Submitted:
26 July 2024
Posted:
26 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
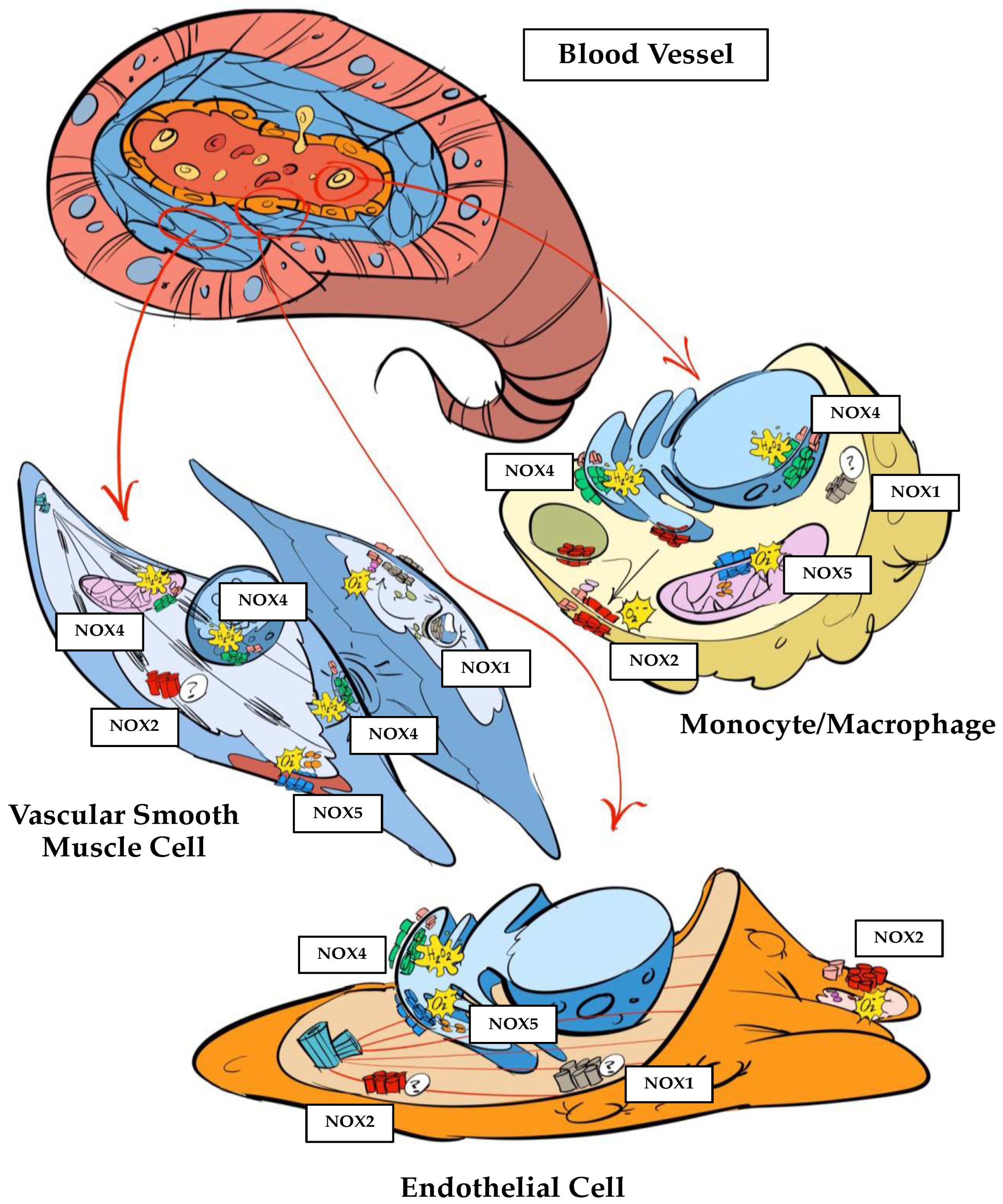
2. NADPH Oxidases in the Vessel Wall
3. NADPH Oxidases in Atherosclerosis
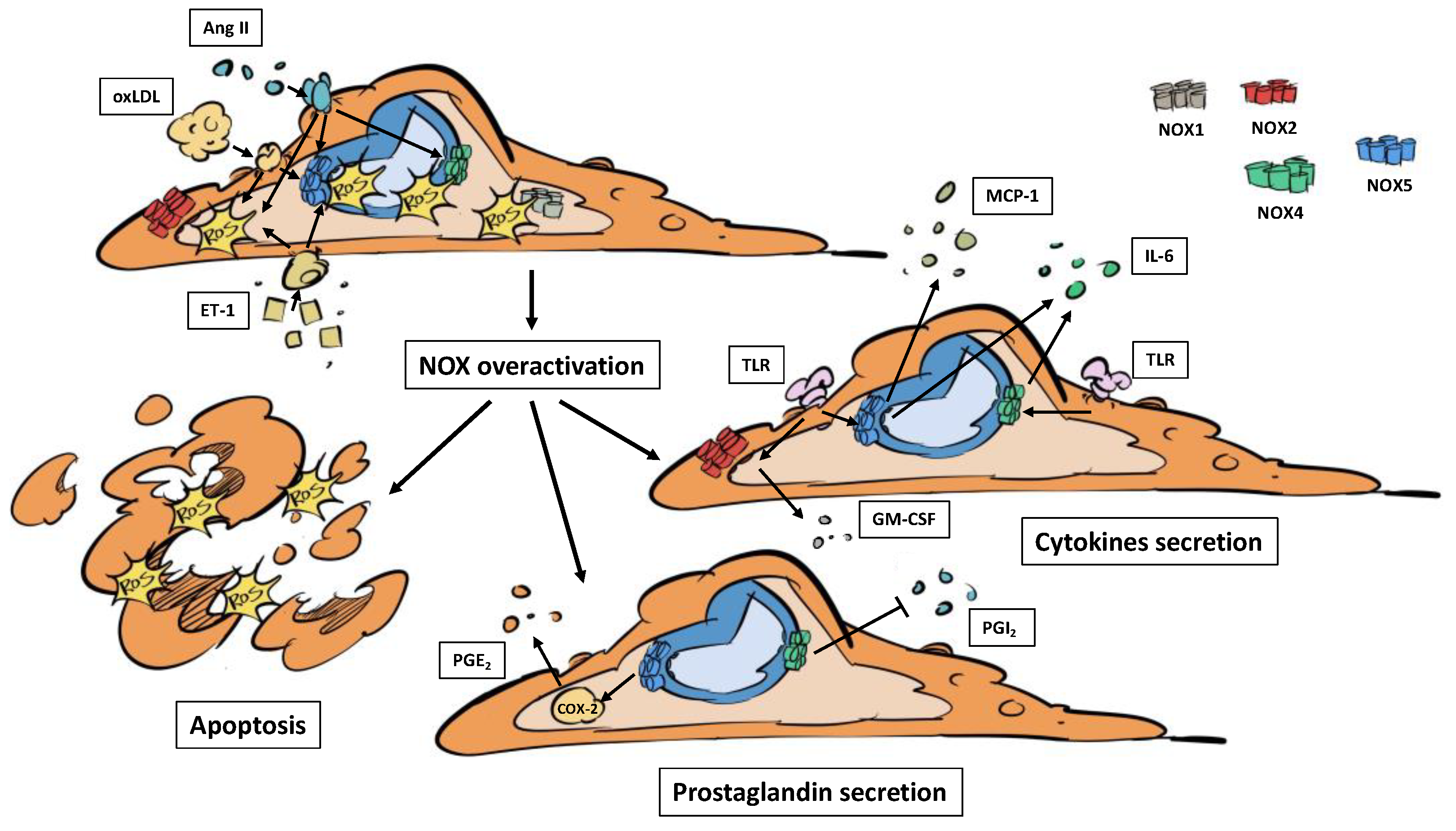
3.1. NADPH Oxidases and Endothelial Dysfunction
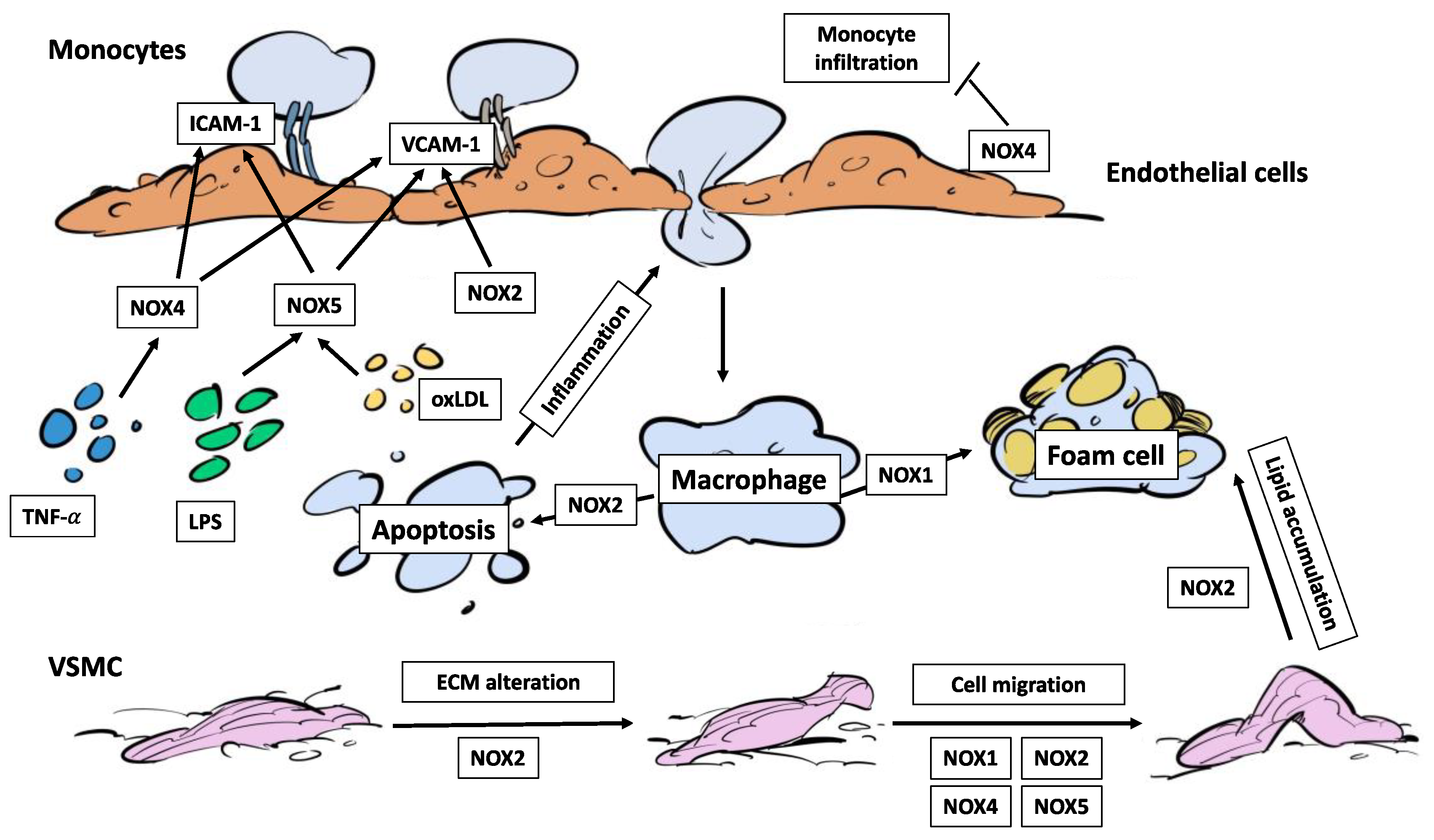
3.2. NADPH Oxidases, Immune Cell Infiltration and Foam Cells
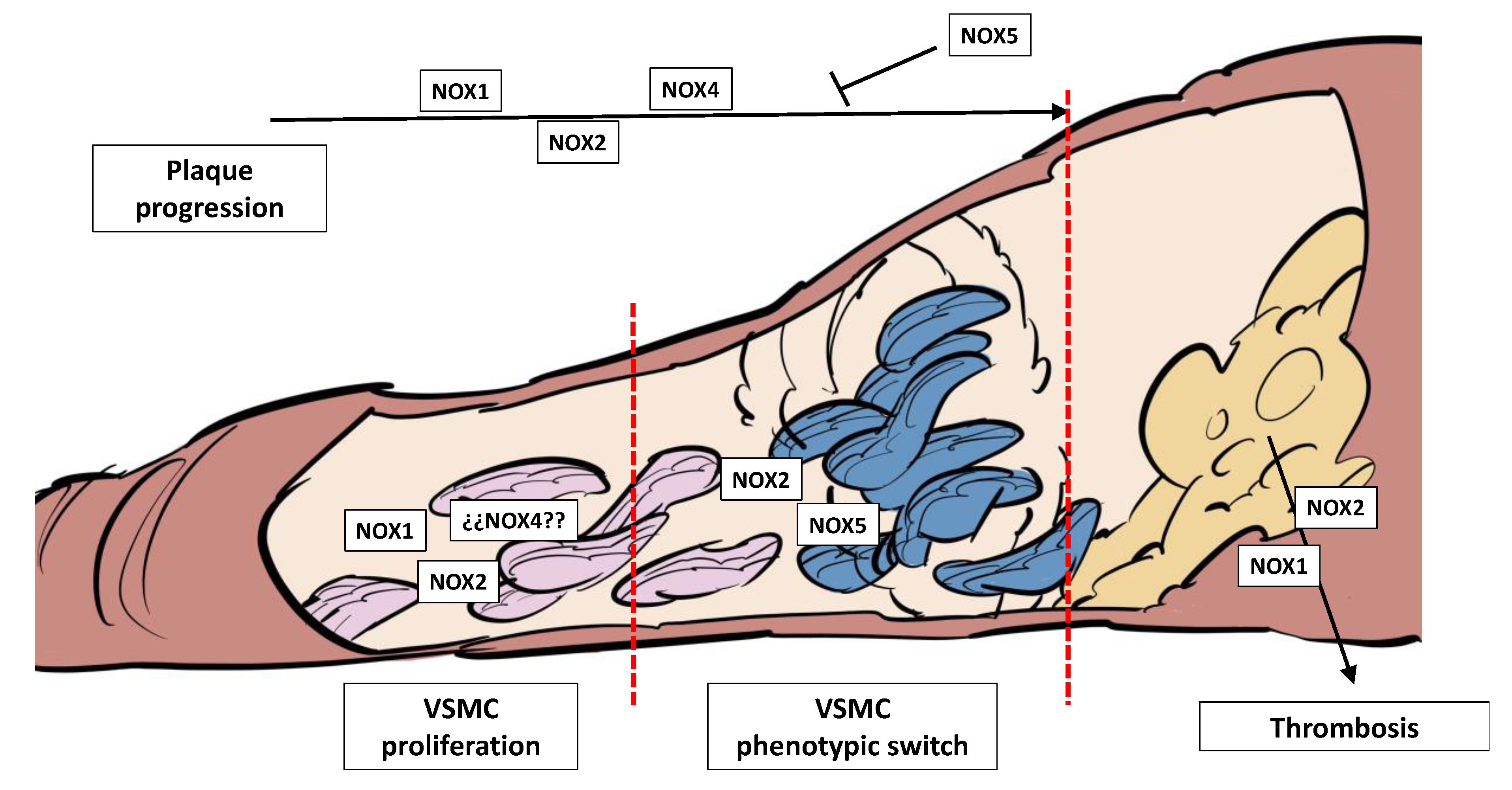
3.3. NADPH Oxidases, Plaque Development, Plaque Rupture, and Thrombosis
4. NADPH Oxidases in Thrombosis and Stroke
4.1. NADPH Oxidases, BBB Disruption and Stroke
4.2. NADPH Oxidases and Immune Infiltration in the Brain
4.3. NADPH Oxidases and Ischemia-Reperfusion Injury
5. Conclusions
- (i)
- More cell type-specific knock-out/knock-in in vivo models would help to improve the current knowledge.
- (ii)
- More integrative studies that deep in the interconnection between different NOXs or their paracrine effects should be performed.
- (iii)
- here is an urgent need to develop isoform-specific NOX inhibitors and study these enzymes as potential therapeutical targets in CVDs.
