Introduction
The harmful impact of air pollution on human health is incontrovertible in the modern era. [
1] Many studies have shown the adverse effects of air pollution on human health. [
2,
3] In particular, it is clear that long-term exposure to air pollution increases the risk of cardiovascular mortality and morbidity, [
4,
5] as well as cancer. [
3] Additionally, air pollution has been shown to increase the incident risk of classical vascular risk factors, such as hypertension and diabetes mellitus. [
6,
7] As air pollution continues to worsen, there is need for studies aimed at reducing the incidence of air pollution-related diseases and damage to the human body.
Dyslipidemia refers to elevated levels of lipids in the blood, particularly low-density lipoprotein (LDL) cholesterol, and is a major risk factor for atherosclerotic cardiovascular disease, including coronary heart disease, stroke, and peripheral arterial disease. Untreated or inadequately managed dyslipidemia can lead to fatal and nonfatal cardiovascular or cerebrovascular events. [
8,
9,
10] Regarding the association of air pollution with dyslipidemia, a previous study showed that long-term air pollution exposure was associated with decreased levels of high-density lipoprotein (HDL) cholesterol. [
11]
Additionally, previous studies on associations between air pollution and the incidence or mortality of various diseases have focused on long-term exposure. [
7,
12,
13,
14,
15] However, air pollution can also have adverse effects on human health with short-term exposure. As expected, previous studies have reported associations between short-term exposure to air pollution and cardiovascular disease, diabetes, and kidney disease. [
16,
17,
18,
19,
20] Therefore, short-term air pollution exposure also may be associated with various diseases including dyslipidemia.
Fruits and vegetables are a major part of a healthy and high-quality diet. A varied diet rich in fruits and vegetables is important for health and development. A previous study has shown that older adults who consume a plant-based dietary pattern rich in whole and refined grains, vegetable oil, fruits, vegetables, and salt-preserved vegetables may reduce the harmful effects of particulate matter (PM
2.5) on cognitive function. [
21] In addition, longitudinal data from a prospective cohort study showed that high PM
2.5 and nitrogen dioxide (NO
2) groups with higher intake of retinol, vitamin A, and cholesterol were associated with a decreased risk of diabetes. [
22]
Accordingly, adequate intake of fruits and vegetables may reduce the risk of metabolic diseases associated with air pollution. However, there has been little study on how the appropriate intake of fruits and vegetables affects the risk of dyslipidemia associated with air pollution exposure, particularly short-term exposure.
Therefore, we hypothesized that short-term air pollution exposure would be associated with an increased incidence of dyslipidemia, and the harmful effects of short-term air pollution on dyslipidemia would be modified by consumption of fruits and vegetables. We investigate the association between short-term air pollution exposure and the incidence of dyslipidemia according to consumption of fruits and vegetables.
Materials and Methods
Ethical Considerations
The study design was reviewed and approved by the Institutional Review Board of Ewha Womans University Seoul Hospital (SEUMC 2024-02-031). This study was conducted in accordance with the tenets of the Declaration of Helsinki.
