Submitted:
26 July 2024
Posted:
26 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Chitosan-Based Gels Preparation
2.2.2. Supercritical Gel Drying
2.2.3. Characterizations
2.2.4. Adsorption Experiments
3. Results
3.1. Aerogel Morphology
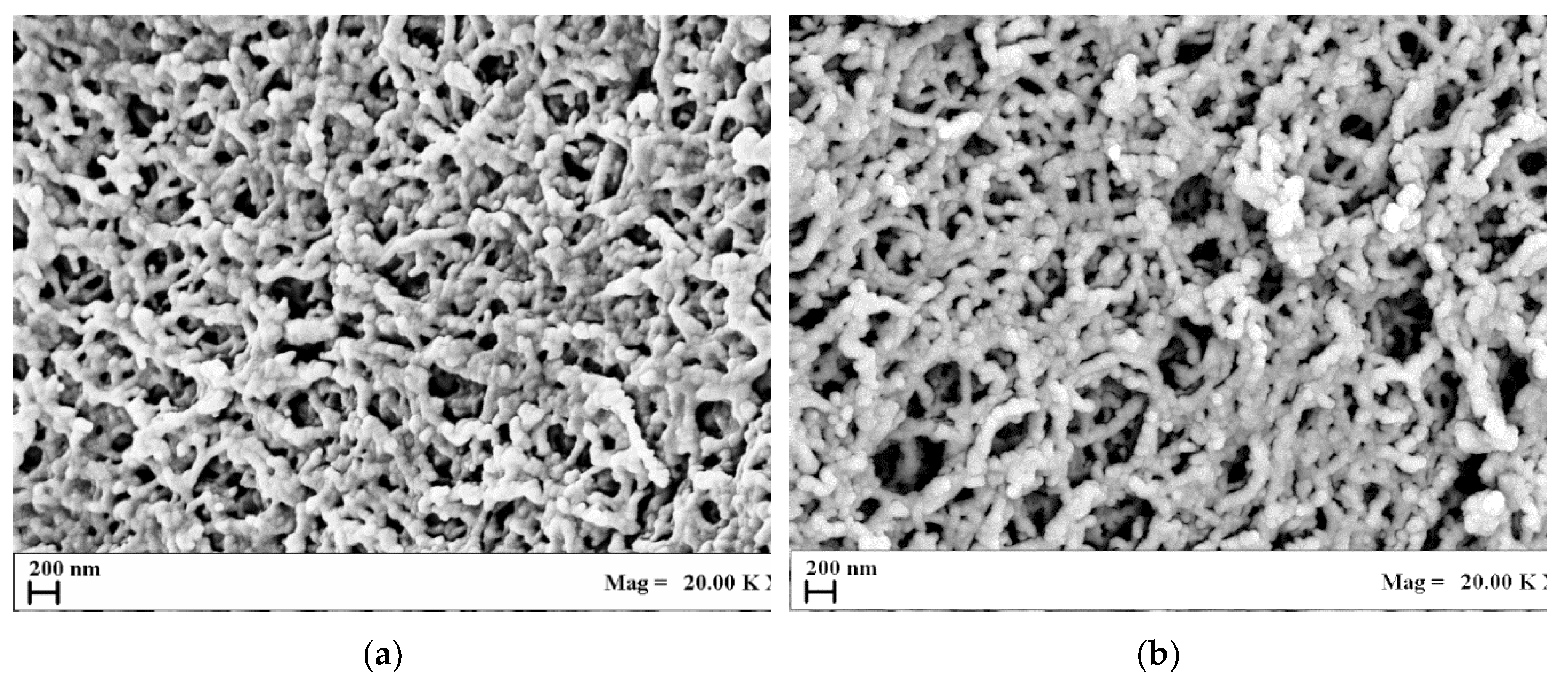
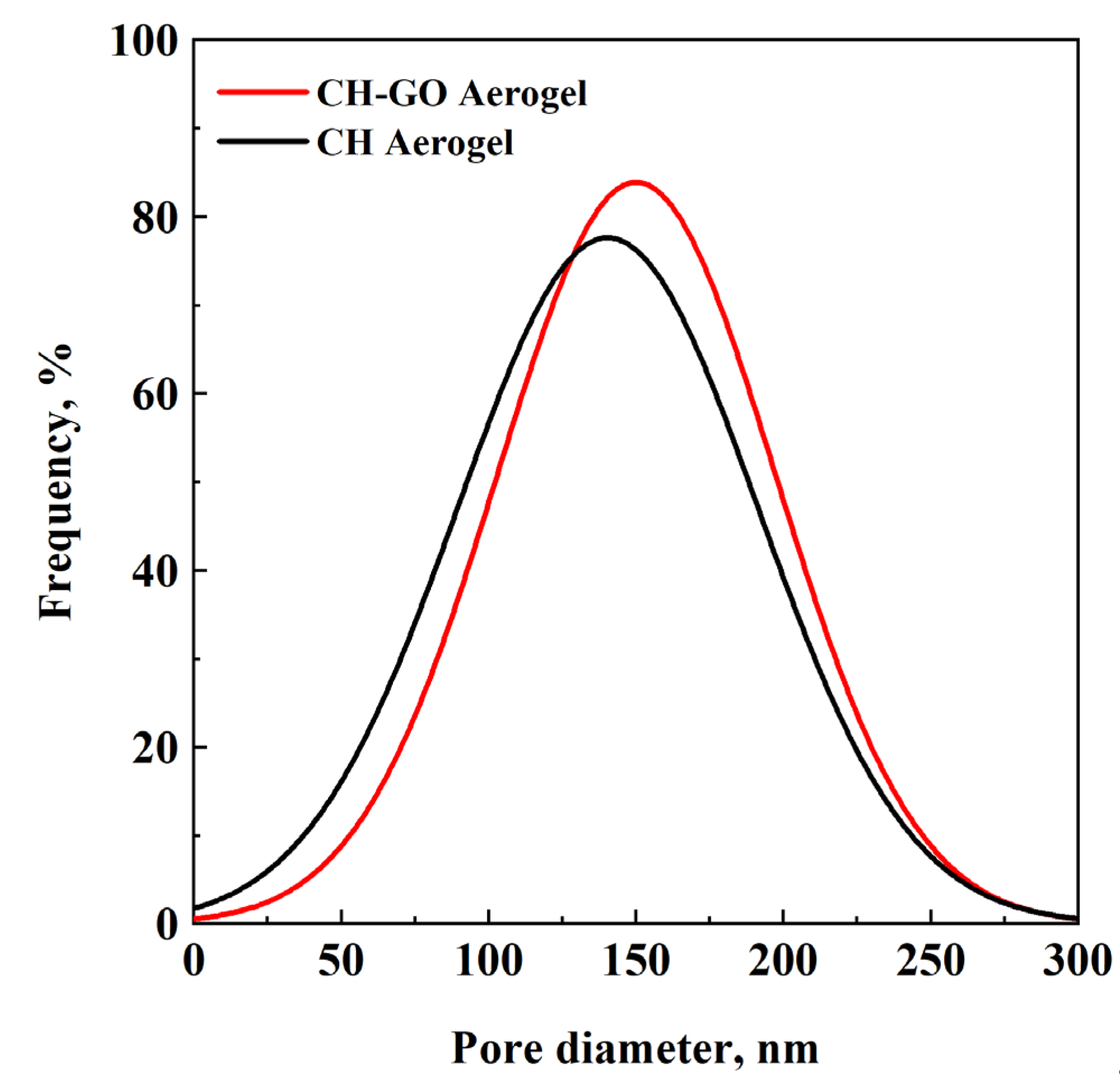
3.2. Effect of Driving Force
3.3. Effect of Adsorbent Dosage
3.4. Adsorption Isotherms
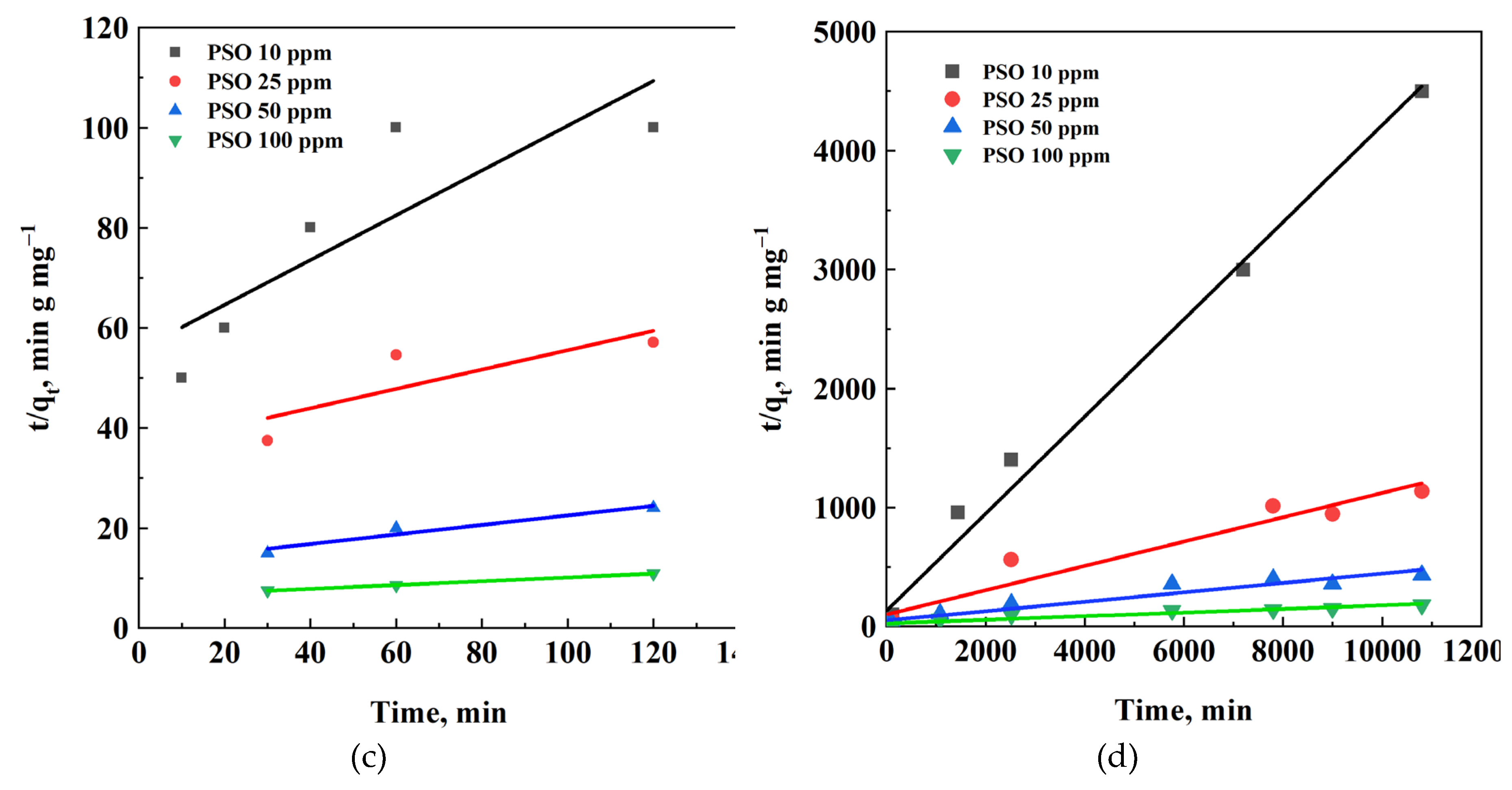
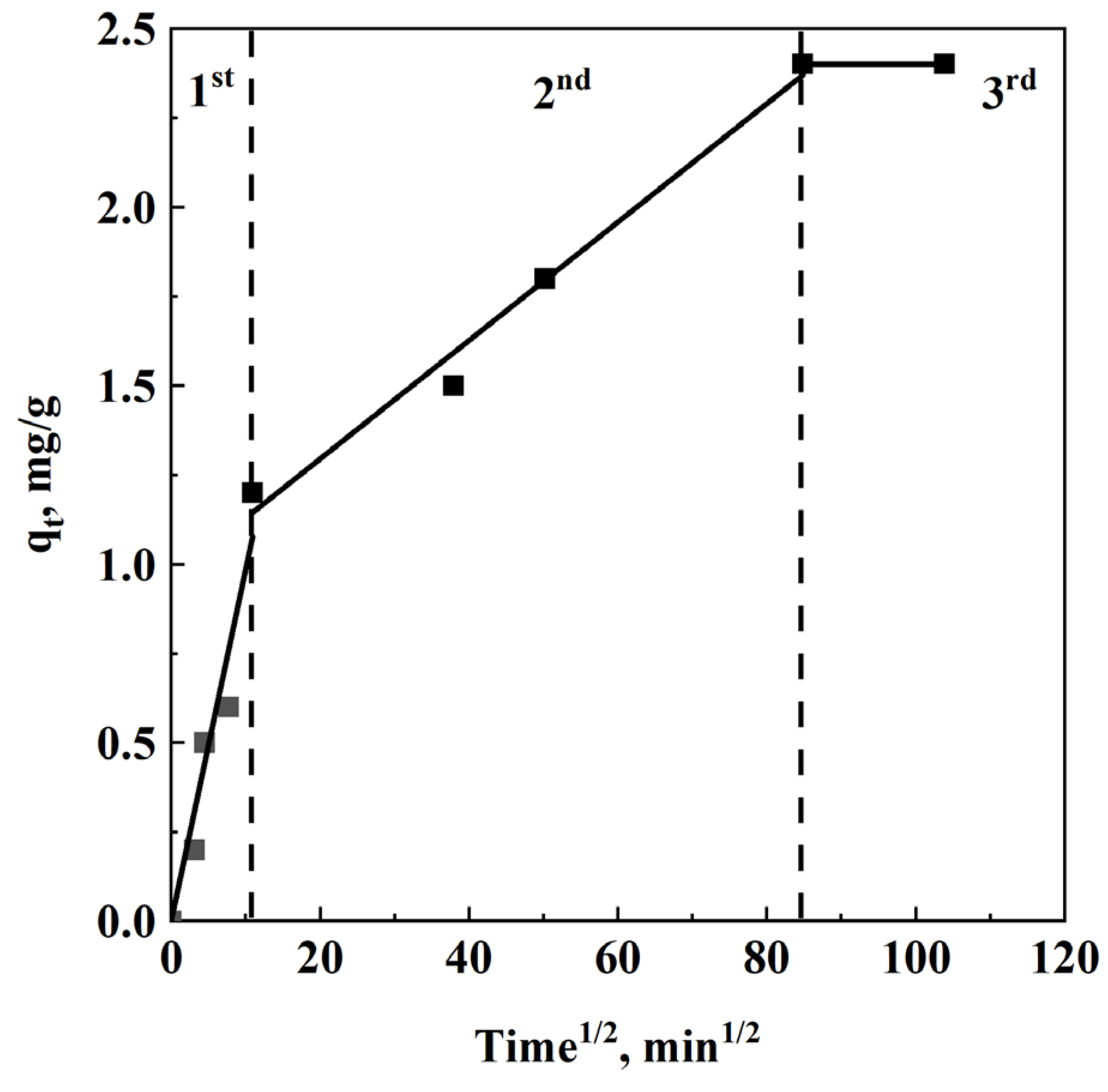
3.5. Kinetic Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A Critical Review on the Treatment of Dye-Containing Wastewater: Ecotoxicological and Health Concerns of Textile Dyes and Possible Remediation Approaches for Environmental Safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic Organic Dyes as Contaminants of the Aquatic Environment and Their Implications for Ecosystems: A Review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef] [PubMed]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of Various Recent Wastewater Dye Removal Methods: A Review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Slama, H.B.; Chenari Bouket, A.; Pourhassan, Z.; Alenezi, F.N.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golińska, P.; Belbahri, L. Diversity of Synthetic Dyes from Textile Industries, Discharge Impacts and Treatment Methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Uddin, F. Environmental Hazard in Textile Dyeing Wastewater from Local Textile Industry. Cellulose 2021, 28, 10715–10739. [Google Scholar] [CrossRef]
