1. Introduction
African swine fever (ASF) is a contagious, lethal hemorrhagic fever that affects both domestic and European wild pigs leading to huge economic losses in the swine industry as well as increased food insecurity [
1,
2]. The causative agent, ASF virus (ASFV), is an enveloped, double-stranded DNA arbovirus now endemic to Sub-Saharan Africa, much of Eastern Europe, Asia and the Central American island of Hispaniola [
3,
4,
5,
6,
7,
8,
9]. As the virus spreads across the globe, many countries have increased their surveillance efforts for early detection of ASF [
10,
11].
RT-PCR is the most sensitive and specific assay available for the detection of ASFV genomic DNA in clinical samples. It is both highly scalable and can be easily automated, making it ideal for use as a screening assay in most diagnostic laboratories. However, the performance of RT-PCR assays relies on the quality of the nucleic acid extracted from samples, which in turn depends on the sample preparation methodology used. Whole blood from ASF suspected live animals as well as spleen tissues from dead animals are the preferred World Organization for Animal Health (WOAH) recommended sample types for ASFV genome detection [
12]. Spleen tissue is highly enriched for ASFV particles in affected swine and is therefore used in diagnostic and surveillance activities in animal health laboratories in many countries [
13,
14]. However, unlike whole blood, which can be used without further processing, spleen samples need to be processed to generate 10% (w/v) homogenates in sterile PBS. Preparation of 10% homogenates involves cutting, weighing, and the process of homogenization which is both time consuming and labor-intensive reducing the throughput and delaying results. Traditionally, homogenization was done using a mortar and pestle. Pestle is a hard, blunt object which is used to grind the sample against a mortar which is a solid container. This is a low-throughput method because only one sample can be processed at a time. The tissue homogenizers have increased the throughput of the homogenization step; however, they are expensive and therefore not accessible to countries with limited resources. Swabbing spleen tissues is a straightforward alternative to tissue homogenization because it directly eliminates the weighing and homogenization steps.
In this study, we compared the use of traditional 10% spleen tissue homogenates to spleen swabs for nucleic acid extraction, and ASFV genome detection by RT-PCR. Spleen tissues collected from pigs experimentally infected with 3 different ASFV strains along with healthy ASF known-negative domestic and wild pigs were used in this study.
2. Materials and Methods
2.1. Sample Origins
Spleen samples used in this study were collected from pigs experimentally inoculated with ASFV p72 genotype I (ASFV Malta’78) and II (ASFV Estonia/2014, and ASFV Georgia 2007/1) viruses at different days post infection (dpi). ASFV Malta’78 is a naturally attenuated moderately virulent strain isolated from Malta in 1978 [
15], kindly provide by Dr. Linda Dixon at the Pirbright Institute, UK. ASFV Estonia/2014 is a naturally attenuated moderately virulent strain isolated in 2014 from Estonia [
16,
17], kindly provided Dr. Sandra Blome, at the FLI, Germany. ASFV Georgia 2007/1 is a highly virulent strain isolated in 2007 from Georgia [
18]. All animal experiments were conducted at the biosafety level 3 facility at the NCFAD, Winnipeg, MB, Canada, under the guidelines of the Canadian Council for Animal Care. The Animal Care Committee at the Canadian Science Centre for Human and Animal Health approved the animal use under the AUDs C-19-012 and C-22-003. The samples were used immediately after collection or were frozen at -80°C. Before use, the frozen spleen samples were thawed at room temperature.
2.2. Preparation of Homogenates and Swabs from Spleen Tissue Samples
For homogenization of spleen tissue, a Precellys® 24 Touch instrument (Bertin technologies, Rockville, MD, USA) was used. Spleen tissue was cut into small pieces using sterile scissors or a scalpel. Approximately 0.1 g of spleen tissue was weighed using a small laboratory scale before being transferred to homogenization tubes, each containing 1 mL of sterile PBS. The tubes were then closed tightly and homogenized at 3 x 10 seconds at 5000 RPM. The homogenate was then transferred to fresh cryovials, spun down at 4 °C, 200 x g for 20 minutes and 55 µl of supernatant from each tube was used for nucleic acid extraction. For the nucleic acid extraction, a MagMax™ Pathogen RNA/DNA kit (Thermo Fisher Scientific, Waltham, MA, USA), was used according to the standard protocol provided by the manufacturer on the MagMax™ KingFisher Apex Deep Well Magnetic Particle Processor (Thermo Fisher Scientific).
