1. Introduction
There is a group of eight large tropical and subtropical colubrid forest snakes, mainly green in color, having elongated and compressed bodies that are typically arboreal in habitat and diurnal in activity. Their diet consists of small mammals (rats, squirrels, bats), birds and their eggs in adults and lizards, frogs, fish and crickets in juveniles. Reproductively they are oviparous, laying from 2–17 eggs in 1–4 clutches per year [
4]. Their distribution encompasses Southeast Asia (including Bhutan, northeast India, southern China, and Taiwan), the Andamans and Philippines, and Sundaland or western Indonesia [
5]. Presently they are all lumped together under the genus
Gonyosoma. They share a number of morphological traits while differing in other characteristics.
Günther's [
6] concept of
Gonyosoma contained three species (
oxycephalum,
frenatum, and
gramineus [
= prasinum]), which was followed by Pope [
7]. However, Boulenger [
8] earlier on lumped many genera, including
Gonyosoma, into his expanded concept of
Elaphe. Dowling [
9] was the first to recognize
Gonyosoma oxycephalum as a separate taxon from the genus
Elaphe although the other species of arboreal Asian ratsnakes related to
Gonyosoma have continued to be treated as members of
Elaphe [
4,
10,
11].
The consolidation of
Gonyosoma began when Chen et al. [
12] combined the following five species into
Gonyosoma based upon one mitochondrial (cyt-b) and five nuclear genes (c-mos, RAG-1, SPTBN1, VIM4, and VIM5):
G. boulengeri [
2],
G. frenatum [
13],
G. margaritatum [
14],
G. oxycephalum [
15], and
G. prasinum [
16]. The species
G. jansenii [
17], for which molecular data was unavailable, was included in
Gonyosoma based upon its similarity to
G. oxycephalum. Their phylogeny utilized both maximum likelihood and Bayesian inference methods, and the time-calibration indicated that the group of species originated in the Early Miocene approximately 20.5 million years ago (MYA) with the separation of
G. oxycephalum from wolfsnakes of the genus
Lycodon (= 38.1 MYA, Eocene
fide Burbrink & Lawson [
18]). The division between the two pairs of sister taxa,
G. margaritatum plus
G. prasinum and
G. boulengeri plus
G. frenatum, occurred ca. 15.8 MYA (= 25 MYA, Late Oligocene
fide Burbrink & Lawson [
18]). The sister taxa
G. margaritatum and
G. prasinum diverged ca. 11.3 MYA with
G. boulengeri and
G. frenatum then separating ca. 7.4 MYA [
12].
Chen et al. [
4] pointed out the three possible taxonomic solutions: 1) consolidate all species into one genus,
Gonyosoma, retaining monophyly but losing taxonomic information, 2) retain
Gonyosoma and consolidate the remaining species in
Gonyophis, also retaining monophyly but with only two genera, 3) retain the genera
Gonyosoma, Gonyophis, Rhynchophis and resurrect
Rhadinophis for
R. prasinus. Chen et al. [
12] selected Solution 1, which involved synonymizing the genera
Gonyophis, Rhynchophis and
Rhadinophis. They did so by remarking that Solution 3 would "provide little evidence for defining the evolutionary history of the related group" and that "other genera of similar age (~20 MYA) often have higher numbers of extant species." We disagree and believe Solution 3 provides not only five clades but best elucidates the evolutionary history of the group. There are numerous colubrid genera that have a similar or fewer number of species that have evolved within the past 20 million years. These include, among others,
Elaphe quatourlineata species group (4 spp., 5.1 Mya, Jablonski et al. [
19] ,
Rhabdophis nuchalis species group (7 spp., 6.2 Mya, Zhu et al. [
20]),
Coronella (2 sp., 10 Mya, Stratakis et al. [
21]),
Zamenis (5 spp., 11.4 Mya, Salvi et al. [
22]),
Cerberus (5 spp., 14 Mya, Alfaro et al. [
23]),
Haldea, Liodytes, Regina, Storeria and
Virginia (1–5 spp. each, 11–14 Mya, McVay et al. [
24]),
Nerodia (10 spp., 15 Mya, McVay et al. [
24]),
Enhydris (6 spp., 16 Mya, Alfaro et al. [
23]),
Natrix (3 spp., 18 Mya, Guicking et al. [
25]),
Crotaphopeltis (5 spp., 21 Mya, Engelbrecht et al. [
26]), and
Spalerosophis (6 spp., 22 Mya, Yadollahvandmiandoab et al. [
27]).
The low number of tissue samples led to the five tested species as belonging to a single clade. Over the past decade more specimens and tissue samples have become available and the most recent phylogenies demonstrate that the currently recognized eight species (including the two newly described forms from China,
G. coeruleum Liu et. al
., [
28], and
G. hainanense Peng et. al., [
29]) segregate into five well supported clades [
28,
29,
30] (Trees 1–3). Since these snakes also exhibit distinct morphological differences, we propose to resurrect three genera that were previously synonymized with
Gonyosoma (
Gonyophis Boulenger [
1],
Rhynchophis Mocquard [
2] and
Rhadinophis [
3]) in addition to proposing a new genus for two species and establishing a new tribe for the group. We believe that by lumping all eight species into a single genus taxonomic and phylogenetic information is lost. We prefer to follow the three rules of nomenclature (e.g. the Taxon naming Criteria or TNC), namely Monophyly, Clade Stability, and Phenotypic Diagnosability (Vences et. al. [
31] and all three criteria are met herein.
2. Materials and Methods
Due to the general dearth of basic systematic data, both morphological and phenotypic, in publications dealing with molecular systematics, we present such data herein for each species (
Table 1) and genus (
Table 2). We collected standard external morphological data for each species, based upon examination of specimens and review of the primary and secondary literature. All measurements were made to the nearest mm, including LOA (overall length), SVL (snout-vent length), and TL (tail length). Relative tail length (RTL) = TL/LOA. In addition, distribution by elevation and some natural history features were noted. Characters of scutellation include dorsal scale rows (ASR = anterior rows one head length behind head, MSR = midbody, PSR = posterior rows one head length in front of vent), carination (S = entirely smooth, k+s = partly keeled and partly smooth), K.R. = keeled scale rows, paired apical scale pits, number of ventrals, cloacal shield (E = entire or undivided, D = divided), number of subcaudals, condition of nasal shield (E = entire or undivided, S = semidivided, D = divided), loreal shield (present or absent and shape), number of supralabials and which ones enter the orbit, number of pre- and postoculars, number of anterior and posterior temporals, number of infralabials and which ones contact the anterior genials, minimum and maximum reported total length in mm, and coloration of body parts (including iris, mouth and tongue). A forward slash separates occurrence of two different conditions and data presented parenthetically indicates rare occurrence. The position of the umbilicus in snakes can be determined (even in some adults) but a fine, light median line covering 3–4 scales and/or faint notches along the midline of said scales. The distance from the anteriormost scale to the vent represents the umbilicus-vent interval and it is calculated as a percentage of the total number of ventrals.
We were unable to obtain a specimen of
G. hainanensis and relied on the recent description of this species [
28] although it is lacking in some respects. There is considerable variation and discrepancy in some characters of this group so we have attempted to sort out the conflicting data. For descriptions of coloration characters, in addition to the literature, we studied photos on the Internet when positive identifications were confirmed. The most comprehensive treatment of
Gonyosoma is by David et al. [
32], who covered four of the eight species (
G. coeruleum, G. frenatum, G. oxycephalum, G. prasinum).
Material examined was from the following institutions: FMNH (Field Museum of Natural History, Chicago, IL), ROM (Royal Ontario Museum, Toronto, Canada), SDSU (Biology Department, San Diego State University, San Diego, CA), and UCM (University of Colorado Museum of Natural History, Boulder, CO). The new genus and tribe names are registered with ZooBank (LSID urn:lsid:zoobank.org:pub:A3F885F0-E466-4EF8-8E8C-639168E011AF).
The purpose of this paper is to provide a synopsis of each genus and species, including the new tribe and a key to the included taxa, supplemented with head figures and range maps, and highlight the distinctive diagnostic features of each, rather than producing an exhaustive work on each taxon, for which numerous other references are available.
3. Taxonomy
3.1. Gonyophis
GONYOPHIS Boulenger, 1891 (Map 1)
Type species: Gonyosoma margaritatus Peters, 1871
Content: monotypic.
Diagnosis: Gonyophis is separable from all other genera by its color and pattern, consisting of an orange or yellow head, multicolored dorsum, and a black tail with orange, yellow or red rings, anterior genials that are longer than posterior genials, and apical scale pits that are close together.
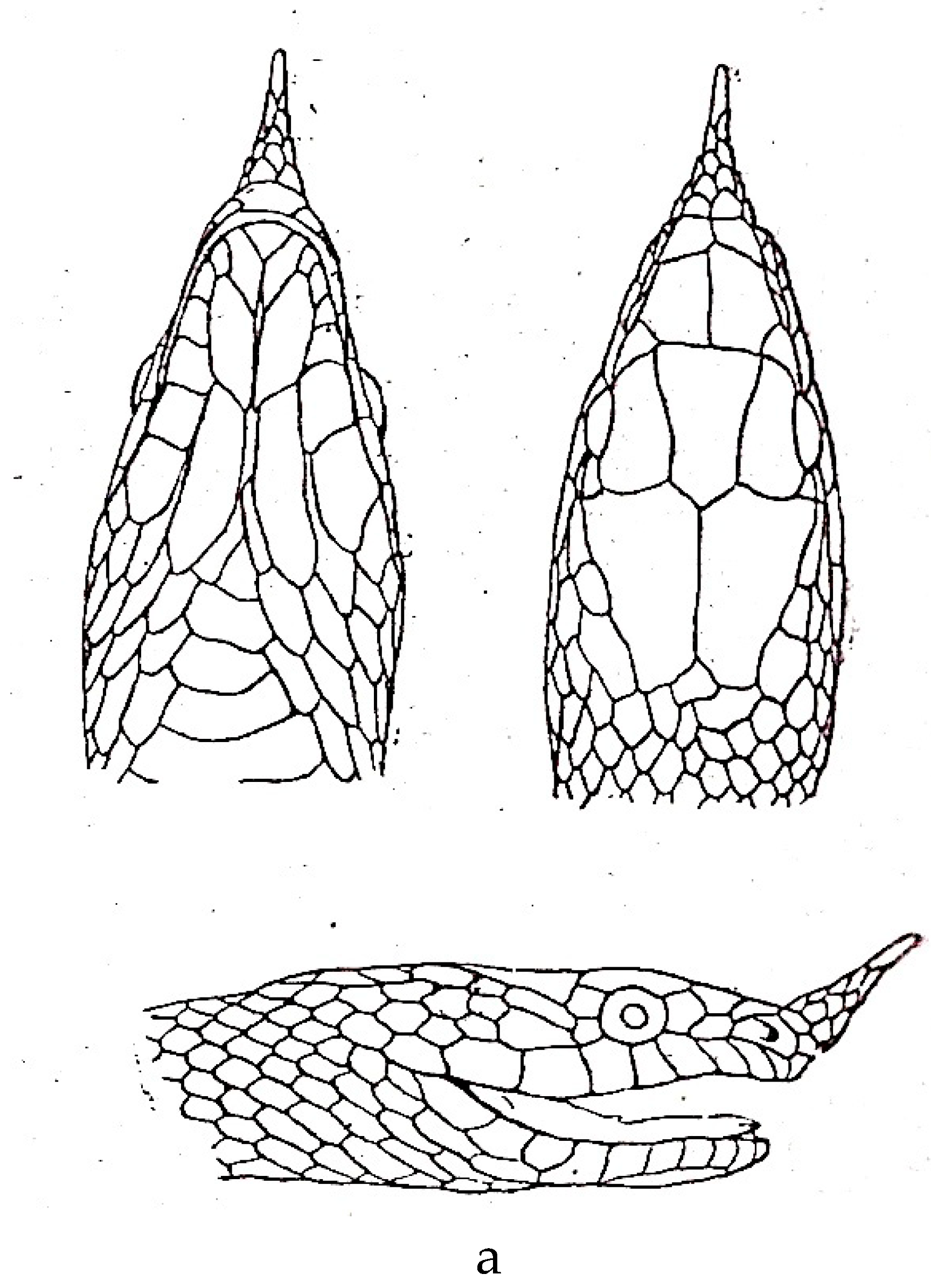
3.1.1. Gonyophis margaritatus
Gonyophis margaritatus (Peters, 1871) (
Figure 1)
Description [
34,
35,
36,
37,
38]: Moderately large snakes with an average adult size 1.0–1.5 m, overall size range 424–1943 mm confirmed but reported maximum length of 2.0 m [
37]; body elongate and laterally compressed in 19-19-15 longitudinal rows (rarely 21 rows anteriorly and 17 rows posteriorly); dorsal scales moderately keeled along the middorsal 7–13 rows (i.e., vertebral row and adjacent 3–6 rows), most distinct anteriorly, faint at midbody, and absent posteriorly, lower 3 to 6 scale rows smooth; elongate apical scale pits spaced closely together, distinctly present in nuchal region, faintly visible at midbody, and invisible posteriorly; scale row reduction involving paravertebral rows; ventrals 230–249, angulate with lateral keels and notches; subcaudals 108–130, also angulate, keeled and notched; cloacal shield divided; head distinct from neck, eye moderate in size with round pupil and black or reddish-brown iris; nasal shield elongate, divided or semidivided with a dorsal suture; preocular one, postoculars 2, supralabials usually 9 (rarely 8 or 10) with three usually entering the orbit (4–6, less commonly 3–5 or 5–7), rarely only two (5–6) entering orbit; temporal formula varies from 2+2+2 to 2+3+3; infralabials 10–12 with the first 5 contacting the anterior genials; anterior genials longer than posterior genials; tail long and slender, 22.6–33.3% total length; 20–23 aglyphous maxillary teeth; umbilicus-vent interval moderate (11.8–13.3% total ventrals); head dorsum, lateral snout and labials bright orange, posterior head and temporal region black (including a broad postocular bar); dorsum black, each scale with a central green, yellow or blue dot, the posterior body and tail encircled by 5–17 orange or yellow rings, oblique and diffuse at midbody but brighter and brick-red, orange or yellow on tail; chin and venter yellow, orangish-yellow or orangish-pink, lateral edges of ventrals with irregular, alternating black marks, subcaudals black, crossed with 5–8 orange/yellow/red rings. Mouth pink, tongue light with black tips.
Natural history [
18,
36,
38,
39,
40,
41]: A rare diurnal, arboreal species inhabiting lowland and montane primary tropical rainforest, often in the forest canopy. An excellent and agile climber, able to scale vertical tree trunks with ease. Presumably it feeds upon small mammals, bats and birds but is only known to feed upon fish in captivity. Specimens have been captured in fish nets so it may frequent habitats in the vicinity of water like
G. jansenii and
G. prasinus. Reproduction unknown, presumed to be oviparous, lacking ontogenetic color change with juveniles exhibiting the same pattern as adults. Although a rare species with limited distribution that is nowhere common, it is listed by the IUCN as a Least Concern species and not included in CITES Appendices. Its major threat is due to deforestation but due to its beautiful coloration it is popular in the pet trade and should be closely monitored. Vernacular name is the Rainbow tree snake or Royal tree snake.