- Verma, Y. Acute Toxicity Assessment of Textile Dyes and Textile and Dye Industrial Effluents Using Daphnia Magna Bioassay. Toxicol. Ind. Health 2008, 24, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Mostafa, M. Textile Dyeing Effluents and Environment Concerns - A Review. J. Environ. Sci. & Natural Resources 2019, 11, 131–144. [Google Scholar] [CrossRef]
- Yadav, A.K.; Jain, C.K.; Malik, D.S. Toxic Characterization of Textile Dyes and Effluents in Relation to Human Health Hazards. J, Sust. Env. Res. 2014, 3, 95–102. [Google Scholar]
- Islam, T.; Repon, Md.R.; Islam, T.; Sarwar, Z.; Rahman, M.M. Impact of Textile Dyes on Health and Ecosystem: A Review of Structure, Causes, and Potential Solutions. Environ. Sci. Pollut. Res. 2022, 30, 9207–9242. [Google Scholar] [CrossRef]
- Sudarshan, S.; Harikrishnan, S.; RathiBhuvaneswari, G.; Alamelu, V.; Aanand, S.; Rajasekar, A.; Govarthanan, M. Impact of Textile Dyes on Human Health and Bioremediation of Textile Industry Effluent Using Microorganisms: Current Status and Future Prospects. J. Appl. Microbiol. 2023, 134, lxac064. [Google Scholar] [CrossRef]
- Tang, K.H.D.; Darwish, N.M.; Alkahtani, A.M.; AbdelGawwad, M.R.; Karácsony, P. Biological Removal of Dyes from Wastewater: A Review of Its Efficiency and Advances. Trop. Aqua. Soil Pollut. 2022, 2, 59–75. [Google Scholar] [CrossRef]
- Donkadokula, N.Y.; Kola, A.K.; Naz, I.; Saroj, D. A Review on Advanced Physico-Chemical and Biological Textile Dye Wastewater Treatment Techniques. Rev. Environ. Sci. Biotechnol. 2020, 19, 543–560. [Google Scholar] [CrossRef]
