Introduction
Premature rupture of membranes (PROM) refers to the rupture of fetal membranes before the onset of labor [
1]. Term PROM complicates approximately 8% of pregnancies, and its most significant maternal consequence is an increased risk of intrauterine infection, which escalates with prolonged membrane rupture. Compared to expectant management for term PROM patients, induction process is a more recommended treatment option [
2,
3].
Induction of labor is determined based on the cervix conditions, as assessed by the Bishop score. In cases where the cervix is deemed favorable, oxytocin is administered intravenously to initiate labor. Conversely, if the cervix is considered unfavorable (Bishop score < 6), prostaglandin or Foley's balloon are commonly employed for cervical ripening. However, in patients with PROM, vaginal manipulation such as Foley’s balloon or vaginal administration of prostaglandin may increase the incidence of infection and potentially result in uterine hyperstimulation, fetal distress, and emergency cesarean section (C-section).
Oxytocin is widely recognized as the most commonly employed induction agent worldwide and has been recommended as the preferred choice in cases of PROM by the American College of Obstetricians and Gynecologists, primarily due to its superior safety profile1. However, oxytocin's efficacy appears to be limited in term PROM patients with an unripened cervix.
Misoprostol, a prostaglandin E1 drug utilized for cervical ripening, demonstrates cost-effectiveness, high efficiency, and global accessibility. While vaginal administration has been extensively studied, limited data exists on oral administration. However, recent evidence suggests that low-dose oral misoprostol exhibits superior rates of vaginal delivery compared to oxytocin, vaginal misoprostol, and dinoprostone PGE2 while also being associated with fewer instances of abnormal fetal heart rate changes and uterine tachysystole [
4,
5,
6]. Similar results were observed in patients with term PROM [
7,
8,
9].
Despite its global prevalence, the utilization of oral low-dose misoprostol for labor induction remains limited in China. Since March 2022, our department has implemented the use of oral low-dose misoprostol for cervical ripening in late gestational women who do not exhibit favorable cervix conditions. Comparative analysis with vaginal misoprostol reveals that oral low-dose misoprostol demonstrates superior efficacy and safety profiles, resulting in a higher rate of vaginal delivery and a lower incidence of fetal stress and uterine hyperstimulation [
10]. After two years of clinical practice, our focus lies on the administration of oral low-dose misoprostol to PROM pregnant women who do not exhibit cervical ripening. In comparison with traditional oxytocin induction of labor, our study aims to ascertain the efficacy and safety of oral low-dose misoprostol for term PROM mothers.
Methods and Materials
This prospective nested case-control study was conducted in a tertiary maternity unit (6000 deliveries per year) in the Gynecology and Obstetrics Unit of Peking University Third Hospital, in China, from March 2020 to February 2024. A retrospective cohort study was conducted over a period of four years, including patients with PROM and unripened cervix after 37 weeks gestation. From March 2020 to February 2022, oxytocin induction was performed in 282 cases, while from March 2022 to February 2024, cervical maturation was achieved using oral low-dose misoprostol solution in 167 cases. This study has been approved by the Ethics Committee of Peking University Third Hospital (Approval No. 245-02). All participants were adequately informed about the labor procedure and provided signed informed consent.
Participants
The inclusion criteria of the study were singleton, primiparous, cephalic presentation, gestational age ≥37 weeks, PROM without rhythmic contractions, no contraindication to prostaglandins, bishop scores < 6 (unripped cervix) and candidate for vaginal delivery. Exclusion criteria were twin pregnancies, fetuses in the breech or transverse position, intrauterine fetal demise or termination of labor due to severe fetal malformation, history of uterine scarring, rhythmic contractions observed and refusal to participate in the study. The diagnosis of PROM was established either through clinical assessment or by employing a pH test paper. Following 37 weeks gestation, as per our departmental protocol, labor induction was initiated if there were no signs of spontaneous labor or absence of regular contractions after more than 2 hours since rupture.
Drugs Administration
Oral misoprostol solution: Pregnant women who met the inclusion criteria were transferred to the delivery room. Prior to administration, confirmation of fetal heart monitoring response type was conducted again to ensure absence of contraindications. The drug was administered by both the midwife and physician, starting with an initial dose of 25 μg followed by subsequent doses of 25 μg every 2 hours. In cases where contraction frequency exceeded 2 times per 10 minutes, administration of the drug was delayed, and daily dosage did not exceed 200 μg. After achieving a cervical Bishop score of ≥6 points, oxytocin infusion was administered if deemed necessary. Specific drug preparation: Dissolve a 200 μg tablet of misoprostol in 200 ml of warm water, ensuring thorough stirring to obtain a well-mixed solution with a concentration of 1 μg/ml. Administer orally to pregnant women at a dosage of 25 ml.
