Submitted:
24 July 2024
Posted:
25 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental
2.1. Lignin sample preparation
2.2. Lignin Characterization
2.3. Activated Carbon Synthesis
2.4. Activated Carbon Characterization
2.5. Carbon Fractional Conversion
3. Results and Discussion
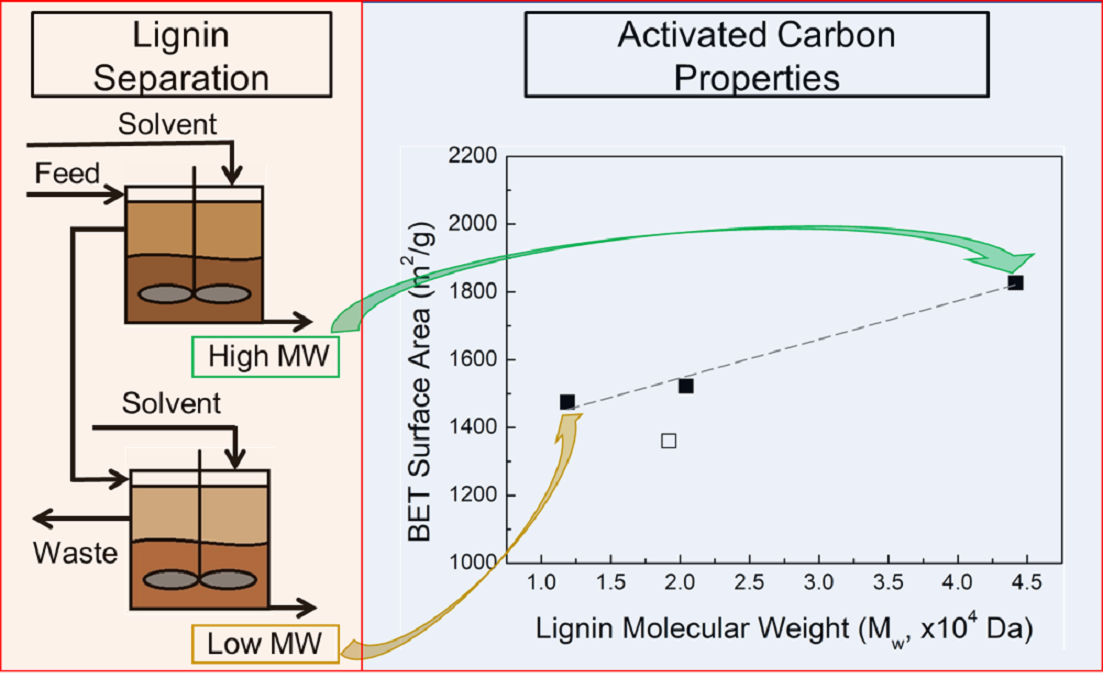
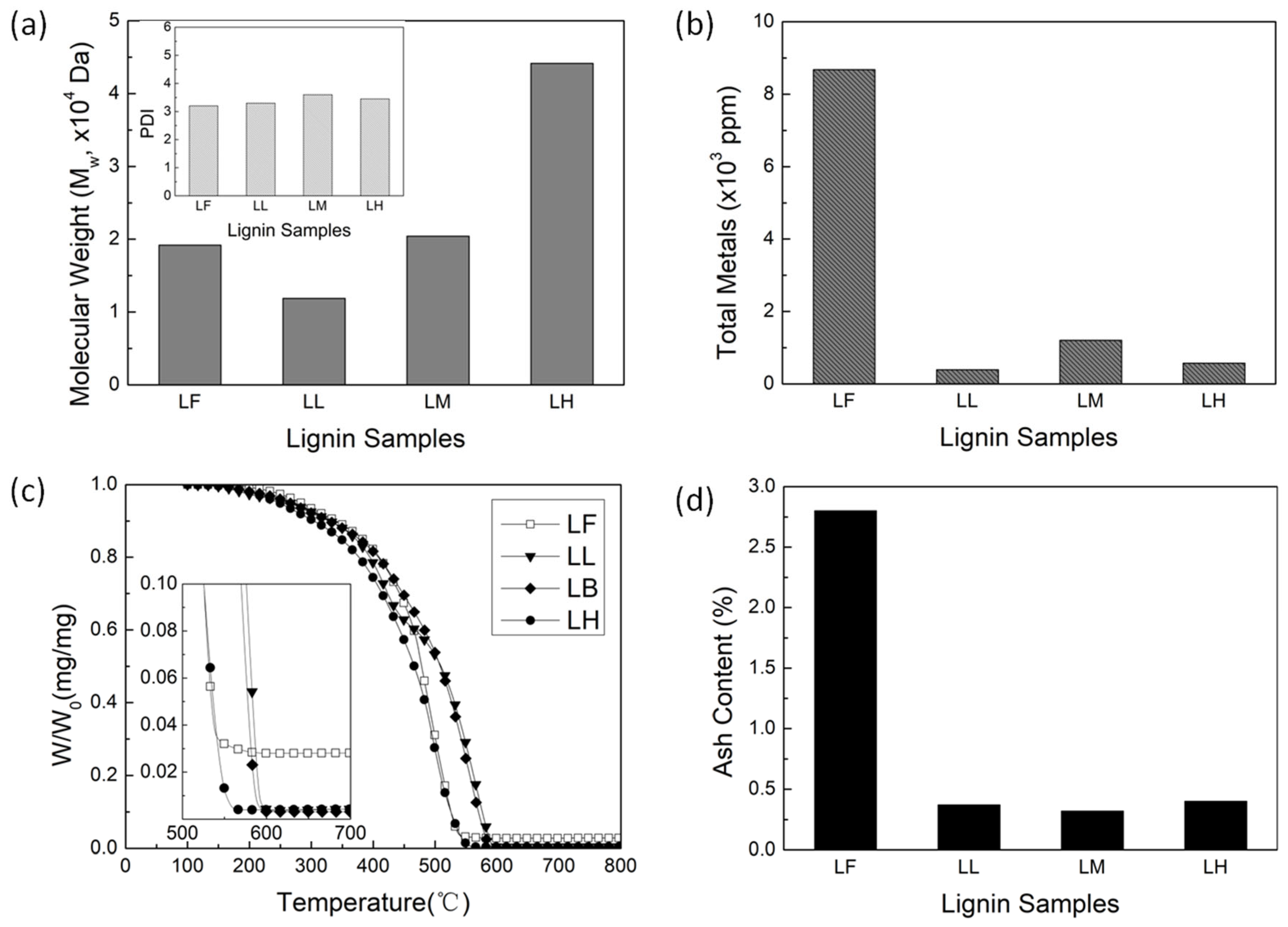
