Submitted:
25 July 2024
Posted:
25 July 2024
You are already at the latest version
Abstract
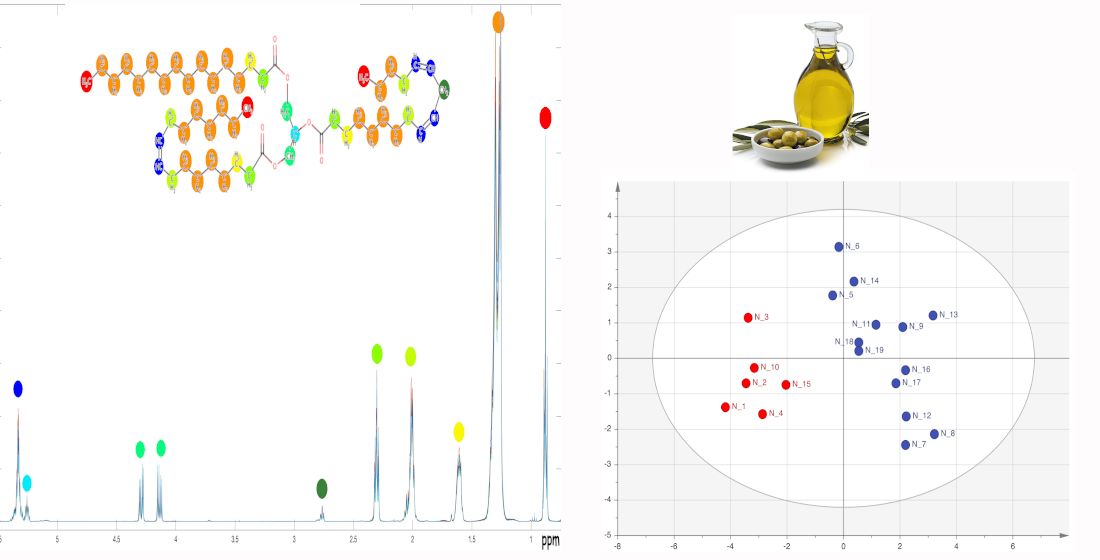
Keywords:
1. Introduction
2. Results
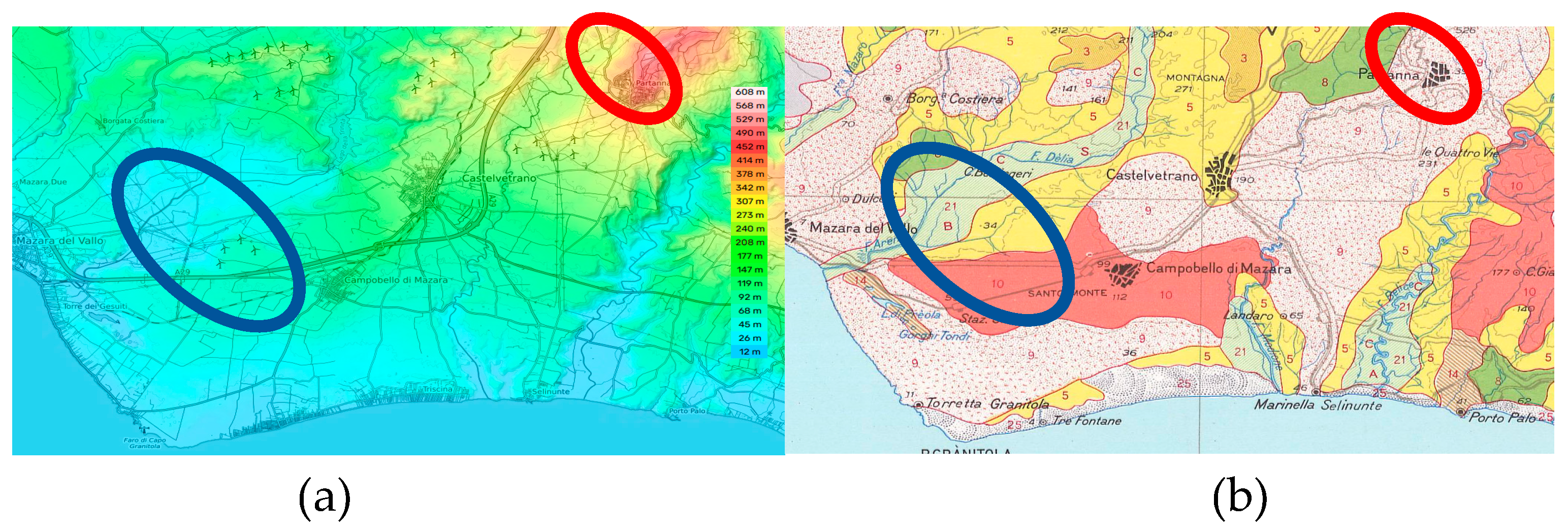
2.1. Sampling Areas
2.2. NMR Metabolic Profile
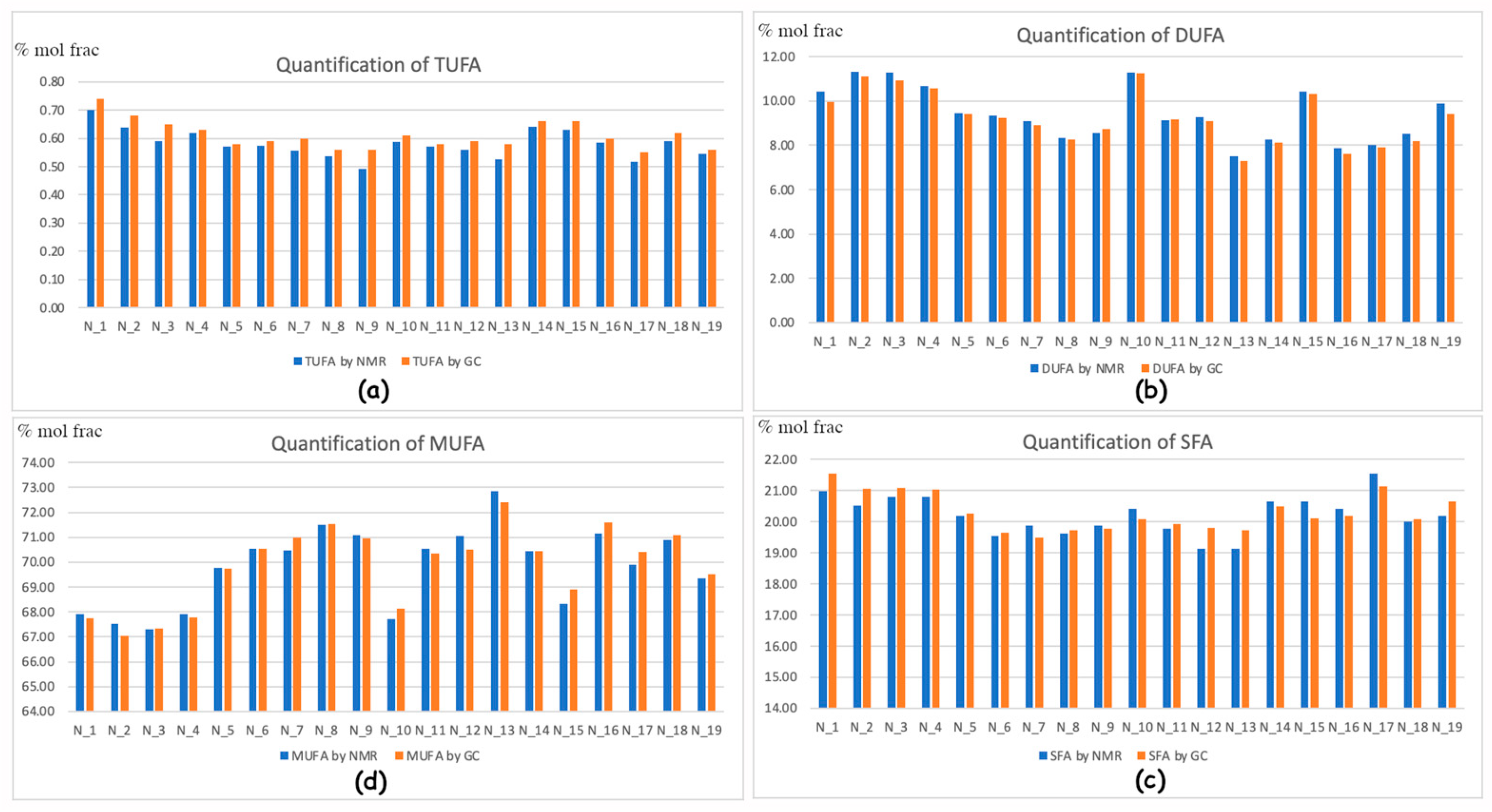
2.3. GC vs NMR Comparison
2.4. Total Phenolic Compounds: NMR vs Folin-Ciocolteau
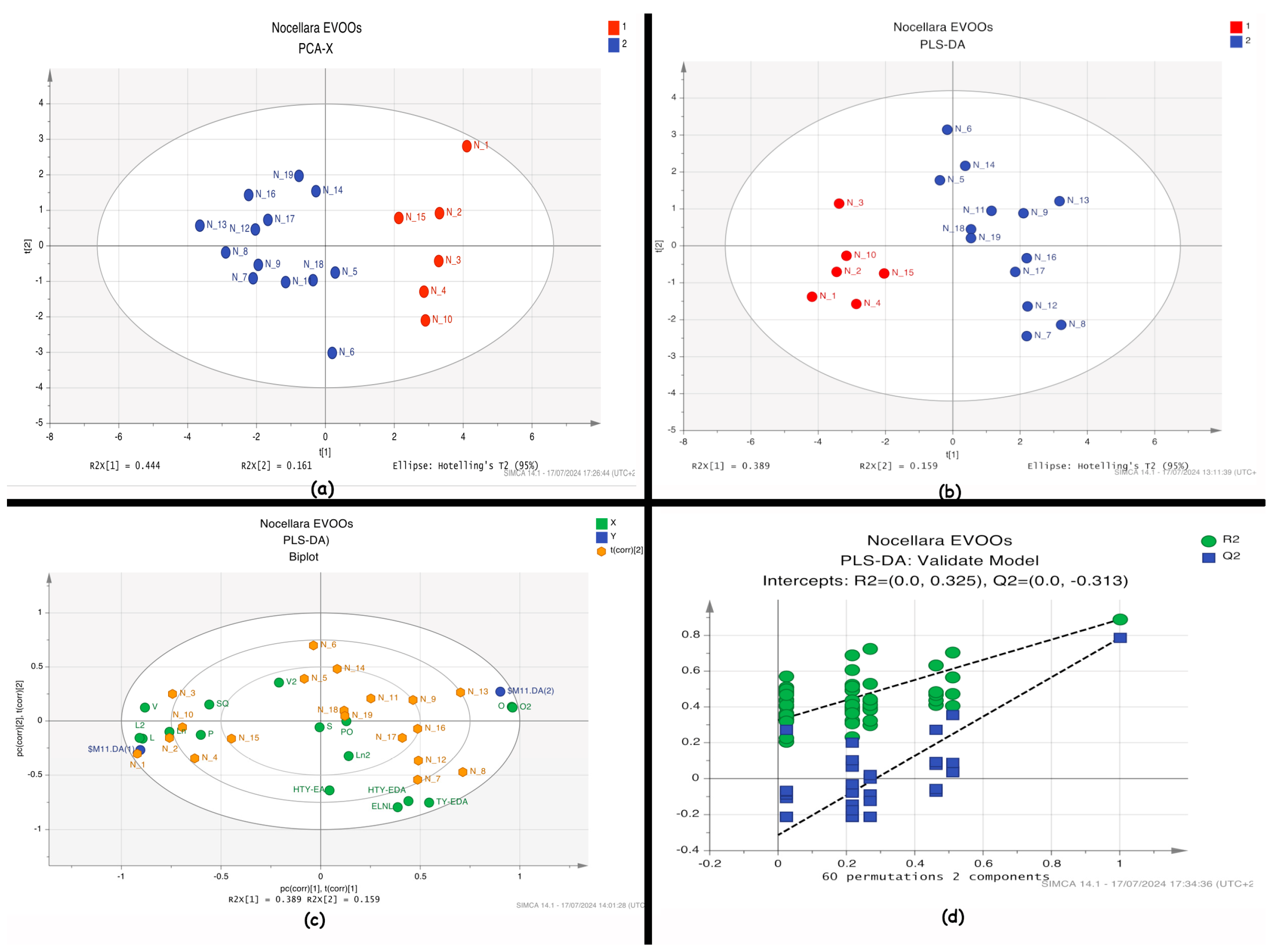
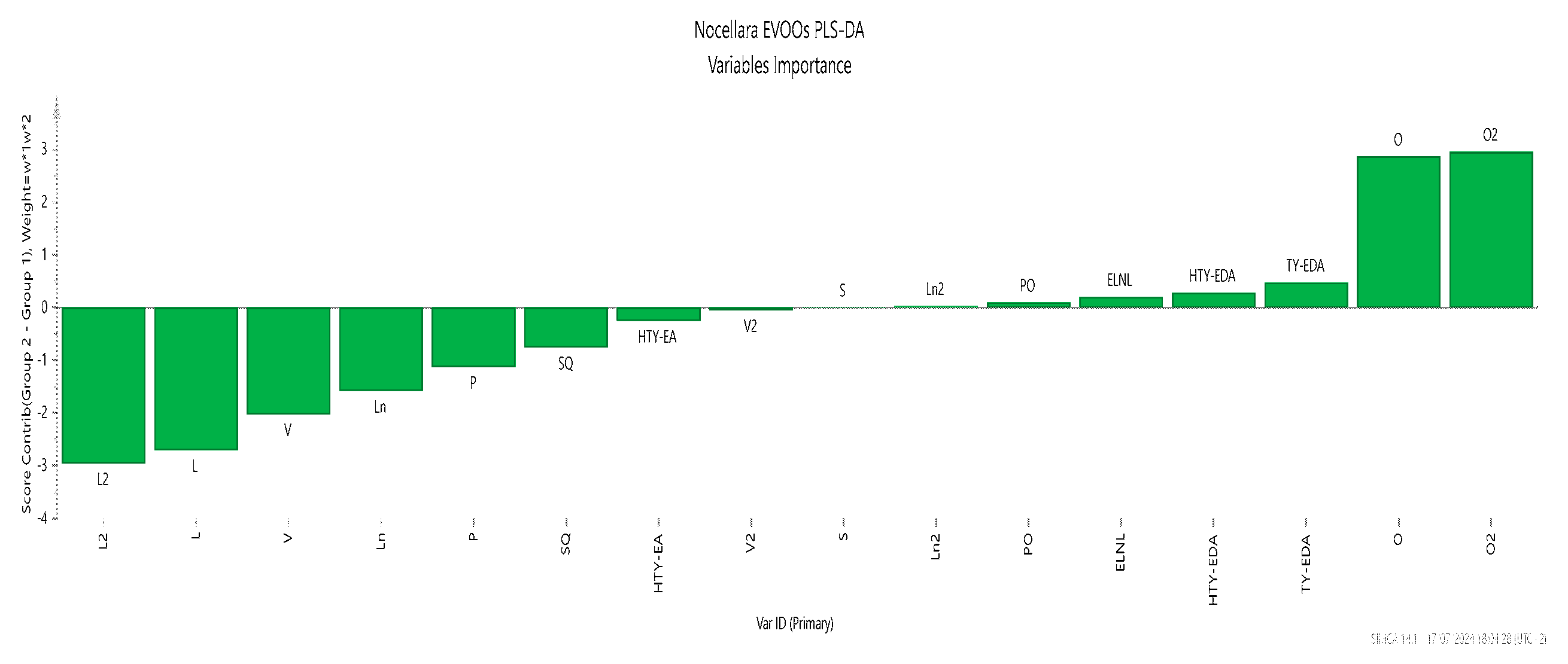
2.5. Statistical Analysis of the Metabolic Profile
3. Discussion
4. Materials and Methods
4.1. Samples
4.2. Chemicals
4.2. NMR Sample Preparation
4.3. NMR Experimental Protocol
- Experiment A: a standard protonic spectrum with 16 scans and a suitable cycling delay for quantitative analysis.
- Experiment B: 1H- DPFGSE (double-pulsed gradient spin echo) spectrum [25] with 32 scans for the detection and quantification of aldehydic phenolic species.
- Experiment C: full-time 1H decoupled 13C spectrum with 32 scans with a suitable recycling delay for quantitative evaluations.[22]
4.4. NMR Acquisition and Processing
4.5. NMR Processing Strategies and Quantification
4.6. Traditional Analytical Essays
4.7. Gas-Chromatographic (GC) Analysis of Fatty Acid Methyl Esters (FAMEs)