Funding
Acknowledgments
Conflicts of Interest
References
- Madamanchi, N.R.; Vendrov, A.; Runge, M.S. Oxidative Stress and Vascular Disease. Arterioscler Thromb Vasc Biol 2005, 25, 29–38. [CrossRef]
- Gimbrone, M.A.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ Res 2016, 118, 620–636. [CrossRef]
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.M.; Aldasoro, M.; Victor, V.M.; Mauricio, M.D. Targeting Early Atherosclerosis: A Focus on Oxidative Stress and Inflammation. Oxid Med Cell Longev 2019, 2019. [CrossRef]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat Rev Mol Cell Biol 2020, 21, 363–383. [CrossRef]
- Holmström, K.M.; Finkel, T. Cellular Mechanisms and Physiological Consequences of Redox-Dependent Signalling. Nat Rev Mol Cell Biol 2014, 15, 411–421. [CrossRef]
- Bedard, K.; Krause, K.H. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiol Rev 2007, 87, 245–313. [CrossRef]
- Ogboo, B.C.; Grabovyy, U. V.; Maini, A.; Scouten, S.; van der Vliet, A.; Mattevi, A.; Heppner, D.E. Architecture of the NADPH Oxidase Family of Enzymes. Redox Biol 2022, 52, 102298. [CrossRef]
- Touyz, R.M.; Briones, A.M. Reactive Oxygen Species and Vascular Biology: Implications in Human Hypertension. Hypertens Res 2011, 34, 5–14. [CrossRef]
- Bánfi, B.; Malgrange, B.; Knisz, J.; Steger, K.; Dubois-Dauphin, M.; Krause, K.H. NOX3, a Superoxide-Generating NADPH Oxidase of the Inner Ear. J Biol Chem 2004, 279, 46065–46072. [CrossRef]
- Bánfi, B.; Maturana, A.; Jaconi, S.; Arnaudeau, S.; Laforge, T.; Sinha, B.; Ligeti, E.; Demaurex, N.; Krause, K.H. A Mammalian H+ Channel Generated through Alternative Splicing of the NADPH Oxidase Homolog NOH-1. Science 2000, 287, 138–142. [CrossRef]
- Kobayashi, S.; Nojima, Y.; Shibuya, M.; Maru, Y. Nox1 Regulates Apoptosis and Potentially Stimulates Branching Morphogenesis in Sinusoidal Endothelial Cells. Exp Cell Res 2004, 300, 455–462. [CrossRef]
- Lassègue, B.; Sorescu, D.; Szöcs, K.; Yin, Q.Q.; Akers, M.; Zhang, Y.; Grant, S.L.; Lambeth, J.D.; Griendling, K.K. Novel Gp91(Phox) Homologues in Vascular Smooth Muscle Cells : Nox1 Mediates Angiotensin II-Induced Superoxide Formation and Redox-Sensitive Signaling Pathways. Circ Res 2001, 88, 888–894. [CrossRef]
- Lee, N.K.; Choi, Y.G.; Baik, J.Y.; Han, S.Y.; Jeong, D.W.; Bae, Y.S.; Kim, N.; Lee, S.Y. A Crucial Role for Reactive Oxygen Species in RANKL-Induced Osteoclast Differentiation. Blood 2005, 106, 852–859. [CrossRef]
- Brown, D.I.; Griendling, K.K. Nox Proteins in Signal Transduction. Free Radic Biol Med 2009, 47, 1239–1253. [CrossRef]
- Hilenski, L.L.; Clempus, R.E.; Quinn, M.T.; Lambeth, J.D.; Griendling, K.K. Distinct Subcellular Localizations of Nox1 and Nox4 in Vascular Smooth Muscle Cells. Arterioscler Thromb Vasc Biol 2004, 24, 677–683. [CrossRef]
- Niu, X.L.; Madamanchi, N.R.; Vendrov, A.E.; Tchivilev, I.; Rojas, M.; Madamanchi, C.; Brandes, R.P.; Krause, K.H.; Humphries, J.; Smith, A.; et al. Nox Activator 1: A Potential Target for Modulation of Vascular Reactive Oxygen Species in Atherosclerotic Arteries. Circulation 2010, 121, 549–559. [CrossRef]
- Tabet, F.; Schiffrin, E.L.; Callera, G.E.; He, Y.; Yao, G.; Östman, A.; Kappert, K.; Tonks, N.K.; Touyz, R.M. Redox-Sensitive Signaling by Angiotensin II Involves Oxidative Inactivation and Blunted Phosphorylation of Protein Tyrosine Phosphatase SHP-2 in Vascular Smooth Muscle Cells from SHR. Circ Res 2008, 103, 149–158. [CrossRef]
- Wendt, M.C.; Daiber, A.; Kleschyov, A.L.; Mülsch, A.; Sydow, K.; Schulz, E.; Chen, K.; Keaney, J.F.; Lassègue, B.; Walter, U.; et al. Differential Effects of Diabetes on the Expression of the Gp91phox Homologues Nox1 and Nox4. Free Radic Biol Med 2005, 39, 381–391. [CrossRef]
- Borregaard, N.; Heiple, J.M.; Simons, E.R.; Clark, R.A. Subcellular Localization of the B-Cytochrome Component of the Human Neutrophil Microbicidal Oxidase: Translocation during Activation. J Cell Biol 1983, 97, 52–61. [CrossRef]
- Van Buul, J.D.; Fernandez-Borja, M.; Anthony, E.C.; Hordijk, P.L. Expression and Localization of NOX2 and NOX4 in Primary Human Endothelial Cells. Antioxid Redox Signal 2005, 7, 308–317. [CrossRef]
- Wang, C.; Zhu, L.; Yuan, W.; Sun, L.; Xia, Z.; Zhang, Z.; Yao, W. Diabetes Aggravates Myocardial Ischaemia Reperfusion Injury via Activating Nox2-Related Programmed Cell Death in an AMPK-Dependent Manner. J Cell Mol Med 2020, 24, 6670–6679. [CrossRef]
- Carnevale, R.; Bartimoccia, S.; Nocella, C.; Di Santo, S.; Loffredo, L.; Illuminati, G.; Lombardi, E.; Boz, V.; Del Ben, M.; De Marco, L.; et al. LDL Oxidation by Platelets Propagates Platelet Activation via an Oxidative Stress-Mediated Mechanism. Atherosclerosis 2014, 237, 108–116. [CrossRef]
- Geiszt, M.; Kopp, J.B.; Várnai, P.; Leto, T.L. Identification of Renox, an NAD(P)H Oxidase in Kidney. Proc Natl Acad Sci U S A 2000, 97, 8010–8014. [CrossRef]
- Ellmark, S.H.M.; Dusting, G.J.; Fui, M.N.; Guzzo-Pernell, N.; Drummond, G.R. The Contribution of Nox4 to NADPH Oxidase Activity in Mouse Vascular Smooth Muscle. Cardiovasc Res 2005, 65, 495–504. [CrossRef]
- Ago, T.; Kitazono, T.; Ooboshi, H.; Iyama, T.; Han, Y.H.; Takada, J.; Wakisaka, M.; Ibayashi, S.; Utsumi, H.; Iida, M. Nox4 as the Major Catalytic Component of an Endothelial NAD(P)H Oxidase. Circulation 2004, 109, 227–233. [CrossRef]
- Lee, C.F.; Qiao, M.; Schröder, K.; Zhao, Q.; Asmis, R. Nox4 Is a Novel Inducible Source of Reactive Oxygen Species in Monocytes and Macrophages and Mediates Oxidized Low Density Lipoprotein-Induced Macrophage Death. Circ Res 2010, 106, 1489–1497. [CrossRef]
- Canugovi, C.; Stevenson, M.D.; Vendrov, A.E.; Hayami, T.; Robidoux, J.; Xiao, H.; Zhang, Y.Y.; Eitzman, D.T.; Runge, M.S.; Madamanchi, N.R. Increased Mitochondrial NADPH Oxidase 4 (NOX4) Expression in Aging Is a Causative Factor in Aortic Stiffening. Redox Biol 2019, 26. [CrossRef]
