Preprint
Article
Uremic Toxins and Inflammation: Metabolic Pathways Affected in Patients Undergoing Renal Replacement Therapies
Altmetrics
Downloads
187
Views
56
Comments
0
A peer-reviewed article of this preprint also exists.
supplementary.xlsx (110.39KB )
Submitted:
25 July 2024
Posted:
25 July 2024
You are already at the latest version
Alerts
Abstract
3.9 million individuals rely on kidney replacement therapy worldwide. They experience height-ened susceptibility to cardiovascular diseases and mortality, alongside an increased risk of in-fections and malignancies, with inflammation being key to explaining this intensified risk. This study utilized semi-targeted metabolomics to explore novel metabolic pathways related to in-flammation in this populations. We collected pre and post-session blood samples of patients who had already undergone one year of chronic hemodialysis. And used liquid chromatography and high resolution mass spectrometry to perform a metabolomic analysis. Afterwards, we employed both univariate (Mann-Whitney test) and multivariate (logistic regression with LASSO regulari-zation) to identify metabolites associated with inflammation. In the univariate analysis, in-dole-3-acetaldehyde, 2-ketobutyric acid, and urocanic acid showed statistically significant de-creases in median concentrations in the presence of inflammation. In the multivariate analysis, metabolites positively associated with inflammation include allantoin, taurodeoxycholic acid, norepinephrine, pyroglutamic acid, and L-hydroorotic acid. Conversely, metabolites showing negative associations with inflammation include benzoic acid, indole-3-acetaldehyde, methio-nine, citrulline, alphaketoglutarate, n-acetyl-ornithine and 3-4-dihydroxibenzeneacetic acid. Non inflamed patients exhibit preserved autophagy and reduced mitochondrial dysfunction. Under-standing inflammation in this group hinges on the metabolism of arginine and the urea cycle. Additionally, the microbiota, particularly uricase-producing bacteria and those metabolizing tryptophan, play critical roles.
Keywords:
Subject: Medicine and Pharmacology - Urology and Nephrology
1. Introduction
Chronic kidney disease (CKD) is an escalating global health threat with an estimated 850 million individuals living with the disease in 2017 and it being the third fastest-growing cause of death [1]. Of those individuals, approximately 3.9 million globally rely on kidney replacement therapy (KRT) indefinitely [1], predominantly through hemodialysis. They face an elevated risk of cardiovascular illness and death and are more prone to infections and malignancies [2]. For instance, in the age group of 40-44 years, there is a notable gap in life expectancy between men undergoing dialysis (10,9 years) and men in the general population (36,5 years), exceeding 25 years (over 30 years for women) [1]. Cardiovascular disease (CVD) impacts over two-thirds of individuals undergoing hemodialysis, representing a significant source of morbidity and contributing to nearly half of all mortality cases [3].
Inflammation serves as a crucial link between chronic kidney disease and cardiovascular issues and is related to the prognosis of this group of patients. For example, inflammation directly results in hemodynamic strain, elevating ventricular wall pressure, initiating myocardial stun, and prolonged exposure ultimately culminates in irreversible myocardial injury [4]. Inflammation in end-stage renal disease (ESRD) patients undergoing KRT arises from various factors such as the exposure to the dialysis membranes, the presence of central venous catheters, sodium and water overload, internal dysbiosis which may lead to uremic toxins accumulations and oxidative stress mediated by mitochondrial dysfunction and cellular senescence [5]. In connection with this, while some medium-sized molecule toxins have been identified as pivotal in this pathology, there are still numerous metabolic pathways yet to be explored [6].
To do this, metabolomics emerges as a promising avenue for unraveling novel pathways within the broader field of ‘omics’ sciences. This discipline employs diverse analytical techniques to identify and quantify small molecules (metabolites) in biological samples, offering insights to the individual phenotype as it accounts for both the genome and the extrinsic factors such as lifestyle, medications and underlying health conditions. This makes it a particularly suitable tool to assess uremic toxins in the body.
Untargeted metabolomics, which focuses on the comprehensive detection and relative quantification of metabolites, but can makethe exact identification of metabolites difficult, has been utilized to investigate the changes in the metabolic profile in hemodialysis patients before and after sessions and comparing different hemodialysis techniques [7,8,9]. Meanwhile, targeted metabolomics, which is used to perform a precise measurement of predefined, much smaller groups of metabolites, has been employed to evaluate alterations in endocabinnoid and oxylipin levels among haemodialysis patients, correlating this changes to inflammatory processes [10].
However, in the present work we decided to employ semitargeted metabolomics to examine the potential biological signature for inflammation in patients who have undergone one year of chronic hemodialysis.
We use an in-house polar compound library to facilitate the identification of measured metabolites within a unified workflow which serves as a bridge between untargeted and targeted procedures. This allows us to broaden the search scope for potential metabolites related to inflammation while accurately identifying them
In this way, we aim to characterize the metabolic profile within this cohort, with a particular emphasis on inflammation-associated metabolites as potential therapeutic target.
2. Results
The study group, with an average age of 68.97 years (SD ± 14.18), comprised 37% females and showed an average height of 165.23 cm (SD ± 8,87) and a mean wight of 75.03 kg (SD ± 16.73). Approximately 54% of the patients were smokers, and an equivalent percentage had type 2 diabetes. Hypertension was prevalent in 93% of the cohort, while dyslipidemia was present in 79%.
The summary of the demographic and clinical characteristics of the sample (n=43) can be found in Table 1.
A semi-targeted metabolic strategy was utilized, employing liquid chromatography and high-resolution mass spectrometry alongside an in-house library of polar compounds. The found metabolites and their mass to charge ratios are available in the supplementary material. Both univariate (Mann-Whitney test) and multivariate (logistic regression with LASSO (Least Absolute Shrinkage and Selection Operator) regularization) analyses were conducted to identify metabolomic variables linked to inflammation.
The analyzed patients had already undergone one year of hemodialysis, and we obtained samples before and after a session to try to identify which metabolites change with the procedure.
A numerical summary of the pre-session samples is provided the Table 2, including the respective medians and interquartile ranges of the variables with significant changes between the two groups, and Figure 1 visually represents this information in a volcano plot.
In the pre-session samples, the median concentrations of indole-3-acetaldehyde (p=0.0023), 2-ketobutyric acid (p=0.0196), and urocanic acid (p=0.0323) significantly decreased in the presence of inflammation, as indicated by a statistically significant Mann-Whitney test. The magnitude of change was greater in carnitine, which saw a decrease in median concentration by a factor of 1.39 in inflamed patients, and lowest in trehalose, with a decrease by only a 0.75-factor.
Table 3 reports the standardized coefficients (SC) and odds-ratio (OR) associated with each metabolite and Figure 2 visually represents the OR obtained in the logistic-LASSO model.
Regarding this multivariate analysis, metabolites showing positive associations with inflammation (inflammation=yes) include allantoin (SC=1.17, OR 3.22), taurodeoxycholic acid (SC=0.43, OR=1.54), norepinephrine (SC=0.4, OR 1.49), pyroglutamic acid (SC=0.38, OR=1.46), and L-hydroorotic acid (SC=0.29, OR=1.34), whereas benzoic acid (SC=-0.97, OR 0.38), indole-3-acetaldehyde (SC=-0.74, OR 0.48), methionine (SC=-0.71, OR 0.49), citrulline (SC=-0.60, OR 0.55), alpha-ketoglutarate (SC=-0.55, OR=0.55), n-acetyl-l-ornithine (SC=-0.53, OR 0.59), and 3-4-dihydroxybenzeneacetic acid (SC=-0.40, OR 0.67) show negative associations. Additionally, n-acetylcarnitine (SC=-0.17, OR 0.85), 3-indolepropionic acid (SC=-0.17, OR 0.85), pantothenate (SC=-0.16, OR 0.85), and cholic acid (SC=-0.15, OR 0.86) exhibit minor negative influences. Odds ratios close to 1 indicate variables with a lesser impact on the model.
A numerical summary of the results obtained in the post-session samples is provided the Table 4, including the respective medians and interquartile ranges of the variables showing significant changes between the two groups, and Figure 3 visually represents this information in a volcano plot.
Regarding this, univariate analysis revealed significant variables that also exhibited a substantial change in the magnitude of their medians: m-coumaric acid showed higher median levels in patients with inflammation compared to those without, while n-acetyllysine and aminoadipic acid had lower median levels in patients with inflammation.
However, when we performed the multivariate analysis in the post-session samples it failed to satisfactorily discriminate between patients with inflammation and those without inflammation, possibly due to the removal of a large portion of metabolites during the hemodialysis technique.
3. Discussion
Our objective was to characterize the metabolic profile observed in one year dialysis-dependent patients before and after undergoing a hemodialysis session. Omics science have seldom been used to specifically identify biomarkers related to inflammation in hemodialysis patients. We conducted a systemic search on PubMed using the terms ‘hemodialysis AND metabolomics AND inflammation’ and we adjusted the publication date to the last 10 years. We obtained 18 results. We dismissed four studies as they did not employ metabolomics or identify novel metabolomic biomarkers related to inflammation, three studies that explored the changes in non-dialysis-dependent patients, an article about a novel research method that did not include results and a review article about aluminum exposure in HD patients. We reviewed the remaining 9 articles:
A study published in 2022 by Holle et al. suggested that gut barrier dysfunction and microbial metabolite imbalances in children with CKD contribute to a proinflammatory immune response as evidenced by stage-dependent elevations in serum TNF-alpha and sCD14, diminished short chain fatty acid production and increased tryptophan metabolites of bacterial origin [11].
A study published by Kim et al in 2021 compared how high-flux and medium cut-off (MCO) dialyzers affect metabolism, showing that MCO produced significant changes in metabolites (lower succinate, higher glutamate) and proteins, potentially improving ocidative stress and nutrition by eliminating medium-sized toxins [12].
Another study published in 2022 investigated endocannabinoid and oxylipin levels in female hemodialysis patients. They had lower levels of EPA and it associated oxylipins and showed elevated levels of metabolites linked to inflammation such as n-acetylethanolamides, monoacylglycerols and kynurenine and creatine [10].
Correspondingly, a study published by Tsalik et al examined the metabolome, proteome and transcriptome of 150 patients with critical illness, including patients receiving chronic HD. They identified elevated levels of phenylacetylglutamine, 4-acetamidobutanoate, and uremic retention substances, linking them to inflammation [13].
Furthermore, a 2022 study identified that elevated levels of the metabolite trimethylamine-N-oxide (TMAO) correlated with increased CRP and dialysate IL-6 in peritoneal dialysis (PD) patients suggesting exacerbated peritoneal inflammation and potentially contributing to peritonitis risk in PD patients [14].
If we consider dialysis in other contexts, such as acute kidney injury (AKI), a 2021 study examined the use of a proteomic platform to analyzed the serum samples from patients with AKI requiring hemodialysis highlighting 119 proteins associated with kidney recovery and inflammation [15].
Similarly, another study found that, in AKI critically ill patients necessitating RRT lower levels of the anti-inflammatory 1-arachidonoyl-lysoPCR and eicosatetraenoyl-lysoPCR were associated with mortality prediction by day 8 and amino acids metabolites by day 28 [16].
A different study published in 2016, found that elevated levels of the metabolite trimethylamine-N-oxide (TMAO) are closely linked with the extent of residual renal function. These levels do not change with hemodialysis but do normalize with renal transplantation [17].
In 2018, researchers used mass spectrometric glycomics to identify biomarkers in serum and dialysate samples from peritoneal dialysis patients. The study found that changes in protein N-glycosylation are linked to PD-related complications such as peritonitis and loss of peritoneal cells [18].
Our article is novel due to the use of semi-targeted metabolomics and the LASSO statistical method, which allows us to create a predictive model that distinguishes between inflamed and non-inflamed patients based on blood metabolites:
In the pre-session group, we identified indole-3-acetaldehyde as a significant variable associated with lack of inflammation in both univariate and multivariate analyses.
