1. Introduction
Laminitis, a common condition in horses, is characterized by the breakdown of the dermal–epidermal junction, resulting in destabilization and potential displacement of the distal phalanx. This pathological process often leads to severe lameness and acute pain, sometimes necessitating euthanasia [
1,
2,
3]. Although the precise etiology remains incompletely understood, evidence suggests that laminitis arises from local manifestations of an exaggerated systemic inflammatory response, leading to compromised blood flow, foot inflammation, endothelial/vascular dysfunction, extracellular matrix degradation, and metabolic perturbations in keratinocytes [
4,
5,
6].
The activation of polymorphonuclear neutrophils in the early stages of laminitis is well-documented [
7]. Our group has specifically demonstrated the presence of both myeloperoxidase (MPO) and elastase, two major proteins from the alpha granules of neutrophils, in the bloodstream, skin, and lamellar tissue of horses with black walnut extract (BWE)-induced laminitis [
8,
9]. Furthermore, recent evidence shows the presence of MPO in lamellar tissue of cases with laminitis induced with the prolonged euglycemic hyperinsulinemic clamp technique [
10]. MPO is a heme-containing peroxidase expressed mainly in neutrophils and to a lesser degree in monocytes. In the presence of hydrogen peroxide and halides, MPO catalyzes the formation of reactive oxygen intermediates, including hypochlorous acid (HOCl). MPO has been demonstrated to be a local mediator of tissue damage and the resulting inflammation in various inflammatory diseases [
11].
Even where mitochondrial dysfunction has not been demonstrated in the foot, energy deficiency has been suggested as an explanation for the disruption of the hemidesmosomes leading to the failure of the dermal-epidermal interface [
12].
The relationships between inflammation, particularly neutrophil activation, either associated with ischemic reperfusion events or not, and mitochondrial dysfunction, are documented across various organs such as the heart, kidneys, and liver [
13].
The hypothesis of an involvement of neutrophil-mediated mitochondrial dysfunction was previously suggested in muscle micro-biopsies from horses after strenuous exercise [
14]. Moreover, in cultured muscle cell lines, it was demonstrated that the potent neutrophil-derived oxidative enzyme myeloperoxidase (MPO) could enter the cell and disturb the electron transfer system resulting in altered ATP production [
15].
In 2014, a clinical study showed a significant decrease of muscle mitochondrial oxidative phosphorylation in horses affected by acute laminitis from divert origins when compared to the values obtained from fit or obese healthy horses [
16].
Recently, He et al. [
17] demonstrated that Neutrophil Extracellular Traps (NETs) induced mitochondrial dysfunction in cardiomyocytes associated with an increase of Reactive Oxygen Species (ROS) release.
Given that neutrophil activation is observed in the early stages of laminitis and that we have recently identified the presence of neutrophil extracellular traps (NETs) in severe clinical cases, we hypothesize a potential correlation between neutrophil activation, MPO activity, NETs release and metabolism dysfunction in equine laminitis [
18].
NETs are extracellular strands of decondensed (unwound) DNA in complex with histones and neutrophil granule proteins, such as MPO and elastase, which were expelled from neutrophils initially to ensnare and kill microbes. Uncontrolled inflammatory responses with excessive NET formation have been found to induce thrombosis and multiple organ failure in sepsis. [
19,
20,
21]. In ischemic-reperfusion injury, Zhang et al. [
22] reported that MPO-DNA complexes were the more cited markers of extracellular trap induction.
In sepsis, a recent metabolomic study confirmed the clinical evidence that patients with septic shock, no survivors exhibited a more profound and persistent dysregulation in protein analytes attributable to neutrophil activation and disruption of mitochondrial-related metabolism than survivors [
23].
Ischemic-reperfusion injury (IRI) takes place during reperfusion by activating inflammation and reactive oxygen species (ROS) production, causing mitochondrial damage and apoptosis of parenchymal cells [
24]. Mesenchymal Stem/Stromal Cells (MSCs) for the treatment of multi-organ IRI is currently considered a valid approach to reducing the injury [
25]. Moreover, Extracellular Vesicles (EVs) derived from human umbilical cord MSCs alleviate rat hepatic IRI by suppressing oxidative stress and neutrophil inflammatory response [
26].
In 2017, Magana-Guerrero demonstrated that amniotic-derived MSCs could interfered with the NET release by neutrophile via a mitochondrial pathway. We recently demonstrated in vitro a significant inhibition on the Net-bound-MPO activity by muscle-derived MSCs and a decrease of ROS production by activated neutrophiles [
27].
Preliminary clinical studies using MSCs in laminitis showed encouraging results. The therapeutic potential is attributed to the unique properties of the MSCs that target damaged tissues, inhibit the immune and inflammatory response, and facilitate repair [
28]. However, the mechanisms of action could be deeply investigated. In our group, we described a minimally invasive technology to obtain muscle-derived Mesenchymal Stem Cells (mdMSCs). These cells have similar properties as MSCs from other sources and showed potent immunomodulatory effects [
29].
Considering the aforementioned points, the aims of this study were: to develop an in vitro model of laminitis using keratinocytes exposed to anoxia-reoxygenation (A/R) in conjunction with activated neutrophils supernatant and to reveal the potential therapeutic effect of mdMSCs on keratinocytes metabolism and the MPO activity.
2. Materials and Methods
2.1. Cells
Keratinocytes (HaCaT) and muscle-derived mesenchymal stem cells (mdMSCs) were purchased from ATCC (USA) and Revatis SA (Aye, Belgium) and cultured in DMEM-F-12 (20 % FBS) and DMEM high glucose with (10% FBS), respectively.
2.2. Preparation and Characterization of the Activated Neutrophil Supernatant
2.2.1. Equine neutrophils were obtained from blood collected by jugular venipuncture on 5 healthy horses on EDTA tubes as described by Pycock et al. [30]. Briefly, the neutrophils were isolated at room temperature (18–22°C) by centrifugation (400 x g, 30 min at 20°C) on a discontinuous percoll density gradient. The polymorphonuclear fraction was collected in PBS counted and diluted into DMEM high glucose + 10% FBS to obtain a suspension of 2 million neutrophils/ml. Neutrophils were stimulated by adding 1µl of cytochalasin B (5 mg/ml) per ml of cell suspension in the medium and incubating them for 30 min at 37°C. Thereafter, 10 µl of fMLP (10-4 M) per ml of cell suspension was added and cell were incubated for 30 min at 37°C. Control conditions were performed in parallel with cell suspensions without the addition of CB and fMLP or with the replacement of the stimulating molecules by DMSO, the solvent used for their solubilisation (Ctrl DMSO). Finally, the cell suspensions were centrifuged for 5 minutes at 600 x g and the supernatants were collected and stored at -20°C for future experiments. Activated neutrophil and non-activated neutrophil supernatants were called ANS and NANS respectively [31]
2.2.2. Active free MPO and active MPO bound to the NET were measured in the supernatants (NANS and ANS) after neutrophil incubation and stimulation according to the techniques described by Franck et al. [32].
