Submitted:
24 July 2024
Posted:
25 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and methods
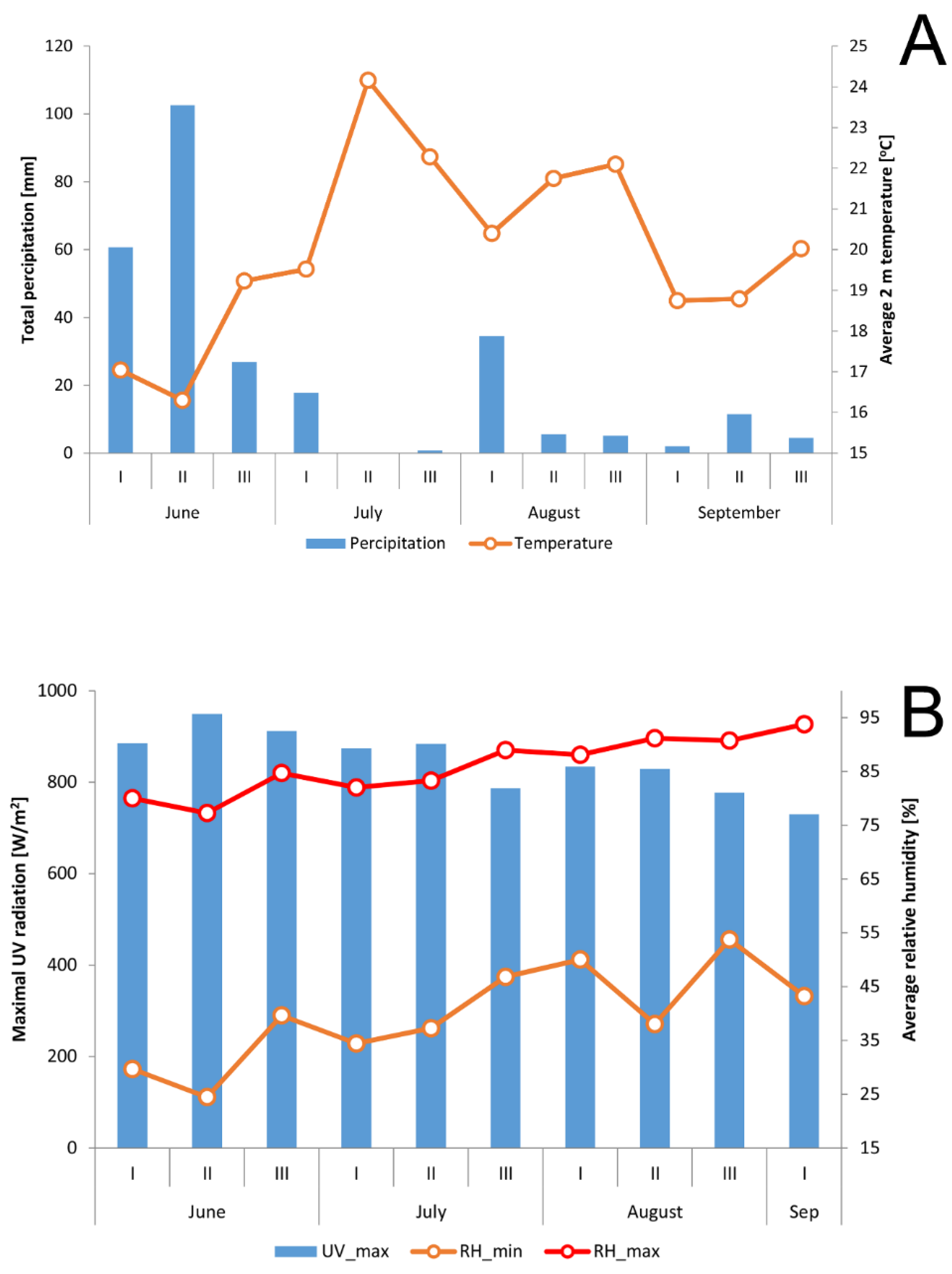
2.1. Climate and Soil
2.2. Plant Material And nets
2.3. Standards and Reagents
2.4. Spectrophotometric Analysis
2.5. Antioxidant Activity
2.6. HPLC Analysis of Particular Anthocyanins
2.7. Statistical Analysis
3. Results
3.1. Organic Acids and Sugars
3.2. Phenolic Content
4. Discussion
4.1. Organic Acids and Sugars
4.2. Polyphenolic Content
4.3. Determination of total and Particular Anthocyanins
4.4. Antioxidant activity
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Mditshwa, A.; Magwaza, L.S.; Tesfay, S.Z. Shade netting on subtropical fruit: Effect on environmental conditions, tree physiology and fruit quality. Sci Hortic. 2019, 256, 108556. [Google Scholar] [CrossRef]
- Stamps, R.H. Differential effects of colored shade nets on three cut foliage crops. Acta Hort. 2008, 770, 169–176. [Google Scholar] [CrossRef]
- Pérez, M.; Plaza, B.M.; Jiménez, S.; Lao, M.T.; Barbero, J.; Bosch, J.L. The radiation spectrum through ornamental net houses and its impact on the climate generated. Acta Hort. 2006, 719, 631–636. [Google Scholar] [CrossRef]
- Stamps, R.H. Use of Colored Shade Netting in Horticulture. Use of Colored Shade Netting in Horticulture. Hort Science Horts. 2009, 44, 239–241. [Google Scholar]
- Hansen, J.; Sato, M.; Ruedy, R.; Lo, K.; Lea, D.W.; Medina-Elizade, M. Global temperature change. Proc Natl Acad Sci. U. S. A. 2006, 103, 14288–14293. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Lee Chen, C.C.; Brillante, L.; Kurtural, S.K. Partial solar radiation exclusion with color shade nets reduce the degradation of organic acids and flavonoids of grape berry (Vitis vinifera L.). J Agric Food Chem. 2017, 65, 10693–10702. [Google Scholar] [CrossRef]
- Berli, F.J.; Bottini, R. UV-B and abscisic acid effects on grape berry maturation and quality. J Berry Res. 2013, 3, 1–14. [Google Scholar] [CrossRef]