The data of the Korean Genome and Epidemiology Study (KoGES) were accessed with approval from the ethical review board following the review and approval of the National Institute of Korea (
https://kdca.go.kr/contents.es?mid=a40504060300).
Study Participants
KoGES is a population-based prospective cohort study that aims to elucidate the associations of lifestyle factors and genetic risk factors with major chronic diseases in Korean adults. Detailed information on KoGES can be found elsewhere. [
23] Among the six cohorts of the KoGES, we used the data of the Cardiovascular Disease Association Study (CAVAS), which recruited 28,337 community dwellers older than 40 years residing in Ganghwa, Goryeong, Namwon, Pyeongchang, Wonju, and Yangpyeong at baseline from 2005 to 2011. In each survey, information on sociodemographic factors, lifestyles, diet, and disease history was obtained by trained interviewers, and anthropometric measurement and urine and blood sampling were conducted by trained technicians. [
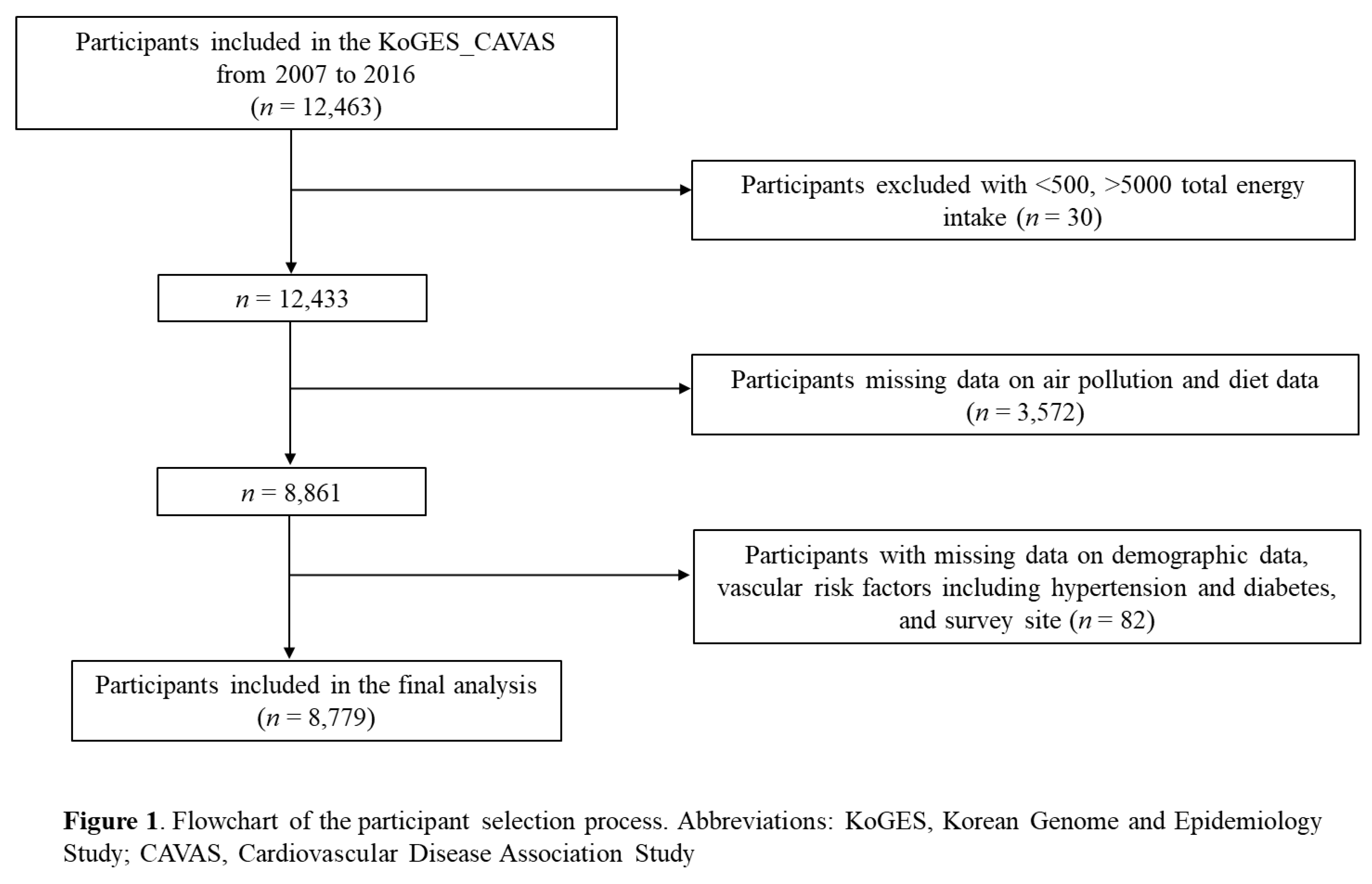
23] Participants for this study were included in the 1st-3rd surveys from 2007 to 2016 with a total of 12,463 participants. Among these participants, those with a total energy intake <500kcal/day (n = 21), and total energy intake >5000 kcal/day (n = 9) were excluded, as were participants with missing data (n = 3,572) on air pollution and diet. In addition, participants (n = 82) without complete information for demographics, vascular risk factors including hypertension and diabetes, and survey site were excluded. Ultimately, a total of 8,779 participants was included in the final analysis (
Figure 1).
Assessment of Air Pollutants
Air pollution exposures were estimated using community multiscale air quality models that integrated meteorological, emissions, and chemical transport models. NO
2 data were evaluated within a 9-km grid using monitoring station data from various locations across Korea. PM data were assessed within a 1-km grid using satellite-derived aerosol optical depth data from NASA's Terra and Aqua satellites, following implementation of nationwide PM
2.5 monitoring in 2015. Additional adjustments for air pollution levels were conducted using the normalized difference vegetation index and meteorological factors through multiple linear regression models. A detailed explanation of the air pollution models can be found elsewhere. [
22,
24]
The residential addresses of each study participant were collected during baseline and follow-up surveys and geocoded using GeoService-Xr software (Geoservice, Seoul, Korea). Air pollution data for the study period (2007–2016) were matched with cohort data by aligning the geocoded addresses with the 1-km grids (for PM
2.5) or 9-km grids (for NO
2) from the air pollution models. [
22]
Short-term exposure to air pollution was defined as the moving average of lag time ranging from “lag 1 day” to “lag 14 days.” To assess low and high exposure to air pollution, we first validated whether the variables followed a normal distribution and then categorized them into two groups based on the mean value: low (< mean) and high (≥ mean). The PM2.5 moving average of lag 0-14 days was divided into two categories: low (<23.3 μg/m3) and high (≥23.3 μg/m3). Similarly, NO2 was categorized as low (<11.2 ppb) and high (≥11.2 ppb).
