1. Global Status of Malaria: Public Health Post-COVID-19
Malaria in humans is caused by species of the
Plasmodium genus, with the most devastating and virulent species being
P. falciparum, a unicellular protozoan parasite capable of intracellular existence. Over the 2000-2019 period, the global malaria death toll declined from 864,000 to 576,000 deaths (World Malaria Report 2023, World Health Organization, WHO). However, this trend was reversed in 2020, with 631,000 deaths recorded worldwide (a 10% increase compared to the number of deaths recorded in 2019), before levelling at around 610,000 deaths over the 2021-2022 period (World Malaria Report 2023, WHO). There were 63,000 deaths reported due to disruptions to essential malaria services during the COVID-19 pandemic (2019-2021) (World Malaria Report 2022, WHO). While there have been reviews on the status of malaria in the Eastern Mediterranean Region (EMR;
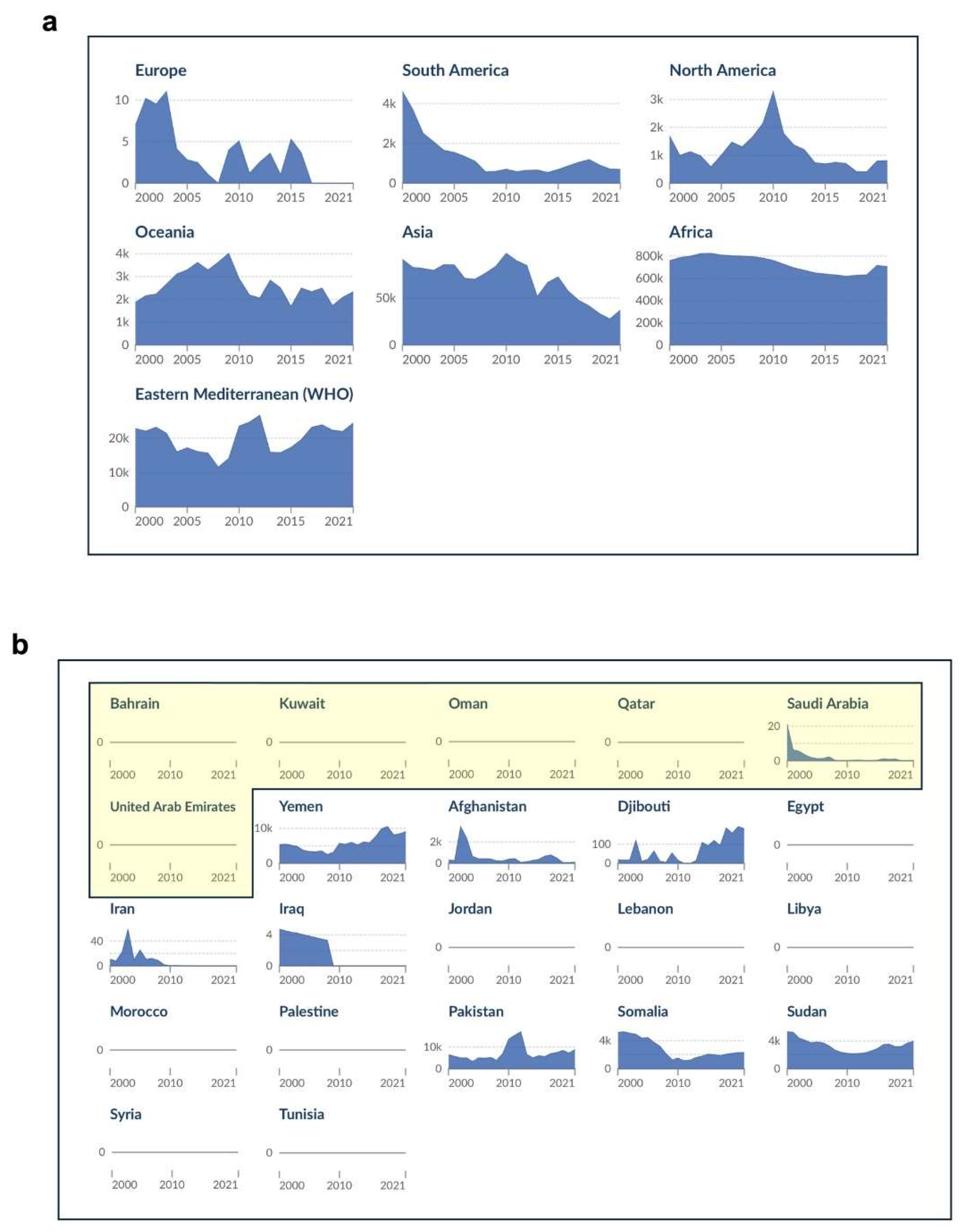
Figure 1a and 1b) based primarily on data prior to the pandemic (Al-Awadhi et al. 2021; Iqbal et al., 2021), this review represents a significant post-COVID-19 update on the status of malaria in this region, with a particular focus on the Gulf Cooperation Council (GCC) countries (Bahrain, Kuwait, Qatar, Oman, Saudi Arabia, and the United Arab Emirates, UAE). Clinical data has been retrieved from publicly available databases for the GCC countries, and systematically collated (
Table 1) and critically analyzed, thereby providing the most up-to-date snapshot of malaria in these countries pre- and post-COVID-19. Hence, in this review we assess the current world-wide status of malaria in terms of public health, prevention and treatment, before providing an Arabian regional perspective, with a critical evaluation of the malaria situation in the GCC countries.
In the EMR as defined by WHO (
Figures 1a and 1b), annual cases of malaria decreased during 2000-2015, from 6.9 million to 4.3 million, before steadily increasing, reaching 8.3 million in 2022, a 92% increase (World Malaria Report 2023, WHO). Mortality due to malaria in the EMR also decreased from 13,600 to 7,500 deaths during 2000-2014, but then increased during 2014-2022 to reach 15,900 deaths, a more than 100% increase. Interestingly, publicly available data retrieved from Our World in Data (“Malaria” by Max Roser and Hannah Ritchie, for the period 2000-2021; ourworldindata.org/malaria;
Figure 1a and 1b), representing estimates of malaria deaths by the Institute of Health Metrics and Evaluation (IHME), were found to be slightly different to the WHO data. While the IHME data show the same overall trends in mortality as the WHO data, the number of deaths were somewhat higher (
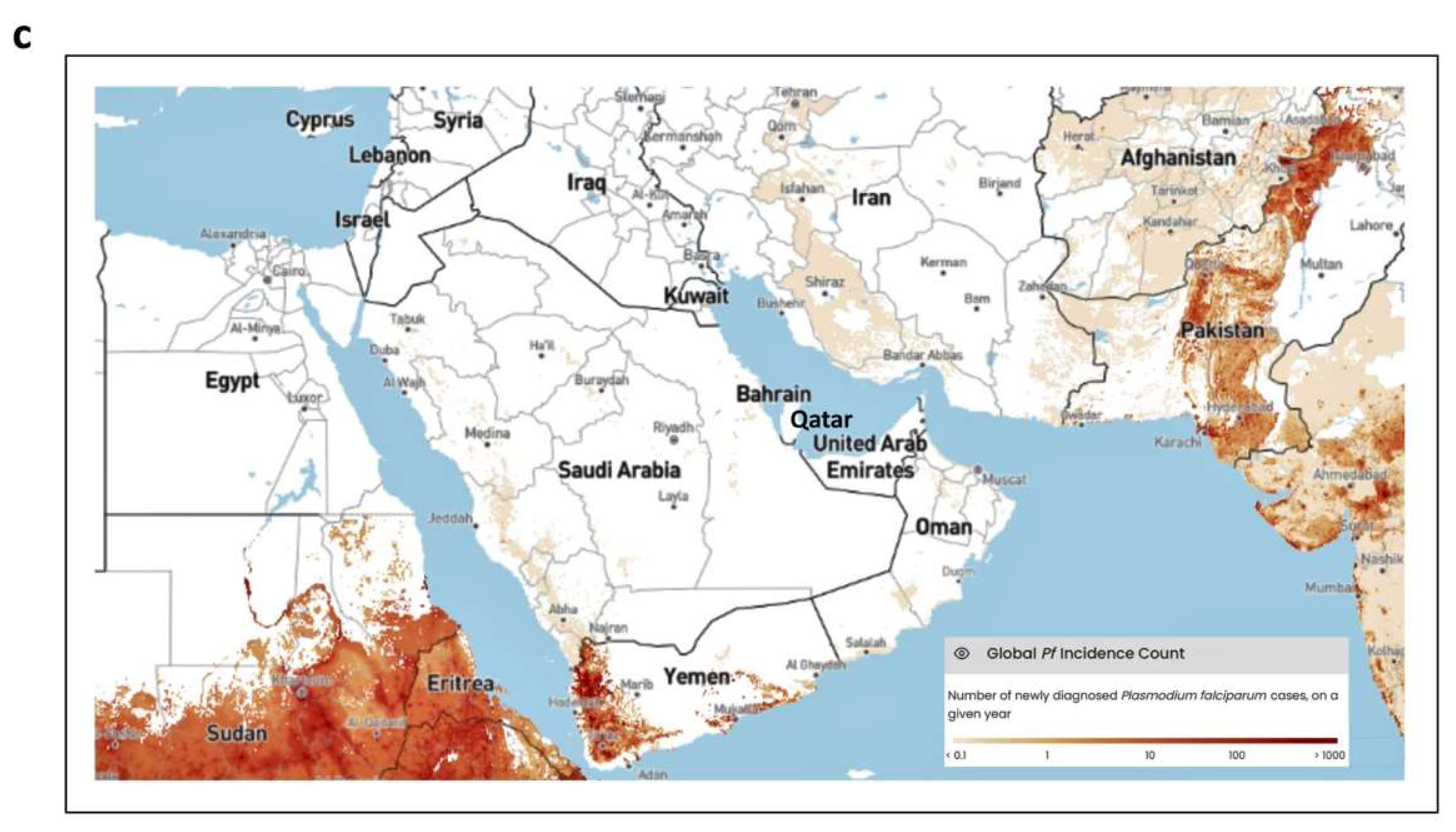
Figures 1a and 1b). While a number of countries in the EMR appear to be free of indigenous malaria, there is still the threat of imported malaria (Al-Awadhi et al., 2021). In the Arabian Peninsula, most of the GCC countries have been declared free of indigenous malaria by the WHO (Bahrain, Kuwait, Qatar, and the UAE; World Malaria Report 2022, WHO); however, imported malaria accounts for a significant number of deaths in this region, and malaria is still endemic in two GCC countries (Saudi Arabia and Oman) and its neighbors (Yemen) (Al-Awadhi et al., 2021) (
Figure 1c).