- Foyer, C.H.; Lelandais, M.; Kunert, K.J. Photooxidative stress in plants. Physiol Plantarum. 1994, 92, 696–717. [Google Scholar] [CrossRef]
- Jansen, M.A.K.; Gaba, V.; Greenberg, B.M. Higher plants and UV-B radiation: Balancing damage, repair and acclimation. Trends Plant Sci. 1998, 3, 131–5. [Google Scholar] [CrossRef]
- Frohnmeyer, H.; Staiger, D. Ultraviolet-B radiation-mediated responses in plants. Balancing damage and protection. Plant Physiol. 2003, 133, 1420–8. [Google Scholar] [CrossRef]
- Bjorn, L.O. Effects of ozone depletion and increased UV-B on terrestrial ecosystems. Int J Environ Stud. 1996, 51, 217–43. [Google Scholar] [CrossRef]
- Scandalios, J.G. Oxygen stress and superoxide dismutases. Plant Physiol. 1993, 101, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Beraud, M.M.; Morales Ulloa, D.M. Shading nets effect on the production and quality of blueberry fruit (Vaccinium corymbosum L.) cv. Brigitta. Sci Agropecuaria 2015, 6, 41–50. [Google Scholar] [CrossRef]
- Aras, S.; Eşitken, A. Physiological effects of photoselective nets in strawberry plant. KSÜ Tarım ve Doğa Derg. 2019, 22, 342–346. [Google Scholar] [CrossRef]
- Szalay, K.; Keller, B.; Rak, R.; Peterfalvi, N.; Kovacs, L.; Soucek, J.; Sillinger, F.; Jung, A. Artificial solar radiation protection of raspberry plantation. Prog. Agric. Eng. Sci. 2020, 16, 141–150. [Google Scholar] [CrossRef]
- Zha, Q.; Yin, X.; Xi, X.; Jiang, A. Colored Shade Nets Can Relieve Abnormal Fruit Softening and Premature Leaf Senescence of “Jumeigui” Grapes during Ripening under Greenhouse Conditions. Plants 2022, 11, 1227. [Google Scholar] [CrossRef] [PubMed]
- Retamales, J.B.; Montecino, J.M.; Lobos, G.A.; Rojas, L.A. Colored Shading Nets Increase Yields and Profitability of Highbush Blueberries. Acta Hort. 770, ISHS 2008, : XXVII International Horticultural Congress - IHC2006.