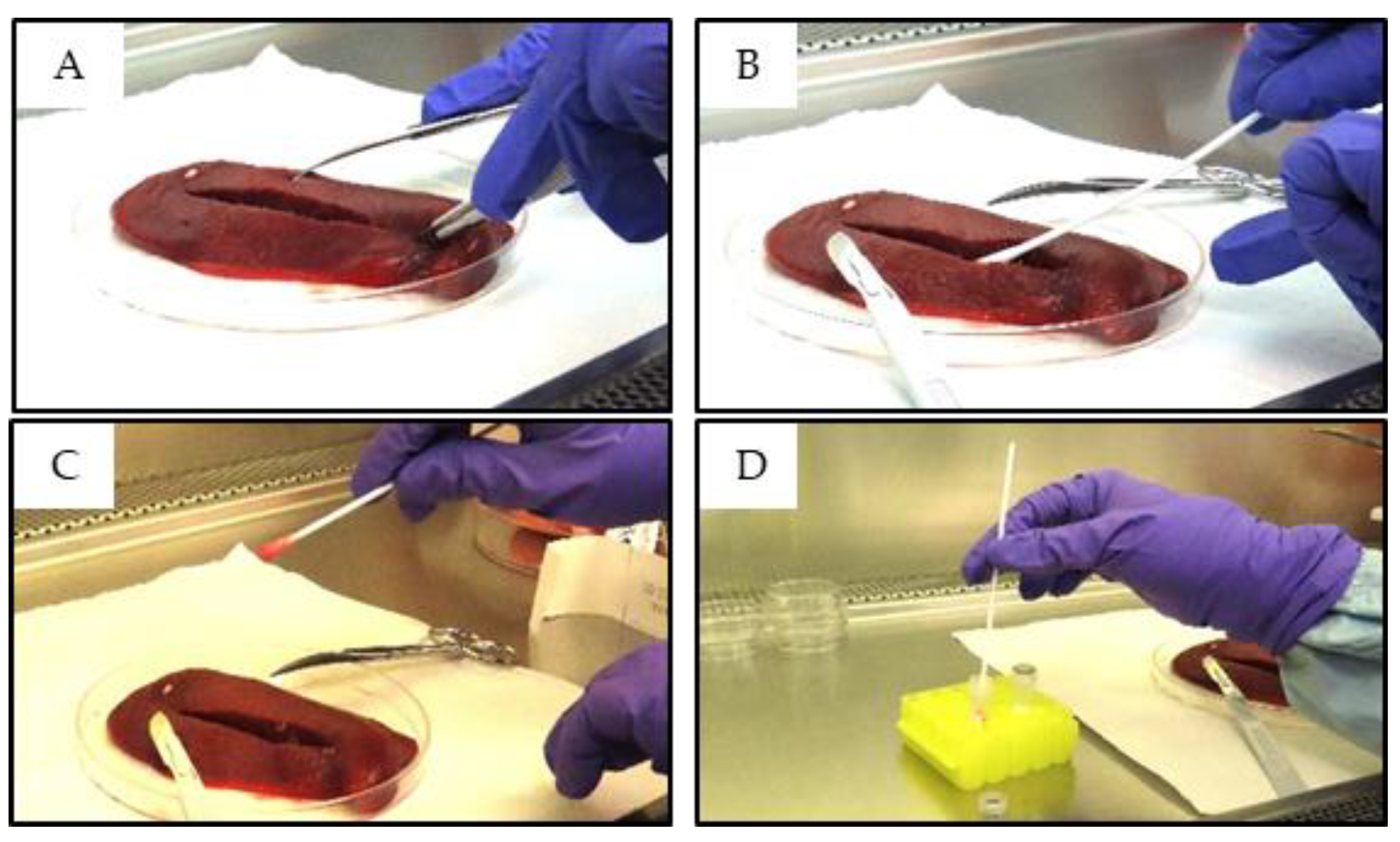
Spleen swabs were collected using sterile polyester-tipped applicator swabs (Puritan #25-806- 1PD, Puritan Medical Products, Falmouth, ME, USA). The spleen was cut open with a sterile scalpel and the cotton swab was inserted into the incision, pressed firmly, and twisted until the tip was fully soaked (
Figure 1). Cutting the spleen was not always necessary since some of the spleen tissues were fragile and the swabs could be inserted directly into the spleen by simply pressing the swab firmly against the tissue. The soaked ends of the swabs were then submerged into cryovials each containing 1 mL of sterile PBS. The excess swab length was snapped off, the cryovials were closed, vortexed for at least 10 seconds and 200 µl of the liquid from each vial was used for nucleic acid extraction, according to the low-cell-count optimized protocol provided by the manufacturer. Following the initial nucleic acid extractions, the remaining samples were stored at -70°C for further testing.
