1. Introduction
One of the rarest forms of inflammatory periodontal disease is the Stage IV grade C localized periodontitis of small child (i.e., pre-puberal localized aggressive periodontitis-LPP/localized juvenile periodontitis) which usually manifests in clinical healthy individuals [
1]. A prevalence of 0.06% for the whites Europeans is cited [
2], but without any reference to the patient’s age (which clinically is of extreme importance since both treatment and communication difficulties related to the young age). This form of periodontal disease has been reported to affect the young age individuals (without reporting any age interval), with an extremely rapid progression of bone and periodontal loss (i.e., an “U” shape bone loss radiological aspect which appears in 2-4 month [
2,
3,
4,
5,
6]) affecting mostly the first molars and incisors [
2,
3,
7,
8,
9,
10,
11,
12,
13].
The onset is insidious in most of the patients, the younger they are, the lower the chance of being noticed and therapeutically intercepted [
1]. The children are complaining of some minor or mild pain/discomfort, and after a while they begin to observe a limited mobility of the affected tooth. At close inspection the young patient shows almost no/low oral plaque deposits or inflammation signs (i.e., pathognomonic signs) around or surrounding the affected tooth/teeth [
1]. In such situations the radiological examination is rarely performed, thus, most of the periodontal loss is missed. In a short period of time (usually a few months) the tooth mobility becomes advanced (i.e., tooth is lost), the periodontal destruction is clinically visible, and the window of opportunity for conservative treatment is missed. To the best of our knowledge there are only few cases reported in literature (mostly for adolescents), and none for pre-puberal children [
1].
The main problem regarding the treatment of this form of periodontitis is related to the correct diagnostic, since the rarity of the disease, the lack of clinical sings and the difficulties in communication with the young patients, thus most of the clinical practitioners dismiss the case. That is why most of them pass unnoticed or misrecognized (mistaken for autoimmune, metabolic, endocrine diseases, etc.) [
1,
7,
14]. It has been reported to affect both dentitions (i.e., pre-puberal periodontitis affecting the first primary molars; juvenile periodontitis affecting the first permanent molars and incisors) [
2], seeming to confirm the genetic familial aggregation [
8]. Nevertheless, other teeth can also be involved (e.g., primary canine) [
1].
Periodontitis is a multifactorial disease, some of the mechanisms being still a debatable subject. This form of localized periodontitis is also multifactorial, with the main mechanism being an abnormal/hyper-immune response to periodontopathic bacteria and showing familial aggregation [
6,
7,
9,
14,
15]. Usually there is a symbiotic relationship between the oral biofilm and bacterial community [
14]. However, for some reasons (not yet identified, supposedly genetic predisposition and/or environmental factors [
8]) an imbalance occurs leading to an aggressive auto-immune response resulting in bone and periodontal loss due to disruptions of the bone metabolism [
1,
7,
14]. The abnormal/hyperimmune response is triggered by both the plaque biofilm (pathogenic bacteria embedded in extracellular polymeric substance with increased resistance to microbial agents and immune defenses) and planktonic counterparts (dispersed pathogenic bacteria with higher virulence) [
6,
16,
17]. Nevertheless, the local oral environment highly influences the bacterial virulence, but the rapid changes in nutrients, oxidative stress, mechanical disruptions of the oral plaque are difficult to be studied and not yet fully understood [
1,
6,
16,
17,
18]. Some studies reported Fusobacterium nucleatum (less periodontal loss if levels are reduced), Porphyromonas gingivalis, Prevotela intermedia, Treponema denticola and Tannerella forsythia as high periodontopathic bacteria [
16,
18]. Other studies reported also Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis to trigger the hyper-immune response [
8,
18]. Blood tests also reported in LPP patients higher levels of B lymphocytes [
1,
7,
19]. Thus, the importance of performing both bacterial and blood test when assessing LPP cases is obvious [
1].
In LPP patients due to genetic predisposition [
1,
15], the hyper-immune response is quick, aggressive and disproportionate, with severe localized inflammation and tissular resorption with significant and visible effects in a very short period of time [
7,
15,
19,
20]. This excessively aggressive hyperimmune response is triggered not only by periodontopathic bacteria levels but also by currently unknown environmental factor [
1,
7,
14,
15,
20].
The treatment of LPP patients is challenging especially due to their young age and difficulties in communicating with them and rapid tissular destruction progression [
1]. The aims are to stop the periodontal loss and conserve the tooth/teeth and regain the bone and periodontal ligament loss, to reduce the periodontopathic bacterial levels in order to reduce the hyper-immune response and regain the oral bacterial balance [
1,
3,
8,
10,
21]. The most important aspect of the treatment is to establish the correct diagnosis based on both clinical and radiological examination and periodontopathic bacterial and blood tests [
1,
8,
10,
21,
22,
23]. The LPP treatment is mostly conservative (scaling, root planning, associated with systemic antibiotics) since the young age of the patients [
1,
3,
8,
10,
13,
21,
23,
24]. The most effectively used antibiotic association is metronidazole and amoxicillin combinations, showing better results with systemic than topical administration [
1,
3,
8,
10,
13,
21,
24,
25]. The “24 hours full-mouth disinfection” concept also improved the clinical prognostic [
3,
11].
The most common LPP misdiagnoses are made with genetic and metabolic disorders, since the young age of the patients, the poor objective symptoms (i.e., especially in the early stages of the LPP disease), rarity of disease and lack of clinical experience. Nevertheless, if properly investigated (radiological examination, periodontopathic bacteria test, and blood count), all the elements necessary for clinical diagnosis begin to appear [
1]. Among bone metabolic diseases hypophosphatasia is one of the common candidates for the misrecognition of LPP. It has a low prevalence of 1 to 300000 in Europe being caused by the deficiency in alkaline phosphatase activity and ALPL gene mutation, involving the calcium and phosphate metabolism [
26]. Two main forms are reported odonto-hypophosphatasia and systemic hypophosphatasia, both related to defective mineralization of bone due to lack of calcium fixation despite a normal absorption [
26,
27]. The odonto-hypophosphatasia displays dental abnormalities, premature loss of primary teeth, dental caries, reduced dentine thickness, reduced alveolar bone, associated with low levels of parathyroid hormone (due to hypercalcemia and hypercalciuria that would lead to the development of hyperphosphatasemia) [
26,
27].
Herein aim was to report the evolution without and with adequate therapy, of a 4-years-old Caucasian girl case, with misrecognized LPP (for half year), localized advanced periodontal loss around lower temporary canines, poor objective symptoms, and atypical localization.