3.1. Lignin Samples for Activated Carbon
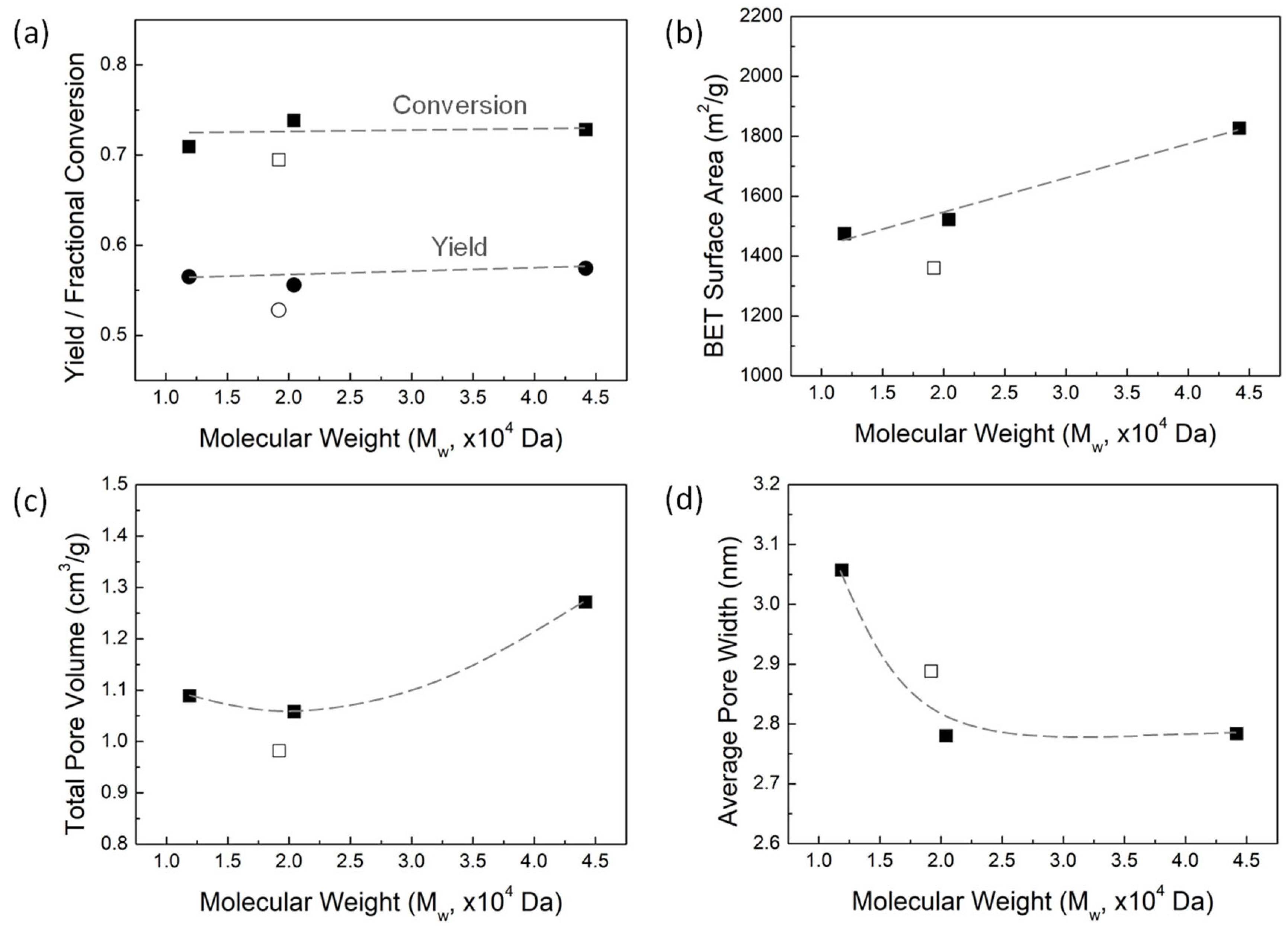
3.2. Activated Carbon Yield and Fractional Conversion
3.3. Pore Structure of Activated Carbon
3.3.1. Effect of MW of Lignin on Pore Properties
3.3.2. Effect of MW of Lignin on Pore-Size Distribution
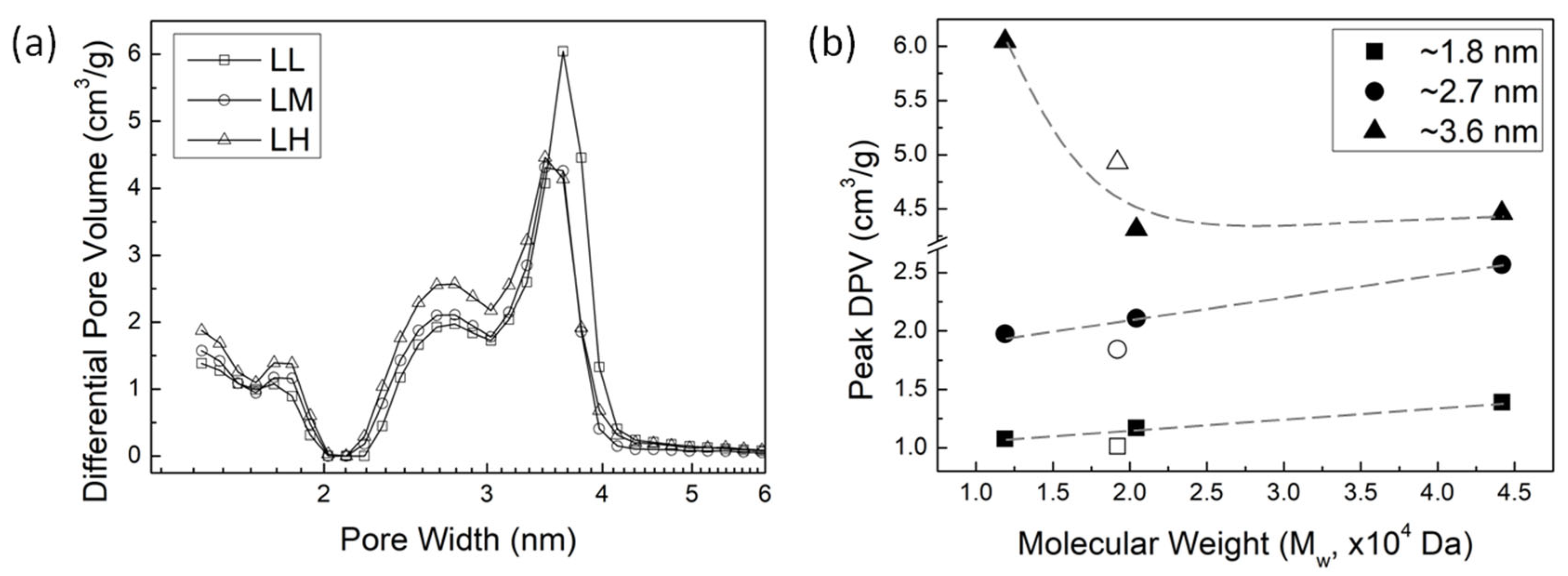
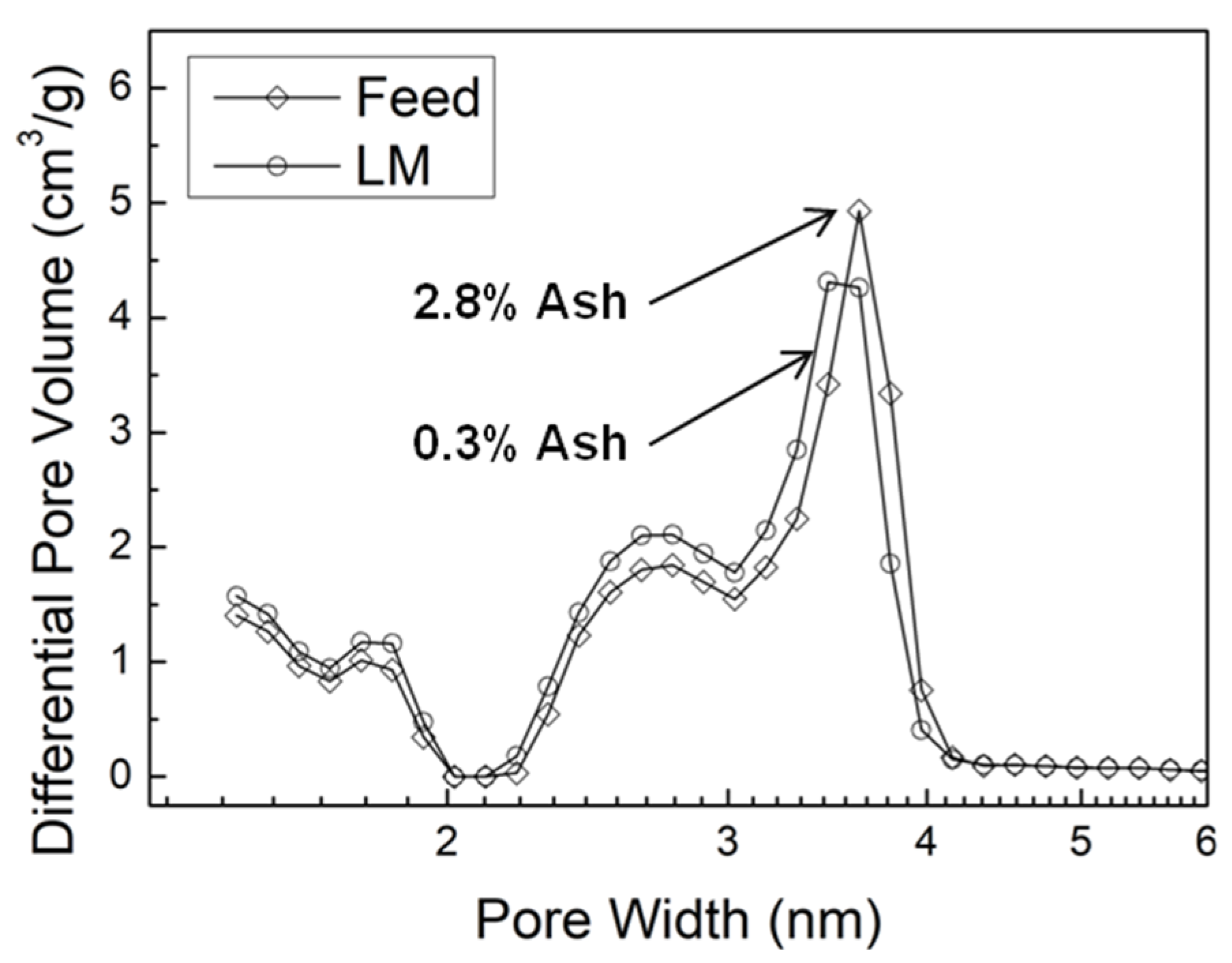