- Singh, P.K.; Singh, R.L. Bio-Removal of Azo Dyes: A Review. Int. J. Appl. Sci. Biotechnol. 2017, 5, 108–126. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Gandhimathi, R.; Ramesh, S.T. Degradation of Dyes from Aqueous Solution by Fenton Processes: A Review. Environ. Sci. Pollut. Res. 2013, 20, 2099–2132. [Google Scholar] [CrossRef]
- Fernandes, N.C.; Brito, L.B.; Costa, G.G.; Taveira, S.F.; Cunha–Filho, M.S.S.; Oliveira, G.A.R.; Marreto, R.N. Removal of Azo Dye Using Fenton and Fenton-like Processes: Evaluation of Process Factors by Box–Behnken Design and Ecotoxicity Tests. Chem.-Biol. Interact. 2018, 291, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Ay, F.; Catalkaya, E.C.; Kargi, F. A Statistical Experiment Design Approach for Advanced Oxidation of Direct Red Azo-Dye by Photo-Fenton Treatment. J. Hazard. Mater. 2009, 162, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Soon, A.N.; Hameed, B.H. Heterogeneous Catalytic Treatment of Synthetic Dyes in Aqueous Media Using Fenton and Photo-Assisted Fenton Process. Desalination 2011, 269, 1–16. [Google Scholar] [CrossRef]
- Garciamontano, J.; Ruiz, N.; Munoz, I.; Domenech, X.; Garciahortal, J.; Torrades, F.; Peral, J. Environmental Assessment of Different Photo-Fenton Approaches for Commercial Reactive Dye Removal. J. Hazard. Mater. 2006, 138, 218–225. [Google Scholar] [CrossRef]
- Cheng, S.; Zhao, S.; Xing, B.; Liu, Y.; Zhang, C.; Xia, H. Preparation of Magnetic Adsorbent-Photocatalyst Composites for Dye Removal by Synergistic Effect of Adsorption and Photocatalysis. J. Cleaner Prod. 2022, 348, 131301. [Google Scholar] [CrossRef]
- Hasanpour, M.; Hatami, M. Photocatalytic Performance of Aerogels for Organic Dyes Removal from Wastewaters: Review Study. J. Mol. Liq. 2020, 309, 113094. [Google Scholar] [CrossRef]