There are a number of factors that are threatening malaria control measures, including but not limited to: antimalarial drug resistance (Balikagala et al., 2021); false negative diagnostic test results due to pfhrp2/3 gene deletions (Feleke et al., 2021); insecticide resistance in mosquitoes to widely used insecticides (pyrethroids, organochlorines, organophosphates and carbamates) (Riveron et al., 2018; Hancock et al., 2020; Venkatesan, 2024); and the emergence of various highly invasive mosquito species, in particular Anopheles stephensi (Ahmed et al., 2021). The potential synergistic negative impact of the emergence of insecticide resistance in invasive species of mosquitoes is of grave concern. Insecticide resistance is acquired mainly through target resistance (e.g. mutant voltage-gated sodium channels, VGSCs, with reduces binding to pyrethroids), metabolic resistance (e.g. increase in detoxification enzymes such as cytochrome P450 monooxygenases, esterases, carboxylesterases and glutathione S-transferases), and penetration resistance (e.g. increase in structural protein involved in cuticle thickness) (Barnes et al., 2017; Riveron et al., 2017; Kouamo et al., 2021). Also of major concern are the emerging signs of antimalarial drug resistance in P. falciparum in Africa and South-East Asia, particularly to first-line drug treatment (artemisinin resistance, which manifests as delayed parasite clearance) and other drugs (antifolates, naphthoquinones, antibiotics like clindamycin and doxycycline and 4-aminoquinolines) (Balikagala et al., 2021). Furthermore, there are only a few novel targets for the development of new antimalarial drugs (Shibeshi et al., 2020), highlighting the need for the identification of further drug targets for antimalarial drug discovery. Elucidation of the structural properties of the protein-protein interactions required for the survival and pathology of the parasite in humans is critical to the continuous development of novel antimalarial drugs.
Similar to antimalarial drug development, advancement in effective malaria vaccine development has also been challenged by the pleiotropic nature of the parasite. Despite such hurdles, in 2021, the first malaria vaccine to be approved and licensed for widespread use was released (RTS,S/AS01; Mosquirix™; Laurens, 2020; Beeson et al., 2022). Furthermore, very recently a second malaria vaccine, R21/Matrix-M™ (R21/MM), was recommended for release (Siddiqui et al., 2023). While these vaccines hold much promise, they are designed for children, and hence further research and development is needed to produce malaria vaccines with broader reach at the population level. These malaria vaccines are critically evaluated later in the section on “Antimalarial drugs and vaccines”.
Given the recent increase in case and mortality rates, and the emerging threats to the control, elimination and ultimately eradication of malaria in regions of the globe with high burden, there is a dire need for a globally integrated approach to this infectious disease (Venkatesan, 2024). Despite the negative impact of COVID-19 on the public health status of malaria, the pandemic has shown what can be done when a disease is viewed as a global threat. Indeed, there is an urgent need for a universal effort in applying more strategic approaches towards the eradication of malaria. In the following sections we evaluate the global status of malaria in terms of prevention and treatment, before focusing on the GCC countries.
2. Malaria Prevention
Since mosquitoes act as vectors, one of the key preventative methods to control the spread of malaria is to reduce the vector population. Several approaches are used to control the malaria vector population, including drainage of swamps, application of pesticides and use of surface coverings (such as oil) to kill mosquito larvae present in standing water bodies. Indoor residual sprays (IRS) and long-lasting insecticidal nets (LLINs) are still the primary approaches to malaria prevention (Corrêa et al., 2019). Despite its effectiveness as a malaria control tool, the use of dichlorodiphenyltrichloroethane (DDT) is controversial because of the persistence of the chemical in the environment (Bouwman et al., 2011). The main insecticides used to control malaria vectors include pyrethroids, organochlorines, carbamates, and organophosphates (Killeen et al., 2017). Of these, pyrethroids pose the least public health concerns as they are bio-degradable (Casida et al., 1975; Cycoń and Piotrowska-Seget, 2016). However, as discussed in the previous section, the consistent use of insecticides leads to resistance to the chemicals by the mosquito vector. The recommended approach to mitigate against mounting insecticide resistance is to make use of IRS or combining IRS with LLINs (West et al., 2014). An alternative approach proposed to prevent insecticide resistance is to mix the active ingredients, and this has been reported to be effective when applied to mosquito bed nets (Pennetier et al., 2008). An additional advantage of this approach is that the active ingredients interact synergistically when applied in combination, such that low doses are highly effective (Pennetier et al., 2008).
Preventative approaches to malaria also involve a combination of vaccines and chemo-preventive approaches, none of which are effective in isolation. Several chemoprevention approaches for malaria have been stipulated in global guidelines, amongst them: intermittent preventive treatments during pregnancy (IPTp), and for infants (IPTi); seasonal malaria chemo-prevention (SMC); and for emergency situations, mass drug administration (MDA) (Plowe, 2022). Effective chemo-preventive approaches are associated not only with reduced disease burden, but also with a reduced incidence of drug resistance.
3. Antimalarial Drugs and Vaccines
Resistance has emerged in P. falciparum to artemisinin (and its derivatives), the globally recommended first-line therapy for malaria. Artemisinin and its derivatives (e.g., artesunate, artemether and arteether), synthesized by the plant Artemisia annua, are able to rapidly clear the parasite by inhibiting protein synthesis. Different research groups have reported that mutations in the P. falciparum Kelch13-encoding gene result in reduced levels of the essential protein PfKelch13, leading to artemisinin resistance (Siddiqui et al., 2021; Paloque et al., 2022). Mutation in PfKelch13 enables the early ring stage of the parasite to avoid going through cell cycle arrest and continue their intra-erythrocytic development when artemisinin is no longer available at effective concentrations (Mok et al., 2015; Mok et al., 2021). Resistance has been shown to develop in six Southeast Asian countries including Cambodia, Myanmar, Thailand, Vietnam, Laos, and southern China (Yunnan, and Guangxi) (Balikagala et al., 2021), and it poses a serious threat to other global regions. This emerging drug resistance has put a spotlight on the fact that there are only a few validated antimalarial drug targets (Shibeshi et al., 2020). One approach to overcoming resistance to artemisinin and other antimalarials has been combination drug therapy using complementary or synergistic drugs that act on distinct targets. Indeed, global guidelines have been released and implemented, involving the use of combinations of antimalarial drugs with distinct targets and mechanisms of action (WHO Guidelines for Malaria, 2022). However, this approach has had mixed success, highlighting that for significant improvement towards treatments where drug resistance is minimized, new antimalarials for combination drug therapies should ideally be derived from novel chemical scaffolds that have novel modes of action (Luo et al., 2023). Furthermore, while there are a number of promising candidate compounds feeding into next-generation antimalarial treatments, many target only the asexual, erythrocytic stage of the parasite’s life cycle. Thus, one logical approach is to identify novel chemotypes targeting the other parasitic stages or multiple stages, a topic that has been extensively reviewed (Luo et al., 2023). Broadly, contemporary approaches involve identification of a protein that is required for growth or survival of the parasite, followed by identification of a chemical series that inhibits the target. A key to antimalarial drug discovery is the subsequent medicinal chemistry efforts, which are designed to enhance binding to the parasite protein while sparing the closest human orthologs. To illustrate this concept, the malarial heat shock proteins (HSPs) are a case in point. The HSPs are a family of molecular chaperones whose expression is upregulated under stress (e.g. heat, low oxygen levels, pH variations, and nutrient deprivation), and which function to prevent the accumulation of toxic levels of misfolded and aggregated proteins under such conditions (Jee, 2016; Edkins and Boshoff, 2021). The major families of HSPs, including HSP10, HSP40, HSP60, HSP70, HSP90, and HSP100, are highly conserved from prokaryotes to eukaryotes, forming an intricate network critical for ensuring protein homeostasis (proteostasis). Consistent with this role, most HSPs have been found to be essential for the growth, survival, viability, and differentiation at all stages of the life cycle of kinetoplastid and apicomplexan parasites, including the malaria parasite (Shonhai et al., 2011; Zhang et al., 2018). Many HSPs are also essential for the pathogenesis and virulence of the malaria parasite (Blatch, 2022). These observations have led to the suggestion that the P. falciparum HSPs (PfHSPs), especially PfHSP40 (Daniyan et al., 2016; Daniyan and Blatch, 2017; Dutta et al., 2021a; Almaazmi et al., 2022), PfHSP70 (Przyborski et al., 2015; Batinovic et al., 2017; Zininga et al., 2017a; Zininga et al., 2017b; Chen et al., 2018; Day et al., 2019; Cortés et al., 2020; Barth et al., 2022; Mrozek et al., 2022), PfHSP90 (Banumathy et al., 2003; Bayih et al., 2016; Wang et al., 2016; Posfai et al., 2018; Shahinas et al., 2013; Seraphim et al., 2019; Stofberg et al., 2021) and their complexes (Botha et al., 2011; Cockburn et al., 2011; Cockburn et al., 2014; Dutta et al., 2021b; Almaazmi et al., 2023; Singh et al., 2023), could be prospective antimalarial drug targets. Unlike many other targets that have been explored, inhibition of these proteins could have broad effects on parasite survival at multiple stages, forming a potentially important addition to the antimalarial armamentarium.
In the preceding paragraphs, we outlined progress towards developing HSPs and their complexes as new targets for antimalarial drug discovery. Ideally, optimized versions of these single-agent compounds might be combined with both emerging and existing drugs. Combination therapies have become a promising tool for reducing burdens on the path to elimination (Luo et al., 2023). Mass drug administration (MDA) is the contemporaneous delivery of a full course of antimalarial medicine at a population level, regardless of whether individuals are infected with malaria or not, before or during high malaria transmission seasons (Eisele, 2019; WHO Guidelines for Malaria, 2022). According to the WHO, MDA use is recommended in settings approaching elimination, where vector control and monitoring are effective, treatment is accessible, and re-infection is minimal (WHO Guidelines for Malaria, 2022). For example, programmatic MDA (pMDA) campaigns with dihydroartemisinin-piperaquine (DHAP) were conducted in the Southern Province of Zambia as a malaria elimination tool (Fraser et al., 2020). Interrupted time-series results with comparison groups, indicated that two rounds of pMDA with DHAP over one year with moderate coverage, resulted in significant short-term reduction of malaria transmission in low transmission areas, but not eradication (Fraser et al., 2020). In recent years, MDA intervention using mosquitocidal drugs has been used in Gambia, combined with antimalarial treatments with ivermectin and DHAP, in an area of moderate malaria, where there is high coverage of insecticide-treated nets and indoor residual spraying. The implementation of MDA was found to be both safe and well-tolerated where there was a high coverage of standard control interventions, resulting in significant reductions in malaria prevalence, and to possibly promote malaria elimination where there was high coverage of vector control interventions (Dabira et al., 2022).