2. Materials and Methods
The herein is an unusual case (given both the young age and the involved tooth) of 4 years-old healthy Caucasian girl with a known history of familial periodontal problems (manifested especially in grandparents generation). The first subjective and objective symptoms were poor, appearing in the early months of 2023 (February-March), with complaints of small to moderate pains in various places of the oral cavity, lasting a few minutes. After a quick intra-oral clinical examination, the local dentist (i.e., a small suburb of München, Germany) dismisses the case since nothing abnormal was seen. The pain complaints continued and in June the family observed that the lower left temporary canine (i.e., 7.3) began to move. The parents returned to the local dentist who, after another quick clinical examination sent the case to München University Hospital (Periodontology Department) [
1].
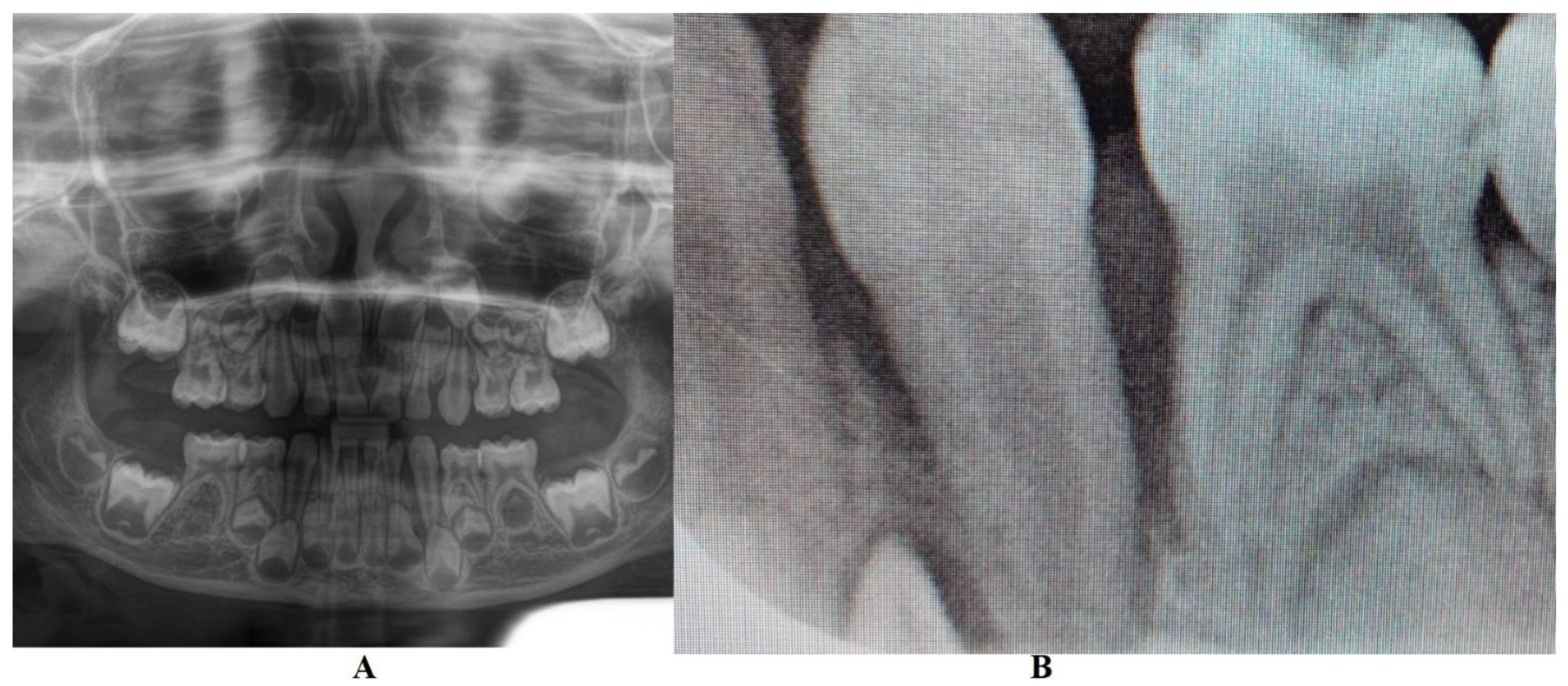
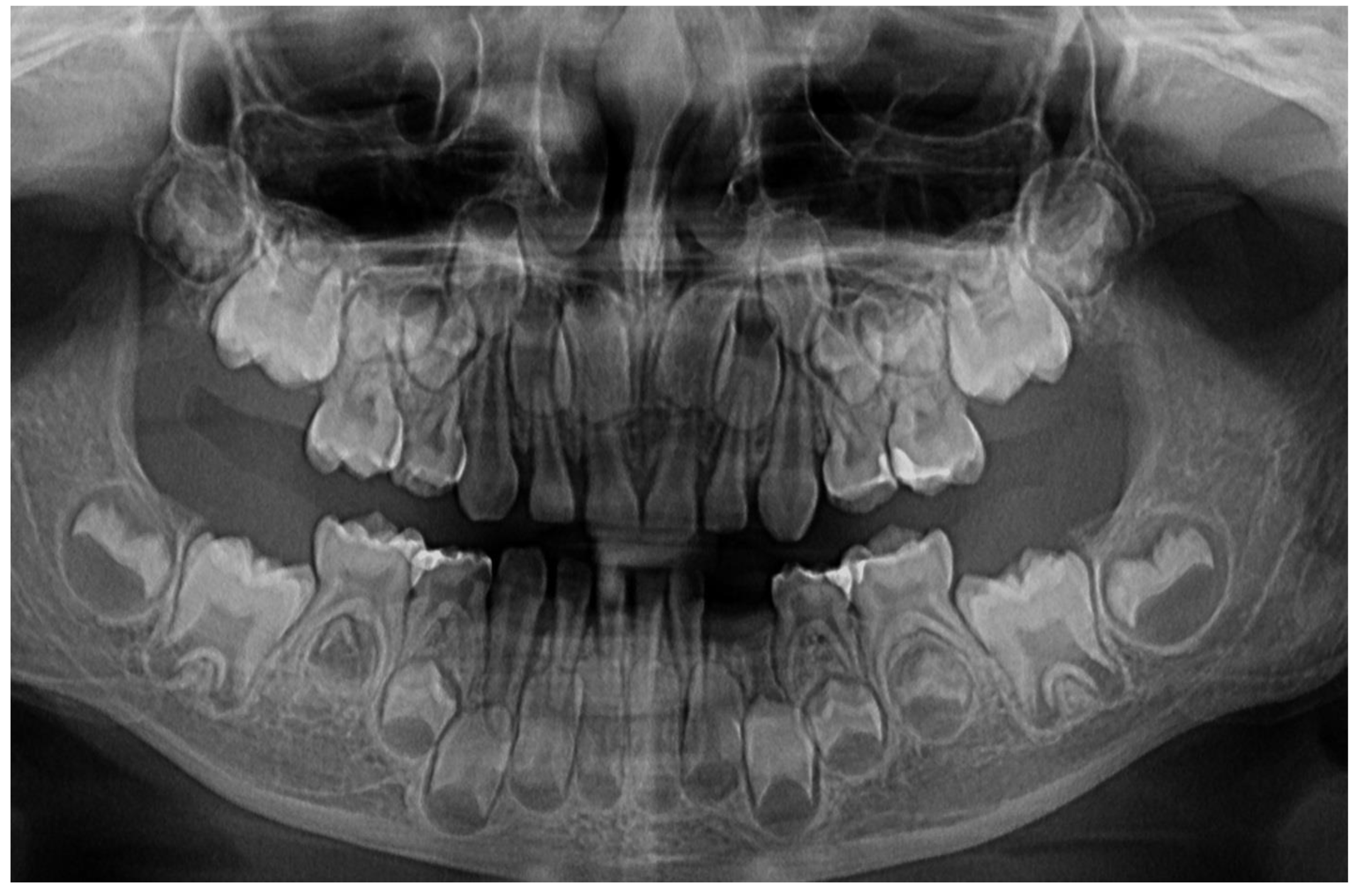
In München University Hospital (Periodontology Department, 14th of June) a full oral clinical and paraclinical examination was performed including x-ray examinations (retro-alveolar and panoramic) (
Figure 1). As a result, the temporary left canine movement and a localized bone loss around the above-mentioned tooth were confirmed, with suspicion of a metabolic disease (i.e., hypophosphatasia/hyperphosphatasia) while the pain complaints were cataloged of unknown cause. No other problems were identified (e.g., orthodontic disorders, hyper-eruption, etc.). The case was dismissed with no prescribed therapy, no clinical diagnosis, and only common oral hygiene instructions. The recommendations were to continue the investigations in the Endocrinology Department for blood and genetic tests [
1].