4.8. Quantification of Total Phenol Content (TPC)
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Appendix A
A.1. NMR Spectra and Assignments
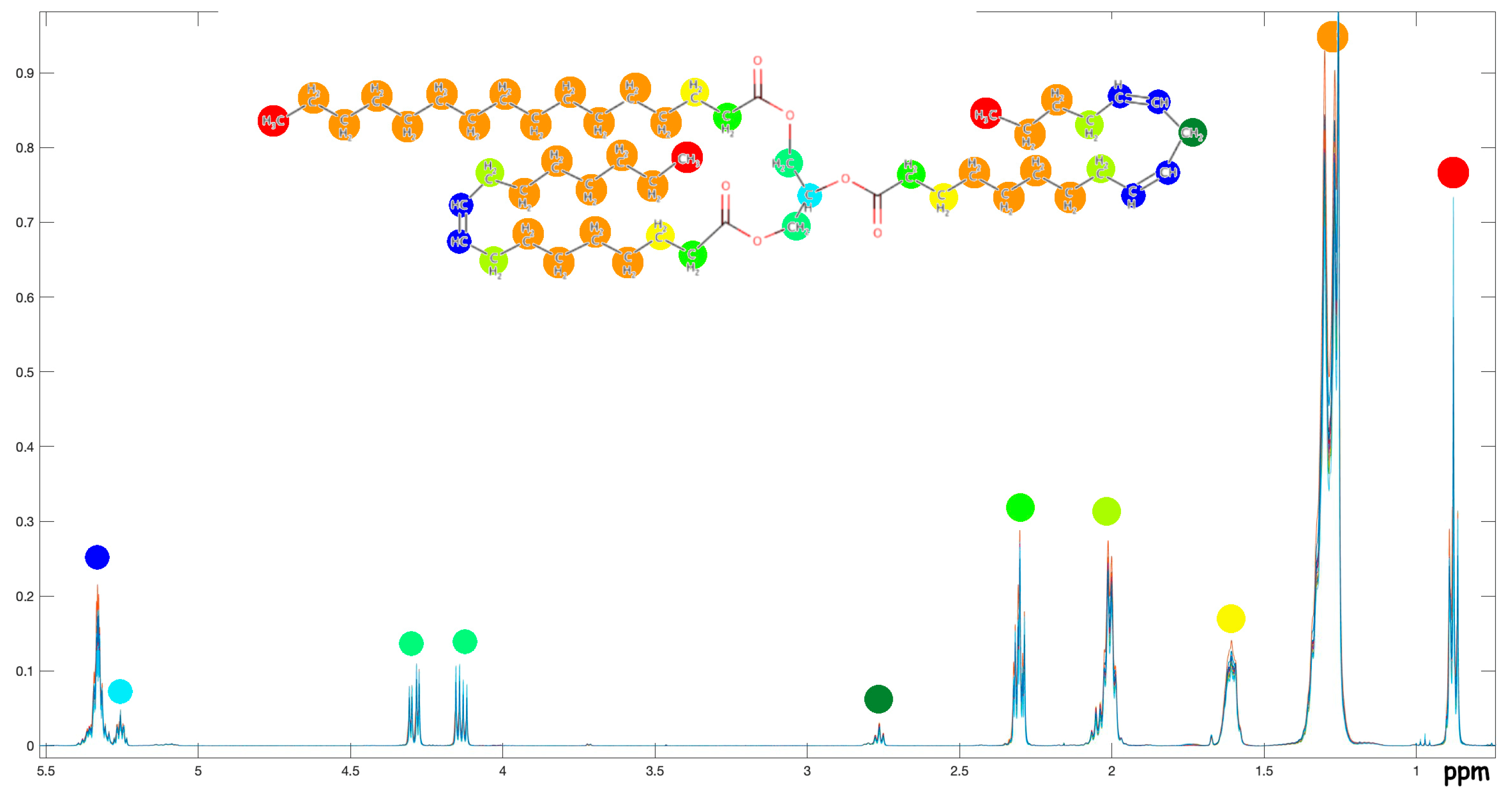
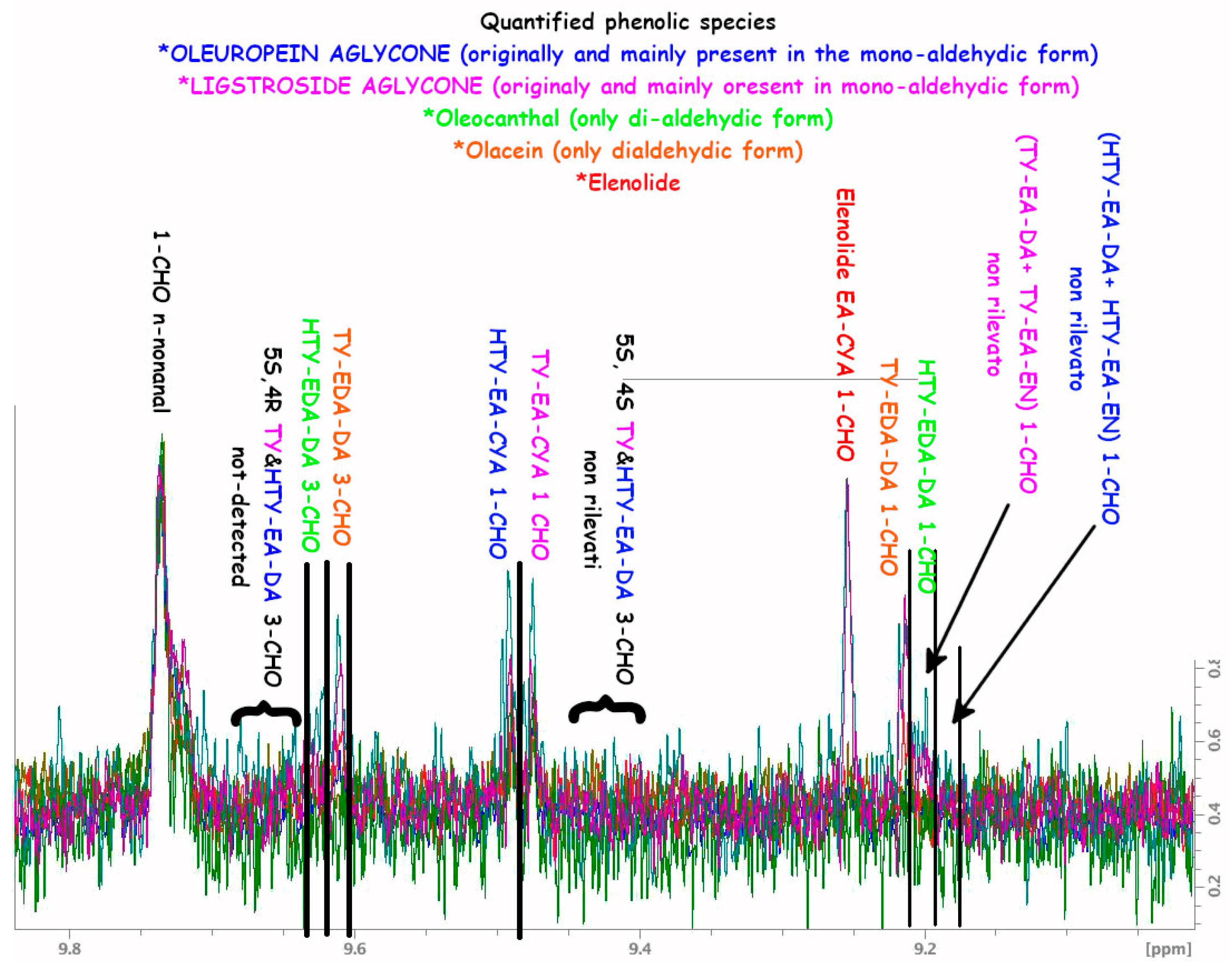
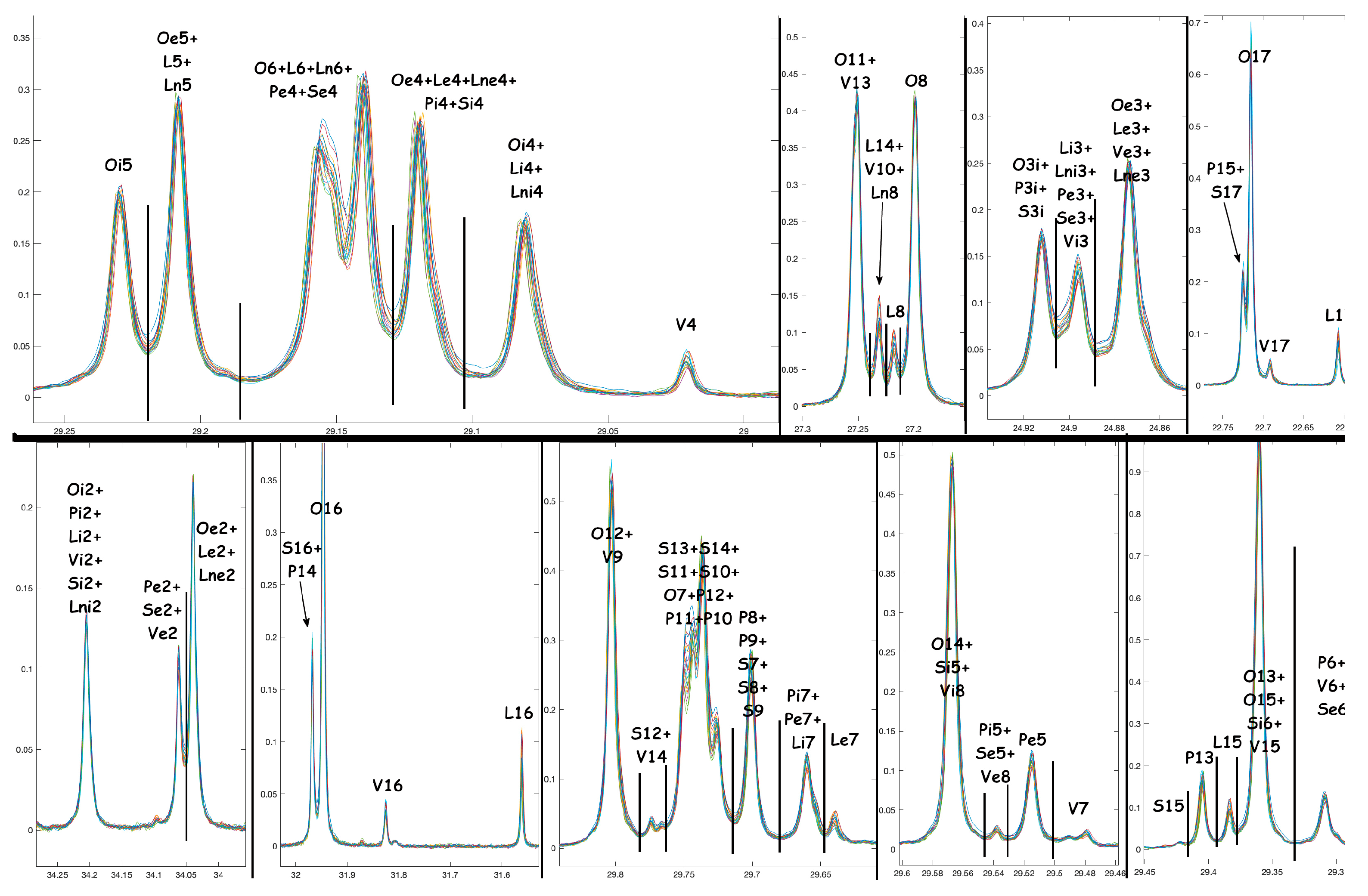
A.2. NMR Tables
| Group 1 | SQ** | Ln | L | O | PO | V | P | S |
|---|---|---|---|---|---|---|---|---|
| SD%* | 4.24 | 4.86 | 0.97 | 0.36 | 9.65 | 5.43 | 0.84 | 5.55 |
| N_1 | 2.74 | 0.70 | 10.41 | 62.78 | 0.47 | 4.66 | 19.31 | 1.67 |
| N_2 | 2.49 | 0.64 | 11.32 | 62.63 | 0.51 | 4.38 | 18.57 | 1.95 |
| N_3 | 2.29 | 0.59 | 11.29 | 62.51 | 0.35 | 4.45 | 18.72 | 2.09 |
| N_4 | 2.31 | 0.62 | 10.68 | 62.94 | 0.70 | 4.26 | 18.96 | 1.84 |
| N_5 | 2.26 | 0.57 | 9.45 | 65.35 | 0.37 | 4.07 | 18.29 | 1.90 |
| N_6 | 2.16 | 0.57 | 9.34 | 65.64 | 0.66 | 4.25 | 17.78 | 1.76 |
| N_7 | 1.81 | 0.56 | 9.10 | 66.44 | 0.56 | 3.46 | 17.79 | 2.09 |
| N_8 | 2.01 | 0.54 | 8.35 | 67.13 | 0.71 | 3.65 | 17.82 | 1.80 |
| N_9 | 2.17 | 0.49 | 8.55 | 66.56 | 0.54 | 4.00 | 17.89 | 1.98 |
| N_10 | 1.98 | 0.59 | 11.27 | 62.57 | 0.55 | 4.61 | 18.56 | 1.86 |
| N_11 | 1.98 | 0.57 | 9.12 | 66.02 | 0.63 | 3.88 | 17.84 | 1.94 |