2.3. Real-Time PCR Detection of ASFV Genomic DNA, β-Actin and Armored Enterovirus RNA.
ASFV genomic DNA in both homogenate and swab samples was detected using two quantitative RT-PCR assays, both targeting the highly conserved region of the ASFV p72 open reading frame. As reported previously, the limit of detection of the Tignon assay is between 5.7 and 57 copies of the ASFV genome while the sensitivity of the Zsak assay is between 1.4 and 8.4 ASFV genome copies [
19,
20]. A separate RT-PCR assay specific for β-actin (Moniwa assay) was included to determine efficient nucleic acid extraction and amplification [
21]. For testing for the presence of PCR inhibitors, an armored enterovirus RNA spike-in (Cat #42050, Assurgent, Inc., Austin TX, USA) was used. All RT-PCR assays were run as singleplex assays and the reactions were prepared using TaqMan™ Fast Virus 1-Step Master Mix (Thermo Fisher Scientific) and was amplified using the Bio-Rad CFX96 instrument (Bio-Rad, Mississauga, ON, Canada), using the recommended cycling conditions (50 ◦C for 5 min; 95°C for 20 s, followed by 95°C for 3 s and 60°C for 30 s) for the TaqMan™ Fast Virus 1-Step Master Mix. To determine C
t values, a positive control well was included while a regression analysis of the positive control alone was used to determine a threshold cutoff for all experimental wells [
22].
2.4. Virus Isolation
Virus isolation was carried out in porcine primary leukocytes (PPLs), following the NCFAD standard operating procedure for ASFV isolation [
23]. Briefly, PPLs were isolated from fresh porcine blood and plated in 24-well plates, 1 mL/well (10
6 WBC/mL with 0.4% v/v RBC). Forty-eight hours later, 11 selected paired homogenate and swab samples along with a no-virus control (#8, 10, 12-14, 41-46) were inoculated (20 uL per well) into PPL cultures. Cultures were then incubated at 37°C in a 5% CO
2 incubator for 7 days and observed daily for the appearance of hemadsorption (HAD). The appearance of HAD was considered a positive indication of isolation. The limit of detection of the NCFAD ASFV isolation protocol is approximately 10
2 tissue culture infectious dose (TCID)
50/ml.
2.5. Virus Titration
A selected number of homogenates and swab samples (#11, 18, 38) were tittered on primary porcine alveolar macrophage (PAM) cultures as previously described [
24]. Briefly, 10-fold dilutions of the isolated viruses were inoculated into 90% confluent primary PAM cells in α-MEM supplemented with 1% Gentamicin, 1% Glutamax and 2% γ-irradiated fetal bovine serum. Following 3 days of incubation at 37 °C and 5% CO
2, the plates were fixed and stained with an anti-ASFV polyclonal pig serum and HRP-conjugated goat anti-pig monoclonal antibody (Cat# 114-035-003; Jackson ImmunoResearch, West Grove, PA, USA).
2.6. Statistical Analysis
The Pearson correlation coefficient between the Ct values for the homogenate and the swab samples was calculated using Graph Pad Prism Software, version 9.0.2 (San Diego, CA, USA). For the comparison of mean Armoured Enterovirus RNA detection, a two-tailed T-test was used.
3. Results and Discussion
In this study, 52 spleen samples collected from pigs experimentally infected with highly and moderately virulent ASFV strains and 20 known negative spleen samples (14 wild boar and 6 domestic pig spleen samples) collected under the ongoing CanSpot Canadian ASF surveillance system (
https://www.animalhealthcanada.ca/canspotasf) were processed in parallel into both 10% (w/v) tissue homogenates and spleen swabs. For nucleic acid extraction, an input volume of 55 µL was maintained for the homogenates while a low-cell-count protocol with an input volume of 200 µL was used for swabs. All samples were tested for the presence of ASFV genomic DNA using both the Tignon and Zsak RT-PCR assays, and for pig ß-Actin (internal sample control) using Moniwa RT-PCR assay.