Assessment of Dietary Factors
Dietary assessments were carried out using a semi-quantified food frequency questionnaire (SQFFQ) during the baseline survey. [
25] Dietary intake data from the participants were collected through a semi-quantitative Food Frequency Questionnaire (FFQ) in the baseline survey.
Participants reported their usual frequencies of consuming 103 food items using nine frequency categories, ranging from 'almost never' to 'three times/day,' along with typical portion sizes ('less than one serving size,' 'one serving size,' or 'more than one serving size'). The daily intake of each food item was converted to grams per day by multiplying the frequency per day by the portion size score. Nutrient intakes, including energy, macronutrients, vitamins, and minerals, were calculated by multiplying the grams per day of food consumed by their respective nutrient contents. [
22,
25,
26,
27]
To evaluate the intake of fruits and vegetables, the groups were divided into six categories: 'non-salted vegetables (NSV),' 'salted vegetables (SV),' 'fruits (F),' 'total vegetables (TV),' 'non-salted vegetables + fruits (NSVF),' and 'total vegetables + fruits (TVF).' The 'NSV' group included fresh and canned vegetables and juices consumed throughout the day, excluding kimchi and pickled vegetables, while the 'SV' group consisted of kimchi and pickled vegetables. The 'F' group encompassed fresh, canned, and dried fruits, as well as juices consumed during the day. The 'TV' group represented the consumption of all vegetables, including kimchi and pickled vegetables, while the 'TVF' group combined the consumption of total vegetables (TV) and fruits (F). The 'NSVF' group represented the combined consumption of non-salted vegetables and fruits. The quantities of food groups were reported in grams per day (g/day). [
28,
29] To assess the low and high intake of fruits and vegetables, we validated whether the variables followed a normal distribution and then categorized them based on the mean value into two groups: low (< mean) and high (≥ mean). The consumption of 'TV' was divided into two categories: low (<186.1 g/day) and high (≥186.1 g/day). Similarly, 'NSV' was categorized as low (<57.4 g/day) and high (≥57.4 g/day), while 'SV' consumption was categorized as low (<110.6 g/day) and high (≥110.6 g/day). For 'F,' the categories were low (<115.9 g/day) and high (≥115.9 g/day). The consumption of 'NSVF' was also categorized as low (<187.9 g/day) and high (≥187.9 g/day), and 'TVF' consumption was categorized as low (<328.6 g/day) and high (≥328.6 g/day).
Assessment of Outcomes
Blood samples were collected from all of the participants in a 10 h fasting state at baseline and follow-up visits and were stored at 4º C until (approximately 1 h later) separated plasma and serum components were aliquoted in 6-10 vials (300-500 uL per vial). The separated fluids were collected and stored at the National Biobank of Korea. [
23,
30] Laboratory evaluations were performed in the same core clinical laboratory, which is accredited and participates annually in inspections and surveys by the Korean Association of Quality Assurance for Clinical Laboratories. [
22,
31] The total cholesterol, HDL cholesterol, and triglycerides levels were measured by the enzymatic colorimetric method (ADVIA 1650-chemistry system, Bayer, Leverkusen, Germany). [
31] LDL cholesterol levels were calculated using the Friedewald formula: LDL cholesterol = total cholesterol – HDL cholesterol – (triglycerides/5). Plasma glucose levels were evaluated using the enzyme method (ADVIA 1650 and ADVIA 1800; Siemens Healthineers, IL, USA). [
22] Dyslipidemia was defined as one or more of the following five criteria according to the National Cholesterol Education Program Adult Treatment Panel III guidelines (NCEP-ATP Ⅲ): total cholesterol ≥240 mg/dl, LDL cholesterol ≥160 mg/dl, HDL cholesterol <40 mg/dl, serum triglycerides ≥200 mg/dl, and physician-reported dyslipidemia. [
32]
Statistical Analysis
The categorical variables were analyzed using the Chi-square test, while the continuous variables were analyzed by the T-test and generalized linear regression (GLM). Significant differences were assessed using GLM analysis, followed by Scheffe's post-hoc comparison test for further evaluation. The dyslipidemia risk was presented as the odds ratio (OR) and 95% confidence interval (CI). We analyzed the associations between short-term air pollution exposure and dyslipidemia using multivariable logistic regression models. We also analyzed the associations between the consumption of fruits and vegetables and dyslipidemia using multivariable logistic regression models. We further evaluated the interaction of short-term air pollution exposure and combined consumption of fruits and vegetables (air pollution × fruits and vegetables) using these multivariable logistic regression models. The multivariable analysis was adjusted for ambient temperature and humidity, total energy intake, age, sex, smoking, drinking, exercise, BMI, and survey site. The short-term exposure to temperature and humidity was defined as the moving average of lag time ranging from “lag 0 days” to “lag 14 days.” BMI was calculated by dividing the weight of the person by the square of their height (kg/m2). Survey sites were categorized into ‘Yangpyeong,’ ‘Namwon,’ ‘Goryeong,’ ‘Wonju,’ ‘Pyeongchang,’ and ‘Ganghwa.’ Job status was categorized into white-collar job, blue-collar job, and others. All statistical analyses were conducted with SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA). A two-tailed p-value < 0.05 was considered statistically significant for all analyses. Most values were rounded to one decimal place.
