known as the NOL-Index
®, ranging from 0 to 100, with a target value of 10 to 25 [
4,
7].
Multiple studies have illustrated the efficacy of this system in detecting perioperative nociception [
8,
9], which can significantly influence analgesic therapy, regardless of patient factors such as sex, age, or BMI [
8]. Furthermore, NOL
® monitoring has been demonstrated to impact postoperative opioid requirements and early postoperative pain, even in correlation of regional anesthesia [
10].
Compared to traditional parameters such as heart rate and blood pressure, this method proves superior during general anesthesia. It can lead to reduced analgesia administration, prevents profound hemodynamic instability and reduces postoperative pain [
7,
11,
12].
Although the efficacy of this approach during general anesthesia has been validated through numerous studies, its impact on epidural anesthesia remains inadequately investigated [
12].
Epidural anesthesia is an established approach for supplementing general anesthesia and is associated with numerous benefits, such as reduced systemic opioid administration, diminished postoperative analgesic needs, and decreased occurrences of postoperative nausea and vomiting [
13,
14]. Nevertheless, there is no uniform implementation for the use of perioperative epidural anesthesia, whether for the choice of drugs used or even the optimal mode of administration (continuous vs. single dose) [
15].
Therefore, this study aimed to conduct a retrospective analysis to assess the impact of perioperative epidural administration of local anesthetics during abdominal surgery on the NOL-Index®, while comparing it with established clinical parameters such as heart rate, blood pressure, and bispectral index. The effects were examined within the initial five minutes following epidural administration.
The investigation encompassed several additional facets. Specifically, it analyzed the ideal range of the NOL-Index®, delineated as falling between 10 and 25, and the repercussions of administering epidurals within or below this threshold. Additionally, the study scrutinized the impacts of varying doses of epidural agents.
2. Materials and Methods
2.1. Ethical Considerations
For this investigation, a retrospective analysis was conducted on 40 patients who underwent combined general anesthesia and epidural anesthesia (thoracic levels between Th6 – 9) for abdominal surgery within the period of July 2022 to July 2023 in the University center of the Johannes-Gutenberg University in Mainz, Germany. The study was registered under the identifier DRKS00029120 on July 1, 2022, with ethics committee approval (No. 2021-16201) obtained from the regional ethics committee of Rhineland Palatine, Mainz, chaired by Dr. Stephan Letzel. Informed consent was not deemed necessary.
2.2. Study Design
All patients received standardized hemodynamic monitoring (invasive blood pressure measurements, heart rate, oxygen saturation) and sedation monitoring utilizing the bispectral index monitoring system (BIS Monitoring System; Medtronic, Minneapolis, USA). Nociception and stress levels were assessed using a dedicated monitoring system (NOL®; PMD-200; Medasense Biometrics Ltd., Ramat Gan, Israel). There was no difference between the patients in terms of the type of steering of the general anesthetic. The primary objective of the study was to assess and compare the impact of epidurally administered local anesthetics (bupivacaine 7.5mg - 25mg) on the NOL-Index®, heart rate (HR), mean arterial blood pressure (MAP), and bispectral index (BIS) at intervals of 0, 1, 3, and 5 minutes.