- Perrotta, I.; Sciangula, A.; Perrotta, E.; Donato, G.; Cassese, M. Ultrastructural Analysis and Electron Microscopic Localization of Nox4 in Healthy and Atherosclerotic Human Aorta. Ultrastruct Pathol 2011, 35, 1–6. [CrossRef]
- Rajaram, R.D.; Dissard, R.; Jaquet, V.; De Seigneux, S. Potential Benefits and Harms of NADPH Oxidase Type 4 in the Kidneys and Cardiovascular System. Nephrol Dial Transplant 2019, 34, 567–576. [CrossRef]
- Bánfi, B.; Molnár, G.; Maturana, A.; Steger, K.; Hegedûs, B.; Demaurex, N.; Krause, K.H. A Ca(2+)-Activated NADPH Oxidase in Testis, Spleen, and Lymph Nodes. J Biol Chem 2001, 276, 37594–37601. [CrossRef]
- Guzik, T.J.; Chen, W.; Gongora, M.C.; Guzik, B.; Lob, H.E.; Mangalat, D.; Hoch, N.; Dikalov, S.; Rudzinski, P.; Kapelak, B.; et al. Calcium Dependent Nox5 NADPH Oxidase Contributes to Vascular Oxidative Stress in Human Coronary Artery Disease. J Am Coll Cardiol 2008, 52, 1803. [CrossRef]
- Pai, W.Y.; Lo, W.Y.; Hsu, T.; Peng, C.T.; Wang, H.J. Angiotensin-(1-7) Inhibits Thrombin-Induced Endothelial Phenotypic Changes and Reactive Oxygen Species Production via NADPH Oxidase 5 Downregulation. Front Physiol 2017, 8. [CrossRef]
- Petsophonsakul, P.; Burgmaier, M.; Willems, B.; Heeneman, S.; Stadler, N.; Gremse, F.; Reith, S.; Burgmaier, K.; Kahles, F.; Marx, N.; et al. Nicotine Promotes Vascular Calcification via Intracellular Ca2+-Mediated, Nox5-Induced Oxidative Stress, and Extracellular Vesicle Release in Vascular Smooth Muscle Cells. Cardiovasc Res 2022, 118, 2196–2210. [CrossRef]
- Manea, A.; Manea, S.A.; Gan, A.M.; Constantin, A.; Fenyo, I.M.; Raicu, M.; Muresian, H.; Simionescu, M. Human Monocytes and Macrophages Express NADPH Oxidase 5; a Potential Source of Reactive Oxygen Species in Atherosclerosis. Biochem Biophys Res Commun 2015, 461, 172–179. [CrossRef]
- Touyz, R.M.; Anagnostopoulou, A.; Rios, F.; Montezano, A.C.; Camargo, L.L. NOX5: Molecular Biology and Pathophysiology. Exp Physiol 2019, 104, 605. [CrossRef]
- Anagnostopoulou, A.; Camargo, L.L.; Rodrigues, D.; Montezano, A.C.; Touyz, R.M. Importance of Cholesterol-Rich Microdomains in the Regulation of Nox Isoforms and Redox Signaling in Human Vascular Smooth Muscle Cells. Sci Rep 2020, 10. [CrossRef]
- Marzaioli, V.; Hurtado-Nedelec, M.; Pintard, C.; Tlili, A.; Marie, J.C.; Monteiro, R.C.; Gougerot-Pocidalo, M.A.; Dang, P.M.C.; El-Benna, J. NOX5 and P22phox Are 2 Novel Regulators of Human Monocytic Differentiation into Dendritic Cells. Blood 2017, 130, 1734–1745. [CrossRef]
- Richter, S.M.; Massman, L.C.; Stuehr, D.J.; Sweeny, E.A. Functional Interactions between NADPH Oxidase 5 and Actin. Front Cell Dev Biol 2023, 11. [CrossRef]
- Marqués, J.; Cortés, A.; Pejenaute, Á.; Zalba, G. Implications of NADPH Oxidase 5 in Vascular Diseases. Int J Biochem Cell Biol 2020, 128. [CrossRef]
- Li, M.; Liu, X.; He, Y.; Zheng, Q.; Wang, M.; Wu, Y.; Zhang, Y.; Wang, C. Celastrol Attenuates Angiotensin II Mediated Human Umbilical Vein Endothelial Cells Damage through Activation of Nrf2/ERK1/2/Nox2 Signal Pathway. Eur J Pharmacol 2017, 797, 124–133. [CrossRef]
- Liang, G.Z.; Cheng, L.M.; Chen, X.F.; Li, Y.J.; Li, X.L.; Guan, Y.Y.; Du, Y.H. ClC-3 Promotes Angiotensin II-Induced Reactive Oxygen Species Production in Endothelial Cells by Facilitating Nox2 NADPH Oxidase Complex Formation. Acta Pharmacol Sin 2018, 39, 1725–1734. [CrossRef]
- Hong, O.K.; Lee, S.S.; Yoo, S.J.; Choi, S.H.; Lee, M.K.; Cha, B.Y.; Kim, M.K.; Baek, K.H.; Song, K.H.; Kwon, H.S. Effects of DA-9801 on the Inflammation and Apoptosis Induced by Angiotensin II in Human Dermal Microvascular Endothelial Cells. J Pharmacol Sci 2021, 145, 52–59. [CrossRef]
- Montezano, A.C.; Burger, D.; Paravicini, T.M.; Chignalia, A.Z.; Yusuf, H.; Almasri, M.; He, Y.; Callera, G.E.; He, G.; Krause, K.H.; et al. Nicotinamide Adenine Dinucleotide Phosphate Reduced Oxidase 5 (Nox5) Regulation by Angiotensin II and Endothelin-1 Is Mediated via Calcium/Calmodulin-Dependent, Rac-1-Independent Pathways in Human Endothelial Cells. Circ Res 2010, 106, 1363. [CrossRef]
- Marqués, J.; Cortés, A.; Pejenaute, Á.; Ansorena, E.; Abizanda, G.; Prósper, F.; Martínez-Irujo, J.J.; Miguel, C. de; Zalba, G. Induction of Cyclooxygenase-2 by Overexpression of the Human NADPH Oxidase 5 (NOX5) Gene in Aortic Endothelial Cells. Cells 2020, 9. [CrossRef]
- Su, E.; Zhao, L.; Yang, X.; Zhu, B.; Liu, Y.; Zhao, W.; Wang, X.; Qi, D.; Zhu, L.; Gao, C. Aggravated Endothelial Endocrine Dysfunction and Intimal Thickening of Renal Artery in High-Fat Diet-Induced Obese Pigs Following Renal Denervation. BMC Cardiovasc Disord 2020, 20. [CrossRef]
- Valente, A.J.; Irimpen, A.M.; Siebenlist, U.; Chandrasekar, B. OxLDL Induces Endothelial Dysfunction and Death via TRAF3IP2: Inhibition by HDL3 and AMPK Activators. Free Radic Biol Med 2014, 70, 117–128. [CrossRef]
- Chen, B.; Zhao, J.; Zhang, S.; Wu, W.; Qi, R. Aspirin Inhibits the Production of Reactive Oxygen Species by Downregulating Nox4 and Inducible Nitric Oxide Synthase in Human Endothelial Cells Exposed to Oxidized Low-Density Lipoprotein. J Cardiovasc Pharmacol 2012, 59, 405–412. [CrossRef]
- Zhao, W.; Li, C.; Gao, H.; Wu, Q.; Shi, J.; Chen, X. Dihydrotanshinone I Attenuates Atherosclerosis in ApoE-Deficient Mice: Role of NOX4/NF-ΚB Mediated Lectin-Like Oxidized LDL Receptor-1 (LOX-1) of the Endothelium. Front Pharmacol 2016, 7. [CrossRef]
- Langbein, H.; Brunssen, C.; Hofmann, A.; Cimalla, P.; Brux, M.; Bornstein, S.R.; Deussen, A.; Koch, E.; Morawietz, H. NADPH Oxidase 4 Protects against Development of Endothelial Dysfunction and Atherosclerosis in LDL Receptor Deficient Mice. Eur Heart J 2016, 37, 1753–1761. [CrossRef]