The breakdown of dietary proteins results in the liberation of tryptophan which is metabolized by gut microbiota in various compounds, including indole-3-acetaldehyde. Tryptophan metabolites have shown diverse beneficial effects on various health conditions, such as modulating immune responses in inflammatory bowel diseases, influencing carcinogenesis though interactions with the gut microbiota, regulating inflammation and metabolism in obesity and metabolic syndrome, limiting nervous system inflammation, contributing to the immune response against infectious diseases, affecting vascular inflammation and cardiovascular health [19]. However, unlike what occurs in our study, indole-3-acetaldehyde has been shown to contribute to inflammation in other studies by modulating the balance between anti-inflammatory regulatory T-cells and pro-inflammatory Th17 responder cells in the gut, while also influencing the adaptive immune response though alterations in TGF- sensing and T cell receptor signaling [20,21].
Although the evidence on whether indole-3-acetaldehyde has beneficial or detrimental effects on inflammation associated with various diseases is unclear, it appears that it has a protective effect on hemodialysis patients according to the results of our study.
In the univariate analysis, the variables identified as associated with lack of inflammation were 2-ketobutyric acid and urocanic acid.
2-ketobutyric acid, also referred to as alpha-ketobutyrate or 2-oxobutyrate, falls within the category of short chain keto acids and derivatives, characterized by ketoacids with alkyl chains containing fewer than 6 carbon atoms. It participates in the metabolic pathways of various amino acids, proponoate metabolism, and C-5 branched dibasic acid metabolism. It is a degradation product of threonine and can be converted into propionyl-coA. Subsequently, propionyl-coA can be transformed into methylmalonyl coA, which feeds into the citric acid cycle as succinyl coA, an intermediate [22].
In a study published in 2022 supplementation with alpha-ketobutyrate extended the lifespan of Caenorhabditis elegans (C.elegans) and delays senescence in fibroblast cells. This effect is likely mediated by an increase in NAD+ via LDH-1, leading to SIR-2.1/SIRT1-dependent enhancement of perixosome function and biogenesis. Increased peroxisome function promotes autophagy, which contributes to longevity and delays cellular senescence [23]. This observation aligns with the findings in our study.
Urocanic acid, derived from histidine breakdown, is abundant in the skin’s outer layer. It absorbs UV light, converting to an immunoregulator. Initially thought to shield against UV-B, further research reveals its role in suppressing hypersensitivity and enhancing skin graft survival. UA’s potential spans from UV protection to immunomodulation [22,24]. Another study observed that human retinal pigment epithelial cells treated with cis-urocanic acid had increased levels of cytoprotection, decreased levels of inflammatory cytokines and lower DNA damage [25].
Although there are no studies showing similar effects of urocanic acid in hemodialysis patients, it seems physiologically plausible that this metabolite is associated with low levels of inflammation in this population.
On the other hand, the multivariate analysis identified the metabolites allantoin, taurodeoxycholic acid, norepinephrine, pyroglutamic acid and L-hydroorotic acid as capable of discriminating patients with an inflammatory profile.
Allantoin is a diureide of glyoxylic acid. Alternatively known as 5-ureidohydantoin or glyoxyldiureide, it is generated though the oxidation of uric acid. Allantoin serves as an indicator of oxygen -free radical load arising from the non-enzymatic interaction between uric acid (UA) and reactive oxygen species (ROS). The creation of allantoin needs myeloperoxidase, superoxide, and hydrogen peroxide. The first changes urate in the blood into allantoin as a final stable product. In the presence of superoxide urate radicals form hydroperoxide and in their absence they are readily reduced to urate. At sites of excess oxidative stress, however, urate radicals will promote detrimental oxidative reactions [26]. Furthermore, it can also arise from uric acid through enzymatic catalysis by uricase, which is not naturally present in humans but may exist in gut bacteria. In end-stage renal disease, higher levels of allantoin are observed, alongside a greater presence of uricase containing bacteria [27].
Finally, a notable correlation has been noted between initial serum concentrations of allantoin and mortality in two extensive human chronic kidney disease cohorts, spanning median follow-up periods of 16.5 and 9.7 years. The mean measured GFR was 30 ml/min and 47 ml/min [28].
Therefore, our study is consistent with previous observations reporting an increase in uricase-producing bacteria at the intestinal level in patients with ESRD. However, it also confirms the role of allantoin as an inflammatory marker.
Taurodeoxycholic acid, synthesized in the liver through the bonding of deoxycholate with taurine, typically exists as a sodium salt. These bile acids are steroid acids [22]. Exogenous administration of taurine conjugated bile acids has been linked to increased vasodilation by enhancing nitric oxide (NO) production from the endothelium and thus offer promising strategies for treating endothelial dysfunction in cardiovascular disease [29]. In a study published in 2020, it was found that in individuals with type 2 diabetes mellitus (T2DM), there is a significant increase in the levels of this molecule compared to age- and gender-matched individuals without T2DM [30]. Consequently, it is possible that the endogenous production of this acid could be related to the physiological response to inflammation and could function as a biomarker in this type of patients, although its exogenous administration has beneficial properties.
Norepinephrine or noradrenalin originating from the adrenal medulla, is the precursor to epinephrine and it serves as the primary transmitter in the majority of postganglionic sympathetic nerve fibers and in the diffuse projection network originating from the locus ceruleus in the brain [22]. According to the European Uremic Toxin Working Group, norepinephrine has been recognized as a uremic toxin [31]. Research has shown that the sympathetic nervous system plays a role in chronic inflammation in conditions such as colitis, multiple sclerosis, systemic sclerosis, lupus erythematosus, and arthritis, both in animal models and humans. The overactivity of this system contributes to the development and the extension of several inflammatory conditions [32]. Hemodialysis is a clear inflammatory condition, and subsequent activation of the sympathetic system could contribute to worsening the situation for these patients.
Pyroglutamic acid, also known as 5-oxoproline, is an organic endogenous acid that can increase with prolonged administration of paracetamol at therapeutic levels, triggering metabolic acidosis characterized by an elevated anion gap. This scenario often arises in individuals facing malnutrition, infections, antibiotic treatment, renal dysfunction, or during pregnancy. Various factors affecting glutathion metabolism can explain this accumulation. Firstly, the depletion of glutathione (due to paracetamol ingestion, malnutrition or pregnancy can ensue an ATP-depleting futile pyroglutamic acid cycle. Secondly, the excretion of the acid can be impaired due to decreased renal excretion [33].
Research published in 2019, revealed elevated pyroglutamic acid and decreased glutathione levels in septic patients, the latter being correlated to severity parameters in critically ill patients with septic shock [34]. A study published in 2024, which explored the causal relationship between gut microbial taxa, human blood metabolites and serum inflammatory markers, revealed that pyroglutamic acid contributes to elevated IL-6 and SAA1 levels [35]. Conversely, another study published two years earlier identified the compound as a potential biomarker for sepsis and systemic inflammatory response syndrome [36]. Considering the impaired renal excretion and chronic inflammation common in dialysis patients, it’s logical for them to exhibit elevated levels of pyroglutamic acid.
L-hydroorotic acid is an alpha amino acid that can be synthesized from ureidosuccinic acid through the catalytic activity of the enzyme CAD protein. Additionally, the conversion of L-dihydroorotic acid and quinone into orotic acid is facilitated by the mitochondrial enzyme dihydroorotate dehydrogenase [22]. It seems unlikely that L-hydroorotic acid would be increased in inflamed individuals due to low activity of the dihydroorotate dehydrogenase enzyme, as inhibition of this enzyme is associated with the improvement of various inflammatory conditions [37,38,39]. However, this inflammatory activity could indeed be explained by the overactivity of the CAD complex, which enables activation of memory T cells and cytokine production through the novo pyrimidine synthesis [40]. Moreover, inhibiting this complex proved to be beneficial in inflammatory situations such as COVID infection [41] or certain types of cancer [42].
Finally, the multivariate analysis identified benzoic acid, indole-3-acetaldehyde, methionine, citrulline, alpha-ketoglutarate, N-acetyl-L-ornithine and 3-4-dihydroxybenzenacetic acid as useful for the identification of non-inflamed patients.
Benzoic acid (BA) is naturally present, both in its free form and as esters in many plant and animal species. Appreciable amounts have been found in most berries. BA ingestion has been found to increase nutrient digestibility and to decrease ammonia nitrogen levels in the gut. Furthermore, it enhances mucosal integrity and promotes intercellular junction formation, as well as influencing immunological responses and microbiota [43]. Hippuric acid is the excreted form of benzoic acid. Benzoic acid undergoes metabolism through butyrate-CoA ligase, producing an intermediate compound called benzoyl-CoA, which is subsequently metabolized by glycine N-acyltransferase to form hippuric acid. The protection that appears to be provided by the presence of benzoic acid could also be related to the decrease in activity of this pathway, which would result in lower levels of hippuric acid. Hippuric acid in advanced CKD has been associated with atherogenesis through the disruption of the endothelial function and renal fibrosis [44,45,46].
Methionine, an indispensable amino acid containing sulfur, plays a crucial role in synthesizing key molecules including antioxidants like gluthatione, along non-essential aminoacids such as carnitine and cysteine, as well as methyl donors like S-adenosyl methionine and vasodilatory intermediates. Eggs, sesame seeds, fish, meat, and cereal grains are among the primary sources of methionine [47]. Methionine supplementation can modulate inflammation and oxidative stress reducing the expression of inflammatory and fibrogenic genes while enhancing endogenous antioxidant capacity and protecting against oxidative damage [48,49,50].
However, research has also indicated that limiting methionine can boost glutathione production curbing oxidative stress and some studies have found that restricting this substance in mice results in decreased oxidative stress, albeit without changes in antioxidant enzyme activity [51,52].
Similarly to what occurs with indole-3- acetaldehyde, it remains uncertain whether methionine has beneficial or detrimental effects on inflammation associated with various diseases, our study indicates a protective effect on hemodialysis patients.
Citrulline is an important metabolite in the arginine metabolism. Asymmetric dimethylarginine (ADMA) is a metabolite derived from arginine and can inhibit nitric oxide synthase (NOS), thereby limiting the production of nitric oxide and potentially leading to vascular complications associated with end-stage renal disease. ADMA is primarily metabolized by dimethylarginine dimethylaminohydrolases (DDAH-1 and DDAH-2) and converted into dimethylamine and citrulline. Patients with kidney dysfunction can have lower concentrations of DDAH-1, which is particularly abundant in the kidney, therefore impeding the further metabolization of citrulline in the arginine-NO pathway. Elevated levels of ADMA are associated with cardiovascular disease, hypertension and immune dysfunction [53]. Therefore, we could argue that patients with increased citrulline have better preserved metabolism of arginine and nitric oxide, and thus, the presence of this metabolite is related to the absence of inflammation. Other studies focusing on patients performing hemodialysis have also found higher citrulline levels. [10] This metabolite has been shown to be significantly reduced after hemodialysis session in adults with ERSD, which may signal an impaired NO synthesis post-procedure [54]. That is possibly the reason why the levels of this metabolite were not significantly associated with the absence of inflammation in the post-hemodialysis examination, as its levels have been reduced to nearly zero. Additionally, citrulline levels can be increased with the exogenous administration of L-carnitine in patients undergoing chronic hemodialysis [55].
Alpha-ketoglutarate (AKG) is an important intermediate of various metabolic pathways including tricarboxylic acid (TCA) cycle, anabolic and catabolic reactions of amino acids, and collagen biosynthesis [56].
Recently there have been several reported health-promoting effects of AKG, including anti-aging, extension of healthy lifespan, anti-osteoporotic, anti-inflammatory, and anticarcinogenic effects, as well as protection against pressure overload cardiomyopathy. These beneficial effects are not only metabolic but also mediated through epigenetic mechanisms [57]. Specifically, it has been recognized as an antioxidant agent, enhancing the activity of other antioxidant enzymes such as SOD, CAT, and gluthation peroxidase. Other antioxidant mechanisms of AKG include the decomposition of hydrogen peroxide and the promotion of mitophagy to clear damaged mitochondria [56]. However, it is important to note that recent research has shown that AKG can induce ROS through autophagy induction [58].