In Endocrinology Department (München University Hospital, 19th of June) the blood test showed (20th of June) only a slight monocytes and lymphocytes increase and neutrophile granulocytes decrease, all other constituents being within age-specific physiological parameters. The urine teste come negative (21st of June). The genetic tests (taken 17th of June, received by parents in late September 2023), come negative disconfirming the initial suspicion of hypophosphatasia [
1].
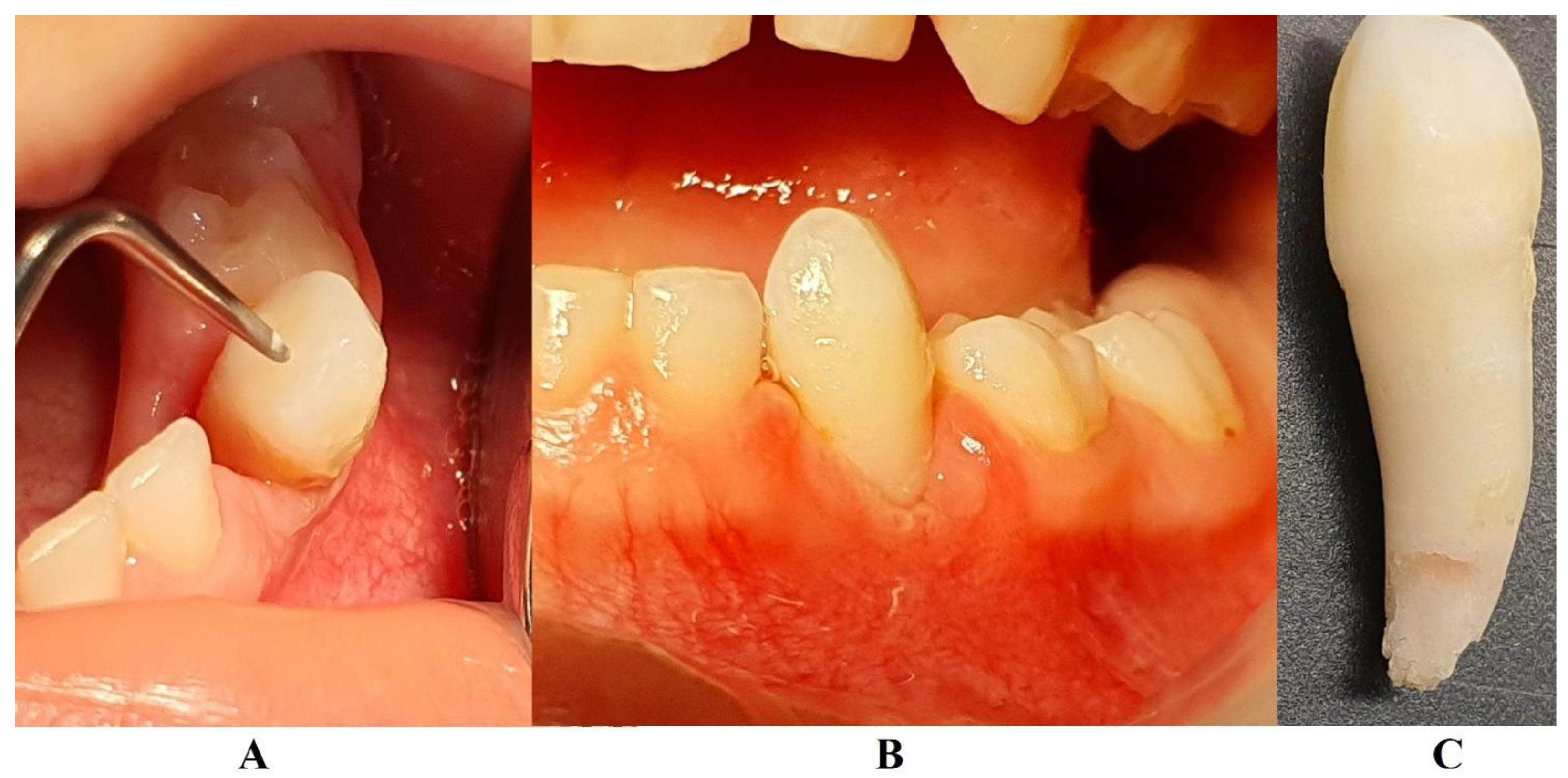
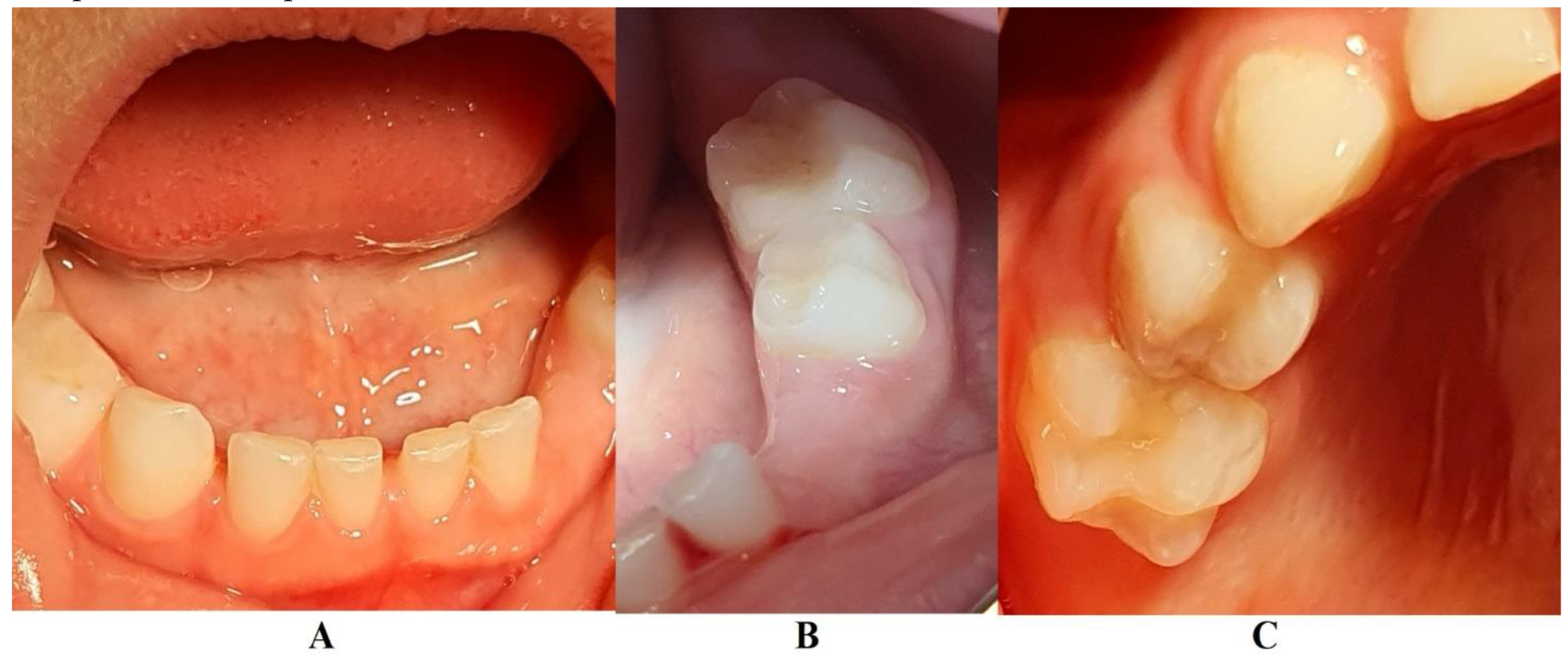
In August 2023, the family consulted another clinician abroad (2nd of August, Klausenburg/Cluj-Napoca - Romania) since parent’s concerns related to deteriorating the local conditions (inflamed periodontium around lower temporary canine and enhanced movement,
Figure 2). The parents presented the entire documentation gathered from previous examinations including paraclinical tests results and x-rays [
1]. After reviewing familial history and clinical and paraclinical examinations (3rd of August) the initial hypophosphatasia/hyperphosphatasia suspicions were dismissed (also confirmed by late September 2023 genetic test negative results) and the Stage IV grade C localized periodontitis/pre-puberal localized aggressive periodontitis-LPP was made. No orthodontic problems were detected during the clinical examination (also confirmed by previous clinical exams), and only the hyper-eruption of the temporary left canine due to advanced periodontal loss and surrounding inflammation (
Figure 2) [
1]. When setting up the LPP diagnosis, the previous x-rays (