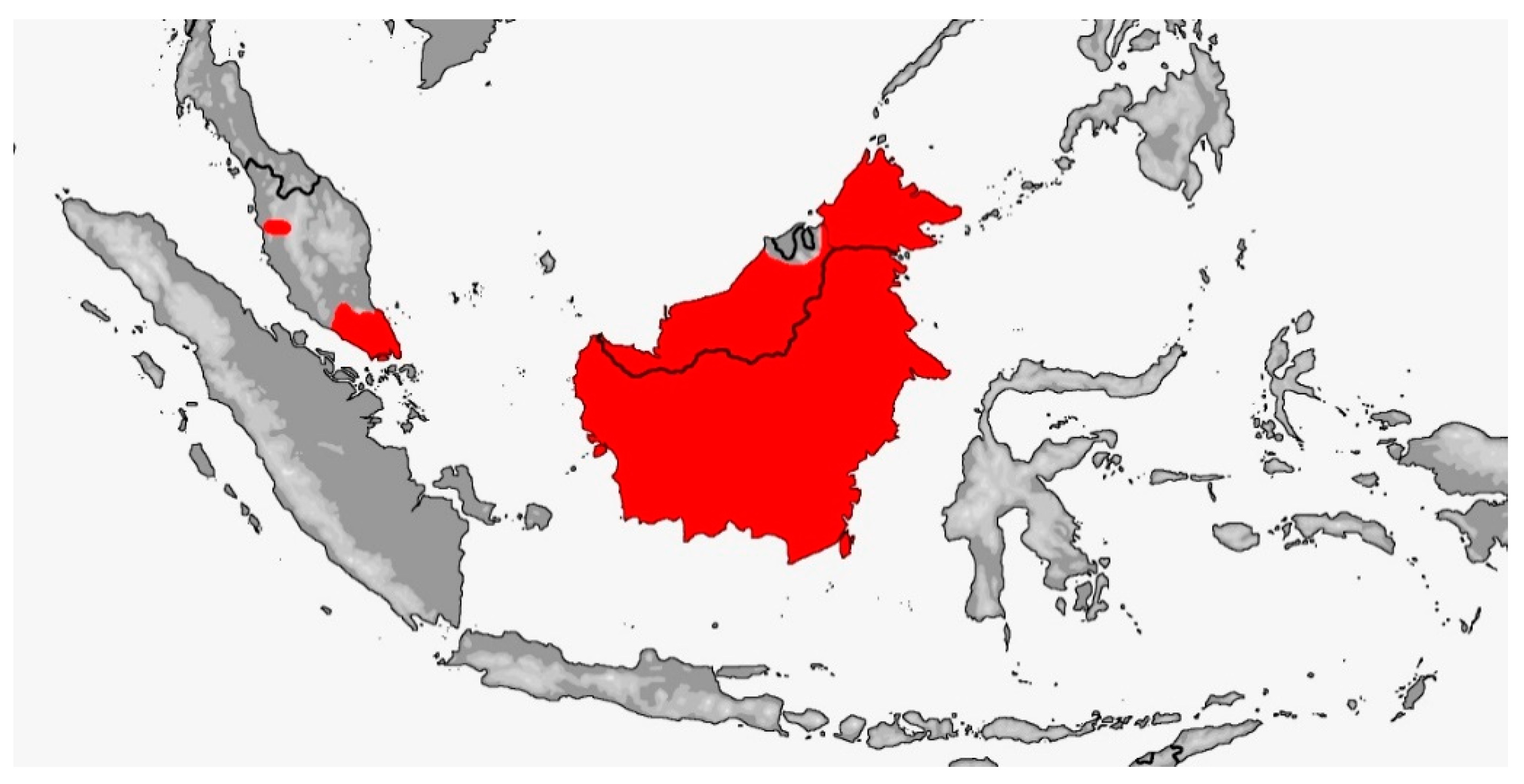
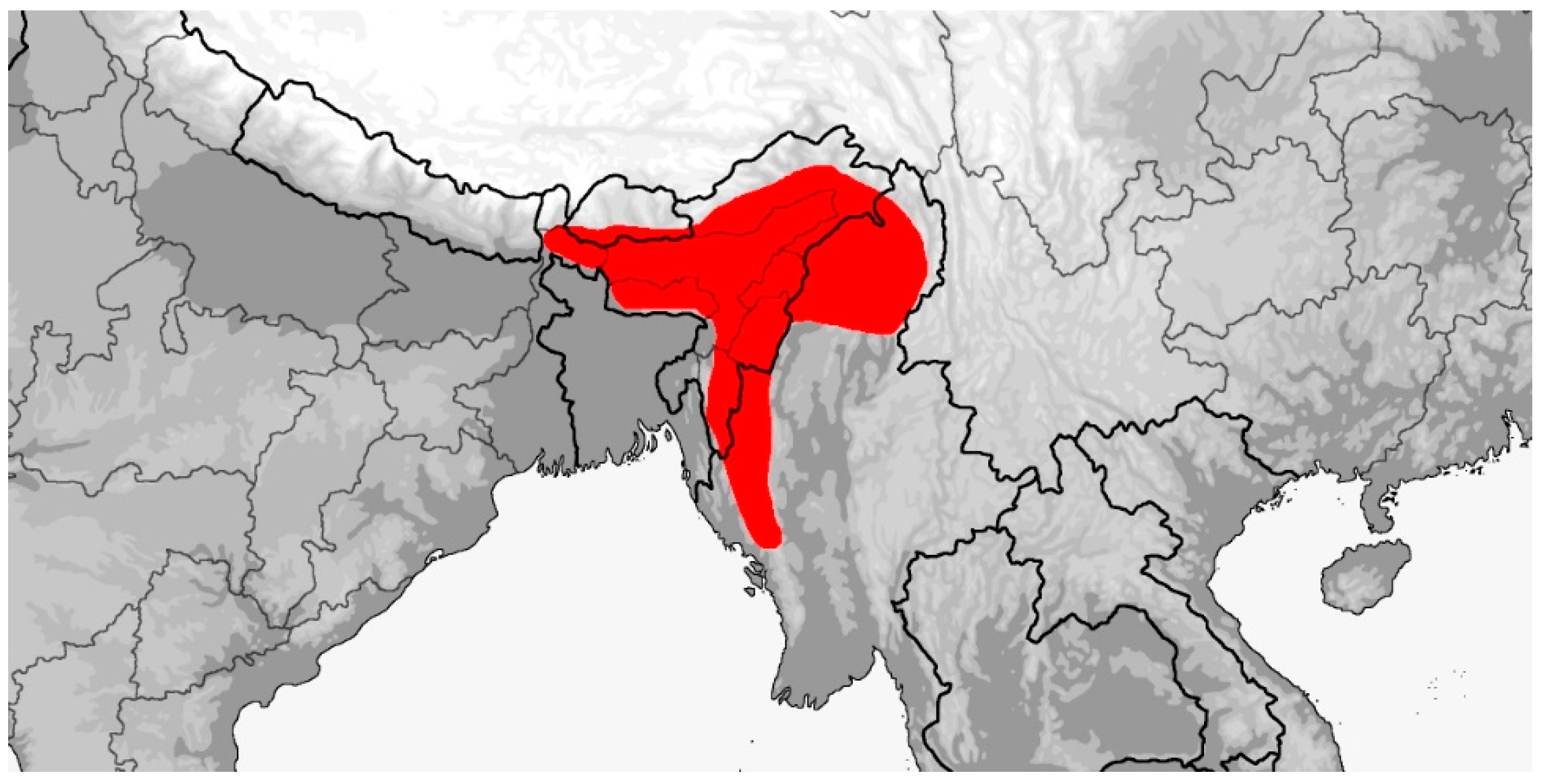
Distribution (Map 1): Indonesia (Kalimantan), East Malaysia (Sabah, Sarawak), West Malaysia (Johor, Kelantan, Pahang, Perak, Selangor), recorded from sea level to 1800 or 2000 m, but found mainly in lowland forests below 700 m [
5]. Now extinct in Singapore.
Map 1.
Map of Gonyophis margaritatus distribution.
Map 1.
Map of Gonyophis margaritatus distribution.
3.2. Gonyosoma
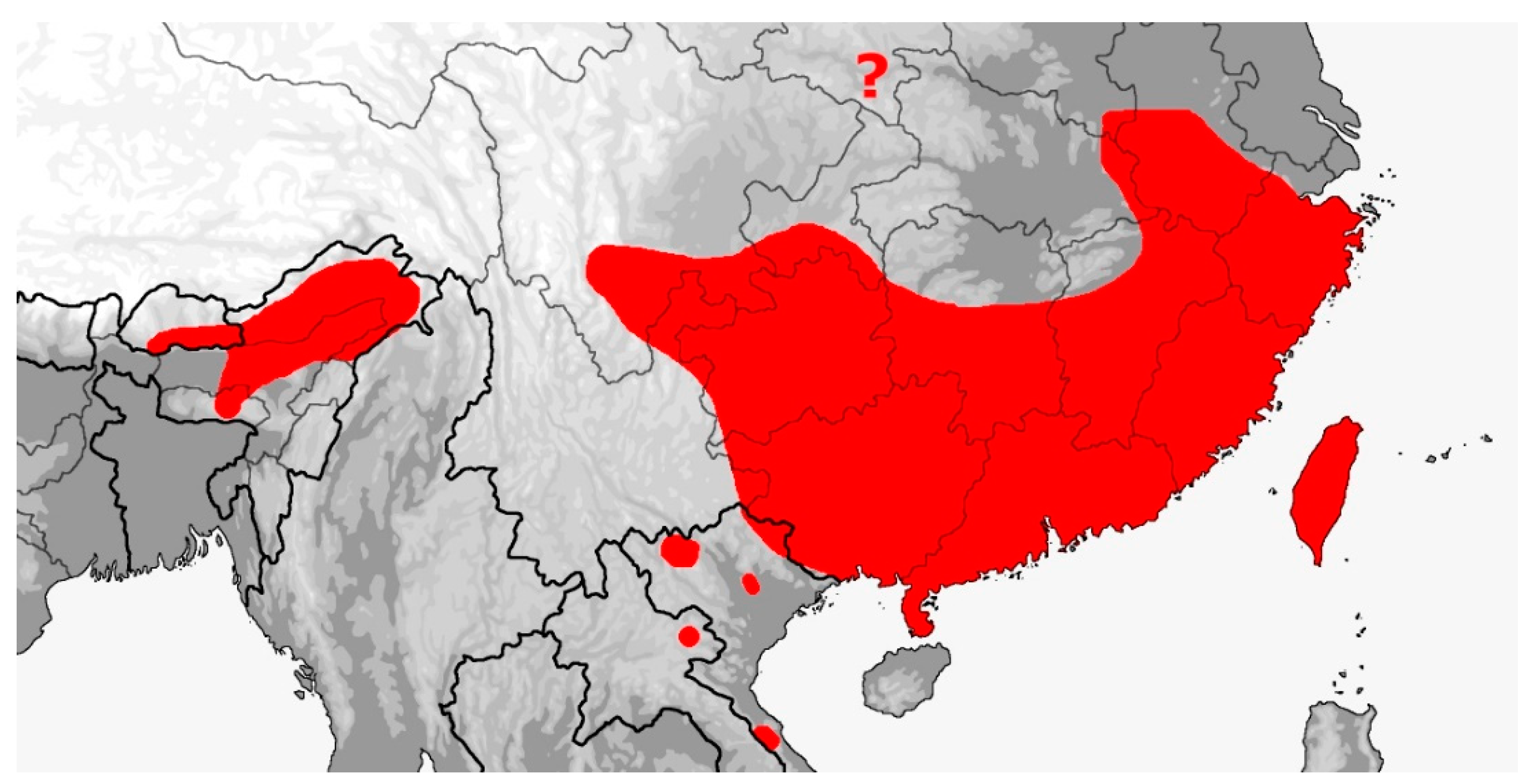
GONYOSOMA Wagler, 1828 (Map 2)
Map 2.
Map of Gonyosoma distribution.
Map 2.
Map of Gonyosoma distribution.
Type species: Coluber oxycephalus Boie, 1827
Content: 2 species – Gonyosoma jansenii Bleeker, 1858 and G. oxycephalum (Boie, 1827).
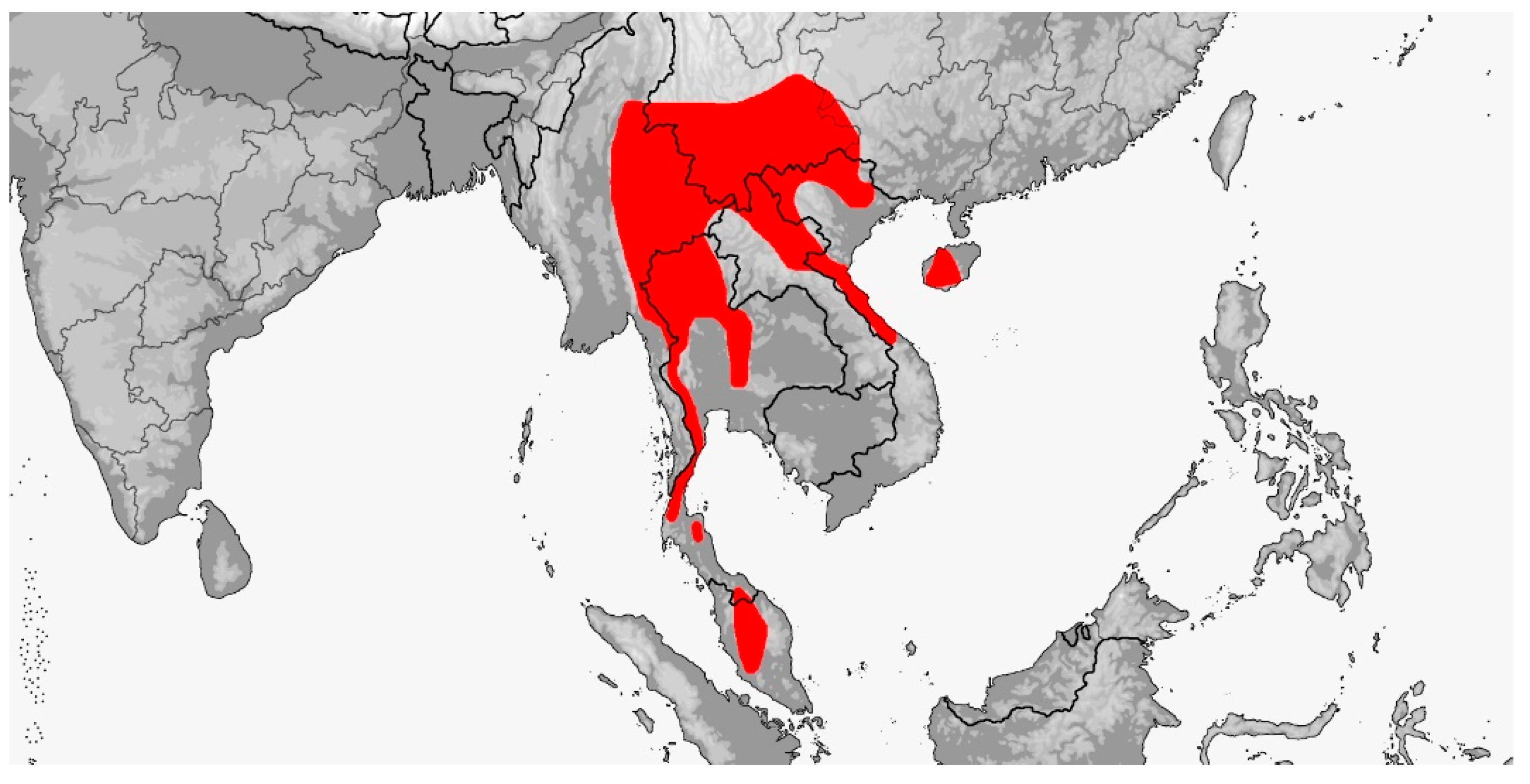
Diagnosis: Gonyosoma is unique among the other genera in having 23–27 midbody scale rows with smooth scales throughout, a red, brown or black tail, a blue tongue with black tips, an avascular, multichambered tracheal lung, and a relatively large left lung.