- Akbari, A.; Sabouri, Z.; Hosseini, H.A.; Hashemzadeh, A.; Khatami, M.; Darroudi, M. Effect of Nickel Oxide Nanoparticles as a Photocatalyst in Dyes Degradation and Evaluation of Effective Parameters in Their Removal from Aqueous Environments. Inorg. Chem. Commun. 2020, 115, 107867. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and Its Removal from Aqueous Solution by Adsorption: A Review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chen, X.; Wu, F.; Ji, Y. High Adsorption Capability and Selectivity of ZnO Nanoparticles for Dye Removal. Colloids Surf. A 2016, 509, 474–483. [Google Scholar] [CrossRef]
- Malek, N.N.A.; Jawad, A.H.; Ismail, K.; Razuan, R.; ALOthman, Z.A. Fly Ash Modified Magnetic Chitosan-Polyvinyl Alcohol Blend for Reactive Orange 16 Dye Removal: Adsorption Parametric Optimization. Int. J. Biol. Macromol. 2021, 189, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Harja, M.; Buema, G.; Bucur, D. Recent Advances in Removal of Congo Red Dye by Adsorption Using an Industrial Waste. Sci. Rep. 2022, 12, 6087. [Google Scholar] [CrossRef] [PubMed]
- Jawad, A.H.; Abdulhameed, A.S.; Mastuli, M.S. Acid-Factionalized Biomass Material for Methylene Blue Dye Removal: A Comprehensive Adsorption and Mechanism Study. J. Taibah Univ. SCI 2020, 14, 305–313. [Google Scholar] [CrossRef]
- El Maguana, Y.; Elhadiri, N.; Benchanaa, M.; Chikri, R. Activated Carbon for Dyes Removal: Modeling and Understanding the Adsorption Process. J. Chem. 2020, 1–9. [Google Scholar] [CrossRef]