Figure 1) were taken into consideration along with girls other paraclinical results. For laboratory confirmation, a periodontopathic bacteria sample was taken (3rd of August). Since the advanced localized periodontal loss and tooth movement only professional hygiene and adjuvant topical applications with amoxicillin and metronidazole association around the inflamed periodontal pocket for a period of 10 days was prescribed. The parents were informed about all these above-mentioned issues, and the most-likely loss of the tooth but also the necessity of instituting a general antibiotic treatment and follow-up of the case. The family left Klausenburg (Romania) 5th of August. The periodontopathic bacteria test came highly positive on the 16th of August 2023 with Fusobacterium nucleatum/periodonticum and Capnocytophaga. The tooth was finally lost in early September (
Figure 2) [
1].
In late October 2023 (i.e., 23rd of October), the temporary lower right canine (i.e., 8.3) showed a localized inflammation of the surrounded periodontium and mobility. They consulted one more time the local dentist (i.e., a small suburb of München, Germany), who confirmed the localized periodontal inflammation around 8.3 and its mobility, but also mobility signs of 5.3, temporary upper right incisors (i.e., 5.2 and 5.1), and temporary lower incisors (i.e., 8.1, 8.2, 71, and 7.2) but no inflamed periodontium. The recommendation was antibiotic treatment with Augmentin of 200 mg (600mg/day, one spoon three times/day) for 10 days, and one week after the treatment ceased a periodontopathic bacterial test was taken, that came with negative results.
