1. Introduction
Already from the early months of the COVID-19 pandemic, it became apparent that the new coronavirus was able to trigger a hyperinflammatory response driving multi-systemic involvement along with respiratory distress [
1,
2]. In this context, there was a need to find immunomodulatory strategies able to dampen lung and systemic inflammation, but at the same time being sufficiently safe for critically ill infected patients [
3]. On April 2020, the Spanish Ministry of Health encouraged the development of pragmatic clinical trials (CT) aimed to reduce mortality, as also to alleviate hospital and intensive care resources saturation. In a short time frame, we set a randomized CT -which results are presented in this article- to assess the efficacy of cyclosporin A (CsA) as add-on therapy to standard of care (SoC) in inpatients with COVID-19 pneumonia requiring oxygen supplementation.
Considering that patients with COVID-19 associated hyperinflammation showed features of macrophage activation syndrome [
2,
4], we postulated that SARS-CoV2 was able to infect myeloid cells, hijacking cell machinery and hindering antiviral responses [
5]. This pathogenic pathway, which is shared by different RNA viruses, can result in inflammation/autoimmunity thereby providing a rationale for the use of immunosuppressants [
6,
7]. In addition, the involvement of RIG-I sensors and mitochondrial antiviral signaling (MAVS) protein in betacoronaviridae recognition pointed to mitochondrial dysfunction as a key event in COVID-19 hyperinflammation [
8,
9,
10].
Along with its cornerstone place in the prevention of graft rejection, CsA is widely used for the treatment of autoimmune and inflammatory disorders. Notwithstanding, the fungus derivate has unique characteristics not limited to its tolerogenic profile. In particular, CsA binding of cyclophilins provides cytoprotection during hypoxia/reperfusion injury and upon the incidence of stressors jeopardizing cellular metabolic processes [
11,
12]. Moreover, inhibition of cyclophilins has been shown to account for an effective counterattack strategy against RNA viruses, including coronaviridae [
13]. Because of the profound lymphocyte depletion which characterizes the acute phase of COVID-19, both of the antiviral properties of CsA as also its good safety profile in patients with HIV were determinant for our decision to launch this trial [
14,
15]. The main purpose of the study was to evaluate the efficacy and safety of CsA as add-on therapy to SoC in improving severity in inpatients with COVID-19 pneumonia and respiratory failure.
2. Materials and Methods
2.1. Study Design
A national, multicentric, open label, low intervention, controlled, randomized study was designed. The study was approved by Ethics Committee and Spanish Medicines Agency (AEMPS) and prospectively registered before recruitment started (NCT04392531). All subjects provided informed consent before entering the trial.
2.2. Study Population
The study was performed in 8 university hospitals across Spain. Potential eligible participants were adults of both sexes with admission criteria because of COVID-19 pneumonia. Detailed inclusion and exclusion criteria are included in Supplementary file 1.
As there were no published studies available at the time of study design to estimate CsA effect, sample size was calculated hypothesizing that adding CsA to SoC would increase the proportion of patients normalizing FiO2 values by day 12 from 70% to 90%. Accepting an alpha risk of 0.05 and a beta risk of 0.2 in a bilateral contrast, 60 subjects were needed in each group to detect as statistically significant the predefined difference.
2.3. Randomization and Study Treatment
Patients fulfilling eligibility criteria were randomly assigned in a 1:1 ratio, to either CsA+SoC or SoC group and stratified by severity level on the day of inclusion. This severity classification was based on British Guideline for oxygen use [
16] and an adaptation from a FiO2-based risk classification for emergency triage (see Supplementary File 2). For stratification purposes, patients were classified in lower (FiO2 requirements < 60%) and higher (FiO2 requirements ≥ 60%) severity level. Patients >80 years were stratified as higher severity level regardless of oxygen requirements. Randomization was centrally performed by using the “blocrank” R package in Coordinating Hospital Statistics Department. No blinding procedures were performed.
2.4. Study Treatment
Patients assigned to CsA+SoC group started treatment with CsA within 24h from inclusion, according to a dosing schedule described in detail in Supplementary file 3. Duration of treatment was 2 and 4 weeks from inclusion for patients stratified as non-severe and severe, respectively.
2.5. Study Procedures
At screening visit, demographics, relevant medical history, COVID-19 related sign and symptoms and CURB-65 [
17] scoring were registered. FiO2 and level of supplemental oxygen requirements were registered daily. In addition, physical examination, vital signs, CsA dosing and compliance, concomitant treatments, complications and adverse events were registered throughout the trial. Routine laboratory parameters including C-reactive protein (CRP), lactate dehydrogenase (LDH), ferritin, creatine phosphokinase (CPK), troponin I, D-dimer were monitored every 48h during admission. Urinalysis (including creatinine and Na), interleukin (IL)-6 levels, lymphocyte subpopulations and CsA plasma concentrations were assessed weekly. Anti SARS-CoV-2 IgG and IgM quantification as well as SARS-CoV-2 detection with PCR techniques were done at baseline, 8th and 15th days of hospitalization and in the end of study (EOS) visit. Chest X ray findings were registered at baseline and in the EOS visit. After discharge, patients were followed by a phone call every 2 days during the first week and every 4 days until EOS visit, to register patient general health status, CsA compliance when applicable, concomitant medications, complications and adverse events. EOS was performed 4 and 6 weeks from admission depending on the severity level reached during hospital stay (lower vs higher), respectively.
2.6. Study Outcomes
According to the study objectives, the primary outcome was the proportion of patients without oxygen support (or who had returned to baseline FiO2 in case of patients with previous oxygen therapy) at day 12 without relapse during follow-up. Secondary outcomes included a) deaths, ICU and hospital stays, and FiO2 change; b) evolution of blood pressure (BP), plasma creatinine and total lymphocyte and CD4 counts; c) adverse events; d) changes in viral load and seroconversion parameters ; e) impact of CsA on the reduction of ferritin, LDH and CRP levels from baseline, peak levels of CPK and CPK and D-dimer, and IL6 levels; f) patient global assessment at EOS.
2.7. Statistical Considerations
For both efficacy and safety assessments, an intention-to-treat analysis was performed including all randomized patients who had received at least one dose of the study medication, and a per protocol analysis including patients who had completed the study. A descriptive analysis of population was performed. The normality of the variables was tested using the Kolmogorov-Smirnov test. To compare differences between treatment groups, Pearson's Chi-square or Student's t-test or the Mann-Whitney test were used depending on the type of variable. Kaplan-Meier survival curves were estimated and compared with the Mantel-Cox test. In addition, Cox regression models were fitted to estimate the Hazard-Ratio together with its confidence interval. An intermediate analysis for the primary outcome was foreseen and performed when 40% of the study population had reached the 8th day of hospitalization. Subgroup analyses by age and severity level were also performed. The statistical package SAS® 9.3 (SAS Institute Inc., Cary, NC, USA) was used to carry out the analysis.
3. Results
3.1. Setting and Study Population
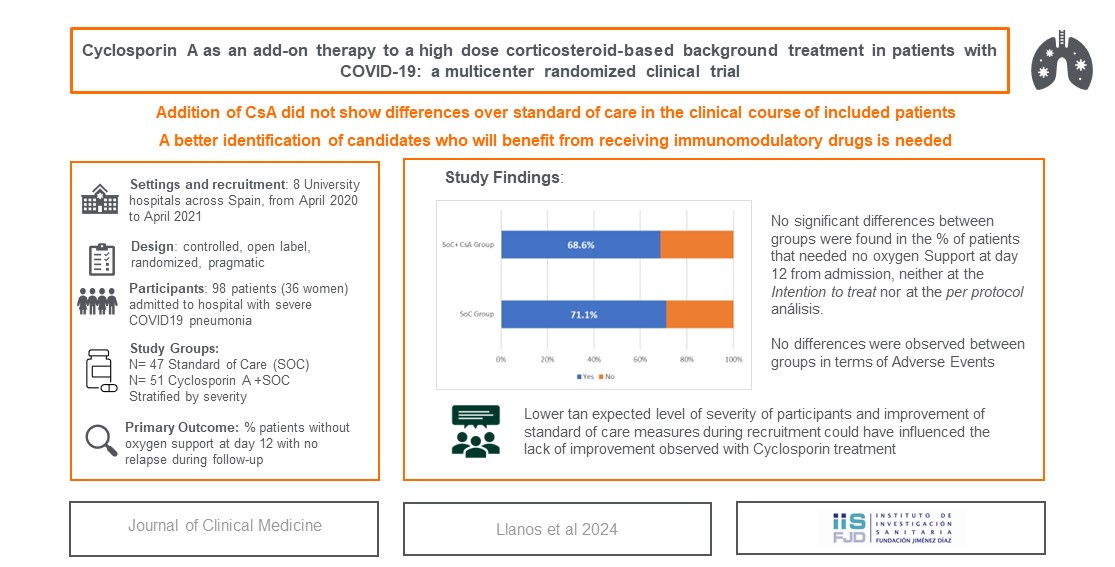
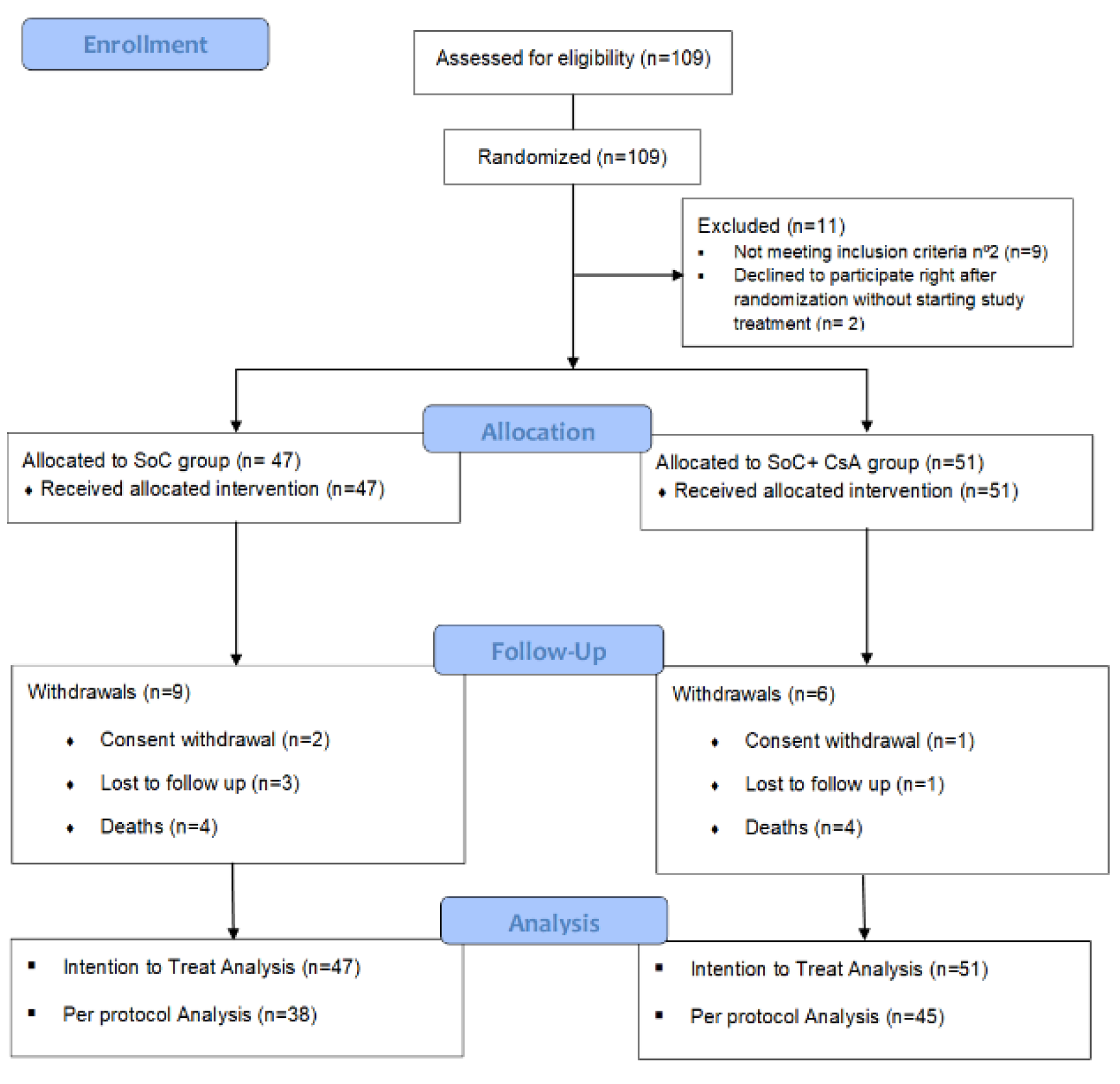
AEMPS approval was received on the 9th of April 2020. 109 patients were included between April 2020 and April 2021 (91% of preplanned sample size). Last center close-out visit was performed in December 2021. All 109 patients were randomized, but 11 of them were not considered for allocation purposes. Thus, 98 patients, 47 in the SoC group and 51 in the CsA+SoC group were considered for the ITT population (See
Figure 1). The majority of the patients were stratified into the “lower severity” stratum (43/47 and 46/51, respectively). 2 patients withdrew consent before reaching day 12, so they could not be included in ITT population for the primary efficacy outcome analysis.
Table 1 shows participants baseline characteristics regarding host dependent features and process related risk factors. No statistically significant differences between groups were found in any of them.
3.2. Study Intervention
Both study groups received similar background treatment according to the SoC protocol elaborated and periodically reviewed by a multidisciplinary committee at Coordinating Hospital (detailed in Supplementary file 3).
In participants assigned to CsA+SoC group, CsA was administered for 15.1 ± 7.5 days, at an average dose of 177 mg/day per patient, drawing a mean cumulative dose of 2686.3 ± 1527 mg [95% CI 2256.8, 3115.8].
With regards to immunomodulating agents, methylprednisolone pulses were administered to 55 participants (25 from SoC group and 30 from CsA+SoC group). In addition, 29 participants (15 in SoC and 14 in CsA+SoC group) received intermediate doses of corticosteroids, whereas administration of rescue medication was done with intravenous 400 mg tocilizumab in 10 subjects (6 from SoC and 4 from CsA+SoC group).
3.3. Primary Outcome
As shown in
Table 2, no significant differences in primary outcome were found between groups for either of the analysis populations. 32 (71,1%) patients from SoC group and 35 (68.6%) from CsA+SoC group reached the primary endpoint in the ITT analysis while proportions drawn in the PP analysis were 76.3% vs 75.6%, respectively. No significant differences were found in the subgroup analysis by age (>65 years) or severity level.
Only 9 participants (6 in the PP analysis) were included in the higher severity category at recruitment. Of them, 1 patient in each treatment arm (25% in SoC group vs 20% in CsA+SoC group) achieved the primary endpoint. Intermediate analysis showed no statistically significant difference between groups for the primary outcome.
3.3. Secondary Outcomes
Results regarding mortality, length of hospital stay and FiO2 evolution are listed in
Table 3. There were 8 deaths during hospitalization, 4 in each of the treatment arms, all attributable to progression of respiratory failure and critical illness-related complications. Hospital length of stay was slightly higher in the CsA+SoC group (10.3 vs 9.4 days), particularly in those patients stratified as lower-severity and in patients above 65 years (8.7 vs 10.4). A total of 15 patients were admitted to the ICU during the episode (5 of them allocated to group A and 10 to group B), of whom only 1 patient was found to meet the primary endpoint. No significant differences were observed in any of these comparisons.
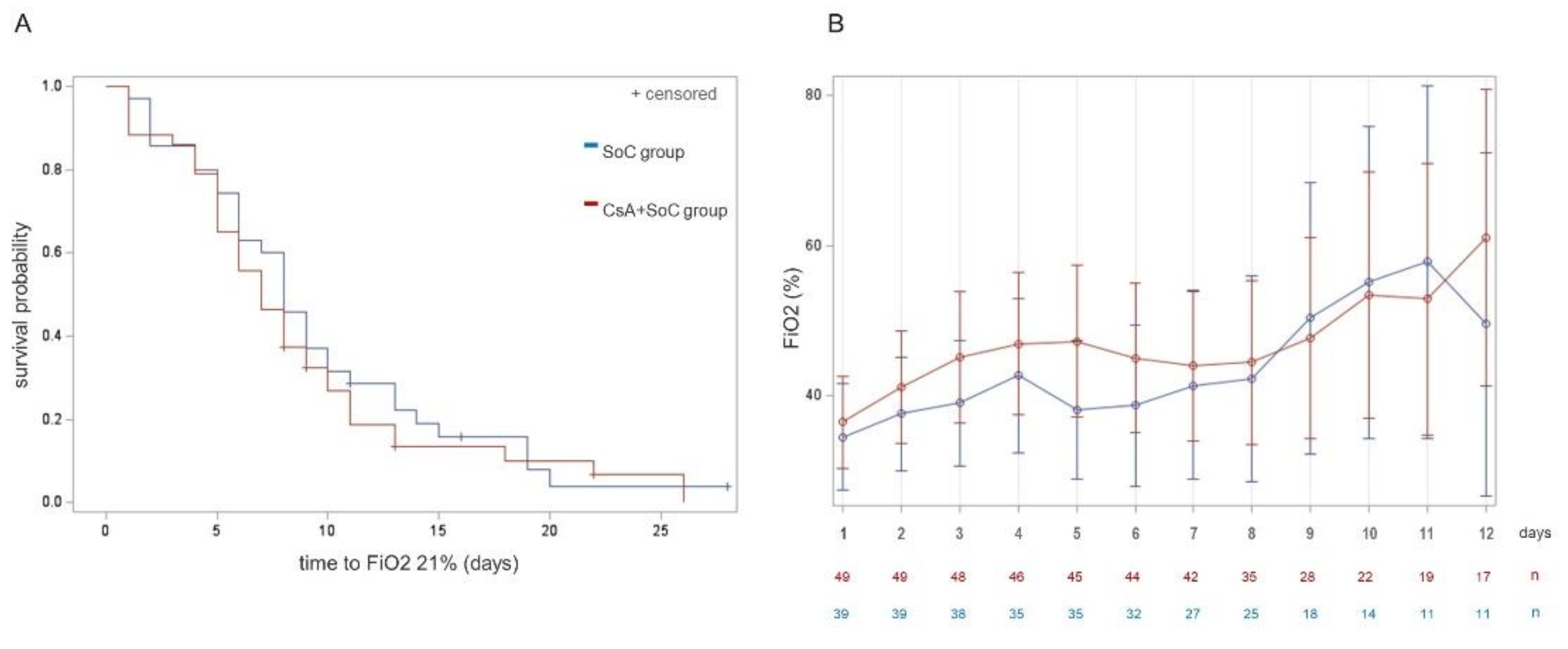
Figure 2 shows time to FiO2 normalization in both arms (A), and mean FiO2 oxygen requirements during the 12 first days of the study in the ward-admitted population (B). Post hoc analyses exploring daily FiO2 requirements were performed in patient subgroups according to FiO2 at enrollment and age subgroups. However, the size of these comparisons was low and none of them yielded significant differences. These results are shown in Supplementary file 4.
3.4. Safety Measures
Systolic blood pressure (SBP) showed a tendency to drop during hospitalization in patients enrolled in SoC group. Conversely, patients in CsA+SoC group showed stable or increasing SBP values, being the difference between arms significant at day 16 (p 0.031). On the other hand, no differences were observed in diastolic BP. Plasma creatinine, which was the principal analytical safety measure, remained stable during admission.
A total of 130 adverse events occurred, most of them of mild intensity (86,2%), 46 in SoC group and 84 in CsA+SoC group. Both the incidence of adverse events (AE) and of treatment related AE were significantly higher in CsA+SoC group (p 0.025 and 0.003, respectively). There were 7 SAEs, 4 of them in patients from SoC group and 3 in CsA+SoC, out of which 2 in each arm were regarded as treatment related. No unexpected AEs were observed. (See
Table 4).
There were 2 readmissions during the study period, one of them accounting for failure to reach primary endpoint and registered as SAE: an episode of asthma exacerbation in a patient from SoC group and the occurrence of acute pancreatitis in a patient from CsA+SoC group. Both patients had a complete recovery.
Likert scale of symptoms and well-being after discharge confirmed improvement in the global population with 86,8% of good-very good answers in SoC group and 88,6% in CsA+SoC group patients at EOS visit. In addition, X ray improvement was stated in 35/36 of SoC group and 43/44 of those in CsA+SoC group.
3.5. Process Related Analytes
Exploratory objectives included time to normalization of relevant laboratory parameters and did not yield significant differences between treatment arms. These comparisons can be found at Supplementary file 5. Briefly, CRP, LDH and ferritin levels were found to decrease over the first 5 days of the trial, whereas D-dimer persisted moderately high during admission. Less movements were observed in levels of the muscle cell markers CPK and Tnp I during the trial. Leucocyte subpopulations were determined, and, in a subgroup of patients, a full analysis of lymphocyte subtype differentiation was included. Of note, the profound depletion of CD4 and CD8 T cells observed in most patients on admission was recovered by day 8 and reconstitution of the different subpopulations was similar between both treatment arms. Lack of enough data at days 15 and 22 hampered comparisons at these time periods. Levels of immunoglobulin classes were within normal range at all time-points and comparable between arms. With respect to seroconversion, around half of the patients had anti SARS-CoV2 positive antibody titers at the study entry (day 1), and the percentage increased to 96% and 81% of IgG anti SARS-CoV2 antibodies at day 8, respectively in SoC and CsA+SoC groups (
Table 5).
4. Discussion
We report here negative results from a randomized controlled trial (RCT) of CsA in patients with COVID-19 pneumonia and respiratory failure on a high-dose corticosteroid background treatment. Even 3 years after the disease outbreak, no definite clues helping select candidates for immunosuppressants have been found. Moreover, solid evidence supporting a role of specific immunosuppressants besides corticosteroids in improving the outcomes of patients with moderate to severe disease is still lacking. Emerging results from different RCT have drawn a small effect size of different strategies, a situation which underscores the need to enroll large numbers of participants. For instance, the RECOVERY Collaborative Group gathered almost 6500 patients to confirm the efficacy of dexamethasone in improving survival of critically ill patients and of those in need of oxygen support [
18]. As for anti-IL6 strategies, their efficacy in improving the principal outcomes of COVID-19 continues to be controversial, according to an updated Cochrane systematic review [
19], in spite of the amount of available data, while the use of other immunosuppressants cannot be recommended at this time [
20,
21,
22].
All clinicians involved in COVID-19 management believe that these agents hold higher efficacy than the one found in the trials, perhaps due to the individualized selection criteria applied in their clinical practice. A plain explanation for RCT failures is that COVID-19 is a complex disease which outcome is determined by a multifactorial background, which includes not only host-intrinsic factors, such as the susceptibility gene variants [
23], but also those related to saturation of health care resources, community transmission and viral load, risk associated to invasive procedures and prolonged hospitalization, timing of referral / consultation, and so forth.
As concerns to our study, even though controllable risk factors were equally distributed between arms, several facts may have contributed to its results, as next discussed.
In the first place, we designed the trial in accordance with the characteristics of patients admitted to Fundación Jiménez Díaz University Hospital during the first weeks of the pandemic. The severity of the condition at that time was extremely high, with almost 30% of admitted patients in need of ventilatory support. In addition, patients usually showed a flaring evolution leading to prolonged hospital stays. At those weeks, a strict lockdown protocol and the use of face masks had an immediate impact on SARS-CoV2 transmission in our environment and most probably on the viral load of the infected, altogether making possible to the initial wave to subside. These circumstances completely changed the scenario for the trial. Indeed, the pandemic course in waves led to a slow and intermittent recruitment pace, in different epidemiological settings, with earlier referrals and better standard of care. The latter included the use of corticosteroids, thromboprophylaxis and specific ventilation procedures which together succeeded in lowering severity. We could effectively observe the impact on these measures in the interim analysis, since our study population did not show the anticipated severity, as also at the end of the trial by a comparatively much lower mortality than the one coming from published data, including the mentioned RECOVERY cohort18. Even though we introduced an amendment to allow inclusion of older, more severe patients (see Supplementary File 7), the post hoc subanalyses of age, comorbidities, or severity of respiratory failure did not help identifying potential candidates for the use of CsA in the study population. Most probably, these subanalyses were hampered by the existence of a group of patients with mild disease, who did not deteriorate during admission and could be rapidly discharged. Notwithstanding, when we plotted the sequential FiO2 requirements, we could identify a signal for a benefit of CsA in preventing a second flare of respiratory failure in a subgroup of patients remaining admitted on day 6 of the trial (as elaborated on Supplementary file 5). This possibility remains highly speculative but led us to explore risk factors for need of hospital care after 1 week of admission as a putative outcome measure in future trials (see Supplementary file 6).
An additional point to raise is that, on the whole, our population did not reach cut-off values of hyperinflammation -except for CRP levels- [
2,
24] which probably defines the target population for immunosuppressant strategies.
Finally, we cannot rule out that the dosing schedule of CsA was insufficient and also that the efficacy of corticosteroids could overshadow that of CsA up to a point. This fact is supported by the trends in normalization of white cell subpopulations during the first week of the trial, which was comparable between arms.
In summary, CsA did not increase therapeutic response over SoC in patients with COVID-19 pneumonia and respiratory failure. We suggest that the target population for this kind of strategy should be carefully selected. In addition, the scenario of inpatients with COVID-19 changed “on the go” soon after the trial started, and outcome measures would have needed to be changed accordingly. We propose the use of a set of criteria predicting risk of prolonged hospital care as a therapeutic objective in future trials, in order to select a target population for immunotherapies.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Supplementary file 1. Eligibility Criteria; Supplementary file 2. Classification of respiratory failure according to oxygen requirement and levels of hospitalization; Supplementary file 3. A. Schematic view of institutional guidelines for the management of inpatients with COVID-19 pneumonia. B. B. Cyclosporin A treatment schedule employed during the trial. C. Criteria for admission of patients with COVID-19 pneumonia throughout the trial; Supplementary file 4. Comparisons between therapeutic arms in daily evolution of FiO2 requirements classifying the sample according to age and baseline FiO2 categories; Supplementary file 5. Evolution of laboratory parameters; Supplementary file 6. Independent risk factors predicting long hospital stays in the study population; Supplementary File 7. Summary of Relevant Protocol Amendments; Supplementary File 8. Study Protocol Current version (in Spanish).
Author Contributions
Conceptualization, Lucía Llanos Jiménez, Germán Peces-Barba, Maria Jesus Rodriguez Nieto and Olga Sánchez-Pernaute; Investigation, Lucía Llanos Jiménez, Beatriz Alvarez-Alvarez, Eva Fonseca Aizpuru, Gloria Pindao Quesada, Francisco J Ruíz-Hornillos, Luis Seijo Maceiras, Ignacio Robles Barrena and Álvaro Mena-de-Cea; Methodology, Lucía Llanos Jiménez, Germán Peces-Barba, Maria Jesus Rodriguez Nieto and Olga Sánchez-Pernaute; Supervision, Lucía Llanos Jiménez and Olga Sánchez-Pernaute; Writing – original draft, Lucía Llanos Jiménez and Olga Sánchez-Pernaute; Writing – review & editing, Germán Peces-Barba and Olga Sánchez-Pernaute.
Funding
This work was supported by a generous donation from Fundación Tatiana Pérez de Guzmán el Bueno. Cyclosporin A was provided by each participating hospital Pharmacy Department at no cost.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Fundación Jimenez Diaz Ethics Committee belonging to FJD Health Research Institute (protocol code EC029-20_FJD-HIE-HRJC-HGV, EudraCT 2020-001262-11; 8th may 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Raw Data and results from statistical analysis are available on request from the corresponding author (lucia.llanos@fjd.es). Data are not publicly available due to patient privacy issues.
Acknowledgments
We would like to express our gratitude to the patients and their families for their willing to participate in the study in such difficult times. We would also like to thank the whole team of health professionals of the participating centers for their dedication to the care of patients during the pandemics. Finally, we thank the Contract Research Organization Alpha Bioresearch, who provided support in regulatory, monitoring, pharmacovigilance, data management and statistical analysis activities for the trial.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Z. Xu, L. Shi, Y. Wang, J. Zhang, L. Huang, C .Zhang, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med., 8 (2020), pp. 420-422. [CrossRef]
- C. Qin, L. Zhou, Z. Hu, S. Zhang, S. Yang, Y. Tao, et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis, 71 (2020), pp. 762-768. [CrossRef]
- P.S. Kim, S.W. Read, A.S. Fauci. Therapy for Early COVID-19: A Critical Need. JAMA, 324 (2020), pp. 2149–2150. [CrossRef]
- M. Merad, J.C. Martin. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol, 20 (2020), pp. 355-362. [CrossRef]
- O. Sanchez-Pernaute , F.I. Romero-Bueno, A. Selva-O'Callaghan. Why choose cyclosporin A as first-line therapy in COVID-19 pneumonia. Reumatol Clin (Engl Ed)., 17 (2021), pp. 556-557. [CrossRef]
- T. Koshiba. Mitochondrial-mediated antiviral immunity. Biochim Biophys Acta, 1833 (2013), pp. 225–232. [CrossRef]
- S.J. Kim, G.H. Syed, M. Khan, W.W. Chiu, M.A. Sohail, R.G. Gish, A. Siddiqui. Hepatitis C virus triggers mitochondrial fission and attenuates apoptosis to promote viral persistence. Proc Natl Acad Sci USA., 111 (2014), pp. 6413-6418. [CrossRef]
- Y. Lei, C.B. Moore, R.M. Liesman, B.P. O'Connor, D.T. Bergstralh, Z.J. Chen, et al. MAVS-mediated apoptosis and its inhibition by viral proteins. PLoS One, 4 (2009), pp. e5466. [CrossRef]
- K.K. Singh, G. Chaubey, J.Y. Chen, P. Suravajhala. Decoding SARS-CoV-2 hijacking of host mitochondria in COVID-19 pathogenesis. Am J Physiol Cell Physiol, 319 (2020), pp. C258-C267. [CrossRef]
- C. Bhowal, S. Ghosh, D. Ghatak, R. De. Pathophysiological involvement of host mitochondria in SARS-CoV-2 infection that causes COVID-19: a comprehensive evidential insight. Mol Cell Biochem, 478 (2023), pp.1325-1343. [CrossRef]
- A.P. Halestrap, S.J. Clarke, S.A. Javadov. Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovasc Res, 61 (2004), pp.372-385. [CrossRef]
- M. Montero, C.D. Lobatón, S. Gutierrez-Fernández, A. Moreno, J. Alvarez. Calcineurin-independent inhibition of mitochondrial Ca2+ uptake by cyclosporin A. Br J Pharmacol, 141 (2004), pp.263-268. [CrossRef]
- Y. Tanaka, Y. Sato, T. Sasaki. Suppression of coronavirus replication by cyclophilin inhibitors. Viruses, 5 (2013), pp. 1250-1260. [CrossRef]
- S. Tavakolpour, T. Rakhshandehroo, E.X. Wei, M. Rashidian. Lymphopenia during the COVID-19 infection: What it shows and what can be learned. Immunol Lett, 225 (2020), pp. 31-32. [CrossRef]
- G.P. Rizzardi, A. Harari, B. Capiluppi, G. Tambussi, K. Ellefsen, D. Ciuffreda, et al. Treatment of primary HIV-1 infection with cyclosporin A coupled with highly active antiretroviral therapy. J Clin Invest, 109 (2002), pp. 681-688. [CrossRef]
- B.R. O'Driscoll, L.S. Howard, J. Earis, V Mak; British Thoracic Society Emergency Oxygen Guideline Group; BTS Emergency Oxygen Guideline Development Group. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax, 72(Suppl1) (2017), pp. ii1-ii90. [CrossRef]
- Jones BE, Jones J, Bewick T, Lim WS, Aronsky D, Brown SM, Boersma WG, van der Eerden MM, Dean NC. CURB-65 pneumonia severity assessment adapted for electronic decision support. Chest. 2011 Jul;140(1):156-163. [CrossRef]
- P. Horby, W.S. Lim, J.R. Emberson, M. Mafham, J.L. Bell, L. Linsell, et al. Dexamethasone in hospitalized patients with covid-19. N Engl J Med, 384 (2021), pp. 693–704. [CrossRef]
- L. Ghosn, R. Assi, T. Evrenoglou, B.S. Buckley, N. Henschke, K. Probyn, et al. Interleukin-6 blocking agents for treating COVID-19: a living systematic review. Cochrane Database Syst Rev, 6 (2023), pp. CD013881. [CrossRef]
- M.K. Han, M. Antila, J.H. Ficker, I. Gordeev, A. Guerreros, A.L. Bernus, et al. Ruxolitinib in addition to standard of care for the treatment of patients admitted to hospital with COVID-19 (RUXCOVID): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Rheumatol, 4 (2022), pp. e351-e361. [CrossRef]
- I. Kralj-Hans, K. Li, A. Wesek, A. Lamorgese, F. Omar, K. Ranasinghe, et al. Leflunomide treatment for patients hospitalised with COVID-19: DEFEAT-COVID randomised controlled trial BMJ Open, 13 (2023), pp. e068179. [CrossRef]
- P. Fanlo, B.D.C. Gracia-Tello, E. Fonseca Aizpuru, J. Álvarez-Troncoso, A. Gonzalez, S. Prieto-González, et al. Efficacy and safety of anakinra plus standard of care for patients with severe COVID-19: A Randomized Phase 2/3 Clinical Trial. JAMA Netw Open, 6 (2023), pp. e237243. [CrossRef]
- E. Pairo-Castineira, K. Rawlik, A.D. Bretherick, T. Qi, Y. Wu, I. Nassiri, et al. GWAS and meta-analysis identifies 49 genetic variants underlying critical COVID-19. Nature, 617 (2023), pp. 764-768. [CrossRef]
- G. Chen, D. Wu, W Guo, Y. Cao, D. Huang, H. Wang, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest, 130 (2020), pp. 2620-2629. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).