Oxytocin Intravenous
The exclusive method employed for cervical ripening and induction of labor in the oxytocin group was through Oxytocin perfusion. A serum pump administered 0.5% low-dose oxytocin perfusion at a rate of 0.5 mL/min, and was doubled every 15minutes until the number of contractions was 3 times or more in 10 minutes with the duration of 40 seconds and appropriate force.
Data Collection
The following clinical data were collected: prenatal information like age, height, pregestational body mass index (BMI), gravidity, gestational weeks, cervical Bishop score, whether complicated with in vitro fertilization-embryo transfer (IVF-ET), gestational diabetes mellitus (GDM) and infection of Group B streptococcus (GBS+); Delivery information like mode of delivery, failed induction of labor, duration from rupture or administration to labor or delivery, duration of labor; delivery outcomes like adverse pregnancy outcomes such as fetal distress, intrauterine infection, postpartum hemorrhage (PPH), postpartum urinary retention, Additionally, rate of episiotomy, birth weight, Apgar score at 1min, and umbilical cord blood pH value were also examined.
Outcome Indicators
The primary and secondary outcomes of the two groups were compared in order to evaluate the efficacy and safety of misoprostol. Primary outcomes were number of vaginal deliveries and failed induction of labor. Failed induction of Labor was defined as a prolonged latent phase or the absence of labor following the administration of oxytocin for a minimum duration of 12 hours [
11]. Secondary outcomes were delivery complications like fetal stress and PPH.
Statistical Analysis
The statistical analyses were performed using GraphPad Prism 9 (GraphPad Software, San Diego, CA). Measurement data with a normal distribution were presented as Mean±SD, and the comparison between two groups was assessed using the Student T-test. Measurement data with a non-normal distribution were described as M (P25-P75), and the comparison between groups was analyzed using the Mann-Whitney U test. Count data were reported as number and percentage, and the comparison between groups was evaluated using Fisher's exact probability. Logistic regression models were adjusted for prenatal information and utilized to analyze induced labor failure and postpartum hemorrhage (PPH). Statistical significance was defined as a P-value <0.05.