ß-Actin was detected in all homogenates, as well as in all spleen swab samples (
Table 1). The mean Ct values for the homogenates was 22.54 ± 1.11, whereas for the swabs, it was 21.07 ± 1.57, suggesting comparable levels of splenic tissue sequestered by the swabs. A similar trend was observed with ASFV genomic detection.
The Tignon assay was able to detect ASFV genomic DNA in 39 out of 52 homogenates (75.0% positivity) prepared from spleen samples collected from pigs experimentally inoculated with ASFV. The same assay was able to detect ASFV genomic DNA in 45/52 (86.5%) of the swab samples collected from the same spleen samples. The mean Ct value for the Tignon assay was 26.35 ± 9.40 for the homogenates and 25.88 ± 8.83 for the swabs.
The Zsak assay was able to detect ASFV genomic DNA in 40 (76.9%) homogenates, and 46 (88.5%) swabs. The mean C
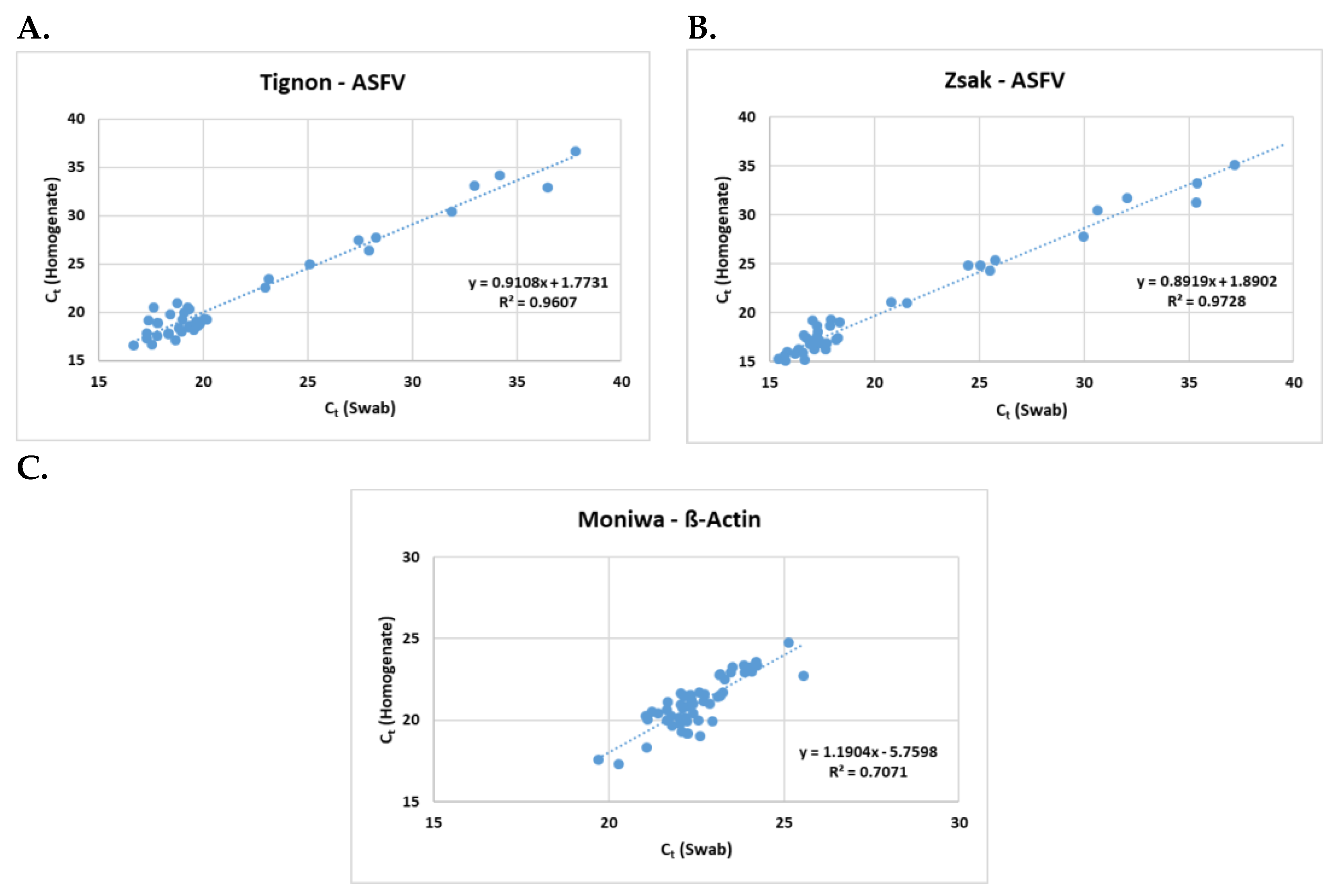
t values were 25.02 ± 9.87 and 24.36 ± 9.15 respectively. A positive correlation (
Figure 2) was observed between the homogenates and the swabs in all three assays (Tignon = 0.96, Zsak = 0.97 and ß-actin = 0.71).