2.3. Data Splitting
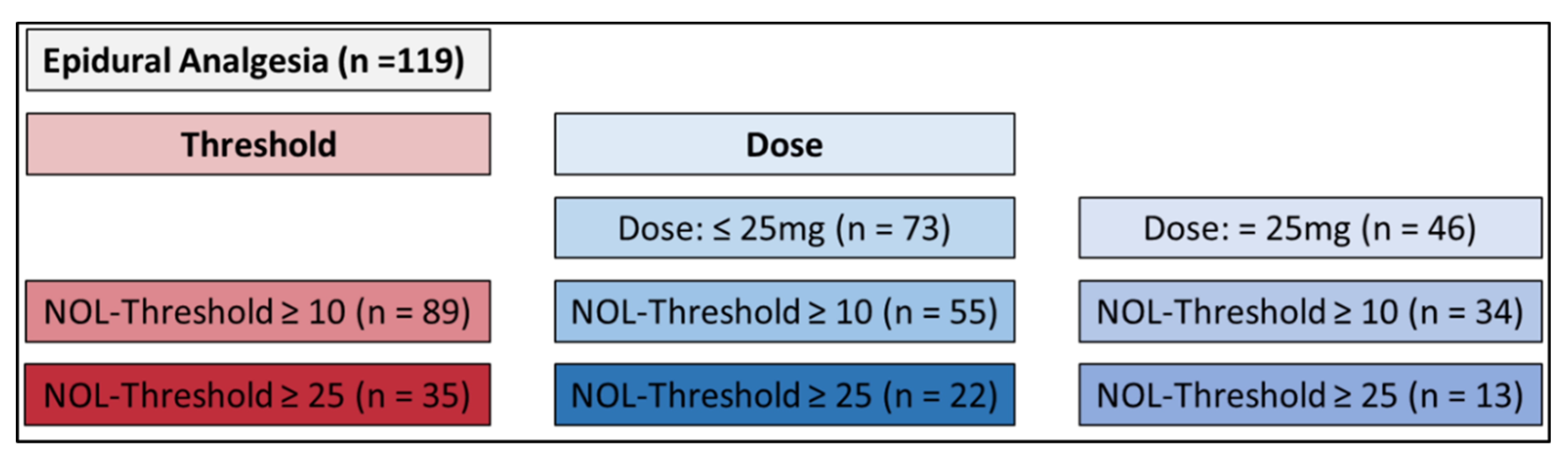
For the purpose of this evaluation, the data set was first split according to a specific protocol.
In the initial phase, all recorded instances of epidural bupivacaine administrations (n=119) were reviewed. Following this, the investigation honed in on instances where the NOL-Level
® at the time of application exceeded a minimum threshold of 10 (n=89), and subsequently, a threshold of at least 25 (n=35). During the third phase, the total number of administered epidural doses was categorized into two groups: a lower 25mg bolus group (n=73) and a 25mg bolus group (n=46). Within each group, subgroups were formed with an NOL-Index
® of at least 10 and at least 25 (lower 25mg: NOL >10 n=55, NOL >25 n=22; 25mg: NOL >10 n=34, NOL >25 n=13) (
Figure 1).
The secondary objective involved comparing the temporal progression of the NOL-Index
® among the various doses of epidural bupivacaine (
Figure 1).
2.4. Statistical Analyses
Statistical analyses were performed using GraphPad Prism 9 software (GraphPad Software, Boston, MA, USA). Given the pilot nature of the analysis, no pre-data collection or statistical planning occurred, and the presented analyses are primarily descriptive.