| N_12 | 2.08 | 0.56 | 9.26 | 66.74 | 0.60 | 3.72 | 17.25 | 1.87 |
| N_13 | 2.22 | 0.53 | 7.51 | 69.04 | 0.44 | 3.36 | 17.57 | 1.55 |
| N_14 | 2.50 | 0.64 | 8.27 | 65.73 | 0.81 | 3.91 | 18.63 | 2.01 |
| N_15 | 2.41 | 0.63 | 10.43 | 63.70 | 0.45 | 4.16 | 18.71 | 1.93 |
| N_16 | 2.26 | 0.59 | 7.86 | 67.05 | 0.44 | 3.66 | 18.37 | 2.04 |
| N_17 | 2.19 | 0.52 | 8.03 | 65.55 | 0.56 | 3.80 | 19.62 | 1.93 |
| N_18 | 2.18 | 0.59 | 8.51 | 65.46 | 1.12 | 4.31 | 18.21 | 1.80 |
| N_19 | 2.29 | 0.55 | 9.89 | 65.73 | 0.06 | 3.57 | 18.18 | 2.02 |
| Group 2 | Ln2 | L2 | O2 | V2 | TY-EDA | HTY-EDA | HTY-EA | ELNL |
| SD%* | 11.34 | 3.22 | 1.3 | 7.34 | 10.46 | 8.62 | 12.43 | 9.32 |
| N_1 | 0.41 | 4.91 | 26.77 | 0.35 | 45.20 | 71.99 | 23.12 | 120.58 |
| N_2 | 0.37 | 5.20 | 27.24 | 0.52 | 23.39 | 70.01 | 23.77 | 92.42 |
| N_3 | 0.33 | 5.24 | 27.10 | 0.66 | n.d. | 25.48 | n.d. | 59.28 |
| N_4 | 0.32 | 4.97 | 27.12 | 0.92 | 29.07 | 95.99 | 46.86 | 211.41 |
| N_5 | 0.32 | 4.33 | 27.90 | 0.78 | 5.10 | 44.30 | 9.85 | 53.21 |
| N_6 | 0.23 | 4.28 | 27.88 | 0.94 | n.d. | 24.39 | n.d. | 9.01 |
| N_7 | 0.32 | 4.31 | 28.20 | 0.51 | 82.33 | 153.88 | 40.14 | 329.83 |
| N_8 | 0.38 | 3.87 | 28.50 | 0.59 | 115.12 | 213.57 | 24.19 | 278.06 |