- Moosavi, S.; Lai, C.W.; Gan, S.; Zamiri, G.; Akbarzadeh Pivehzhani, O.; Johan, M.R. Application of Efficient Magnetic Particles and Activated Carbon for Dye Removal from Wastewater. ACS Omega 2020, 5, 20684–20697. [Google Scholar] [CrossRef]
- Bal, G.; Thakur, A. Distinct Approaches of Removal of Dyes from Wastewater: A Review. Mater. Today: Proc. 2022, 50, 1575–1579. [Google Scholar] [CrossRef]
- Solayman, H.M.; Hossen, Md.A.; Abd Aziz, A.; Yahya, N.Y.; Leong, K.H.; Sim, L.C.; Monir, M.U.; Zoh, K.-D. Performance Evaluation of Dye Wastewater Treatment Technologies: A Review. J. Environ. Chem. Eng. 2023, 11, 109610. [Google Scholar] [CrossRef]
- Zhao, S.; Malfait, W.J.; Guerrero-Alburquerque, N.; Koebel, M.M.; Nyström, G. Biopolymer Aerogels and Foams: Chemistry, Properties, and Applications. Angew Chem. Int. Ed. 2018, 57, 7580–7608. [Google Scholar] [CrossRef] [PubMed]
- Idumah, C.I.; Ezika, A.C.; Okpechi, V.U. Emerging Trends in Polymer Aerogel Nanoarchitectures, Surfaces, Interfaces and Applications. Surf. Interfaces 2021, 25, 101258. [Google Scholar] [CrossRef]
- Shi, H.; Cui, J.; Shen, H.; Wu, H. Preparation of Silica Aerogel and Its Adsorption Performance to Organic Molecule. Adv. Mater. Sci. Eng. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Basak, S.; Singhal, R.S. The Potential of Supercritical Drying as a “Green” Method for the Production of Food-Grade Bioaerogels: A Comprehensive Critical Review. Food Hydrocolloids 2023, 141, 108738. [Google Scholar] [CrossRef]