Results
Baseline Characteristics of the Study Participants
Table 1 presents the baseline characteristics of controls and cases of dyslipidemia. Participants with dyslipidemia were more likely to be current smokers, non-alcohol drinkers, and from the Goryeong survey site than were the controls. In addition, participants with dyslipidemia were more likely to have a higher BMI and percentage of energy from carbohydrates than the controls. On the other hand, participants with dyslipidemia consumed a lower percentage of energy from protein and fat and had lower TV and SV consumption compared to the controls. Although no significant difference was observed between the control group and cases of dyslipidemia, consumption of NSV, F, NSVF, and TVF was proportionally lower among individuals with dyslipidemia compared to controls.
Association of Short-Term Air Pollution Exposure with the Risk of Dyslipidemia
In analyses for the associations between short-term PM
2.5 concentrations and dyslipidemia, the moving average of lag 9, 10, 11, 12, 13, 14 days was associated with a higher risk of dyslipidemia [OR = 1.008, 95% CI: 1.001, 1.015 for lag 9 days; OR = 1.009, 95% CI: 1.002, 1.017 for lag 10 days; OR = 1.010, 95% CI: 1.003, 1.018 for lag 11 days; OR = 1.010, 95% CI: 1.003, 1.018 for lag 12 days; OR = 1.011, 95% CI: 1.003, 1.019 for lag 13 days; and OR = 1.012, 95% CI: 1.003, 1.020 for lag 14 days]. There were no significant associations between the moving average of lag times of NO
2 and dyslipidemia (
Table 2).
Association of Fruits and Vegetables Consumption with the Risk of Dyslipidemia
Higher intakes of TV, NSV and SV were associated with a decreased risk of dyslipidemia compared with lower intakes [OR = 0.89, 95% CI: 0.82, 0.98 for high intake of TV (≥186.1g/day); OR = 0.90, 95% CI: 0.82, 0.98 for high intake of NSV (≥57.4g/day); and OR = 0.89, 95% CI: 0.81, 0.98 for high intake of SV (≥110.6g/day)]. No significant difference in fruit consumption was observed, but higher intake of TVF was associated with a decreased risk of dyslipidemia compared with lower intake [OR = 0.91, 95% CI: 0.83, 0.99 for high intake of TVF (≥328.6g/day)] (
Table 3).
Effect Modification by Vegetable Consumption on the Association between Short-Term Air Pollution Exposure and the Risk of Dyslipidemia
For vegetable consumption within strata of short-term PM2.5 and NO2 concentrations, the high-PM
2.5 groups (≥23.3 μg/m
3) who had a higher intake of TV (≥186.1 g/day) were associated with a decreased risk of dyslipidemia compared to participants who had a lower intake of TV [OR = 0.88, 95% CI: 0.78, 0.99]. In addition, the high-PM
2.5 groups (≥23.3 μg/m
3) with a higher intake of SV (≥110.6 g/day) were associated with a decreased risk of dyslipidemia compared to participants with a lower intake of SV [OR = 0.86, 95% CI: 0.76, 0.97]. However, no interaction was found for short-term air pollution exposure and vegetable consumption on the risk of dyslipidemia (
Table 4).
We performed a post-hoc comparison test of characteristics by PM
2.5 concentrations and TV consumption groups using GLM analysis. Compared to those with high short-term PM
2.5 concentrations (≥23.3 μg/m³) and low TV consumption (<186.1 g/day), individuals in the other groups had a relatively higher proportion of blue-collar jobs (74.8%, p = 0.003) and current alcohol consumption (43.4%, p < 0.0001), were younger (60.9 ± 9.4 years, p < 0.0001), and had a higher BMI (24.4 ± 3.2 kg/m², p = 0.0004). Although no significant difference was observed in the post-hoc comparison test, individuals with high short-term PM
2.5 concentrations (≥23.3 μg/m³) and high TV consumption (≥186.1 g/day) had a lower prevalence of dyslipidemia (59.8% vs. 58.4%), lower LDL-cholesterol levels (146.6 ± 34.8 mg/dL vs. 146.9 ± 36.0 mg/dL), and higher HDL-cholesterol levels (47.3 ± 13.0 mg/dL vs. 46.5 ± 11.9 mg/dL) compared to those with high short-term PM
2.5 concentrations (≥23.3 μg/m³) and low TV consumption (<186.1 g/day) (
Supplementary Table 1).