Distribution: Southeast Asia (from Myanmar and Vietnam southwards), Malay Archipelago, from 20–1400 m [
5].
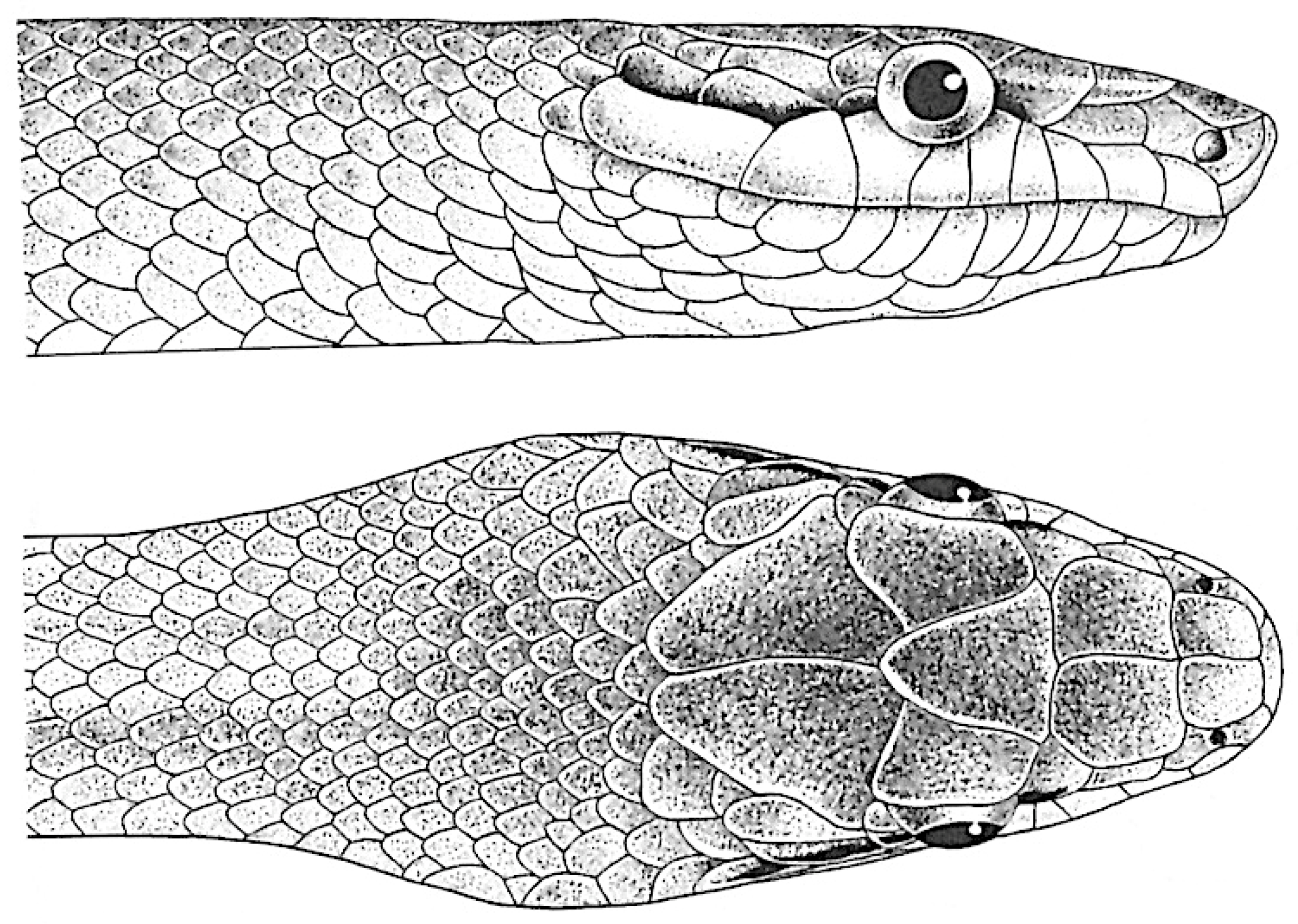
3.2.1. Gonyosoma jansenii
Gonyosoma jansenii Bleeker, 1858 (
Figure 2)
Synonym: Allophis (Elaphis) nigricaudus Peters, 1872
Diagnosis:
Gonyosoma jansenii is separable from
G. oxycephalum by its tail and subcaudal coloration (black vs. red, brown or gray), anterior temporals (1 vs. 2), and internasal proportions (subequal length and width vs. length twice the width). The only unique character of
G. jansenii among the other four genera is the black color of the tail and subcaudals. See Lang and Vogel [
40] for the most detailed description.
Description [
4,
36,
43,
44,
45]: Description: Large snakes with an average adult size 1.5–2.0 m, overall size range 420–2374 mm; head distinct from neck, body elongate and laterally compressed with oblique scale rows, 21–23 rows anteriorly, 23–25 midbody rows, and 15–17 (rarely 13) rows posteriorly; dorsal scales entirely smooth; apical scale pits elongated and paired, large and distinct throughout; scale row reduction involving paravertebral rows; ventrals 245–257, angulate with lateral keels; subcaudals 130–140, angulate and keeled; divided cloacal shield; internasal length greater than width, frontal moderate in size with tapered sides, large preocular in contact with frontal, loreal elongate, twice as long as deep; eye moderate in size with round pupil and yellow or orange iris; nasal shield elongate, divided or semidivided with a ventral suture; preocular one, postoculars 2, supralabials 9–10 with 5th to 7th entering orbit (rarely only 6th to 7th contacting eye); anterior temporals 1 (rarely 2), middle temporals 2–3, posterior temporals 3, normally 1+2+3 or 1+3+3; infralabials 11–13 with the 1st to 5th contacting the anterior pair of chin shields; posterior genials longer than anterior pair; tail long and slender, 22.8–24.8% total length; dentition unknown; umbilicus-vent interval moderate (16.7% total ventrals); no ontogenetic color change from juvenile to adult; dorsal color variable, head yellowish-olive, gray or black, lacking a distinct black pre- and postocular bar, dorsum olive or yellowish-brown , turning black posteriorly, with a narrow, discrete ventrolateral stripe on posterior body, not continuing onto tail, venter yellow to black, tail black above and below, mouth pink, tongue blue with black tips.
Natural history [
4,
36,
42,
45,
46]: Inhabits primary rainforest, both lowland and montane, in addition disturbed areas and mangroves, often in association with rivers and domestic dwellings. Semiarboreal and diurnal, it is a shy but active hunter with a diet that includes birds and mammals. Reproduction is oviparous with 2–9 eggs in a clutch with up to 4 clutches per year, neonate size 420–560 mm. This species inflates its neck vertically in a defensive posture, made possible by its tracheal lung. A rarely encountered snake, it is listed by the IUCN as a Least Concern species and not included in CITES Appendices. Vernacular name is Black-tailed ratsnake or Jansen's racer.
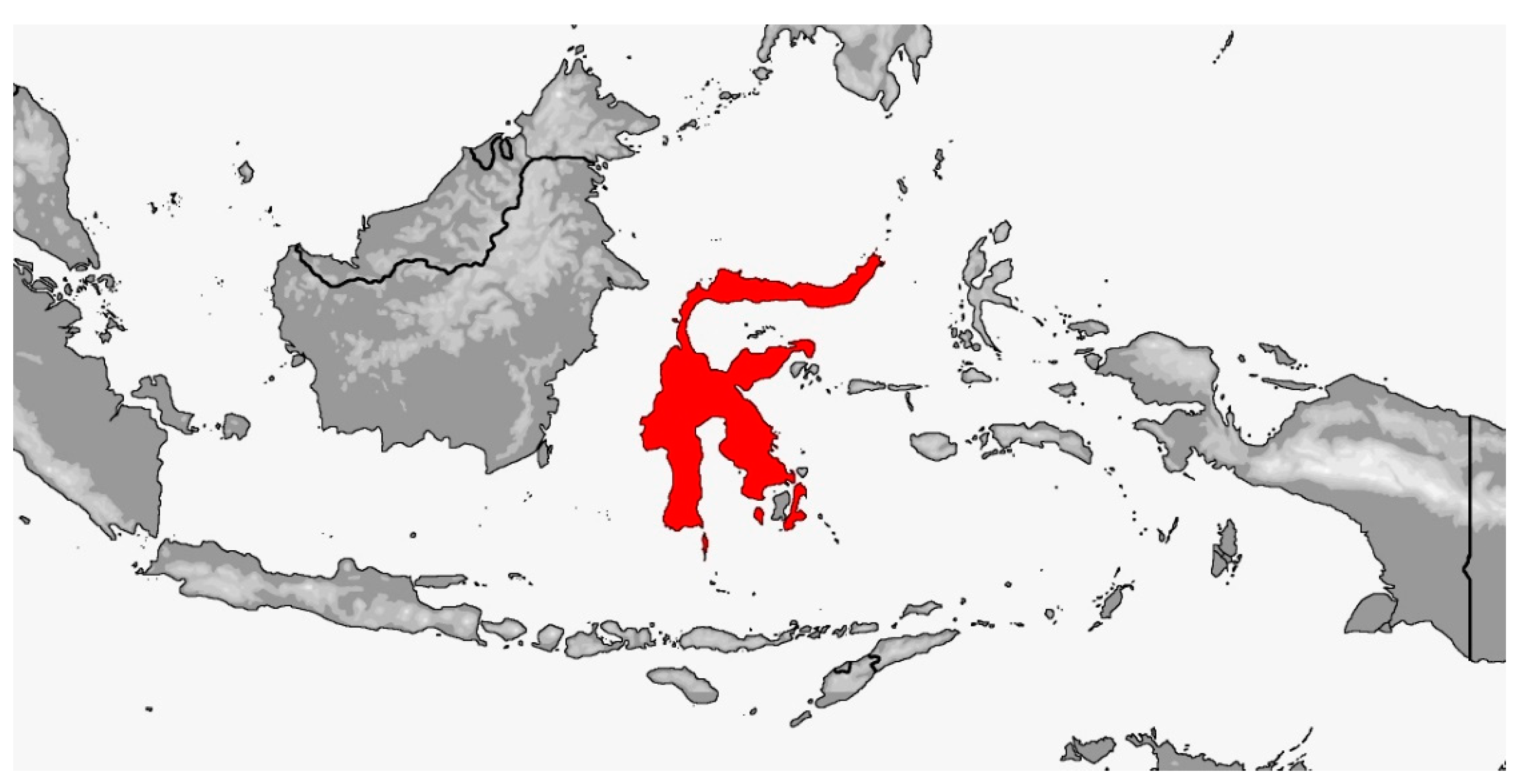
Distribution (Map 3): Indonesia (Sulawesi, incl. nearby islands Buton, Kabaena, and Selayar), from 50–1000 m [
5].
Map 3.
Map of Gonyosoma jansenii distribution.
Map 3.
Map of Gonyosoma jansenii distribution.
3.2.2. Gonyosoma oxycephalum
Gonyosoma oxycephalum (Boie, 1827) (
Figure 3)
Synonyms:
Gonyosoma viride Wagler, 1828 [
48];
Herpetodryas oxycephalus Schlegel, 1837 [
49];
Alopecophis chalybeus Gray, 1849 [
50];
Aepidea robusta Hallowell, 1861 [
51]; Coluber deroyi Werner, 1923 [
52];
Coluber floweri Werner, 1925 [
53];
Coluber janseni elegans Werner, 1926. [
54]
Diagnosis: Gonyosoma oxycephalum is distinguished from G. jansenii by its tail and subcaudal coloration (red, brown or gray vs. black), anterior temporals (2 vs. 1), and internasal proportions (length twice the width vs. length and width subequal). Gonyosoma oxycephalum is unique among the other four genera in anterior and midbody scale rows (23–27 vs. 19–21), dorsal scales (entirely smooth vs. partly keeled), a red, gray or brown tail (vs. green), a multichambered tracheal lung (vs. absent), a large left lung (vs. tiny left lung).
Description [
32,
41,
45,
56,
55,
57,
58,
59,
61]: Large snakes with an average adult size 1.6–2.0 m, overall size range 240–2400 mm; head distinct from neck, body elongate and laterally compressed with oblique scale rows, 23–27 rows anteriorly, 23–25 (rarely 27) midbody rows, and 15–17 rows posteriorly; dorsal scales completely smooth; large, elongate, faintly visible paired apical scale pits present throughout body; scale row reduction involving paravertebral rows; ventrals 229–263, angulate with lateral keels and notches; angulate subcaudals 120–157; cloacal shield divided by an oblique suture; internasal length greater than width, frontal moderate in size with tapered sides, loreal elongate, twice as long as high, large preocular in contact with frontal; eye moderate in size with round pupil and blue, green, yellowish-green or gray iris; nasal shield elongate, divided, semidivided with a dorsal or ventral suture, or entire; preocular one, postoculars 2, supralabials usually 9–11 (rarely 7–8) with three (4th–6th, 5th–7th, 6th–8th) normally entering the orbit, less commonly only two (4th-5th. 5th–6th, 6th–7th); anterior temporals 2 (rarely 1,3), middle temporals 2 (rarely 4,5), posterior temporals 3; infralabials 12–15 with the first 5 (rarely 6 or 7) contacting the anterior genials; posterior chin shields longer than anterior pair; tail long and slender, 20.9–27.7% total length; maxillary teeth 20–25, palatine teeth 12, pterygoid teeth 13–15, and dentary teeth 24–26; umbilicus-vent interval moderate (14.2–18.7% total ventrals); neonates are typically green, rarely reddish-brown, normally retaining that color as adults with a red, brown, olive or green head; typically a dark green, light green or yellowish-green body with or without a broad, distinct yellow ventrolateral stripe consisting of the lateral edges of the ventrals (lateral to keels and notches) but dorsum color variable in some areas; venter green, yellowish-green or yellow; dorsal tail red, yellowish-brown or gray, usually lacking a ventrolateral stripe; subcaudals light with a median dark zigzag stripe; a weak pre- and postocular streak may be present or absent, the latter bolder than the former; mouth pink with a blue tongue with black tips. Dorsal coloration of some specimens may be variable with a gray or grayish-brown dorsum in Java, an orange dorsum in Sulawesi, or a yellow dorsum in Thailand and the Philippines. A multichambered tracheal lung with 15–20 air cells present, allowing the snakes to inflate their necks and produce sound. The vestigial left lung is large for colubroids (5.6–7.5% SVL).