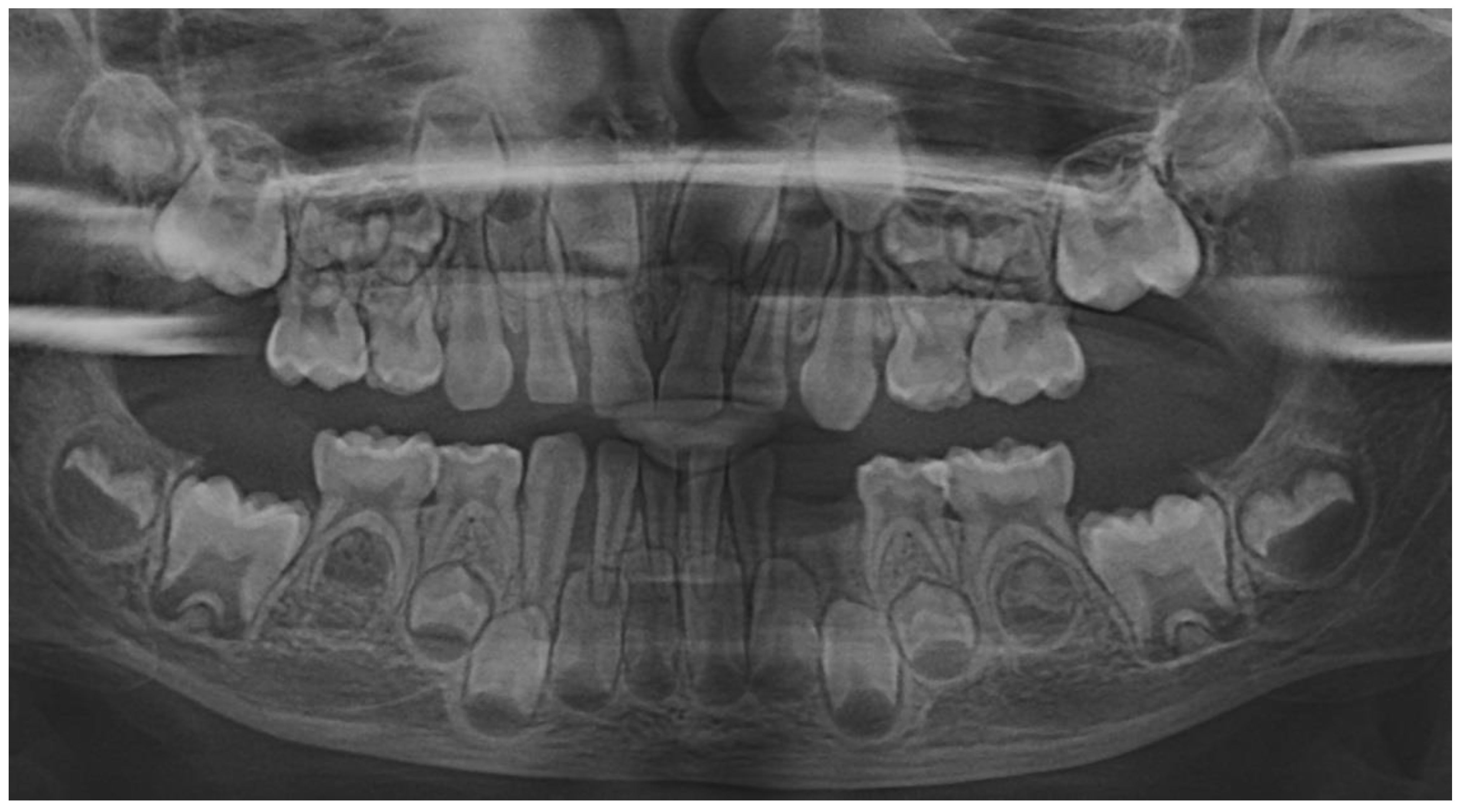
The family kept in touch with the clinician who diagnosed the LPP disorder informing about the case evolution, and in late December 2023 the family returned to Klausenburg (Cluj-Napoca – Romania). The panoramic x-ray (
Figure 3) confirmed the presumed advanced periodontal loss around 8.3, but also bone loss around upper temporary canine and incisors (i.e., 5.3, 5.2 and 5.1), when compared with previous panoramic from June 2023 (
Figure 1). Another investigated aspect was the lower left region of previous troubles, with no radiological signs of definitive canine sufferance (i.e., 3.3) and visible signs of bone regeneration. The oral clinical examination confirmed the lack of dental plaque and calculus, the advanced mobility of temporary lower canine, a light mobility of the other above-mentioned teeth and healing of the lost temporary lower left canine area (
Figure 4). Another periodontopathic bacterial test was taken to reflect the latest bacterial types. Nevertheless, an oral professional cleaning was performed and based on previous LPP positive diagnosis, positive periodontopathic bacterial test and clinical evolution, a systemic antibiotic treatment was prescribed. The prescribed antibiotic (girl’s weight was about 15 kg) association was of Augmentin of 500 mg (1g/day, 1table twice a day) and Metronidazole of 250mg (500 mg/day, 1 table twice a day, but with the need to adjust the dosage if allergy/overdosed signs were to be displayed) for a period of 10 day, a periodontopathic bacterial test 2-3 months after the treatment ceased.
The family returned home and the young patient (who turned 5 years old by now) started the antibiotic treatment immediately in early January (i.e., 8th of January) 2024, remaining in contact with the Klausenburg clinician. Metronidazole daily dosage (despite being at the upper limit) induced vomiting after the second day (i.e., 10th of January), and it was decided to reduce by half the daily dosage to 250 mg/day (from initial 500 mg/day), fragmented in two (125 mg twice a day, taken together with the Augmentin dose). After this dosage adjustment no other incident appeared, finishing the treatment on the 18th of January.
The periodontopathic bacterial test taken before the antibiotic prescription (i.e., late December 2023) came highly positive with five types of bacteria (when compared with only two types in the previous test received 16th of August 2023). Thus, besides the two types present in first test (i.e., 16th of August 2023) Fusobacterium nucleatum/periodonticum and Capnocytophaga already present in the oral cavity (and responsible for the loss of 7.3 and localized periodontal destruction) who remained highly positive, another three types showed up mildly positive: Treponema denticola, Eubacterium nodatum, Eikenella corrodens. Confirming not only the clinical worsening of the oral condition (
Figure 3 and
Figure 4), seen during oral examination (late December 2023) but also the chosen antibiotic association scheme.
Meanwhile (18th of January) the immunology test came (taken 23rd of November 2023) positive with high levels of IgE (i.e., 828 U/ml when physiological normal is below 60 U/ml) and a light increase for CPR (i.e., 1.2 mg/dl instead of 1 mg/dl), reticulocytes (i.e., 17 Promille instead of 15 Promille), MPV (11.5 ft instead of 11 ft), eosinophile granulocytes (4 % instead of 3 %) and monocytes (0.9 G/l instead of 0.77 G/l), confirming one more the initial diagnosis of LPP.