Discussion
Our findings suggest that vegetable consumption is an important dietary factor in prevention of dyslipidemia, which was associated with short-term air pollution exposure.
Despite this study showing no interaction between short-term air pollution exposure and vegetable consumption on the risk of dyslipidemia, individuals in high PM2.5 groups (≥23.3 μg/m3) who consumed higher TV (≥186.1 g/day) and SV (≥110.6 g/day) had a significantly lower risk of dyslipidemia compared to those with lower intakes of these vegetables.
The American Heart Association indicates that PM
2.5 exposure has a substantial effect on morbidity and mortality. In particular, as levels of PM
2.5 exposure increase, there is a corresponding increase in cardiovascular disease. [
33] Moreover, several studies have shown that PM
2.5 exposure can increase the likelihood of metabolic diseases and has an adverse impact on public health. [
34,
35] While previous studies have associated the risks of hypertension, diabetes, and metabolic syndrome with increased exposure to air pollutants, they studies mainly focused on the association with long-term exposure. [
6,
7,
12,
13,
14,
36,
37] Our study showed the importance of the association between short-term PM
2.5 exposure and an increased risk of dyslipidemia, particularly in an individual-level dataset.
We identified that higher intakes of TV (≥186.1 g/day), NSV (≥57.4 g/day), SV (≥110.6 g/day), and TVF (≥328.6 g/day) were associated with a decreased risk of dyslipidemia. Meanwhile, no association was found between short-term air pollution exposure and consumption of fruits alone on the incidence risk of dyslipidemia. Though fruits and vegetables are important for a healthy diet as good sources of vitamins, minerals, and fiber, fruits have high sugar contents. In previous studies, consumption of strawberries, cantaloupe, and fruit juice had a positive association with type 2 diabetes. To determine the effects of these fruits, more studies are needed to evaluate the associations between consumption of individual fruit varieties and health outcomes. [
38,
39] On the other hand, research highlights that consumption of vegetables has an inverse relationship with obesity and metabolic syndrome. [
40,
41,
42] In addition, more vegetable-rich dietary patterns were associated with decreased risk of obesity and metabolic syndrome. [
43,
44,
45,
46] Our study results were consistent with the findings of these previous studies and suggested additional information regarding the details of the vegetables, including both salted and non-salted vegetables, for the association of short-term air pollution with dietary factors.
A previous study demonstrated an inverse association between the Mediterranean diet (characterized by high TV excluding potatoes, nuts, legumes, fish, whole grains, monounsaturated fatty acids to saturated fatty acids ratio, and alcohol intake) and the risk of cardiovascular disease mortality in relation to long-term exposure to air pollutants in a large prospective US cohort. [
21] Furthermore, a significant interaction effect has been reported between the animal foods pattern (characterized by high intake of animal blood and organs, preserved eggs, and processed meat products) and exposure to air pollution on gestational diabetes mellitus. [
47] A recent study has also shown that sufficient intake of dietary nutrients, such as retinol, vitamin A, and cholesterol, is inversely associated with the occurrence of diabetes mellitus in a scenario where air pollution levels are reduced. [
22] Healthy dietary factors, such as consuming high levels of vegetables, are associated with a diet that is rich in vitamins, minerals, fiber, and antioxidants. [
48] Our findings demonstrate consistent associations between air pollution exposure, dietary factors, and their relationships with health outcomes.
Although the present study cannot confirm the interaction between short-term air pollution exposure and vegetable consumption, we suggest that vegetable consumption be considered an important factor in the prevention of dyslipidemia associated with short-term air pollution exposure. Although our study does not offer a precise mechanism, it suggests that exposure to air pollution may induce oxidative stress-related inflammatory responses in various parts of the body, particularly the respiratory system, leading to pathological changes and impaired metabolic function. Furthermore, air pollution exposure can impact biological pathways, such as NF-KB, by depositing a range of toxic substances that trigger oxidative stress-related inflammatory responses. The health-promoting compounds in vegetables have the potential to alleviate the development of metabolic diseases through their antioxidant and anti-inflammatory activity. [
1,
6,
22]
Our study had several limitations that need to be considered. First, the data used in this study were derived from a community-based cohort of non-institutionalized adults, primarily consisting of individuals with early-stage disease. Lifestyle factors such as physical activity, dietary habits, and nutrition may not have as significant an impact on the treatment of severe cases. Therefore, caution should be exercised when generalizing our results to patients with severe health conditions, including those who are hospitalized or undergoing intensive treatments. Second, the follow-up survey was conducted in only 6 of the 11 total communities where air pollution data were available, potentially introducing selection bias, a common issue in many prospective cohort studies. [
49] Third, due to the nature of our cross-sectional data analysis, we were unable to establish causality in the associations observed. Future studies should aim to investigate and confirm the causal factors contributing to dyslipidemia.