Natural history [
4,
35,
38,
40,
45,
60,
62,
63,
64,
62,
66,
67]: Inhabits a variety of primary and secondary forest habitats from tropical humid evergreen forests to secondary and open forests, including also mangroves, marshes, riverbanks and even disturbed areas and rural gardens. Preference for vegetation and branches near or overhanging fresh or brackish water. Activity is mainly diurnal but also crepuscular and nocturnal at times. Adults strictly arboreal, excepting Javanese snakes that are mainly terrestrial, with juveniles being more semiarboreal in nature. Diet consists mainly of birds and bats, the latter caught in flight near cave entrances, although bird eggs and arboreal mammals like tree rats and tree squirrels are also eaten; juveniles feed upon lizards and cave crickets. Reproduction is oviparous with mating occurring in the trees and 2–12 eggs in a clutch, 1–4 annual clutches, neonates measuring 229–560 mm. As a defensive strategy, this snake will inflate its neck region ventrally, exposing the black interstitial skin beneath. This is assisted by the presence of its tracheal lung, which has numerous inflatable air sacs. According to the IUCN it is a Least Concern species and not included in CITES Appendices. Vernacular name is Red-tailed ratsnake or Red-tailed racer.
Distribution (Map 4): Brunei, Cambodia, India (Andaman Islands), Indonesia (Bali, Bangka, Belitung, Java, Kalimantan, Karimata Islands, Legundi, Lombok, Natuna Islands, Nias, Panaitan, Riau Islands, Sebuku, Sumatra), Laos, Malaysia (Sabah, Sarawak, West Malaysia [incl. Langkawi Archipelago, Seribuat Archipelago (incl. Tioman)]), Myanmar, Philippines (Babuyan Islands [Calayan, Camiguin Norte], Balabac, Batan Islands [Batan, Itbayat, Sabtang], Bohol, Camiguin Sur, Dinagat, Dumaran, Lubang, Luzon, Mindanao, Mindoro, Negros, Palawan, Panay, Samar, Sibuyan, Sulu Islands [Bongao]), Singapore, Thailand, Vietnam, recorded from 20–1400 m [
5].
Map 4.
Map of Gonyosoma oxycephalum distribution.
Map 4.
Map of Gonyosoma oxycephalum distribution.
3.3. Rhadinophis
RHADINOPHIS Vogt, 1922 (Map 4)
Type species: Herpetodryas frenatus Gray 1853.
Content: monotypic.
Diagnosis: Rhadinophis is distinguished from all other Rynchophiini by the absence of a loreal shield (fused with prefrontal), 8 supralabials, and a frontal that is narrow with parallel sides.
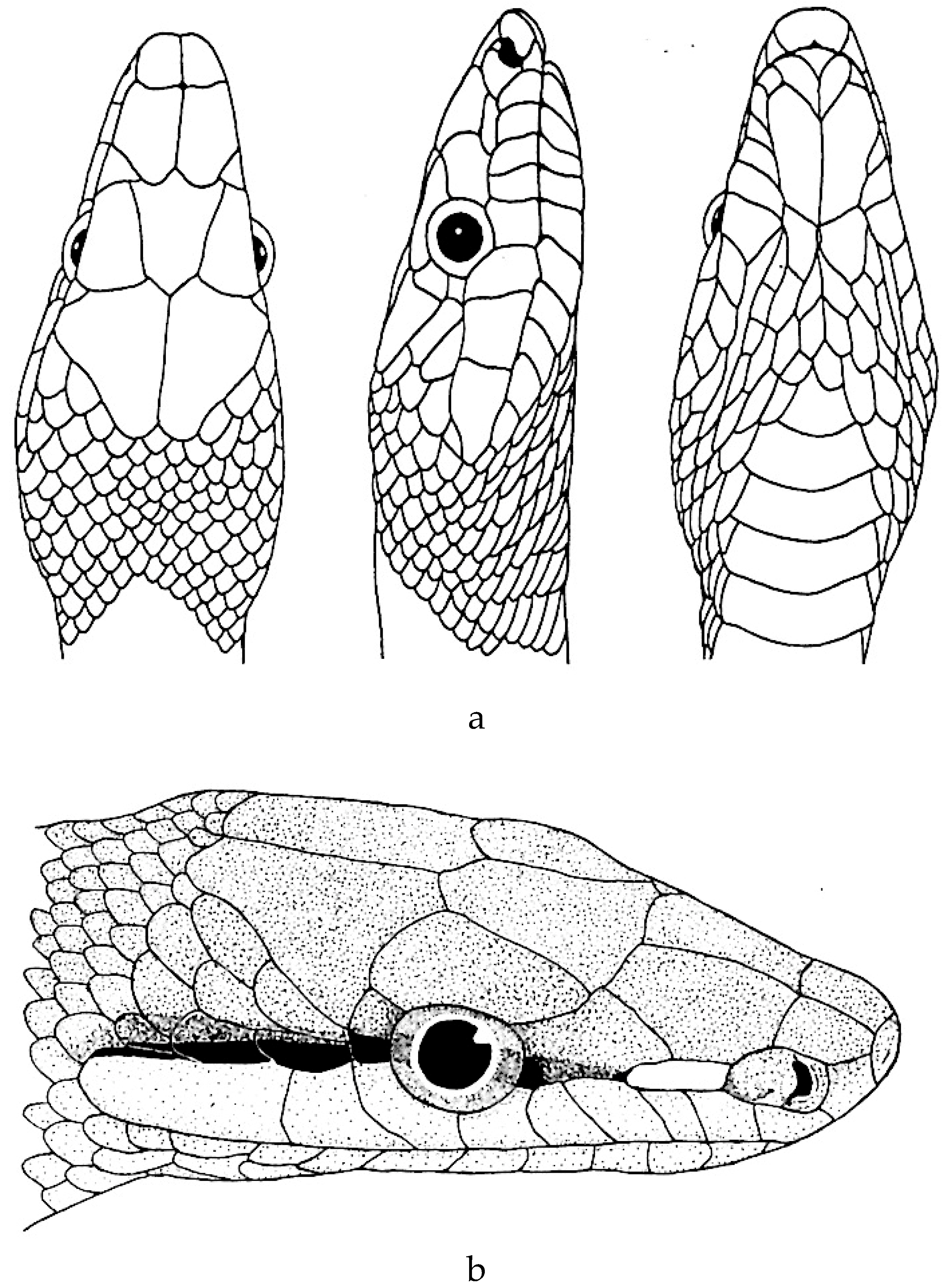
3.3.1. Rhadinophis frenatus
Rhadinophis frenatus (Gray 1853) (
Figure 4)
Synonyms:
Rhadinophis melli Vogt, 1922 [
3];
Gonyosoma caldwelli Schmidt, 1925 [
69];
Chrysopelea ornata lungchuanensis Hu et. al., 1958 [
70].
Description [
32,
71,
72,
73]: Moderate sized snakes with an average adult size 0.75–1.0 m, overall size range 120–1475 mm; head distinct from neck, body elongate and laterally compressed, dorsal scale rows arranged in longitudinal rows in 19 (rarely 21)-19 (rarely 17)-15 (rarely 13,15) rows; 3 middorsal scale rows (vertebral and paravertebral) feebly keeled, lower 8 rows smooth; paired apical scale pits small and more rounded than elongate but indistinct at midbody, absent in nuchal and cloacal region; scale row reduction involving paravertebral rows; ventrals 198–235, angulate and notched; subcaudals 108–149, angulate; cloacal shield divided; internasal length greater than width, frontal narrow with parallel borders; nasal shield divided; loreal absent, fused with prefrontal; preocular not in contact with frontal; eye moderate in size with round pupil and golden yellow iris; preocular one, postoculars 2, supralabials usually 8 (rarely 9) with three (3rd–5th or 4th–6th) entering the orbit; temporal formula varies from 2+2+2 to 2+3+3 (rarely 1 or 3 anterior temporals and 4 posterior temporals); infralabials 9–11 with the first 5 (rarely 6) contacting the anterior genials; posterior genials longer than anterior genials; tail long and slender, 23.7–31.0% total length; maxillary teeth 19–25, palatine teeth 10–13, pterygoid teeth 21, and dentary teeth 21–22; umbilicus-vent interval moderate (14.1% total ventrals); ontogenetic color/pattern change from juveniles to adults with neonates being gray, brown or olive with black crossbars and outlining of head shields; adults green (head, body and tail) with a broken ventrolateral light stripe broken up by dark triangles every 2–3 ventrals, tail also with a ventrolateral stripe, venter yellowish-green to white, subcaudals with a median zigzag line; head with a weak to strong preocular and postocular bar, mouth interior pink to purple.
Natural history [
4,
61,
68,
74,
75,
76]: Occurs in tropical and subtropical lowland and montane evergreen forests. A diurnal snake that is arboreal, semiarboreal and terrestrial, it sometimes inhabits low brush and bamboo thickets, woodpiles, rock crevices, and agricultural areas. Diet consists of rodents, birds and lizards, killed by constriction, with juveniles feeding upon fish and tadpoles. The concave preocular region suggests that this snake has binocular vision. Reproduction is oviparous with 6–12 eggs in a clutch with hatchlings 120–150 in length. Although rare it is listed by the IUCN as a Least Concern species and not included in CITES Appendices. Vernacular name is Assam Trinket snake or Khasi Hills ratsnake.
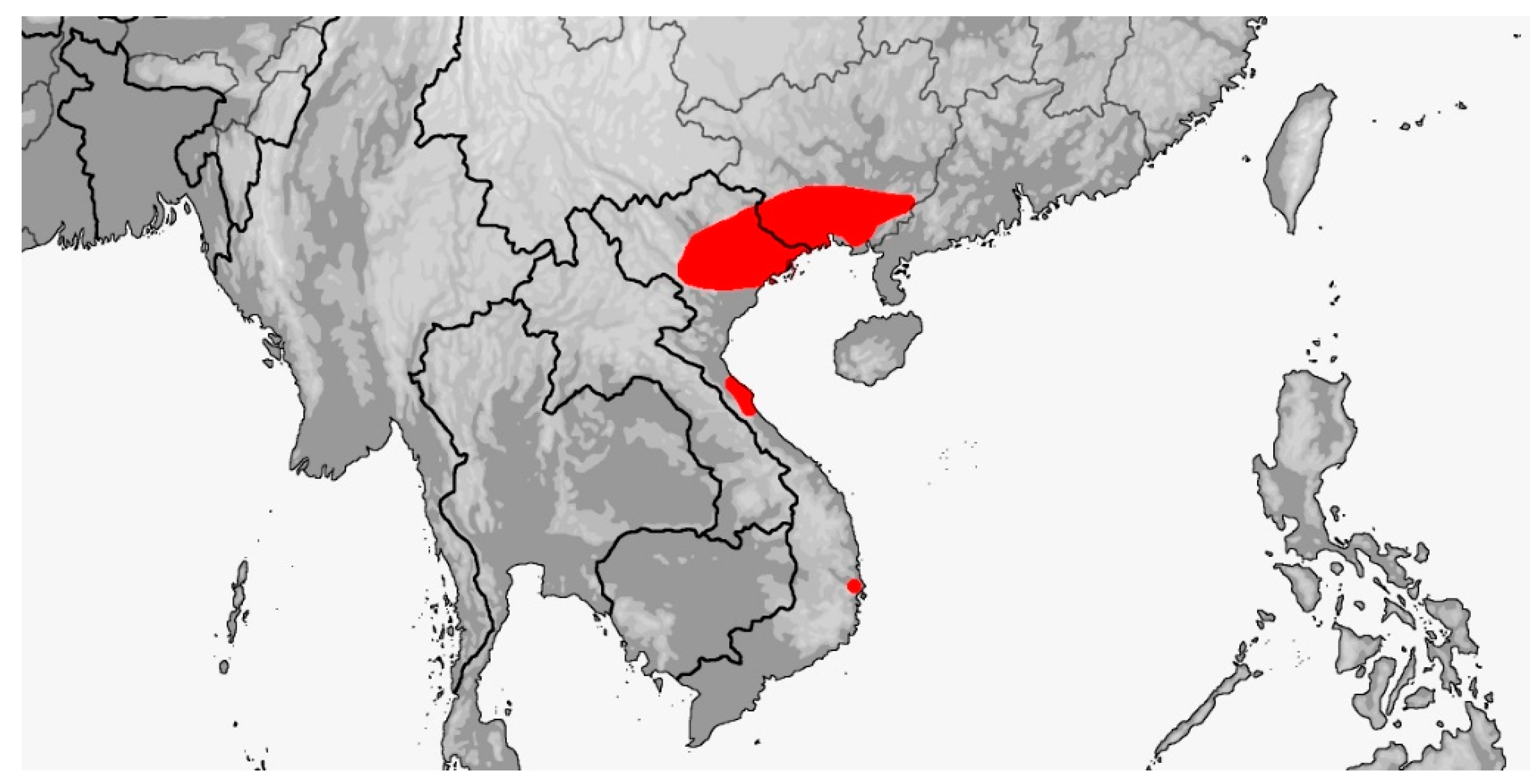
Distribution (Map 5): Bhutan, China (Anhui, Chongqing, Fujian, Guangdong, Guangxi, Guizhou, Henan, Hubei, Hunan, Jiangxi, Shaanxi, Sichuan, Yunnan, Zhejiang), India (Arunachal Pradesh, Assam, Meghalaya), Laos, Taiwan, Vietnam, from 170–2800 m. Presumably also in Myanmar [
5].
Map 5.
Map of Rhadinophis frenatus distribution.
Map 5.
Map of Rhadinophis frenatus distribution.
3.4. Rhynchophis
RHYNCHOPHIS Mocquard, 1897 (Map 6)
Map 6.
Map of Rhynchophis distribution.
Map 6.
Map of Rhynchophis distribution.
Type species: Rhynchophis boulengeri Mocquard, 1897
Content: 2 species – Rhynchophis boulengeri Mocquard, 1897 and R. hainanensis (Peng et. al., 2021)
Diagnosis: Rhynchophis is unique among the other four genera with a snout having a projecting rostral appendage of 6–10 small scales (vs. absent), frontal shape (subtriangular vs. not subtriangular), and genial proportion (posterior genials longer than anterior pair vs. otherwise).
Distribution: China (Guangxi, Hainan), Vietnam (incl. Ha Long Bay Islands: Cat Ba Island), from sea level to 2000 m.