3. Results
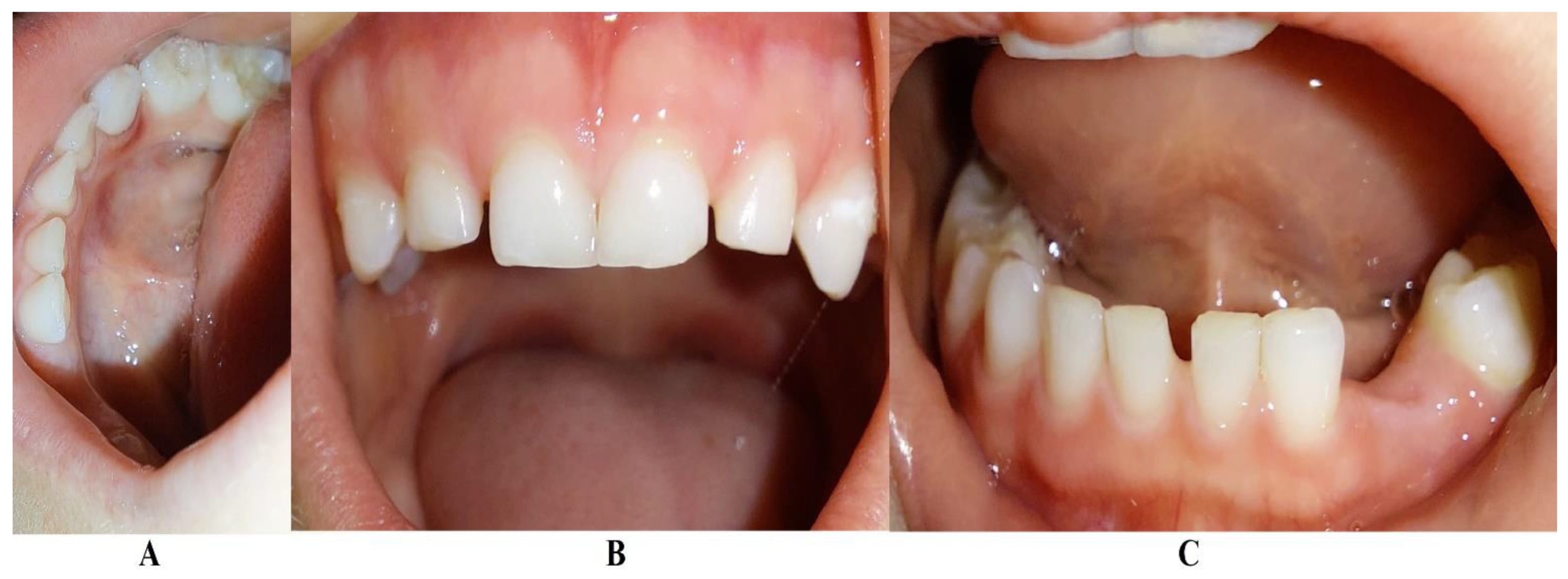
In early May 2024 (i.e., 15th of May 2024) the family returned to Klausenburg (Cluj-Napoca – Romania), for a three-month control and evaluation. The clinical examination showed physiological mobility of all previous involved teeth, with no signs of periodontal inflammation and no plaque/calculus (
Figure 5). No reports of pain complaints were reported (The last one being reported in October-November 2023). The panoramic radiological examination (
Figure 6) showed a spectacular and visible periodontal regeneration and gain around 8.3 (that previously, in late December 2023, showed high periodontal loss), with visible periodontal gain around the upper and lower incisors. The definitive teeth showed physiological normality (especially the 3.3, closer to the periodontal loss site). A periodontopathic bacteria test was also taken (17th of May). The results (31st of May) revealed the presence of only “precursor germs of highly pathogenic ones” but with “no detectable highly pathogenic germs”. Nevertheless, the revealed precursor germs were those of Fusobacterium nucleatum/periodonticum, Campylobacter rectus, Eubacterium nodatum, Eikenella corrodens, and Capnocytophaga spp. The results at three months were considered to be favorable, meanwhile a 6 month’s control was scheduled for the autumn of 2024. However, up to early August 2024 (when herein report was written) no LPP reactivation signs showed up and no more pain complaints were reported.
4. Discussion
Herein report describes a case of Stage IV grade C localized periodontitis (pre-puberal localized aggressive periodontitis/LPP) to a 4-years-old Caucasian girl, as well as its evolution over a period of one and half year (i.e., early January 2023 up to early August 2024). No similar case was found in current research flow [
1].
The unusualness of this case is due to several aspects. The first is related to the extremely young age of the patient (i.e., 4 years-old in February 2023, temporary dentition), with an insidious onset, and apparently no familial aggregation or history. The second aspect is related to the misdiagnose to metabolic diseases (i.e., hypophosphatasia/hyperphosphatasia) despite the initial radiographical (i.e.,
Figure 1) and clinical examination that suggested a clear picture of an unusual LPP (e.g., the lower left canine involvement). The third aspect is related to the lack of therapeutic measures that rapidly set the course for the periodontal and 7.3 loss, in an interval of around 7 months (
Figure 2) and the further progression involving other oral sites (
Figure 3 and
Figure 4). The fourth aspect is related to the evolution under adequate treatment (despite no written report to guide it) and the periodontal gain over a period of around 8 months following the antibiotic therapy (
Figure 5 and
Figure 6).
The young age of the patient (4-years-old) led to a superficial consideration of oral pain reports (for a period of 5 months, February-June) backed up by no clinical elements of concern found by the first dentist who approached the case. However, no mobility tests reported for the temporary teeth and especially no radiographical examination were taken (even for preventive dentistry reasons) despite the repeated reported pain complaints.
In order to be able to correctly diagnose herein case, it must be emphasized that LPP is a very rare form of periodontal disease usually with familial aggregation (despite here, where this aspect was negative) [
1,
2,
3,
7,
8,
9,
10,
11,
12,
13,
21,
22,
23,
24,
25]. The pathogenic mechanism relies on a hyper-immune disproportionate aggressive response to periodontopathic bacteria [
6,
16,
17,
18] found in the oral cavity that lead to rapid bone metabolism disruptions soon followed by periodontal and tooth loss [
1,
2,
6,
7,
8,
9,
10,
14,
15,
19,
20]. There is no available treatment of the condition [
1]. Moreover, it is not fully understood and recognized (since almost no scientific data are available), and from this point of view herein report brings new data to clinical treatment field [
1]. Since no changes in how the hyper-immune mechanism can be performed (especially in a 4-years-old), the only possibility resides in managing the trigger factor (i.e., the amount of periodontopathic bacteria found in the oral cavity) [
1]. To achieve this, the bacterial types and their amount must be investigated.
The clinical examination as well as the complementary radiological one could point out the LPP diagnosis, despite the unusual involvement and limitation to temporary canines (the literature reports the involvement of molars and incisors) [
2,
3,
7,
8,
9,
10,
11,
12,
13]. The initial “U” shape bone loss [
2,
3,
4,
5,
6] and limitation to 7.3 site can easily exclude a general metabolic disease (where usually there is no such limitation). The hypophosphatasia/hyperphosphatasia misdiagnosis (i.e., June 2023) can be easily rejected since the blood count and urine test displayed no such signs (i.e., high phosphate serum levels, low levels of parathyroid hormone due to hypercalcemia and hypercalciuria) [
26,
27]. The initial blood test (20th of June 2023) revealed a slight increase in monocytes and lymphocytes, and a small decrease in neutrophile granulocytes, perfectly normal in case localized inflammation due to bacterial infection.
The rapid periodontal loss which characterizes the LPP means that the treatment window of opportunity is very tight. In this case a period of 5 months was lost, with no diagnosis, and no treatment, which lead to rapid periodontal loss and temporary left canine loss.
The only practical approach in such cases is to address the triggering factor, i.e. the large number of bacteria that trigger the aggressive hyper-immune response [
6,
16,
17,
18]. Thus, a simple periodontopathic bacteria test can easily confirm the LPP diagnosis (as it did here, in August 2023). Based on the bacterial type a proper antibiotic association can be established (usually the amoxicillin with metronidazole) [
1,
3,
8,
10,
13,
23,
25]. Nevertheless, due to the young age of the patient, the dosage must be approached with care. Another aspect that needs to be addressed with elegance and tact is communication with the parents, because of the previous negative experiences in dealing with the case (i.e., varying degrees of mistrust). A rational scientific evidence-based approach might help.