What is the status of antimalarial vaccines, and their role in antimalarial MDA? The first malaria vaccine to be released, RTS,S/AS01, is recommended for young children (five to six months old, with a booster at two years old) living in moderate-to-high malaria transmission locations (RTS,S Clinical Trials Partnership, 2015; Beeson et al., 2022). RTS,S/AS01 is a genetically engineered fusion protein-based vaccine (also referred to as a subunit vaccine) which targets the pre-erythrocytic stage of the P. falciparum life cycle. The fusion protein consists of the central tandem repeat region (R) and C-terminal T-cell epitopes (T) of the P. falciparum circumsporozoite protein, PfCSP, fused to the S antigen of the hepatitis B virus (S). This fusion protein (RTS) and the hepatitis B surface antigen, HBsAg, (S) are co-produced in a yeast (Saccharomyces cerevisiae) expression system, and formulated with the adjuvant AS01 (Zavala, 2022). RTS,S/AS01 clinical trials in malaria-endemic African countries reported only a modest decrease in malaria cases for test versus control groups (clinical efficacy), with ~55% efficacy in young children (5–17 months), and ~31–26% efficacy in infants (6–12-weeks) (Beeson et al., 2022; Zavala, 2022). Nevertheless, this vaccine appears to be safe, readily delivered, and already making a notable positive impact on protection from malaria among young children at the community level (Beeson et al., 2022). Indeed, the vaccine is reported to have already reached approximately 2 million children in three countries in Africa (Ghana, Kenya and Malawi). However, despite the promising potential of RTS,S/AS01, it does not meet the WHO threshold clinical efficacy (75%), and it offers only modest, short-lived and strain-specific protection (RTS,S Clinical Trials Partnership, 2015; Neafsey et al., 2015; Laurens, 2020). The recently approved second malaria vaccine, R21/Matrix-M™ (R21/MM), offers greater clinical efficacy, having exceeded the WHO efficacy threshold. R21/MM is also a subunit-based vaccine targeting the pre-erythrocytic stage of the parasite lifecycle, and designed for the prevention of malaria in young children (Datoo et al., 2021). This vaccine comprises R21, a PfCSP central tandem repeat region fused to the N-terminus of the HBsAg, co-produced with HBsAg in a yeast (Hansenula polymorpha) expression system, and formulated in the adjuvant Matrix-M (MM). R21/MM clinical trials in malaria-endemic African countries have demonstrated 74% (low dose) and 77% (high dose) efficacy in young children (5 and 17 months) (Datoo et al., 2022; Siddiqui et al., 2023). While there are now two malaria vaccines for young children, one released and licenced (RTS,S/AS01), and one approved for release (R21/MM), there is still currently no malaria vaccine available for older children, adolescents or adults at risk of malaria. Mass drug administration is proposed to interrupt transmission permanently if combined with mass vaccination (Tun et al., 2017). For example, the RTS,S/AS01 vaccine was shown to be safe and immunogenic for healthy adult Thai volunteers without adverse effects when administered in combination with DP and primaquine (Von Seidlein et al., 2020). The results of these interventions support the combined use of multiple antimalarial drugs in concert with malaria vaccines to accelerate the elimination of malaria. However, further large-scale intervention-research studies are necessary to improve the application of vaccine-drug strategies, as well as innovative approaches to next generation vaccines to produce malaria vaccines with greater reach across the life span.
4. Malaria in the GCC Countries
The History of Malaria in the Region
The GCC countries are all located in the Arabian Peninsula (
Figure 1c), and for more than 30 years they have shared several common characteristics such as a dry hot climate, high income generated from an oil-based economy leading to rapid development, and modern healthcare and infrastructure (Al-Awadhi et al., 2021). In addition, these countries have large and dynamic expatriate populations feeding into their workforce. In most of the GCC countries, the expatriate population outnumber its citizens (Iqbal et al., 2021). The majority of the expatriates are from malaria-endemic developing countries within Africa (e.g. Ethiopia, Nigeria and Sudan;
Table 1) and south/southeast Asia (e.g. Afghanistan, Bangladesh, India, Nepal, Pakistan, Philippines and Sri Lanka;
Table 1) (Al-Awadhi et al., 2021; Iqbal et al., 2021). On an annual basis, the GCC countries welcome millions of new expatriates and expatriates returning from visits to their home countries, thereby increasing the risk of imported malaria entering the region (Iqbal et al., 2021). Since the 1950s, the GCC countries have increased their investments into malarial control leading to the interruption of local malaria transmission. This successfully eliminated malaria (malaria free status) in all the GCC countries, apart from Oman and Saudi Arabia (Al-Rumhi et al., 2020;
Table 1). Oman still suffers from periodic outbreaks of malaria, while in Saudi Arabia, isolated clusters of indigenous malaria prevail. Hence, the GCC countries have shifted policy toward a malaria-free Arabian Peninsula, with an emphasis on approaches that prevent reintroduction of malaria (Regional Malaria Action Plan 2016-2020: Towards a Malaria-Free Region, WHO). None of the GCC countries is among the 25 high malaria burden countries, and since 2010 there have been zero, or close to zero, annually reported malarial deaths in this region (Our World in Data, IHME, 2022; World Malaria Report 2022, WHO;
Figure 1b).
Malaria Status by Country
Bahrain: According to the WHO, Bahrain was declared malaria-free in 2012 (
Table 1). However, the Bahrain Ministry of Health reported the national eradication of malaria in 1979 (Al-Awadhi et al., 2021). There have been limited research reports dedicated to the status of malaria in Bahrain (Ismaeel et al., 2004); however, a number of articles reviewing malaria epidemiology in the Eastern Mediterranean Region have included Bahrain (Al-Awadhi et al., 2021; Iqbal et al., 2021). Furthermore, since the year 2000, the Bahrain Ministry of Health has published health statistics, which include epidemiological data on malaria cases (
Table 1). The previous reports indicated malaria cases of 1,572 for the 1992-2001 period (Ismaeel et al., 2004), and 133 in 2017 (Iqbal et al., 2021). Over the 2018-2020 period, the trend of declining malaria cases has continued with only 15 cases reported in 2020 (
Table 1). As reported previously, all malaria cases appear to be imported among expatriates originating mostly from Pakistan and India, with the predominant causative agent being
P. vivax. The last time Bahrain nationals presented with malaria was 2018 (6 out of a total of 53 malaria cases;
Table 1); however, these are most likely citizens returning from travel in malaria endemic countries.
Kuwait: The WHO declared Kuwait as malaria-free in 1979 (
Table 1). Ever since malaria was declared to be eradicated from Kuwait in 1963, no indigenous cases have been reported. However, several studies reported that despite precautions, imported malaria cases have been identified among expatriate families travelling from regions on the Indian subcontinent, Southeast Asia, and Africa where malaria is endemic (Iqbal et al., 2000; Iqbal et al., 2003; Iqbal et al., 2020; Sher and Latif, 2023). Nevertheless, the number of malaria cases has steadily decreased from 1,400 (in 1993) to less than 200 (in 2019) due to a highly effective malaria control program managed by the Ministry of Health, Kuwait (Sher and Latif, 2023;
Table 1). While the majority of early reports (1985 to 2000) of malaria cases were single infections with
P. falciparum and
P. vivax, recent studies have revealed that the majority of current malaria cases in Kuwait were caused by mixed infections of both
P. falciparum and
P. vivax parasites, and that nearly all these mixed-infection cases were among travelers from India, the largest expatriate group in Kuwait (Iqbal et al., 2020; Sher and Latif 2023).
Oman: Oman is still officially considered a malaria endemic country, and while malaria has been a major public health issue since the 1960s, national eradication programs have considerably reduced the number of indigenous cases, which are now approaching zero on an ongoing basis in recent years (
Table 1). In 1975, a malaria training program was initiated (Snow et al, 2013). This program had multiple key objectives and measures to reduce malarial cases in Oman, such as the establishment of malaria training centers, identification of vector sources and vector control using larvivorous fish,
Aphanius dispar, and continuous tracking of larval control and the widespread use of larvicides. Furthermore, during the 1973-1979 period, in specific high endemic areas, weekly chloroquine prophylaxis treatment was offered to school children. Despite these measures, Oman remained endemic with the number of malaria cases escalating to almost 33,000 in 1990 (Hassan et al., 2017). In 1991, the Omani Ministry of Health launched the National Malaria Eradication Programme (NMEP), with the aim of reducing parasite incidence to 1/10,000 population by the year 2000, by robust vector control, and early identification, management and curing of active cases (Simon et al., 2017). Over the 1994-2004 period, indigenous cases decreased from 4415 to zero, with no local transmission reported between 2004 and 2006 (Simon et al., 2017; Al Mukhaini et al., 2023). However, since 2007, due to the continuous existence of the vector, local transmission has been reported in small outbreaks (Simon et al., 2017; Al Mukhaini et al., 2023). In 2016, malaria control efforts were further increased in Oman, by enhancing malaria surveillance as a fundamental approach for better monitoring and assessment of the effectiveness of interventions (Global Technical Strategy for Malaria 2016-2030, 2021 update, WHO). Analysis of publicly available malaria infection data for the 2018-2022 period (
Table 1), revealed that the annual number of imported malaria cases was significant but steadily dropping, while the number of indigenous malaria cases was virtually zero for the last three years (Table1). Interestingly, the predominant malaria species has significantly changed over the years, somewhat mirroring the changes in the origin of imported cases. In the 1988-1999 period, the majority of malaria cases were due to infection by
P. falciparum, and was attributed to imported cases from East Africa. Over the 2000-2017 period, there was a shift to predominantly
P. vivax cases due to an increasing imported workforce from India (Al Mukhaini et al., 2023). However, since 2018
P. falciparum cases have again predominated, most likely due to an influx of immigrants from East Africa (Al Mukhaini et al., 2023). In 2022, a total of 259 cases were recorded (with only one indigenous case), mostly resulting from infection by
P. vivax (52.5%), followed by
P. falciparum (42.5%) (
Table 1).