- da Silva, J.F.; Alves, J. V.; Silva-Neto, J.A.; Costa, R.M.; Neves, K.B.; Alves-Lopes, R.; Carmargo, L.L.; Rios, F.J.; Montezano, A.C.; Touyz, R.M.; et al. Lysophosphatidylcholine Induces Oxidative Stress in Human Endothelial Cells via NOX5 Activation - Implications in Atherosclerosis. Clin Sci (Lond) 2021, 135, 1845–1858. [CrossRef]
- Sipkens, J.A.; Hahn, N.; van den Brand, C.S.; Meischl, C.; Cillessen, S.A.G.M.; Smith, D.E.C.; Juffermans, L.J.M.; Musters, R.J.P.; Roos, D.; Jakobs, C.; et al. Homocysteine-Induced Apoptosis in Endothelial Cells Coincides with Nuclear NOX2 and Peri-Nuclear NOX4 Activity. Cell Biochem Biophys 2013, 67, 341–352. [CrossRef]
- Cortés, A.; Pejenaute, Á.; Marqués, J.; Izal, Í.; Cenoz, S.; Ansorena, E.; Martínez-Irujo, J.J.; Miguel, C.; Zalba, G. Nadph Oxidase 5 Induces Changes in the Unfolded Protein Response in Human Aortic Endothelial Cells and in Endothelial-Specific Knock-in Mice. Antioxidants 2021, 10. [CrossRef]
- Marqués, J.; Fernández-Irigoyen, J.; Ainzúa, E.; Martínez-Azcona, M.; Cortés, A.; Roncal, C.; Orbe, J.; Santamaría, E.; Zalba, G. NADPH Oxidase 5 (NOX5) Overexpression Promotes Endothelial Dysfunction via Cell Apoptosis, Migration, and Metabolic Alterations in Human Brain Microvascular Endothelial Cells (HCMEC/D3). Antioxidants (Basel) 2022, 11. [CrossRef]
- Schuett, J.; Schuett, H.; Oberoi, R.; Koch, A.K.; Pretzer, S.; Luchtefeld, M.; Schieffer, B.; Grote, K. NADPH Oxidase NOX2 Mediates TLR2/6-Dependent Release of GM-CSF from Endothelial Cells. FASEB J 2017, 31, 2612–2624. [CrossRef]
- Park, H.S.; Chun, J.N.; Jung, H.Y.; Choi, C.; Bae, Y.S. Role of NADPH Oxidase 4 in Lipopolysaccharide-Induced Proinflammatory Responses by Human Aortic Endothelial Cells. Cardiovasc Res 2006, 72, 447–455. [CrossRef]
- Maloney, E.; Sweet, I.R.; Hockenbery, D.M.; Pham, M.; Rizzo, N.O.; Tateya, S.; Handa, P.; Schwartz, M.W.; Kim, F. Activation of NF-KappaB by Palmitate in Endothelial Cells: A Key Role for NADPH Oxidase-Derived Superoxide in Response to TLR4 Activation. Arterioscler Thromb Vasc Biol 2009, 29, 1370–1375. [CrossRef]
- Masai, N.; Tatebe, J.; Yoshino, G.; Morita, T. Indoxyl Sulfate Stimulates Monocyte Chemoattractant Protein-1 Expression in Human Umbilical Vein Endothelial Cells by Inducing Oxidative Stress through Activation of the NADPH Oxidase-Nuclear Factor-ΚB Pathway. Circ J 2010, 74, 2216–2224. [CrossRef]
- Jha, J.C.; Dai, A.; Holterman, C.E.; Cooper, M.E.; Touyz, R.M.; Kennedy, C.R.; Jandeleit-Dahm, K.A.M. Endothelial or Vascular Smooth Muscle Cell-Specific Expression of Human NOX5 Exacerbates Renal Inflammation, Fibrosis and Albuminuria in the Akita Mouse. Diabetologia 2019, 62, 1712–1726. [CrossRef]
- Rius, C.; Company, C.; Piqueras, L.; Cerdá-Nicolás, J.M.; González, C.; Servera, E.; Ludwig, A.; Morcillo, E.J.; Sanz, M.J. Critical Role of Fractalkine (CX3CL1) in Cigarette Smoke-Induced Mononuclear Cell Adhesion to the Arterial Endothelium. Thorax 2013, 68, 177–186. [CrossRef]
- Muzaffar, S.; Jeremy, J.Y.; Angelini, G.D.; Shukla, N. NADPH Oxidase 4 Mediates Upregulation of Type 4 Phosphodiesterases in Human Endothelial Cells. J Cell Physiol 2012, 227, 1941–1950. [CrossRef]
- Murdoch, C.E.; Chaubey, S.; Zeng, L.; Yu, B.; Ivetic, A.; Walker, S.J.; Vanhoutte, D.; Heymans, S.; Grieve, D.J.; Cave, A.C.; et al. Endothelial NADPH Oxidase-2 Promotes Interstitial Cardiac Fibrosis and Diastolic Dysfunction through Proinflammatory Effects and Endothelial-Mesenchymal Transition. J Am Coll Cardiol 2014, 63, 2734–2741. [CrossRef]
- Sun, H.J.; Zhao, M.X.; Liu, T.Y.; Ren, X.S.; Chen, Q.; Li, Y.H.; Kang, Y.M.; Zhu, G.Q. Salusin-β Induces Foam Cell Formation and Monocyte Adhesion in Human Vascular Smooth Muscle Cells via MiR155/NOX2/NFκB Pathway. Sci Rep 2016, 6. [CrossRef]
- Cook-Mills, J.M.; Marchese, M.E.; Abdala-Valencia, H. Vascular Cell Adhesion Molecule-1 Expression and Signaling During Disease: Regulation by Reactive Oxygen Species and Antioxidants. Antioxid Redox Signal 2011, 15, 1607. [CrossRef]
- Wang, M.; Murdoch, C.E.; Brewer, A.C.; Ivetic, A.; Evans, P.; Shah, A.M.; Zhang, M. Endothelial NADPH Oxidase 4 Protects against Angiotensin II-Induced Cardiac Fibrosis and Inflammation. ESC Heart Fail 2021, 8, 1427–1437. [CrossRef]
- Xia, F.; Wang, C.; Jin, Y.; Liu, Q.; Meng, Q.; Liu, K.; Sun, H. Luteolin Protects HUVECs from TNF-α-Induced Oxidative Stress and Inflammation via Its Effects on the Nox4/ROS-NF-ΚB and MAPK Pathways. J Atheroscler Thromb 2014, 21, 768–783. [CrossRef]
- Escudero, P.; De Marañón, A.M.; Collado, A.; Gonzalez-Navarro, H.; Hermenegildo, C.; Peiró, C.; Piqueras, L.; Sanz, M.J. Combined Sub-Optimal Doses of Rosuvastatin and Bexarotene Impair Angiotensin II-Induced Arterial Mononuclear Cell Adhesion through Inhibition of Nox5 Signaling Pathways and Increased RXR/PPARα and RXR/PPARγ Interactions. Antioxid Redox Signal 2015, 22, 901–920. [CrossRef]
- Cortés, A.; Marqués, J.; Pejenaute, Á.; Ainzúa, E.; Ansorena, E.; Abizanda, G.; Prósper, F.; de Miguel, C.; Zalba, G. Endothelial NOX5 Overexpression Induces Changes in the Cardiac Gene Profile: Potential Impact in Myocardial Infarction? J Physiol Biochem 2023, 79, 787–797. [CrossRef]
- Wang, H.; Albadawi, H.; Siddiquee, Z.; Stone, J.M.; Panchenko, M.P.; Watkins, M.T.; Stone, J.R. Altered Vascular Activation Due to Deficiency of the NADPH Oxidase Component P22phox. Cardiovascular Pathology 2014, 23, 35–42. [CrossRef]
- Valente, A.J.; Yoshida, T.; Murthy, S.N.; Sakamuri, S.S.V.P.; Katsuyama, M.; Clark, R.A.; Delafontaine, P.; Chandrasekar, B. Angiotensin II Enhances AT1-Nox1 Binding and Stimulates Arterial Smooth Muscle Cell Migration and Proliferation through AT1, Nox1, and Interleukin-18. Am J Physiol Heart Circ Physiol 2012, 303, 282–296. [CrossRef]
- Moraes, J.A.; Frony, A.C.; Dias, A.M.; Renovato-Martins, M.; Rodrigues, G.; Marcinkiewicz, C.; Assreuy, J.; Barja-Fidalgo, C. Alpha1beta1 and Integrin-Linked Kinase Interact and Modulate Angiotensin II Effects in Vascular Smooth Muscle Cells. Atherosclerosis 2015, 243, 477–485. [CrossRef]