3.4.1. Rhynchophis boulengeri
Rhynchophis boulengeri (Mocquard, 1897) (
Figure 5)
Synonym: Proboscidophis versicolor Fan, 1931 [
79]
Diagnosis: Rhynchophis boulengeri is separable from R. hainanensis by the presence of a single loreal (vs. two) and a dark postocular bar. The only unique characters of R. boulengeri among the Rhynchophiini are the nasal appendage and a subtriangular frontal (shared with R. hainanensis).
Description [
10,
80,
81,
82]: Moderate sized snakes with an average adult size 0.8–1.0 m, overall size range 170–1630/1700 mm; head distinct from neck, body elongate and laterally compressed, dorsal scale rows arranged longitudinally in 19 anterior rows, 19 (rarely 17,18) midbody rows, and 15 (rarely 13) posterior rows, with and without some keeling; middorsal 3–7 rows with weakly keeled scales, lower 6–8 rows entirely smooth; elongate apical scale pits throughout, paired, large and parallel in orientation but indistinct; scale row reduction involving paravertebral rows; ventrals 207–227, angulate, keeled and notched; subcaudals 101–133, angulate; cloacal shield divided; internasal length equal to internasal width, frontal subtriangular; nasal shield divided or semidivided with a superior suture; elongate loreal present; eye moderate in size with round pupil and reddish-brown iris; one large preocular in contact with frontal, postoculars 2, supralabials normally 9 (rarely 10) with three (4th–6th or 5th–7th) entering the orbit; temporal formula varies from 2+2+3 to 2+3+3 (rarely one anterior temporal); infralabials 10–11 with the first 5 (rarely 6) contacting the anterior genials; posterior genials longer than anterior genials; tail long and slender, 20.6–30.2% total length; maxillary teeth 16–22 and dentary teeth 25; umbilicus-vent interval moderate (13.1–13.7% total ventrals); ontogenetic color/pattern change from juveniles to adults with neonates being gray or brown with black markings; adults green (head, body and tail) with a narrow, distinct ventrolateral light stripe that continues onto tail, venter pale green, subcaudals dark with a solid green stripe; head with a weak pre- and postocular stripe, mouth interior pink, tongue pink.
Natural history [
39,
45,
83,
84,
85,
86,
87,
88,
89]: Inhabits the lower and middle layers of primary subtropical and tropical monsoon rainforests, particularly near streams and lakes, but can also be found in degraded forests. Captive specimens spend a lot of time in water, especially juveniles. Arboreal and semiarboreal in habits, it is both diurnal and nocturnal, with most activity during the first half of the night. Diet consists mostly of rodents and small mammals with juveniles preferring lizards and fish. Captive juveniles may be cannibalistic. Reproduction is oviparous with 2–17 eggs in an annual clutch, the females often guarding the eggs after oviposition, with neonates measuring 170–385 mm. Listed by the IUCN as a Least Concern species and not included in CITES Appendices. Vernacular name is Rhinoceros ratsnake or Vietnamese horned snake.
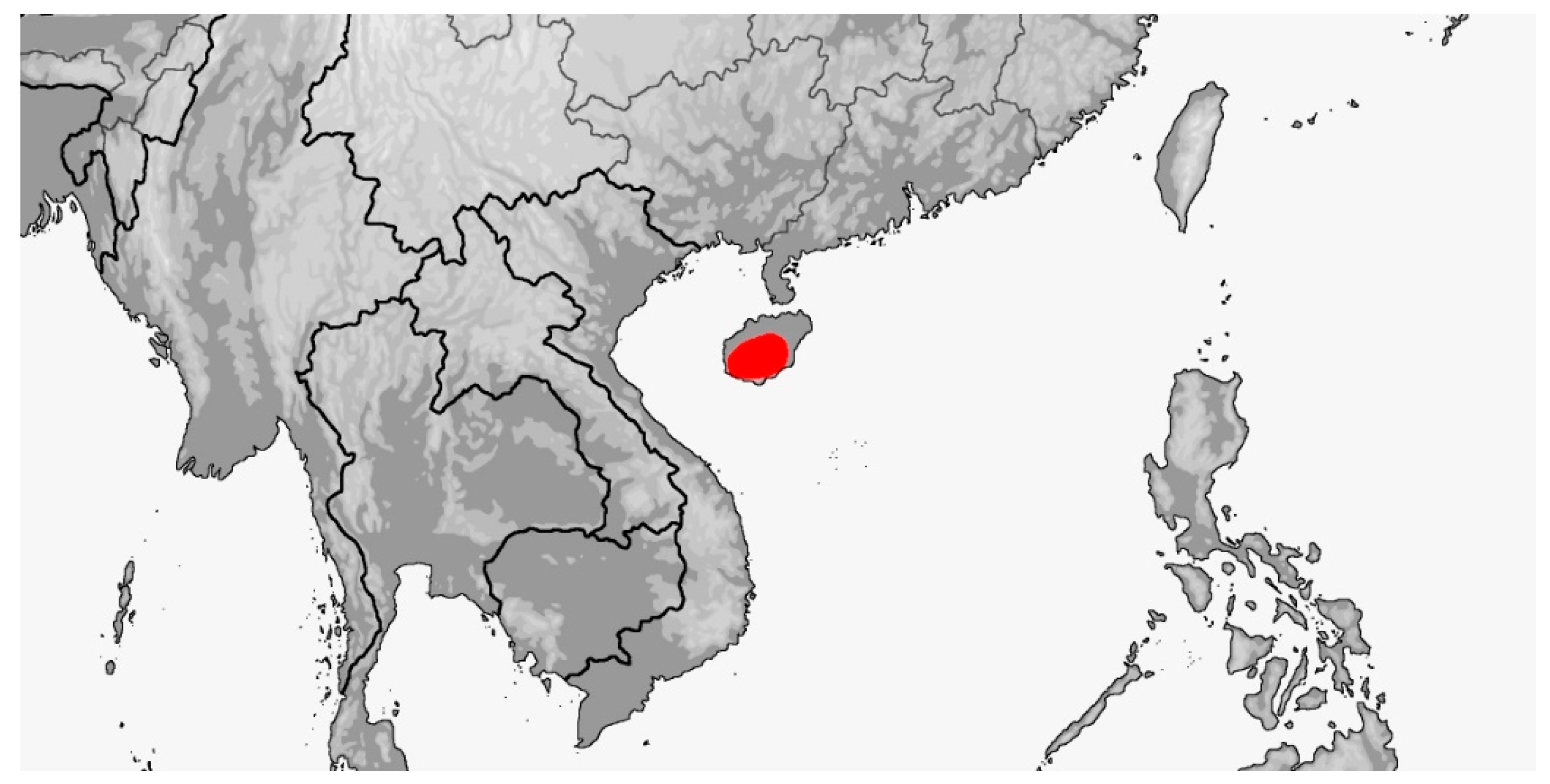
Distribution (Map 7): China (Guangxi), Vietnam (incl. Ha Long Bay Islands: Cat Ba Island), documented from sea level to 2000 m [
5].
Map 7.
Map of Rhynchophis boulengeri distribution.
Map 7.
Map of Rhynchophis boulengeri distribution.
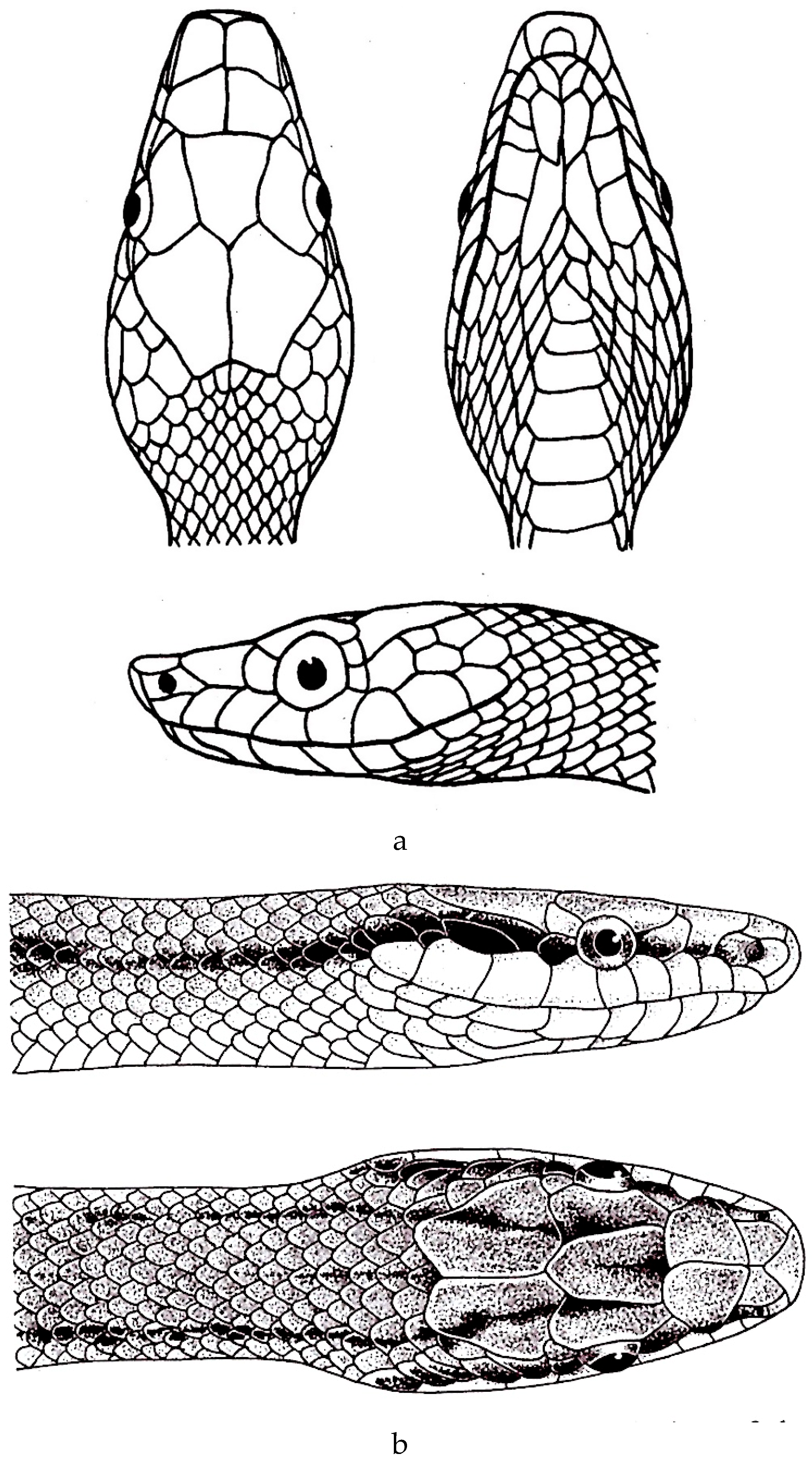
3.4.2. Rhynchophis hainanensis
Rhynchophis hainanensis (Peng et. al., 2021) (
Figure 6)
Diagnosis: Rhynchophis hainanensis can be distinguished from R. boulengeri by the presence of two loreals (vs. one) and absence of a dark postocular bar (vs. present). It is unique among all other genera in the presence of a nasal appendage and a subtriangular frontal (shared with R. boulengeri).
Description [
29]: Moderate sized snakes with an average adult size 0.7–0.9 m, overall size range 718–1229 mm; head distinct from neck, body elongate and laterally compressed, dorsal scale rows arranged in longitudinal rows in 19-19-15 (rarely 13) rows, with and without some weak keeling; apical scale pits unknown; ventrals 216–221, angulate and keeled; subcaudals 122–133, angulate and keeled; cloacal shield divided; internasal length equal to internasal width, frontal subtriangular; nasal shield semidivided with a dorsal suture; loreal divided into two shields; eye moderate in size with round pupil and brown iris; one large preocular contacting frontal, 2 postoculars, supralabials 9 with three 4th, 5th and 6th in contact with the eye; temporal formula 2+2+3; infralabials 10–12 with 1st to 5th in contact with the anterior chin shields; posterior genials longer than anterior genials; tail long and slender, 22.0–32.5% total length; dentition unknown; neonates gray with ontogenetic variation that turns green in adults; dorsal head, body and tail green, venter and subcaudals green, a yellow ventrolateral stripe present but not on tail; lacking pre- and postocular dark streaks; mouth interior and tongue unknown.
Natural history [
29]: Little is known about this recently described species. Inhabits subtropical rainforests, particularly valleys with streams. Arboreal with nocturnal activity recorded (presumably also diurnal like
R. boulengeri). Feeds upon mice in captivity. Oviparous with 6 eggs per clutch. Its conservation status by the IUCN and CITES has not yet been evaluated. Vernacular name is Hainan rhinoceros ratsnake.
Distribution (Map 8): China (Hainan). Currently known only from the Diaoluoshan Mountains (type locality) and the Jianfengling Mountains, from 80–900 m [
5].
Map 8.
Map of Rhynchophis hainanensis distribution.
Map 8.
Map of Rhynchophis hainanensis distribution.
3.5. Verdigrophis
VERDIGROPHIS gen. nov. (Map 9)
Map 9.
Map of Verdigrophis distribution.
Map 9.
Map of Verdigrophis distribution.
Type species: Coluber prasinus Blyth, 1855
Content: 2 species – Verdigrophis coeruleus (Liu et. al., 2021) and V. prasinus (Blyth, 1858).
Diagnosis: The only unique character of Verdigrophis among the Rhynchophiini is the anterior and posterior genials subequal in length.
Etymology: The generic name is derived from the Latin verdigris, meaning bluish-green, greenish-blue, or shades between green and blue, in reference to the coloration of the dorsum and iris. Verdigris is also based upon the Old French term vert-de-Grèce, which refers to the turquoise pigment resulting from the oxidation of copper.
Distribution: Southeast Asia (from Bhutan and S. China to West Malaysia), 75–2560 m [
5].
3.5.1. Verdigrophis coeruleus
Verdigrophis coeruleus (Liu et. al., 2021) (
Figure 7)
Diagnosis: The only unique characters of Verdigrophis coeruleus among the other genera are a blue iris and gray mouth interior. Verdigrophis coeruleus differs from V. prasinus by its ventrolateral stripe (white vs. yellow), cloacal shield (divided vs. entire), iris color (blue, greenish-blue or bluish-green vs. greenish-yellow or brownish-yellow), tongue color (brownish-yellow vs. reddish-brown), and mouth interior (gray vs. pink).