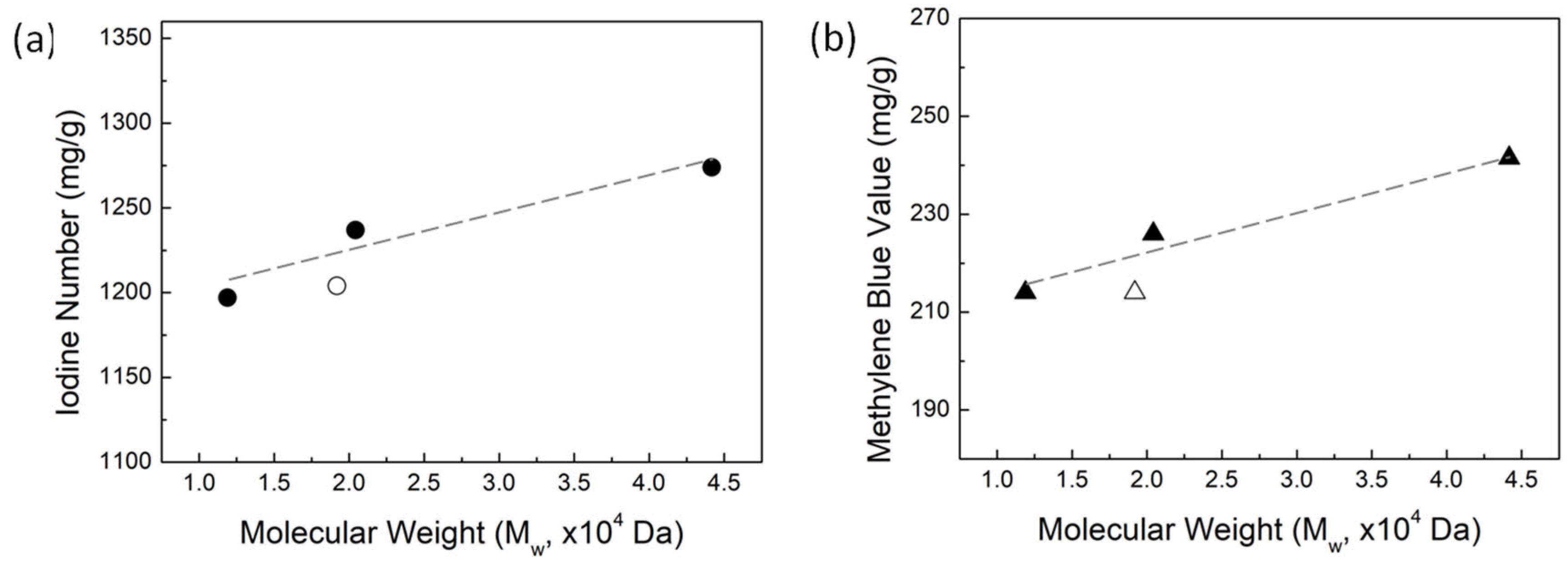
3.4. Aqueous Adsorption Capacity of Activated Carbon
4. Conclusions
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statista. Available online: https://www.statista.com/statistics/217179/global‐biofuels‐market‐size (access on 14 July 2024).
- Luque, R.; Herrero-Davila, L.; Campelo, J.M.; Clark, J.H.; Hidalgo, J.M.; Luna, D.; Marinas, J.M.; Romero, A.A. Biofuels: A Technological Perspective. Energy & Environmental Science 2008, 1, 542–564.
- Demirbas, A. Progress and Recent Trends in Biofuels. Progress in energy and combustion science 2007, 33, 1–18. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic Biomass: A Sustainable Platform for the Production of Bio-Based Chemicals and Polymers. Polymer chemistry 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Bimetallic Catalysts for Upgrading of Biomass to Fuels and Chemicals. Chemical Society Reviews 2012, 41, 8075–8098. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Industrial & engineering chemistry research 2009, 48, 3713–3729.