Results
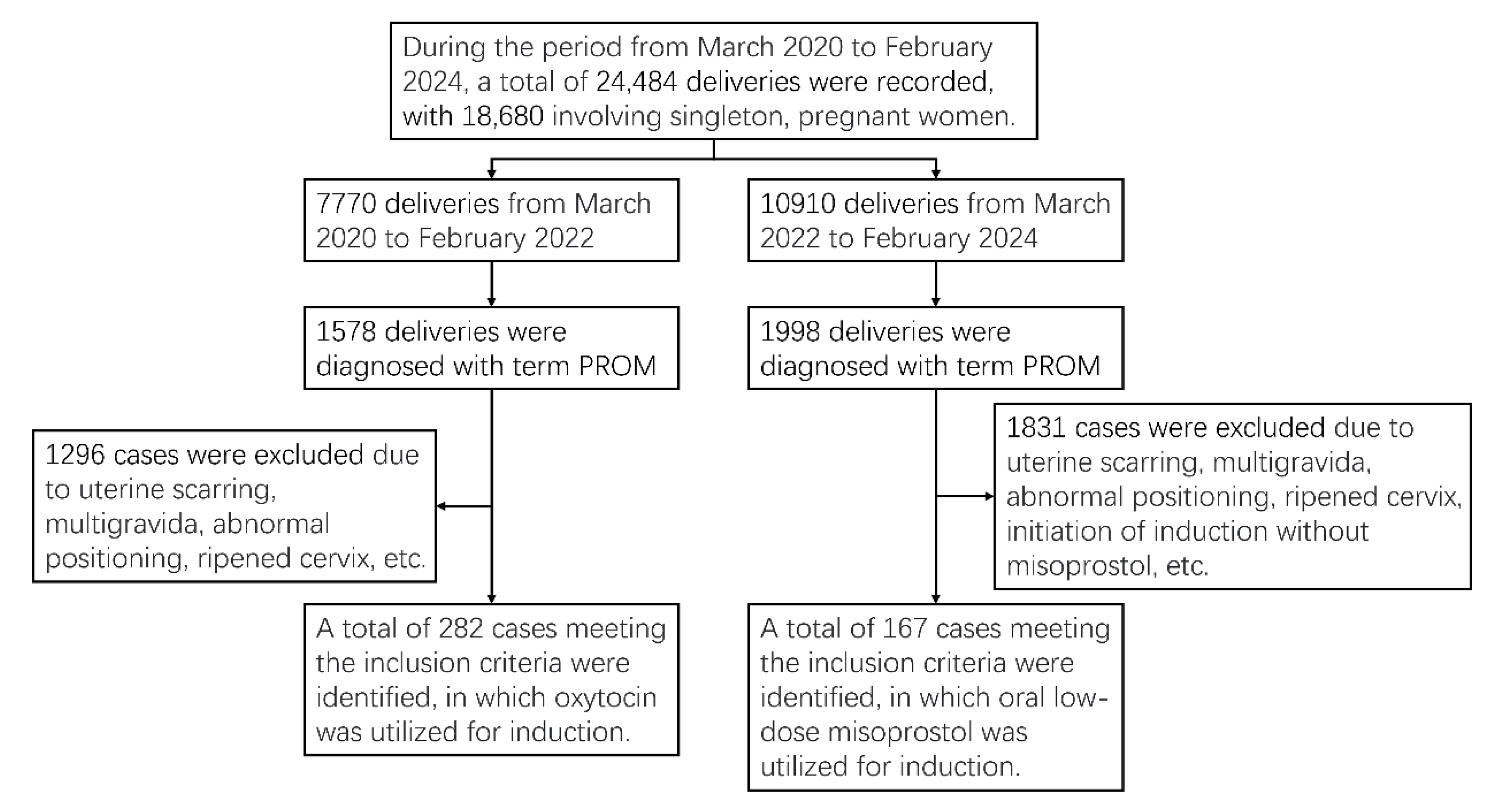
Participant flow is outlined in
Figure 1. During the period from March 2020 to February 2024, a total of 24,484 deliveries were recorded, with 18,680 involving singleton pregnant women. A total of 282 and 167 PROM cases meeting the inclusion criteria were identified, in which oxytocin or oral low-dose misoprostol were utilized for induction. The prenatal information, delivery information, and delivery outcomes were compared between the oxytocin group and misoprostol group, as presented in
Table 1,
Table 2 and
Table 3.
Comparison of Delivery Outcomes between Two Groups
In terms of delivery outcomes, as presented in
Table 3, there were no significant differences in the rates of uterine hyperstimulation, fetal distress, suspected intrauterine infection, pathological abnormalities of the placenta, newborn body weight, pH of cord blood, and postpartum blood loss between the two groups. However, the incidence of postpartum hemorrhage (PPH) >500ml within 24 hours after delivery and severe PPH (blood loss ≥1000ml within 24 hours after delivery) was significantly higher in the oxytocin administration group compared to the oral misoprostol group. Additionally, a higher incidence of postpartum urinary retention (PPUR) was observed in the misoprostol group.
Logistic Analysis of Vaginal Delivery, Failed Induction of Labor and Postpartum Hemorrhage
The logistic regression model was established with vaginal delivery and failed induction of labor as the dependent variables, while independent variables included oral misoprostol administration, age, pregnancy weight gain, pregestational BMI, gravidity, gestational weeks, IVF-ET status, GDM, infection of GBS, Bishop score and newborn body weight. As shown in
Table 4, concerning vaginal delivery outcomes, pregestational BMI (
aOR: 0.93,
95% CI: 0.87~0.99) and IVF-ET status (
aOR: 0.30,
95% CI: 0.17~0.52) exhibited a negative association with successful vaginal delivery. Conversely, for failed labor induction outcomes, misoprostol administration demonstrated a negative relationship (
aOR 0.46,
95% CI: 0.21~0.95), while IVF-ET showed a positive association (
aOR: 3.12,
95%CI: 1.49~6 .43). These findings suggest that oral misoprostol induction for term PROM may reduce the occurrence of failed labor induction whereas IVF-ET is associated with an increased risk of failed labor induction.