- Li, X.; Wang, H.F.; Li, X.X.; Xu, M. Contribution of Acid Sphingomyelinase to Angiotensin II-Induced Vascular Adventitial Remodeling via Membrane Rafts/Nox2 Signal Pathway. Life Sci 2019, 219, 303–310. [CrossRef]
- Quesada, I.M.; Lucero, A.; Amaya, C.; Meijles, D.N.; Cifuentes, M.E.; Pagano, P.J.; Castro, C. Selective Inactivation of NADPH Oxidase 2 Causes Regression of Vascularization and the Size and Stability of Atherosclerotic Plaques. Atherosclerosis 2015, 242, 469–475. [CrossRef]
- Haurani, M.J.; Cifuentes, M.E.; Shepard, A.D.; Pagano, P.J. Nox4 Oxidase Overexpression Specifically Decreases Endogenous Nox4 MRNA and Inhibits Angiotensin II-Induced Adventitial Myofibroblast Migration. Hypertension 2008, 52, 143–149. [CrossRef]
- Lee, S.H.; Park, D.W.; Park, S.C.; Park, Y.K.; Hong, S.Y.; Kim, J.R.; Lee, C.H.; Baek, S.H. Calcium-Independent Phospholipase A2beta-Akt Signaling Is Involved in Lipopolysaccharide-Induced NADPH Oxidase 1 Expression and Foam Cell Formation. J Immunol 2009, 183, 7497–7504. [CrossRef]
- Lee, J.G.; Lim, E.J.; Park, D.W.; Lee, S.H.; Kim, J.R.; Baek, S.H. A Combination of Lox-1 and Nox1 Regulates TLR9-Mediated Foam Cell Formation. Cell Signal 2008, 20, 2266–2275. [CrossRef]
- Csányi, G.; Feck, D.M.; Ghoshal, P.; Singla, B.; Lin, H.; Nagarajan, S.; Meijles, D.N.; Al Ghouleh, I.; Cantu-Medellin, N.; Kelley, E.E.; et al. CD47 and Nox1 Mediate Dynamic Fluid-Phase Macropinocytosis of Native LDL. Antioxid Redox Signal 2017, 26, 886–901. [CrossRef]
- Yvan-Charvet, L.; Pagler, T.A.; Seimon, T.A.; Thorp, E.; Welch, C.L.; Witztum, J.L.; Tabas, I.; Tall, A.R. ABCA1 and ABCG1 Protect against Oxidative Stress-Induced Macrophage Apoptosis during Efferocytosis. Circ Res 2010, 106, 1861–1869. [CrossRef]
- Yu, P.; Han, W.; Villar, V.A.M.; Yang, Y.; Lu, Q.; Lee, H.; Li, F.; Quinn, M.T.; Gildea, J.J.; Felder, R.A.; et al. Unique Role of NADPH Oxidase 5 in Oxidative Stress in Human Renal Proximal Tubule Cells. Redox Biol 2014, 2, 570–579. [CrossRef]
- Vendrov, A.E.; Sumida, A.; Canugovi, C.; Lozhkin, A.; Hayami, T.; Madamanchi, N.R.; Runge, M.S. NOXA1-Dependent NADPH Oxidase Regulates Redox Signaling and Phenotype of Vascular Smooth Muscle Cell during Atherogenesis. Redox Biol 2019, 21. [CrossRef]
- Al Ghouleh, I.; Rodríguez, A.; Pagano, P.J.; Csányi, G. Proteomic Analysis Identifies an NADPH Oxidase 1 (Nox1)-Mediated Role for Actin-Related Protein 2/3 Complex Subunit 2 (ARPC2) in Promoting Smooth Muscle Cell Migration. Int J Mol Sci 2013, 14, 20220–20235. [CrossRef]
- Abhijit, S.; Bhaskaran, R.; Narayanasamy, A.; Chakroborty, A.; Manickam, N.; Dixit, M.; Mohan, V.; Balasubramanyam, M. Hyperinsulinemia-Induced Vascular Smooth Muscle Cell (VSMC) Migration and Proliferation Is Mediated by Converging Mechanisms of Mitochondrial Dysfunction and Oxidative Stress. Mol Cell Biochem 2013, 373, 95–105. [CrossRef]
- Zhao, Q.; Zhang, J.; Wang, H. PGC-1α Limits Angiotensin II-Induced Rat Vascular Smooth Muscle Cells Proliferation via Attenuating NOX1-Mediated Generation of Reactive Oxygen Species. Biosci Rep 2015, 35. [CrossRef]
- Zhang, J.; Chen, C.; Li, L.; Zhou, H.J.; Li, F.; Zhang, H.; Yu, L.; Chen, Y.; Min, W. Endothelial AIP1 Regulates Vascular Remodeling by Suppressing NADPH Oxidase-2. Front Physiol 2018, 9. [CrossRef]
- Pietrowski, E.; Bender, B.; Huppert, J.; White, R.; Luhmann, H.J.; Kuhlmann, C.R.W. Pro-Inflammatory Effects of Interleukin-17A on Vascular Smooth Muscle Cells Involve NAD(P)H- Oxidase Derived Reactive Oxygen Species. J Vasc Res 2011, 48, 52–58. [CrossRef]
- Fernandes, D.C.; Wosniak, J.; Gonçalves, R.C.; Tanaka, L.Y.; Fernandes, C.G.; Zanatta, D.B.; de Mattos, A.B.M.; Strauss, B.E.; Laurindo, F.R.M. PDIA1 Acts as Master Organizer of NOX1/NOX4 Balance and Phenotype Response in Vascular Smooth Muscle. Free Radic Biol Med 2021, 162, 603–614. [CrossRef]
- Meng, D.; Lv, D.D.; Fang, J. Insulin-like Growth Factor-I Induces Reactive Oxygen Species Production and Cell Migration through Nox4 and Rac1 in Vascular Smooth Muscle Cells. Cardiovasc Res 2008, 80, 299–308. [CrossRef]
- Camargo, L.L.; Montezano, A.C.; Hussain, M.; Wang, Y.; Zou, Z.; Rios, F.J.; Neves, K.B.; Alves-Lopes, R.; Awan, F.R.; Guzik, T.J.; et al. Central Role of C-Src in NOX5- Mediated Redox Signalling in Vascular Smooth Muscle Cells in Human Hypertension. Cardiovasc Res 2022, 118, 1359–1373. [CrossRef]
- Furmanik, M.; Chatrou, M.; Van Gorp, R.; Akbulut, A.; Willems, B.; Schmidt, H.; Van Eys, G.; Bochaton-Piallat, M.L.; Proudfoot, D.; Biessen, E.; et al. Reactive Oxygen-Forming Nox5 Links Vascular Smooth Muscle Cell Phenotypic Switching and Extracellular Vesicle-Mediated Vascular Calcification. Circ Res 2020, 127, 911–927. [CrossRef]
- Gray, S.P.; Di Marco, E.; Okabe, J.; Szyndralewiez, C.; Heitz, F.; Montezano, A.C.; De Haan, J.B.; Koulis, C.; El-Osta, A.; Andrews, K.L.; et al. NADPH Oxidase 1 Plays a Key Role in Diabetes Mellitus-Accelerated Atherosclerosis. Circulation 2013, 127, 1888–1902. [CrossRef]
- Ouerd, S.; Idris-Khodja, N.; Trindade, M.; Ferreira, N.S.; Berillo, O.; Coelho, S.C.; Neves, M.F.; Jandeleit-Dahm, K.A.; Paradis, P.; Schiffrin, E.L. Endothelium-Restricted Endothelin-1 Overexpression in Type 1 Diabetes Worsens Atherosclerosis and Immune Cell Infiltration via NOX1. Cardiovasc Res 2021, 117, 1144–1153. [CrossRef]
- Serino, A.; Zhao, Y.; Hwang, J.; Cullen, A.; Deeb, C.; Akhavan, N.; Arjmandi, B.; Salazar, G. Gender Differences in the Effect of Blackberry Supplementation in Vascular Senescence and Atherosclerosis in ApoE-/- Mice. J Nutr Biochem 2020, 80. [CrossRef]