The anticipated progression of LPP under lack of treatment and its consequences must be communicated to the family (as here, in August 2023, and unfortunately correctly anticipated) since the legal consequences could be significant. Nevertheless, the treatment must also be based on scientific data and under completely understanding of the pathogenic mechanisms (i.e., previous incorrect prescription both in terms of antibiotic type and dosage, October 2023, Augmentin 600 mg/day). The periodontopathic bacterial test recommendation (to be repeated after a period of two-three months after the antibiotic ceases) has a strong scientific point and must be followed. If to repeat the test a week after the antibiotic stops (as incorrectly was performed), the test will only show a biased result (i.e., as it did herein for the October 2023 test).
The natural periodontal gain observed in this case is important from the prognosis point of view. It was observed that the site of the lost 7.3 showed periodontal gain (i.e.,
Figure 3, the panoramic form early January 2024), in the absence of the correct antibiotic association (since solely Augmentin did not cover all pathological bacterial types). However, it must be emphasized that it cannot be predicted the impact on this matter of the above-mentioned incorrect antibiotic treatment performed in October 2023 (i.e., no signs of other periodontal gain on upper and lower incisors and only progression of the periodontal loss around the 8.3). Nevertheless, significant periodontal gain was observed on the last panoramic (
Figure 6, May 2024) after the correct antibiotic association.
The aim of the treatment was only to minimize the triggering factor (the bacterial amount) of the aggressive hyper-immune response in order to minimize the periodontal loss of LPP (which was achieved), and to maintained it for a variable period of time (in order to buy time). To completely remove the periodontopathic bacteria in this case seems to be not possible (as the evolution showed) both due to unknow nature of the LPP disease, mechanism, bacterial origin, and proliferation and not the last the lack of knowledge related to this subject.
5. Conclusions
The rapid diagnosis of LPP is essential in order not to miss the treatment window of opportunity, to preserve/minimize the periodontium/periodontal loss.
The diagnosis should be essentially based on the clinical and radiological examination and supported by paraclinical test. Moreover, repeated pain complaints (even from a 4-year-old) should be taken seriously and not easily dismissed.
The periodontopathic bacterial test is essential to have a workable antibiotic association protocol, as well as assessing the LPP progression.
The herein periodontal gain proves that even at a young age under adequate treatment LPP can be kept under control providing correct and physiological development from both orthodontic and periodontic point of view.
6. Clinical and Scientifical Significance
Since the rarity of the Stage IV grade C localized periodontitis/pre-puberal localized aggressive periodontitis-LPP (0.06%) and the difficulty related to following the clinical case, there are no available data in the current research flow about pre-puberal cases. Moreover, little is known regarding the adequate workable treatment (for pre-puberal) as well as the evolution with and without treatment. Another aspect is related to the knowledge needed to establish the correct clinical diagnosis. Herein, is the first to addresses the above-mentioned issues, assessing the evolution of a 4-year-old Caucasian girl with LPP (misdiagnosed with metabolic disease for almost half a year), with periodontal loss due to lack of proper treatment for about a year, and then the favorable evolution with correct treatment for more than 8 moths.
Author Contributions
Conceptualization: R.A.M.; Methodology: R.A.M.; Software: R.A.M.; Validation: R.A.M., and C.D.O.; Formal analysis: R.A.M.; Investigation: R.A.M.; Resources: R.A.M.; Data Curation: R.A.M.; Writing-original draft preparation: R.A.M.; Writing-review and editing: R.A.M., and C.D.O.; Visualization- Supervision- Project administration: R.A.M., and C.D.O.; Funding acquisition: R.A.M., and C.D.O. All authors have read and agreed to the published version of the manuscript.
Funding
The authors were the funders of this research project.
Institutional Review Board Statement
The study was conducted in accordance with the 362 Declaration of Helsinki. It does not require approval by the Institutional Review Board or 363 Ethics Committee since the current Regulation of the Ethics Committee of the Higher 364 Institute of Health stipulates that projects with epidemiological, medico-social and 365 evaluative contents need evaluation, approval and monitoring of trial protocols only if 366 they contain personal data according to the legislative decrees on clinical trials and 367 function of the ethics committees (decreto legislativo 24 giugno 2003, n.211; decreto 368 ministeriale 8 febbraio 2013). The official definition of “personal data” is given by the 369 Medicina 2023, 59, x FOR PEER REVIEW 12 of 14 National Data Protection Authority (Garante per la Protezione dei Dati Personali, 370
https://www.garanteprivacy.it/home/diritti/cosa-intendiamo-per-dati-personali— 371 Regolamento (UE) 2016/679 art.9 (accessed on 07 May 2023). The term “personal data” 372 includes information about first and last name, images, social security code, IP address 373 and license plate number. The images and radiographs in the manuscript are anonymized 374 for this reason. Parents gave oral informed consent to participate in the study. However, this manuscript is part of a larger study involving periodontics and orthodontics issues, which research protocol has been approved by the Ethical Committee of the University of Medicine (158/2.04.2018).
Informed Consent Statement
Oral informed consent has been obtained from the parents of the children to publish this paper.