AKG is widely considered safe and bioavailable, making oral or intravenous supplementation of AKG an effective therapeutic approach [56]. Additionally, SGLT inhibitors have been shown to increase renal levels of AKG, which may be another factor to explain their cardiovascular benefits [59].
In summary, it makes sense that AKG is associated with reduced inflammation in hemodialysis patients, and furthermore, it suggests a potential therapeutic target in these individuals.
It is remarkable that although alpha-ketoglutarate can be formed from several aminoacids one of the pathways starts in histidine. Histidine is an essential amino acid that is obtained from meat and other high protein products. Histidine is oxidized and deaminated to urocanic acid by the enzyme histidase. Urocanic acid is subsequently hydrolyzed to form N-formiminoglutamate (FIGu). This compound binds to tetrahydrofolate, releasing glutamate. Finally, glutamate is degraded to alpha-ketoglutarate [60].
Both urocanic acid and alpha-ketoglutarate help us distinguish the group of non-inflamed patients, suggesting that the activation of this metabolic pathway may confer some protection to patients on hemodialysis. At this stage of kidney disease, the protein needs of patients increase to 1,3 g/kg/day, so changes in the diet could help explain these observations. In Figure 4, we observe the relationship of urocanic acid, alphaketoglutarate and 2-oxybutyrate with the Krebs cycle, the increase in autophagy, mitochondrial biogenesis, and their connection to the reduction of the inflammatory environment.
Within the ornithine cycle, ornithine serves as the direct substrate and n-acetylornithine is a N-acetylation derivative, which can also serve as a precursor of ornithine [22]. The ornithine cycle is a pivotal amino acid metabolic pathway linked to various diseases such as infection [61], cancer [62] and hypertension [63]. A study published in 2021 found that n-acetyl-ornithine was positively correlated with multiple cytokines and coagulation indices in COVID-19 patients, especially those with severe cases [64]. Currently, there are no cases in literature that associate this metabolite with protective characteristics against inflammation. However, other studies suggest that oxidative stress upregulates the transcription of enzymes such as ornithine decarboxylase which produces an increase in polyamines, such as spermine and spermidine [65]. Diabetic rats have lower levels of spermine and spermidine, which cause heart damage and inflammation, leading to fibrosis. Spermidine treatment showed promising results in reversing this harmful processes [66]. Another study found that ornithine, through its conversion to putrescine via Arg 1 and ornithine decarboxylase, plays a critical role in enhancing continual efferocytosis by macrophages. This metabolic pathway supports the optimal clearance of apoptotic cells and promotes the resolution of tissue injury [67]. Therefore, the fact that N-acetylornithine levels are associated with the absence of inflammation could indicate a more conserved polyamine metabolic pathway and, consequently, a better response to inflammation.
A 2022 study, 3-4-dihydroxybenzeneacetic acid also known as 3,4-dihydroxyphenylacetic acid or DOPAC/DHPAA demonstrated a partial reversal of pro-inflammatory microRNA responses in human intestinal epithelial cells exposed in cranberry extract [68]. Conversely, another study published in 2020, highlights the anti-inflammatory potential of epicatechin and dihydroxybenzoic acid from cocoa in alleviating diabetic kidney inflammation through targeted cellular pathways, contrasting with the lack of similar effects observed with 3,4-dihydroxyphenylacetic acid [69].
Finally, DOPAC/DHPAA is a metabolite of dopamine and its reduction indicates dopamine dysfunction in Parkinson’s disease. In an article published in 2023, it was found that empagliflozin treatment restored this metabolite levels which suggest a potential stabilization of dopaminergic function [70].
In the post-session samples, m-coumaric acid levels were higher in inflamed patients compared to non-inflamed patients, whereas n-acetyllysine and aminoadipic acid levels were lower in inflamed patients.
M-coumaric acid, alternatively referred to as 3-coumarate, is a hydroxycinnamic acid. Its presence in human biofluids is influenced by dietary intake, particularly from whole grain consumption [22]. In an in vitro study published in 2011 it was found that m-coumaric acid had pro-oxidative and pro-glycative properties, suggesting a potential detrimental role under conditions involving high levels of glycation inducing agents like methylglyoxal [71]. Moreover, in a study performed in rats showed that metabolites of coumaric acids like dihydro-3-coumaric acid may play a role in Alzheimer’s disease [72].
N-acetyllysine is a n-acetylaminoacid which can be classified as uremic toxins if present in high concentrations in serum of plasma contributing to kidney damage, cardiovascular disease, and neurological deficits if not properly eliminated by the kidneys [73]. Nonetheless, newer studies have found that posttranslational modification such as acetylation of histone proteins within chromatin are dynamically regulated in response to DNA damage. Acetylated lysine residues serve as a binding site for acetyl-lysine reader proteins, which interpret acetylation signals during DNA damage and have a pivotal role in genome maintenance and repair processes critical for cellular health and disease prevention [74], which would be consistent with our results.
Aminoadipic acid, also known as alpha-aminoadipic acid and L-alpha-aminoadipic acid has been identified as a marker for metabolic dysfunction and diabetes risk [75]. It has been found to correlate with other inflammatory diseases such as gout [76], ischemic heart failure [77] and community-acquired pneumonia [78]. However, in another study published in 2019, it was used to impair the function of astrocytes, revealing the role these cells play in sustaining neuroinflammation and contributing to dopaminergic neuronal loss in a model of Parkinson’s disease [79]. In any case, as can be seen in Figure 3, the clinical magnitude difference for distinguishing the non-inflamed group was very small for this metabolite and was not confirmed in the multivariate analysis.
The fact that dialysis was able to reduce the concentration of many of the metabolites studied makes the analysis in this group of patients difficult.
In comparison with our previous study analyzing inflammation associated metabolites in stage 5 non-dialysis dependent CKD patients, we continue to observe the significance of mitochondrial energy metabolism. However, in this case, patients with preserved metabolic pathways that promote autophagy and mitochondrial biogenesis appear to be more protected against inflammation. Additionally, the metabolism of arginine, the urea cycle and the microbiota, which interacts with several metabolic pathways seem to play an important role [80].
This study has several limitations. Firstly, our data only indicate trends or changes in response intensities rather than precise concentrations. Secondly, we opted to use an in-house library of polar compounds to streamline results and interpretation, which may have led to the omission of some metabolites. Lastly, due to time and budget constraints we were limited in our sample size, restricting the scope for multiple comparisons and subgroup analysis.
Due to this limitations, future research should expand these findings to validate identified biomarkers and explore their clinical utility in larger patient cohorts, potentially paving the way for personalized therapeutic interventions aimed at mitigating inflammation in hemodialysis patients.
4. Materials and Methods
Experimental Design and Cohort
Our objective was to investigate the influence of various metabolites on inflammation in patients who had undergone one year of chronic hemodialysis. We conducted an observational prospective, single-center study, where we gathered the patient’s clinical and analytical data and information about the hemodialysis sessions.
Initially, we conducted a descriptive analysis of the study population to characterize its demographics. Subsequently, we explored the metabolomic profiles of the patients before and after the hemodialysis session through the use of liquid chromatography and high-resolution mass spectrometry
The inclusion criteria comprised age over 18 years and to have been undergoing hemodialysis sessions for more than a one year at the Hospital La Fe or at a peripheral dialysis center affiliated with it. Exclusion criteria included suboptimal initiation due to clinical instability (defined as acute decompensation requiring hospitalization, requiring temporal CVC insertion or unexpected and unwanted change in techniques).
We collected data about age, gender, height, weight, BMI, cardiovascular risk factors, urea, uric acid, corrected calcium for serum albumin, phosphate, sodium, potassium, chloride, CRP, hemoglobin, leucocytes and platelets. Regarding the hemodialysis session, we collected data on the type of technique employed, the access used, the residual diuresis at the start of the procedure, the average interdialysis weight gain, and the Kt/V to ensure efficacy.
Metabolomics Analysis
The processing of the samples was similar to that conducted in the article published by our group this year [80].
The serum centrifuged from the samples was collected and stored at -80ºC and subsequently, a procedure for protein precipitation and the extraction of polar compounds was performed by adding 180 microliters of cold methanol to 20 microliters of the serum. After a double centrifugation at 13000 g (10 min, 4ºC), 20 microliters of the supernatant were transferred to a 96-well plate for liquid chromatography coupled to mass spectrometry analysis. Additionally, 70 microliters of water and 10 microliters of the standard mix solution (reserpine, leucine, enkephalin, phenylanalanine-d5, 20 microM) were added to each sample. Quality control samples were prepared by mixing 10 microliters from each serum sample. Blank samples were prepared by replacing serum with ultrapure water to identify potential artifacts. Finally, plasma samples, QCs, and blanks were injected into the chromographic system. To minimize intrabatch variability, there was a random injection order and an analysis of quality samples every six plasma samples, with blank analysis conducted at the end of the sequence.
Ultra-Performance Liquid Chromatography-High Resolution Mass Spectrometry (UPLC-HRMS) analysis was conducted using and Orbitrap QExactive spectrometer (Thermofisher) couple to a Ultra-Performance Liquid Chromatography (UPLC) system. Chromatographic separation utilized a Xbridge BEH Amide column (150x2mm, 2.5 microliter particle size; Waters) with a runtime of 25 minutes, an injection volume of 5 microliters, and a column temperature set a 25ºC. The autosampler was maintained at 4ºC, and a flow rate of 105 microliters/min was achieved using water and 10 mM ammonium acetate as Mobile Phase A, and acetonitrile (ACN) as Mobile Phase B. The gradient protocol included various proportions of Mobile Phase B, starting at 90% ad gradually decreasing to 0% over the course of 25 minutes.
Electrospray ionization was utilized in both positive and negative modes (ESI +/-) for full mas acquisition, with a resolving power of 140,000. Two events were employed: one covering mass ranges from 70-700 Da, and another covering mass ranges from 700-1700 Da. Data were acquired in centroid mode.
Data preprocessing involved converting raw data to mzXML format using mass converter, followed by processing in EI-MAVEN software to generate a peak table containing m/Z values, retention times, and intensity of polar compounds. Peak areas were extracted and annotated using an in-house polar compound library. Data from positive and negative modes were combined for statistical analysis.
Before conducting statistical analysis, data quality was assesessed by evaluating internal standards stability and coefficients of variation (CV) of quality control samples. Molecular features with CV > 30% were excluded from the data matrix, and normalization method (LOESS) was applied to mitigate intrabatch variability resulting from technical differences. The filtered peak table was then used for subsequent statistical analysis.
Statistical Analysis
Patients with CRP values ≤ 2 mg/L were categorized as non-inflamed, while those with CRP > 2 mg/L were considered inflamed. We determined this value cut point based on the identification of CRP levels between 1-3 mg/L as the grey zone of inflammation, where cardiovascular risk could possibly increase. [81] All the patients underwent a system based medical and basic physical examination before the sample examination to ensure the influence of clearly infectious events was minimal.
Initially, we conducted a univariate analysis to explore which metabolomic variables correlated with the presence of inflammation. This involved identifying metabolites significantly upregulated with a fold change of 1,5 in the inflammatory group compared to the non-inflammatory group using a non-parametric Mann-Whitney test (log2(foldchange)=log2(MInflammation=1/Minflammation=0).
For the multivariate analysis, we employed a logistic regression model, suited for binary response variables, applying LASSO regularization. LASSO (Least Absolute Shrinkage and Selection Operator) is a regularization technique aiding in variable selection and regularization by shrinking some regression coefficients towards zero, thus facilitating a more interpretable model. This algorithm optimized the coefficients of logistic regression for each variable (standardized) to enhance performance, specifically reducing classification errors. This was achieved by progressively diminishing coefficients using a multiplier parameter in the algorithm’s cost function until they reached zero, effectively eliminating the corresponding variable. The extent of coefficient minimization and elimination was determined by an additional parameter called lambda. A higher lambda value led to a greater coefficient penalization, reducing their values to zero, and consequently, reducing the number of variables included in the model. The optimal lambda value was determined using a leave-one-out cross-validation. Various models were fitted, with one group of subjects excluded in each iteration to estimate their classification into the ‘inflamed=no’ or ‘inflamed=yes’ group. The error for each case was calculated, and the lambda value minimizing this error was considered the optimal choice. In this model, k was set to 50, implying one patient was excluded for estimation. In each iteration, with a focus on minimizing classification errors. Standardized coefficients and Odds Ratio were computed for each variable. ORs represent the multiplier for the probability of belonging to the inflammation=yes group compared to inflammation no assuming a one standard deviation increase in the variable, as we use standardized coefficients.