Qatar: In 1970, Qatar eliminated malaria transmission, with the WHO declaring it malaria-free in 2012 (
Table 1). A number of studies (Al-Kuwari et al., 2009; Farag et al., 2018; Al-Rumhi et al., 2020) and recent reviews of the malaria situation in Qatar (Al-Awadhi et al 2021; Iqbal et al., 2021), have reported that all malaria cases have been travel-related, largely due to expatriates who have visited their home countries which are malaria-endemic, but also Qatari nationals who have visited malaria-endemic countries. The two predominant malaria vectors in Qatar were
An. stephensi and
An. multicolor which were eliminated by malaria elimination programs and intense vector control (Al-Kuwari et al., 2009). A study employing molecular diagnostic techniques reported that between 2013 to 2016, most cases of imported malaria were due to
P. vivax, while
P. falciparum and mixed
P. falciparum/P. vivax infections occurred far less frequently (Al-Rumhi et al., 2020).
P. vivax infections originated in the Indian subcontinent, whereas
P. falciparum infections primarily originated from Africa. The study also demonstrated a high genetic variability of
P. falciparum strains and the incidence of mutations in the PfKelch13-encoding gene, and other genes linked to drug resistance in malaria parasites. Scientists fear that there is a risk of the re-establishment of drug-resistant malaria in Qatar and other GCC nations due to the increasing introduction of multi-drug resistant
P. falciparum (Al-Rumhi et al., 2020).
Saudi Arabia: Saudi Arabia is still officially classified as endemic for malaria. In 1998, Saudi Arabia faced a major malaria outbreak, but due to intensive federal malaria control programs, the number of reported indigenous malaria cases sharply declined (Shibl et al., 2012; Coleman et al., 2014; El Hassan et al., 2015). Over the past two decades, extensive measures have been introduced to reduce indigenous malaria. Analysis of publicly available data for the 2018-2022 period (
Table 1) revealed that while the number of imported malaria cases per annum was significant, the number of indigenous malaria cases has been relatively low (Table1). Indeed, by 2021, Saudi Arabia achieved its inaugural year of reporting zero indigenous malaria cases, which was maintained through 2022 (
Table 1). The majority of reported indigenous malaria cases appear to originate from endemic countries bordering Saudi Arabia, which were not part of the GCC (El Hassan et al., 2015; Alshahrani et al., 2016; Al Zahrani et al., 2018; Alshahrani et al., 2019). A study conducted in south-western Saudi Arabia, reported that the vast majority of cases were due to
P. falciparum (98%), with very few cases caused by mixed
P. falciparum/P. vivax infection (1.85%), and no detection of
P. vivax cases (Bin Dajem, 2015). Another study investigated the type of malaria parasite in western Saudi Arabia (the Makkah region), where millions of immigrants annually visit to perform the Islamic pilgrimage, Haj, and reported that 95% of malaria cases were among non-Saudi nationals. Most of the malaria cases were caused by
P. falciparum (67%) and
P. vivax (32%), with the majority (62%) of cases originating from immigrants traveling from India, Pakistan and Nigeria (Memish et al., 2014). Antimalarial drug resistance alleles have been reported in Saudi Arabia, especially mutations associated with chloroquine resistance and anti-folate drug resistance, suggesting that improvements were needed in future antimalarial drug regimens (Soliman et al., 2018; Dafalla et al., 2020; Madkhali et al., 2020).
United Arab Emirates (UAE): Since 2007, the UAE has been declared by the WHO as malaria-free (
Table 1). However, imported malaria cases have been reported annually among working expatriates or returning travelers from endemic countries (Al-Awadhi et al., 2021;
Table 1). A study performed in a Dubai hospital between 2008 and 2010, reported 629 malaria cases, where most of the patients (90.1%) were from Pakistan or India, 7% were from sub-Saharan Africa and none of the reported cases were Emirati citizens. The study showed that most of the cases (78%) were caused by
P. vivax followed by
P. falciparum and only 2% of infections were caused by mixed
P. vivax/P. falciparum (Nilles et al., 2014; Al-Awadhi et al., 2021). The UAE Ministry of Health and Prevention has implemented national strategies to maintain a malaria-free status, including early malaria detection and treatment of imported cases, workforce training in malaria prevention and control, and enhanced vector control. These measures appear to be effective, as publicly available data for a five-year period (2018-2022;
Table 1), indicated that the number of imported malaria cases has been steadily decreasing year-on-year, while the number of indigenous malaria cases has been zero for the entire period (Table1). Interestingly, the majority of imported malaria cases are reported from the Emirates of Abu Dhabi and Dubai (
Table S1), which is perhaps not surprising given that they are major cities, channeling millions of travelers into the UAE every year through their international airports. Strategic preventative measures could be implemented, such as compulsory pre-screening of travelers on route to the UAE prior to departure from malaria endemic countries. National strategies such as these, informed by tried and tested COVID-19 infection control procedures, could potentially result in a substantial reduction in future imported malaria cases.
Impact of Mosquito Vector Insecticide Resistance in the Region
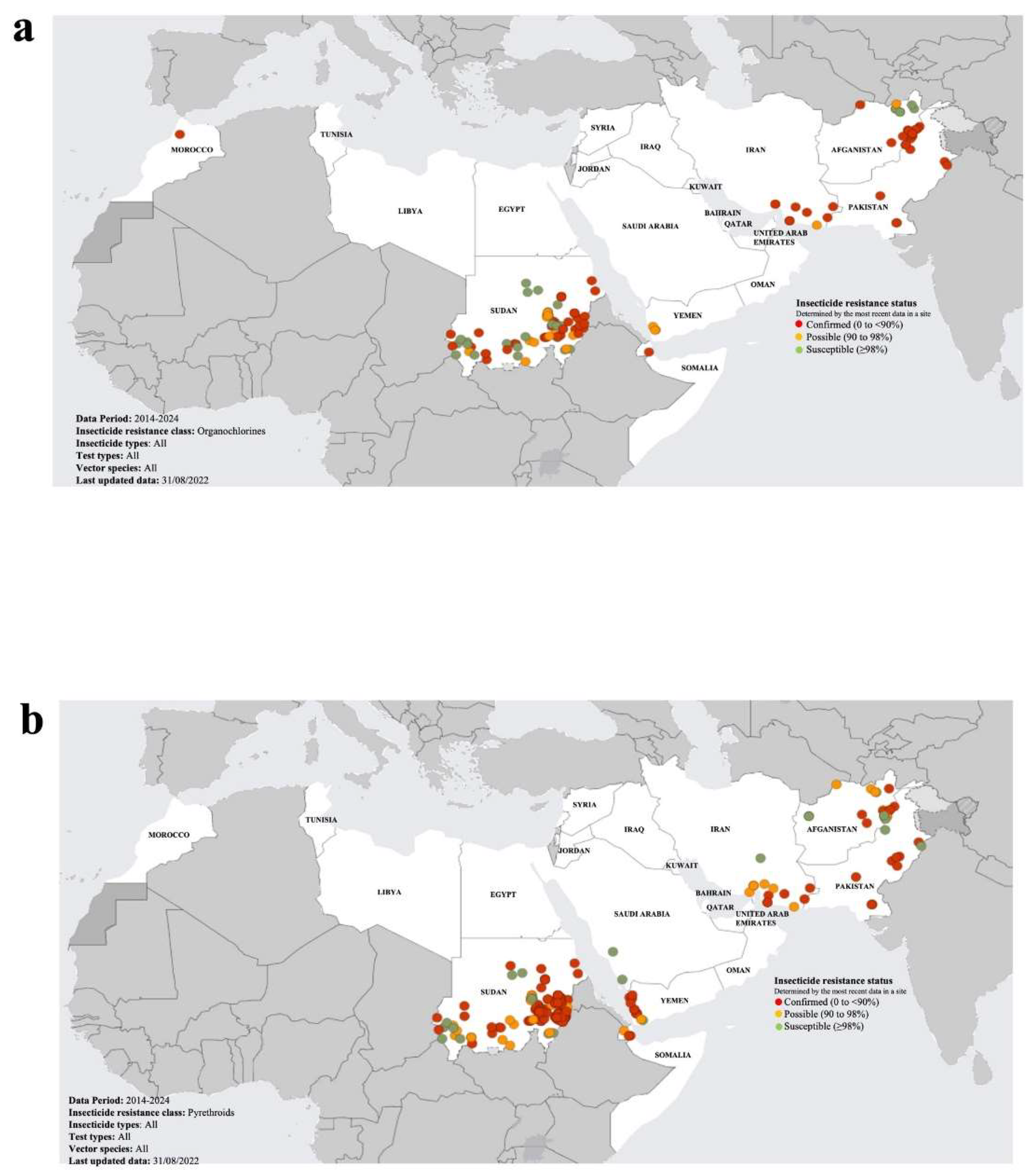
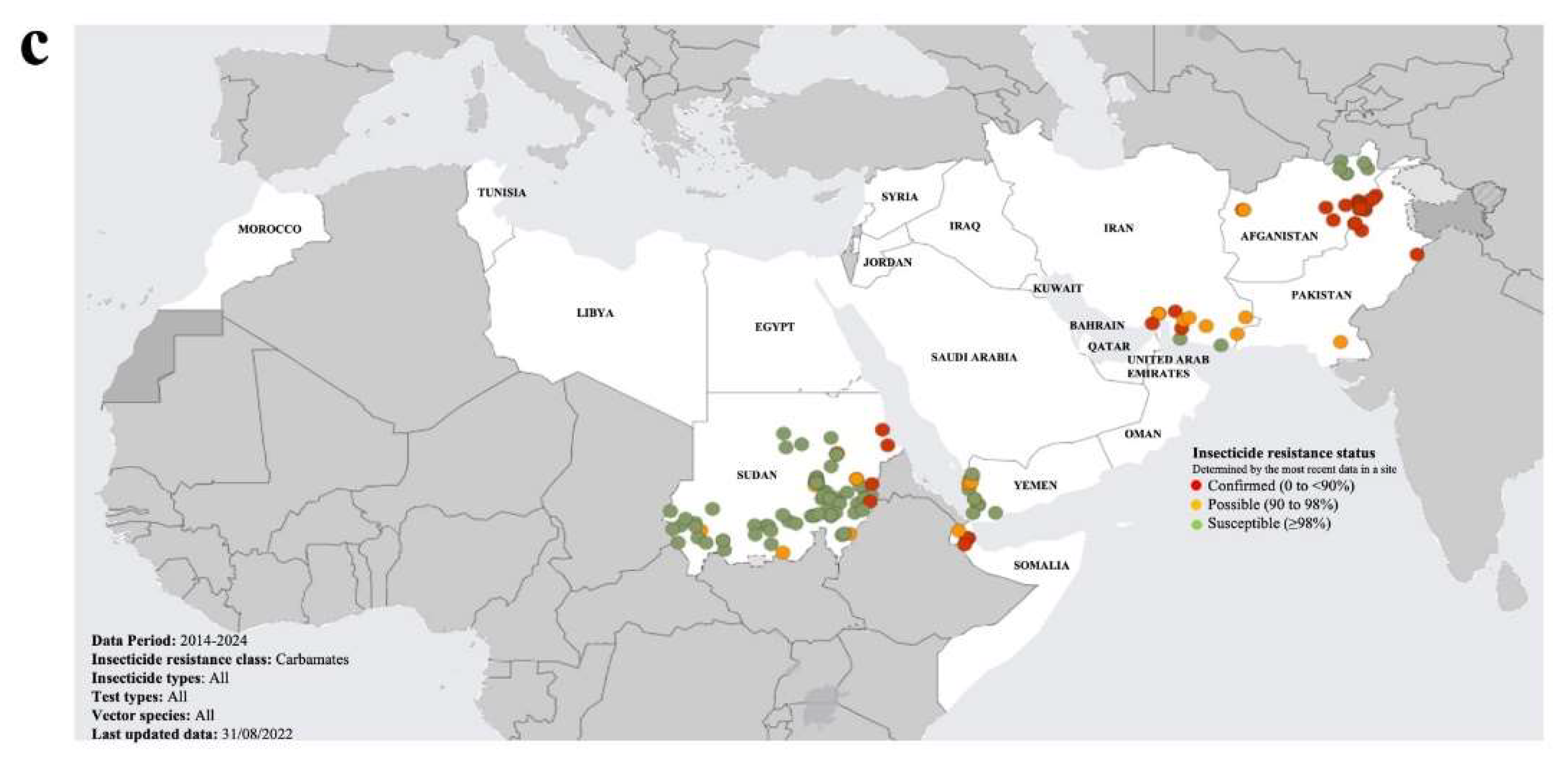
Over the last two decades, insecticide resistance in the highly invasive malaria vector,
Anopheles stephensi, has spread widely across most endemic countries, posing a substantial threat to successfully control of malaria (Riveron et al., 2018; World Malaria Report 2023, WHO). This is of major concern to the GCC countries, given they are under constant risk of the re-introduction of
Anopheles species (including species previously undetected in the region), particularly from neighbouring countries with compromised malaria vector control measures (Al-Eryani et al., 2023). The WHO have released two “Vector Alerts” (WHO Vector Alerts, 2019 and 2022), highlighting the threat of the spread of
An. stephensi into regions where it has not previously been detected, notably countries in the Horn of Africa and Yemen (Malaria Threats Map, Global Malaria Programme, World Health Organization, WHO 2024; apps.who.int/malaria/maps/threats/; accessed 20 March 2024;
Figure 2). The presence of
An. stephensi was not reported in Yemen until 2021, although it may have been resident but not detected (Al-Eryani et al., 2023). Furthermore, the vector was found to be resistance to multiple insecticides (pyrethroids, organochlorines, organophosphates and carbamates) (