2.2.3. Measurement of Active MPO by SIEFED
The SIEFED (specific immuno-extraction followed by enzymatic detection) uses an immobilized primary antibody (polyclonal rabbit anti-MPO IgG antibody) coated onto the microplate wells. The non-diluted supernatant was added to the wells and incubated for 2 hours at 37◦C in darkness to allow the capture of MPO by the antibodies. After removing the sample and three washing, the substrate (H2O2) and co-substrates (nitrite and Amplex Red) were added to reveal the peroxidase activity of MPO as evidenced by the oxidation of Amplex Red into its fluorescent adduct resorufin. In more detail, the peroxidase activity of MPO was monitored by adding 100 µL of a 40 µM Amplex red solution freshly prepared in 50 mM phosphate buffer, pH 7.4, supplemented with 10 µM H2O2 and 10 mM sodium nitrite. Fluorescence was measured at the excitation and emission wavelengths of 544 and 590 nm, respectively for 30 min at 37◦C with the fluorescent plate reader (Fluoroskan Ascent, Fisher, Merelbeke, Belgium). The fluorescence value was directly proportional to the quantity of active MPO in the sample. MPO concentrations were calculated in reference to a calibration curve carried out with purified equine MPO ranging from 2 to 140 ng/ml.
2.2.4. NET-bound-MPO activity MPO was measured in the supernatant after neutrophil incubation and stimulation according to the techniques described in Storms et al. [18]). The NET released by the neutrophils was captured by anti-histone H3 (citrulline R2 + R8 + R17; anti-H3Cit) antibodies as performed in Franck et al. (2021). Then the presence of active MPO bound to the NET was detected in the same way as the SIEFED assay. This technique used an immobilized primary rabbit anti-H3Cit antibody (0.5 μg/ml) diluted with 20 mM PBS buffer coated onto a transparent 96-well microplate to capture NET. After removal of the coating solution, the plates were incubated (150 min, 22◦C) with blocking buffer (PBS buffer with 5 g/L of BSA) and washed four times with PBS buffer with 0.1% Tween 20. The plates were then dried for 3 hours at 22◦C and conserved in a dry atmosphere in a hermetic bag at 4◦C until use. The samples were loaded into the wells of the anti-H3Cit coated microplate in duplicate and incubated for 2 hours at 37◦C. Then, the supernatants were removed, and the wells were washed four times with a PBS solution containing 0.1% Tween 20 before active MPO was measured. For the revelation of the peroxidase activity of MPO bound to NET, sodium nitrite and Amplex Red solution were added as described above (SIEFED) and fluorescence was measured over 30 min with the fluorescent plate reader (Fluoroskan Ascent, Fisher, Merelbeke, Belgium). To evaluate the level of active MPO bound to the NET, a calibration curve ranging from 2 to 140 ng/ml was performed parallelly with purified equine MPO but using wells coated with polyclonal rabbit anti-MPO IgG antibody.
2.3. Effect of NANS and ANS on HaCaT Metabolism in Normoxia and in Anoxia
HaCaT were seeded in 2 transparent 96 microwell plates (10,000 cells/well) for 24h in DMEM high glucose with (10% FBS) to let them adhere. Afterwards the medium was removed, the wells were washed once with HBSS then the plate was divided into 5 parts to add medium alone, medium containing cisplatin (1.10-3M) as cytotoxic control, medium prepared with non-activated (NANS) and activated (ANS) neutrophils. A control assay was performed with the supernatant obtained with neutrophils in presence of DMSO (Ctrl DMSO). In all tested conditions, the HaCaT medium was used without red phenol. One plate was exposed to anoxia with a controlled oxygen level at 0.2% and the other one to normoxia (18.6 % oxygen) for 48 h. After incubation, the plate incubated under anoxia was transferred to normoxia with the other plate and incubated during 24h. The medium from the wells of the two plates was removed and the wells were washed once with HBSS before the addition of 100 µl HBSS and 10 µl MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium). MTS used as metabolic viability-based assays was from Promega (CellTiter 96 Aqueous non-radioactive cell proliferation assay). Just after MTS addition a first reading of the absorbance at 490 nm was made with Multiskan Ascent spectrophotometer (Thermo labsystem, Finland) then every 1 h till 4 h to follow the evolution of the absorbance.
2.4. Effect of mdMSCs on HaCaT Metabolism
HaCaT were seeded as above in two transparent plates for 24h but some wells were left empty. At cell adherence, the medium was removed, the cells were washed once with HBSS then the wells containing HaCaT were divided into 3 parts to add medium (without red phenol), medium containing cisplatin (1.10-3M) as cytotoxic control and the medium prepared with activated neutrophil (ANS). One plate was exposed to anoxia with controlled oxygen level at 0.2% and the other one to normoxia (18.6 % oxygen level) for 48 h. Afterwards, the medium from the wells was removed and the wells were washed once with HBSS before the addition of 20,000 mdMSCs/well in all wells containing HaCaT to achieve a 50% HaCaT medium and 50% mdMSCs medium ratio. In the wells previously let empty mdMSCs were also added in the presence of the mixed medium in order to have a control with mdMSCs alone. The plates are then placed in normoxia for 24h. Subsequently, the medium from the wells of the two plates was removed and the wells were washed once with HBSS before the addition of 100 µl HBSS and 10 µl MTS for the measurement of cell metabolism. Just after MTS addition a first reading of the absorbance at 490 nm was made with Multiskan Ascent spectrophotometer (Thermo labsystem, Finland) then every 1h till 4h to follow the evolution of the absorbance. For result interpretation, the metabolic response of mdMSCs alone is subtracted from that of HaCaT + mdMSCs.
2.5. HaCaT-MPO Activity and Immunolocalization
2.5.1. HaCaT Incubation with ANS
The experiment was performed with a 6-well cell culture plate (CellStar, Greiner bio-one) seeded with 100,000 HaCaT per well. ANS obtained after CB/fMLP was diluted 4 or 8 times with the HaCaT medium and then added into the wells to have a final volume of 2 ml. Each dilution was tested twice. In the two remaining wells no ANS was added. After ANS addition, HaCaT were incubated for 2h in the incubator (37°C, 5 % CO2) then the medium was removed and the wells were rinsed 3 times with 1.5 mL DPBS, before the measurement of the in situ MPO activity.
2.5.2. HaCaT Incubation with MPO
The experiment was performed with a 6-well cell culture plate (CellStar, Greiner bio-one) seeded with 100.000 HaCaT per well. To HaCaT adherent cells, a 100 µg/ml stock solution of equine MPO was added to have in two wells 250 ng/ml MPO and in two other wells 500 ng/ml MPO. In the two remaining wells no MPO was added. After MPO addition, HaCaT were incubated for 2h in the incubator (37°C, 5 % CO2). After incubation, the medium was removed and the wells were rinsed 3 times with 1.5 mL DPBS, before the measurement of the in situ MPO activity or the immunological detection of MPO.
2.5.3. Measurement of the HaCaT-MPO Activity
After washing, the in-situ peroxidase activity of MPO was monitored by adding 1 mL of a 40 μM Amplex red solution freshly prepared in 50 mM phosphate buffer, pH 7.4, supplemented with 10 μM H
2O
2 and 10 mM sodium nitrite according to Franck et al. [
26]. The fluorescence development was monitored during 30 min (37 °C) with a Fluoroskan Ascent (Thermo Fisher Scientific, Waltham, MA, USA) set at 544 nm and 590 nm for the excitation and emission wavelengths, respectively. Total fluorescence was directly proportional to the amount of active MPO in the sample.