- Moradi, S.; Zamani, Z.; Fatahi Moghadam, M.R.; Saba, M.K. Combination effects of preharvest tree net-shading and postharvest fruit treatments with salicylic acid or hot water on attributes of pomegranate fruit. Sci Hortic. 2022, 304, 111257. [Google Scholar] [CrossRef]
- Rigo, A.; Vianello, F.; Clementi, G.; Rossetto, M.; Scarpa, M.; Vrhovšek, U. Contribution of proanthocyanidins to the peroxy radical scavenging capacity of some Italian red wines. J Agric Food Chem 2000, 48, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Vrhovsek, U.; Mattivi, F.; Watterhouse, A.L. Analysis of red wine phenolics: comparison of HPLC and spectrophotometric methods. Vitis 2001, 40, 87–91. [Google Scholar]
- Lisjak, K.; Lelova, Z.; Žigon, U.; Bolta, Š.V.; Teissedre, P.-L. and Vanzo, A. Effect of extraction time on content, composition and sensory perception of proanthocyanidins in wine-like medium and during industrial fermentation of Cabernet Sauvignon. J Sci Food Agric 2020, 100, 1887–1896. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, E.; Berset, C.M. Use of free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Shaw, D.V. 1988. Genotypic variation and genotypic correlation for sugars and organic acids of strawberries. J. Am. Soc. Hort. Sci. 1988, 113, 770–774. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Giongo, L.; Mattivi, F.; Roberto, V. A survey of ellagitannin content in raspberry and blackberry cultivars grown in Trentino (Italy). Eur. Food Res. Technol. 2008, 226, 817–824. [Google Scholar] [CrossRef]
- Kafkas, E.; Ko§ar, M.; Turemi§, N.; Ba§er, K.H.C. Analysis of sugars, organic acids and vitamin C contents of blackberry genotypes from Turkey. Food Chem. 2006. Food Chem. 2006, 2006. 97, 732–736. [Google Scholar] [CrossRef]
- Poyrazoğlu, E.; Gökmen, V.; Artιk, N. Organic acids and phenolic compounds in pomegranates (Punica granatum L.) grown in Turkey. J. Food Compos. Anal. 2002, 15, 567–575. [Google Scholar] [CrossRef]
- Haro-Maza, J.F.; Guerrero-Beltrán, J.A. Ultraviolet-C Light Effect on the Physicochemical and Antioxidant Properties of Blackberry, Blueberry, and Raspberry Nectars. J. Food Res. 2016, 5, 11. [Google Scholar] [CrossRef]
- Frías-Moreno, M.N.; Parra-Quezada, R.A.; González-Aguilar, G.; Ruíz-Canizales, J.; Molina-Corral, F.J.; Sepulveda, D.R.; Salas-Salazar, N.; Olivas, G.I. Quality, Bioactive Compounds, Antioxidant Capacity, and Enzymes of Raspberries at Different Maturity Stages, Effects of Organic vs. Conventional Fertilizatio. Foods 2021, 10, 953. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, G.; Sun, L.; Song, X.; Bao, Y.; Luo, T.; Wang, J. Comprehensive Evaluation of 24 Red Raspberry Varieties in Northeast China Based on Nutrition and Taste. Foods, 2022, 11, 3232. [Google Scholar] [CrossRef] [PubMed]
- Šne, E.; Kampuse, S.; Berņa, E. The composition of sugars and sugar-acid ratio of highbush blueberry varieties grown in Latvia. Research for Rural Development. International Scientific Conference 2011, 1, 140–144. [Google Scholar]