The sign of the coefficients indicates how changes in variables affect the probability of inflammation. Positive coefficients imply a higher likelihood of inflammation, while negative coefficients decrease the probability. Variables with larger absolute standardized coefficients hold higher impact in the model, as reflected in their odds ratios (OR= e^coefficient).
5. Conclusions
Our study introduces novel findings through the application of semi-targeted metabolomics and the LASSO statistical method, enabling the creation of a predictive model distinguishing inflamed hemodialysis patients. Non-inflamed patients seem to have a preserved autophagy and less mitochondrial dysfunction. Moreover, the metabolism of arginine and therefore the urea cycle are pivotal to the understanding of inflammation in this group of patients. Finally, the microbiota, along with uricase producing bacterial and those that metabolize tryptophan play a crucial role.
These results allow us to identify metabolic pathways related to inflammation in hemodialysis patients, thereby identifying potential therapeutic targets and points of optimization in renal replacement therapy.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1: Polar metabolites.
Author Contributions
Conceptualization, M.P-F, M.R-M and J.H-J; methodology, M.P-F M.R-M, J.A.L. and A.B-T.;.; software, M.R-M, J.A.L. and A.B-T..; formal analysis, M.R-M, J.A.L. and A.B-T.; investigation, M.P-F; writing—original draft preparation, M.P-F.; writing—review and editing, M.P-F, M.R-M, J.A.L., I.V-B and A.B-T .; visualization, M.P-F, M.R-M, J.A.L., I.V-B, A.B-T, J.H-J, R.D-S, A. S-O and P.S-P; supervision, J.H-J..; funding acquisition, M.P-F. All authors have read and agreed to the submitted version of the manuscript.
Funding
Part of the study has been funded by the Valencian Society of Nephrology through the María Isabel Burches Nephrology Research Scholarship. The funder had no role in the design, data collection, data analysis, and reporting of this study.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Hospital Universitario y Politécnico la Fe (protocol code 2019-232-1 and date of approval 29th January 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data on which the article is based are available as Supplementary Materials.
Acknowledgments
We want to express our gratitude to the patients who participated and the dedicated nursing and medical staff of the Dialysis units and centers within the Department of Nephrology at Hospital Universitari I Politècnic la Fe for their generous and wholehearted support throughout the study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bello, A. K., Okpechi, I. G., Osman, M. A., Cho, Y., Htay, H., Jha, V., Wainstein, M., & Johnson, D. W. (2022). Epidemiology of haemodialysis outcomes. Nature Reviews Nephrology, 18(6), Article 6. [CrossRef]
- Betjes, M. G. H. (2013). Immune cell dysfunction and inflammation in end-stage renal disease. Nature Reviews. Nephrology, 9(5), 255-265. [CrossRef]
- Thompson, S., James, M., Wiebe, N., Hemmelgarn, B., Manns, B., Klarenbach, S., Tonelli, M., & Alberta Kidney Disease Network. (2015). Cause of Death in Patients with Reduced Kidney Function. Journal of the American Society of Nephrology: JASN, 26(10), 2504-2511. [CrossRef]
- Zuidema, M. Y., & Dellsperger, K. C. (2012). Myocardial Stunning with Hemodialysis: Clinical Challenges of the Cardiorenal Patient. Cardiorenal Medicine, 2(2), 125-133. [CrossRef]
- Wang, Y., & Gao, L. (2022). Inflammation and Cardiovascular Disease Associated With Hemodialysis for End-Stage Renal Disease. Frontiers in Pharmacology, 13, 800950. [CrossRef]
- Cobo, G., Lindholm, B., & Stenvinkel, P. (2018). Chronic inflammation in end-stage renal disease and dialysis. Nephrology Dialysis Transplantation, 33(Suppl 3), iii35-iii40. [CrossRef]
- Kalim, S., Wald, R., Yan, A. T., Goldstein, M. B., Kiaii, M., Xu, D., Berg, A. H., Clish, C., Thadhani, R., Rhee, E. P., & Perl, J. (2018). Extended Duration Nocturnal Hemodialysis and Changes in Plasma Metabolite Profiles. Clinical Journal of the American Society of Nephrology: CJASN, 13(3), 436-444. [CrossRef]
- Velenosi, T. J., Thomson, B. K. A., Tonial, N. C., RaoPeters, A. A. E., Mio, M. A., Lajoie, G. A., Garg, A. X., House, A. A., & Urquhart, B. L. (2019). Untargeted metabolomics reveals N, N, N-trimethyl-L-alanyl-L-proline betaine (TMAP) as a novel biomarker of kidney function. Scientific Reports, 9(1), 6831. [CrossRef]
- Sirich, T. L., Aronov, P. A., Fullman, J., Nguyen, K., Plummer, N. S., & Meyer, T. W. (2017). Untargeted mass spectrometry discloses plasma solute levels poorly controlled by hemodialysis. PLOS ONE, 12(11), e0188315. [CrossRef]
- Watkins, B. A., Friedman, A. N., Kim, J., Borkowski, K., Kaiser, S., Fiehn, O., & Newman, J. W. (2022). Blood Levels of Endocannabinoids, Oxylipins, and Metabolites Are Altered in Hemodialysis Patients. International Journal of Molecular Sciences, 23(17), 9781. [CrossRef]
- Holle, J., Bartolomaeus, H., Löber, U., Behrens, F., Bartolomaeus, T. U. P., Anandakumar, H., Wimmer, M. I., Vu, D. L., Kuhring, M., Brüning, U., Maifeld, A., Geisberger, S., Kempa, S., Schumacher, F., Kleuser, B., Bufler, P., Querfeld, U., Kitschke, S., Engler, D., … Müller, D. (2022). Inflammation in Children with CKD Linked to Gut Dysbiosis and Metabolite Imbalance. Journal of the American Society of Nephrology: JASN, 33(12), 2259-2275. [CrossRef]
- Kim, H. J., Seong, E. Y., Lee, W., Kim, S., Ahn, H.-S., Yeom, J., Kim, K., Kwon, C. H., & Song, S. H. (2021). Comparative analysis of therapeutic effects between medium cut-off and high flux dialyzers using metabolomics and proteomics: Exploratory, prospective study in hemodialysis. Scientific Reports, 11(1), 17335. [CrossRef]
- Tsalik, E. L., Willig, L. K., Rice, B. J., van Velkinburgh, J. C., Mohney, R. P., McDunn, J. E., Dinwiddie, D. L., Miller, N. A., Mayer, E. S., Glickman, S. W., Jaehne, A. K., Glew, R. H., Sopori, M. L., Otero, R. M., Harrod, K. S., Cairns, C. B., Fowler, V. G., Rivers, E. P., Woods, C. W., … Langley, R. J. (2015). Renal systems biology of patients with systemic inflammatory response syndrome. Kidney International, 88(4), 804-814. [CrossRef]
- Zhang, L., Xie, F., Tang, H., Zhang, X., Hu, J., Zhong, X., Gong, N., Lai, Y., Zhou, M., Tian, J., Zhou, Z., Xie, L., Hu, Z., Zhu, F., Jiang, J., & Nie, J. (2022). Gut microbial metabolite TMAO increases peritoneal inflammation and peritonitis risk in peritoneal dialysis patients. Translational Research, 240, 50-63. [CrossRef]
- Daniels, J. R., Ma, J. Z., Cao, Z., Beger, R. D., Sun, J., Schnackenberg, L., Pence, L., Choudhury, D., Palevsky, P. M., Portilla, D., & Yu, L.-R. (2021). Discovery of Novel Proteomic Biomarkers for the Prediction of Kidney Recovery from Dialysis-Dependent AKI Patients. Kidney360, 2(11), 1716-1727. [CrossRef]
- Sun, J., Cao, Z., Schnackenberg, L., Pence, L., Yu, L.-R., Choudhury, D., Palevsky, P. M., Portilla, D., & Beger, R. D. (2021). Serum metabolite profiles predict outcomes in critically ill patients receiving renal replacement therapy. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 1187, 123024. [CrossRef]
- Missailidis, C., Hällqvist, J., Qureshi, A. R., Barany, P., Heimbürger, O., Lindholm, B., Stenvinkel, P., & Bergman, P. (2016). Serum Trimethylamine-N-Oxide Is Strongly Related to Renal Function and Predicts Outcome in Chronic Kidney Disease. PloS One, 11(1), e0141738. [CrossRef]
- Ferrantelli, E., Farhat, K., Ederveen, A. L. H., Reiding, K. R., Beelen, R. H. J., van Ittersum, F. J., Wuhrer, M., & Dotz, V. (2018). Effluent and serum protein N-glycosylation is associated with inflammation and peritoneal membrane transport characteristics in peritoneal dialysis patients. Scientific Reports, 8(1), 979. [CrossRef]
- Su, X., Gao, Y., & Yang, R. (2022). Gut Microbiota-Derived Tryptophan Metabolites Maintain Gut and Systemic Homeostasis. Cells, 11(15), 2296. [CrossRef]
- Wojciech, L., Png, C. W., Koh, E. Y., Kioh, D. Y. Q., Deng, L., Wang, Z., Wu, L.-Z., Hamidinia, M., Tung, D. W., Zhang, W., Pettersson, S., Chan, E. C. Y., Zhang, Y., Tan, K. S., & Gascoigne, N. R. (2023). A tryptophan metabolite made by a gut microbiome eukaryote induces pro-inflammatory T cells. The EMBO Journal, 42(21), e112963. [CrossRef]
- Shang, H., Huang, C., Xiao, Z., Yang, P., Zhang, S., Hou, X., & Zhang, L. (2023). Gut microbiota-derived tryptophan metabolites alleviate liver injury via AhR/Nrf2 activation in pyrrolizidine alkaloids-induced sinusoidal obstruction syndrome. Cell & Bioscience, 13(1), 127. [CrossRef]
- Wishart, D. S., Guo, A., Oler, E., Wang, F., Anjum, A., Peters, H., Dizon, R., Sayeeda, Z., Tian, S., Lee, B. L., Berjanskii, M., Mah, R., Yamamoto, M., Jovel, J., Torres-Calzada, C., Hiebert-Giesbrecht, M., Lui, V. W., Varshavi, D., Varshavi, D., … Gautam, V. (2022). HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Research, 50(D1), D622-D631. [CrossRef]
- Wu, N., Ma, Y.-C., Gong, X.-Q., Zhao, P.-J., Jia, Y.-J., Zhao, Q., Duan, J.-H., & Zou, C.-G. (2023). The metabolite alpha-ketobutyrate extends lifespan by promoting peroxisomal function in C. elegans. Nature Communications, 14(1), 240. [CrossRef]
- Boo, Y. C. (2020). Emerging Strategies to Protect the Skin from Ultraviolet Rays Using Plant-Derived Materials. Antioxidants (Basel, Switzerland), 9(7), 637. [CrossRef]
- Korhonen, E., Piippo, N., Hytti, M., Kaarniranta, K., & Kauppinen, A. (2023). Cis-urocanic acid improves cell viability and suppresses inflammasome activation in human retinal pigment epithelial cells. Biochemical Pharmacology, 216, 115790. [CrossRef]
- Meotti, F. C., Jameson, G. N. L., Turner, R., Harwood, D. T., Stockwell, S., Rees, M. D., Thomas, S. R., & Kettle, A. J. (2011). Urate as a physiological substrate for myeloperoxidase: Implications for hyperuricemia and inflammation. The Journal of Biological Chemistry, 286(15), 12901-12911. [CrossRef]
- Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD - PubMed. (s. f.). Recuperado 9 de abril de 2024, de https://pubmed.ncbi.nlm.nih.