2.5.4. Detection of HaCaT-MPO by Immunocytology
After the incubation of HaCaT with human MPO or/and with mdMSCs as described above, the medium was removed, the wells were washed 3 times with 1.5 mL of DPBS and the cells were fixed with a cold commercial 4% PFA solution (Thermo Scientific) for 10 min. Thereafter, a cell permeabilization was done by incubating the cell during 10 min with 0.1% Tritron X-100. After 3 washings with 1.5 ml DPBS, a circular zone at the bottom of the wells was delimited with a hydrophobic slide marker. This zone was immuno-stained for human MPO using the horseradish peroxidase/diaminobenzidine ABC detection immunohistochemistry (IHC) kit (Abcam) and according to the protocol with slight modifications. Successively the steps were: hydrogen peroxide block (10 min, RT), protein block (10 min, RT), incubation (1h, 37°C) with the rabbit primary antibody against human MPO (Abcam 9535, diluted 1:100 x with 20 mM PBS pH 7.4 + 0.5% bovine serum albumin and 0.1% Tween 20), biotinylated goat (15 min, RT), streptavidin peroxidase (15 min, RT), DAB chromogen substrate (10 min, RT), hematoxylin solution (Merck) (45 sec). Between each step 3 washings with DPBS were performed (3 x 3 min). Two ml of DPBS buffer were added before the observation with light microscopy using a Zeiss Axioskop microscope, and all photographs were obtained using the same light intensity and shutter speed.
2.6. Effects of mdMSCs on HaCaT-MPO Activity
As in point 5.2, the experiment was performed with a 6-well cell culture plate (CellStar, Greiner bio-one) seeded with 100,000 HaCaT per well. Adherent cells were preincubated with 250 ng/ml and 500 ng/ml equine MPO for 2h then the excess of MPO was removed by 3 washing with PBS. Following the washing step, a mix (v/v, 1/1) of DMEM high glucose and DMEM-F12 was added in the 3 upper wells, while in the 3 bottom ones 200,000 mdMSCs in 1 mL DMEM-F12 were added to HaCaT cells previously covered by 1 ml DMEM high glucose. Thereafter, the culture plate was incubated for 24 h in the incubator (37°C, 5 % CO2). After removal of the medium, the wells were washed 3 times with 1.5 mL DPBS before the measurement of the in situ MPO activity.