- Li, C.; Dang, Q.; Yang, Q.; Chen, D.; Zhu, H.; Chen, J.; Liu, R.; Wang, X. Study of the Microstructure of Chitosan Aerogel Beads Prepared by Supercritical CO 2 Drying and the Effect of Long-Term Storage. RSC Adv. 2022, 12, 21041–21049. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, A.; Baldino, L.; Misol, A.; Cardea, S.; Del Valle, E.M.M. Role of Rheological Properties on Physical Chitosan Aerogels Obtained by Supercritical Drying. Carbohydr. Polym. 2020, 233, 115850. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent Advances on the Removal of Dyes from Wastewater Using Various Adsorbents: A Critical Review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Dassanayake, R.S.; Acharya, S.; Abidi, N. Recent Advances in Biopolymer-Based Dye Removal Technologies. Molecules 2021, 26, 4697. [Google Scholar] [CrossRef]
- Sadiq, A.C.; Olasupo, A.; Ngah, W.S.W.; Rahim, N.Y.; Suah, F.B.M. A Decade Development in the Application of Chitosan-Based Materials for Dye Adsorption: A Short Review. Int. J. Biol. Macromol. 2021, 191, 1151–1163. [Google Scholar] [CrossRef]
- Leudjo Taka, A.; Klink, M.J.; Yangkou Mbianda, X.; Naidoo, E.B. Chitosan Nanocomposites for Water Treatment by Fixed-Bed Continuous Flow Column Adsorption: A Review. Carbohydr. Polym. 2021, 255, 117398. [Google Scholar] [CrossRef]
- Crini, G.; Badot, P.-M. Application of Chitosan, a Natural Aminopolysaccharide, for Dye Removal from Aqueous Solutions by Adsorption Processes Using Batch Studies: A Review of Recent Literature. Prog. Polym. Sci. 2008, 33, 399–447. [Google Scholar] [CrossRef]
- Robati, D.; Mirza, B.; Rajabi, M.; Moradi, O.; Tyagi, I.; Agarwal, S.; Gupta, V.K. Removal of Hazardous Dyes-BR 12 and Methyl Orange Using Graphene Oxide as an Adsorbent from Aqueous Phase. Chem. Eng. J. 2016, 284, 687–697. [Google Scholar] [CrossRef]