Figure 2). Yemen has recently become the centre of increasingly acute conflict and upheaval, resulting in the displacement of segments of the population into make-shift urban dwellings, which has inadvertently created conditions favourable to the spread of the malaria vector (Allan et al., 2023). This has seriously compromised the capacity of Yemen to control the spread of malaria, and hence its proximity to the GCC countries poses a major threat to the spread and re-establishment of malaria in this largely malaria-free region. In particularly, the threat of the spread of insecticide resistant and invasive mosquito species across the Horn of Africa and through Yemen to the GCC countries, requires that stricter cross-border vector surveillance and control measures are established in the Arabian Peninsula.
Impact of Climate Change on Malaria Cases in the Region
The Arabian Peninsula is highly vulnerable to the negative impacts of climate change, particularly the adverse effects on the control of infectious diseases (Waha et al., 2017). The spread of malaria relies on a strong ecosystem between humans, the environment and infected mosquitoes, and the degree of malaria distribution and seasonal activity are highly sensitive to climactic factors (Costello et al., 2009). Given that mosquitoes are poikilothermic, variation in temperature, humidity and rainfall can significantly affect mosquito prevalence, successful development of the malaria parasite within the vector, and malaria transmission (Carrington et al., 2013; Shapiro et al., 2017; Mordecai et al., 2019). From a risk framework perspective, there is a high likelihood that climate change will cause an increase in malaria infections not only in endemic areas, but also current malaria-free regions. Modelling of potential future world-wide climate change scenarios indicate conditions in tropical highlands could favor the spread of malaria, socioeconomic factors not-withstanding (Caminade et al., 2014). In the next 10 years, GCC countries are predicted to suffer from extreme heat waves (Salimi and Al-Ghamdi, 2020), and severe water constraints (Bayram and Öztürk, 2021). There are data gaps regarding climate dynamics and disease populations across the GCC, reflecting an under-representation in global health forecast research for this region (Rawson et al., 2023), and highlighting the urgent need for such public health research in the near future.
5. Conclusion
While global malaria cases and deaths fell significantly during the 2000-2015 period, progress has since slowed, particularly in the EMR, which has experienced an overall increase in malaria cases and deaths. The emergence of widespread resistance in P. falciparum to first-line treatment drugs, such as artemisinin, has highlighted the need for new antimalarial drugs, and smarter approaches, including better combination therapies and mass drug administration. Furthermore, the threat of the spread of insecticide resistant and invasive mosquitoes from endemic to non-endemic regions, requires that stricter cross-border vector surveillance and control measures are established. The recent successes of the first malaria vaccine (RTS,S/AS01), and the approval of a second (R21/MM), have renewed the promise of widely applicable vaccines, which provide long-term protection, and are affordable and accessible in malaria endemic areas. In the Arabian Peninsula, while most of the GCC countries have been declared free of indigenous malaria (Bahrain, Kuwait, Qatar, and the United Arab Emirates), malaria is still endemic in two GCC countries (Saudi Arabia and Oman) and their neighbors (Yemen). The GCC region is poised in the near future to move towards malaria-free status for all member states. Preventative measures have proven highly successful in population-level control of malaria, while the targeted treatment of malaria infected patients will continue to be an important approach for preventing deaths. However, evolving environmental conditions such as climate change with increasing average temperatures, increased insecticide resistance and invasiveness of the mosquito vector, and increased resistance of the malaria parasite to first-line treatment drugs, mean that malaria should still be considered a major threat to public health in the GCC region. Therefore, further strengthening of current malaria-control frameworks, with well-developed prevention, surveillance, monitoring, diagnosis and treatment regimes, will be required before the entire GCC region can achieve malaria-free status. A region is only as safe as its “weakest link”, and hence, as the world learnt from the COVID-19 pandemic, borderless, collective intent leads to robust solutions to public health threats.
Conflict of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ORCID: Tanveer Ahmad (orcid.org/0000-0001-5910-2309); Shaikha Y Almaazmi (orcid.org/0000-0002-8145-7998); Sahar Arafa (orcid.org/0000-0001-7003-5477); Gregory L Blatch (orcid.org/0000-0003-0778-8577); Tanima Dutta (orcid.org/0000-0002-0882-2602); Jason E Gestwicki (orcid.org/0000-0002-6125-3154); Robert A Keyzers (orcid.org/0000-0002-7658-7421); Addmore Shonhai (orcid.org/0000-0003-3203-0602); Harpreet Singh (orcid.org/0000-0001-7202-3912).
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
All authors listed have made a direct, and intellectual contribution to the work and approved the manuscript for publication
Funding
This work was supported by a Higher Colleges of Technology (UAE) Interdisciplinary Research Grant (grant number 213471).
References
- Ahmed, A.; Khogali, R.; Elnour, M.A.B.; Nakao, R.; Salim, B.; Elhaj, H. Emergence of the invasive malaria vector Anopheles stephensi in Khartoum State, Central Sudan. Parasites & Vectors 2021, 14, 511. [Google Scholar]
- Al Mukhaini, S.K.; Mohammed, O.A.; Gerbers, S.; Al Awaidy, S.T. The Progress Towards National Malaria Elimination: The Experience of Oman. Oman Med J. 2023, 38, e500–e500. [Google Scholar] [CrossRef] [PubMed]
- Al Zahrani, M.H.; Omar, A.I.; Abdoon, A.M.O.; Ibrahim, A.A.; Alhogail, A.; Elmubarak, M.; Elamin, Y.E.; AlHelal, M.A.; Alshahrani, A.M.; Abdelgader, T.M.; et al. Cross-border movement, economic development and malaria elimination in the Kingdom of Saudi Arabia. BMC Med. 2018, 16, 98. [Google Scholar] [CrossRef]
- Al-Awadhi, M.; Ahmad, S.; Iqbal, J. Current Status and the Epidemiology of Malaria in the Middle East Region and Beyond. Microorganisms 2021, 9, 338. [Google Scholar] [CrossRef] [PubMed]
- Al-Eryani, S.M.; Irish, S.R.; Carter, T.E.; Lenhart, A.; Aljasari, A.; Montoya, L.F.; Awash, A.A.; Mohammed, E.; Ali, S.; Esmail, M.A.; et al. Public health impact of the spread of Anopheles stephensi in the WHO Eastern Mediterranean Region countries in Horn of Africa and Yemen: need for integrated vector surveillance and control. Malar. J. 2023, 22, 187. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuwari, M.G. (2009). Epidemiology of imported malaria in Qatar. Journal of Travel Medicine, 16, 119-122.