- Hu, J.-R., Coresh, J., Inker, L. A., Levey, A. S., Zheng, Z., Rebholz, C. M., Tin, A., Appel, L. J., Chen, J., Sarnak, M. J., & Grams, M. E. (2018). Serum metabolites are associated with all-cause mortality in chronic kidney disease. Kidney international, 94(2), 381-389. [CrossRef]
- Guizoni, D. M., Vettorazzi, J. F., Carneiro, E. M., & Davel, A. P. (2020). Modulation of endothelium-derived nitric oxide production and activity by taurine and taurine-conjugated bile acids. Nitric Oxide, 94, 48-53. [CrossRef]
- Choucair, I., Nemet, I., Li, L., Cole, M. A., Skye, S. M., Kirsop, J. D., Fischbach, M. A., Gogonea, V., Brown, J. M., Tang, W. H. W., & Hazen, S. L. (2020). Quantification of bile acids: A mass spectrometry platform for studying gut microbe connection to metabolic diseases. Journal of Lipid Research, 61(2), 159-177. [CrossRef]
- Duranton, F., Cohen, G., De Smet, R., Rodriguez, M., Jankowski, J., Vanholder, R., Argiles, A., & European Uremic Toxin Work Group. (2012). Normal and pathologic concentrations of uremic toxins. Journal of the American Society of Nephrology: JASN, 23(7), 1258-1270. [CrossRef]
- Pongratz, G., & Straub, R. H. (2023). Chronic Effects of the Sympathetic Nervous System in Inflammatory Models. Neuroimmunomodulation, 30(1), 113-134. [CrossRef]
- Hunter, R. W., Lawson, C., Galitsiou, E., Gifford, F., & Neary, J. J. (2016). Pyroglutamic acidosis in association with therapeutic paracetamol use. Clinical Medicine, 16(6), 524-529. [CrossRef]
- Gamarra, Y., Santiago, F. C., Molina-López, J., Castaño, J., Herrera-Quintana, L., Domínguez, Á., & Planells, E. (2019). Pyroglutamic acidosis by glutathione regeneration blockage in critical patients with septic shock. Critical Care (London, England), 23(1), 162. [CrossRef]
- Liu, Y., Zhu, Q., Guo, G., Xie, Z., Li, S., Lai, C., Wu, Y., Wang, L., & Zhong, S. (2024). Causal associations of genetically predicted gut microbiota and blood metabolites with inflammatory states and risk of infections: A Mendelian randomization analysis. Frontiers in Microbiology, 15, 1342653. [CrossRef]
- Lu, G., Zhou, J., Yang, T., Li, J., Jiang, X., Zhang, W., Gu, S., & Wang, J. (2022). Landscape of Metabolic Fingerprinting for Diagnosis and Risk Stratification of Sepsis. Frontiers in Immunology, 13, 883628. [CrossRef]
- Li, D., Lu, X., Xu, G., Liu, S., Gong, Z., Lu, F., Xia, X., Jiang, J., Wang, H., Zou, F., & Ma, X. (2023). Dihydroorotate dehydrogenase regulates ferroptosis in neurons after spinal cord injury via the P53-ALOX15 signaling pathway. CNS Neuroscience & Therapeutics, 29(7), 1923-1939. [CrossRef]
- Liu, Y., Tan, S., Wu, Y., & Tan, S. (2022). The Emerging Role of Ferroptosis in Sepsis. DNA and Cell Biology, 41(4), 368-380. [CrossRef]
- Zhang, S., Zhao, D., Yang, Z., Wang, F., Yang, S., & Wang, C. (2024). Circulating mitochondria promoted endothelial cGAS-derived neuroinflammation in subfornical organ to aggravate sympathetic overdrive in heart failure mice. Journal of Neuroinflammation, 21(1), 27. [CrossRef]
- Claiborne, M. D., Sengupta, S., Zhao, L., Arwood, M. L., Sun, I.-M., Wen, J., Thompson, E. A., Mitchell-Flack, M., Laiho, M., & Powell, J. D. (2022). Persistent CAD activity in memory CD8+ T cells supports rRNA synthesis and ribosomal biogenesis required at rechallenge. Science Immunology, 7(71), eabh4271. [CrossRef]
- Qin, C., Rao, Y., Yuan, H., Wang, T.-Y., Zhao, J., Espinosa, B., Liu, Y., Zhang, S., Savas, A. C., Liu, Q., Zarinfar, M., Rice, S., Henley, J., Comai, L., Graham, N. A., Chen, C., Zhang, C., & Feng, P. (2022). SARS-CoV-2 couples evasion of inflammatory response to activated nucleotide synthesis. Proceedings of the National Academy of Sciences of the United States of America, 119(26), e2122897119. [CrossRef]
- Tu, H.-F., Ko, C.-J., Lee, C.-T., Lee, C.-F., Lan, S.-W., Lin, H.-H., Lin, H.-Y., Ku, C.-C., Lee, D.-Y., Chen, I.-C., Chuang, Y.-H., Del Caño-Ochoa, F., Ramón-Maiques, S., Ho, C.-C., Lee, M.-S., & Chang, G.-D. (2021). Afatinib Exerts Immunomodulatory Effects by Targeting the Pyrimidine Biosynthesis Enzyme CAD. Cancer Research, 81(12), 3270-3282. [CrossRef]
- Mao, X., Yang, Q., Chen, D., Yu, B., & He, J. (2019). Benzoic Acid Used as Food and Feed Additives Can Regulate Gut Functions. BioMed Research International, 2019, 5721585. [CrossRef]
- Sun, B., Wang, X., Liu, X., Wang, L., Ren, F., Wang, X., & Leng, X. (2020). Hippuric Acid Promotes Renal Fibrosis by Disrupting Redox Homeostasis via Facilitation of NRF2-KEAP1-CUL3 Interactions in Chronic Kidney Disease. Antioxidants (Basel, Switzerland), 9(9), 783. [CrossRef]
- Huang, M., Wei, R., Wang, Y., Su, T., Li, P., & Chen, X. (2018). The uremic toxin hippurate promotes endothelial dysfunction via the activation of Drp1-mediated mitochondrial fission. Redox Biology, 16, 303-313. [CrossRef]
- Shang, F., Wang, S.-C., Hsu, C.-Y., Miao, Y., Martin, M., Yin, Y., Wu, C.-C., Wang, Y.-T., Wu, G., Chien, S., Huang, H.-D., Tarng, D.-C., Shiu, Y.-T., Cheung, A. K., Huang, P.-H., Chen, Z., & Shyy, J. Y.-J. (2017). MicroRNA-92a Mediates Endothelial Dysfunction in CKD. Journal of the American Society of Nephrology: JASN, 28(11), 3251-3261. [CrossRef]
- Navik, U., Sheth, V. G., Khurana, A., Jawalekar, S. S., Allawadhi, P., Gaddam, R. R., Bhatti, J. S., & Tikoo, K. (2021). Methionine as a double-edged sword in health and disease: Current perspective and future challenges. Ageing Research Reviews, 72, 101500. [CrossRef]
- Błaszczyk, I., Grucka-Mamczar, E., Kasperczyk, S., & Birkner, E. (2009). Influence of methionine upon the concentration of malondialdehyde in the tissues and blood of rats exposed to sodium fluoride. Biological Trace Element Research, 129(1-3), 229-238. [CrossRef]
- Li, H., Cai, L., Liang, M., Wang, Z., Zhang, Y., Wu, Q., & Yang, L. (2020). Methionine augments endogenous antioxidant capacity of rice protein through stimulating MSR antioxidant system and activating Nrf2-ARE pathway in growing and adult rats. European Food Research and Technology, 246(5), 1051-1063. [CrossRef]
- Kumar, A., Pathak, R., Palfrey, H. A., Stone, K. P., Gettys, T. W., & Murthy, S. N. (2020). High levels of dietary methionine improves sitagliptin-induced hepatotoxicity by attenuating oxidative stress in hypercholesterolemic rats. Nutrition & Metabolism, 17(1), 2. [CrossRef]
- Liu, G., Yu, L., Fang, J., Hu, C.-A. A., Yin, J., Ni, H., Ren, W., Duraipandiyan, V., Chen, S., Al-Dhabi, N. A., & Yin, Y. (2017). Methionine restriction on oxidative stress and immune response in dss-induced colitis mice. Oncotarget, 8(27), 44511-44520. [CrossRef]
- Maddineni, S., Nichenametla, S., Sinha, R., Wilson, R. P., & Richie, J. P. (2013). Methionine restriction affects oxidative stress and glutathione-related redox pathways in the rat. Experimental Biology and Medicine, 238(4), 392-399. [CrossRef]
- Shah, V. O., Townsend, R. R., Feldman, H. I., Pappan, K. L., Kensicki, E., & Vander Jagt, D. L. (2013). Plasma Metabolomic Profiles in Different Stages of CKD. Clinical Journal of the American Society of Nephrology : CJASN, 8(3), 363-370. [CrossRef]
- Heredia Martinez, A., Rosa Diez, G., Ferraris, V., Coccia, P. A., Ferraris, J. R., Checa, A., Wheelock, C. E., Lundberg, J. O., Weitzberg, E., Carlström, M., & Krmar, R. T. (2020). Removal of nitrate and nitrite by hemodialysis in end-stage renal disease and by sustained low-efficiency dialysis in acute kidney injury. Nitric Oxide: Biology and Chemistry, 98, 33-40. [CrossRef]
- Kuwasawa-Iwasaki, M., Io, H., Muto, M., Ichikawa, S., Wakabayashi, K., Kanda, R., Nakata, J., Nohara, N., Tomino, Y., & Suzuki, Y. (2020). Effects of L-Carnitine Supplementation in Patients Receiving Hemodialysis or Peritoneal Dialysis. Nutrients, 12(11), 3371. [CrossRef]
- Guo, L., Chen, S., Ou, L., Li, S., Ye, Z.-N., & Liu, H.-F. (2022). Disrupted Alpha-Ketoglutarate Homeostasis: Understanding Kidney Diseases from the View of Metabolism and Beyond. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy, 15, 1961-1974. [CrossRef]
- Nakanishi, T., & Kuragano, T. (2024). Growing concerns about using hypoxia-inducible factor prolyl hydroxylase inhibitors for the treatment of renal anemia. Clinical Kidney Journal, 17(3), sfae051. [CrossRef]
- Zhang, J., Zhou, B., Sun, R., Ai, Y., Cheng, K., Li, F., Wang, B., Liu, F., Jiang, Z., Wang, W., Zhou, D., Chen, H., & Wu, Q. (2021). The metabolite α-KG induces GSDMC-dependent pyroptosis through death receptor 6-activated caspase-8. Cell Research, 31(9), 980-997. [CrossRef]
- Onishi, A., Fu, Y., Patel, R., Darshi, M., Crespo-Masip, M., Huang, W., Song, P., Freeman, B., Kim, Y. C., Soleimani, M., Sharma, K., Thomson, S. C., & Vallon, V. (2020). A role for tubular Na+/H+ exchanger NHE3 in the natriuretic effect of the SGLT2 inhibitor empagliflozin. American Journal of Physiology - Renal Physiology, 319(4), F712-F728. [CrossRef]
- Todd A. Swanson (Author), Sandra I. Kim (Author), Ph.D. Glucksman, Marc J. (Author),. (s. f.). BRS Biochemistry, Molecular Biology, and Genetics (5th ed.).