Description [
28,
32]: Moderate sized snakes with an average adult size 0.8–1.0 m, overall size range 220–1192 mm; head distinct from neck, body elongate and laterally compressed, dorsal scale rows normally arranged in 19-19-15 longitudinal rows (rarely 20 anterior rows, 15 or 17 midbody rows, and 13 or 17 posterior rows); 5–11 scale rows feebly keeled, lower 4– 6 rows smooth; apical scale pits paired, large, and widely-spaced anteriorly and at midbody but absent posteriorly; scale row reduction involving paravertebral rows; ventrals 181–224, angulate, keeled and presumably notched; subcaudals 88–128; cloacal shield divided; internasal width greater than length, frontal broad with tapered sides; nasal shield semidivided with a ventral suture; loreal absent, fused with prefrontal; preocular not in contact with frontal; moderate sized eye with round pupil and a blue or greenish-blue iris; preocular one, postoculars 2, supralabials usually 9 (rarely 8,10) with three the 3rd–5th entering the orbit; anterior temporals 1–2, middle temporals 2(rarely 1,3) and posterior temporals 2–3; infralabials 9–11 with the first 5 contacting the anterior genials; both pairs of genials equal in length; tail long and slender, 22.4–27.5% total length; 20–21 maxillary teeth; umbilicus-vent interval unknown; dorsum of head, body and tail are green, bluish-green or blue, lacking a ventrolateral stripe on venter and subcaudals, venter pale green to bluish-green, iris blue, greenish-blue or bluish-green (juveniles with yellow iris), interior of mouth gray, tongue brownish-yellow with black tips.
Natural history [
29,
32,
95]: It inhabits a variety of primary and secondary rainforests, from tropical humid evergreen forests to subtropical deciduous montane forests. Preference for moist areas with big trees and tangled vegetation near rivers and streams but also in bamboo thickets, shrubs and bushes. An agile and swift species, diurnal and crepuscular, mainly arboreal and sleeping in the trees at night but occasionally can be found on the ground. Diet includes small mammals like rodents, which are constricted, and presumably also birds and lizards. Reproduction is oviparous with 3–11 eggs/clutch and neonates measuring 200–280 mm. Its conservation status by the IUCN and CITES has not yet been evaluated. Vernacular name is Blue ratsnake.
Distribution (Map 10): China (Guangxi, Guizhou, Hainan, Sichuan, Yunnan), Laos, Malaysia (West Malaysia), Myanmar, Thailand, Vietnam, from 250–1650 m [
5].
Map 10.
Map of Verdigrophis coeruleus distribution.
Map 10.
Map of Verdigrophis coeruleus distribution.
3.5.2. Verdigrophis prasinus
Verdigrophis prasinus (Blyth, 1854) (
Figure 8)
Synonym: Gonyosoma gramineum Günther, 1864
Diagnosis: Verdigrophis prasinus is unique among the other four genera by its entire cloacal shield. It is distinguished from V. coeruleus by its iris color (yellowish-green vs. blue), tongue color (reddish-brown vs. brownish-yellow), mouth interior (pink vs. gray), and the cloacal shield (entire vs. divided).
Description [
32]: Moderate sized snakes with an average adult size 0.9–1.2 m, overall size range 150–1355/1500 mm; head distinct from neck, body elongate and laterally compressed, dorsal scale longitudinally arranged in 19-19-15 rows typically but there are rarely 17 or 21 rows anteriorly, 17 at midbody, and 13,14 or 17 rows posteriorly); 7–9 midbody scale rows feebly keeled, lowermost 5– 6 rows smooth; paired apical scale pits large, elongate and widely-spaced; scale row reduction involving paravertebral rows; ventrals 186–209, angulate, keeled and notched; subcaudals 91–116, angled, keeled and notched; cloacal shield entire; internasal width greater than length, frontal broad with tapered sides; nasal shield divided or semidivided with a ventral suture; loreal single (rarely absent); preocular not in contact with frontal; moderate sized eye with round pupil and a blue or greenish-yellow or olive iris; preocular one, postoculars 2, supralabials usually 9 (rarely 8) with three the 3rd–5th (rarely 4th–5th) entering the orbit; anterior temporals 1–2 (rarely 3), middle temporals 1–2 (rarely 3) and posterior temporals 2 (rarely 3); infralabials 9–10 (rarely 12) with the first 5–6 contacting the anterior genials; both pairs of chin shields subequal in length; tail long and slender, 19.4–26.7% total length; maxillary teeth 19–23, dentary teeth 25; umbilicus-vent interval 13.7–14.4% total ventrals; lacking ontogenetic color change, juveniles are green, head and body dorsum green, bluish-green or blue, tail green, venter pale greenish-yellow, yellow or white, subcaudals dark, lacking ventrolateral stripe on venter and subcaudals, no pre- or postocular bar, iris greenish-yellow or brownish-yellow, mouth interior pink, tongue reddish-brown with black tips.
Natural history [
4,
36,
45,
61,
74,
90,
91,
92,
93,
94,
95]: Inhabits tropical evergreen and broadleaf forests, both lowland and submontane, including deciduous montane forests. An arboreal species that is usually found in trees or foliage associated with bodies of water, such as bamboo clusters, bushes and cane breaks. It is reported to be diurnal, crepuscular and even nocturnal. Diet consists mainly of small mammals (rodents and shrews) but also includes birds, lizards and snakes. Reproduction is oviparous with mating observed throughout the year and 3–14 eggs per clutch, which are produced once or twice per year, neonates ranging in length from 150–280 mm. Listed by the IUCN as a Least Concern species and not included in CITES
Appendices. Vernacular name is Green bush ratsnake, Green tree racer, or Green mountain racer.
Distribution (Map 11): Bhutan, India (Arunachal Pradesh, Assam, Manipur, Meghalaya, Mizoram, Nagaland, West Bengal), Myanmar. Expected to occur in Tibet, China, from 75–2560 m. A population of uncertain taxonomic status in southern Laos and Central Vietnam has also been assigned to this species [
5].
Map 11.
Map of Verdigrophis prasinus distribution.
Map 11.
Map of Verdigrophis prasinus distribution.
3.6. Rhynchophiini
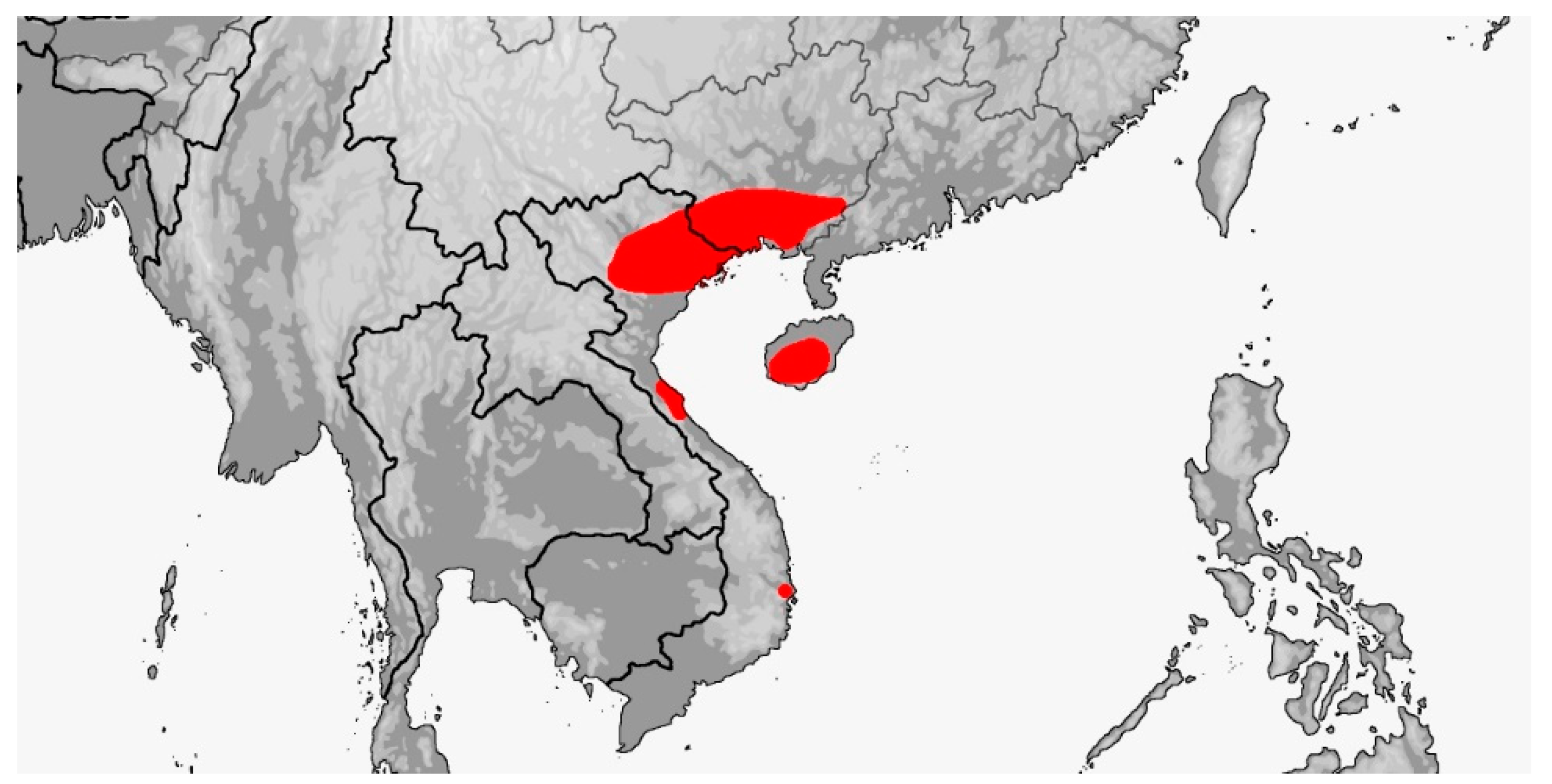
Tribe Rhynchophiini trib. nov. (Map 12)
Map 12.
Map of Rhynchophiini distribution.
Map 12.
Map of Rhynchophiini distribution.
Type genus: Rhynchophis Mocquard, 1897
Content: Five genera (Gonyophis, Gonyosoma, Rhadinophis, Rhynchophis, and Verdigrophis) containing eight species.
Etymology: Although the name Gonyosoma is the oldest among the group of five genera, we feel that the tribal name should be based upon the most conspicuous genus, which is Rhynchophis with its distinctive, projecting nasal appendage.
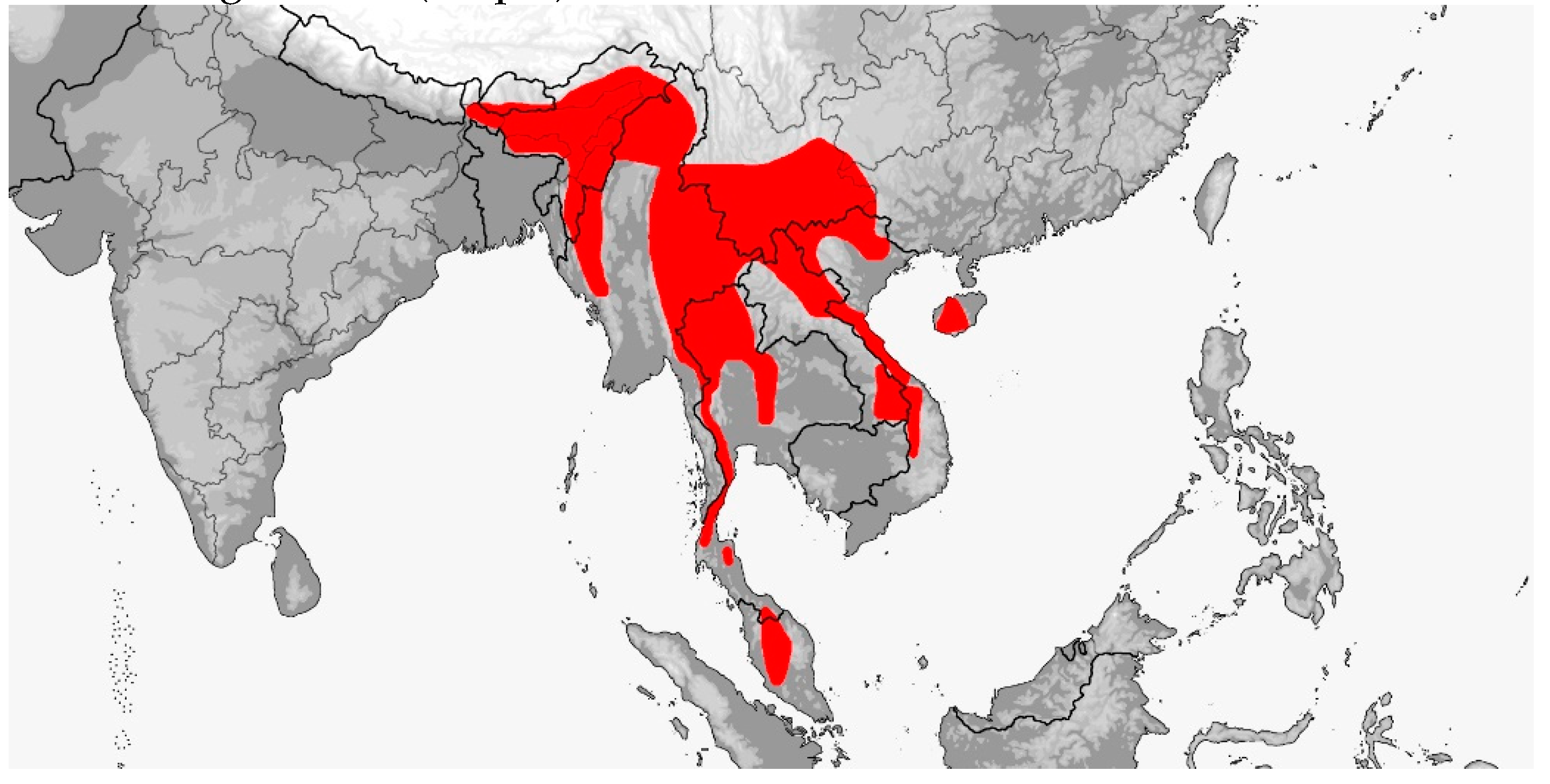
Description: Large sized snakes (adults 1.5–2.4 m); head distinct from neck, body elongate and laterally compressed with feebly keeled and/or smooth dorsal scales, in 17–27 longitudinal (rarely oblique) rows at midbody and paravertebral scale row reduction posteriorly to 13–17 rows, paired apical scale pits present, large and elongate in shape and faintly visible to distinct; ventrals 181–263, angulate with lateral keels and notches; angulate and keeled subcaudals 88–157; cloacal shield divided (entire in one species); eye moderate with round pupil and distinctly colored iris; nasal entire, divided or semidivided with a dorsal or ventral suture; preocular one, postoculars 2, anterior temporals 1–2 (rarely 3), middle temporals 2–3 (rarely 1, 4–5), posterior temporals 2–3 (rarely 4); supralabials usually 8–10 (but ranging from 7–11) with three (rarely two) entering the orbit; infralabials usually 9–12 (rarely 13–15) with the first 5 (rarely 6–7) contacting the anterior genials; anterior/posterior genial length ratio variable; tail relatively long (19–33% total length) and prehensile; umbilicus-vent interval moderate (12–19% total ventrals); maxillary dentition aglyphous with 19–23 subequal-sized teeth, 10–13 palatine teeth, 13–21 pterygoid teeth, and 21–26 dentary teeth. Coloration is variable although most specimens are uniformly some shade of green with black, white or blue interstitial skin that is exposed during inflation. Color pattern of the head, dorsum, tail, venter, subcaudals, iris, mouth and tongue varying to some degree. The loreal shield is also variable from typically being single to divided or even absent. The Rhynchophiini are arboreal or semiarboreal forest inhabitants, ranging from primary humid tropical rainforests to secondary mixed deciduous subtropical forests, often found in vegetation associated with water; diurnal, crepuscular and even nocturnal in activity; swift and agile climbers with a prehensile tail; having a diet of mainly mammals and birds that are killed by constriction; and reproductively oviparous with small clutches, usually less than 10 eggs, but with up to four clutches laid per year.