- Judkins, C.P.; Diep, H.; Broughton, B.R.S.; Mast, A.E.; Hooker, E.U.; Miller, A.A.; Selemidis, S.; Dusting, G.J.; Sobey, C.G.; Drummond, G.R. Direct Evidence of a Role for Nox2 in Superoxide Production, Reduced Nitric Oxide Bioavailability, and Early Atherosclerotic Plaque Formation in ApoE-/- Mice. Am J Physiol Heart Circ Physiol 2010, 298. [CrossRef]
- Schürmann, C.; Rezende, F.; Kruse, C.; Yasar, Y.; Löwe, O.; Fork, C.; Van De Sluis, B.; Bremer, R.; Weissmann, N.; Shah, A.M.; et al. The NADPH Oxidase Nox4 Has Anti-Atherosclerotic Functions. Eur Heart J 2015, 36, 3447–3456. [CrossRef]
- Yu, W.; Xiao, L.; Que, Y.; Li, S.; Chen, L.; Hu, P.; Xiong, R.; Seta, F.; Chen, H.; Tong, X. Smooth Muscle NADPH Oxidase 4 Promotes Angiotensin II-Induced Aortic Aneurysm and Atherosclerosis by Regulating Osteopontin. Biochim Biophys Acta Mol Basis Dis 2020, 1866. [CrossRef]
- Vlad, M.L.; Manea, S.A.; Lazar, A.G.; Raicu, M.; Muresian, H.; Simionescu, M.; Manea, A. Histone Acetyltransferase-Dependent Pathways Mediate Upregulation of NADPH Oxidase 5 in Human Macrophages under Inflammatory Conditions: A Potential Mechanism of Reactive Oxygen Species Overproduction in Atherosclerosis. Oxid Med Cell Longev 2019, 2019. [CrossRef]
- Ho, F.; Watson, A.M.D.; Elbatreek, M.H.; Kleikers, P.W.M.; Khan, W.; Sourris, K.C.; Dai, A.; Jha, J.; Schmidt, H.H.H.W.; Jandeleit-Dahm, K.A.M. Endothelial Reactive Oxygen-Forming NADPH Oxidase 5 Is a Possible Player in Diabetic Aortic Aneurysm but Not Atherosclerosis. Sci Rep 2022, 12. [CrossRef]
- Petheő, G.L.; Kerekes, A.; Mihálffy, M.; Donkó, Á.; Bodrogi, L.; Skoda, G.; Baráth, M.; Hoffmann, O.I.; Szeles, Z.; Balázs, B.; et al. Disruption of the NOX5 Gene Aggravates Atherosclerosis in Rabbits. Circ Res 2021, 128, 1320–1322. [CrossRef]
- Cho, D.I.; Ahn, M.J.; Cho, H.H.; Cho, M.; Jun, J.H.; Kang, B.G.; Lim, S.Y.; Yoo, S.J.; Kim, M.R.; Kim, H.S.; et al. ANGPTL4 Stabilizes Atherosclerotic Plaques and Modulates the Phenotypic Transition of Vascular Smooth Muscle Cells through KLF4 Downregulation. Exp Mol Med 2023, 55, 426–442. [CrossRef]
- Wang, Y.; Liu, X.Y.; Zhao, W.X.; Li, F.D.; Guo, P.R.; Fan, Q.; Wu, X.F. NOX2 Inhibition Stabilizes Vulnerable Plaques by Enhancing Macrophage Efferocytosis via MertK/PI3K/AKT Pathway. Redox Biol 2023, 64. [CrossRef]
- Hofmann, A.; Frank, F.; Wolk, S.; Busch, A.; Klimova, A.; Sabarstinski, P.; Gerlach, M.; Egorov, D.; Kopaliani, I.; Weinert, S.; et al. NOX4 MRNA Correlates with Plaque Stability in Patients with Carotid Artery Stenosis. Redox Biol 2022, 57. [CrossRef]
- Vara, D.; Tarafdar, A.; Celikag, M.; Patinha, D.; Gulacsy, C.E.; Hounslea, E.; Warren, Z.; Ferreira, B.; Koeners, M.P.; Caggiano, L.; et al. NADPH Oxidase 1 Is a Novel Pharmacological Target for the Development of an Antiplatelet Drug without Bleeding Side Effects. FASEB J 2020, 34, 13959–13977. [CrossRef]
- Delaney, M.K.; Kim, K.; Estevez, B.; Xu, Z.; Stojanovic-Terpo, A.; Shen, B.; Ushio-Fukai, M.; Cho, J.; Du, X. Differential Roles of the NADPH-Oxidase 1 and 2 in Platelet Activation and Thrombosis. Arterioscler Thromb Vasc Biol 2016, 36, 846–854. [CrossRef]
- Sonkar, V.K.; Kumar, R.; Jensen, M.; Wagner, B.A.; Sharathkumar, A.A.; Miller, F.J.; Fasano, M.B.; Lentz, S.R.; Buettner, G.R.; Dayal, S. Nox2 NADPH Oxidase Is Dispensable for Platelet Activation or Arterial Thrombosis in Mice. Blood Adv 2019, 3, 1272–1284. [CrossRef]
- Vara, D.; Mailer, R.K.; Tarafdar, A.; Wolska, N.; Heestermans, M.; Konrath, S.; Spaeth, M.; Renné, T.; Schröder, K.; Pula, G. NADPH Oxidases Are Required for Full Platelet Activation In Vitro and Thrombosis In Vivo but Dispensable for Plasma Coagulation and Hemostasis. Arterioscler Thromb Vasc Biol 2021, 41, 683. [CrossRef]
- Zhang, B.; Li, J. Phoenixin-14 Protects Human Brain Vascular Endothelial Cells against Oxygen-Glucose Deprivation/Reoxygenation (OGD/R)-Induced Inflammation and Permeability. Arch Biochem Biophys 2020, 682. [CrossRef]
- Hwang, J.S.; Cha, E.H.; Ha, E.; Park, B.; Seo, J.H. GKT136901 Protects Primary Human Brain Microvascular Endothelial Cells against Methamphetamine-Induced Blood-Brain Barrier Dysfunction. Life Sci 2020, 256. [CrossRef]
- Kuriakose, M.; Younger, D.; Ravula, A.R.; Alay, E.; Rama Rao, K. V.; Chandra, N. Synergistic Role of Oxidative Stress and Blood-Brain Barrier Permeability as Injury Mechanisms in the Acute Pathophysiology of Blast-Induced Neurotrauma. Sci Rep 2019, 9. [CrossRef]
- Kleinschnitz, C.; Grund, H.; Wingler, K.; Armitage, M.E.; Jones, E.; Mittal, M.; Barit, D.; Schwarz, T.; Geis, C.; Kraft, P.; et al. Post-Stroke Inhibition of Induced NADPH Oxidase Type 4 Prevents Oxidative Stress and Neurodegeneration. PLoS Biol 2010, 8. [CrossRef]
- Jackman, K.A.; Miller, A.A.; Drummond, G.R.; Sobey, C.G. Importance of NOX1 for Angiotensin II-Induced Cerebrovascular Superoxide Production and Cortical Infarct Volume Following Ischemic Stroke. Brain Res 2009, 1286, 215–220. [CrossRef]
- Alwjwaj, M.; Kadir, R.R.A.; Bayraktutan, U. Outgrowth Endothelial Progenitor Cells Restore Cerebral Barrier Function Following Ischaemic Damage: The Impact of NOX2 Inhibition. Eur J Neurosci 2022, 55, 1658–1670. [CrossRef]
- Namyen, J.; Permpoonputtana, K.; Nopparat, C.; Tocharus, J.; Tocharus, C.; Govitrapong, P. Protective Effects of Melatonin on Methamphetamine-Induced Blood-Brain Barrier Dysfunction in Rat Model. Neurotox Res 2020, 37, 640–660. [CrossRef]
- Yang, F.; Wang, Z.; Wei, X.; Han, H.; Meng, X.; Zhang, Y.; Shi, W.; Li, F.; Xin, T.; Pang, Q.; et al. NLRP3 Deficiency Ameliorates Neurovascular Damage in Experimental Ischemic Stroke. J Cereb Blood Flow Metab 2014, 34, 660–667. [CrossRef]
- Casas, A.I.; Geuss, E.; Kleikers, P.W.M.; Mencl, S.; Herrmann, A.M.; Buendia, I.; Egea, J.; Meuth, S.G.; Lopez, M.G.; Kleinschnitz, C.; et al. NOX4-Dependent Neuronal Autotoxicity and BBB Breakdown Explain the Superior Sensitivity of the Brain to Ischemic Damage. Proc Natl Acad Sci U S A 2017, 114, 12315–12320. [CrossRef]