2.7. Statistical Analysis
At least, five independent experiments were taken into consideration for the figures and statistical analysis. For the figures, some data were expressed as mean standard deviation (SD) and given in relative values (%) by reference to control groups taken as 100%. As some populations or data series did not follow a Gaussian distribution, a nonparametric test was used: a two-tail Mann–Whitney test was performed (Graph Pad, InStat, San Diego, CA, USA). A p-value < 0.05 was considered significant.
3. Results
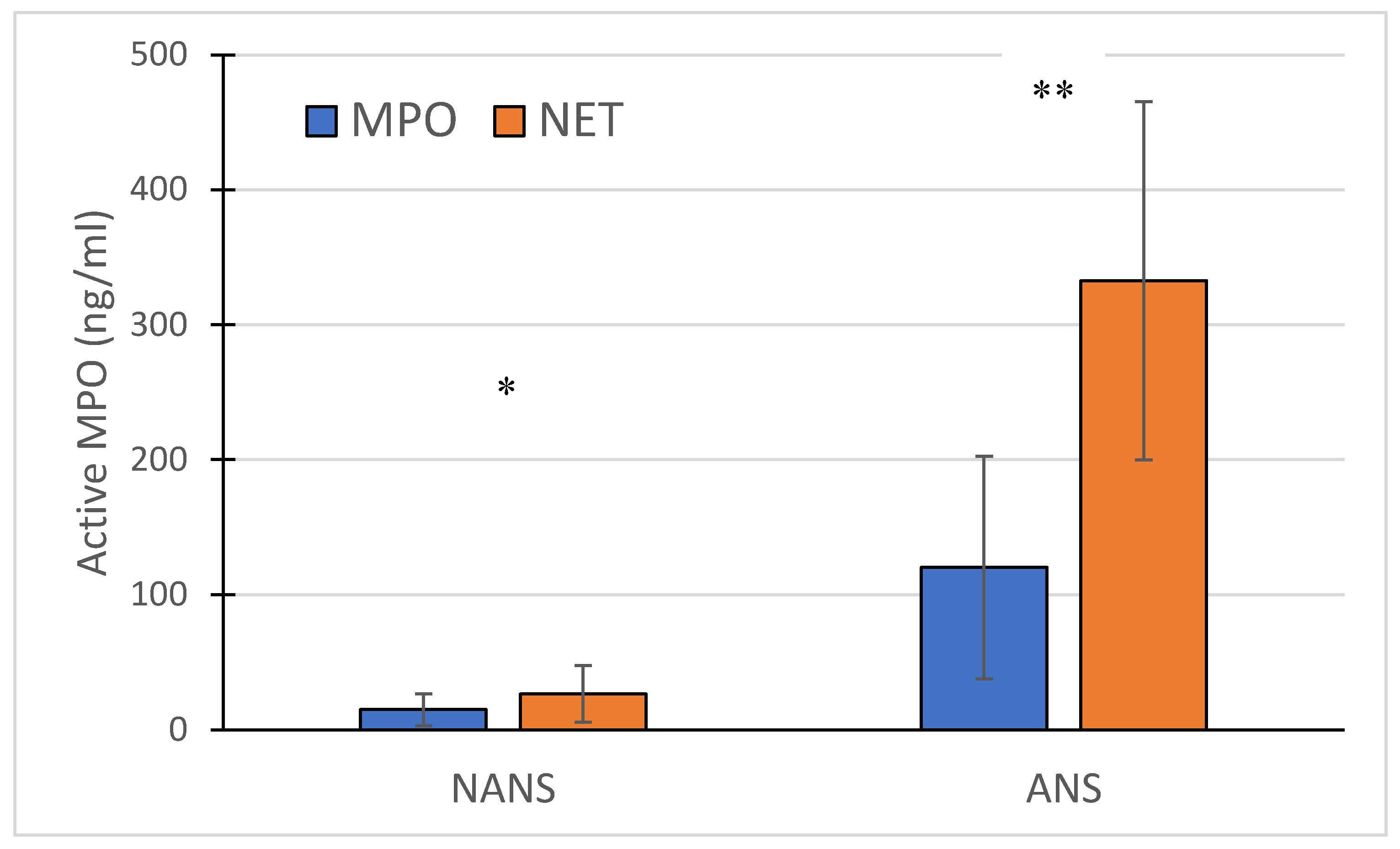
3.1. Free MPO and NET-Bound MPO Released by Neutrophils
The concentration of free active MPO and NET-bound MPO significantly increased in the ANS compared to the NANS. Additionally, active MPO appears to be primarily associated with NETs (
Figure 1)
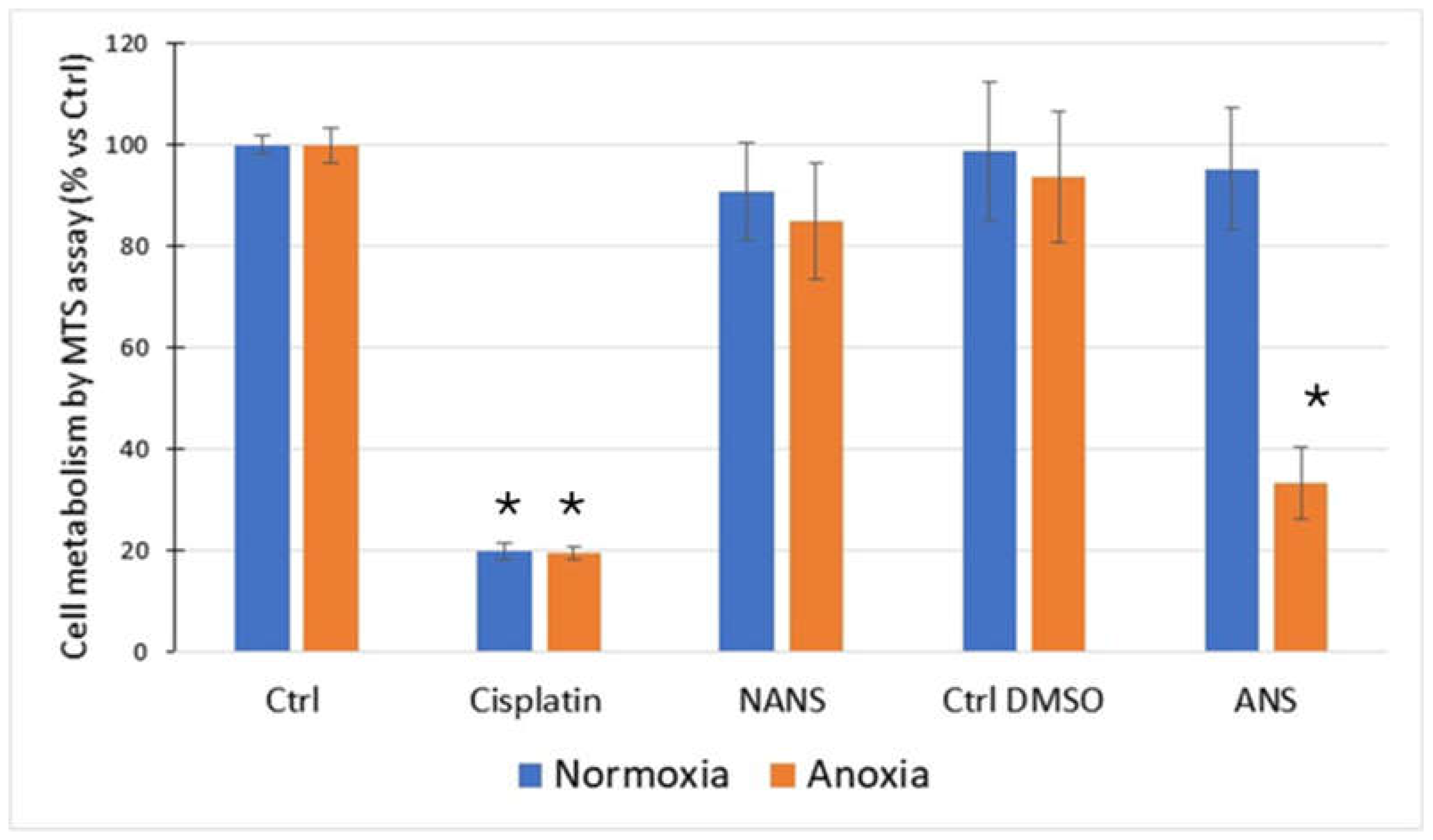
3.2. Effect of Normoxia and Anoxia on HaCaT Metabolism in the Presence of Neutrophil Supernatants
HaCaT cells exposed to anoxia-reoxygenation, in the presence of ANS, exhibited a significant decrease in their metabolic activity compared to the control (normoxia) and the non-activated condition. Cisplatin used as a positive toxicity control showed a strong decrease in metabolic activity (
Figure 2).
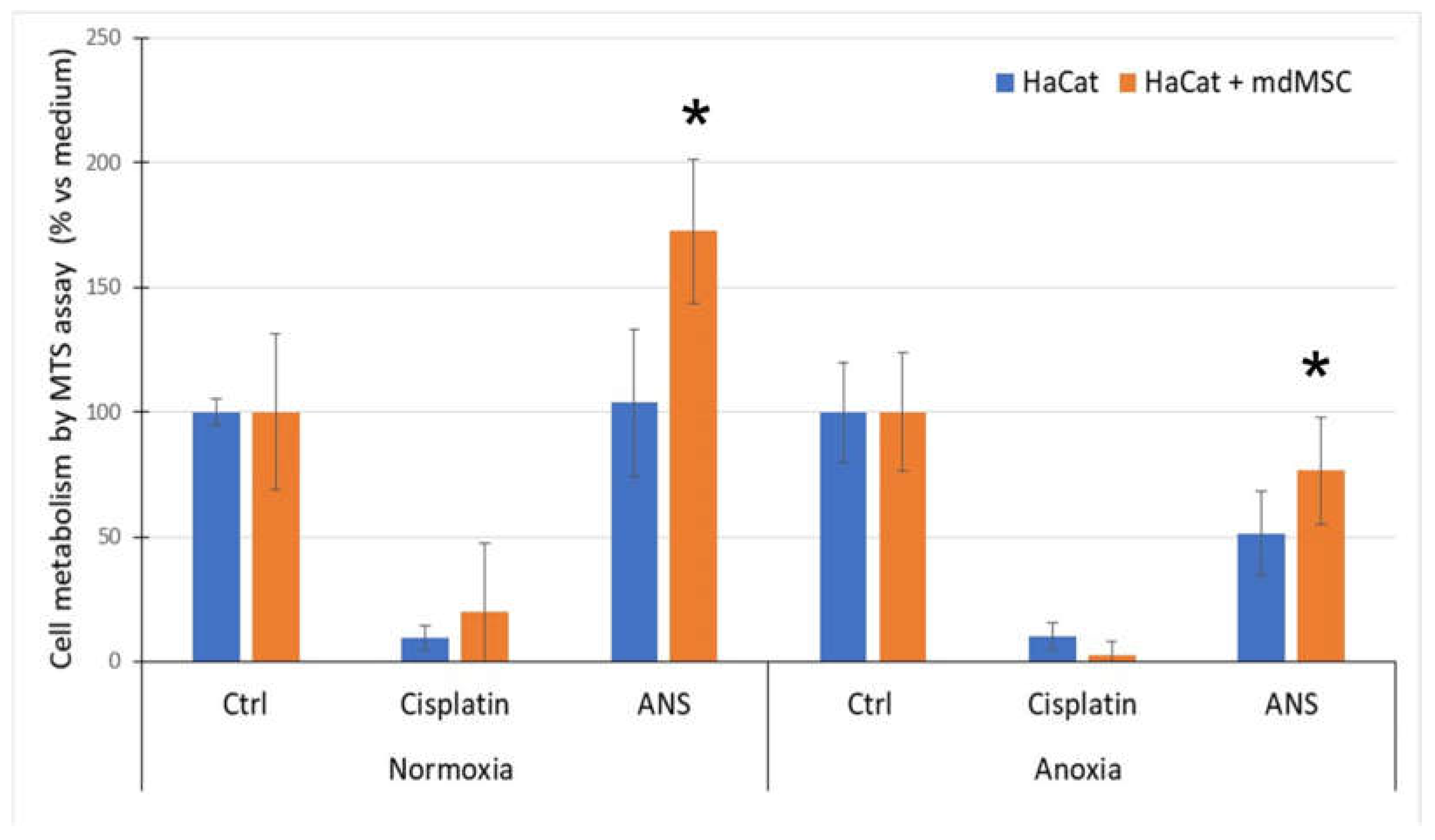
3.3. Effect of mdMSC on HaCaT Metabolism Submitted to Normoxia or Anoxia with ANS
MdMSCs co-cultured with HaCaT showed a significant increase of the HaCaT metabolism in normoxia and a protective effect against stress conditions induced by A/R in the presence of ANS (
Figure 3). No significant improvement was observed when mdMSCs were put in contact with HaCaT cells pre-treated with Cisplatin.