- Kaye, F.; Susanna, H.H.; Janette, C.B. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008, 31, 2281–2283. [Google Scholar]
- Zheng, J.; Heikki, K.; Kaisa, L.; Baoru, Y. Sugars, sugar alcohols, fruit acids, and ascorbic acid in wild Chinese sea buckthorn (Hippophaë rhamnoides ssp. sinensis) with special reference to influence of latitude and altitude. Food Res. Int. 2011, 44, 2018–2026. [Google Scholar] [CrossRef]
- Zhang, J.; Nie, J.; Li, J.; Zhang, H.; Li, Y.; Saqib, F.; Syed Asim Shah Bacha, S.A.S.; Wang, J. Evaluation of sugar and organic acid composition and their levels in highbush blueberries from two regions of China. J. Integr. Agric. 2020, 19, 2352–2361. [Google Scholar] [CrossRef]
- Gutierrez, E.; Velasco, A. G.; Lucas, J. A.; Gutierrez-Mañero, F. J.; Ramos-Solano, B. The Flavonol-Anthocyanin Pathway in Blackberry and Arabidopsis: State of the Art. Flavonoids-from biosynthesis to human health. 2017. [Google Scholar] [CrossRef]
- Shomali, A.; Das, S.; Arif, N.; Sarraf, M.; Zahra, N.; Yadav, V.; Aliniaeifard, S.; Chauhan, D.K.; Hasanuzzaman, M. Diverse Physiological Roles of Flavonoids in Plant Environmental Stress Responses and Tolerance. Plants (Basel). 2022, 11, 3158. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lin, H.S. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage, J. Agric. Food Chem., 2000, 48, 140–146. [Google Scholar] [CrossRef]
- Anttonen, J.M.; Karjalainen, R.O. Environmental and genetic variation of phenolic compounds in red raspberry. J Food Chomp. Anal. 2005, 18, 759–769. [Google Scholar] [CrossRef]
- Pantelidis, G.E.; Vasilakakis, M.; Manganaris, G.A.; Diamantidis, G.R. Antioxidant capacity, phenol, anthocyanin and ascorbic acid contents in raspberries, blackberries, red currants, gooseberries and Cornelian cherries. Food Chem. 2007, 102, 777–783. [Google Scholar] [CrossRef]
- Chen, L.; Xin, X.; Zhang, H.; Yuan, Q. ; Phytochemical properties and antioxidant capacities of commercial raspberry varieties. J. Funct. Foods. 2013, 5, 508–515. [Google Scholar] [CrossRef]
- Sariburun, E.; Şahin, S.; Demir, C.; Türkben, C.; Uylaşer, V. Phenolic Content and Antioxidant Activity of Raspberry and Blackberry Cultivars. J Food Sci. 2010, 75, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Amakura, Y.; Umino, Y.; Tsuji, S.; Tonogai, Y. Influence of jam processing on the radical scavenging activity and phenolic content in berries. J Agric Food Chem 2000, 48, 6292–7. [Google Scholar] [CrossRef]
- De Ancos, B.; Gonzales, E.; Cano, M.P. Differentiation of raspberry varieties accord- ´ ing to anthocyanin composition. Z Lebensm Unters Forsch A. 1999, 208, 33–8. [Google Scholar] [CrossRef]
- Wang, S.; Lin, H. Antioxidant activity in fruits and leaves of blackberry, raspberry and strawberry varies with cultivar and developmental stage. J Agric Food Chem. 2000, 2000. 48, 140–6. [Google Scholar] [CrossRef]