The repeated measures underwent one-way ANOVA analysis, with time-point comparisons being performed utilizing the Holm-Sidak test. P-values less than 0.05 were considered statistically significant. For better comparability of the different NOL-Index® values in the figures, these are given as percentages. All figures depicted boxplots featuring the median and quartiles. The plot illustrates the interquartile range and demonstrates the percentage relative to the baseline. In order to compare the various doses, a One-Sample t-test and Wilcoxon test were conducted.
3. Results
3.1. Included Measurements
This retrospective data analysis comprised a cohort of 40 patients, encompassing 119 instances of local anesthetic administration during abdominal surgery. The measurement was divided up as described above.
3.1. Comparative Analysis
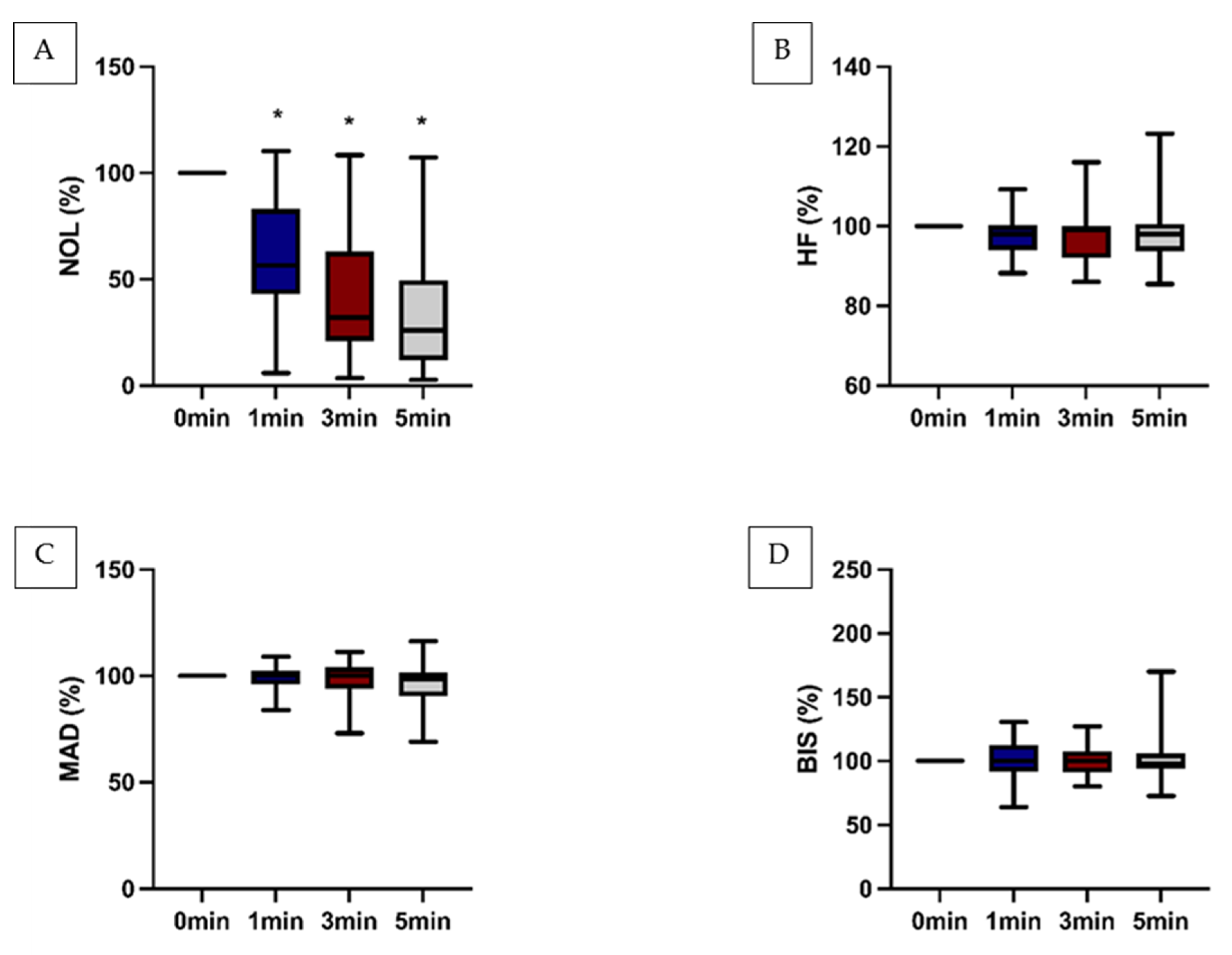
It was noted that the NOL-Index
® exhibited a significant decrease within the assessed timeframe following the administration the epidural analgesics. Conversely, conventional clinical parameters displayed no discernible changes and failed to mirror the observed effect. Notably, this effect was particularly pronounced when the NOL-Index
® surpassed the threshold of 25 (
Figure 2).
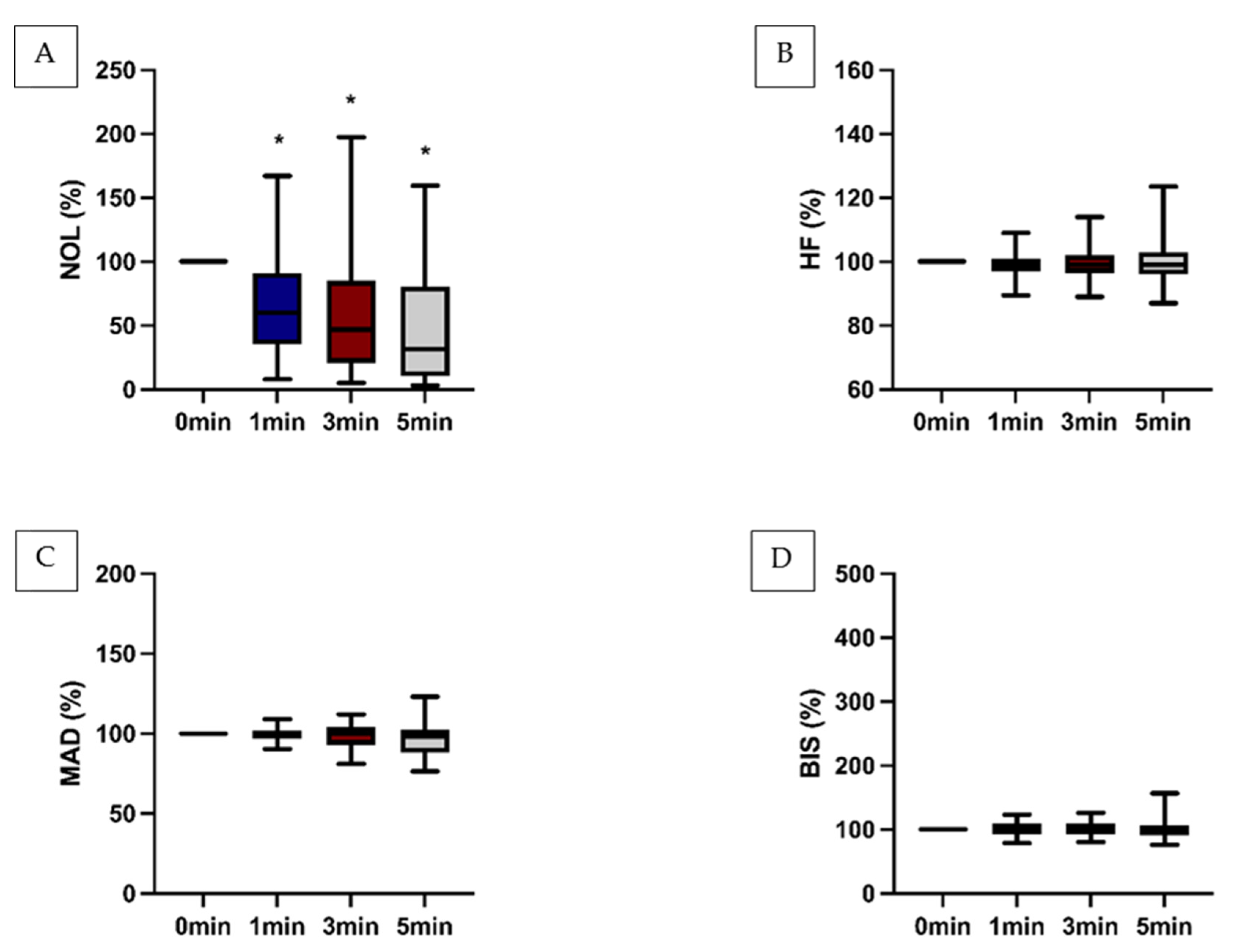
However, the impact of antinociceptive therapy was also evident when this parameter exceeded 10 (
Figure 3) or when the NOL-Index
® was less than 10 (
Table 1).
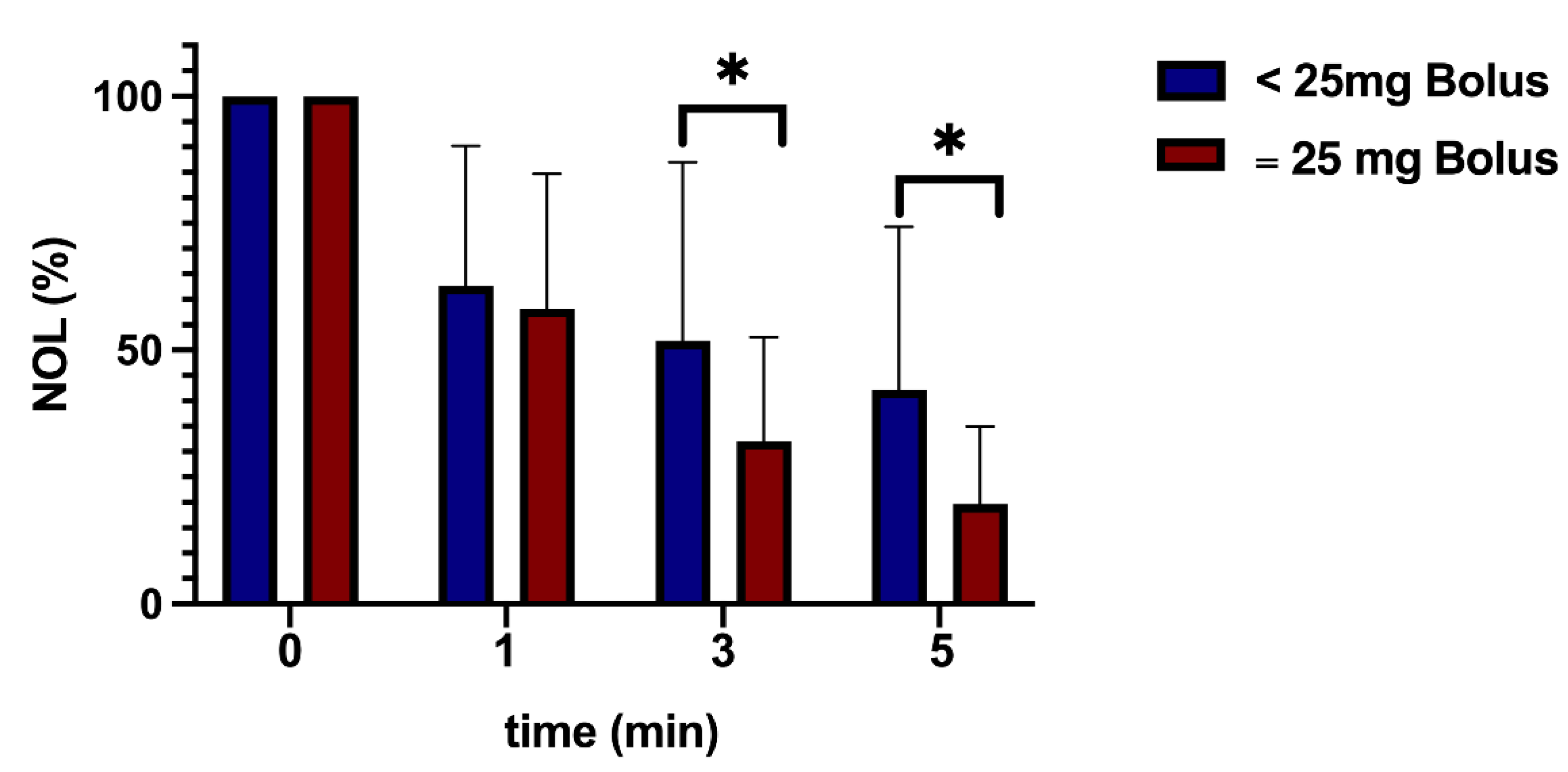
3.2. Dose Analysis