Data Availability Statement
All needed data are displayed herein.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Moga, R. A.; Olteanu, C. D.; Delean, A. G. , Case Report of a 4-Year-Old Girl with Stage IV Grade C Localized Periodontitis (Pre-Puberal Localized Aggressive Periodontitis) Affected by Misrecognition and Late Diagnosis. Journal of clinical medicine 2024, 13. [Google Scholar] [CrossRef] [PubMed]
- Merchant, S. N.; Vovk, A.; Kalash, D.; Hovencamp, N.; Aukhil, I.; Harrison, P.; Zapert, E.; Bidwell, J.; Varnado, P.; Shaddox, L. M. , Localized aggressive periodontitis treatment response in primary and permanent dentitions. Journal of periodontology 2014, 85, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Kim, T. J.; Littlejohn, C. G.; Richey, K. H.; Falsafi, N.; Li, C.; Wang, T. J.; Lander, B.; Chang, Y. C. , A Modern Approach to Treat Molar/Incisor Pattern Periodontitis-Review. Journal of clinical medicine 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Moga, R. A.; Olteanu, C. D.; Buru, S. M.; Botez, M. D.; Delean, A. G. Cortical and Trabecular Bone Stress Assessment during Periodontal Breakdown-A Comparative Finite Element Analysis of Multiple Failure Criteria. Medicina (Kaunas, Lithuania) 2023, 59. [Google Scholar] [CrossRef]
- Moga, R.-A.; Olteanu, C. D.; Delean, A. G. , Investigating the Ability of the Tooth and Surrounding Support Tissues to Absorb and Dissipate Orthodontic Loads during Periodontal Breakdown—Finite Elements Analysis. Applied Sciences 2024, 14, 1041. [Google Scholar] [CrossRef]
- Gonçalves, P. F.; Huang, H.; McAninley, S.; Alfant, B.; Harrison, P.; Aukhil, I.; Walker, C.; Shaddox, L. M. , Periodontal treatment reduces matrix metalloproteinase levels in localized aggressive periodontitis. Journal of periodontology 2013, 84, 1801–1808. [Google Scholar] [CrossRef]
- Harris, T. H.; Wallace, M. R.; Huang, H.; Li, H.; Mohiuddeen, A.; Gong, Y.; Kompotiati, T.; Harrison, P.; Aukhil, I.; Shaddox, L. M. , Association of P2RX7 functional variants with localized aggressive periodontitis. Journal of periodontal research 2020, 55, 32–40. [Google Scholar] [CrossRef]
- Teughels, W.; Dhondt, R.; Dekeyser, C.; Quirynen, M. , Treatment of aggressive periodontitis. Periodontology 2000 2014, 65, 107–133. [Google Scholar] [CrossRef]
- Papapanou, P. N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D. H.; Flemmig, T. F.; Garcia, R.; Giannobile, W. V.; Graziani, F.; Greenwell, H.; Herrera, D.; Kao, R. T.; Kebschull, M.; Kinane, D. F.; Kirkwood, K. L.; Kocher, T.; Kornman, K. S.; Kumar, P. S.; Loos, B. G.; Machtei, E.; Meng, H.; Mombelli, A.; Needleman, I.; Offenbacher, S.; Seymour, G. J.; Teles, R.; Tonetti, M. S. , Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. Journal of clinical periodontology 2018, 45 Suppl 20, S162–s170. [Google Scholar]
- Guha Biswas, P.; Mohan, A.; Kandaswamy, E. , Treatment of Periodontitis Affecting Human Primary Teeth-A Systematic Review. Dentistry journal 2023, 11. [Google Scholar] [CrossRef]
- Păunica, S.; Giurgiu, M. C.; Ciongaru, D. N.; Pădure, C. E.; Albu Ș, D.; Pițuru, S. M.; Dumitriu, A. S. Clinical Aspects and Therapeutic Management of an Aggressive Manifestation of Stage III Grade C Periodontitis in a Female Teenager. Diagnostics (Basel, Switzerland) 2023, 13. [Google Scholar] [CrossRef]
- Skalerič, E.; Petelin, M.; Gašpirc, B. , Antimicrobial photodynamic therapy in treatment of aggressive periodontitis (stage III, grade C periodontitis): A comparison between photodynamic therapy and antibiotic therapy as an adjunct to non-surgical periodontal treatment. Photodiagnosis and photodynamic therapy 2023, 41, 103251. [Google Scholar] [CrossRef] [PubMed]
- Branco-de-Almeida, L. S.; Velsko, I. M.; de Oliveira, I. C. V.; de Oliveira, R. C. G.; Shaddox, L. M. , Impact of Treatment on Host Responses in Young Individuals with Periodontitis. Journal of dental research 2023, 102, 473–488. [Google Scholar] [CrossRef]
- Harris, T. H.; Wallace, M. R.; Huang, H.; Li, H.; Shaddox, L. M. , Associations of P2RX7 Functional Diplotypes with Localized Aggressive Periodontitis. JDR clinical and translational research 2019, 4, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Shaddox, L. M.; Spencer, W. P.; Velsko, I. M.; Al-Kassab, H.; Huang, H.; Calderon, N.; Aukhil, I.; Wallet, S. M. , Localized aggressive periodontitis immune response to healthy and diseased subgingival plaque. Journal of clinical periodontology 2016, 43, 746–753. [Google Scholar] [CrossRef]
- Ding, Q.; Tan, K. S. , The Danger Signal Extracellular ATP Is an Inducer of Fusobacterium nucleatum Biofilm Dispersal. Frontiers in cellular and infection microbiology 2016, 6, 155. [Google Scholar] [CrossRef]
- Skurska, A.; Dolinska, E.; Pietruska, M.; Pietruski, J. K.; Dymicka, V.; Kemona, H.; Arweiler, N. B.; Milewsk, R.; Sculean, A. , Effect of nonsurgical periodontal treatment in conjunction with either systemic administration of amoxicillin and metronidazole or additional photodynamic therapy on the concentration of matrix metalloproteinases 8 and 9 in gingival crevicular fluid in patients with aggressive periodontitis. BMC oral health 2015, 15, 63. [Google Scholar]
- Ding, Q.; Quah, S. Y.; Tan, K. S. , Secreted adenosine triphosphate from Aggregatibacter actinomycetemcomitans triggers chemokine response. Molecular oral microbiology 2016, 31, 423–434. [Google Scholar] [CrossRef]
- Berglundh, T.; Wellfelt, B.; Liljenberg, B.; Lindhe, J. , Some local and systemic immunological features of prepubertal periodontitis. Journal of clinical periodontology 2001, 28, 113–120. [Google Scholar] [CrossRef]
- Meng, H.; Xu, L.; Li, Q.; Han, J.; Zhao, Y. , Determinants of host susceptibility in aggressive periodontitis. Periodontology 2000 2007, 43, 133–159. [Google Scholar] [CrossRef]
- Morikawa, T.; Ishii, T.; Goto, H.; Motegi, E.; Nishii, Y. , A Case of Orthodontic Treatment for Generalized Aggressive Periodontitis. The Bulletin of Tokyo Dental College 2021, 62, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Putrino, A.; Caputo, M.; Galeotti, A.; Marinelli, E.; Zaami, S. Type I Dentin Dysplasia: The Literature Review and Case Report of a Family Affected by Misrecognition and Late Diagnosis. Medicina (Kaunas, Lithuania) 2023, 59. [Google Scholar] [CrossRef] [PubMed]
- Motta, A. , Orthodontic treatment in the presence of aggressive periodontitis. Dental press journal of orthodontics 2021, 26, e21bbo6. [Google Scholar] [CrossRef] [PubMed]
- Khattri, S.; Kumbargere Nagraj, S.; Arora, A.; Eachempati, P.; Kusum, C. K.; Bhat, K. G.; Johnson, T. M.; Lodi, G. , Adjunctive systemic antimicrobials for the non-surgical treatment of periodontitis. The Cochrane database of systematic reviews 2020, 11, Cd012568. [Google Scholar] [PubMed]
- Casarin, R. C.; Peloso Ribeiro, E. D.; Sallum, E. A.; Nociti, F. H., Jr.; Gonçalves, R. B.; Casati, M. Z. , The combination of amoxicillin and metronidazole improves clinical and microbiologic results of one-stage, full-mouth, ultrasonic debridement in aggressive periodontitis treatment. Journal of periodontology 2012, 83, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Martos-Moreno, G. A.; Calzada, J.; Couce, M. L.; Argente, J. , [Hypophosphatasia: Clinical manifestations, diagnostic recommendations and therapeutic options]. Anales de pediatria 2018, 88, e1–e356. [Google Scholar] [CrossRef]
- Silve, C. , Hereditary hypophosphatasia and hyperphosphatasia. Current opinion in rheumatology 1994, 6, 336–339. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).