- Sellappan, S.; Akoh, C.C.; Krewer, G. Phenolic compounds and antioxidant capacity of Georgia-grown blueberries and blackberries. J Agric Food Chem. 2002, 50, 2432–8. [Google Scholar] [CrossRef]
- Wada, L.; Ou, B. Antioxidant activity and phenolic content of oregon caneberries. J Agric Food Chem 2002, 50, 3495–500. [Google Scholar] [CrossRef]
- Benvenutı, S.; Pellatı, F.; Melegarı, M.; Bertelli, D. Polyphenols, anthocyanins, ascorbic acid, and radical scavenging activity of rubus, ribes, and aronia. J Food Sci Food Chem Toxicol. 2004, 69, 164–9. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J.; Kucharska, A.Z.; Sokół-Łętowska, A.; Fecka, I. Characterization of Phenolic Compounds of Thorny and Thornless Blackberries. J. Agr. Food Chem. 2015, 63, 3012–3021. [Google Scholar] [CrossRef]
- Fan-Chiang, H.; Wrolstad, R. Anthocyanin pigment composition of blackberries. J. Food Sci. 2005, 70, 198–202. [Google Scholar] [CrossRef]
- Lee, J.; Dossett, M.; Finn, C.E. Rubus fruit phenolics research: The good, the bad, and the confusing. Food Chem. 2012, 130, 785–796. [Google Scholar] [CrossRef]
- Wu, X.; Prior, RL. Systematic identification and characterization of anthocyanins by HPLC-ESI-MS/MS in common foods in the United States: fruits and berries. J Agric Food Chem. 2005, 53, 2589–2599. [Google Scholar] [CrossRef]
- Chen, F.; Sun, Y.; Zhao, G.; Liao, X.; Hu, X.; Wu, J.; Wang, Z. Optimization of ultrasound-assisted extraction of anthocyanins in red raspberries and identification of anthocyanins in extract using high-performance liquid chromatography–mass spectrometry. Ultrason Sonochem. 2007, 14, 767–78. [Google Scholar] [CrossRef] [PubMed]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z.; Miller, A.R.; Scheerens, J.C. Modified 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,20-diphenyl-1-picrylhydrazyl (DPPH) methods. J Agric Food Chem. 2006, 54, 1151–7. [Google Scholar] [CrossRef] [PubMed]
- Sariburun, E.; Şahin, S.; Demir, C.; Türkben, C.; Uylaşer, V. Phenolic Content and Antioxidant Activity of Raspberry and Blackberry Cultivars. J. Food Sci., 2010, 75, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Guedes, M. N. S.; Pio, R.; Maro, L. A. C.; Lage, F. F.; Abreu, C. M. P.; de, Saczk, A. A. Antioxidant activity and total phenol content of blackberries cultivated in a highland tropical climate. Acta Scient. Agr. 2017, 39, 43. [Google Scholar] [CrossRef]
- Maurer, T.; Tonello, C.; Machado, B.; Trentin, T.; Bertol, C.; Lângaro, N.; Chiomento, J. L. Phytochemical potential of berries: An overview. LADEE 2023, 4, 11–28. [Google Scholar] [CrossRef]
- Wolske, E.; Chatham, L.; Juvik, J.; Branham, B. Berry Quality and Anthocyanin Content of 'Consort' Black Currants Grown under Artificial Shade. Plants (Basel). 2021, 10, 766. [Google Scholar] [CrossRef]
- Pallotti, L.; Silvestroni, O.; Dottori, E.; Lattanzi, T.; Lana, V. Effects of shading nets as a form of adaptation to climate change on grapes production: a review. OENO One 2023, 57. [Google Scholar] [CrossRef]