- Molla, A.; Li, Y.; Mandal, B.; Kang, S.G.; Hur, S.H.; Chung, J.S. Selective Adsorption of Organic Dyes on Graphene Oxide: Theoretical and Experimental Analysis. Appl. Surf. Sci. 2019, 464, 170–177. [Google Scholar] [CrossRef]
- Mishra, A.K.; Ramaprabhu, S. Functionalized Graphene Sheets for Arsenic Removal and Desalination of Sea Water. Desalination 2011, 282, 39–45. [Google Scholar] [CrossRef]
- Zhu, W.; Jiang, X.; Liu, F.; You, F.; Yao, C. Preparation of Chitosan—Graphene Oxide Composite Aerogel by Hydrothermal Method and Its Adsorption Property of Methyl Orange. Polymers 2020, 12, 2169. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Song, G.; Li, A.; Wang, J.; Wang, H.; Sun, Y.; Ding, G. Graphene Oxide-Chitosan Composite Aerogel for Adsorption of Methyl Orange and Methylene Blue: Effect of pH in Single and Binary Systems. Colloids Surf., A 2022, 641, 128595. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, T.; Ren, Y.; Wang, Y.; Yu, R.; Wang, J.; Tu, Q. Preparation of Chitosan Crosslinked with Metal-Organic Framework (MOF-199)@aminated Graphene Oxide Aerogel for the Adsorption of Formaldehyde Gas and Methyl Orange. Int. J. Biol. Macromol. 2021, 193, 2243–2251. [Google Scholar] [CrossRef] [PubMed]
- Benjelloun, M.; Miyah, Y.; Akdemir Evrendilek, G.; Zerrouq, F.; Lairini, S. Recent Advances in Adsorption Kinetic Models: Their Application to Dye Types. Arabian J. Chem. 2021, 14, 103031. [Google Scholar] [CrossRef]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption Kinetic Modeling Using Pseudo-First Order and Pseudo-Second Order Rate Laws: A Review. Cleaner Engineering and Technology 2020, 1, 100032. [Google Scholar] [CrossRef]