- Lercher, A., Bhattacharya, A., Popa, A. M., Caldera, M., Schlapansky, M. F., Baazim, H., Agerer, B., Gürtl, B., Kosack, L., Májek, P., Brunner, J. S., Vitko, D., Pinter, T., Genger, J.-W., Orlova, A., Pikor, N., Reil, D., Ozsvár-Kozma, M., Kalinke, U., … Bergthaler, A. (2019). Type I Interferon Signaling Disrupts the Hepatic Urea Cycle and Alters Systemic Metabolism to Suppress T Cell Function. Immunity, 51(6), 1074-1087.e9. [CrossRef]
- Lee, J. S., Adler, L., Karathia, H., Carmel, N., Rabinovich, S., Auslander, N., Keshet, R., Stettner, N., Silberman, A., Agemy, L., Helbling, D., Eilam, R., Sun, Q., Brandis, A., Malitsky, S., Itkin, M., Weiss, H., Pinto, S., Kalaora, S., … Erez, A. (2018). Urea Cycle Dysregulation Generates Clinically Relevant Genomic and Biochemical Signatures. Cell, 174(6), 1559-1570.e22. [CrossRef]
- Zheng, H.-K., Zhao, J.-H., Yan, Y., Lian, T.-Y., Ye, J., Wang, X.-J., Wang, Z., Jing, Z.-C., He, Y.-Y., & Yang, P. (2018). Metabolic reprogramming of the urea cycle pathway in experimental pulmonary arterial hypertension rats induced by monocrotaline. Respiratory Research, 19, 94. [CrossRef]
- Li, T., Ning, N., Li, B., Luo, D., Qin, E., Yu, W., Wang, J., Yang, G., Nan, N., He, Z., Yang, N., Gong, S., Li, J., Liu, A., Sun, Y., Li, Z., Jia, T., Gao, J., Zhang, W., … Wang, H. (2021). Longitudinal Metabolomics Reveals Ornithine Cycle Dysregulation Correlates With Inflammation and Coagulation in COVID-19 Severe Patients. Frontiers in Microbiology, 12, 723818. [CrossRef]
- Smirnova, O. A., Isaguliants, M. G., Hyvonen, M. T., Keinanen, T. A., Tunitskaya, V. L., Vepsalainen, J., Alhonen, L., Kochetkov, S. N., & Ivanov, A. V. (2012). Chemically induced oxidative stress increases polyamine levels by activating the transcription of ornithine decarboxylase and spermidine/spermine-N1-acetyltransferase in human hepatoma HUH7 cells. Biochimie, 94(9), 1876-1883. [CrossRef]
- Wei, C., Xu, J., Liu, Y., Qadir, J., Zhang, S., & Yuan, H. (2023). Exogenous Spermidine Alleviates Diabetic Myocardial Fibrosis Via Suppressing Inflammation and Pyroptosis in db/db Mice. Balkan Medical Journal, 40(5), 333-343. [CrossRef]
- Yurdagul, A., Subramanian, M., Wang, X., Crown, S. B., Ilkayeva, O., Darville, L., Kolluru, G. K., Rymond, C. C., Gerlach, B. D., Zheng, Z., Kuriakose, G., Kevil, C. G., Koomen, J. M., Cleveland, J. L., Muoio, D. M., & Tabas, I. (2020). Macrophage Metabolism of Apoptotic Cell-Derived Arginine Promotes Continual Efferocytosis and Resolution of Injury. Cell metabolism, 31(3), 518-533.e10. [CrossRef]
- Lofft, Z., Taibi, A., Massara, P., Tokar, T., Paetau-Robinson, I., Khoo, C., & Comelli, E. M. (2022). Cranberry Proanthocyanidin and Its Microbial Metabolite 3,4-Dihydroxyphenylacetic Acid, but Not 3-(4-Hydroxyphenyl)-Propionic Acid, Partially Reverse Pro-Inflammatory microRNA Responses in Human Intestinal Epithelial Cells. Molecular Nutrition & Food Research, 66(8), e2100853. [CrossRef]
- Cilleros, D. Á., López-Oliva, M. E., Martín, M. Á., & Ramos, S. (2020). (−)-Epicatechin and the colonic metabolite 2,3-dihydroxybenzoic acid protect against high glucose and lipopolysaccharide-induced inflammation in renal proximal tubular cells through NOX-4/p38 signalling. Food & Function, 11(10), 8811-8824. [CrossRef]
- Mohammed, N. N., Tadros, M. G., & George, M. Y. (2024). Empagliflozin repurposing in Parkinson’s disease; modulation of oxidative stress, neuroinflammation, AMPK/SIRT-1/PGC-1α, and wnt/β-catenin pathways. Inflammopharmacology, 32(1), 777-794. [CrossRef]
- Wu, C.-H., Huang, H.-W., Lin, J.-A., Huang, S.-M., & Yen, G.-C. (2011). The proglycation effect of caffeic acid leads to the elevation of oxidative stress and inflammation in monocytes, macrophages and vascular endothelial cells. The Journal of Nutritional Biochemistry, 22(6), 585-594. [CrossRef]
- Favero, F., Barberis, E., Gagliardi, M., Espinoza, S., Contu, L., Gustincich, S., Boccafoschi, F., Borsotti, C., Lim, D., Rubino, V., Mignone, F., Pasolli, E., Manfredi, M., Zucchelli, S., Corà, D., & Corazzari, M. (2022). A Metabologenomic approach reveals alterations in the gut microbiota of a mouse model of Alzheimer’s disease. PLoS ONE, 17(8), e0273036. [CrossRef]
- Vanholder, R., Baurmeister, U., Brunet, P., Cohen, G., Glorieux, G., Jankowski, J., & European Uremic Toxin Work Group. (2008). A bench to bedside view of uremic toxins. Journal of the American Society of Nephrology: JASN, 19(5), 863-870. [CrossRef]
- Zaware, N., & Zhou, M.-M. (2017). Chemical Modulators for Epigenome Reader Domains as Emerging Epigenetic Therapies for Cancer and Inflammation. Current opinion in chemical biology, 39, 116-125. [CrossRef]
- Wang, T. J., Ngo, D., Psychogios, N., Dejam, A., Larson, M. G., Vasan, R. S., Ghorbani, A., O’Sullivan, J., Cheng, S., Rhee, E. P., Sinha, S., McCabe, E., Fox, C. S., O’Donnell, C. J., Ho, J. E., Florez, J. C., Magnusson, M., Pierce, K. A., Souza, A. L., … Gerszten, R. E. (2013). 2-Aminoadipic acid is a biomarker for diabetes risk. The Journal of Clinical Investigation, 123(10), 4309-4317. [CrossRef]
- Li, Y., Han, X., Tong, J., Wang, Y., Liu, X., Liao, Z., Jiang, M., & Zhao, H. (2023). Analysis of Metabolites in Gout: A Systematic Review and Meta-Analysis. Nutrients, 15(14), 3143. [CrossRef]
- Halade, G. V., Kain, V., Tourki, B., & Jadapalli, J. K. (2019). Lipoxygenase drives lipidomic and metabolic reprogramming in ischemic heart failure. Metabolism: Clinical and Experimental, 96, 22-32. [CrossRef]
- Zhou, B., Lou, B., Liu, J., & She, J. (2020). Serum metabolite profiles as potential biochemical markers in young adults with community-acquired pneumonia cured by moxifloxacin therapy. Scientific Reports, 10(1), 4436. [CrossRef]
- O’Neill, E., Chiara Goisis, R., Haverty, R., & Harkin, A. (2019). L-alpha-aminoadipic acid restricts dopaminergic neurodegeneration and motor deficits in an inflammatory model of Parkinson’s disease in male rats. Journal of Neuroscience Research, 97(7), 804-816. [CrossRef]
- Peris-Fernández, M., Roca-Marugán, M., Amengual, J. L., Balaguer-Timor, Á., Viejo-Boyano, I., Soldevila-Orient, A., Devesa-Such, R., Sánchez-Pérez, P., & Hernández-Jaras, J. (2024). Uremic Toxins and Inflammation: Metabolic Pathways Affected in Non-Dialysis-Dependent Stage 5 Chronic Kidney Disease. Biomedicines, 12(3), 607. [CrossRef]
- Kalantar-Zadeh, K. (2007). Inflammatory Marker Mania in Chronic Kidney Disease: Pentraxins at the Crossroad of Universal Soldiers of Inflammation. Clinical Journal of the American Society of Nephrology, 2(5), 872. [CrossRef]
Figure 1.
Volcano plot of metabolomic variables. The horizontal dashed line represents the limit of statistical significance (corresponding to p-value = 0.05); values above it denote variables with significantly different medians. The vertical dashed lines represent a log2(foldchange) of -0.6 and +0.6. Values above + 0.6 represent variables with a median for inflammation=yes that is 1.5 times in greater magnitude than for inflammation=no Values below -0.6 represent variables with a median for inflammation=no that is 1.5 times greater in magnitude than for inflammation=yes.
Figure 1.
Volcano plot of metabolomic variables. The horizontal dashed line represents the limit of statistical significance (corresponding to p-value = 0.05); values above it denote variables with significantly different medians. The vertical dashed lines represent a log2(foldchange) of -0.6 and +0.6. Values above + 0.6 represent variables with a median for inflammation=yes that is 1.5 times in greater magnitude than for inflammation=no Values below -0.6 represent variables with a median for inflammation=no that is 1.5 times greater in magnitude than for inflammation=yes.
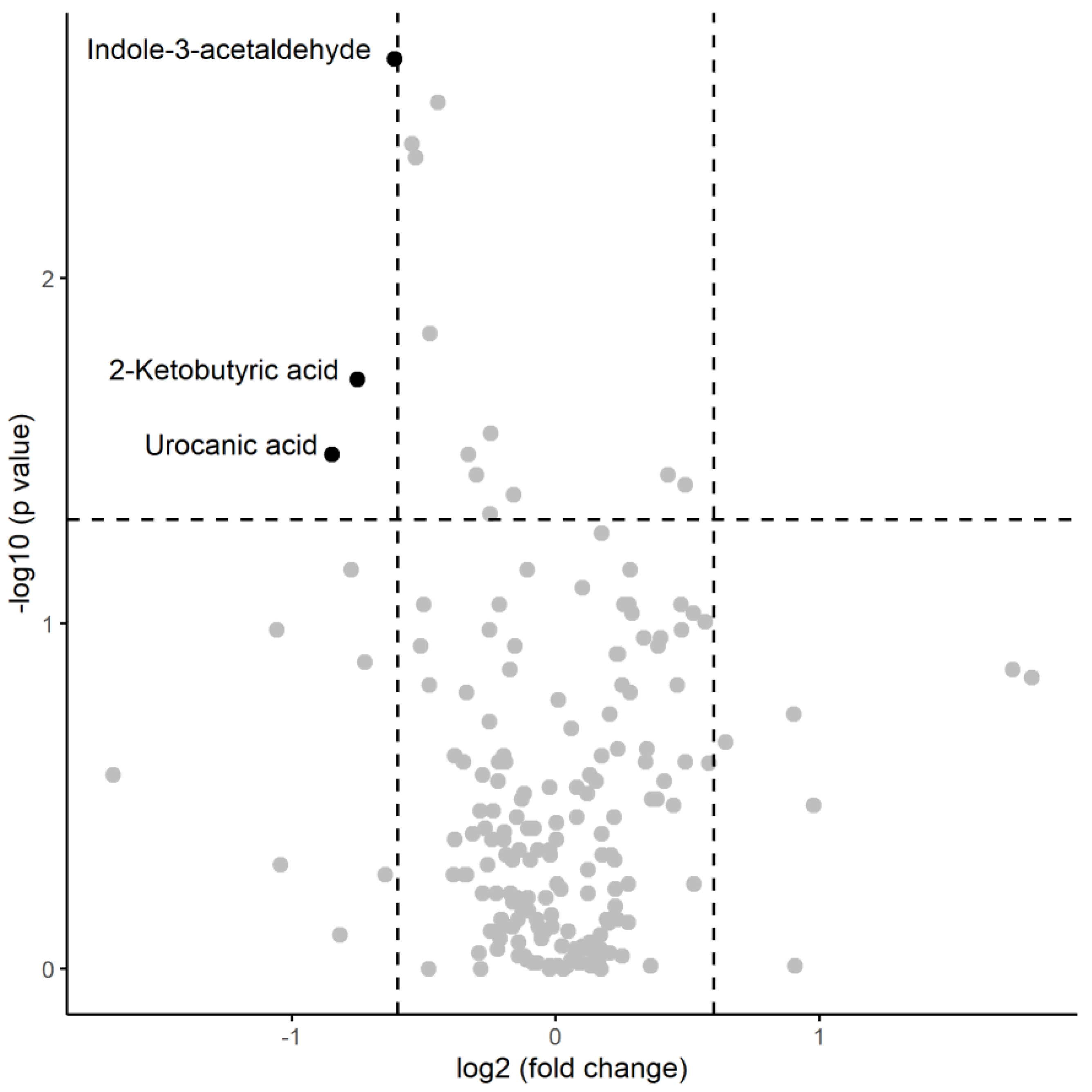
Figure 2.