ASFV genomic DNA was not detected in any of known negative samples from the 14 wild boar and 6 domestic pig spleen samples by both the Tignon and Zsak assays while ß-Actin was detected in all samples demonstrating that both ASFV assays are specific (
Table S1).
Both the Tignon and Zsak assays failed to detect ASFV genomic DNA in six homogenate samples compared to their paired spleen swab samples. Those swab samples (#6, 10, 27, 28, 30 and 31 for Tignon, and # 10, 27, 28, 30, 31 and 33 for Zsak) had very low ASFV genome levels and therefore the discrepancy could be due to the difference in input sample volumes (55 µl for homogenates and 200 µl for swabs) used for nucleic acid extractions. To address this, 10 paired homogenate and swab samples were selected, and nucleic acid was extracted from homogenate samples using the low-cell-count nucleic acid extraction protocol which uses 200 µL input. Despite the same input volume being used, swabs remained more sensitive than homogenates (
Table 2). Both Tignon and Zsak assays failed to detect ASFV genomic DNA in the homogenate from sample 27. Likewise, the Tignon assay failed to detect in the homogenate from sample 28 while the Zsak assay in the homogenates from samples 30 and 31.
The decreased sensitivity observed with homogenates could also be due to presence of PCR inhibitors in extracted nucleic acids [
25]. The presence of PCR inhibitors is the most common reason for poor PCR performance when DNA yield is sufficient. PCR inhibitors can derive either from the original sample or from sample preparation prior to PCR [
26]. Possible effects of PCR inhibitors in nucleic acid extracted from homogenate versus swabs, was evaluated by spiking the nucleic acid extracts from a selected number of homogenates and swab samples with armored enterovirus RNA (
Table S2). The mean C
t values from the armored enterovirus RNA RT-PCR for homogenates and spleen swabs were comparable (29.26 ± 2.49 and 28.48 ± 1.96 for homogenates and swabs respectively).
Another potential contributor to the observed increase in sensitivity in swabs compared to homogenates could be that the swabs are able to better capture and concentrate the splenic cells including red blood cells with high affinity to ASFV [
27]. To determine, if ASFV positive spleen swabs contain comparable or slightly higher levels of intact ASFV particles, a selected number of ASFV genome positive spleen swabs and paired tissue homogenate samples were inoculated into primary PPL cultures. ASFV was isolated from both sample types, however hemadsorption (HAD) was clearer and more abundant in PPL cultures inoculated with spleen swab samples, compared to those inoculated with the paired homogenate samples. HAD was observed in 4 out of 11 PPL cultures inoculated with swab samples compared to 3 out of 11 PPL cultures inoculated with homogenate samples (
Table S3 and
Figure S1). Ten-fold dilutions of three spleen swab samples with variable C
t values and paired homogenate samples (
Table 3) were also inoculated onto PAM cells to determine the ASFV titers for each sample type. Consistent with the RT-PCR and ASFV isolation data, spleen swabs had slightly higher viral titers than homogenates.
Collectively, the results from this study show that the spleen swab samples provide comparable, if not slightly increased sensitivity compared to the spleen tissue homogenates routinely used for ASFV genomic detection. Swabbing spleen tissue requires less hands-on time and offers a straightforward methodology for clinical sample preparation for ASFV genomic DNA detection. The exact mechanism for the increased sensitivity is not clear but could simply be that the swabs cover more splenic surface area than tissue homogenization.