- Bajwa, D.; Pourhashem, G.; Ullah, A.H.; Bajwa, S. A Concise Review of Current Lignin Production, Applications, Products and Their Environmental Impact. Industrial Crops and Products 2019, 139, 111526. [Google Scholar] [CrossRef]
- Doherty, W.O.; Mousavioun, P.; Fellows, C.M. Value-Adding to Cellulosic Ethanol: Lignin Polymers. Industrial crops and products 2011, 33, 259–276. [Google Scholar] [CrossRef]
- Tardy, B.L.; Lizundia, E.; Guizani, C.; Hakkarainen, M.; Sipponen, M.H. Prospects for the Integration of Lignin Materials into the Circular Economy. Materials Today 2023, 65, 122–132. [Google Scholar] [CrossRef]
- Gregorich, N.; Ding, J.; Thies, M.C.; Davis, E.M. Novel Composite Hydrogels Containing Fractionated, Purified Lignins for Aqueous-Based Separations. Journal of Materials Chemistry A 2021, 9, 1025–1038. [Google Scholar] [CrossRef]
- Henry, C.; Nejad, M. Lignin-Based Low-Density Rigid Polyurethane/Polyisocyanurate Foams. Industrial & Engineering Chemistry Research 2023, 62, 6865–6873.
- Tejado, A.; Pena, C.; Labidi, J.; Echeverria, J.; Mondragon, I. Physico-Chemical Characterization of Lignins from Different Sources for Use in Phenol–Formaldehyde Resin Synthesis. Bioresource technology 2007, 98, 1655–1663. [Google Scholar] [CrossRef]
- Jin, J.; Ding, J.; Klett, A.; Thies, M.C.; Ogale, A.A. Carbon Fibers Derived from Fractionated–Solvated Lignin Precursors for Enhanced Mechanical Performance. ACS Sustainable Chemistry & Engineering 2018, 6, 14135–14142.
- Marsh, H.; Reinoso, F.R. Activated Carbon; Elsevier, 2006.
- Kanhere, S.V.; Tindall, G.W.; Ogale, A.A.; Thies, M.C. Carbon Fibers Derived from Liquefied and Fractionated Poplar Lignins: The Effect of Molecular Weight. Iscience 2022, 25. [Google Scholar] [CrossRef]
- Danish, M.; Ahmad, T. A Review on Utilization of Wood Biomass as a Sustainable Precursor for Activated Carbon Production and Application. Renewable and Sustainable Energy Reviews 2018, 87, 1–21. [Google Scholar] [CrossRef]
- Ahmadpour, A.; Do, D.D. The Preparation of Active Carbons from Coal by Chemical and Physical Activation. Carbon 1996, 34, 471–479. [Google Scholar] [CrossRef]
- Ioannidou, O.; Zabaniotou, A. Agricultural Residues as Precursors for Activated Carbon Production—A Review. Renewable and sustainable energy reviews 2007, 11, 1966–2005. [Google Scholar] [CrossRef]
- Jain, A.; Balasubramanian, R.; Srinivasan, M.P. Hydrothermal Conversion of Biomass Waste to Activated Carbon with High Porosity: A Review. Chemical Engineering Journal 2016, 283, 789–805. [Google Scholar] [CrossRef]
- Yahya, M.A.; Al-Qodah, Z.; Ngah, C.Z. Agricultural Bio-Waste Materials as Potential Sustainable Precursors Used for Activated Carbon Production: A Review. Renewable and sustainable energy reviews 2015, 46, 218–235. [Google Scholar] [CrossRef]
- Tekinalp, H.L.; Cervo, E.G.; Fathollahi, B.; Thies, M.C. The Effect of Molecular Composition and Structure on the Development of Porosity in Pitch-Based Activated Carbon Fibers. Carbon 2013, 52, 267–277. [Google Scholar] [CrossRef]
- Dias, J.M.; Alvim-Ferraz, M.C.; Almeida, M.F.; Rivera-Utrilla, J.; Sánchez-Polo, M. Waste Materials for Activated Carbon Preparation and Its Use in Aqueous-Phase Treatment: A Review. Journal of environmental management 2007, 85, 833–846. [Google Scholar] [CrossRef]
- Nor, N.M.; Lau, L.C.; Lee, K.T.; Mohamed, A.R. Synthesis of Activated Carbon from Lignocellulosic Biomass and Its Applications in Air Pollution Control—a Review. Journal of Environmental Chemical Engineering 2013, 1, 658–666. [Google Scholar]
- Bhatnagar, A.; Hogland, W.; Marques, M.; Sillanpää, M. An Overview of the Modification Methods of Activated Carbon for Its Water Treatment Applications. Chemical Engineering Journal 2013, 219, 499–511. [Google Scholar] [CrossRef]
- Roy, G.M. Activated Carbon Applications in the Food and Pharmaceutical Industries; Routledge, 2023.