3.4. Active MPO from ANS and Purified Equine MPO are Captured by HaCaT
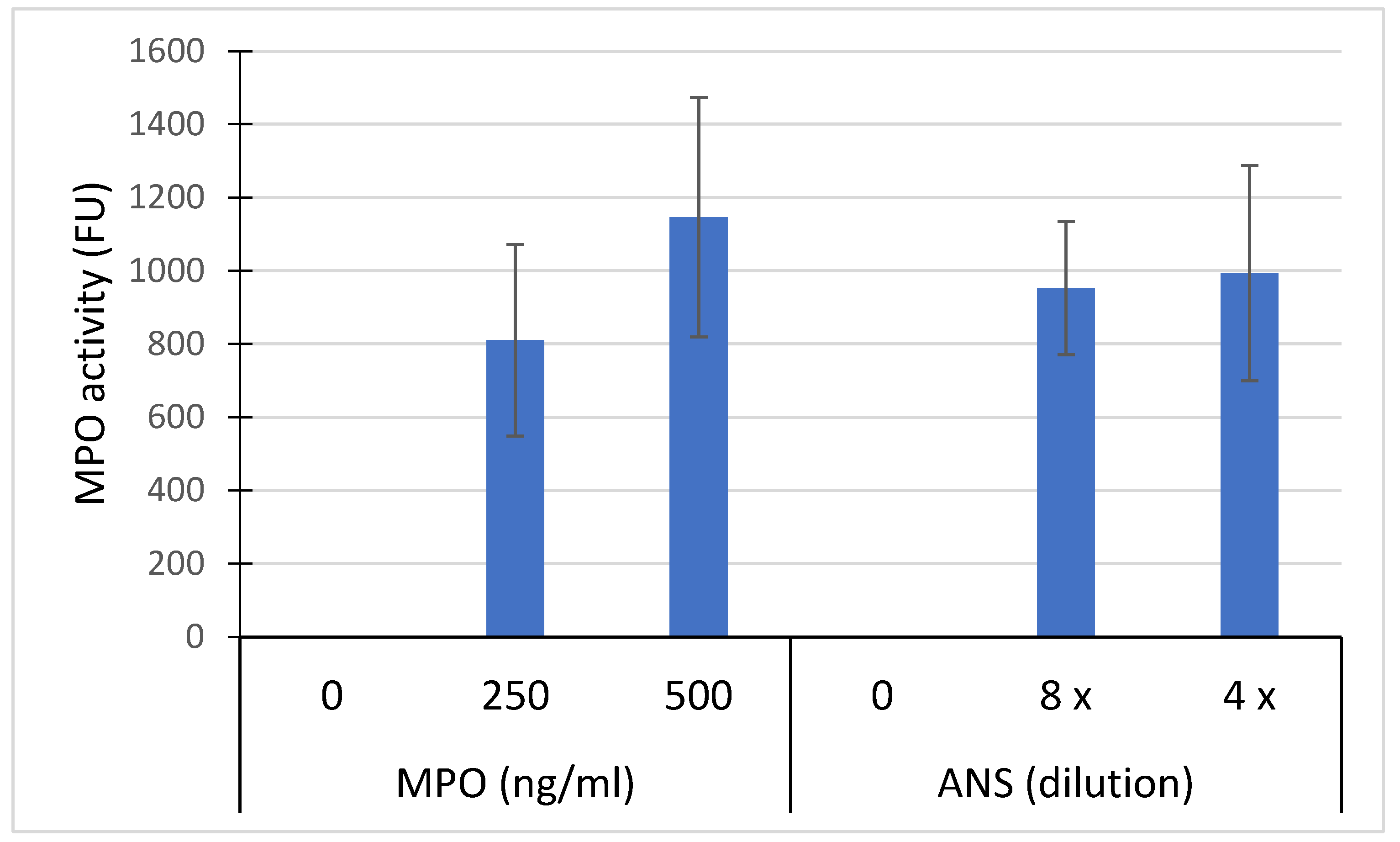
Despite washing of HaCaT cells exposed for 2h with ANS or purified MPO, an in situ peroxidase activity was measured. This activity appeared more dose-dependent for purified MPO than for ANS (Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Figure Fig 4). The capture of MPO by HaCat was confirmed by IHC as shown in
Figure 5 where MPO appeared included in the cytosol with a perinuclear localization observed in some cells.
Figure 4.
MPO activity measured on adherent HaCaT cells (100,000/ml) after their incubation with 250 ng/ml and 500 ng/ml of total equine MPO or with ANS diluted 4 and 8 times into the medium. After their incubation with MPO, cells were washed 3 times with PBS buffer before the in situ measurement of the peroxidase activity. Results are express in fluorescence units Mean +/- SD (n= 5 independent experiments).
Figure 4.
MPO activity measured on adherent HaCaT cells (100,000/ml) after their incubation with 250 ng/ml and 500 ng/ml of total equine MPO or with ANS diluted 4 and 8 times into the medium. After their incubation with MPO, cells were washed 3 times with PBS buffer before the in situ measurement of the peroxidase activity. Results are express in fluorescence units Mean +/- SD (n= 5 independent experiments).
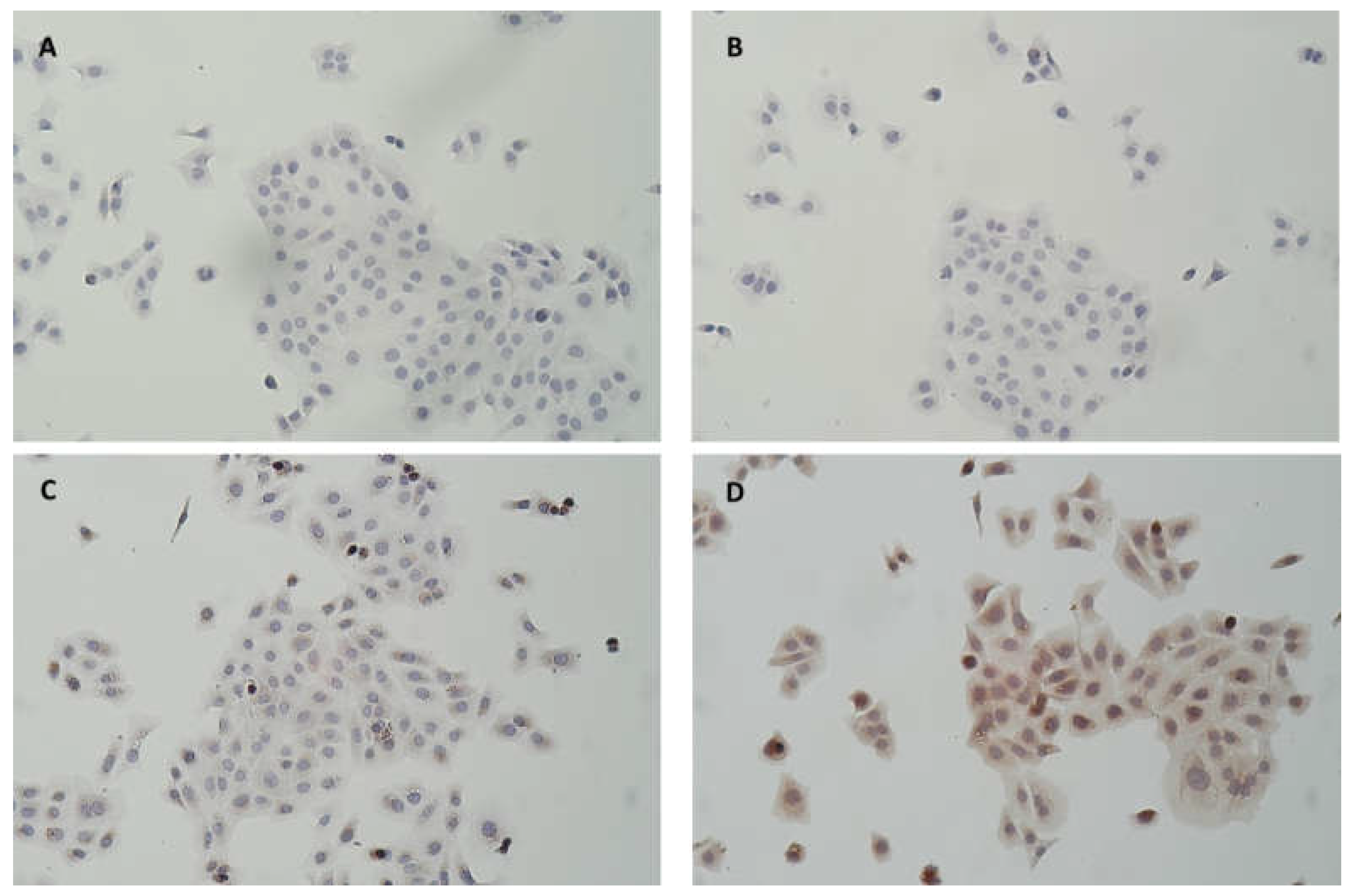
Figure 5.