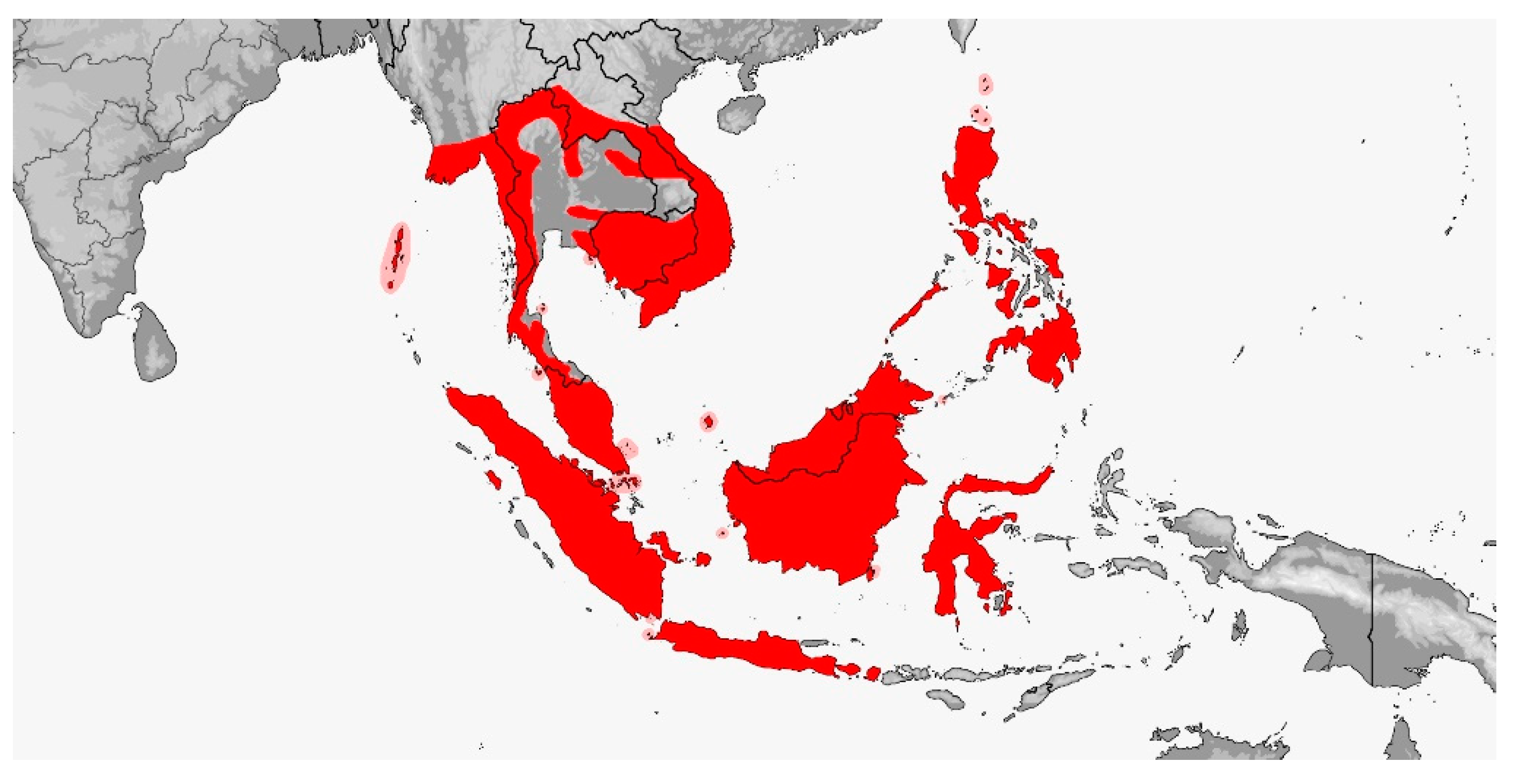
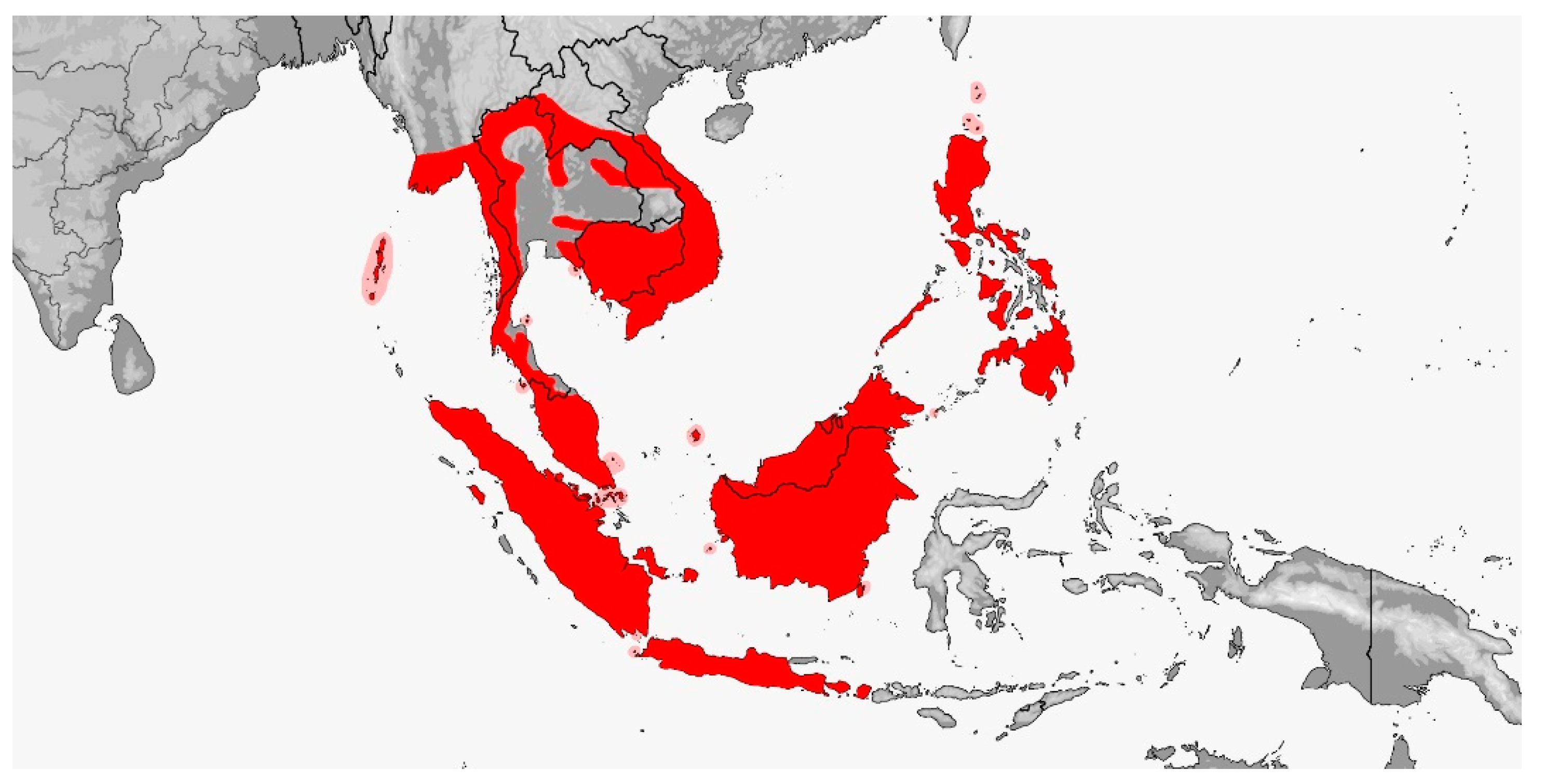
Distribution (Map 12): From Bhutan and northeastern India to southeastern China and Taiwan, thence southward throughout Southeast Asia, the Andamans and Philippines to Sundaland Indonesia, from sea level to 2800 m [
5].
3.7. Rhynchophiini Key
| Key to the genera and species of the tribe Rhynchophiini |
| |
| 1a. Rostral appendage present, subtriangular frontal, internasal length |
| equals width |
2 (Rhynchophis) |
| 1b. Rostral appendage absent, frontal not subtriangular, internasal length |
| not equal in width |
3 |
| 2a. One elongate loreal, pre- and postocular bars present, ventrolateral |
| light stripe present on tail, temporals usually 2+3+3 |
Rhynchophis boulengeri |
| 2b. Two normal loreals, pre- and postocular bars absent, ventrolateral |
| light stripe absent on tail, temporals usually 2+2+3 |
Rhynchophis hainanensis |
| 3a. Midbody scale rows 23–27, scales entirely smooth, preocular contacts |
| frontal, umbilicus-vent interval >14% total ventrals, tail uniformly black, |
| red or brown, tracheal lung present |
4 (Gonyosoma) |
| 3b. Midbody scale rows 17–19, scales partly/feebly keeled, preocular |
| not contacting frontal, umbilicus-vent interval < 14% total ventrals, tail |
| uniformly green or black with orange rings, tracheal lung absent |
5 |
| 4a. Tail red to brown, ventrolateral light stripe broad, iris green, blue, or gray, |
| subcaudals light with median zigzag line, usually two anterior temporals |
Gonyosoma oxycephalum |
| 4b. Tail black, ventrolateral light stripe absent or narrow, iris yellow or orange, |
| subcaudals uniformly black, usually one anterior temporal |
Gonyosoma janseni |
| 5a. Loreal shield absent, supralabials 8, internasal length greater than width, |
| posterior genials longer than anterior genials, iris yellow, ontogenetic |
| color change from juveniles to adult, frontal narrow with parallel sides |
| |
Rhadinophis frenatus |
| 5b. Loreal shield present, supralabials 9, internasal width greater than length, poste rior genials not longer than anterior genials, iris not yellow; no ontogenetic color change from juveniles to adult, frontal broad with tapered sides |
6 |
| 6a. Head orange, dorsum multicolored with orange and black rings |
| posteriorly, ventrals > 230, ventrolateral light stripe absent on body and tail; |
| anterior genials longer than posterior genials |
Gonyophis margaritatus |
| 6b. Head green or blue, ventrals < 225, spotted ventrolateral stripe |
| present on body; anterior genials equal in length to posterior genials |
| 7 (Verdigrophis) |
| 7a. Cloacal shield entire, ventrolateral stripe yellow, iris greenish-yellow or olive, mouth pink, tongue reddish-brown |
Verdigrophis prasinus |
| 7b. Cloacal shield divided, ventrolateral stripe white, iris blue or greenish-blue, |
| mouth grey, tongue brownish-yellow |
Verdigrophis coeruleus |
| |
|
|
4. Discussion
The Miocene Epoch, 5.3–23.03 Mya, was a period that saw the decline (Aniliidae, Boidae, Pythonidae, Tropidophiidae) or extinction (Anomalophiidae, Nigerophhidae, Paleophiidae, Russellophiidae) of Eurasian henophidian snakes and the rise or modernization of caenophidians (Colubridae, Elapidae, Viperidae) [
96,
97]. According to Chen et al. [
12], the origin of the Rhynchophiidae can be traced to late Early Miocene or early Middle Miocene, approximately 20.47 Mya (CI = 18.0–27.95 Mya). In an earlier study Burbrink & Lawson [
18] placed the origin of the
Gonyosoma jansenii and
G. oxycephalum clade at 38.1 Mya (CI = 37.1–39.2 Mya) in the Eocene and
Elaphe frenata and
E. prasina at 24.8 Mya (CI = 24.2–25.5 Mya). The nearest relatives of the Rhynchophiini appear to be
Coelognathus, which is the basal sister group, and the Lycodontini and Boigini, the two sister clades of the Rhynchophiini (Figueroa et al. [
98].
Gonyophis and
Verdigrophis are sister taxa and that clade is sister group to the clade containing
Rhynchophis and
Rhadinophis, with
Gonyosoma being the ancestral sister group to the other four clades.
Among the Rhynchophini, Gonyosoma is unique in comparison with the other four genera in the number of scale rows (23–25 vs. 19), carination of dorsal scales (entirely smooth vs. partly keeled), and a multicameral tracheal lung (present vs. absent), Rhadinophis is unique from the other genera in the loreal shield (absent vs. present), number of supralabials (8 vs. 9–11), and frontal shape (narrow with parallel sides vs. moderate to broad with tapered sides), Rhynchophis is unique among the other genera in the rostral appendage (present vs. absent), frontal shape (subtriangular vs. not subtriangular), and internasal shape (length and width subequal vs. length or width greater than other dimension), Gonyophis is unique among the other genera in genial proportions (anterior longer than posterior vs. posterior longer than anterior), dorsal head color (orange or yellow vs. green, brown or maroon), and tail color and pattern (black with orange/yellow rings vs. uniformly green), and Verdigrophis is unique among the other four genera in genial proportions (anterior and posterior length subequal vs. anterior or posterior longer than other pair). We suggest that the recognition of each of the five clades of the Rhynchophiini provides the most taxon information for the phylogenetic history, relationships, and taxonomy of the group. Subsuming all eight species into one genus is less informative and ignores the distinctiveness of the genera.
Material examined
Gonyophis margaritatus – FMNH 138677, 243941; Gonyosoma jansenii – UCM 57840; G. oxycephalum – FMNH 14909, 180107, SDSU uncatalogued; Rhynchophis boulengeri – ROM 26999, 34730; Rhadinophis frenatus – FMNH 22345, 24941, 172322; Verdigrophis coeruleus – FMNH 128282; V. prasinus – SDSU uncatalogued.
Author Contributions
Conceptualization, all authors; Methodology, all authors; Formal Analysis, all authors; Investigations, all authors; Visualization, R.M. and E.H.; Data Curation, V.W.; Writing–Original Draft Preparation, V.W.; Writing–Review and Editing, V.W. and R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data used in this study are presented in the tables and text.
Acknowledgments
Thanks are due to the curators and staff of FMNH (H. Marx, R.F. Inger, A. Resetar, R.G. Kamei and J. Mata), ROM (D. Evans and A.J. Lathrop), SDSU (R. Etheridge), and UCM (E. Braker) for the loan of material that forms the basis of this report. Also, VW is indebted to James Hanken and Stevie Kennedy-Gold (MCZ) for approval of the loans and workspace to study the snakes. Emma Hsiao expertly produced the figures of Rhynchophis hainanensis and Verdigrophis coeruleus from photographs.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Bio 1
Van Wallach (Independent Researcher). Education: B.S. in Zoology (SDSU, 1976); M.S. in Zoology & Physiology (LSU, 1991), and Ph.D. in Biology (Northeastern University, 1998). Employment: Retired from C.A. position in Herpetology at Harvard University (MCZ, 1989–2011), Louisiana State University (LSUMZ, 1985–1989), and San Diego Natural History Museum (SDMNH, 1982–1984). Interests: Snake systematics, biodiversity, taxonomy, comparative morphology, nomenclature, identification, and dicephalic snakes. Taxa described: 54 species, 15 genera, and 2 families. Membership: SSAR and CHS.