Swabbing tissues as a sample preparation methodology for virus detection, has been explored for several other animal viruses. During an equine influenza outbreak in New South Wales, Australia, complications, and delays associated with homogenizing tissue samples were avoided by directly swabbing freshly cut tissues [
28]. This approach along with the incorporation of an influenza specific RT-PCR assay helped laboratories to dramatically increase the number of samples screened during the outbreak. In a relatively small study conducted by Errington
et al., spleen swabs were tested for the detection of bovine viral diarrhea virus (BVDV) and porcine reproductive and respiratory syndrome virus (PRRSV) [
27]. The results demonstrated that the spleen swabs were a useful alternative to the traditional tissue lysis approach for the detection of both viruses. In a more recent study, Okwasiimire
et al., compared diaphragm meat juice and muscle swab samples to spleen and spleen swab samples for the detection of ASFV nucleic acid [
29]. The samples were collected from pigs with signs of ASFV infection at slaughterhouses near Kampala, Uganda. In this study a high correlation (r = 0.8728) was observed between spleen tissue and spleen swab samples. Out of 493 samples tested, the positivity in spleen swabs (48.9%) was slightly lower than for spleen tissues (50.1%). The difference could be due to many reasons including the type of swabs used, the increased volume of storage medium (2-3 ml versus 1 ml in our study) and the use of different nucleic acid extraction methodology (column-based vs magnetic based extraction) used in the study. Spleen swabs are not yet approved by the WOAH; however, the USDA approved the use of spleen swabs for ASF detection in the USA [
30].
4. Conclusions
Early detection of an ASF incursion is critical to prevent the severe economic and social impacts related to loss of animals, loss of trade and re-attaining a disease-free status [
31]. To achieve this, many nations have implemented passive surveillance programs. Whole blood from live pigs and spleen samples from dead pigs are the preferred WOAH recommended sample types for ASFV genome detection by RT-PCR followed by virus isolation. While whole blood can be used directly in nucleic acid extraction protocols, current laboratory standard operating procedures for spleen tissue samples require processing into 10% homogenates which is both time consuming and requires additional resources, significantly reducing throughput. In this study, we have shown that the spleen swabs are a better alternative to spleen tissue homogenates for both ASFV genome detection and virus isolation. Increased efficiency in sample processing, preparation and testing is critical for expanding ongoing surveillance and maintaining diagnostic throughput in the event of an outbreak. The use of spleen swabs instead of spleen tissue homogenates will not only allow animal health laboratories to reduce the costs associated with reporting but also streamline and increase the diagnostic throughput for ASF detection.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1: RTPCR results for the 20 known negative samples; Table S2: RT-PCR results for the test for PCR inhibition using Armored RNA Enterovirus spike-in nucleic acids; Table S3: Results for the inoculation of 11 selected paired homogenates and swab samples into PPL cultures; Figure S1: Brightfield image of HAD formation 2 days following inoculation of PPL cultures with paired sample 46.
Author Contributions
Conceptualization, A.A. and C.J.C; methodology, C.C., K.G.; software, C.C.; validation, A.A., and K.G.; formal analysis, A.A., C.J.C.; resources, A.A.; data curation, C.C.; writing—original draft preparation, C.C.; writing—review and editing, A.A., C.J.C, K.G; visualization, C.C.; supervision, A.A.; project administration, A.A; funding acquisition, A.A.. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the CFIA ASF supplementary funds, USA National Pork Board projects 19-194 and 20-152 and USDA.