Furthermore, PPH is a significant complication during delivery and exhibited notable variations when different drugs were employed for induction. Consequently, an additional logistic regression model was constructed with the occurrence of PPH>500ml as the dependent variable and independent variables including oral misoprostol or oxytocin administration along with basic information. As depicted in
Table 5, positive associations were observed between age (
aOR: 1.17, 95%CI: 1.07~1.27), gestational weeks (
aOR: 1.35, 95%CI: 1.02~1.80), IVF-ET (aOR:2.43, 95%CI:1.32~4.48), and PPH incidence rates; conversely, a negative correlation was identified between misoprostol administration and PPH occurrence (aOR=0.49, 95%CI:0 .27~0 .85). These findings suggest that advanced age, prolonged gestational weeks, and IVF-ET are associated with an increased risk of PPH, while the utilization of oral misoprostol for induction may potentially mitigate the prevalence of PPH in patients with PROM.
Discussion
Despite its off-label use for late gestational induction of labor in China and other regions, misoprostol has gained widespread acceptance in obstetrics. According to the national guidelines on labor induction published in 2014, vaginal administration remains the recommended route for misoprostol usage; however, numerous complications including uterine hyperstimulation, fetal heart rate changes, placental abruption, emergency operations, and amniotic fluid embolism have been reported following its vaginal administration. Meanwhile, the national guideline recommends the use of misoprostol only in pregnant women with intact membranes, limiting its administration via vaginal route for mothers with premature rupture of membranes. Following the 37th week of gestation, the American College of Obstetricians and Gynecologists (ACOG) recommends oxytocin administration for labor induction in cases of PROM. However, clinical practice has revealed suboptimal efficacy and unsatisfactory maternal experiences with oxytocin. Consequently, there is an urgent need for alternative and more effective methods to induce labor, particularly in individuals with an unripe cervix condition.
Although the national guideline on labor induction has not been updated, numerous published studies have demonstrated the superior efficacy of oral low-dose misoprostol over vaginal administration; however, its prevalence in China remains limited. This study aims to evaluate the effectiveness and safety of oral low-dose misoprostol for term PROM mothers with an unripe cervix condition.
Several comparative studies have been conducted on the use of oral misoprostol for labor induction in term PROM pregnant women. One research by Leila Pourali et al [
8] demonstrated that sublingual administration of 25 ug misoprostol every 4 hours for labor induction in PROM cases resulted in improved neonatal outcomes and greater efficacy compared to oxytocin. The latest study conducted by Rania et al. [
9] demonstrated that the administration of oral misoprostol tablets at a dose of 25ug every four hours for labor induction in PROM resulted in significantly shorter induction-delivery intervals compared to the oxytocin group, particularly among multiparous women. In another study [
12], the utilization of oral misoprostol solution at a dosage of 20ug every 2 hours demonstrated comparable maternal complications and neonatal outcomes when compared to the standard intravenous oxytocin administration.
The administration of a 25 ug misoprostol solution every 2 hours in our study adhered to the recommendations set forth by the Society of Obstetricians and Gynecologists of Canada (SOGC) [
13] and the National Institute for Health and Care Excellence (NICE) [
14]. In line with the aforementioned findings, our study revealed no statistically significant differences in the rates of labor onset and vaginal delivery between the groups receiving oral misoprostol and oxytocin. Furthermore, there were no discernible disparities in the incidence of uterine hyperstimulation, fetal distress or neonatal asphyxia between the two groups. Conversely, administration of oral misoprostol was associated with a shorter overall duration of labor. Consequently, our results suggest that oral misoprostol is both safe and demonstrates superior efficacy compared to oxytocin infusion for labor induction in term PROM pregnant women.
Although there is a lack of randomized trials comparing criteria for failed labor induction, consistent observational data suggest that if the maternal and fetal conditions allow, administration of oxytocin should be continued for at least 12 to 18 hours after membrane rupture before considering an induction of labor as unsuccessful due to nonprogression into the active phase [
15]. According to the definition, the failed induction cases in our study adhere to the criteria, even within the misoprostol group, wherein oxytocin perfusion is initiated after 24 hours of misoprostol administration or upon improvement of cervical condition. Our study demonstrated that although no statistically significant differences in the incidence of failed induction were observed between the two groups, logistic regression analysis suggests that oral misoprostol may potentially mitigate the risk of unsuccessful labor induction. Additionally, high birth weight of the newborn and IVF-ET were identified as other significant risk factors for failed induction. It is reasonable to hypothesize that infants classified as large for gestational age (LGA) are more prone to experiencing a higher incidence of cesarean section and dystocia. Furthermore, IVF-ET gestation is associated with advanced maternal age and various social and mental factors which can impact successful vaginal deliveries.