Graphical depiction of the OR obtained in the Logistic-LASSO model. ORs close to 1 (red vertical line) indicate variables with lesser impact on the model.
Figure 2.
Graphical depiction of the OR obtained in the Logistic-LASSO model. ORs close to 1 (red vertical line) indicate variables with lesser impact on the model.
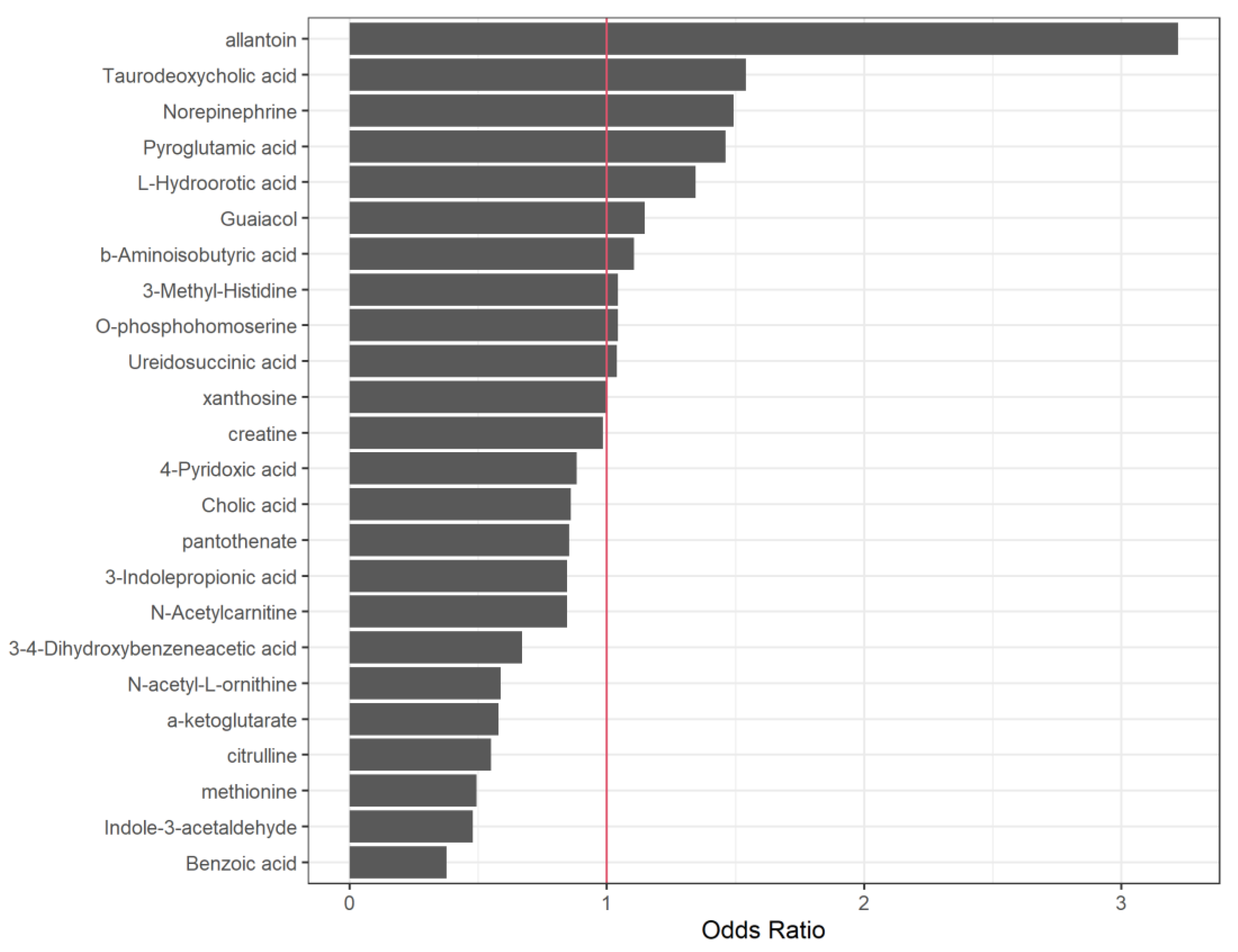
Figure 3.
Volcano plot of metabolomic variables. The horizontal dashed line represents the limit of statistical significance (corresponding to p-value = 0.05); values above it denote variables with significantly different medians. The vertical dashed lines represent a log2(foldchange) of -0.6 and +0.6. Values above + 0.6 represent variables with a median for inflammation=yes that is 1.5 times in greater magnitude than for inflammation=no Values below -0.6 represent variables with a median for inflammation=no that is 1.5 times greater in magnitude than for inflammation=yes.
Figure 3.
Volcano plot of metabolomic variables. The horizontal dashed line represents the limit of statistical significance (corresponding to p-value = 0.05); values above it denote variables with significantly different medians. The vertical dashed lines represent a log2(foldchange) of -0.6 and +0.6. Values above + 0.6 represent variables with a median for inflammation=yes that is 1.5 times in greater magnitude than for inflammation=no Values below -0.6 represent variables with a median for inflammation=no that is 1.5 times greater in magnitude than for inflammation=yes.
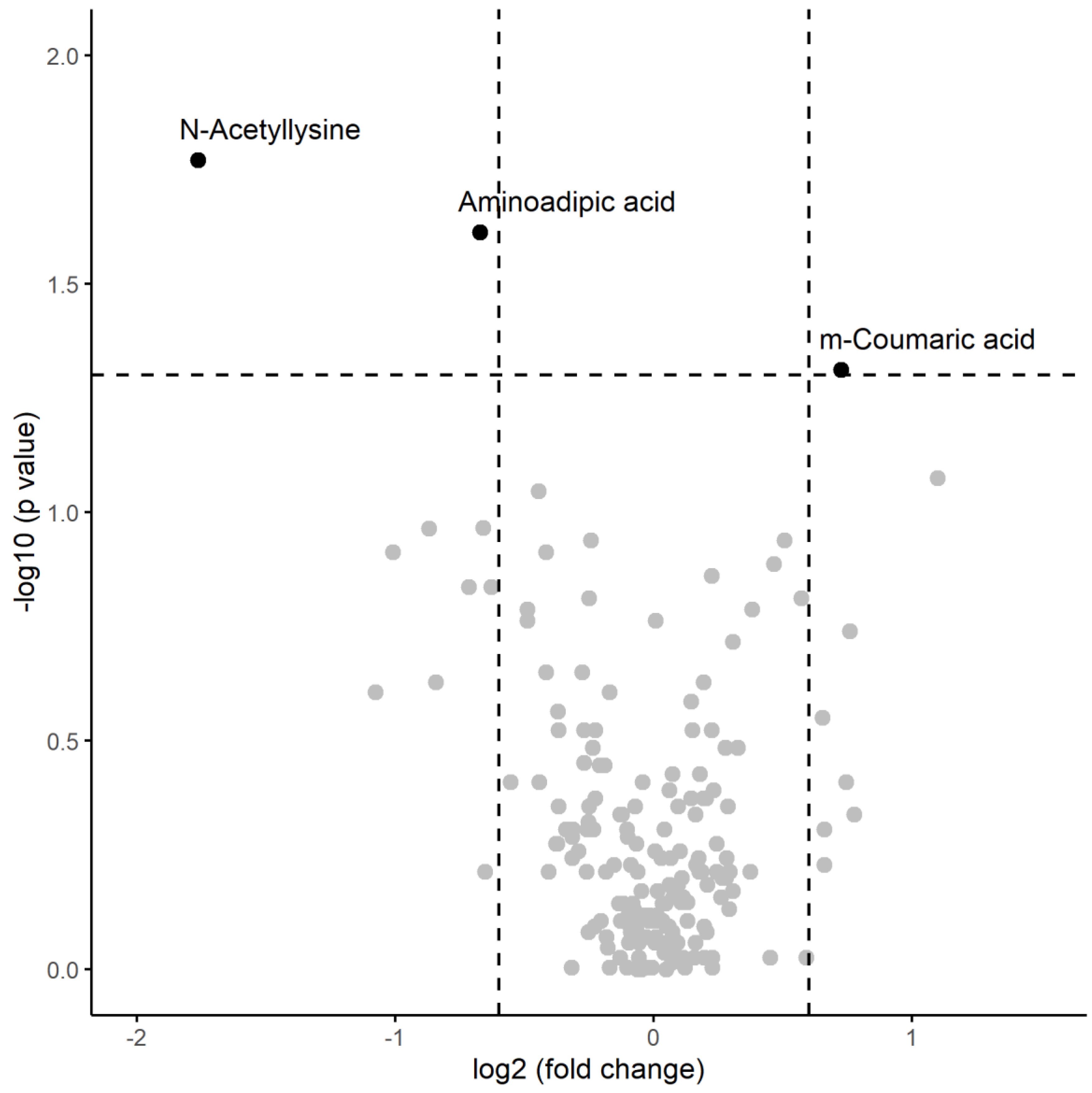
Figure 4.
Relationship of urocanic acid, alpha-ketoglutarate, and 2-oxobutyrate with the Krebs cycle, autophagy and mitochondrial biogenesis.
Figure 4.
Relationship of urocanic acid, alpha-ketoglutarate, and 2-oxobutyrate with the Krebs cycle, autophagy and mitochondrial biogenesis.
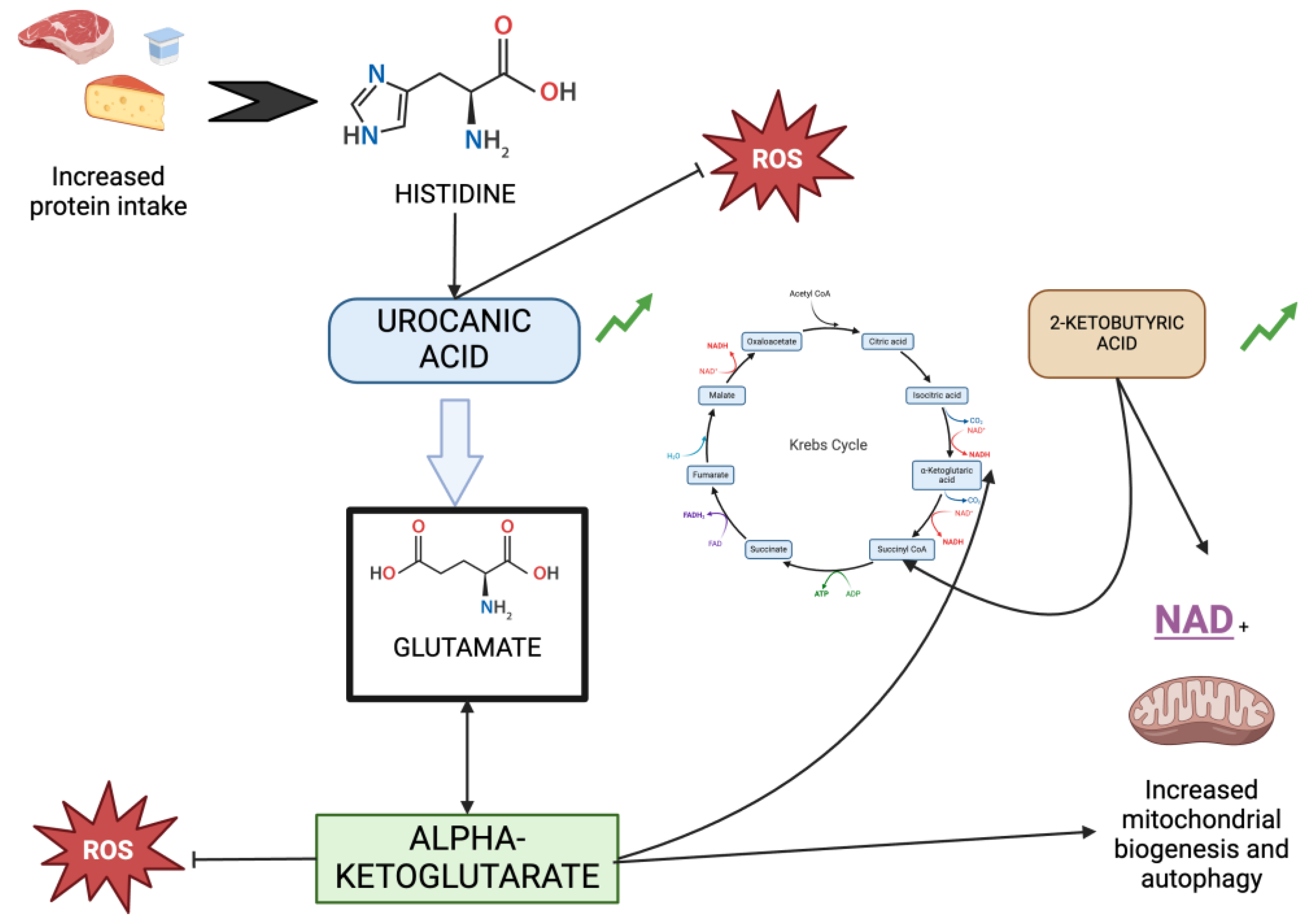
Figure 5.