| N_9 | 0.37 | 3.89 | 28.33 | 0.74 | 39.70 | 119.85 | 7.39 | 159.78 |
| N_10 | 0.34 | 5.01 | 27.08 | 0.91 | 8.71 | 27.13 | 40.31 | 73.01 |
| N_11 | 0.32 | 4.23 | 28.21 | 0.58 | 43.17 | 61.82 | 12.62 | 89.72 |
| N_12 | 0.41 | 4.19 | 28.24 | 0.49 | 83.02 | 157.32 | 18.29 | 292.24 |
| N_13 | 0.34 | 3.83 | 28.80 | 0.37 | 22.70 | 13.85 | 34.45 | 18.08 |
| N_14 | 0.44 | 3.87 | 28.28 | 0.74 | 10.81 | 45.08 | 4.00 | 97.75 |
| N_15 | 0.35 | 4.95 | 27.51 | 0.53 | 31.52 | 111.53 | 10.08 | 211.25 |
| N_16 | 0.41 | 3.60 | 28.73 | 0.59 | 44.12 | 81.18 | 38.82 | 223.21 |
| N_17 | 0.38 | 3.66 | 28.66 | 0.64 | 52.14 | 141.61 | 22.74 | 270.57 |
| N_18 | 0.37 | 4.18 | 28.07 | 0.72 | 40.34 | 81.39 | 21.34 | 189.95 |
| N_19 | 0.39 | 4.42 | 28.22 | 0.30 | 23.78 | 62.30 | 8.74 | 117.39 |
| Samples | Free acidity (FFA) (%) |
Peroxydes (meqO2/Kg) | K232 | K270 | ∆K |
|---|---|---|---|---|---|
| N_1 | 0,1 | 3 | 1,85 | 0,13 | -0,001 |
| N_2 | 0,1 | 4 | 1,37 | 0,15 | 0,000 |
| N_3 | 0,3 | 6 | 1,62 | 0,12 | 0,000 |
| N_4 | 0,1 | 5 | 1,49 | 0,10 | 0,000 |
| N_5 | 0,2 | 5 | 1,38 | 0,12 | 0,000 |
| N_6 | 0,1 | 5 | 1,41 | 0,08 | -0,001 |
| N_7 | 0,2 | 3 | 1,18 | 0,08 | 0,000 |
| N_8 | 0,1 | 3 | 1,29 | 0,08 | -0,001 |