- Serp, P.; Figueiredo, J.L. Carbon Materials for Catalysis; John Wiley & Sons, 2008; ISBN 0-470-17885-X.
- Frackowiak, E. Carbon Materials for Supercapacitor Application. Physical chemistry chemical physics 2007, 9, 1774–1785. [Google Scholar] [CrossRef]
- Sevilla, M.; Mokaya, R. Energy Storage Applications of Activated Carbons: Supercapacitors and Hydrogen Storage. Energy & Environmental Science 2014, 7, 1250–1280.
- Abioye, A.M.; Ani, F.N. Recent Development in the Production of Activated Carbon Electrodes from Agricultural Waste Biomass for Supercapacitors: A Review. Renewable and sustainable energy reviews 2015, 52, 1282–1293. [Google Scholar] [CrossRef]
- Grand View Research. Avaulable online: https://www.grandviewresearch.com/industry-analysis/activated-carbon-market (accessed on 14 July 2024).
- Carrott, P.J.M.; Carrott, M.R. Lignin–from Natural Adsorbent to Activated Carbon: A Review. Bioresource technology 2007, 98, 2301–2312. [Google Scholar]
- Wu, C.; Tindall, G.; Fitzgerald, C.; Thies, M.; Roberts, M. Decoupling the Role of Lignin, Cellulose/Hemi-Cellulose, and Ash on ZnCl2-Activated Carbon Pore Structure.
- Thies, M.C.; Klett, A.S.; Bruce, D.A. Solvent and Recovery Process for Lignin. US10053482B2, 2018.
- Klett, A.S.; Chappell, P.V.; Thies, M.C. Recovering Ultraclean Lignins of Controlled Molecular Weight from Kraft Black-Liquor Lignins. Chemical communications 2015, 51, 12855–12858. [Google Scholar] [CrossRef]
- Blasi, C.D.; Branca, C.; Galgano, A. Products and Global Weight Loss Rates of Wood Decomposition Catalyzed by Zinc Chloride. Energy & fuels 2008, 22, 663–670.
- Branca, C.; Di Blasi, C.; Galgano, A. Pyrolysis of Corncobs Catalyzed by Zinc Chloride for Furfural Production. Industrial & engineering chemistry research 2010, 49, 9743–9752.
- Di Blasi, C.; Branca, C.; Galgano, A.; Zenone, F. Modifications in the Thermicity of the Pyrolysis Reactions of ZnCl2-Loaded Wood. Industrial & Engineering Chemistry Research 2015, 54, 12741–12749.
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic Biomass Pyrolysis Mechanism: A State-of-the-Art Review. Progress in energy and combustion science 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Khalili, N.R.; Campbell, M.; Sandi, G.; Golaś, J. Production of Micro-and Mesoporous Activated Carbon from Paper Mill Sludge: I. Effect of Zinc Chloride Activation. Carbon 2000, 38, 1905–1915. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Zheng, C.; Lee, D.H.; Liang, D.T. In-Depth Investigation of Biomass Pyrolysis Based on Three Major Components: Hemicellulose, Cellulose and Lignin. Energy & fuels 2006, 20, 388–393.
- Siengchum, T.; Isenberg, M.; Chuang, S.S. Fast Pyrolysis of Coconut Biomass–An FTIR Study. Fuel 2013, 105, 559–565. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Liou, T.-H. Development of Mesoporous Structure and High Adsorption Capacity of Biomass-Based Activated Carbon by Phosphoric Acid and Zinc Chloride Activation. Chemical engineering journal 2010, 158, 129–142. [Google Scholar] [CrossRef]
- Nakano, T. Synthesis, Structure and Function of π-Stacked Polymers. Polymer journal 2010, 42, 103–123. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