In conclusion, our study revealed an association between higher short-term PM2.5 levels and an elevated risk of dyslipidemia, while also suggesting the potential of vegetable consumption to reduce this risk. Our results imply that including vegetables in the diet may have a significant association with decreased risk of dyslipidemia associated with short-term exposure to air pollution.
Supplementary Materials
Table S1. Characteristics of the study participants by PM2.5 concentrations and TV consumption.
Author Contributions
Conceptualization: Shin MK, Song TJ. Data curation: Shin MK. Formal analysis: Shin MK. Funding acquisition: Song TJ. Investigation: Shin MK. Methodology: Shin MK, Kwag YR, Song TJ. Software: Shin MK. Validation: Kwag YR, Song TJ. Visualization: Shin MK, Song TJ. Writing - original draft: Shin MK, Song TJ. Writing - review & editing: Shin MK, Kwag YR, Song TJ.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No.2022R1C1C1004772). This work was also supported by an Institute of Information & Communications Technology Planning & Evaluation (IITP) grant funded by the Korean government (MSIT) (RS-2022-II220621, Development of artificial intelligence technology that provides dialog-based multi-modal explainability) and a Korea Evaluation Institute of Industrial Technology (KEIT) grant funded by the Korean government. This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, South Korea (grant number: RS-2023-00262087 to TJS). The funding source had no role in the design, conduct, or reporting of this study.
Acknowledgments
We thank the study participants and researchers of CAVAS. This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2022R1C1C1004772). This work was also supported by an Institute of Information & Communications Technology Planning & Evaluation (IITP) grant funded by the Korean government (MSIT) (RS-2022-II220621, Development of artificial intelligence technology that provides dialog-based multi-modal explainability) and a Korea Evaluation Institute of Industrial Technology (KEIT) grant funded by the Korean government. This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Korea (grant number: RS-2023-00262087 to TJS). The funding source had no role in the design, conduct, or reporting of this study.
Disclosure
The authors have no potential conflicts of interest to disclose.
References
- Organization, W.H. Ambient air pollution: a global assessment of exposure and burden of disease. World Health Organization 2016, https://iris.who.int/handle/10665/250141, 121.
- Dominski FH; Lorenzetti Branco JH; Buonanno G; Stabile L; Gameiro da Silva M; A, A. Effects of air pollution on health: A mapping review of systematic reviews and meta-analyses. Environ Res 2021, 201, 111487. [CrossRef] [PubMed]
- Manisalidis I; Stavropoulou E; Stavropoulos A; E., B. Environmental and Health Impacts of Air Pollution: A Review. Front Public Health 2020, 8, 14. [CrossRef]
- Jia Y; Lin Z; He Z; Li C; Zhang Y; Wang J; Liu F; Li J; Huang K; Cao J; et al. Effect of Air Pollution on Heart Failure: Systematic Review and Meta-Analysis. Environ Health Perspect 2023, 131, 76001. [CrossRef] [PubMed]
- Bont JD; Jaganathan S; Dahlquist M; Persson Å; Stafoggia M; P., L. Ambient air pollution and cardiovascular diseases: An umbrella review of systematic reviews and meta-analyses. J Intern Med 2022, 291, 779-800. [CrossRef] [PubMed]
- Kim KN; Kim JH; Jung, K.; YC., H. Associations of air pollution exposure with blood pressure and heart rate variability are modified by oxidative stress genes: A repeated-measures panel among elderly urban residents. Environ Health 2016, 15, 47. [CrossRef] [PubMed]
- Andersen ZJ; Raaschou-Nielsen O; Ketzel M; Jensen SS; Hvidberg M; Loft S; Tjønneland A; Kim O; M., S. Diabetes incidence and long-term exposure to air pollution: a cohort study. Diabetes Care 2012, 35, 92-98.
- Park HJ; Chang YK; Lee HJ; Hong IS; TJ., S. Association of total cholesterol variability with risk of venous thromboembolism: A nationwide cohort study. PLoS One 2023, 18, e0289743.
- Chang YK; Eom SJ; Kim MJ; TJ., S. Medical Management of Dyslipidemia for Secondary Stroke Prevention: Narrative Review. . Medicina (Kaunas) 2023, 59, 776. [CrossRef]
- Song TJ; Chang YK; Shin MJ; Heo JH; YJ., K. Low levels of plasma omega 3-polyunsaturated fatty acids are associated with cerebral small vessel diseases in acute ischemic stroke patients. Nutr Res 2015, 35, 368-374. [CrossRef]
- Bell G; Mora S; Greenland P; Tsai M; Gill E; JD., K. Association of Air Pollution Exposures With High-Density Lipoprotein Cholesterol and Particle Number: The Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol 2017, 37, 976-982. [CrossRef]
- Fuks KB; Weinmayr G; Basagaña X; Gruzieva O; Hampel R; Oftedal B; Sørensen M; Wolf K; Aamodt G; Aasvang GM; et al. Long-term exposure to ambient air pollution and traffic noise and incident hypertension in seven cohorts of the European study of cohorts for air pollution effects (ESCAPE). Eur Heart J 2017, 38, 983-990.