The results of our study also revealed a significant finding that the incidence of postpartum hemorrhage is lower in cases where oral misoprostol was administered. Furthermore, logistic regression analysis further supports the notion that oral misoprostol administration may effectively mitigate the risk for PPH. It has been reported that prolonged exposure to high doses of oxytocin during labor is associated with severe postpartum hemorrhage (PPH) due to uterine atony [
16]. A recent study conducted by Braund S, et al. [
17] has demonstrated an association between labor induction and an increased risk of PPH, with the quantity of administered oxytocin playing a significant role in this relationship. Bernitz et al [
18] have also illustrated an increased risk of PPH associated with prenatal administration of high doses of oxytocin infusion. At the molecular level, this phenomenon is referred to as desensitization of oxytocin receptors (OTRs) [
19]. Balki et al [
20] discovered that pretreatment with oxytocin attenuates oxytocin-induced contractility in human myometrium, even after a rest period of up to 90 minutes following oxytocin administration.
The incidence of postpartum urinary retention (PPUR) was also observed as a significant difference between the two groups. Several prognostic factors associated with a high risk of PPUR have been identified [
21]
, [
22], including the mode and duration of labor, presence of perineal trauma, method of anesthesia or analgesia, patient's BMI, and baby's birth weight. However, there is limited reporting on the association between induction of labor and the mode of induction with PPUR. Despite an uncertain pathophysiology for PPUR, caution should be exercised regarding oral misoprostol induction in patients with PROM.
There are still several limitations in our study. Firstly, it is important to note that this was a single-center study conducted at a tertiary general hospital in Beijing. The patient population primarily consisted of individuals with perinatal complications and some transferal patients from lower-level hospitals [
23]. Therefore, future studies in China should consider conducting multi-center studies and randomized clinical trials to enhance the generalizability of the findings. Secondly, it is worth mentioning that different medical staff members may have varying interpretations of Bishop scores, which can impact the predictive value of successful induction. To minimize errors, it is recommended that the fixed individuals consistently assess the Bishop scores. Lastly, for comprehensive evaluation of drug safety, it is essential to include data on adverse reactions such as rapid heart rate, fever, gastrointestinal reactions etc., which were not captured in this study. Future research designs should incorporate relevant observation indicators to address this limitation.
Conclusion
Oral low-dose misoprostol has demonstrated both safe and superior efficacy to oxytocin infusion for labor induction and may potentially reduce the incidence of failed induction in term PROM pregnant women with unfavorite cervix condition. Additionally, oral low-dose misoprostol was associated with a decreased risk of postpartum hemorrhage but an increased rate of postpartum urinary retention.
Funding
The project was funded by the National Key Research and Development Program of China (2021YFC2701500).
Data availability
On request.
Author Contributions
YANG Yike: project design, data collection, data sorting, statistical analysis, thesis writing; YU Zhiheng: project design, data analysis, thesis modification; SHI Huifeng: statistical analysis and guidance; WANG Yan and ZHAO Yangyu: project design, research guidance. All authors approved the submission.
Acknowledgments
The authors would like to acknowledge support from the National Key Research and Development Program of China.
Competing interests
The authors declare no competing interests.