- Cheung, W.H.; Szeto, Y.S.; McKay, G. Intraparticle Diffusion Processes during Acid Dye Adsorption onto Chitosan. Bioresour. Technol. 2007, 98, 2897–2904. [Google Scholar] [CrossRef]
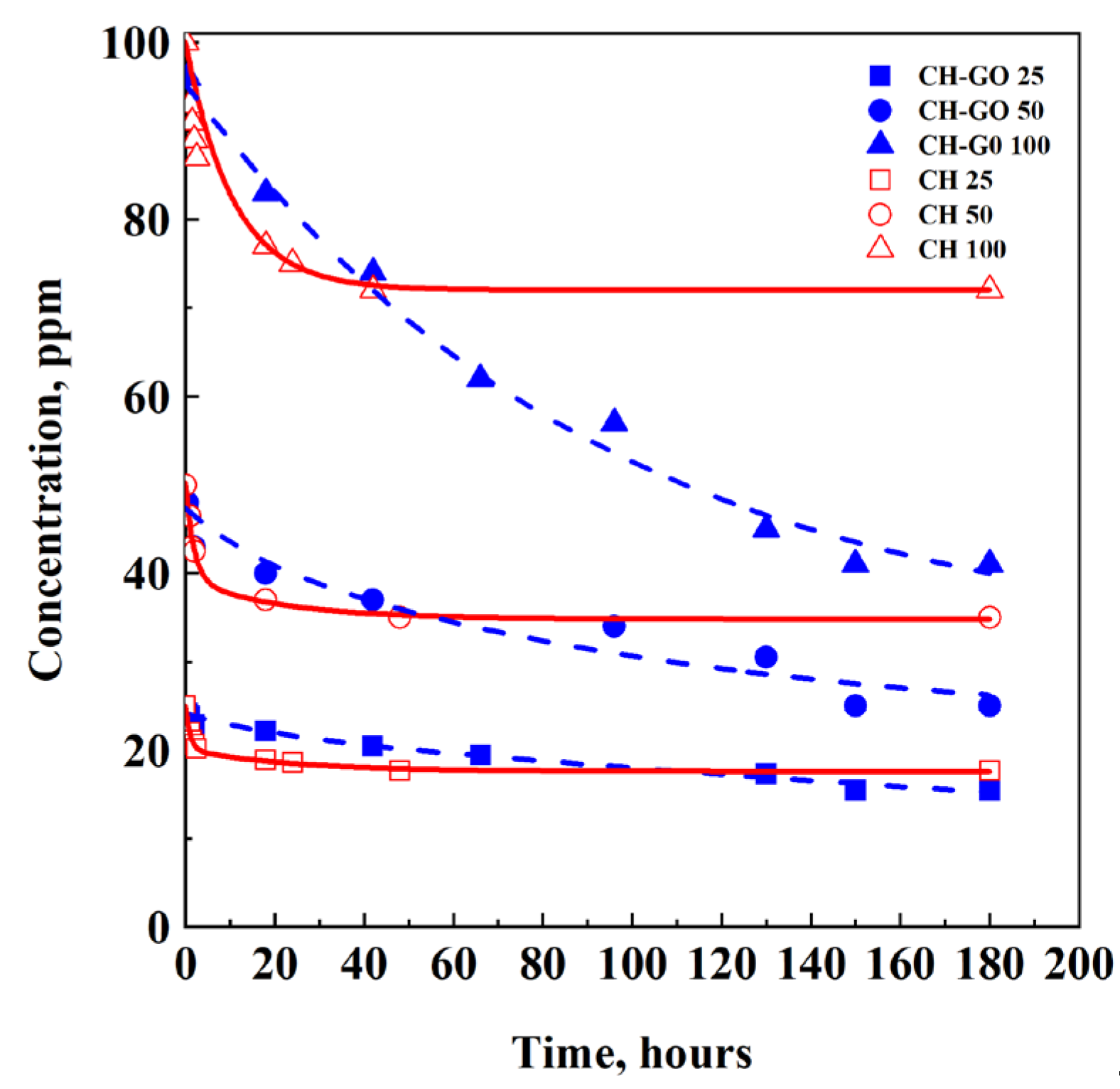
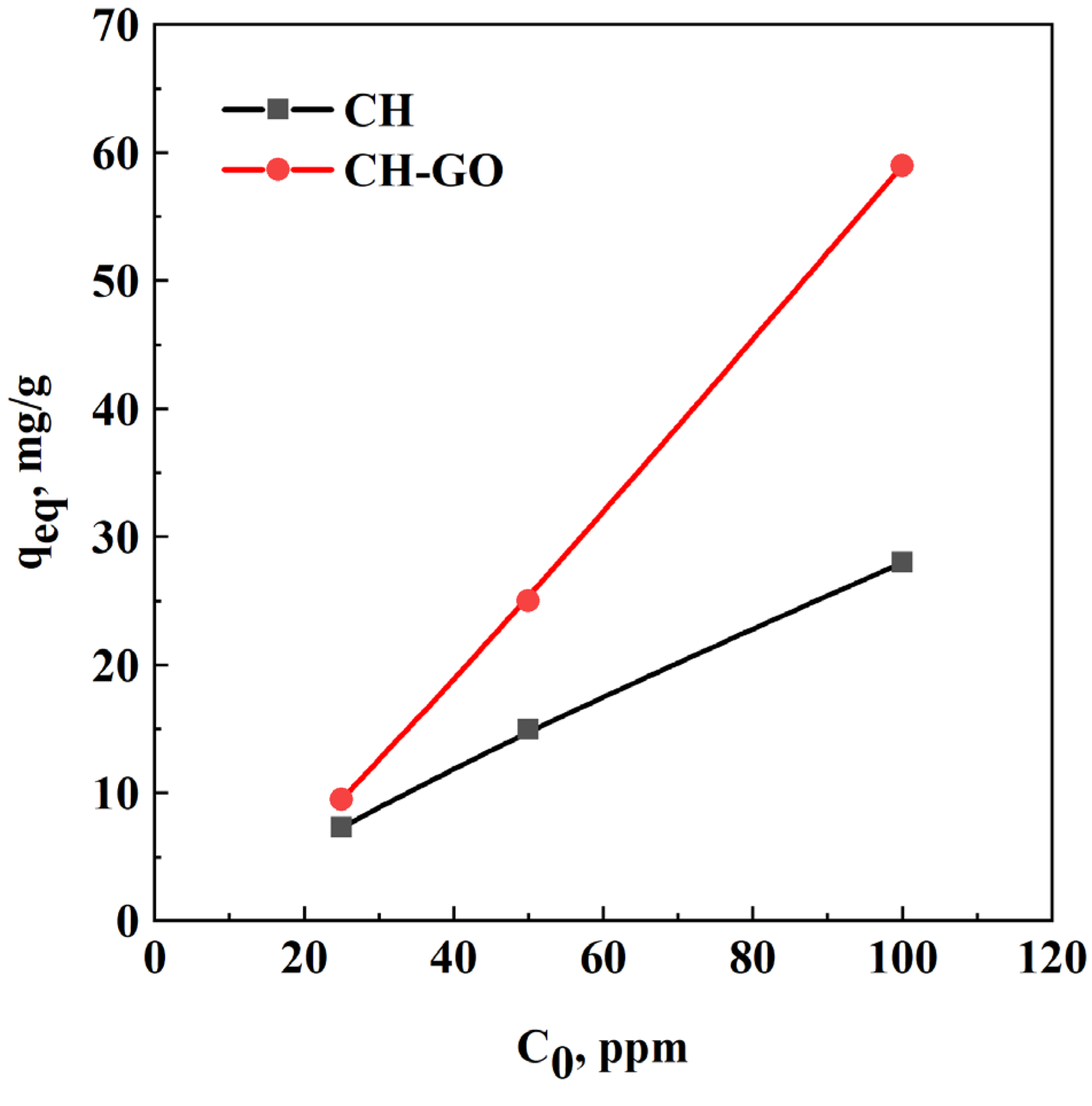
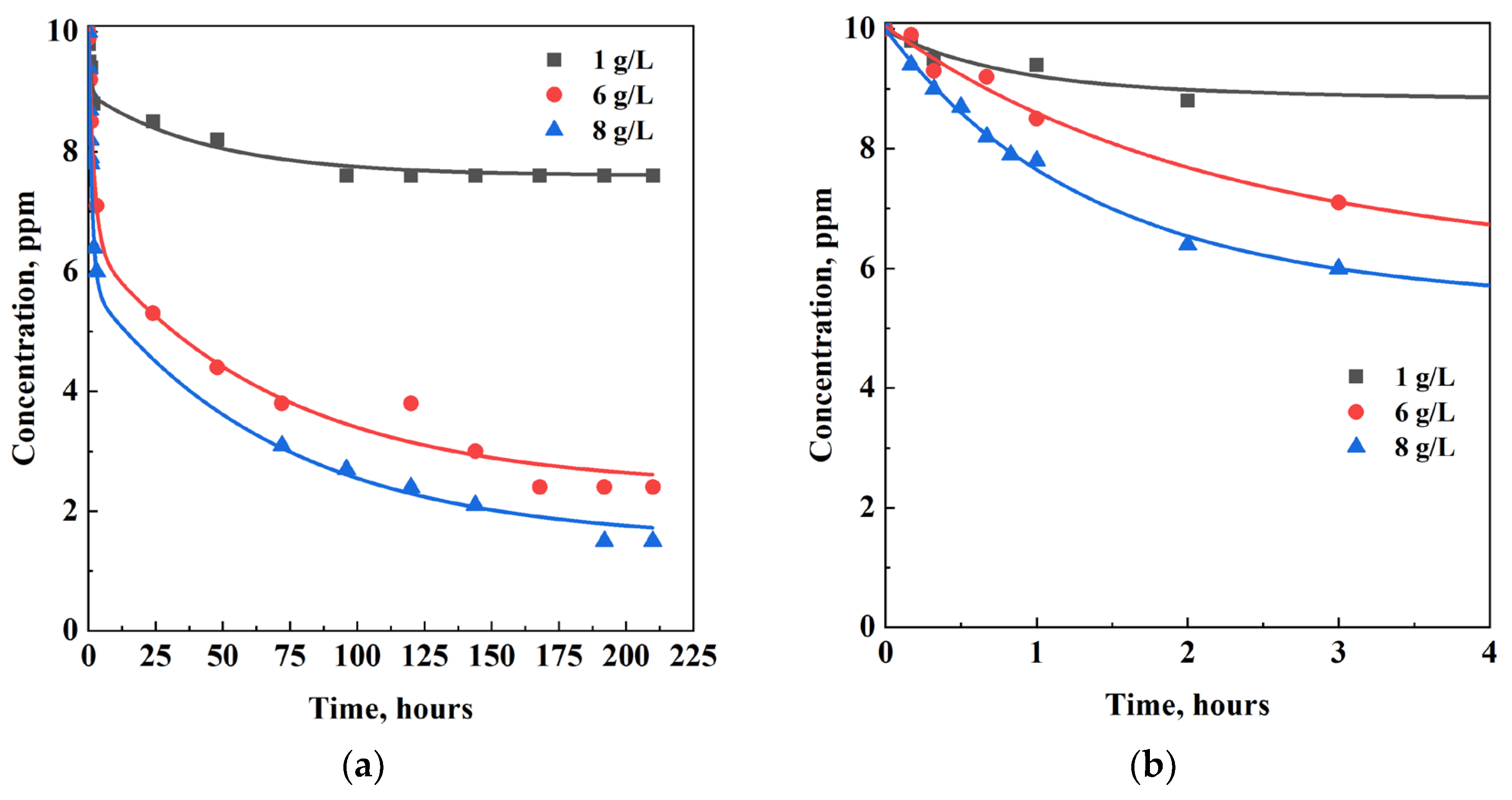
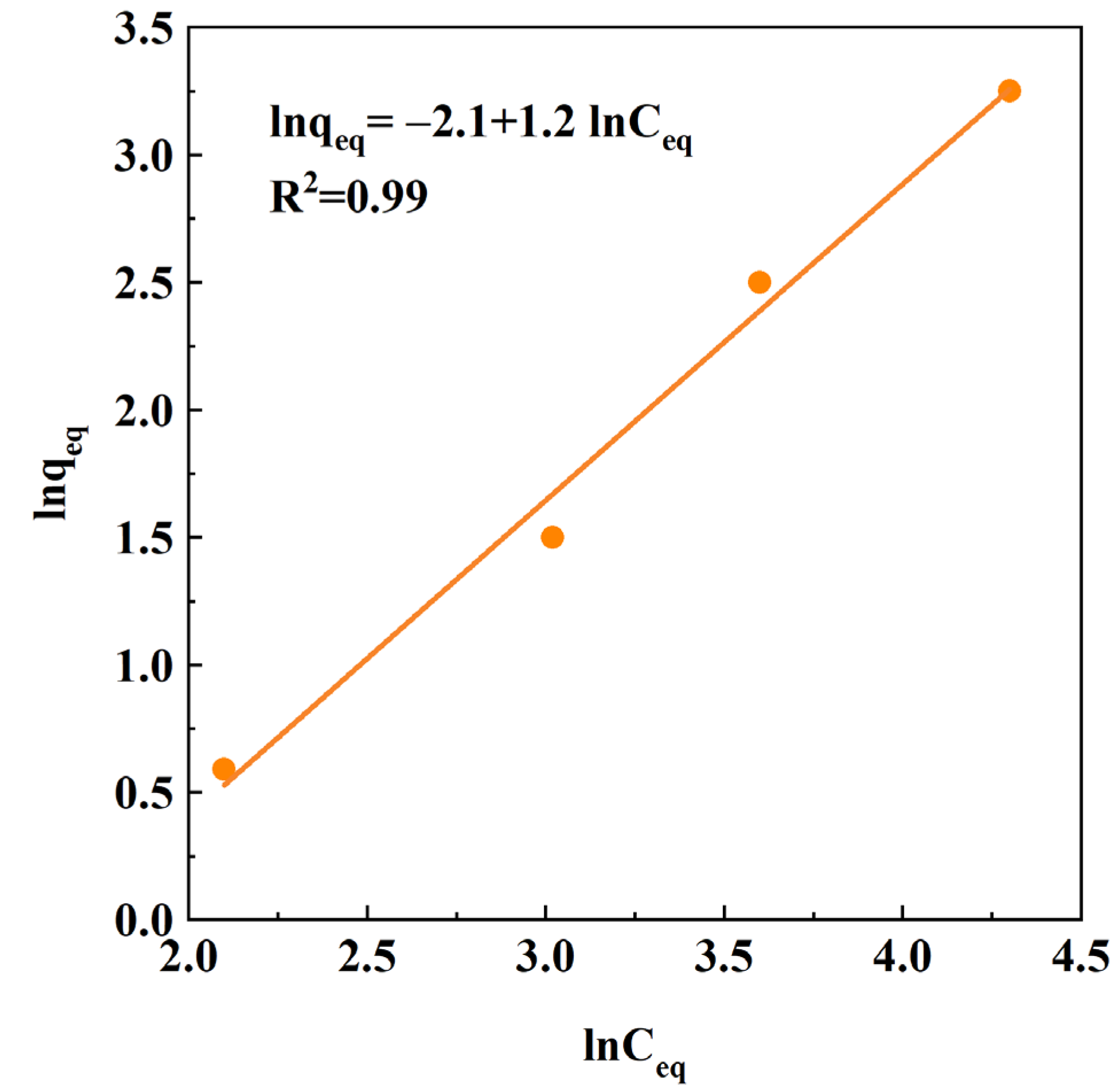
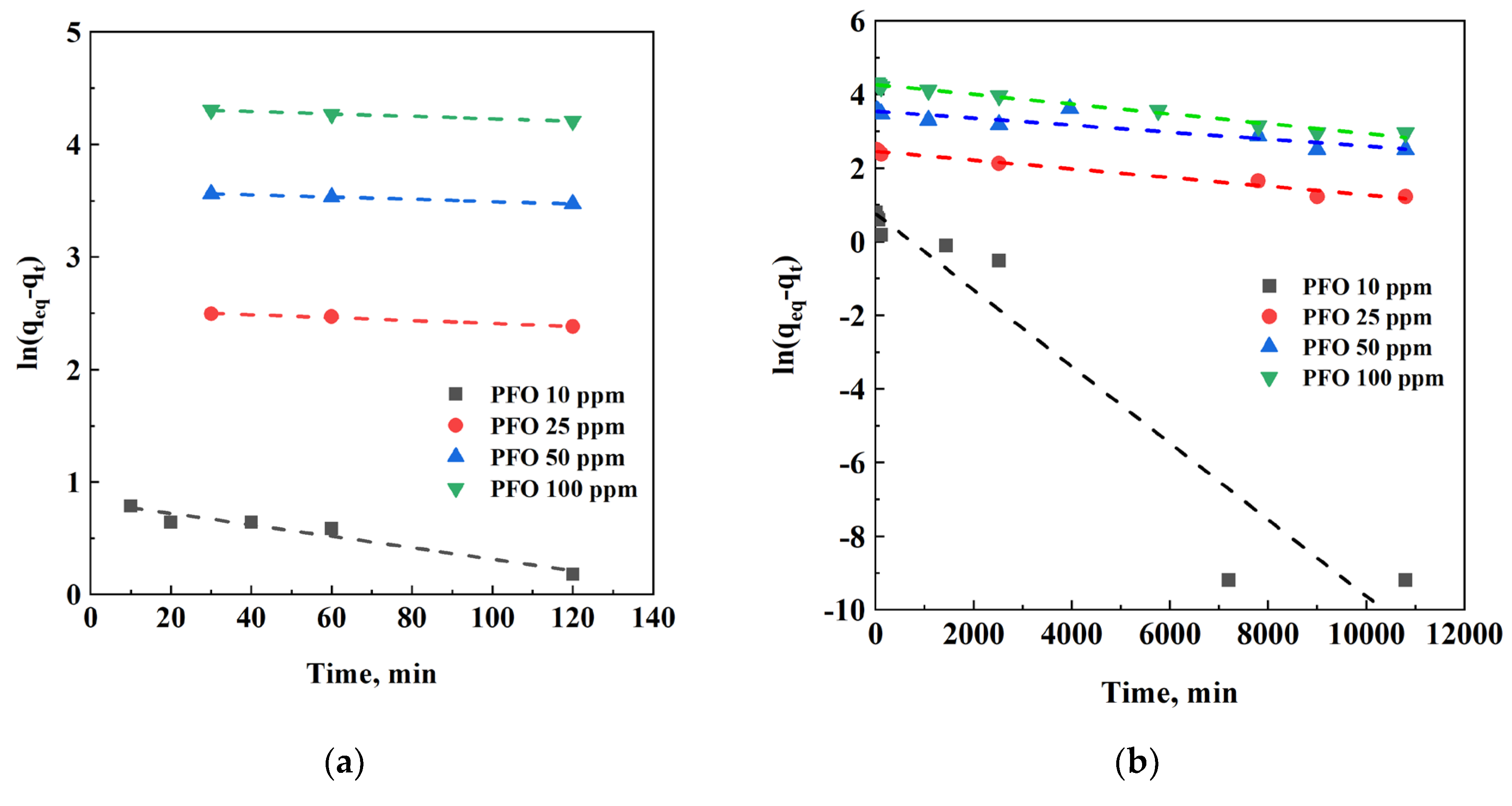
| C0, ppm | Adsorbent | Ceq, ppm | qeq, mg/g | η, % |
|---|---|---|---|---|
| 25 | CH | 17.7 | 7.3 | 29.2 |
| CH-GO | 15.5 | 9.5 | 38.0 | |
| 50 | CH | 35.0 | 15.0 | 30.0 |
| CH-GO | 25.0 | 25.0 | 50.0 | |
| 100 | CH | 72.0 | 28.0 | 28.0 |
| CH-GO | 41.0 | 59.0 | 59.0 |
| CH-GO dosage, g/L | Ceq, ppm | qeq, mg/g | η, % |
|---|---|---|---|
| 1 | 7.6 | 2.4 | 24 |
| 6 | 2.4 | 1.3 | 76 |
| 8 | 1.5 | 1.1 | 85 |
| 120 min | Global | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C0, ppm | 10 | 25 | 50 | 100 | 10 | 25 | 50 | 100 | |
| PFO | k1, min-1 | 0.0051 | 0.0013 | 0.0009 | 0.00108 | 0.0010 | 0.00011 | 9.53 E-05 | 0.00013 |
| qeq,calc, mg/g | 2.27 | 12.66 | 36.28 | 76.38 | 2.16 | 11.62 | 64.69 | 71.32 | |
| R2 | 0.938 | 0.985 | 0.999 | 0.991 | 0.930 | 0.974 | 0.822 | 0.983 | |
| PSO | k2, g (mg min)-1 | 0.0036 | 0.0010 | 0.0007 | 0.0002 | 0.0012 | 0.0001 | 3.32 E-05 | 9.43 E-06 |
| qeq,calc, mg/g | 2.24 | 5.17 | 10.50 | 26.29 | 2.45 | 9.79 | 25.08 | 64.94 | |
| R2 | 0.731 | 0.689 | 0.937 | 0.999 | 0.993 | 0.950 | 0.928 | 0.919 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