Institutional Review Board Statement
The samples used in this study were collected from animal experiments conducted under AUD C-19-012 and C-22-003, approved by the Animal Care Committee at the Canadian Science Centre for Human and Animal Health. All animal studies were conducted under the guidelines of the Canadian Council for Animal Care.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Authors like to thank Amy Snow, Kathleen Hooper McGrevy and Charles Nfon at the Canadian Food inspection Agency for their insightful input during the review process.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Penrith, M.L. Current status of African swine fever. CABI Agric. Biosci. 2020, 1, 1–26. [Google Scholar] [CrossRef]
- Ruiz-Saenz, J.; Diaz, A.; Bonilla-Aldana, D.K.; Rodríguez-Morales, A.J.; Martinez-Gutierrez, M.; Aguilar, P.V. African swine fever virus: A re-emerging threat to the swine industry and food security in the Americas. Front. Microbiol. 2022, 13, 1011891. [Google Scholar] [CrossRef]
- Gaudreault, N.N.; Madden, D.W.; Wilson, W.C.; Trujillo, J.D.; Richt, J.A. African Swine Fever Virus: An Emerging DNA Arbovirus. Front. Vet. Sci. 2020, 7, 215. [Google Scholar] [CrossRef] [PubMed]
- Adedeji, A.J.; Luka, P.D.; Atai, R.B.; Olubade, T.A.; Hambolu, D.A.; Ogunleye, M.A.; Muwanika, V.B.; Masembe, C. First-Time Presence of African Swine Fever Virus Genotype II in Nigeria. Genome Announc. 2021, 10, e0035021. [Google Scholar] [CrossRef]
- Oļševskis, E.; Guberti, V.; Seržants, M.; Westergaard, J.; Gallardo, C.; Rodze, I.; Depner, K. African swine fever virus introduction into the EU in 2014: Experience of Latvia. Res. Veter- Sci. 2016, 105, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Le, V.P.; Jeong, D.G.; Yoon, S.-W.; Kwon, H.-M.; Trinh, T.B.N.; Nguyen, T.L.; Bui, T.T.N.; Oh, J.; Kim, J.B.; Cheong, K.M.; et al. Outbreak of African Swine Fever, Vietnam, 2019. Emerg. Infect. Dis. 2019, 25, 1433–1435. [Google Scholar] [CrossRef]
- Sun, E.; Huang, L.; Zhang, X.; Zhang, J.; Shen, D.; Zhang, Z.; Wang, Z.; Huo, H.; Wang, W.; Huangfu, H.; et al. Genotype I African swine fever viruses emerged in domestic pigs in China and caused chronic infection. Emerg. Microbes Infect. 2021, 10, 2183–2193. [Google Scholar] [CrossRef]
- Gonzales, W.; Moreno, C.; Duran, U.; Henao, N.; Bencosme, M.; Lora, P.; Reyes, R.; Núñez, R.; De Gracia, A.; Perez, A.M. African swine fever in the Dominican Republic. Transbound. Emerg. Dis. 2021, 68, 3018–3019. [Google Scholar] [CrossRef] [PubMed]
- OIE-WAHIS Immediate Notification: African Swine Fever Virus Haiti, Reported on 2021-09-19, report ID IN_151732. Available online: https://wahis.oie.int/#/report-info?reportId=39928.
- Gervasi, V.; Marcon, A.; Bellini, S.; Guberti, V. Evaluation of the Efficiency of Active and Passive Surveillance in the Detection of African Swine Fever in Wild Boar. Veter- Sci. 2019, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Cappai, S.; Rolesu, S.; Feliziani, F.; Desini, P.; Guberti, V.; Loi, F. Standardized Methodology for Target Surveillance against African Swine Fever. Vaccines 2020, 8, 723. [Google Scholar] [CrossRef]
- WOAH (2024). Addressing African swine fever: Protocols and Guidelines for Laboratory Diagnosis. Paris, 38 pp., DOI [https://doi.org/10.20506/asf.3475]. Licence: CC BY-SA 3.0 IGO.
- Greig, A. Pathogenesis of African swine fever in pigs naturally exposed to the disease. J. Comp. Pathol. 1972, 82, 73–79. [Google Scholar] [CrossRef]
- Blome, S.; Gabriel, C.; Beer, M. Pathogenesis of African swine fever in domestic pigs and European wild boar. Virus Res. 2012, 173, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, P.; Lawman, M.; Johnston, R. African swine fever in Malta, 1978. Veter- Rec. 1980, 106, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Nurmoja, I.; Schulz, K.; Staubach, C.; Sauter-Louis, C.; Depner, K.; Conraths, F.J.; Viltrop, A. Development of African swine fever epidemic among wild boar in Estonia - two different areas in the epidemiological focus. Sci. Rep. 2017, 7, 12562–12562. [Google Scholar] [CrossRef] [PubMed]