- Allan, R.; Weetman, D.; Sauskojus, H.; Budge, S.; Bin Hawail, T.; Baheshm, Y. Confirmation of the presence of Anopheles stephensi among internally displaced people’s camps and host communities in Aden city, Yemen. Malar. J. 2023, 22, 1. [Google Scholar] [CrossRef]
- Almaazmi, S.Y.; Kaur, R.P.; Singh, H.; Blatch, G.L. The Plasmodium falciparum exported J domain proteins fine-tune human and malarial Hsp70s: pathological exploitation of proteostasis machinery. Front. Mol. Biosci. 2023, 10, 1216192. [Google Scholar] [CrossRef] [PubMed]
- Almaazmi, S.Y.; Singh, H.; Dutta, T.; Blatch, G.L. Exported J domain proteins of the human malaria parasite. Front. Mol. Biosci. 2022, 9, 978663. [Google Scholar] [CrossRef] [PubMed]
- Al-Rumhi, A.; Al-Hashami, Z.; Al-Hamidhi, S.; Gadalla, A.; Naeem, R.; Ranford-Cartwright, L.; Pain, A.; Sultan, A.A.; Babiker, H.A. Influx of diverse, drug resistant and transmissible Plasmodium falciparum into a malaria-free setting in Qatar. BMC Infect. Dis. 2020, 20, 1–10. [Google Scholar] [CrossRef]
- Alshahrani, A.M.; Abdelgader, T.M.; Saeed, I.; Al-Akhshami, A.; Al-Ghamdi, M.; Al-Zahrani, M.H.; El Hassan, I.; Kyalo, D.; Snow, R.W. The changing malaria landscape in Aseer region, Kingdom of Saudi Arabia: 2000–2015. Malar. J. 2016, 15, 538. [Google Scholar] [CrossRef]
- Alshahrani, A.M.; Abdelgader, T.M.; Saeed, I.; Al-Akhshami, A.; Al-Ghamdi, M.; Al-Zahrani, M.H.; Snow, R.W. Risk associated with malaria infection in Tihama Qahtan, Aseer Region, Kingdom of Saudi Arabia: 2006–2007. Malaria Control & Elimination 2019, 5, 144. [Google Scholar]
- Balikagala, B.; Fukuda, N.; Ikeda, M.; Katuro, O.T.; Tachibana, S.-I.; Yamauchi, M.; Opio, W.; Emoto, S.; Anywar, D.A.; Kimura, E.; et al. Evidence of Artemisinin-Resistant Malaria in Africa. New Engl. J. Med. 2021, 385, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Banumathy, G.; Singh, V.; Pavithra, S.R.; Tatu, U. Heat Shock Protein 90 Function Is Essential for Plasmodium falciparum Growth in Human Erythrocytes. J. Biol. Chem. 2003, 278, 18336–18345. [Google Scholar] [CrossRef] [PubMed]
- Barnes, K.G.; Irving, H.; Chiumia, M.; Mzilahowa, T.; Coleman, M.; Hemingway, J.; Wondji, C.S. Restriction to gene flow is associated with changes in the molecular basis of pyrethroid resistance in the malaria vector Anopheles funestus. Proc. Natl. Acad. Sci. 2017, 114, 286–291. [Google Scholar] [CrossRef]
- Barth, J.; Schach, T.; Przyborski, J.M. HSP70 and their co-chaperones in the human malaria parasite P. falciparum and their potential as drug targets. Front. Mol. Biosci. 2022, 9, 968248. [Google Scholar] [CrossRef]
- Batinovic, S.; McHugh, E.; Chisholm, S.A.; Matthews, K.; Liu, B.; Dumont, L.; Charnaud, S.C.; Schneider, M.P.; Gilson, P.R.; de Koning-Ward, T.F.; et al. An exported protein-interacting complex involved in the trafficking of virulence determinants in Plasmodium-infected erythrocytes. Nat. Commun. 2017, 8, 16044. [Google Scholar] [CrossRef] [PubMed]
- Bayih, A.G.; Folefoc, A.; Mohon, A.N.; Eagon, S.; Anderson, M.; Pillai, D.R. In vitro and in vivo anti-malarial activity of novel harmine-analog heat shock protein 90 inhibitors: a possible partner for artemisinin. Malar. J. 2016, 15, 579. [Google Scholar] [CrossRef]
- Bayram, H.; Öztürk, A.B. (2021). Global climate change, desertification, and its consequences in Turkey and the Middle East. In: Climate Change and Global Public Health, pp. 445–458. Humana Press, New York, NY.
- Beeson, J.G.; Kurtovic, L.; Valim, C.; Asante, K.P.; Boyle, M.J.; Mathanga, D.; Dobano, C.; Moncunill, G. The RTS,S malaria vaccine: Current impact and foundation for the future. Sci. Transl. Med. 2022, 14, eabo6646. [Google Scholar] [CrossRef] [PubMed]
- Bin Dajem, S.M. Molecular investigation of mixed malaria infections in southwest Saudi Arabia. Saudi Medical Journal 2015, 36, 248–251. [Google Scholar] [CrossRef]
- Blatch, G.L. Plasmodium falciparum Molecular Chaperones: Guardians of the Malaria Parasite Proteome and Renovators of the Host Proteome. Front. Cell Dev. Biol. 2022, 10, 921739. [Google Scholar] [CrossRef]
- Botha, M.; Chiang, A.N.; Needham, P.G.; Stephens, L.L.; Hoppe, H.C.; Külzer, S.; Przyborski, J.M.; Lingelbach, K.; Wipf, P.; Brodsky, J.L.; et al. Plasmodium falciparum encodes a single cytosolic type I Hsp40 that functionally interacts with Hsp70 and is upregulated by heat shock. Cell Stress Chaperon- 2011, 16, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Bouwman, H.; van den Berg, H.; Kylin, H. DDT and Malaria Prevention: Addressing the Paradox. Environ. Health Perspect. 2011, 119, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Caminade, C.; Kovats, S.; Rocklov, J.; Tompkins, A.M.; Morse, A.P.; Colón-González, F.J.; Stenlund, H.; Martens, P.; Lloyd, S.J. Impact of climate change on global malaria distribution. Proceedings of the National Academy of Sciences of the United States of America 2014, 111, 3286–3291. [Google Scholar] [CrossRef] [PubMed]
- Carrington, L.B.; Armijos, M.V.; Lambrechts, L.; Barker, C.M.; Scott, T.W. Effects of Fluctuating Daily Temperatures at Critical Thermal Extremes on Aedes aegypti Life-History Traits. PLOS ONE 2013, 8, e58824. [Google Scholar] [CrossRef] [PubMed]
- Casida, J.E.; Ueda, K.; Gaughan, L.C.; Jao, L.T.; Soderlund, D.M. Structure-biodegradability relationships in pyrethroid insecticides. Arch. Environ. Contam. Toxicol. 1975, 3, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Murillo-Solano, C.; Kirkpatrick, M.G.; Antoshchenko, T.; Park, H.-W.; Pizarro, J.C. Repurposing drugs to target the malaria parasite unfolding protein response. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Cockburn, I.L.; Boshoff, A.; Pesce, E.-R.; Blatch, G.L. Selective modulation of plasmodial Hsp70s by small molecules with antimalarial activity. Biol. Chem. 2014, 395, 1353–1362. [Google Scholar] [CrossRef]
- Cockburn, I.L.; Pesce, E.-R.; Pryzborski, J.M.; Davies-Coleman, M.T.; Clark, P.G.; Keyzers, R.A.; Stephens, L.L.; Blatch, G.L. Screening for small molecule modulators of Hsp70 chaperone activity using protein aggregation suppression assays: inhibition of the plasmodial chaperone PfHsp70-1. Biol. Chem. 2011, 392, 431–8. [Google Scholar] [CrossRef]
- Coleman, M.; Al-Zahrani, M.H.; Coleman, M.; Hemingway, J.; Omar, A.; Stanton, M.C.; Thomsen, E.K.; Alsheikh, A.A.; Alhakeem, R.F.; McCall, P.J.; et al. () A country on the verge of malaria elimination–the Kingdom of Saudi Arabia. PLoS ONE 2014, 9, e105980:1–e105980:8. [Google Scholar] [CrossRef]
- Corrêa, A.P.S.A.; Galardo, A.K.R.; Lima, L.A.; Câmara, D.C.P.; Müller, J.N.; Barroso, J.F.S.; Lapouble, O.M.M.; Rodovalho, C.M.; Ribeiro, K.A.N.; Lima, J.B.P. Efficacy of insecticides used in indoor residual spraying for malaria control: an experimental trial on various surfaces in a “test house”. Malar. J. 2019, 18, 345. [Google Scholar] [CrossRef]
- Cortés, G.T.; Wiser, M.F.; Gómez-Alegría, C.J. Identification of Plasmodium falciparum HSP70-2 as a resident of the Plasmodium export compartment. Heliyon 2020, 6, e04037. [Google Scholar] [CrossRef] [PubMed]
- Costello, A.; Abbas, M.; Allen, A.; Ball, S.; Bell, S.; Bellamy, R.; Friel, S.; Groce, N.; Johnson, A.; Kett, M.; Lee, M. Managing the health effects of climate change: lancet and University College London Institute for Global Health Commission. The Lancet 2009, 373, 1693–1733. [Google Scholar] [CrossRef] [PubMed]
- Cycoń, M.; Piotrowska-Seget, Z. Pyrethroid-Degrading Microorganisms and Their Potential for the Bioremediation of Contaminated Soils: A Review. Front. Microbiol. 2016, 7, 1463. [Google Scholar] [CrossRef]
- Dabira, E.D.; Soumare, H.M.; Conteh, B.; Ceesay, F.; O Ndiath, M.; Bradley, J.; Mohammed, N.; Kandeh, B.; Smit, M.R.; Slater, H.; et al. Mass drug administration of ivermectin and dihydroartemisinin–piperaquine against malaria in settings with high coverage of standard control interventions: a cluster-randomised controlled trial in The Gambia. Lancet Infect. Dis. 2021, 22, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Dafalla, O.M.; Alzahrani, M.; Sahli, A.; Al Helal, M.A.; Alhazmi, M.M.; Noureldin, E.M.; Mohamed, W.S.; Hamid, T.B.; Abdelhaleem, A.A.; Hobani, Y.A.; et al. Kelch 13-propeller polymorphisms in Plasmodium falciparum from Jazan region, southwest Saudi Arabia. Malar. J. 2020, 19, 1–9. [Google Scholar] [CrossRef]
- Daniyan, M.O.; Blatch, G.L. Plasmodial Hsp40s: New Avenues for Antimalarial Drug Discovery. Curr. Pharm. Des. 2017, 23, 4555–4570. [Google Scholar] [CrossRef] [PubMed]
- Daniyan, M.O.; Boshoff, A.; Prinsloo, E.; Pesce, E.-R.; Blatch, G.L. The Malarial Exported PFA0660w Is an Hsp40 Co-Chaperone of PfHsp70-x. PLOS ONE 2016, 11, e0148517. [Google Scholar] [CrossRef] [PubMed]
- Datoo, M.S.; Natama, H.M.; Somé, A.; Bellamy, D.; Traoré, O.; Rouamba, T.; Tahita, M.C.; Ido, N.F.A.; Yameogo, P.; Valia, D.; et al. Efficacy and immunogenicity of R21/Matrix-M vaccine against clinical malaria after 2 years' follow-up in children in Burkina Faso: a phase 1/2b randomised controlled trial. Lancet Infectious Diseases 2022, 22, 1728–1736. [Google Scholar] [CrossRef] [PubMed]
- Day, J.; Passecker, A.; Beck, H.P.; Vakonakis, I. The Plasmodium falciparum Hsp70-x chaperone assists the heat stress response of the malaria parasite. FASEB Journal 2019, 33, 14611. [Google Scholar] [CrossRef]
- Dutta, T.; Pesce, E.R.; Maier, A.G.; Blatch, G.L. Role of the J Domain Protein Family in the Survival and Pathogenesis of Plasmodium falciparum. Advances in Experimental Medicine and Biology 2021, 1340, 97–123. [Google Scholar]
- Dutta, T.; Singh, H.; Gestwicki, J.E.; Blatch, G.L. Exported plasmodial J domain protein, PFE0055c, and PfHsp70-x form a specific co-chaperone-chaperone partnership. Cell Stress Chaperon- 2020, 26, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Edkins, A.L.; Boshoff, A. (2021). General Structural and Functional Features of Molecular Chaperones. Advances in Experimental Medicine and Biology, 1340, 11–73.