- Eze IC; Schaffner E; Fischer E; Schikowski T; Adam M; Imboden M; Tsai M; Carballo D; von Eckardstein A; Künzli N; et al. Long-term air pollution exposure and diabetes in a population-based Swiss cohort. Environ Int 2014, 70, 95-105. [CrossRef] [PubMed]
- Eze IC; Schaffner E; Foraster M; Imboden M; von Eckardstein A; Gerbase MW; Rothe T; Rochat T; Künzli N; Schindler C; N., P.-H. Long-Term Exposure to Ambient Air Pollution and Metabolic Syndrome in Adults. PLoS One 2015, 10, e0130337.
- Andersen ZJ; Hvidberg M; Jensen SS; Ketzel M; Loft S; Sørensen M; Tjønneland A; Kim O; O., R.-N. Chronic obstructive pulmonary disease and long-term exposure to traffic-related air pollution: a cohort study. Am J Respir Crit Care Med 2011, 183, 455-461. [CrossRef]
- Orellano P; Reynoso J; Quaranta N; Bardach A; A., C. Short-term exposure to particulate matter (PM(10) and PM(2.5)), nitrogen dioxide (NO(2)), and ozone (O(3)) and all-cause and cause-specific mortality: Systematic review and meta-analysis. Environ Int 2020, 142, 105876. [CrossRef] [PubMed]
- Lee WH; Prifti K; Kim H; Kim EJ; Yang JY; Min JE; Park JY; Kim YC; Lee JP; ML., B. Short-term Exposure to Air Pollution and Attributable Risk of Kidney Diseases: A Nationwide Time-series Study. Epidemiology 2022, 33, 17-24. [CrossRef]
- Ni Y; Tracy RP; Cornell E; Kaufman JD; Szpiro AA; Campen MJ; S., V. Short-term exposure to air pollution and biomarkers of cardiovascular effect: A repeated measures study. Environ Pollut 2021, 279, 116893. [CrossRef]
- Xu R; Tian Q; Lu W; Yang Z; Ye Y; Li Y; Lin Q; Wang Y; Fan Z; Liu T; et al. Association of short-term exposure to air pollution with recurrent ischemic cerebrovascular events in older adults. Int J Hyg Environ Health 2022, 240, 113925. [CrossRef]
- Wu C; Yan Y; Chen X; Gong J; Guo Y; Zhao Y; Yang N; Dai J; Zhang F; H., X. Short-term exposure to ambient air pollution and type 2 diabetes mortality: A population-based time series study. Environ Pollut 2021, 289, 117886. [CrossRef]
- Zhu A; Chen H; Shen J; Wang X; Li Z; Zhao A; Shi X; Yan L; Zeng Y; Yuan C; JS., J. Interaction between plant-based dietary pattern and air pollution on cognitive function: a prospective cohort analysis of Chinese older adults. Lancet Reg Health West Pac 2022, 20, 100372.
- Shin MK; KN., K. Association between long-term air pollution exposure and development of diabetes among community-dwelling adults: Modification of the associations by dietary nutrients. Environ Int 2023, 174, 107908. [CrossRef] [PubMed]
- Kim YJ; group, H.B.K. Cohort Profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int J Epidemiol 2017, 46, e20. [CrossRef]
- Woo HD; Song DS; Choi SH; Park JK; Lee K; Yun HY; Choi DR; Koo YS; HY., P. Integrated dataset of the Korean Genome and Epidemiology Study cohort with estimated air pollution data. Epidemiol Health 2022, 44, e2022071. [CrossRef]
- Ahn Y; Kwon E; Shim J; Park M; Joo Y; Kimm K; Park C; D., K. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur J Clin Nutr 2007, 61, 1435-1441. [CrossRef]
- Shin MK; Kwak SH; Park YM; Jung JY; Kim YS; YA., K. Association between Dietary Patterns and Chronic Obstructive Pulmonary Disease in Korean Adults: The Korean Genome and Epidemiology Study. Nutrients 2021, 13, 4348. [CrossRef] [PubMed]
- Shin MK; KN., K. Association Between Instant Coffee Consumption and the Development of Chronic Obstructive Pulmonary Disease: Results From a Community-Based Prospective Cohort. J Korean Med Sci 2024, 39, e1. [CrossRef]
- Kim KN; MK., S. Feeding characteristics in infancy affect fruit and vegetable consumption and dietary variety in early childhood. Nutr Res Pract 2023, 17, 307-315. [CrossRef]
- Lee EK; Choi JH; YR, H. Intake of fruits and vegetables may modify the risk of cataract in Korean males: data from Korean National Health and Nutrition Examination Survey. J Nutr Health 2018, 51, 423-432. [CrossRef]
- Cho SY; Hong EJ; Nam JM; Han BK; OK., P. Opening of the national biobank of Korea as the infrastructure of future biomedical science in Korea. Osong Public Health Res Perspect 2012, 3, 177-184. [CrossRef]
- Kanmani S; Kwon MJ; Shin MK; MK., K. Association of C-Reactive Protein with Risk of Developing Type 2 Diabetes Mellitus, and Role of Obesity and Hypertension: A Large Population-Based Korean Cohort Study. Sci Rep 2019, 14, 4573.