The analysis of varying epidural doses demonstrated a marked decrease in nociception signals within the high-dose group (administered with 25mg Bupivacaine). This effect was particularly notable in the subgroup adhering to a threshold of 25, exhibiting statistical significance (
Figure 4). During the initial five minutes following epidural administration, no significant hemodynamic response was observed in any group (
Table 1).
4. Discussion
This retrospective study presents novel findings indicating the Nociception Level (NOL
®) Index as a highly efficacious method for illustrating the antinociceptive impact of epidural local anesthetics. The NOL-Index
® exhibited in this context a notably superior ability to demonstrate this effect compared to conventional parameters such as heart rate, mean arterial blood pressure, or bispectral index (BIS
®), which did not exhibit significant effects. The significant decrease was especially pronounced if the anesthetics were administered when the NOL-Index
® was over the value of 25 [
16]. This is congruent with the recommended range between 10 and 25 where values above 25 are associated with high stress and nociception [
17]. In previous studies, the NOL-Index
® has consistently shown superior performance compared to HR and MAP in distinguishing between noxious and non-noxious stimuli, as evidenced in various events such as intubation or skin incision [
18,
19]. For patients undergoing Video-Assisted Thoracoscopic Surgery (VATS), comparable results have been documented, with the NOL
® demonstrating greater efficacy in evaluating different events [
20]. This study is particularly noteworthy as it showcases the effectiveness of the NOL
® system when used in conjunction with epidural anesthesia, suggesting a potentially valuable combination [
20].
The use of the NOL-Index
® in this context could provide clarity and potentially improve patient safety by facilitating the development of an optimized approach. In daily clinical practice, there is considerable heterogeneity in the management of perioperative epidural analgesia, with no standardized guidelines or instructions for optimal use. Diverse perspectives exist regarding the selection of initial drug, dosage, and co-analgesics, with no standardized guidelines or recommendations available. The literature remains inconclusive regarding optimal practices in this regard [
21].
Numerous studies have shown the potential of the NOL-Index
® for this purpose. Investigation of this context was notably focused within the domain of general anesthesia. For instance, reducing NOL
® usage has led to decreased perioperative opioid consumption and shortened extubation times [
22]. The opioid-sparing effect can be characterized by a reduction of 22% [
23]. Although the opioid-sparing effect was measurable in most instances, there were studies where it was not observed. Conversely, in these trials, NOL
® demonstrated a positive effect on postoperative pain outcomes [
5].