- Eisele, T.P. Mass drug administration can be a valuable addition to the malaria elimination toolbox. Malar. J. 2019, 18, 281. [Google Scholar] [CrossRef] [PubMed]
- El Hassan, I.M.; Sahly, A.; Alzahrani, M.H.; Alhakeem, R.F.; Alhelal, M.; Alhogail, A.; Alsheikh, A.A.; Assiri, A.M.; ElGamri, T.B.; Faragalla, I.A.; et al. () Progress toward malaria elimination in Jazan Province, Kingdom of Saudi Arabia: 2000–2014. Malaria Journal 2015, 14, 444:1–444:10. [Google Scholar] [CrossRef] [PubMed]
- Farag, E.; Bansal, D.; Chehab, M.A.H.; Al-Dahshan, A.; Bala, M.; Ganesan, N.; Al Abdulla, Y.A.; Al Thani, M.; Sultan, A.A.; Al-Romaihi, H. Epidemiology of Malaria in the State of Qatar, 2008-2015. Mediterranean Journal of Hematology and Infectious Diseases 2018, 10, e2018050. [Google Scholar] [CrossRef] [PubMed]
- Feleke, S.M.; Reichert, E.N.; Mohammed, H.; Brhane, B.G.; Mekete, K.; Mamo, H.; Petros, B.; Solomon, H.; Abate, E.; Hennelly, C.; et al. Plasmodium falciparum is evolving to escape malaria rapid diagnostic tests in Ethiopia. Nat. Microbiol. 2021, 6, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Fraser, M.; Miller, J.M.; Silumbe, K.; Hainsworth, M.; Mudenda, M.; Hamainza, B.; Moonga, H.; Kawesha, E.C.; Mercer, L.D.; Bennett, A.; et al. Evaluating the Impact of Programmatic Mass Drug Administration for Malaria in Zambia Using Routine Incidence Data. J. Infect. Dis. 2020, 225, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Hancock, P.A.; Hendriks, C.J.M.; Tangena, J.-A.; Gibson, H.; Hemingway, J.; Coleman, M.; Gething, P.W.; Cameron, E.; Bhatt, S.; Moyes, C.L. Mapping trends in insecticide resistance phenotypes in African malaria vectors. PLOS Biol. 2020, 18, e3000633. [Google Scholar] [CrossRef] [PubMed]
- Hassan, K.S. World Malaria Day: Our story with malaria in Oman. Sultan Qaboos University Medical Journal 2017, 17, e133–e134. [Google Scholar] [CrossRef] [PubMed]
- IHME. Our World in Data (www.ourworldindata.org/malaria; accessed 17 July 2023). Institute of Health Metrics and Evaluation (IHME).2022.
- Iqbal, J.; Ahmad, S.; Sher, A.; Al-Awadhi, M. Current Epidemiological Characteristics of Imported Malaria, Vector Control Status and Malaria Elimination Prospects in the Gulf Cooperation Council (GCC) Countries. Microorganisms 2021, 9, 1431. [Google Scholar] [CrossRef]
- Iqbal, J.; Al-Awadhi, M.; Ahmad, S. Decreasing trend of imported malaria cases but increasing influx of mixed P. falciparum and P. vivax infections in malaria-free Kuwait. PLOS ONE 2020, 15, e0243617. [Google Scholar] [CrossRef]
- Iqbal, J.; Al-Ali, F.; Sher, A.; Hira, P.R. Imported malaria in Kuwait (1985-2000). J. Travel Med. 2003, 10, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Sher, A.; Hira, P.R.; Al-Anezi, A.A. (2000) Risk of introduction of drug-resistant malaria in a non- endemic country, Kuwait: A real threat? Med Princ Pract, 9, 125–130.
- Ismaeel, A.Y.; Senok, A.C.; Al-Khaja, K.A.J.; Botta, G.A. Status of malaria in the Kingdom of Bahrain: a 10-year review. J. Travel Med. 2004, 11, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Jee, H. Size dependent classification of heat shock proteins: a mini-review. J. Exerc. Rehabilitation 2016, 12, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Killeen, G.F.; Masalu, J.P.; Chinula, D.; Fotakis, E.A.; Kavishe, D.R.; Malone, D.; Okumu, F. Control of Malaria Vector Mosquitoes by Insecticide-Treated Combinations of Window Screens and Eave Baffles. Emerg. Infect. Dis. 2017, 23, 782–789. [Google Scholar] [CrossRef]
- Kouamo, M.F.M.; Ibrahim, S.S.; Hearn, J.; Riveron, J.M.; Kusimo, M.; Tchouakui, M.; Ebai, T.; Tchapga, W.; Wondji, M.J.; Irving, H.; et al. Genome-Wide Transcriptional Analysis and Functional Validation Linked a Cluster of Epsilon Glutathione S-Transferases with Insecticide Resistance in the Major Malaria Vector Anopheles funestus across Africa. Genes 2021, 12, 561. [Google Scholar] [CrossRef] [PubMed]
- Laurens, M.B. RTS,S/AS01 vaccine (Mosquirix™): an overview. Human Vaccines & Immunotherapeutics 2020, 16, 480–489. [Google Scholar]
- Luo, A.-P.; Giannangelo, C.; Siddiqui, G.; Creek, D.J. Promising antimalarial hits from phenotypic screens: a review of recently-described multi-stage actives and their modes of action. Front. Cell. Infect. Microbiol. 2023, 13, 1308193. [Google Scholar] [CrossRef]
- Madkhali, A.M.; Al-Mekhlafi, H.M.; Atroosh, W.M.; Ghzwani, A.H.; Zain, K.A.; Abdulhaq, A.A.; Ghailan, K.Y.; Anwar, A.A.; Eisa, Z.M. Increased prevalence of pfdhfr and pfdhps mutations associated with sulfadoxine-pyrimethamine resistance in Plasmodium falciparum isolates from Jazan Region, Southwestern Saudi Arabia: important implications for malaria treatment policy. Malaria Journal 2020, 19, 446. [Google Scholar] [CrossRef]
- Memish, Z.A.; Alzahrani, M.; Alhakeem, R.F.; Bamgboye, E.A.; Smadia, H.N. Toward malaria eradication in Saudi Arabia: evidence from 4-year surveillance in Makkah. Ann. Saudi Med. 2014, 34, 153–158. [Google Scholar] [CrossRef]
- Mok, S.; Ashley, E.A.; Ferreira, P.E.; Zhu, L.; Lin, Z.; Yeo, T.; Chotivanich, K.; Imwong, M.; Pukrittayakamee, S.; Dhorda, M.; et al. Population transcriptomics of human malaria parasites reveals the mechanism of artemisinin resistance. Science 2015, 347, 431–435. [Google Scholar] [CrossRef]
- Mok, S.; Stokes, B.H.; Gnädig, N.F.; Ross, L.S.; Yeo, T.; Amaratunga, C.; Allman, E.; Solyakov, L.; Bottrill, A.R.; Tripathi, J.; et al. Artemisinin-resistant K13 mutations rewire Plasmodium falciparum’s intra-erythrocytic metabolic program to enhance survival. Nat. Commun. 2022, 12, 1–15. [Google Scholar] [CrossRef]
- Mordecai, E.A.; Caldwell, J.M.; Grossman, M.K.; Lippi, C.A.; Johnson, L.R.; Neira, M.; Rohr, J.R.; Ryan, S.J.; Savage, V.; Shocket, M.S.; Sippy, R. () Thermal biology of mosquito-borne disease. Ecology Letters 2019, 22, 1690–708. [Google Scholar] [CrossRef] [PubMed]
- Mrozek, A.; Antoshchenko, T.; Chen, Y.; Zepeda-Velázquez, C.; Smil, D.; Kumar, N.; Lu, H.; Park, H.-W. A non-traditional crystal-based compound screening method targeting the ATP binding site of Plasmodium falciparum GRP78 for identification of novel nucleoside analogues. Front. Mol. Biosci. 2022, 9, 956095. [Google Scholar] [CrossRef] [PubMed]
- Neafsey, D.E.; Juraska, M.; Bedford, T.; Benkeser, D.; Valim, C.; Griggs, A.; Lievens, M.; Abdulla, S.; Adjei, S.; Agbenyega, T.; et al. Genetic Diversity and Protective Efficacy of the RTS,S/AS01 Malaria Vaccine. New Engl. J. Med. 2015, 373, 2025–2037. [Google Scholar] [CrossRef]
- Nilles, E.J.; Alosert, M.; Mohtasham, M.A.; Saif, M.; Sulaiman, L.; Seliem, R.M.; Kotlyar, S.; Dziura, J.D.; Al-Najjar, F.J. Epidemiological and Clinical Characteristics of Imported Malaria in the United Arab Emirates. J. Travel Med. 2014, 21, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Paloque, L.; Coppée, R.; Stokes, B.H.; Gnädig, N.F.; Niaré, K.; Augereau, J.-M.; Fidock, D.A.; Clain, J.; Benoit-Vical, F. Mutation in the Plasmodium falciparum BTB/POZ Domain of K13 Protein Confers Artemisinin Resistance. Antimicrob. Agents Chemother. 2022, 66, e0132021. [Google Scholar] [CrossRef] [PubMed]
- Pennetier, C.; Costantini, C.; Corbel, V.; Licciardi, S.; Dabiré, R.K.; Lapied, B.; Chandre, F.; Hougard, J.-M. Mixture for Controlling Insecticide-Resistant Malaria Vectors. Emerg. Infect. Dis. 2008, 14, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Plowe, C.V. Malaria chemoprevention and drug resistance: a review of the literature and policy implications. Malar. J. 2022, 21, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Posfai, D.; Eubanks, A.L.; Keim, A.I.; Lu, K.-Y.; Wang, G.Z.; Hughes, P.F.; Kato, N.; Haystead, T.A.; Derbyshire, E.R. Identification of Hsp90 Inhibitors with Anti-Plasmodium Activity. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Przyborski, J.M.; Diehl, M.; Blatch, G.L. Plasmodial HSP70s are functionally adapted to the malaria parasite life cycle. Front. Mol. Biosci. 2015, 2, 34. [Google Scholar] [CrossRef]
- Rawson, T.; Doohan, P.; Hauck, K.; Murray, K.A.; Ferguson, N. Climate change and communicable diseases in the Gulf Cooperation Council (GCC) countries. Epidemics 2023, 42, 100667. [Google Scholar] [CrossRef] [PubMed]
- Riveron, J.M.; Tchouakui, M.; Mugenzi, L.; Menze, B.D.; Chiang, M.C.; Wondji, C.S. Insecticide resistance in malaria vectors: an update at a global scale. In Towards malaria elimination-a leap forward. IntechOpen. 2018. [CrossRef]
- RTS,S Clinical Trials Partnership Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 2015, 386(9988), 31–45. [CrossRef] [PubMed]
- Salimi, M.; Al-Ghamdi, S.G. Climate change impacts on critical urban infrastructure and urban resiliency strategies for the Middle East. Sustain. Cities Soc. 2019, 54, 101948. [Google Scholar] [CrossRef]
- Seraphim, T.V.; Chakafana, G.; Shonhai, A.; Houry, W.A. Plasmodium falciparum R2TP complex: driver of parasite Hsp90 function. Biophys. Rev. 2019, 11, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Shahinas, D.; Folefoc, A.; Taldone, T.; Chiosis, G.; Crandall, I.; Pillai, D.R. A Purine Analog Synergizes with Chloroquine (CQ) by Targeting Plasmodium falciparum Hsp90 (PfHsp90). PLOS ONE 2013, 8, e75446. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, L.L.M.; Whitehead, S.A.; Thomas, M.B. Quantifying the effects of temperature on mosquito and parasite traits that determine the transmission potential of human malaria. PLOS Biol. 2017, 15, e2003489–e2003489. [Google Scholar] [CrossRef] [PubMed]
- Sher, A.; Latif, S.A. (2023) Increasing trend of mixed infections of Plasmodium falciparum and Plasmodium vivax among imported malaria cases in Kuwait, a non-endemic country. Open Access Library Journal, 10, e9733.