- Cortés, A.; Solas, M.; Pejenaute, Á.; Abellanas, M.A.; Garcia-Lacarte, M.; Aymerich, M.S.; Marqués, J.; Ramírez, M.J.; Zalba, G. Expression of Endothelial Nox5 Alters the Integrity of the Blood-Brain Barrier and Causes Loss of Memory in Aging Mice. Antioxidants 2021, 10. [CrossRef]
- Qiu, Y.M.; Zhang, C.L.; Chen, A.Q.; Wang, H.L.; Zhou, Y.F.; Li, Y.N.; Hu, B. Immune Cells in the BBB Disruption After Acute Ischemic Stroke: Targets for Immune Therapy? Front Immunol 2021, 12. [CrossRef]
- Eum, S.Y.; Andras, I.; Hennig, B.; Toborek, M. NADPH Oxidase and Lipid Raft-Associated Redox Signaling Are Required for PCB153-Induced Upregulation of Cell Adhesion Molecules in Human Brain Endothelial Cells. Toxicol Appl Pharmacol 2009, 240, 299–305. [CrossRef]
- Lelli, A.; Gervais, A.; Colin, C.; Chéret, C.; de Almodovar, C.R.; Carmeliet, P.; Krause, K.H.; Boillée, S.; Mallat, M. The NADPH Oxidase Nox2 Regulates VEGFR1/CSF-1R-Mediated Microglial Chemotaxis and Promotes Early Postnatal Infiltration of Phagocytes in the Subventricular Zone of the Mouse Cerebral Cortex. Glia 2013, 61, 1542–1555. [CrossRef]
- Zhu, Y.; Haddad, Y.; Yun, H.J.; Geng, X.; Ding, Y. Induced Inflammatory and Oxidative Markers in Cerebral Microvasculature by Mentally Depressive Stress. Mediators Inflamm 2023, 2023. [CrossRef]
- Li, R.; Yuan, Q.; Su, Y.; Chopp, M.; Yan, T.; Chen, J. Immune Response Mediates the Cardiac Damage after Subarachnoid Hemorrhage. Exp Neurol 2020, 323. [CrossRef]
- Kim, S.W.; Davaanyam, D.; Seol, S.I.; Lee, H.K.; Lee, H.; Lee, J.K. Adenosine Triphosphate Accumulated Following Cerebral Ischemia Induces Neutrophil Extracellular Trap Formation. Int J Mol Sci 2020, 21, 7668. [CrossRef]
- Zhang, Y.; Wei, X.; Liu, L.; Liu, S.; Wang, Z.; Zhang, B.; Fan, B.; Yang, F.; Huang, S.; Jiang, F.; et al. TIPE2, a Novel Regulator of Immunity, Protects against Experimental Stroke. J Biol Chem 2012, 287, 32546–32555. [CrossRef]
- Jin, R.; Song, Z.; Yu, S.; Piazza, A.; Nanda, A.; Penninger, J.M.; Granger, D.N.; Li, G. Phosphatidylinositol-3-Kinase Gamma Plays a Central Role in Blood-Brain Barrier Dysfunction in Acute Experimental Stroke. Stroke 2011, 42, 2033–2044. [CrossRef]
- Tuo, Y.H.; Liu, Z.; Chen, J.W.; Wang, Q.Y.; Li, S.L.; Li, M.C.; Dai, G.; Wang, J.S.; Zhang, Y.L.; Feng, L.; et al. NADPH Oxidase Inhibitor Improves Outcome of Mechanical Reperfusion by Suppressing Hemorrhagic Transformation. J Neurointerv Surg 2017, 9, 492–498. [CrossRef]
- Yingze, Y.; Zhihong, J.; Tong, J.; Yina, L.; Zhi, Z.; Xu, Z.; Xiaoxing, X.; Lijuan, G. NOX2-Mediated Reactive Oxygen Species Are Double-Edged Swords in Focal Cerebral Ischemia in Mice. J Neuroinflammation 2022, 19. [CrossRef]
- Choi, D.H.; Kim, J.H.; Lee, K.H.; Kim, H.Y.; Kim, Y.S.; Choi, W.S.; Lee, J. Role of Neuronal NADPH Oxidase 1 in the Peri-Infarct Regions after Stroke. PLoS One 2015, 10. [CrossRef]
- McCann, S.K.; Dusting, G.J.; Roulston, C.L. Nox2 Knockout Delays Infarct Progression and Increases Vascular Recovery through Angiogenesis in Mice Following Ischaemic Stroke with Reperfusion. PLoS One 2014, 9. [CrossRef]
- Zuo, M.L.; Wang, A.P.; Song, G.L.; Yang, Z.B. MiR-652 Protects Rats from Cerebral Ischemia/Reperfusion Oxidative Stress Injury by Directly Targeting NOX2. Biomed Pharmacother 2020, 124. [CrossRef]
- Liu, H.; Wei, X.; Kong, L.; Liu, X.; Liu, X.; Cheng, L.; Yan, S.; Zhang, X.; Chen, L. NOD2 Is Involved in the Inflammatory Response after Cerebral Ischemia-Reperfusion Injury and Triggers NADPH Oxidase 2-Derived Reactive Oxygen Species. Int J Biol Sci 2015, 11, 525. [CrossRef]
- Zhang, T.; Han, H.; Zhou, Y.; Liu, Z.; Ma, T.; Cao, X. MicroRNA-454 Modulates the Oxidative Stress and Neuronal Apoptosis after Cerebral Ischemia/Reperfusion Injury via Targeting NADPH Oxidase 4 (NOX4). J Biochem Mol Toxicol 2022, 36. [CrossRef]
- Suzuki, Y.; Hattori, K.; Hamanaka, J.; Murase, T.; Egashira, Y.; Mishiro, K.; Ishiguro, M.; Tsuruma, K.; Hirose, Y.; Tanaka, H.; et al. Pharmacological Inhibition of TLR4-NOX4 Signal Protects against Neuronal Death in Transient Focal Ischemia. Sci Rep 2012, 2. [CrossRef]
- Hu, Z.Y.; Yang, Z.B.; Zhang, R.; Luo, X.J.; Peng, J. The Protective Effect of Vitexin Compound B-1 on Rat Cerebral I/R Injury through a Mechanism Involving Modulation of MiR-92b/NOX4 Pathway. CNS Neurol Disord Drug Targets 2023, 22, 137–147. [CrossRef]
- Dai, Y.; Zhang, H.; Zhang, J.; Yan, M. Isoquercetin Attenuates Oxidative Stress and Neuronal Apoptosis after Ischemia/Reperfusion Injury via Nrf2-Mediated Inhibition of the NOX4/ROS/NF-ΚB Pathway. Chem Biol Interact 2018, 284, 32–40. [CrossRef]
- Lu, P.; Zhang, C.C.; Zhang, X.M.; Li, H.G.; Luo, A.L.; Tian, Y.K.; Xu, H. Down-Regulation of NOX4 by Betulinic Acid Protects against Cerebral Ischemia-Reperfusion in Mice. J Huazhong Univ Sci Technolog Med Sci 2017, 37, 744–749. [CrossRef]
- Qin, Y.Y.; Li, M.; Feng, X.; Wang, J.; Cao, L.; Shen, X.K.; Chen, J.; Sun, M.; Sheng, R.; Han, F.; et al. Combined NADPH and the NOX Inhibitor Apocynin Provides Greater Anti-Inflammatory and Neuroprotective Effects in a Mouse Model of Stroke. Free Radic Biol Med 2017, 104, 333–345. [CrossRef]
- Li, H.; Wang, Y.; Feng, D.; Liu, Y.; Xu, M.; Gao, A.; Tian, F.; Zhang, L.; Cui, Y.; Wang, Z.; et al. Alterations in the Time Course of Expression of the Nox Family in the Brain in a Rat Experimental Cerebral Ischemia and Reperfusion Model: Effects of Melatonin. J Pineal Res 2014, 57, 110–119. [CrossRef]
- Casas, A.I.; Kleikers, P.W.M.; Geuss, E.; Langhauser, F.; Adler, T.; Busch, D.H.; Gailus-Durner, V.; De Angelis, M.H.; Egea, J.; Lopez, M.G.; et al. Calcium-Dependent Blood-Brain Barrier Breakdown by NOX5 Limits Postreperfusion Benefit in Stroke. J Clin Invest 2019, 129, 1772. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