Photomicrograph of HaCaT pretreated (B, D) or not (A, C) for 2h with 500 ng/ml human MPO. After 3 washings with DPBS to remove the medium with MPO, cells were directly fixed and further stained following an immunohistochemical protocol. A and B without primary MPO antibody, C and D with primary MPO antibody (× 100).
Figure 5.
Photomicrograph of HaCaT pretreated (B, D) or not (A, C) for 2h with 500 ng/ml human MPO. After 3 washings with DPBS to remove the medium with MPO, cells were directly fixed and further stained following an immunohistochemical protocol. A and B without primary MPO antibody, C and D with primary MPO antibody (× 100).
3.5. mdMSCS Added to Pre-Treated MPO HaCaT Cells Inhibit the In Situ Activity of MPO
After the washing of HaCaT pre-treated 2h with MPO, mdMSCs were added to HaCaT and cultivated for 24 h to let them adhere. After this incubation period, the mix of adherent cell populations (HaCaT + mdMSC) was washed 3 times with PBS and the in situ activity of MPO was revealed as above. First, we noticed a strong decrease in the in situ MPO activity 24h after their initial contact with MPO. This decrease appeared superior to 80% for HaCaT cells pre-treated with 500 ng/ml MPO (supplementary material). An additional decrease of MPO activity was observed when HaCaT were cocultured with mdMSCs compared to HaCaT cell alone. This decrease appeared significant (p < 0.01) when HaCaT cells were exposed to 500 ng/ml equine MPO (
Table 1).
4. Discussion
The primary objective of this study has been achieved. We have successfully developed a straightforward in vitro model to explore laminitis by subjecting a continuous-keratinocyte cell line (HaCaT) to an activated neutrophil supernatant (ANS) following a period of anoxia and subsequent reoxygenation. The MTS tetrazolium assay was used in this study to evaluate the activity of cellular metabolism by measuring the activities of mitochondrial NAD(P)H oxidoreductases or cytoplasmic esterases [
33,
34]. This test is currently one of the most widely used methods to assess drug toxicity [
35]. In our study cisplatin was used as cytotoxic molecule control to evaluate the stress intensity.
When cells were exposed to ANS and anoxia followed by reoxygenation a significant decrease of HaCaT metabolism was observed. Such decrease was not observed in cells staying in normoxic conditions. ANS was obtained according to the method of Franck et al. [
31] using a combination of CB and fMLP mimicking a more physiological process for neutrophil stimulation and degranulation of active MPO. Our results showed that ANS contained an important concentration of free active MPO but also a more important NET bound active MPO in comparison to the supernatant obtained with non-stimulated neutrophils (NANS).
Herein, the inhibition of HaCaT metabolism upon exposure to ANS was observed specifically following a period of anoxia followed by reoxygenation. It is widely recognized that IRI arises from mitochondrial dysfunction, leading to the production of reactive oxygen species such as superoxide anion, hydroxyl radical, and hydrogen peroxide [
36,
37]. The latter serves as a substrate for MPO, generating additional potent oxidants that can contribute to exacerbate mitochondrial dysfunction. Our results are in accordance with Ceusters et al. [
15] who demonstrated that MPO enters in myoblasts and contributes to the exacerbation of mitochondrial dysfunction during an anoxia-reoxygenation period. It is important to note that ANS likely contains various other products released by activated neutrophils, such as cytokines, lipid mediators, and other proteolytic enzymes able to participate in tissue injury [
38]. In addition, He et al. [
17] have indicated that NET release can also disrupt the mitochondrial function of targeted cells.
Interestingly, the addition of mdMSCs during the reoxygenation phase exhibited a protective effect, leading to a restoration of cellular metabolic activity in HaCaT cells treated with ANS and previously submitted anoxia suggesting a beneficial effect of mdMSCs in the restoration of mitochondrial function during stress condition as often described in the literature [
39]. However, an increase in mitochondrial function was also observed in cell submitted to ANS without an anoxia period. Indeed, mitochondrial transfer was described under both physiological and pathological conditions. MSCs can transfer entire mitochondria or fragments via extracellular vesicles (EVs) or nanotubules [
40]. The mdMSCs mechanism of action may involve modulation of cellular respiratory function. Indeed, preliminary results presented at the Berlin congress suggest the ability of mdMSCs to transfer mitochondria in HaCat cells. However, the recovery of metabolic activity could also be due to the effect of mdMSCs on NETs present in the medium. Magana-Guerrero et al. [
41] demonstrated that amniotic-derived MSCs inhibited NET release by interfering with neutrophil mitochondria. Furthermore, their study showed that the inhibition of NETs, reduction in ROS levels, and loss of mitochondrial membrane potential were reversed by MSCs overexpressing TSG-6, an important factor that inhibits the TNF/NF-kB signalling pathway.
Additionally, to its pivotal role in the regulation of the energetic metabolism, the administration of MSCs is increasingly recognized as a valid strategy for treating multi-organ IRI, attenuating cytokine storms associated with acute respiratory distress syndrome (ARDS), systemic inflammatory response syndrome, and sepsis [
25]. These beneficial effects are attributed in part to the immunomodulatory properties of mdMSCs. Our previous studies have demonstrated that in vitro, mdMSCs exhibit significant decrease of neutrophil modulation involving an inhibition of ROS production, active MPO release and NET-bound MPO activity [
27].