- Shibeshi, M.A.; Kifle, Z.D.; Atnafie, S.A. Antimalarial Drug Resistance and Novel Targets for Antimalarial Drug Discovery. Infect. Drug Resist. 2020, ume 13, 4047–4060. [Google Scholar] [CrossRef]
- Shibl, A.; Senok, A.; Memish, Z. Infectious diseases in the Arabian Peninsula and Egypt. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases 2012, 18, 1068–1080.
- Shonhai, A.; Maier, A.G.; Przyborski, J.M.; Blatch, G.L. Intracellular Protozoan Parasites of Humans: The Role of Molecular Chaperones in Development and Pathogenesis. Protein Pept. Lett. 2011, 18, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.J.; Bhardwaj, J.; Saxena, J.; Jahan, S.; Snoussi, M.; Bardakci, F.; Badraoui, R.; Adnan, M. A Critical Review on Human Malaria and Schistosomiasis Vaccines: Current State, Recent Advancements, and Developments. Vaccines 2023, 11, 792. [Google Scholar] [CrossRef]
- Siddiqui, F.A.; Liang, X.; Cui, L. Plasmodium falciparum resistance to ACTs: Emergence, mechanisms, and outlook. Int. J. Parasitol. Drugs Drug Resist. 2021, 16, 102–118. [Google Scholar] [CrossRef]
- Simon, B.; Sow, F.; Al Mukhaini, S.K.; Al-Abri, S.; Ali, O.A.; Bonnot, G.; Bienvenu, A.-L.; Petersen, E.; Picot, S. An outbreak of locally acquired Plasmodium vivax malaria among migrant workers in Oman. Parasite 2017, 24, 25–25. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Almaazmi, S.Y.; Dutta, T.; Keyzers, R.A.; Blatch, G.L. In silico identification of modulators of J domain protein-Hsp70 interactions in Plasmodium falciparum: a drug repurposing strategy against malaria. Front. Mol. Biosci. 2023, 10, 1158912. [Google Scholar] [CrossRef] [PubMed]
- Snow, R.W.; Amratia, P.; Zamani, G.; Mundia, C.W.; Noor, A.M.; Memish, Z.A.; Al Zahrani, M.H.; Al Jasari, A.; Fikri, M.; Atta, H. The malaria transition on the Arabian Peninsula: progress toward a malaria-free region between 1960-2010. Advances in Parasitology 2013, 82, 205–251. [Google Scholar] [PubMed]
- Soliman, R.H.; Garcia-Aranda, P.; Elzagawy, S.M.; Hussein, B.E.; Mayah, W.W.; Martin Ramirez, A.; Ta-Tang, T.H.; Rubio, J.M. Imported and autochthonous malaria in West Saudi Arabia: results from a reference hospital. Malaria Journal 2018, 17, 286. [Google Scholar] [CrossRef] [PubMed]
- Stofberg, M.L.; Caillet, C.; de Villiers, M.; Zininga, T. Inhibitors of the Plasmodium falciparum Hsp90 towards selective antimalarial drug design: The past, present and future. Cells 2021, 10, 2849. [Google Scholar] [CrossRef] [PubMed]
- Tun, S.T.T.; von Seidlein, L.; Pongvongsa, T.; Mayxay, M.; Saralamba, S.; Kyaw, S.S.; Chanthavilay, P.; Celhay, O.; Nguyen, T.D.; Tran, T.N.-A.; et al. Towards malaria elimination in Savannakhet, Lao PDR: mathematical modelling driven strategy design. Malar. J. 2017, 16, 483–483. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, P. The 2023 WHO World malaria report. Lancet Microbe 2024, 5, e214–e214. [Google Scholar] [CrossRef] [PubMed]
- von Seidlein, L.; Hanboonkunupakarn, B.; Jittamala, P.; Pongsuwan, P.; Chotivanich, K.; Tarning, J.; Hoglund, R.M.; Winterberg, M.; Mukaka, M.; Peerawaranun, P.; et al. Combining antimalarial drugs and vaccine for malaria elimination campaigns: a randomized safety and immunogenicity trial of RTS,S/AS01 administered with dihydroartemisinin, piperaquine, and primaquine in healthy Thai adult volunteers. Hum. Vaccines Immunother. 2020, 16, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Waha, K.; Krummenauer, L.; Adams, S.; Aich, V.; Baarsch, F.; Coumou, D.; Fader, M.; Hoff, H.; Jobbins, G.; Marcus, R.; et al. Climate change impacts in the Middle East and Northern Africa (MENA) region and their implications for vulnerable population groups. Reg. Environ. Chang. 2017, 17, 1623–1638. [Google Scholar] [CrossRef]
- Wang, T.; Mäser, P.; Picard, D. Inhibition of Plasmodium falciparum Hsp90 Contributes to the Antimalarial Activities of Aminoalcohol-carbazoles. J. Med. Chem. 2016, 59, 6344–6352. [Google Scholar] [CrossRef]
- West, P.A.; Protopopoff, N.; Wright, A.; Kivaju, Z.; Tigererwa, R.; Mosha, F.W.; Kisinza, W.; Rowland, M.; Kleinschmidt, I. Indoor Residual Spraying in Combination with Insecticide-Treated Nets Compared to Insecticide-Treated Nets Alone for Protection against Malaria: A Cluster Randomised Trial in Tanzania. PLOS Med. 2014, 11, e1001630. [Google Scholar] [CrossRef] [PubMed]
- WHO (2017) Regional Malaria Action Plan 2016-2020: Towards a Malaria-Free Region. World Health Organization, Regional Office for the Eastern Mediterranean (applications.emro.who.int/docs/EMROPUB_2017_EN_19546.pdf; accessed 17 July 2023).
- WHO (2019) Vector alert: Anopheles stephensi invasion and spread: Horn of Africa, the Republic of the Sudan and surrounding geographical areas, and Sri Lanka: information note. World Health Organization, Geneva (iris.who.int/handle/10665/326595).
- WHO (2021) Global Technical Strategy for Malaria 2016–2030, 2021 Update. World Health Organization, Geneva (www.who.int/publications/i/item/9789240031357; accessed 17 July 2023).
- WHO (2022) Guidelines for Malaria. World Health Organization, Geneva (reliefweb.int/report/world/who-guidelines-malaria-3-june-2022; accessed 17 July 2023).
- WHO (2022) Vector alert: Anopheles stephensi invasion and spread in Africa and Sri Lanka: information note. World Health Organization, Geneva (www.who.int/publications/i/item/9789240067714).
- WHO (2022) World Malaria Report. World Health Organization, Geneva (www.who.int/teams/global-malaria-programme/reports; accessed 17 July 2023).
- WHO (2023) World Malaria Report. World Health Organization, Geneva (www.who.int/teams/global-malaria-programme/reports; accessed 20 March 2024).
- WHO (2023) Countries and Territories Certified Malaria-Free by WHO. World Health Organization, Geneva (www.who.int/teams/global-malaria-programme/elimination/countries-and-territories-certified-malaria-free-by-who; accessed 17 July 2023).
- Yoon, S.-J.; Kim, Y.-E.; Kim, E.-J. Why They Are Different: Based on the Burden of Disease Research of WHO and Institute for Health Metrics and Evaluation. BioMed Res. Int. 2018, 2018, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Zavala, F. RTS,S: The first malaria vaccine. Journal of Clinical Investigation 2022, 132, e156588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, C.; Otto, T.D.; Oberstaller, J.; Liao, X.; Adapa, S.R.; Udenze, K.; Bronner, I.F.; Casandra, D.; Mayho, M.; et al. Uncovering the essential genes of the human malaria parasite Plasmodium falciparum by saturation mutagenesis. Science 2018, 360, eaap7847. [Google Scholar] [CrossRef] [PubMed]
- Zininga, T.; Pooe, O.J.; Makhado, P.B.; Ramatsui, L.; Prinsloo, E.; Achilonu, I.; Dirr, H.; Shonhai, A. Polymyxin B inhibits the chaperone activity of Plasmodium falciparum Hsp70. Cell Stress Chaperon- 2017, 22, 707–715. [Google Scholar] [CrossRef]
- Zininga, T.; Ramatsui, L.; Makhado, P.B.; Makumire, S.; Achilinou, I.; Hoppe, H.; Dirr, H.; Shonhai, A. (−)-Epigallocatechin-3-Gallate Inhibits the Chaperone Activity of Plasmodium falciparum Hsp70 Chaperones and Abrogates Their Association with Functional Partners. Molecules 2017, 22, 2139. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).