References
- Prelabor Rupture of Membranes. Obstetrics & Gynecology (2020) 135: e80-e97. [CrossRef]
- Middleton, P.; Shepherd, E.; Flenady, V.; McBain, R.D.; Crowther, C.A. Planned early birth versus expectant management (waiting) for prelabour rupture of membranes at term (37 weeks or more). Cochrane Database of Systematic Reviews 2017. [Google Scholar] [CrossRef] [PubMed]
- Hannah, M.E.; et al. Induction of Labor Compared with Expectant Management for Prelabor Rupture of the Membranes at Term. New England Journal of Medicine (1996) 334, 1005-1010. [CrossRef]
- Kumar, N.; Haas, D.M.; Weeks, A.D. Misoprostol for labour induction. Best Practice & Research Clinical Obstetrics & Gynaecology (2021) 77, 53-63. [CrossRef]
- Pergialiotis, V.; et al. Efficacy and safety of oral and sublingual versus vaginal misoprostol for induction of labour: a systematic review and meta-analysis. Archives of Gynecology and Obstetrics (2022) 308, 727-775. [CrossRef]
- Kerr, R.S.; et al. Low-dose oral misoprostol for induction of labour. Cochrane Database of Systematic Reviews (2021) 2021. [CrossRef]
- Padayachee, L.; Kale, M.; Mannerfeldt, J.; Metcalfe, A. Oral Misoprostol for Induction of Labour in Term PROM: A Systematic Review. (2020) Journal of Obstetrics and Gynaecology Canada 42, 1525-1531.e1521. [CrossRef]
- Pourali, L.; et al. Induction of labour in term premature rupture of membranes; oxytocin versus sublingual misoprostol; a randomised clinical trial. Journal of Obstetrics and Gynaecology (2017) 38, 167-171. [CrossRef]
- Ahmed, R.H.M.; Sweed, M.S.E.; El-Bishry, G.A.; Hassan, R.K. Oxytocin Versus Oral Misoprostol for Induction of Labor in Pregnant Women with Term Prelabor Rupture of Membranes: a Randomized Clinical Trial. Reproductive Sciences (2023) 30, 3507-3514. [CrossRef]
- Yike, Y.; et al. Effectiveness and safety of low-dose oral misoprostol solution for cervical ripening in the third trimester. Zhong Hua Wei Chan Yi Xue Za Zhi (2024) 27, 24-32. [CrossRef]
- Kawakita, T.; et al. Duration of Oxytocin and Rupture of the Membranes Before Diagnosing a Failed Induction of Labor. Obstetrics & Gynecology (2016) 128, 373-380. [CrossRef]
- Mbaluka, C.M.; Kamau, K.; Karanja, J.G.; Mugo, N. EFFECTIVENESS AND SAFETY OF 2-HOURLY 20 MCG ORAL MISOPROSTOL SOLUTION COMPARED TO STANDARD INTRAVENOUS OXYTOCIN IN LABOUR INDUCTION DUE TO PRE-LABOUR RUPTURE OF MEMBRANES AT TERM: A RANDOMISED CLINICAL TRIAL AT KENYATTA NATIONAL HOSPITAL. East African medical journal (2014) 91, 303-310.
- Robinson, d.; et al. Guideline No. 432c: Induction of Labour. (2023) Journal of Obstetrics and Gynaecology Canada 45, 70-77.e73. [CrossRef]
- in Inducing labour (National Institute for Health and Care Excellence (NICE), 2021 Nov 4).
- Ayala, N.K.; Rouse, D.J. Failed induction of labor. Am J Obstet Gynecol (2024) 230, S769-s774. [CrossRef]
- Grotegut, C.A.; Paglia, M.J.; Johnson, L.N.; Thames, B.; James, A.H. Oxytocin exposure during labor among women with postpartum hemorrhage secondary to uterine atony. Am J Obstet Gynecol (2011) 204, 56.e51-56. [CrossRef]
- Braund, S.; et al. Induction of labor and risk of postpartum hemorrhage in women with vaginal delivery: A propensity score analysis. International Journal of Gynecology & Obstetrics (2023) 164, 732-740. [CrossRef]
- Bernitz, S.; et al. Association of oxytocin augmentation and duration of labour with postpartum haemorrhage: A cohort study of nulliparous women. Midwifery (2023) 123. [CrossRef]
- Kelly, E.; Bailey, C.P.; Henderson, G. Agonist-selective mechanisms of GPCR desensitization. British journal of pharmacology (2008) 153 Suppl 1, S379-388. [CrossRef]
- Balki, M.; Ramachandran, N.; Lee, S.; Talati, C. The Recovery Time of Myometrial Responsiveness After Oxytocin-Induced Desensitization in Human Myometrium In Vitro. Anesthesia and analgesia (2016) 122, 1508-1515. [CrossRef]
- Ain, Q.U.; Shetty, N.; K; S. Postpartum urinary retention and its associated obstetric risk factors among women undergoing vaginal delivery in tertiary care hospital. Journal of gynecology obstetrics and human reproduction (2021) 50, 101837. [CrossRef]
- Nutaitis, A.C.; et al. Postpartum urinary retention: an expert review. Am J Obstet Gynecol (2023) 228, 14-21. [CrossRef]
- Yang, Y.; et al. Characteristics and treatment for severe postpartum haemorrhage in different midwifery hospitals in one district of Beijing in China: an institution-based, retrospective cohort study. (2024) BMJ Open 14. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).