Our finding suggested that active MPO played a significant role in the pathogenesis of laminitis. We observed its presence in lamellar tissue of horse hooves submitted to experimental laminitis and in clinical case where active NET-bound MPO was also measured and detected in lamellae [
9,
18]. Thus, using an in vitro culture cells model we investigated whether MPO could enter into keratinocytes cells and if the supplementation of mdMSCs in this model could modulate the activity of MPO. We first demonstrated that equine MPO as well as MPO from ANS was captured by HaCaT cells and that this MPO remained active. IHC confirmed the presence of MPO intracellularly mainly in a perinuclear manner. Ceusters et al. [
15] also observed a perinuclear localization of MPO after its entrance into myoblasts.
Remarkably, the coculture of HaCaT during 24h with mdMSCs induced a decrease of the activity of MPO captured compared to HaCaT alone. These effects are likely attributed to specific compounds released by mdMSCs and internalized by HaCAT cells to inhibit MPO activity intracellularly. Previously, our research suggested that the secretum of mdMSCs can effectively suppress MPO activity [
27].
Our study has certain limitations that merit consideration. Firstly, HaCat cells, being a continuous line of keratinocytes, may not fully represent the behaviour of primary keratinocytes. Therefore, it would be beneficial to validate our findings using primary keratinocytes to ensure the robustness of our model. Secondly, while our current model provides a simplified in vitro representation of laminitis, future advancements could involve the development of a more sophisticated organ-on-chip model. This advanced model could incorporate additional components such as endothelial cells and a basement membrane, along with equine keratinocytes, to better mimic the complex physiological environment of the lamellar tissue.
5. Conclusions
Our study sheds light on the potential mechanisms underlying the recovery of HaCaT metabolic activity. While the transfer of mitochondria may play a role, our findings highlight the significant anti-inflammatory properties of mdMSCs, implicating the NETs, MPO activity, and ROS generation. These findings hint at a promising direction for future research in cell therapy for laminitis, where mdMSCs show promise in mitigating inflammation and potentially restoring keratinocyte metabolism.
Author Contributions
All the co-authors participated in the writing and reviewing of the manuscript. D.S. and N.S.: Main investigators responsible for the project and in charge of the follow-up of each assay. J.C and C.S: Provided expertise in equine laminitis and performed the muscle microbiopsies. A.N., T.F., and A.M.: Conducted lab manipulations and adapted protocols based on the results. H.G. and J.D.: Managed the production and quality control of the mdMSCs and participated in the experimental protocols.
Funding
This work was supported by a grant of Wallonia (Lamitrans).
Institutional Review Board Statement
Muscle micro-biopsy protocol for producing MSC was approved by the ethic comity of the University of Liege (N° 21-2421).
Informed Consent Statement
Not Applicable.
Data Availability Statement
All data generated or analysed during this study are included in this published article.
Conflicts of Interest
D. Serteyn and J. Ceusters are the co-inventors of a patent related to muscle-derived stem cells. This patent is licensed by the University of Liège to Revatis, a Spin-Off company where D. Serteyn and J. Ceusters serve as scientific advisors. The other co-authors declare no conflicts of interest.
References
- Menzies-Gow, N.J.; Stevens, K.; Barr, A.; Camm, I.; Pfeiffer, D.; Marr, C.M. Severity and outcome of equine pasture-associated laminitis managed in first opinion practice in the UK. Vet Rec. 2010, 167, 364–9. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.M.; Bailey, S.R. A review of recent advances and current hypotheses on the pathogenesis of acute laminitis. Equine Vet J. 2012, 44, 752–61. [Google Scholar] [CrossRef] [PubMed]
- Leise, B. The role of neutrophils in equine laminitis. Cell Tissue Res. 2018, 371, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Peroni, J.F.; Harrison, W.E.; Moore, J.N.; Graves, J.E.; Lewis, S.J.; Krunkosky, T.M.; Robertson, T.P. Black walnut extract-induced laminitis in horses is associated with heterogeneous dysfunction of the laminar microvasculature. Equine Vet J. 2005, 37, 546–51. [Google Scholar] [CrossRef] [PubMed]
- Hurley, D.J.; Parks, R.J.; Reber, A.J.; Donovan, D.C.; Okinaga, T.; Vandenplas, M.L.; Peroni, J.; Moore, J. Dynamic changes in circulating leukocytes during the induction of equine laminitis with black walnut extract. Vet Immunol Immunopathol. 2006, 110, 195–206. [Google Scholar] [CrossRef]
- Loftus, J.P.; Belknap, J.K.; Stankiewicz, K.M.; Black, S.J. Laminar xanthine oxidase, superoxide dismutase and catalase activities in the prodromal stage of black-walnut induced equine laminitis. Equine Vet J. 2007, 39, 48–53. [Google Scholar] [CrossRef] [PubMed]
- de la Rebière, G.; Serteyn, D. The role of activated neutrophils in the early stage of equine laminitis. Vet J. 2011, 189, 27–33. [Google Scholar] [CrossRef]
- Riggs, L.M.; Franck, T.; Moore, J.N.; Krunkosky, T.M.; Hurley, D.J.; Peroni, J.F.; de la Rebière, G.; Serteyn, D.A. Neutrophil myeloperoxidase measurements in plasma, laminar tissue, and skin of horses given black walnut extract. Am J Vet Res. 2007, 68, 81–6. [Google Scholar] [CrossRef] [PubMed]
- de la Rebière, G.; Riggs, L.M.; Moore, J.N.; Franck, T.; Deby-Dupont, G.; Hurley, D.J.; Serteyn, D. Equine neutrophil elastase in plasma, laminar tissue, and skin of horses administered black walnut heartwood extract. Vet Immunol Immunopathol. 2010, 135, 181–7. [Google Scholar] [CrossRef] [PubMed]
- Storms, N.; Medina Torres, C.; Franck, T.; Sole Guitart, A.; de la Rebière, G.; Serteyn, D. Presence of Myeloperoxidase in Lamellar Tissue of Horses Induced by an Euglycemic Hyperinsulinemic Clamp. Front Vet Sci. 2022, 9, 846835. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aratani, Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Arch Biochem Biophys. 2018, 640, 47–52. [Google Scholar] [CrossRef] [PubMed]
- French, K.R.; Pollitt, C.C. Equine laminitis: loss of hemidesmosomes in hoof secondary epidermal lamellae correlates to dose in an oligofructose induction model: an ultrastructural study. Equine Veterinary Journal 2004, 36, 230–235. [Google Scholar] [CrossRef]