- Zani, L.; Forth, J.H.; Forth, L.; Nurmoja, I.; Leidenberger, S.; Henke, J.; Carlson, J.; Breidenstein, C.; Viltrop, A.; Höper, D.; et al. Deletion at the 5’-end of Estonian ASFV strains associated with an attenuated phenotype. Sci. Rep. 2018, 8, 6510. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, R.J.; Michaud, V.; Heath, L.; Hutchings, G.; Oura, C.; Vosloo, W.; Dwarka, R.; Onashvili, T.; Albina, E.; Dixon, L.K. African Swine Fever Virus Isolate, Georgia, 2007. Emerg. Infect. Dis. 2008, 14, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Tignon, M.; Gallardo, C.; Iscaro, C.; Hutet, E.; Van der Stede, Y.; Kolbasov, D.; De Mia, G.M.; Le Potier, M.-F.; Bishop, R.P.; Arias, M.; et al. Development and inter-laboratory validation study of an improved new real-time PCR assay with internal control for detection and laboratory diagnosis of African swine fever virus. J. Virol. Methods 2011, 178, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Zsak, L.; Borca, M.V.; Risatti, G.R.; Zsak, A.; French, R.A.; Lu, Z.; Kutish, G.F.; Neilan, J.G.; Callahan, J.D.; Nelson, W.M.; et al. Preclinical Diagnosis of African Swine Fever in Contact-Exposed Swine by a Real-Time PCR Assay. J. Clin. Microbiol. 2005, 43, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Moniwa, M.; Clavijo, A.; Li, M.; Collignon, B.; Kitching, R.P. Performance of a Foot-and-Mouth Disease virus Reverse Transcription-Polymerase Chain Reaction with Amplification Controls between Three Real-Time Instruments. J. Veter- Diagn. Investig. 2007, 19, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Oura, C.A.L.; Arias, M.; Register, K.B. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. 2023, Ch.3.9.1. African Swine fever (Infection with African Swine fever Virus).
- Goonewardene, K.B.; Chung, C.J.; Goolia, M.; Blakemore, L.; Fabian, A.; Mohamed, F.; Nfon, C.; Clavijo, A.; Dodd, K.A.; Ambagala, A. Evaluation of oral fluid as an aggregate sample for early detection of African swine fever virus using four independent pen-based experimental studies. Transbound. Emerg. Dis. 2021, 68, 2867–2877. [Google Scholar] [CrossRef]
- Carrascosa, A.L.; Bustos, M.J.; de Leon, P. Methods for Growing and Titrating African Swine Fever Virus: Field and Laboratory Samples. Curr. Protoc. Cell Biol. 2011, 53, 26–14. [Google Scholar] [CrossRef] [PubMed]
- Sidstedt, M.; Rådström, P.; Hedman, J. PCR inhibition in qPCR, dPCR and MPS—mechanisms and solutions. Anal. Bioanal. Chem. 2020, 412, 2009–2023. [Google Scholar] [CrossRef] [PubMed]
- Hedman, J.; Knutsson, R.; Ansell, R.; Rådström, P.; Rasmusson, B. Pre-PCR Processing in Bioterrorism Preparedness: Improved Diagnostic Capabilities for Laboratory Response Networks. Biosecurity Bioterrorism: Biodefense Strat. Pr. Sci. 2013, 11 Supp 1, S87–S101. [Google Scholar] [CrossRef] [PubMed]
- Errington, J.; Jones, R.M.; Sawyer, J. Use of tissue swabbing as an alternative to tissue dissection and lysis prior to nucleic acid extraction and real-time polymerase chain reaction detection of Bovine viral diarrhea virus and Porcine reproductive and respiratory syndrome virus. J. Veter- Diagn. Investig. 2014, 26, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, P.; Davis, R.; Gu, X.; Frost, M. Application of high-throughput systems for the rapid detection of DNA and RNA viruses during the Australian equine influenza outbreak. Aust. Veter- J. 2011, 89 Supp 1, 38–39. [Google Scholar] [CrossRef] [PubMed]
- Okwasiimire, R.; Nassali, A.; Ndoboli, D.; Ekakoro, J.E.; Faburay, B.; Wampande, E.; Havas, K.A. Comparison of diaphragm meat juice and muscle swab samples to spleen and spleen swab samples for the detection of African swine fever viral nucleic acid. J. Veter- Diagn. Investig. 2023, 35, 145–152. [Google Scholar] [CrossRef]
- National Veterinary Services Laboratories. NAHLN sample chart for regulatory submitters. Document NVSL-WI-0729.08. USDA, 2021.
- Goonewardene, K.B.; Onyilagha, C.; Goolia, M.; Le, V.P.; Blome, S.; Ambagala, A. Superficial Inguinal Lymph Nodes for Screening Dead Pigs for African Swine Fever. Viruses 2022, 14, 83. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).