- Šavikin, K.; Mikulič-Petkovšek, M.; Djordjević, B.; Zdunić, G.; Janković, T.; Djurović, D.; Veberič, R. Influence of shading net on polyphenol profile and radical scavenging activity in different varieties of black currant berries. Sci. Hortic. 2013, 160, 20–28. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Lee Chen, C.C.; Brillante, L.; Kurtural, S.K. Partial Solar Radiation Exclusion with Color Shade Nets Reduces the Degradation of Organic Acids and Flavonoids of Grape Berry (Vitis vinifera L.). J. Agric. Food Chem. 2017, 65, 10693–10702. [Google Scholar] [CrossRef]
| Species | Treatment | Acetic | Citric | D-gluconic | Lactic | Malic | Total acidity | pH | D-glc + D-fru |
|---|---|---|---|---|---|---|---|---|---|
| [g/kg] | [g/kg] | [g/kg] | [g/kg] | [g/kg] | [g/kg] | [g/kg] | |||
| Year 2022 | |||||||||
| Blackberry | without shading | 0.02±0.01 b | 0.92±0.13 | 0.20±0.03 a | 0.04±0.00 | 2.14±0.02 b | 11.90±0.49 b | 3.23±0.01 a | 63.00±1.44 a |
| Year 2023 | |||||||||
| Blackberry | without shading | 0.03±0.01 b | - | 0.05±0.01 b | 0.01±0.00 | 3.08±0.04 a | 9.19±0.11 c | 3.33±0.05 a | 36.98±3.21 c |
| shading | 0.03±0.00 b | - | 0.05±0.00 b | - | 3.12±0.06 a | 11.31±0.07 b | 3.06±0.04 b | 48.34±2.51 b | |
| Raspberry | without shading | 0.01±0.00 b | - | 0.03±0.00 b | - | 0.67±0.09 c | 21.93±2.03 a | 3.02±0.03 b | 38.83±1.56 c |
| shading | 0.11±0.02 a | - | 0.04±0.01 b | - | 0.72±0.10 c | 20.19±3.21 a | 3.10±0.01 b | 39.48±1.71 c | |
| a Different letters next to mean values indicate statistical differences according to the post hoc Tukey’s test at the level of P<0.05 | |||||||||
| Species | Treatment | A420(violet) | A520(blue) | A620(orange) |
|---|---|---|---|---|
| Year 2022 | ||||
| Blackberry | without shading | 4.74±0.69 a | 6.14±0.81 c | 1.99±0.11 a |
| Year 2023 | ||||
| Blackberry | without shading | 3.33±0.10 b | 4.43±0.27 d | 0.53±0.31 b |
| shading | 1.93±0.22 c | 3.98±0.41 d | 0.16±0.02 d | |
| Raspberry | without shading | 3.15±0.29 b | 10.00±2.01 a | 0.18±0.00 d |
| shading | 3.21±0.34 b | 9.83±2.21 b | 0.32±0.02 c |
| Species | Treatment | Total anthocyanins | Cya-3-O-glc | Cya-3-O-soph.b | Total polyphenols | LM proAc | HM proAd | IC50 |
|---|---|---|---|---|---|---|---|---|
| [mg/g FW]a | [mg/g FW] | [mg/g FW] | [mg/g FW] | [mg/g FW] | [mg/g FW] | [mg] | ||
| Year 2022 | ||||||||
| Blackberry | without shading | 469.0±83.81 c | 0.74±0.05 b | n.d. | 278.0±14.50 a | 445.1±10.11 b | 868.0±14.71 b | 87.1±2.1 b |
| Year 2023 | ||||||||
| Blackberry | without shading | 457.5±17.22 c | 0.97±0.06 b | n.d. | 294.3±23.93 a | 512.7±31.24 a | 931.5±21.09 a | 94.0±12.30 a |
| shading | 323.1±10.05 d | 1.22±0.09 a | n.d. | 256.6±12.97 ab | 462.9±14.53 b | 872.4±13.2 b | 92.1±9.10 a | |
| Raspberry | without shading | 626.2±21.43 a | 0.21±0.03 a | 0.48±0.09 b | 223.5±31.81 b | 388.0±22.92 c | 772.9±30.0 c | 84.1±10.0 b |
| shading | 558.1±41.92 b | 0.28±0.04 a | 0.66±0.08 a | 217.4±13.99 b | 365.2±12.41 c | 713.6±13.21 c | 82.1±11.4 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