| N_9 | 0,2 | 4 | 1,31 | 0,10 | -0,003 |
| N_10 | 0,3 | 6 | 1,46 | 0,10 | -0,003 |
| N_11 | 0,2 | 7 | 1,51 | 0,13 | -0,001 |
| N_12 | 0,2 | 6 | 1,38 | 0,12 | 0,000 |
| N_13 | 0,2 | 5 | 1,44 | 0,10 | -0,001 |
| N_14 | 0,2 | 5 | 1,36 | 0,09 | -0,001 |
| N_15 | 0,3 | 7 | 1,53 | 0,14 | 0,001 |
| N_16 | 0,1 | 5 | 1,55 | 0,10 | -0,006 |
| N_17 | 0,2 | 8 | 1,51 | 0,10 | -0,001 |
| N_18 | 0,2 | 3 | 1,42 | 0,09 | -0,002 |
| N_19 | 0,2 | 4 | 1,49 | 0,10 | -0,004 |
References
- Esposto, S.; Taticchi, A.; Urbani, S.; Selvaggini, R.; Veneziani, G.; Di Maio, I.; Sordini, B.; Servili, M. Effect of Light Exposure on the Quality of Extra Virgin Olive Oils According to Their Chemical Composition. Food Chem 2017, 229, 726–733. [Google Scholar] [CrossRef]
- Boskou, D.; Tsimidou, M.; Blekas, G. Olive Oil: Chemistry and Technology, Second ed.; 2006. [Google Scholar]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Gammazza, A.M.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int J Mol Sci 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Martín-Peláez, S.; Covas, M.I.; Fitó, M.; Kušar, A.; Pravst, I. Health Effects of Olive Oil Polyphenols: Recent Advances and Possibilities for the Use of Health Claims. Mol Nutr Food Res 2013, 57, 760–771. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.; Xu, Z.; Liu, J.; Li, T.; Yang, Z.; Ding, C. Quality, Composition, and Antioxidant Activity of Virgin Olive Oil from Introduced Varieties at Liangshan. LWT 2017, 78, 226–234. [Google Scholar] [CrossRef]
- Kouka, P.; Priftis, A.; Stagos, D.; Angelis, A.; Stathopoulos, P.; Xinos, N.; Skaltsounis, A.L.; Mamoulakis, C.; Tsatsakis, A.M.; Spandidos, D.A.; et al. Assessment of the Antioxidant Activity of an Olive Oil Total Polyphenolic Fraction and Hydroxytyrosol from a Greek Olea Europea Variety in Endothelial Cells and Myoblasts. Int J Mol Med 2017, 40, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Andrewes, P.; Busch, J.L.H.C.; De Joode, T.; Groenewegen, A.; Alexandre, H. Sensory Properties of Virgin Olive Oil Polyphenols: Identification of Deacetoxy-Ligstroside Aglycon as a Key Contributor to Pungency. J Agric Food Chem 2003, 51, 1415–1420. [Google Scholar] [CrossRef]
- II of the European Parliament and of the Council as Regards Marketing Standards for Olive Oil, and Repealing Commission Regulation (EEC) No 2568/91 and Commission Implementing Regulation (EU) No 29/2012; 2022.