- RJ., L. The National Cholesterol Education Program Adult Treatment Panel III guidelines. J Manag Care Pharm 2003, 9, 2-5.
- Aryal A; Harmon AC; TR., D. Particulate matter air pollutants and cardiovascular disease: Strategies for intervention. Pharmacol Ther 2021, 223, 107890. [CrossRef] [PubMed]
- Krittanawong C; Qadeer YK; Hayes RB; Wang Z; Virani S; Thurston GD; CJ., L. PM2.5 and Cardiovascular Health Risks. Curr Probl Cardiol 2023, 48, 101670. [CrossRef] [PubMed]
- Balti EV; Echouffo-Tcheugui JB; Yako YY; AP., K. Air pollution and risk of type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin Pract 2014, 106, 161-172. [CrossRef] [PubMed]
- Shin MK; Kim KN; Bae SH; Kim MJ; Kim JH; Kwon HJ; Hwang SS; Kim HE; YJ, A. Study on the Health Effects of Climate Change among the Elderly. Public Health Weekly Report 2023, 16, 1165-1177, doi:10.56786/PHWR.2023.16.33.1. [CrossRef]
- Hwang MJ; Kim JH; Koo YS; Yun HY; HK., C. Impacts of ambient air pollution on glucose metabolism in Korean adults: a Korea National Health and Nutrition Examination Survey study. Environ Health 2020, 19, 70. [CrossRef] [PubMed]
- Muraki I; Imamura F; Manson JE; Hu FB; Willett WC; van Dam RM, S.Q. Fruit consumption and risk of type 2 diabetes: results from three prospective longitudinal cohort studies. BMJ 2013, 347, f5001. [CrossRef] [PubMed]
- Wang PY; Fang JC; Gao ZH; Zhang C; SY., X. Higher intake of fruits, vegetables or their fiber reduces the risk of type 2 diabetes: A meta-analysis. J Diabetes Investig 2016, 7, 56-69. [CrossRef] [PubMed]
- Pepe RB; Lottenberg AM; Fujiwara CTH; Beyruti M; Cintra DE; Machado RM; Rodrigues A; Jensen NSO; Caldas APS; Fernandes AE; et al. Position statement on nutrition therapy for overweight and obesity: nutrition department of the Brazilian association for the study of obesity and metabolic syndrome (ABESO-2022). Diabetol Metab Syndr 2023, 15, 124. [CrossRef]
- Zhang Y; DZ., Z. Associations of vegetable and fruit consumption with metabolic syndrome. A meta-analysis of observational studies. Public Health Nutr 2018, 21, 1693-1703. [CrossRef]
- Tian Y; Su L; Wang J; Duan X; X., J. Fruit and vegetable consumption and risk of the metabolic syndrome: a meta-analysis. Public Health Nutr 2018, 21, 756-765. [CrossRef] [PubMed]
- Khalil M; Shanmugam H; Abdallah H; John Britto JS; Galerati I; Gómez-Ambrosi J; Frühbeck G; P., P. The Potential of the Mediterranean Diet to Improve Mitochondrial Function in Experimental Models of Obesity and Metabolic Syndrome. . Nutrients 2022, 14, 3112. [CrossRef] [PubMed]
- Lee KW; Kang MS; Lee SJ; Kim HR; Jang KA; D., S. Prospective Associations between Dietary Patterns and Abdominal Obesity in Middle-Aged and Older Korean Adults. Foods 2023, 12, 2148. [CrossRef] [PubMed]
- Fabiani R; Naldini G; M., C. Dietary Patterns and Metabolic Syndrome in Adult Subjects: A Systematic Review and Meta-Analysis. . Nutrients 2019, 11, 2056. [CrossRef] [PubMed]
- Kim SA; Joung HJ; SA., S. Dietary pattern, dietary total antioxidant capacity, and dyslipidemia in Korean adults. Nutr J 2019, 18, 37. [CrossRef]
- Hehua Z; Yang X; Qing C; Shanyan G; Z., Y. Dietary patterns and associations between air pollution and gestational diabetes mellitus. Environ Int 2021, 147, 106347. [CrossRef]
- Society, T.K.N. Dietary Reference Intakes for Koreans (KDRIs). 2015.
- Group, C.-C.F.E.N.S. Cohort Profile: The French E3N Cohort Study. Int J Epidemiol 2015, 44, 801-809.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).