- Shepherd, H.M.; Gauthier, J.M.; Terada, Y.; Li, W.; Krupnick, A.S.; Gelman, A.E.; KreiselD. Updated Views on Neutrophil Responses in Ischemia-Reperfusion Injury. Transplantation. 2022, 106, 2314–2324. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Franck, T.; Votion, D.M.; Ceusters, J.; De La Rebière de Pouyade, G.; Mouithys-Mickalad, A.; Niesten, A.; Fraipont, A.; VANErck, E.; Goachet, A.G.; Robert, C.; Serteyn, D. Specific immuno-extraction followed by enzymatic detection (SIEFED) of myeloperoxidase and mitochondrial complex I in muscular microbiopsies: preliminary results in endurance horses. Equine Vet J Suppl. 2010, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Ceusters, J.D.; Mouithys-Mickalad, A.A.; Franck, T.J.; Derochette, S.; Vanderplasschen, A.; Deby-Dupont, G.P.; Serteyn, D.A. Effect of myeloperoxidase and anoxia/reoxygenation on mitochondrial respiratory function of cultured primary equine skeletal myoblasts. Mitochondrion. 2013, 13, 410–6. [Google Scholar] [CrossRef] [PubMed]
- Serteyn, D.; Pouyade, G.R.; Sandersen, C.; Salciccia, A.; Grulke, S. Muscle Mitochondrial Dysfunction in Horses Affected by Acute Laminitis. Bioenergetics 2014, 3, 1000120. [Google Scholar] [CrossRef]
- He, L.; Liu, R.; Yue, H.; Zhang, X.; Pan, X.; Sun, Y.; Shi, J.; Zhu, G.; Qin, C.; Guo, Y. Interaction between neutrophil extracellular traps and cardiomyocytes contributes to atrial fibrillation progression. Signal Transduct Target Ther. 2023, 8, 279. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Storms, N.; de la Rebière, G.; Franck, T.; Mouithys Mickalad, A.; Sandersen, C.; Ceusters, J.; Serteyn, D. Neutrophil extracellular traps and active myeloperoxidase concentrate in lamellar tissue of equids with naturally occurring laminitis. Vet Immunol Immunopathol. 2024, 270, 110738. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, O.; Borregaard, N. Neutrophil extracellular traps — the dark side of neutrophils. J. Clin. Investig. 2016, 126, 1612–1620. [Google Scholar] [CrossRef]
- Delgado-Rizo, V.; Martínez-Guzmán, M.; Iñiguez-Gutierrez, L.; García-Orozco, A.; Alvarado-Navarro, A.; Fafutis-Morris, M. Neutrophil Extracellular Traps and Its Implications in Inflammation: An Overview. Front. Immunol. 2017, 8, 81. [Google Scholar] [CrossRef]
- Tablin, A.; Li, R. A comparative review of neutrophil extracellular traps in sepsis Front. Vet. Sci. 2018, 5, 291. [Google Scholar]
- Zhang, F.; Li, Y.; Wu, J.; Zhang, J.; Cao, P.; Sun, Z.; Wang, W. The role of extracellular traps in ischemia reperfusion injury. Front Immunol. 2022, 13, 1022380. [Google Scholar] [CrossRef]
- Jennaro, T.S.; Puskarich, M.A.; Evans, C.R.; Karnovsky, A.; Flott, T.L.; McLellan, L.A.; Jones, A.E.; Stringer, K.A. Sustained Perturbation of Metabolism and Metabolic Subphenotypes Are Associated With Mortality and Protein Markers of the Host Response. Crit Care Explor. 2023, 5, e0881. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miceli, V.; Bulati, M.; Gallo, A.; Iannolo, G.; Busà, R.; Conaldi, P.G.; Zito, G. Role of Mesenchymal Stem/Stromal Cells in Modulating Ischemia/Reperfusion Injury: Current State of the Art and Future Perspectives. Biomedicines. 2023, 11, 689. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rowart, P.; Erpicum, P.; Detry, O.; Weekers, L.; Grégoire, C.; Lechanteur, C.; Briquet, A.; Beguin, Y.; Krzesinski, J.M.; Jouret, F. Mesenchymal Stromal Cell Therapy in Ischemia/Reperfusion Injury. J Immunol Res. 2015, 2015, 602597. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yao, J.; Zheng, J.; Cai, J.; Zeng, K.; Zhou, C.; Zhang, J.; Li, S.; Li, H.; Chen, L.; He, L.; Chen, H.; Fu, H.; Zhang, Q.; Chen, G.; Yang, Y.; Zhang, Y. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate rat hepatic ischemia-reperfusion injury by suppressing oxidative stress and neutrophil inflammatory response. FASEB J. 2019, 33, 1695–1710. [Google Scholar] [CrossRef] [PubMed]
- Franck, T.; Ceusters, J.; Graide, H.; Mouithys-Mickalad, A.; Serteyn, D. Muscle Derived Mesenchymal Stem Cells Inhibit the Activity of the Free and the Neutrophil Extracellular Trap (NET)-Bond Myeloperoxidase. Cells 2021, 10, 3486. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barrett, J.; MacDonald, S. Use of Biologics and Stem Cells in the Treatment of Other Inflammatory Diseases in the Horse, Veterinary Clinics of North America: Equine Practice, Volume 39, Issue 3, 2023, Pages 553-563. [CrossRef]
- Ceusters, J.; Lejeune, J.P.; Sandersen, C.; Niesten, A.; Lagneaux, L.; Serteyn, D. From skeletal muscle to stem cells: an innovative and minimally-invasive process for multiple species. Sci Rep. 2017, 7, 696. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pycock, J.F.; Allen, W.E.; Morris, T.H. Rapid, single-step isolation of equine neutrophils on a discontinuous Percoll density gradient. Res Vet Sci. 1987, 42, 411–2. [Google Scholar] [CrossRef] [PubMed]
- Franck, T.; Kohnen, S.; de la Rebière, G.; Deby-Dupont, G.; Deby, C.; Niesten, A.; Serteyn, D. Activation of equine neutrophils by phorbol myristate acetate or N-formyl-methionyl-leucyl-phenylalanine induces a different response in reactive oxygen species production and release of active myeloperoxidase. Vet Immunol Immunopathol. 2009, 130, 243–50. [Google Scholar] [CrossRef] [PubMed]
- Franck, T.; Kohnen, S.; Boudjeltia, K.Z.; Van Antwerpen, P.; Bosseloir, A.; Niesten, A.; Gach, O.; Nys, M.; Deby-Dupont, G.; Serteyn, D. A new easy method for specific measurement of active myeloperoxidase in human biological fluids and tissue extracts. Talanta. 2009, 80, 723–9. [Google Scholar] [CrossRef] [PubMed]
- Braissant, O.; Astasov-Frauenhoffer, M.; Waltimo, T.; Bonkat, G. A review of methods to determine viability, vitality, and metabolic rates in microbiology. Front Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int J Mol Sci. 2021, 22, 12827. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stockert, J.C.; Horobin, R.W.; Colombo, L.L.; Blázquez-Castro, A. Tetrazolium salts and formazan products in cell biology: viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochem. 2018, 120, 159–67. [Google Scholar] [CrossRef]
- Marin, W.; Marin, D.; Ao, X.; Liu, Y. Mitochondria as a therapeutic target for cardiac ischemia-reperfusion injury (Review). Int J Mol Med. 2021, 47, 485–499. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, R.; Zhang, C.; Xiang, Z.; Lin, T.; Ling, J.; Hu, H. Role of mitochondria in renal ischemia-reperfusion injury. FEBS J. 2024. [Google Scholar] [CrossRef] [PubMed]
- Burn, G.L.; Foti, A.; Marsman, G.; Patel, D.F.; Zychlinsky, A. The Neutrophil. Immunity 2021, 54, 1377–1391. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.M.; Nakhle, J.; Griessinger, E.; Vignais, M.L. Intercellular mitochondria trafficking highlighting the dual role of mesenchymal stem cells as both sensors and rescuers of tissue injury. Cell Cycle. 2018, 17, 712–721. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, D.; Gao, Y.; Liu, J.; Huang, Y.; Yin, J.; Feng, Y.; Shi, L.; Meloni, B.P.; Zhang, C.; Zheng, M.; Gao, J. Intercellular mitochondrial transfer as a means of tissue revitalization. Signal Transduct Target Ther. 2021, 6, 65. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Magaña-Guerrero, F.S.; Domínguez-López, A.; Martínez-Aboytes, P.; Buentello-Volante, B.; Garfias, Y. Human Amniotic Membrane Mesenchymal Stem Cells inhibit Neutrophil Extracellular Traps through TSG-6. Sci Rep. 2017, 7, 12426. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).