- Bella, G.; Rotondo, A. Theoretical Prediction of 13C NMR Spectrum of Mixed Triglycerides by Mean of GIAO Calculations to Improve Vegetable Oils Analysis. Chem Phys Lipids 2020, 232. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.S.; Mendonça, M.A.; Pinho, D.M.M.; Resck, I.S.; Suarez, P.A.Z. Chromatographic Analyses of Fatty Acid Methyl Esters by HPLC-UV and GC-FID; 2012; Vol. 23. [Google Scholar]
- Ammar, S.; Kelebek, H.; Zribi, A.; Abichou, M.; Selli, S.; Bouaziz, M. LC-DAD/ESI-MS/MS Characterization of Phenolic Constituents in Tunisian Extra-Virgin Olive Oils: Effect of Olive Leaves Addition on Chemical Composition. Food Research International 2017, 100, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Aracama, A.; Goicoechea, E.; Guillén, M.D. Direct Study of Minor Extra-Virgin Olive Oil Components without Any Sample Modification. 1H NMR Multisupression Experiment: A Powerful Tool. Food Chem 2017, 228, 301–314. [Google Scholar] [CrossRef]
- Ruiz-Aracama, A.; Goicoechea, E.; Guillén, M.D. Direct Study of Minor Extra-Virgin Olive Oil Components without Any Sample Modification. 1H NMR Multisupression Experiment: A Powerful Tool. Food Chem 2017, 228, 301–314. [Google Scholar] [CrossRef]
- Simmler, C.; Napolitano, J.G.; McAlpine, J.B.; Chen, S.N.; Pauli, G.F. Universal Quantitative NMR Analysis of Complex Natural Samples. Curr Opin Biotechnol 2014, 25, 51–59. [Google Scholar] [CrossRef]
- Cevallos-Cevallos, J.M.; Reyes-De-Corcuera, J.I.; Etxeberria, E.; Danyluk, M.D.; Rodrick, G.E. Metabolomic Analysis in Food Science: A Review. Trends Food Sci Technol 2009, 20, 557–566. [Google Scholar] [CrossRef]
- Salvo, A.; Rotondo, A.; La Torre, G.L.; Cicero, N.; Dugo, G. Determination of 1,2/1,3-Diglycerides in Sicilian Extra-Virgin Olive Oils By1H-NMR over a One-Year Storage Period. Nat Prod Res 2017, 31, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Laghi, L.; Picone, G.; Capozzi, F. Nuclear Magnetic Resonance for Foodomics beyond Food Analysis. TrAC - Trends in Analytical Chemistry 2014, 59, 93–102. [Google Scholar] [CrossRef]
- Marcone, M.F.; Wang, S.; Albabish, W.; Nie, S.; Somnarain, D.; Hill, A. Diverse Food-Based Applications of Nuclear Magnetic Resonance (NMR) Technology. Food Research International 2013, 51, 729–747. [Google Scholar] [CrossRef]
- Liland, K.H. Multivariate Methods in Metabolomics - from Pre-Processing to Dimension Reduction and Statistical Analysis. TrAC - Trends in Analytical Chemistry 2011, 30, 827–841. [Google Scholar] [CrossRef]
- Mannina, L.; Marini, F.; Gobbino, M.; Sobolev, A.P.; Capitani, D. NMR and Chemometrics in Tracing European Olive Oils: The Case Study of Ligurian Samples. Talanta 2010, 80, 2141–2148. [Google Scholar] [CrossRef]
- Mannina, L.; Dugo, G.; Salvo, F.; Cicero, L.; Ansanelli, G.; Calcagni, C.; Segre, A. Study of the Cultivar-Composition Relationship in Sicilian Olive Oils by GC, NMR, and Statistical Methods. J Agric Food Chem 2003, 51, 120–127. [Google Scholar] [CrossRef]
- Rotondo, A.; Mannina, L.; Salvo, A. Multiple Assignment Recovered Analysis (MARA) NMR for a Direct Food Labeling: The Case Study of Olive Oils. Food Anal Methods 2019, 12, 1238–1245. [Google Scholar] [CrossRef]
- Trygg, J.; Wold, S. Orthogonal Projections to Latent Structures (O-PLS). J Chemom 2002, 16, 119–128. [Google Scholar] [CrossRef]
- Rotondo, A.; La Torre, G.L.; Dugo, G.; Cicero, N.; Santini, A.; Salvo, A. Oleic Acid Is Not the Only Relevant Mono-Unsaturated Fatty Ester in Olive Oil. Foods 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Klikarová, J.; Rotondo, A.; Cacciola, F.; Česlová, L.; Dugo, P.; Mondello, L.; Rigano, F. The Phenolic Fraction of Italian Extra Virgin Olive Oils: Elucidation Through Combined Liquid Chromatography and NMR Approaches. Food Anal Methods 2019, 12, 1759–1770. [Google Scholar] [CrossRef]
- Pérez, M.; Dominguez-López, I.; Lamuela-Raventós, R.M. The Chemistry Behind the Folin-Ciocalteu Method for the Estimation of (Poly)Phenol Content in Food: Total Phenolic Intake in a Mediterranean Dietary Pattern. J Agric Food Chem 2023, 71, 17543–17553. [Google Scholar] [CrossRef] [PubMed]