Metabolic pathways involving citrulline and n-acetylornithine highlighting their roles in arginine metabolism and polyamine synthesis influencing inflammation and tissue health.
Figure 5.
Metabolic pathways involving citrulline and n-acetylornithine highlighting their roles in arginine metabolism and polyamine synthesis influencing inflammation and tissue health.
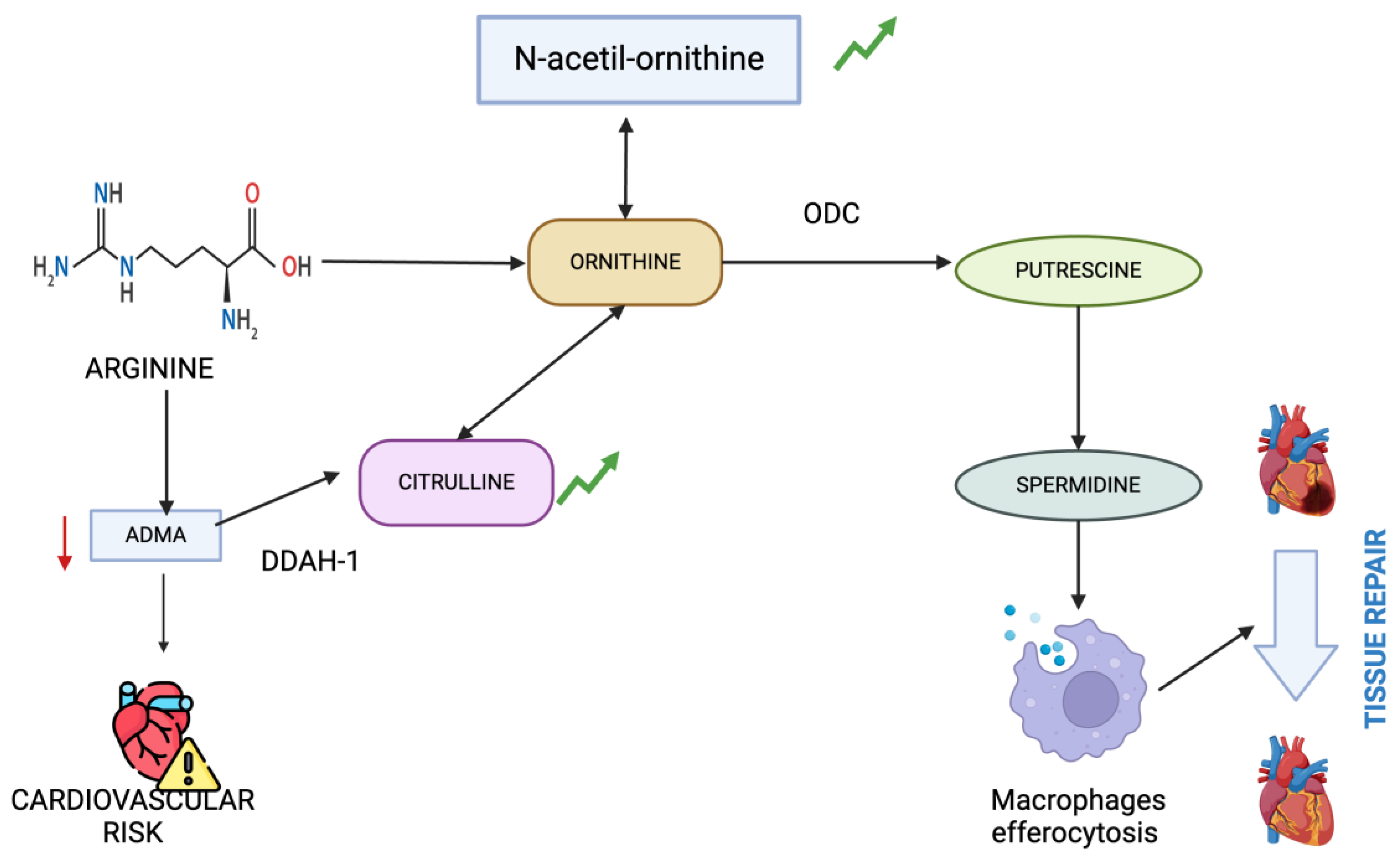
Table 1.
Demographic characteristics of the sample.
| Variable | Mean/frequency |
|---|---|
| Age (years) (mean ± SD) | 68.97 ± 14.18 |
| Female gender, n (%) | 16 (37%) |
| Height (cm) (mean ± SD) | 165.23 ± 8.87 |
| Weight (kg) (mean ± SD) | 75.03 ± 16.73 |
| BMI (mean ± SD) | 27.75 ± 4.76 |
| Smoker, n (%) | 23 (53,5%) |
| Type 2 diabetes, n (%) | 21 (48,8%) |
| Hypertension, n (%) | 40 (93%) |
| Dyslipidemia, n (%) | 34 (79,1%) |
| Urea (mg/dl) (mean ± SD) | 88.70 ± 25.59 |
| Uric acid (mg/dl) (mean ± SD) | 4.97 ± 1.22 |
| Corrected calcium for serum albumin (mg/dl) (mean ± SD) | 9.13 ± 0.77 |
| Phosphate (mg/dl) (mean ± SD) | 4.26 ± 1.13 |
| Sodium (mEq/L) (mean ± SD) | 139.84 ± 2.98 |
| Potassium (mEq/L) (mean ± SD) | 4.70 ± 0.69 |
| Chloride (mEq/L) (mean ± SD) | 106 ± 4.33 |
| CRP (mg/L) (mean ± SD) | 14.24 ± 57.29 |
| Hemoglobin (g/dl) (mean ± SD) | 9.5 ± 1.35 |
| Leukocytes (× 109/L) (mean ± SD) | 7.03 ± 2.30 |
| Platelets (× 109/L) (mean ± SD) | 198.53 ± 62.55 |
| AVF as a venous access, n (%) | 24 (55,8%) |
| Hemodiafiltration as KRT, n (%) | 32 (74,4%) |
| Average interdialysis weight gain (cc) (mean ± SD) | 1622,5 ± 1018,67 |
| Kt/V (mean ± SD) | 1,7 ± 0,5 |
| Residual diuresis (cc) (mean ± SD) | 2097,67 ± 765,08 |
| Inflammed patients defined as CRP ≥ 2 mg/L, n (%) | 23 (67%) |
Table 2.
Numerical summary of metabolomic variables with significant differences in their medians based on inflammation=no and inflammation=yes.
Table 2.
Numerical summary of metabolomic variables with significant differences in their medians based on inflammation=no and inflammation=yes.
| Variable | Median inflammation =No | IQR Inflammation =No | Median Inflammation =Yes | IQR inflammation =Yes | Mann-Whitney statistic | p-value |
|---|---|---|---|---|---|---|
| Indole-3-acetaldehyde | 1190130 | 579521 | 778141 | 467437 | 316 | 0.0023 |
| tryptophan | 2988287 | 2263933 | 2191984 | 1182744 | 313 | 0.0031 |
| methionine | 937980 | 238906 | 641991 | 319903 | 310 | 0.0041 |
| Benzoic acid | 42742 | 18539 | 29556 | 14609 | 309 | 0.0045 |
| carnitine | 82227848 | 73058260 | 58997524 | 53464650 | 295 | 0.0145 |
| 2-Ketobutyric acid | 872432 | 494891 | 517984 | 433389 | 291 | 0.0196 |
| alanine | 7982122 | 1829652 | 6727464 | 1840257 | 286 | 0.0281 |
| Urocanic acid | 15909 | 15612 | 8828 | 13043 | 284 | 0.0323 |
| 2-Methyl-3-ketovaleric acid | 65164816 | 25911640 | 51769852 | 22772580 | 284 | 0.0323 |
| trehalose | 74993 | 45349 | 100659 | 51935 | 123 | 0.0370 |
| D-2-Aminobutyric acid | 467418 | 132337 | 379133 | 248834 | 282 | 0.0370 |
| N-Acetyl-L-phenylalanine | 50225 | 36626 | 70619 | 56563 | 124 | 0.0396 |
| tyrosine | 2760424 | 852724 | 2470091 | 1027556 | 280 | 0.0423 |
| N-Acetylcarnitine | 31762998 | 18670840 | 26701374 | 14508230 | 278 | 0.0481 |
Table 3.
Variables, standardized coefficients, and Odds Ratios obtained (LASSO).
| Variables | Coef.estandarizados | Odds Ratio |
|---|---|---|
| Allantoin | 1.1695281 | 3.2204726 |
| Benzoic acid | -0.9747088 | 0.3773022 |
| Indole-3-acetaldehyde | -0.7354908 | 0.4792702 |
| methionine | -0.7067793 | 0.4932302 |
| Citrulline | -0.5962893 | 0.5508519 |
| a-ketoglutarate | -0.5466127 | 0.5789074 |
| N-acetyl-L-ornithine | -0.5330003 | 0.5868416 |
| Taurodeoxycholic acid | 0.4326940 | 1.5414044 |
| 3-4-Dihydroxybenzeneacetic acid | -0.4005447 | 0.6699550 |
| Norepinephrine | 0.4000314 | 1.4918716 |
| Pyroglutamic acid | 0.3795375 | 1.4616085 |
| L-Hydroorotic acid | 0.2959347 | 1.3443824 |
| N-Acetylcarnitine | -0.1682766 | 0.8451200 |
| 3-Indolepropionic acid | -0.1674606 | 0.8458099 |
| pantothenate | -0.1585006 | 0.8534225 |
| Cholic acid | -0.1503721 | 0.8603877 |
| Guaiacol | 0.1375204 | 1.1474251 |
| 4-Pyridoxic acid | -0.1233154 | 0.8839848 |
| b-Aminoisobutyric acid | 0.1005446 | 1.1057730 |
| 3-Methyl-Histidine | 0.0429894 | 1.0439269 |
| O-phosphohomoserine | 0.0415483 | 1.0424235 |
| Ureidosuccinic acid | 0.0387375 | 1.0394976 |
| creatine | -0.0143093 | 0.9857926 |
| xanthosine | 0.0020708 | 1.0020730 |
Final del formulario.
Table 4.
Numerical summary of metabolomic variables in post-session with significant differences in their medians based on inflammation=no and inflammation=yes.
Table 4.
Numerical summary of metabolomic variables in post-session with significant differences in their medians based on inflammation=no and inflammation=yes.
| Variable | Median inflammation =No | IQR Inflammation =No | Median Inflammation =Yes | IQR inflammation =Yes | Mann-Whitney statistic | p-value |
|---|---|---|---|---|---|---|
| N-Acetyllysine | 5267 | 9939 | 1550 | 3945 | 248 | 0.0170 |
| Aminoadipic acid | 33806 | 29710 | 21197 | 20038 | 244 | 0.0244 |
| m-Coumaric acid | 60008 | 50430 | 99180 | 82241 | 101 | 0.0487 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated







