Submitted:
24 July 2024
Posted:
25 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Bioinformatics Characteristics
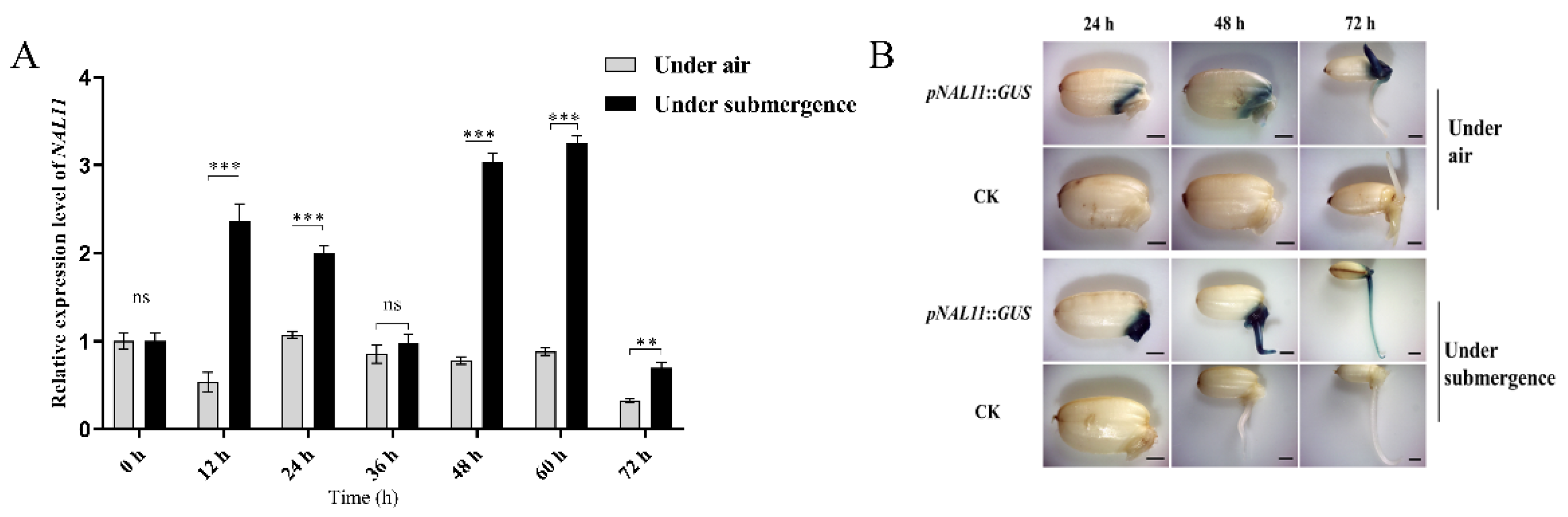
2.2. The Expression of NAL11 Was Induced during Seed Germination under Submerged Conditions
2.3. Knockout Lines Exhibited Longer Coleoptiles during Seed Germination under Submerged Conditions
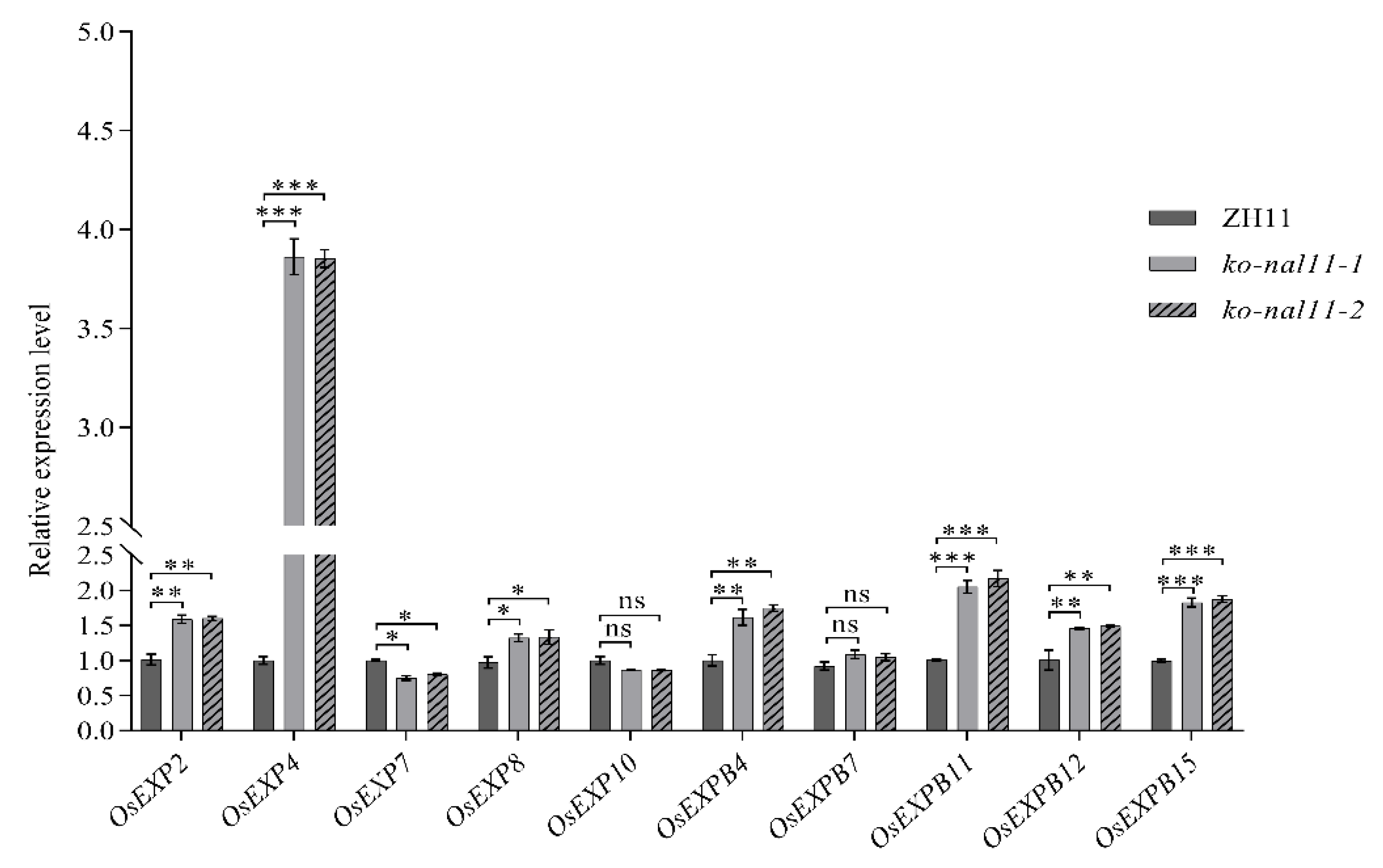
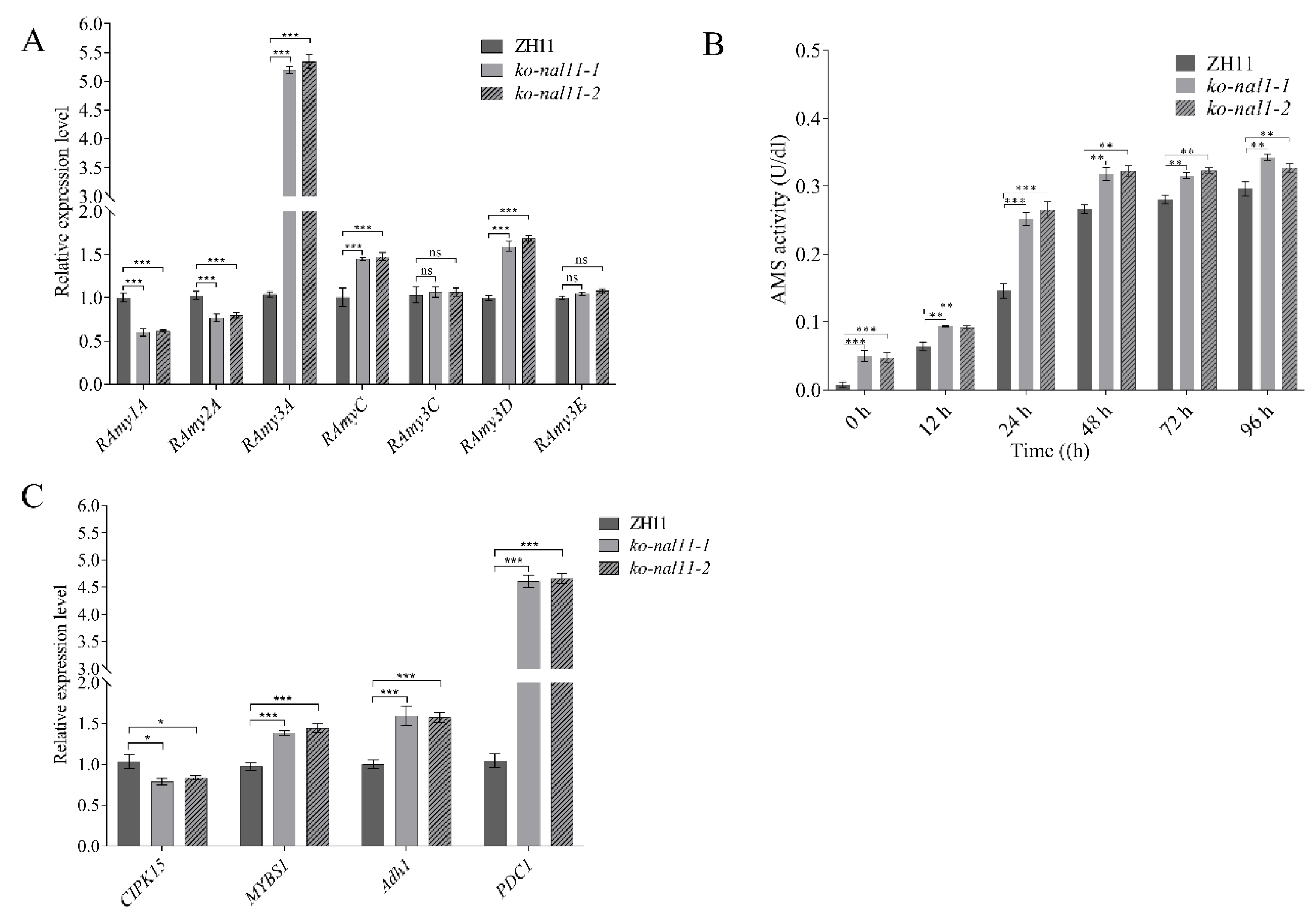
2.4. Knockout of NAL11 Affected Sugar and Energy Pathways under Submerged Conditions
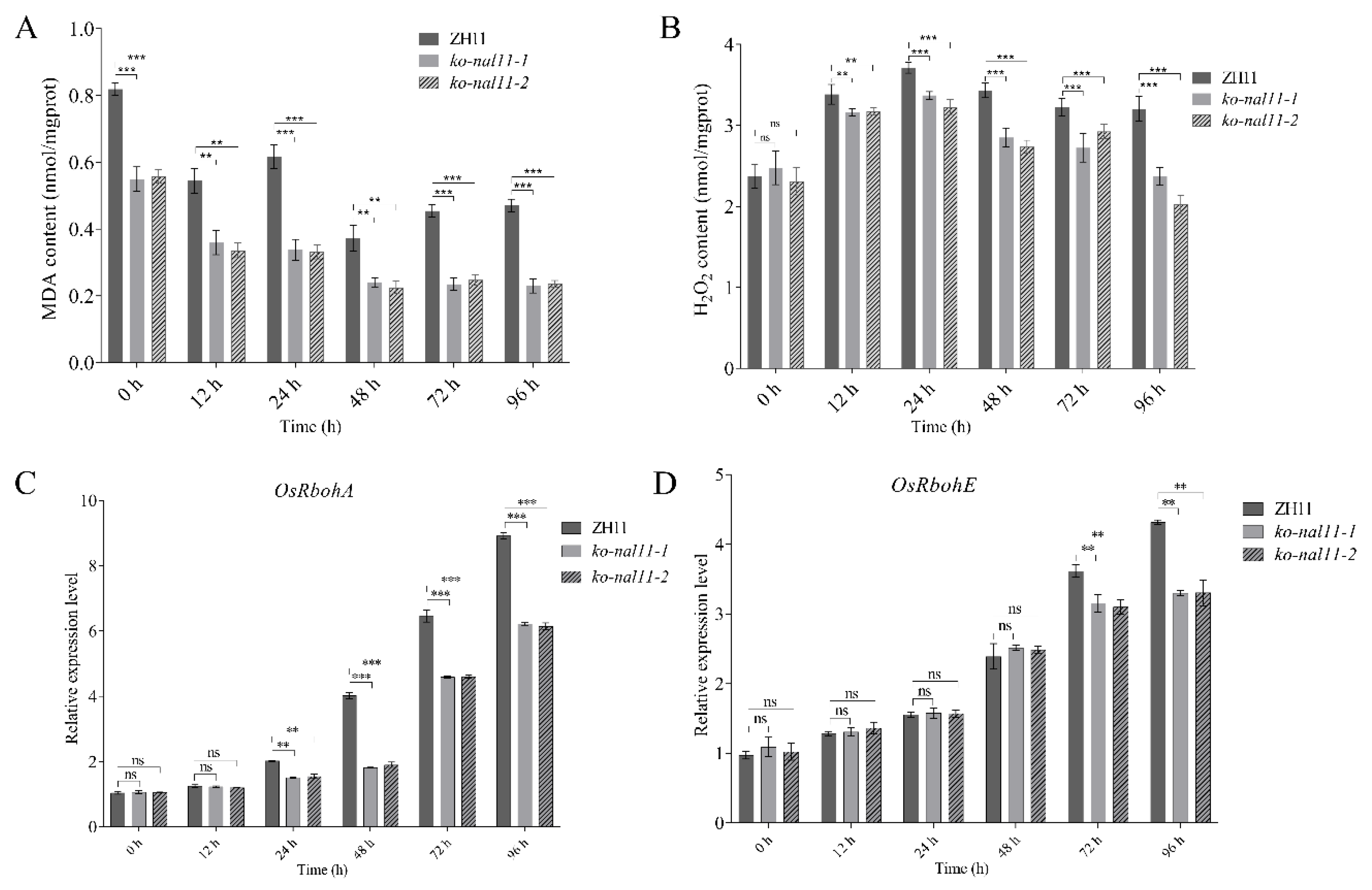
2.5. Knockout of NAL11 Affects ROS Levels and the Expression of Some Stress-Related Genes under Submerged Conditions
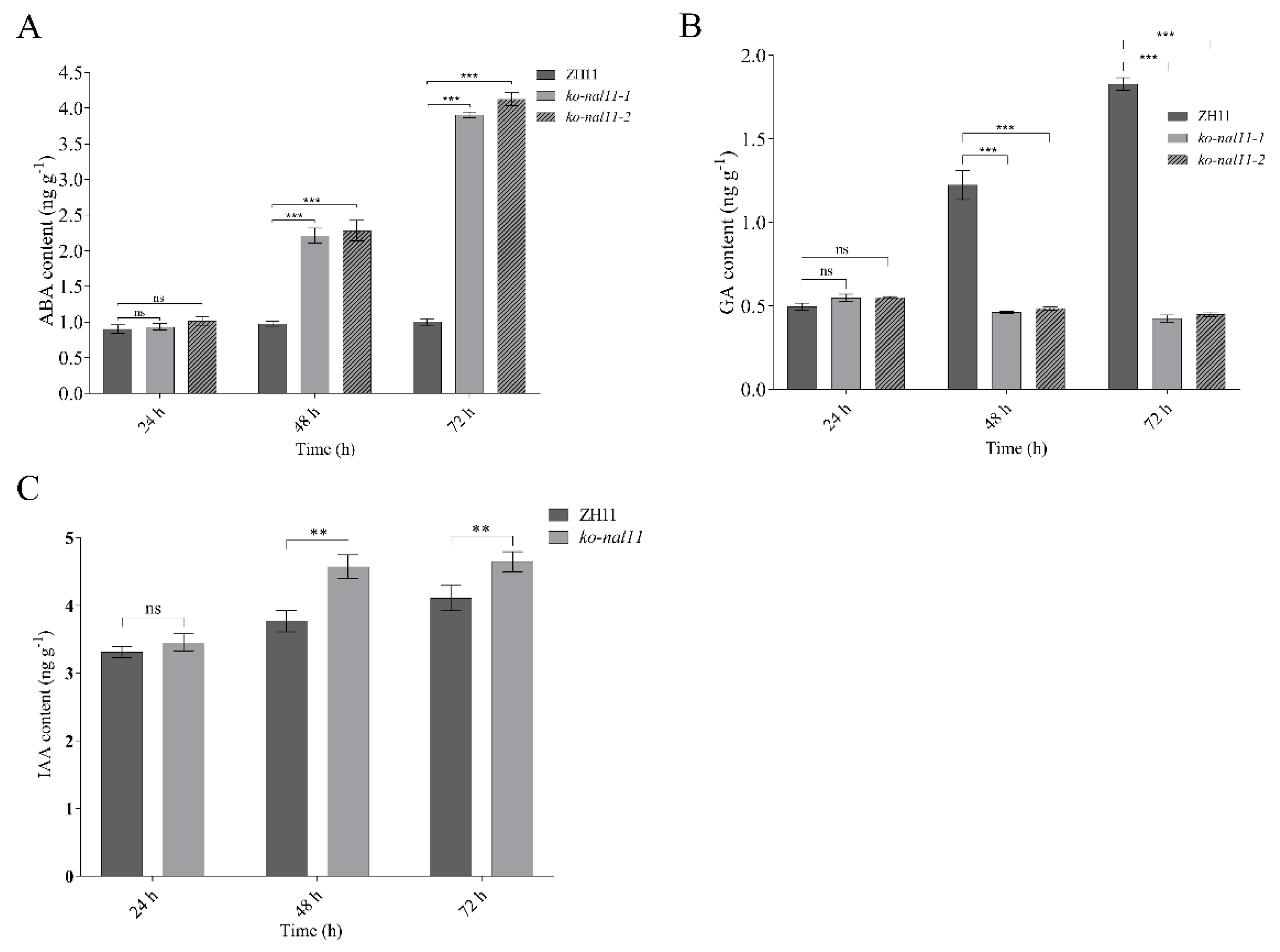
2.6. NAL11 Is Involved in the Phytohormone-Mediated Regulatory Pathway
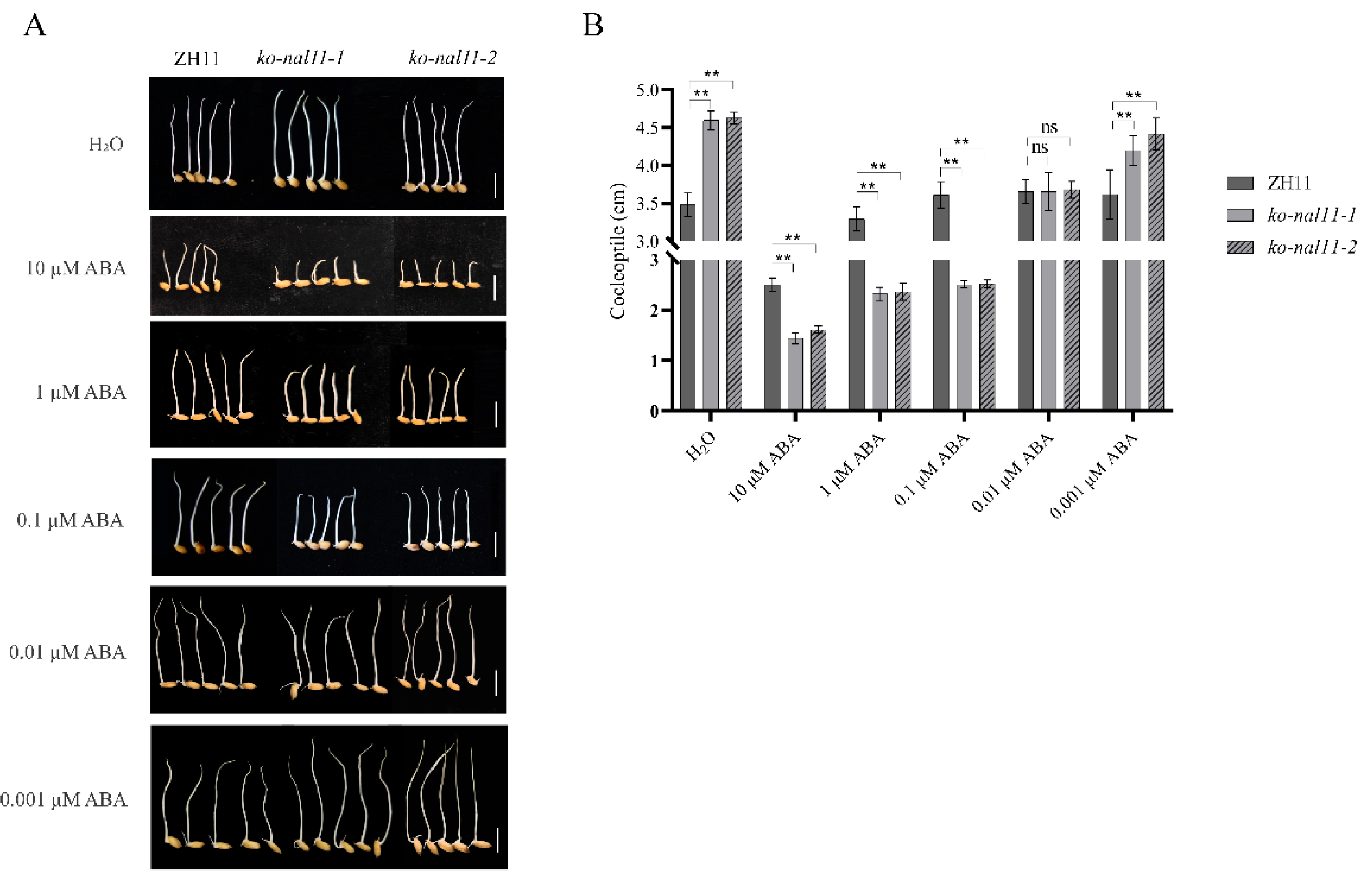
2.7. Knockout Lines Are Sensitive to ABA
3. Discussion
4. Materials and methods
4.1. Bioinformatics Analysis of NAL11
4.2. Plant Materials and Growth Conditions
4.3. Construction of Transgenic Plants
4.4. Evaluation of Germination Rate and Coleoptile Length
4.5. Germination Test by Exogenous Application of ABA
4.6. Measurement of Endogenous ABA, GA and IAA Levels
4.7. Analysis of Physiological Parameters Related to Submergence Stress
4.8. RNA Extraction and Analysis of Gene Expression
4.9. Statistical Analysis
5. Conclusion
Supplementary Materials
Author information
Funding
Data Availability Statement
Conflicts of Interest
References
- Long, Q.; Qiu, S.; Man, J.; Ren, D.; Xu, N.; Luo, R. OsAAI1 Increases Rice Yield and Drought Tolerance Dependent on ABA-Mediated Regulatory and ROS Scavenging Pathway. Rice 2023, 16, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, T.; Pelayo, M.A.F.; Trijatmiko, K.R.; Gabunada, L.F.M.; Alam, R.; Jimenez, R.; Mendioro, M.S.; Slamet-Loedin, I.H.; Sreenivasulu, N.; Bailey-Serres, J.; et al. A trehalose-6-phosphate phosphatase enhances anaerobic germination tolerance in rice. Nat. Plants 2015, 1, 15124. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, G.; Cui, Z.; Kong, X.; Yu, X.; Gui, R.; Han, Y.; Li, Z.; Lang, H.; Hua, Y.; et al. Regain flood adaptation in rice through a 14-3-3 protein OsGF14h. Nat. Commun. 2022, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Perata, P.; Alpi, A. Plant responses to anaerobiosis. Plant Sci. 1993, 93, 1–17. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Voesenek, L. Flooding Stress: Acclimations and Genetic Diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Guo, Z.; Luo, L.; Gao, Q.; Xiao, W.; Wang, J.; Wang, H.; Chen, Z.; Guo, T. Identification of QTL and candidate genes involved in early seedling growth in rice via high-density genetic mapping and RNA-seq. Crop. J. 2020, 9, 360–371. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Lee, S.C.; Brinton, E. Waterproofing Crops: Effective Flooding Survival Strategies. Plant Physiol. 2012, 160, 1698–1709. [Google Scholar] [CrossRef] [PubMed]
- Pucciariello, C. Molecular Mechanisms Supporting Rice Germination and Coleoptile Elongation under Low Oxygen. Plants 2020, 9, 1037. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Lu, X.; Liu, H.; Zhao, Q.; Ye, G. Mesocotyl elongation, an essential trait for dry-seeded rice (Oryza sativa L.): a review of physiological and genetic basis. Planta 2019, 251, 27. [Google Scholar] [CrossRef]
- Choi, D.; Lee, Y.; Cho, H.-T.; Kende, H.; Ht, C. Regulation of Expansin Gene Expression Affects Growth and Development in Transgenic Rice Plants. Plant Cell 2003, 15, 1386–1398. [Google Scholar] [CrossRef]
- Lasanthi-Kudahettige, R.; Magneschi, L.; Loreti, E.; Gonzali, S.; Licausi, F.; Novi, G.; Beretta, O.; Vitulli, F.; Alpi, A.; Perata, P. Transcript Profiling of the Anoxic Rice Coleoptile. Plant Physiol. 2007, 144, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Loreti, E.; Yamaguchi, J.; Alpi, A.; Perata, P. Sugar modulation of α-amylase genes under anoxia. Annals of Botany 2003, 91, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Perata, P.; Geshi, N.; Yamaguchi, J.; Akazawa, T. Effet of anoxia on the induction of α-amylase in cereal seeds. Planta 1993, 191, 402–408. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kugimiya, T. Preferential Induction of Alcohol Dehydrogenase in Coleoptiles of Rice Seedlings Germinated in Submergence Condition. Biol. Plant. 2003, 46, 153–155. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Forde, B.G.; Davies, W.J. The Biphasic Root Growth Response to Abscisic Acid in Arabidopsis Involves Interaction with Ethylene and Auxin Signalling Pathways. Front. Plant Sci. 2017, 8, 1493–1493. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-W.; Chen, P.-W.; Lu, C.-A.; Chen, S.; Ho, T.-H.D.; Yu, S.-M. Coordinated Responses to Oxygen and Sugar Deficiency Allow Rice Seedlings to Tolerate Flooding. Sci. Signal. 2009, 2, ra61–ra61. [Google Scholar] [CrossRef]
- Takahashi, H.; Saika, H.; Matsumura, H.; Nagamura, Y.; Tsutsumi, N.; Nishizawa, N.K.; Nakazono, M. Cell division and cell elongation in the coleoptile of rice alcohol dehydrogenase 1-deficient mutant are reduced under complete submergence. Ann. Bot. 2011, 108, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Xu, N.; Chu, Y.; Chen, H.; Li, X.; Wu, Q.; Jin, L.; Wang, G.; Huang, J. Rice transcription factor OsMADS25 modulates root growth and confers salinity tolerance via the ABA–mediated regulatory pathway and ROS scavenging. PLOS Genet. 2018, 14, e1007662. [Google Scholar] [CrossRef]
- Brookbank, B.P.; Patel, J.; Gazzarrini, S.; Nambara, E. Role of Basal ABA in Plant Growth and Development. Genes 2021, 12, 1936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cai, Z.; Wang, X. The primary signaling outputs of brassinosteroids are regulated by abscisic acid signaling. Proc. Natl. Acad. Sci. 2009, 106, 4543–4548. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Liu, J.; Weng, H.; Yuan, X.; Xiao, W.; Wang, H. A long noncoding RNA derived from lncRNA-mRNA networks modulates eeed vigor. Int. J. Mol. Sci. 2022, 23, 9472. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhu, Z.; Chern, M.; Yin, J.; Yang, C.; Ran, L.; Cheng, M.; He, M.; Wang, K.; Wang, J.; et al. A Natural Allele of a Transcription Factor in Rice Confers Broad-Spectrum Blast Resistance. Cell 2017, 170, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, W.; Zhang, Y.; Zhan, Q.; Liu, K.; Botella, J.R.; Bai, L.; Song, C. Abscisic acid-induced cytoplasmic translocation of constitutive photomorphogenic 1 enhances reactive oxygen species accumulation through the HY5-ABI5 pathway to modulate seed germination. Plant, Cell Environ. 2022, 45, 1474–1489. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Z.; Sun, T.; Wang, D.; Wang, Z.; Zhang, C.; Que, Y.; Guo, J.; Xu, L.; Su, Y. Sugarcane ScDREB2B-1 l Nicotiana benthamiana by regulating the ABA signal, ROS level and stress-related gene expression. Int. J. Mol. Sci. 2022, 23, 9557. [Google Scholar] [CrossRef] [PubMed]
- Postiglione, A.E.; Muday, G.K. The Role of ROS Homeostasis in ABA-Induced Guard Cell Signaling. Front. Plant Sci. 2020, 11, 968. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.V.S.; Yadav, S.K.; Verma, R.K.; Shrivastava, S.; Ghimire, O.; Pushkar, S.; Rao, M.V.; Kumar, T.S.; Chinnusamy, V. The abscisic acid receptor OsPYL6 confers drought tolerance to indica rice through dehydration avoidance and tolerance mechanisms. J. Exp. Bot. 2020, 72, 1411–1431. [Google Scholar] [CrossRef]
- Kurup, S.; Jones, H.D.; Holdsworth, M.J. Interactions of the developmental regulator ABI3 with proteins identied from developing arabidopsis seeds. Plant J. 2000, 21, 143–155. [Google Scholar] [CrossRef]
- Khan, N.A.; Nazar, R.; Iqbal, N.; Anjum, N.A. Phytohormones and abiotic stress tolerance in plants; Anjum, N.A., Ed.; Springer Science & Business Media, 2012. [Google Scholar]
- Gallei, M.; Luschnig, C.; Friml, J. Auxin signaling in growth: schrödinger's cat out of the bag. Curr Opin Plant Biol. 2020, 53, 43–49. [Google Scholar] [CrossRef]
- Nghi, K.; Tagliani, A.; Mariotti, L.; Weits, D.; Perata, P.; Pucciariello, C. Auxin is required for the long coleoptile trait in japonica rice under submergence. New Phytol. 2021, 229, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Vinocur, B.; Shoseyov, O.; Altman, A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004, 9, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Matsuba, S.; Funatsuki, H.; Kawaguchi, K.; Saruyama, H.; Tanida, M.; Sato, Y. Over-expression of a small heat shock protein, sHSP17.7, confers both heat tolerance and UV-B resistance to rice plants. Mol. Breed. 2004, 13, 165–175. [Google Scholar] [CrossRef]
- Zhao, H.; Jan, A.; Ohama, N.; Kidokoro, S.; Soma, F.; Koizumi, S.; Mogami, J.; Todaka, D.; Mizoi, J.; Shinozaki, K.; et al. Cytosolic HSC70s repress heat stress tolerance and enhance seed germination under salt stress conditions. Plant, Cell Environ. 2021, 44, 1788–1801. [Google Scholar] [CrossRef] [PubMed]
- Krishna, P.; Gloor, G. The Hsp90 family of proteins in Arabidopsis thaliana. Cell Stress Chaperones 2001, 6, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Knowlton, A.A.; Wasser, J.S.; Li, Q.; Lin, X.; Yang, X.; Weber, R.E.; Ostojic, H.; Fago, A.; Dewilde, S.; et al. Expression of heat shock proteins in turtle and mammal hearts: relationship to anoxia tolerance. Am. J. Physiol. Integr. Comp. Physiol. 2000, 278, R209–R214. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Xie, Y.; Yu, S.; Yang, J.; Chen, S.; Yuan, X.; Guo, T.; Wang, H.; Liu, Y.; Chen, C.; et al. The DnaJ domain-containing heat-shock protein NAL11 determines plant architecture by mediating gibberellin homeostasis in rice (Oryza sativa). New Phytol. 2023, 237, 2163–2179. [Google Scholar] [CrossRef] [PubMed]
- Magneschi, L.; Kudahettige, R.L.; Alpi, A.; Perata, P. Expansin gene expression and anoxic coleoptile elongation in rice cultivars. J. Plant Physiol. 2009, 166, 1576–1580. [Google Scholar] [CrossRef]
- Asatsuma, S.; Sawada, C.; Itoh, K.; Okito, M.; Kitajima, A.; Mitsui, T. Involvement of α-Amylase I-1 in Starch Degradation in Rice Chloroplasts. Plant Cell Physiol. 2005, 46, 858–869. [Google Scholar] [CrossRef]
- Hwang, Y.-S.; Thomas, B.; Rodriguez, R. Differential expression of rice α-amylase genes during seedling development under anoxia. Plant Mol. Biol. 1999, 40, 911–920. [Google Scholar] [CrossRef]
- Jackson, M.B.; Ram, P.C. Physiological and Molecular Basis of Susceptibility and Tolerance of Rice Plants to Complete Submergence. Ann. Bot. 2003, 91, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-H.; Wang, P.-Q.; Zhang, P.-P.; Nie, X.-M.; Li, B.-B.; Tai, L.; Liu, W.-T.; Li, W.-Q.; Chen, K.-M. NADPH Oxidases: The Vital Performers and Center Hubs during Plant Growth and Signaling. Cells 2020, 9, 437. [Google Scholar] [CrossRef]
- Hewage, K.A.H.; Yang, J.; Wang, D.; Hao, G.; Yang, G.; Zhu, J. Chemical Manipulation of Abscisic Acid Signaling: A New Approach to Abiotic and Biotic Stress Management in Agriculture. Adv. Sci. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.; Zhu, G.; Liu, Y.; Zhang, A.; Li, Y.; Liu, R.; Shi, L.; Jia, L.; Zhang, J. Ascorbic acid and reactive oxygen species are involved in the inhibition of seed germination by abscisic acid in rice seeds. J. Exp. Bot. 2011, 63, 1809–1822. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: the new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Liao, K.; Du, H.; Xu, Y.; Song, H.; Li, X.; Xiong, L. A stress-responsive NAC transcription factor SNAC3 confers heat and drought tolerance through modulation of reactive oxygen species in rice. J. Exp. Bot. 2015, 66, 6803–6817. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Li, X.; Hicks, L.M.; Xiong, L. A Raf-Like MAPKKK Gene DSM1 Mediates Drought Resistance through Reactive Oxygen Species Scavenging in Rice. Plant Physiol. 2009, 152, 876–890. [Google Scholar] [CrossRef] [PubMed]
- Panda, D.; Mishra, S.S.; Behera, P.K. Drought Tolerance in Rice: Focus on Recent Mechanisms and Approaches. Rice Sci. 2021, 28, 119–132. [Google Scholar] [CrossRef]
- Aleem, M.; Aleem, S.; Sharif, I.; Wu, Z.; Aleem, M.; Tahir, A.; Atif, R.M.; Cheema, H.M.; Shakeel, A.; Lei, S.; Yu, D.; Wang, H.; Kaushik, P.; Alyemeni, M.N.; Bhat, J.A.; Ahmad, P. Characterization of SOD and GPX gene families in the Soybeans in response to drought and salinity stresses. Antioxidants 2022, 11, 460. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Hnilickova, H.; Kraus, K.; Vachova, P.; Hnilicka, F. Salinity stress affects photosynthesis, malondialdehyde formation, and proline content in Portulaca oleracea L. Plants 2021, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Miro, B.; Ismail, A.M. Tolerance of anaerobic conditions caused by flooding during germination and early growth in rice (Oryza sativa L.). Front. Plant Sci. 2013, 4, 269. [Google Scholar] [CrossRef] [PubMed]
- Perata, P.; Guglielminetti, L.; Alpi, A. Mobilization of Endosperm Reserves in Cereal Seeds under Anoxia. Ann. Bot. 1997, 79, 49–56. [Google Scholar] [CrossRef]
- Banti, V.; Loreti, E.; Novi, G.; Santaniello, A.; Alpi, A.; Perata, P. Heat acclimation and cross-tolerance against anoxia in Arabidopsis. Plant Cell Environ. 2008, 31, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-A.; Lim, E.-K.; Yu, S.-M. Sugar Response Sequence in the Promoter of a Rice α-Amylase Gene Serves as a Transcriptional Enhancer. J. Biol. Chem. 1998, 273, 10120–10131. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-W.; Chen, P.-W.; Lu, C.-A.; Chen, S.; Ho, T.-H.D.; Yu, S.-M. Coordinated Responses to Oxygen and Sugar Deficiency Allow Rice Seedlings to Tolerate Flooding. Sci. Signal. 2009, 2, ra61–ra61. [Google Scholar] [CrossRef]
- Ismail, A.M.; Ella, E.S.; Vergara, G.V.; Mackill, D.J. Mechanisms associated with tolerance to flooding during germination and early seedling growth in rice (Oryza sativa L.). Annu. Bot. 2008, 103, 197–209. [Google Scholar] [CrossRef]
- Gibbs, J.; Morrell, S.; Valdez, A.; Setter, T.; Greenway, H. Regulation of alcoholic fermentation in coleoptiles of two rice cultivars differing in tolerance to anoxia. J. Exp. Bot. 2000, 51, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Quimio, C.A.; Torrizo, L.B.; Setter, T.L.; Ellis, M.; Grover, A.; Abrigo, E.M.; Oliva, N.P.; Ella, E.S.; Carpena, A.L.; Ito, O.; et al. Enhancement of Submergence Tolerance in Transgenic Rice Overproducing Pyruvate Decarboxylase. J. Plant Physiol. 2000, 156, 516–521. [Google Scholar] [CrossRef]
- Lee, K.; Chen, J.J.W.; Wu, C.; Chang, H.; Chen, H.; Kuo, H.; Lee, Y.; Chang, Y.; Chang, H.; Shiue, S.; et al. Auxin plays a role in the adaptation of rice to anaerobic germination and seedling establishment. Plant, Cell Environ. 2022, 46, 1157–1175. [Google Scholar] [CrossRef]
- Singh, P.; Sinha, A.K. A Positive Feedback Loop Governed by SUB1A1 Interaction with MITOGEN-ACTIVATED PROTEIN KINASE3 Imparts Submergence Tolerance in Rice. Plant Cell 2016, 28, 1127–1143. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, X.; Jin, S.; Liu, X.; Zhu, L.; Nie, Y.; Zhang, X. Overexpression of Rice NAC Gene SNAC1 Improves Drought and Salt Tolerance by Enhancing Root Development and Reducing Transpiration Rate in Transgenic Cotton. PLOS ONE 2014, 9, e86895. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, T.; Pelayo, M.A.F.; Trijatmiko, K.R.; Gabunada, L.F.M.; Alam, R.; Jimenez, R.; Mendioro, M.S.; Slamet-Loedin, I.H.; Sreenivasulu, N.; Bailey-Serres, J.; et al. A trehalose-6-phosphate phosphatase enhances anaerobic germination tolerance in rice. Nat. Plants 2015, 1, 15124. [Google Scholar] [CrossRef] [PubMed]
- Gallei, M.; Luschnig, C.; Friml, J. Auxin signaling in growth: schrödinger's cat out of the bag. Current Opinion in Plant Biology 2020, 53, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Nghi, K.N.; Tagliani, A.; Mariotti, L.; Weits, D.A.; Perata, P.; Pucciariello, C. Auxin is required for the long coleoptile trait in japonica rice under submergence. New Phytol. 2020, 229, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Miao, R.; Yuan, W.; Wang, Y.; Garcia-Maquilon, I.; Dang, X.; Li, Y.; Zhang, J.; Zhu, Y.; Rodriguez, P.L.; Xu, W. Low ABA concentration promotes root growth and hydrotropism through relief of ABA INSENSITIVE 1-mediated inhibition of plasma membrane H + -ATPase 2. Sci. Adv. 2021, 7, eabd4113. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Zhou, L.; Chen, W.; Ye, N.; Xia, J.; Zhuang, C. Overexpression of a microRNA-targeted NAC transcription factor improves drought and salt tolerance in Rice via ABA-mediated pathways. Rice 2019, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hou, Y.; Qiu, J.; Wang, H.; Wang, S.; Tang, L.; Tong, X.; Zhang, J. Abscisic acid promotes jasmonic acid biosynthesis via a ‘SAPK10-bZIP72-AOC’ pathway to synergistically inhibit seed germination in rice (Oryza sativa). New. Phytol. 2020, 228, 1336–1353. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, W.; He, Y.; Adegoke, T.V.; Ying, J.; Tong, X.; Li, Z.; Tang, L.; Wang, H.; Zhang, J.; et al. bZIP72 promotes submerged rice seed germination and coleoptile elongation by activating ADH1. Plant Physiol. Biochem. 2021, 169, 112–118. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; Xia, R. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant. 2023, 16, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Ma, X.; Zhu, Q.; Zeng, D.; Li, G.; Liu, Y.-G. CRISPR-GE: A Convenient Software Toolkit for CRISPR-Based Genome Editing. Mol. Plant 2017, 10, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.-X.; Yin, X.-X.; Li, S.; Peng, Y.-T.; Yan, X.-L.; Chen, C.; Hassan, B.; Zhou, S.-X.; Pu, M.; Zhao, J.-H.; et al. miR167d-ARFs Module Regulates Flower Opening and Stigma Size in Rice. Rice 2022, 15, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xie, X.; Ma, X.; Li, J.; Chen, J.; Liu, Y.-G. DSDecode: A Web-Based Tool for Decoding of Sequencing Chromatograms for Genotyping of Targeted Mutations. Mol. Plant 2015, 8, 1431–1433. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Yang, J.; Li, D.; Peng, Z.; Xia, A.; Yang, M.; Luo, L.; Huang, C.; Wang, J.; Wang, H.; et al. Dynamic genome-wide association analysis and identification of candidate genes involved in anaerobic germination tolerance in rice. Rice 2021, 14, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Ma, J.; Zhai, H.; Xin, P.; Chu, J.; Qiao, Y.; Han, L. CHR729 Is a CHD3 Protein That Controls Seedling Development in Rice. PLOS ONE 2015, 10, e0138934. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Luo, L.; Cui, H.; Wang, H.; Guo, T.; Liu, Y.; Wang, J.; Huang, M.; Yang, G.; Chen, Z.; et al. Characterization and Fine Mapping of a Leaf Wilt Mutant, m3, Induced by Heavy Ion Irradiation of Rice. Crop. Sci. 2019, 59, 2679–2688. [Google Scholar] [CrossRef]
- Chen, T.; Luo, L.; Zhao, Z.; Wang, H.; Chen, C.; Liu, Y.; Li, X.; Guo, T.; Xiao, W. Fine mapping and candidate gene analysis of qGL10 affecting rice grain length. Crop. J. 2023, 11, 540–548. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Huang, L.; Hua, K.; Xu, R.; Zeng, D.; Wang, R.; Dong, G.; Zhang, G.; Lu, X.; Fang, N.; Wang, D.; Duan, P.; Zhang, B.; Liu, Z.; Li, N.; Luo, Y.; Qian, Q.; Yao, S.; Li, Y. The LARGE2-APO1/APO2 regulatory module controls panicle size and grain number in rice. Plant Cell. 2021, 33, 1212–1228. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