- Rigakou, A.; Diamantakos, P.; Melliou, E.; Magiatis, P. S-(E)-Elenolide: A New Constituent of Extra Virgin Olive Oil. J Sci Food Agric 2019, 99, 5319–5326. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, A.; Salvo, A.; Giuffrida, D.; Dugo, G.; Rotondo, E. NMR Analysis of Aldehydes in Sicilian Extra-Virgin Olive Oils by DPFGSE Techniques. AAPP Atti della Accademia Peloritana dei Pericolanti, Classe di Scienze Fisiche, Matematiche e Naturali 2011, 89. [Google Scholar] [CrossRef]
- Deiana, P.; Santona, M.; Dettori, S.; Culeddu, N.; Dore, A.; Molinu, M.G. Multivariate Approach to Assess the Chemical Composition of Italian Virgin Olive Oils as a Function of Variety and Harvest Period. Food Chem 2019, 300. [Google Scholar] [CrossRef] [PubMed]
- Piravi-Vanak, Z.; Ghasemi, J.B.; Ghavami, M.; Ezzatpanah, H.; Zolfonoun, E. The Influence of Growing Region on Fatty Acids and Sterol Composition of Iranian Olive Oils by Unsupervised Clustering Methods. JAOCS, Journal of the American Oil Chemists’ Society 2012, 89, 371–378. [Google Scholar] [CrossRef]
- Lechhab, T.; Lechhab, W.; Cacciola, F.; Salmoun, F. Sets of Internal and External Factors Influencing Olive Oil (Olea Europaea L.) Composition: A Review. European Food Research and Technology 2022, 248, 1069–1088. [Google Scholar] [CrossRef]
- Dag, A.; Harlev, G.; Lavee, S.; Zipori, I.; Kerem, Z. Optimizing Olive Harvest Time under Hot Climatic Conditions of Jordan Valley, Israel. European Journal of Lipid Science and Technology 2014, 116, 169–176. [Google Scholar] [CrossRef]
- Culeddu, N.; Chessa, M.; Bandino, G.; Sedda, P.; Zurru, R.; Anedda, R.; Motroni, A.; Molinu, M.G.; Dettori, S.; Santona, M. Classification of Monovarietal Sardinian Extra Virgin Olive Oils by 1H NMR Metabolomic. European Journal of Lipid Science and Technology 2017, 119. [Google Scholar] [CrossRef]
- Castejón, D.; Mateos-Aparicio, I.; Molero, M.D.; Cambero, M.I.; Herrera, A. Evaluation and Optimization of the Analysis of Fatty Acid Types in Edible Oils by 1H-NMR. Food Anal Methods 2014, 7, 1285–1297. [Google Scholar] [CrossRef]
- Dugo, G.; Rotondo, A.; Mallamace, D.; Cicero, N.; Salvo, A.; Rotondo, E.; Corsaro, C. Enhanced Detection of Aldehydes in Extra-Virgin Olive Oil by Means of Band Selective NMR Spectroscopy. Physica A: Statistical Mechanics and its Applications 2015, 420, 258–264. [Google Scholar] [CrossRef]
- Barison, A.; Da Silva, C.W.P.; Campos, F.R.; Simonelli, F.; Lenz, C.A.; Ferreira, A.G. A Simplemethodology for the Determination of Fatty Acid Composition in Edible Oils through 1H NMR Spectroscopy. Magnetic Resonance in Chemistry 2010, 48, 642–650. [Google Scholar] [CrossRef]
- Rotondo, A.; Salvo, A.; Gallo, V.; Rastrelli, L.; Dugo, G. Quick Unreferenced NMR Quantification of Squalene in Vegetable Oils. European Journal of Lipid Science and Technology 2017, 119. [Google Scholar] [CrossRef]
- International Olive Council. Coi/t.20/Doc. No 33/Rev.1. Determination of Fatty Acid Methyl Esters by Gas Chromatography Available online: http://www.internationaloliveoil.org/.
- Dordevic, D.; Dordevic, S.; Ćavar-Zeljković, S.; Kulawik, P.; Kushkevych, I.; Tremlová, B.; Kalová, V. Monitoring the Quality of Fortified Cold-Pressed Rapeseed Oil in Different Storage Conditions. European Food Research and Technology 2022, 248, 2695–2705. [Google Scholar] [CrossRef]
- Eriksson, L.; Trygg, J.; Wold, S. CV-ANOVA for Significance Testing of PLS and OPLS® Models. In Proceedings of the Journal of Chemometrics; John Wiley and Sons Ltd, 2008; Vol. 22, pp. 594–600. [Google Scholar]

| Metabolites | code |
| Squalene molecular % | SQ |
| Linolenate esters % | Ln |
| Linoleates esters % | L |
| Oleic esters % | O |
| Palmitoleic esters % | PO |
| cis-vaccenic esters % | V |
| palmitate esters % | P |
| sterarate esters | S |
| Internal* Linolenate esters % | Ln2 |
| Internal* Linoleates esters % | L2 |
| Internal* Oleic esters % | O2 |
| Internal* cis-vaccenic esters % | V2 |
| Oleocanthal | TY-EDA |
| Olaceine | HTY-EDA |
| Ligstroside aglycone (all the derivates) | TY-EA |
| Oleuropein aglycone (all the derivates) | HTY-EA |
| Elenolide | ELNL |
| total Phenolic species | TPH |
| Members | Correct | 1 | 2 | No class (YPred <= 0) | |
|---|---|---|---|---|---|
| 1 | 6 | 100% | 6 | 0 | 0 |
| 2 | 13 | 100% | 0 | 13 | 0 |
| No class | 0 | 0 | 0 | 0 | |
| Total | 19 | 100% | 6 | 13 | 0 |
| Fisher's prob. | 3.7e-005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