These findings suggest the potential applicability of NOL® for guiding analgesic therapy and its potential transferability to the context of epidural anesthesia.
The secondary analysis of this study elucidates the impact of varying epidural doses of Bupivacaine on NOL
® and other parameters included in the study. In this regard, the NOL-Index
® exhibited a significantly faster decrease in the high-dose group. Comparable effects were observed following the assessment of standardized tetanic stimuli with varying doses of remifentanil. The NOL-Index
® proved effective in discerning this effect [
24]. This particular feature of the NOL-Index
® can have a significant impact on the efficacy of analgesic therapy, as it can help prevent both underdosing and overdosing [
5].
This study possesses several limitations, with the primary concern being its retrospective nature. Additionally, it lacks involvement in a sizable prospective randomized trial featuring distinct groups, where the integration of the Nociception Level (NOL®) index is incorporated as an intervention within a designated group. Hence, the study was not specifically designed to yield these outcomes with robust statistical power. This is especially crucial for analyzing the epidural dosage, given the limitation of the relatively small number of analyzed applications. However, the noteworthy level of significance observed underscores the informative quality of the results.
An added constraint of this study design is the evaluation of the Nociception Level (NOL
®) index only within the subsequent five minutes following analgesic administration. On one hand, the duration may not be optimal due to the 20-minute onset time of bupivacaine. On the other hand, intraoperative events have the potential to confound the analysis within this observational window, necessitating a shorter analysis time [
25]. This accounts for the substantial error bars depicted in the graphs and could be mitigated by waiting for a more precisely defined timeframe.
Another limitation of this study is the fact that nociceptive stimuli, upon cerebral processing, lead to a sympathetic stress reaction. This sympathetic response is notably attenuated by an epidural catheter, which directly influences the NOL-Index
®. This effect is also discernible in established clinical parameters such as heart rate and arterial pressure [
26]. For optimized evaluation, a comparison with more precise methods, such as direct cerebral assessment of nociceptive stimuli or the direct hormone activity of stress, would be necessary [
27].
5. Conclusions
In conclusion, this retrospective study represents the first demonstration of the effectiveness of the NOL-Index® in assessing nociceptive effects following epidural administrations within the first few minutes, showing superiority over conventional clinical parameters. Furthermore, the study illustrated the sensitivity of the NOL-Index® in distinguishing between different dose effects.
Supplementary Materials: The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization: A.Z., R.R. and EV.G.; Methodology: DJ.R..; formal analysis, DJ.R., S.W., K.M. and J.H.; Investigation: DJ.R. S.W. and K.M.; Data curation: S.W. and K.M.; Writing—original draft preparation: A.Z.; Writing—review and editing, J.H. J.K., M.S. and R.R.; Visualization: A.Z.; Supervision: A.Z.; R.R. and EV.G.; Project administration: A.Z. and R.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
The study was registered under the identifier DRKS00029120 on July 1, 2022, with ethics committee approval (No. 2021-16201) obtained from the regional ethics committee of Rhineland Palatine, Mainz, Germany, chaired by Dr. Stephan Letzel. Informed consent was not deemed necessary.
Data Availability Statement
The original contributions presented in the study are included in the article further inquiries (raw data) can be directed to the corresponding authors.
Acknowledgments
The study is part of the doctoral thesis of Katharina Mackert and Sophia Woldt. During the preparation of this work the authors used GPT-3.5/OpenAI in order to improve language and readability. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Conflicts of Interest
Alexander Ziebart and Eva-Verena Griemert gave paid lectures for Medtronic. All other authors have no conflicts of interest to declare The study was fundet by the Department of Anaesthesiology, University Medical Centre, Johannes Gutenberg-University Mainz, Germany. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Fuchs-Buder, T.; Romero, C.S.; Lewald, H.; Lamperti, M.; Afshari, A.; Hristovska, A.M.; Schmartz, D.; Hinkelbein, J.; Longrois, D.; Popp, M.; et al. Peri-operative management of neuromuscular blockade: A guideline from the European Society of Anaesthesiology and Intensive Care. Eur J Anaesthesiol 2023, 40, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.E.; Taraday, J.K.; Kharasch, E.D. Bispectral index monitoring during sedation with sevoflurane, midazolam, and propofol. Anesthesiology 2001, 95, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Edry, R.; Recea, V.; Dikust, Y.; Sessler, D.I. Preliminary Intraoperative Validation of the Nociception Level Index: A Noninvasive Nociception Monitor. Anesthesiology 2016, 125, 193–203. [Google Scholar] [CrossRef]
- Stöckle, P.A.; Julien, M.; Issa, R.; Décary, E.; Brulotte, V.; Drolet, P.; Henri, M.; Poirier, M.; Latulippe, J.F.; Dorais, M.; et al. Validation of the PMD100 and its NOL Index to detect nociception at different infusion regimen of remifentanil in patients under general anesthesia. Minerva Anestesiol. 2018, 84, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Meijer, F.; Honing, M.; Roor, T.; Toet, S.; Calis, P.; Olofsen, E.; Martini, C.; van Velzen, M.; Aarts, L.; Niesters, M.; et al. Reduced postoperative pain using Nociception Level-guided fentanyl dosing during sevoflurane anaesthesia: a randomised controlled trial. British journal of anaesthesia 2020, 125, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Shahiri, T.S.; Richebé, P.; Richard-Lalonde, M.; Gélinas, C. Description of the validity of the Analgesia Nociception Index (ANI) and Nociception Level Index (NOL) for nociception assessment in anesthetized patients undergoing surgery: a systematized review. Journal of clinical monitoring and computing 2022, 36, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Nitzschke, R.; Fischer, M.; Funcke, S. [Nociception monitoring : Method for intraoperative opioid control?]. Anaesthesist 2021, 70, 735–752. [Google Scholar] [CrossRef] [PubMed]
- Ruetzler, K.; Montalvo, M.; Rotem, O.M.; Ekrami, E.; Rössler, J.; Duran, J.A.A.; Dahan, A.; Gozal, Y.; Richebe, P.; Farhang, B.; et al. Generalizability of nociception level as a measure of intraoperative nociceptive stimulation: A retrospective analysis. Acta Anaesthesiol Scand 2023, 67, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Ruemmler, R.; Moravenova, V.; Al-Butmeh, S.; Fukui-Dunkel, K.; Griemert, E.V.; Ziebart, A. A novel non-invasive nociceptive monitoring approach fit for intracerebral surgery: a retrospective analysis. PeerJ 2024, 12, e16787. [Google Scholar] [CrossRef] [PubMed]
- Neumann, C.; Gehlen, L.; Weinhold, L.; Straßberger-Nerschbach, N.; Soehle, M.; Kornilov, E.; Thudium, M. Influence of Intraoperative Nociception during Hip or Knee Arthroplasty with Supplementary Regional Anaesthesia on Postoperative Pain and Opioid Consumption. Medicina (Kaunas) 2023, 59. [Google Scholar] [CrossRef]
- Ruetzler, K.; Montalvo, M.; Rotem, O.M.; Ekrami, E.; Rössler, J.; Duran, J.A.A.; Dahan, A.; Gozal, Y.; Richebe, P.; Farhang, B.; et al. Generalizability of nociception level as a measure of intraoperative nociceptive stimulation: A retrospective analysis. Acta anaesthesiologica Scandinavica 2023. [Google Scholar] [CrossRef] [PubMed]
- Fuica, R.; Krochek, C.; Weissbrod, R.; Greenman, D.; Freundlich, A.; Gozal, Y. Reduced postoperative pain in patients receiving nociception monitor guided analgesia during elective major abdominal surgery: a randomized, controlled trial. Journal of clinical monitoring and computing 2023, 37, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Shui, M.; Zhao, D.; Xue, Z.; Wu, A. Impact of Spinal/Epidural Anesthesia Versus General Anesthesia on Perioperative Outcomes in Patients Undergoing Lumbar Spine Surgery: An Updated Systematic Review and Meta-analysis. Clin Spine Surg 2023, 36, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Behem, C.R.; Wegner, J.C.; Pinnschmidt, H.O.; Greiwe, G.; Graessler, M.F.; Funcke, S.; Nitzschke, R.; Trepte, C.J.C.; Haas, S.A. Effect of thoracic epidural anesthesia on postoperative outcome in major liver surgery: a retrospective cohort study. Langenbecks Arch. Surg. 2023, 408, 168. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, T.; Weibel, S.; Steinfeldt, T.; Sitter, M.; Meybohm, P.; Kranke, P. Intraoperative management of combined general anesthesia and thoracic epidural analgesia: A survey among German anesthetists. Acta Anaesthesiol Scand 2021, 65, 1490–1496. [Google Scholar] [CrossRef] [PubMed]
- Ben-Israel, N.; Kliger, M.; Zuckerman, G.; Katz, Y.; Edry, R. Monitoring the nociception level: a multi-parameter approach. J Clin Monit Comput 2013, 27, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Ghiyasinasab, M.; Morisson, L.; Laferrière-Langlois, P.; Geraldo-Demers, M.A.; Gélinas, C.; Nadeau-Vallée, M.; Verdonck, O.; Lahrichi, N.; Richebé, P. Identification of the intraoperative antinociceptive effect of intravenous fentanyl using the Nociception Level (NOL) index versus clinical parameters in patients undergoing gynecological laparoscopic surgery: A secondary analysis of the NOLGYN study. Anaesth Crit Care Pain Med 2022, 41, 101102. [Google Scholar] [CrossRef] [PubMed]
- Martini, C.H.; Boon, M.; Broens, S.J.; Hekkelman, E.F.; Oudhoff, L.A.; Buddeke, A.W.; Dahan, A. Ability of the nociception level, a multiparameter composite of autonomic signals, to detect noxious stimuli during propofol-remifentanil anesthesia. Anesthesiology 2015, 123, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Sbeghen, V.; Verdonck, O.; McDevitt, J.; Zaphiratos, V.; Brulotte, V.; Loubert, C.; Tanoubi, I.; Drolet, P.; Belanger, M.E.; Fortier, L.P.; et al. A randomized controlled trial comparing nociception level (NOL) index, blood pressure, and heart rate responses to direct laryngoscopy versus videolaryngoscopy for intubation: the NOLint project. Can J Anaesth 2021, 68, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Bollag, L.; Jelacic, S.; Delgado Upegui, C.; Wu, C.; Richebe, P. The nociception level index (NOL) response to intubation and incision in patients undergoing video-assisted thoracoscopic surgery (VATS) with and without thoracic epidural analgesia. A pilot study. F1000Res 2018, 7, 875. [Google Scholar] [CrossRef]
- Gulamani, A.; Rehman, A.; Nazir, M.; Shabbir, Z. Intraoperative Epidural Analgesia Practices And Their Outcomes In Major Abdominal Surgeries At A Tertiary Care Hospital In Karachi. J Pak Med Assoc 2023, 73, 1587–1591. [Google Scholar] [CrossRef] [PubMed]
- Jiao, B.; Chen, M.; Wang, W.; Chen, C. The opioid-sparing effect of nociception level (NOL) index monitoring for adult patients undergoing surgery: A systematic review and meta-analysis. Asian J Surg 2023, 46, 1731–1732. [Google Scholar] [CrossRef] [PubMed]
- Espitalier, F.; Idrissi, M.; Fortier, A.; Bélanger, M.; Carrara, L.; Dakhlallah, S.; Rivard, C.; Brulotte, V.; Zaphiratos, V.; Loubert, C.; et al. “Impact of Nociception Level (NOL) index intraoperative guidance of fentanyl administration on opioid consumption, postoperative pain scores and recovery in patients undergoing gynecological laparoscopic surgery. A randomized controlled trial”. J Clin Anesth 2021, 75, 110497. [Google Scholar] [CrossRef] [PubMed]
- Renaud-Roy, E.; Stöckle, P.A.; Maximos, S.; Brulotte, V.; Sideris, L.; Dubé, P.; Drolet, P.; Tanoubi, I.; Issa, R.; Verdonck, O.; et al. Correlation between incremental remifentanil doses and the Nociception Level (NOL) index response after intraoperative noxious stimuli. Can J Anaesth 2019, 66, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- Urbanek, B.; Duma, A.; Kimberger, O.; Huber, G.; Marhofer, P.; Zimpfer, M.; Kapral, S. Onset time, quality of blockade, and duration of three-in-one blocks with levobupivacaine and bupivacaine. Anesth Analg 2003, 97, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Wattwil, M.; Sundberg, A.; Arvill, A.; Lennquist, C. Circulatory changes during high thoracic epidural anaesthesia--influence of sympathetic block and of systemic effect of the local anaesthetic. Acta Anaesthesiol Scand 1985, 29, 849–855. [Google Scholar] [CrossRef]
- Feng, Y.; Chang, P.; Liu, J.; Zhang, W.S. Effects and mechanisms of perioperative medications on the hypothalamic pituitary adrenal response to surgical injury: A narrative review. J Clin Anesth 2024, 94, 111367. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).