Author Bio 2
Rune Midtgaard (Independent Researcher). Currently employed as an educator at Terrariet in Vissenbjerg, Denmark, a reptile zoo founded in 1973. Former zoo board member (2005-2010) and webmaster (2003-2013) at the same place. Graduated from Marselisborg Teacher College (now Aarhus Teacher College), Aarhus, Denmark, in 1988. Work experience primarily as a biology and math teacher (8
th-10
th grade). Author and editor of RepFocus (
www.repfocus.dk), a website dedicated to the taxonomy, distribution, and bibliography of the reptiles of the world, first launched in 2019. Interested in various fields of research, primarily those related to his website, i.e., herpetological taxonomy and distribution. Self-taught nature photographer with images displayed at the online gallery Nature's Window (
www.repfocus.dk/natureswindow). Member of SSAR and formerly DGHT and NHF.
Author Bio 3
Emma Hsiao (Independent Researcher). Freelance illustrator and undergraduate student at Brown University, studying Biology and Visual Art ('25). She has previously worked in the Irschick Lab at UMass Amherst, Liu Lab at Harvard University, and Witman Lab at Brown University, where she contributed to photogrammetry, digital 3D specimen creation, figure illustration, and science outreach projects. Her research interests lie in herpetology and ecology. .
References
- Boulenger, G.A. On new or little-known Indian and Malayan reptiles and batrachians. Ann. Mag. Nat. Hist., London (Ser. 6) 1891, 8, 288–292. [Google Scholar] [CrossRef]
- Mocquard, F. 1897. Notes herpetologiques. Bull. Mus. Nat. Hist. Nat., Paris 1897, 3, 211–217. [Google Scholar] [CrossRef]
- Vogt, T. 1922. Beiträge zur Fauna Sinica. Zur Reptilien- und Amphibienfauna Südchinas. Mell, R., Ed.; Archiv Natur., Berlin 1922, 88A, 135–146. [Google Scholar]
- Schulz, K.-D. A monograph of the colubrid snakes of the genus Elaphe Fitzinger. Koeltz Scientific Books, Havlickuv Brod, Czech Republic, 1996; pp. 1–439.
- Midtgaard, R. RepFocus – A survey of the reptiles of the world. 2023; Available online: www.repfocus.dk.
- Günther, A.C.L.G. 1864. The reptiles of British India. Robert Hardwicke, London, 1864; pp. 1–452.
- Pope, C.H. The reptiles of China. American Museum of Natural History, New York, USA, 1935; pp. 1–604.
- Boulenger, G.A. Catalogue of the snakes in the British Museum (Natural History). Volume II, containing the conclusion of the Colubridae aglyphae. British Museum (Natural History), London, 1894; pp. 1–382.
- Dowling, H.G. A taxonomic study of the ratsnakes. VI. Validation of the genera Gonyosoma Wagler and Elaphe Fitzinger. Copeia 1958, 1958, 29–40. [Google Scholar] [CrossRef]
- Smith, M.A. Fauna of British India. Reptilia and Amphibia. Vol. 3. Ophidia. Taylor & Francis, London, U.K., 1943; pp. 1–583.
- Staszko, R.; Walls, J.G. Rat snakes: a hobbyist's guide to Elaphe and kin. T.F.H. Publications, Neptune City, USA, 1994; pp. 1–208.
- Chen, X. , McKelvy A.D.; Grismer L.L.; Matsui, M.; Nishikawa, K.; Burbrink, F.T. The phylogenetic position and taxonomic status of the rainbow tree snake Gonyophis margaritatus (Peters, 1871) (Squamata: Colubridae). Zootaxa 2014, 3881, 532–548. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.E. Descriptions of some undescribed species of reptiles collected by Dr. Joseph Hooker, in the Khasia Mountains, E Bengal and Sikkim, Himalaya. Ann. Mag. Nat. Hist., London 1853, (Ser. 2) 12, 386–392.
- Peters, W.C.H. 1871. Über neue Reptilien aus Ostafrica und Sarawak (Borneo), vorzüglich aus der Sammlung des Hrn. Marquis J. Doria zu Genua. Monats. König. Akad. Wissen. Berlin 1871 1871, 566–581.
- Boie, F. Bemerkungen über Merrem's Versuch eines Systems der Amphibien. 1te Lieferung: Ophidier. Isis v. Oken, Jena 1827, 20, 508–566. [Google Scholar]
- Blyth, E. Notices and descriptions of various reptiles, new or little known. J. Asiatic Soc. Bengal (Nat. Hist.), Calcutta 1858, 23, 287–302. [Google Scholar]
- Bleeker, P. de. Gonyosoma jansenii Blkr., eene nieuwe slang van Manado. Natuurk. Tijds. Nederl. Indië, Batavia (Sér. 4) 1858, 16, 242. [Google Scholar]
- Burbrink, F.T.; Lawson, R. How and when did Old World ratsnakes disperse into the New World? Mol. Phylo. Evol. 2007, 43, 173–189. [Google Scholar] [CrossRef]
- Jablonski, D., Ribeiro-Júnior, M.A.; Simonov, E.; Soltys, K.; Meiri S. A new, rare, small-ranged, and endangered mountain snake of the genus Elaphe from the southern Levant. Sci. Report., 2023. [CrossRef]
- Zhu, G.-X.; Yang, S.-J.; Savitzky, A.H.; Cheng, Y.-Q.; Mori, A.; Ding, L.; Rao, D.-Q.; Wang, Q. Cryptic diversity and phylogeography of the Rhabdophis nuchalis group (Squamata: Colubridae). Mol. Phylo. Evol. 2022, 166, 107325. [Google Scholar] [CrossRef]
- Stratakis, M.; Koutmanis, I.; Ilgaz, C.; Jablonski, D.; Kukushkin, O.V.; Crnobrnja-Isailovic, J.; Carretero, M.A.; Liuzzi, C.; Kumlutas, Y.; Lymberakis, P.; Poulakakis, N. Evolutionary divergence of the smooth snake (Serpentes, Colubridae): the role of the Balkans and Anatolia. Zool. Scripta 2022, 51, 310–329. [Google Scholar] [CrossRef]
- Salvi, D.; Mendes, J.; Carranza, S.; Harris, D.J. Evolution, biogeography and systematics of the western Palearctic Zamenis snakes. Zool. Scripta 2018, 47, 441–461. [Google Scholar] [CrossRef]
- Alfaro, M.E.; Karns, D.R.; Voris, H.K.; Brock, C.D.; Stuart, B.L. Phylogeny, evolutionary history, and biogeography of Oriental-Australian rear-fanged water snakes (Colubroidea: Homalopsidae) inferred from mitochondrial and nuclear DNA sequences. Mol. Phylo. Evol. 2008, 46, 576–593. [Google Scholar] [CrossRef] [PubMed]
- McVay, J.D.; Flores-Villela, O.; Carstens, B. Diversification of North American natricine snakes. Biol. J. Linn. Soc., London 2015, 116, 1–12. [Google Scholar] [CrossRef]
- Guicking, D.; Lawson, R.; Joger, U.; Wink, M. Evolution and phylogeny of the genus Natrix (Serpentes: Colubridae). Biol. J. Linn. Soc., London 2006, 87, 127–143. [Google Scholar] [CrossRef]
- Engelbrecht, H.M.; Branch, W.R.; Tolley, K.A. Snakes on an African plain: the radiation of Crotaphopeltis and Philothamnus into open habitat (Serpentes: Colubridae). Peer J. 2021, 9, e11728. [Google Scholar] [CrossRef] [PubMed]
- Yadollahvandmiandoab, R.; Koroiva, R.; Bashirichelkasari, N.; Mesquita, D.O. Phylogenetic relationships and divergence times of the poorly known genus Spalerosophis (Serpentes: Colubridae). Organ. Diver. Evol. 2023, 23, 415–423. [Google Scholar] [CrossRef]
- Liu, S.; Hou, M.; Lwin, Y.H.; Wang, Q.; Rao, D. A new species of Gonyosoma Wagler, 1828 (Serpentes, Colubridae), previously confused with G. prasinum (Blyth, 1854). Evol. Syst. 2021, 5, 129–139. [Google Scholar] [CrossRef]
- Peng, L.-F.; Zhang, Y.; Huang, S.; Burbrink, F.T.; Chen, J.-M.; Hou, M.; Zhu, Y.-W.; Yang, H.; Wang, J.-C. A new snake species of the genus Gonyosoma Wagler, 1828 (Serpentes: Colubridae) from Hainan Island, China. Zool. Res. 2021, 42, 487–491. [Google Scholar] [CrossRef]
- Lalrinsanga, H.T.L.; Decemson, H.; Mathipi, V.; Tanpuii, L.; Muansanga, L.; Biakzuala, L. Contributions to the morphology and molecular phylogenetics of Gonyosoma prasinum (Blyth, 1854) (Reptilia: Squamata: Colubridae) from Mizoram, India. Hamadryad 2022, 39, 96–103. [Google Scholar]
- Vences, M.; Guayasamin, J.M.; Miralles, A.; Riva, I. de la. To name or not to name: criteria to promote economy of chance in Linnean classification schemes. Zootaxa 2013, 3636, 201–244. [Google Scholar] [CrossRef]
- David, P.; Teynié, A.; Vogel, G. The snakes of Laos. Edition Chimaira, Frankfurt am Main, Germany. 2023; pp. 1–960.
- Peters, W.C.H. Über eine neue von Hrn. Dr. A.B. Meyer auf Luzon entdeckte Art von Eidechsen (Lygosoma (Hinulia) leucospilos) und eine von demselben in Nordcelebes gefundene neue Schlangengattung (Allophis nigricaudus). Monats. König. Akad. Wissen. Berlin 1872, 1872, 684–687. [Google Scholar]
- . Rooij, N. de. The reptiles of the Indo-Australian Archipelago. II. Ophidia. E.J. Brill, Leiden, The Netherlands, 1917; pp. 1–334.
- Tweedie, M.W.F. The snakes of Malaya, 3rd ed.; Singapore National Printers, Singapore, 1983; pp. 1–167.
- Schulz, K.-D.; Gumprecht, A. 2013. Undercover in the rainforest canopy: the rainbow tree snake Gonyophis margaritatus. In Old World ratsnakes, Schulz, K.-D., Ed.; Bushmaster Publications, Berg, Germany, 2013; pp. 395–402.
- Stuebing, R.B.; Inger, R.F.; Lardner, B. A field guide to the snakes of Borneo, 2nd ed.; Natural History Publications (Borneo), Kota Kinabalu, Malaysia, 2014; pp. 1–310 pp.
- Charlton, T. A guide to snakes of peninsular Malaysia and Singapore. Natural History Publications (Borneo), Kota Kinabalu, Malaysia. 2020; pp. 1–300.
- Das, I. A field guide to the reptiles of South-East Asia. New Holland Publishers, London, U.K., 2010; pp. 1– 376.
- Malkmus, R.; Manthey, U.; Vogel, G.; Hoffmann, P.; Kosuch, J. Amphibians & reptiles of Mount Kinabalu (North Borneo). A.R.G. Gantner Verlag, Ruggell, Liechtenstein, 2002; pp. 1–424.
- Iskandar, D., Jenkins, H.; Das, I.; Auliya, M.; Inger, R.F.; Lilley R. Gonyophis margaritatus. IUCN Red List of Threatened Species 2012; e.T192044A2032534.
- Lang, R. de; Vogel, G. The snakes of Sulawesi. Edition Chimaira, Frankfurt am Main, Germany, 2005; pp. 1–312.
- Taylor, E.H. The serpents of Thailand and adjacent waters. Univ. Kansas Sci. Bull., 1965, 45, 609–1096. [Google Scholar]
- Tepedelen, K.; Smith, H.M. Natural history notes: Serpentes: Elaphe janseni (Celebes black-tailed ratsnake). Maximum size. Herp. Rev., 1998, 29, 241. [Google Scholar]
- Fesser, R. Forty years of husbandry and breeding of Old World ratsnakes – a summary. In Old World ratsnakes; Schulz, K.-D., Ed., Bushmaster Publications, Berg, Germany, 2013; pp. 417–422.
- Rusli, N.; Gillespie, G. Gonyosoma jansenii. IUCN Red List of Threatened Species 2021; Available online: e.T104839453A104853990.
- Nutaphand, W. "Snakes in Thailand." Amarin Printing & Publishing Company, Bangkok, 2001; pp. 1–2001. [in Thai].
- Wagler, J.G. 1828. Auszüge aus seinem Systema Amphibiorum. Isis v. Oken, Leipzig 1828, 21, 740–744. [Google Scholar]
- Schlegel, H. Essai sur la physionomie des serpens. I. Partie générale. II. Partie descriptive. Atlas. Arnz & Comp., Leiden, The Netherlands, 1837; pp. 1–251+606.
- Gray, J.E. Description of three new genera and species of snakes. Ann. Mag. Nat. Hist., London 1849, (Ser. 2) 4, 246–248. [CrossRef]
- Hallowell, E. Report upon the Reptilia of the North Pacific Exploring Expedition, under the command of Capt. John Rogers, U.S.N. Proc. Acad. Nat. Sci. Philadelphia 1861 12, 480–510.
- Werner, F. Neue Schlangen des Naturhistorischen Museums in Wien. Ann. Naturhist. Mus. Wien 1923, 36, 160–166. [Google Scholar]
- Werner, F. Neue oder wenig bekannte Schlangen aus dem Wiener naturhistorischen Staatsmuseum (2. Teil.) Sitz. Akad. Wiss. Wien (Math.-Nat.), 1925, 134, 45–66. [Google Scholar]
- Werner, F. Neue oder wenig bekannte Schlangen aus dem Wiener naturhistorischen Staatsmuseum (III. Teil). Sitz. Akad. Wiss. Wien (Math.-Nat.), 1926, 135, 243–257. [Google Scholar]
- Taylor, E.H. The snakes of the Philippine Islands. Bureau of Printing, Manila, Philippines, 1922; pp. 1–312.
- Iskandar, D.T. A new color variation of Gonyosoma oxycephalum from central Java. The Snake 1987, 1987 19, 129–132. [Google Scholar]
- Lillywhite, H.B.; Ellis, T.M. Extrapulmonary air sacs in an arboreal snake (abstract). Amer. Zool. 1991 31, 38A.
- Wallach, V. 1998. The lungs of snakes. In Biology of the Reptilia, Gans C.; Gaunt, A.S., Eds.; Vol. 19 (Morphology G). Society for the Study of Amphibians and Reptiles, Athens, USA, 1998; pp. 93–295.
- McKay, J.L. A field guide to the amphibians and reptiles of Bali. Krieger Publishing Company, Malabar, USA, 2006; pp. 1–138.
- Pickersgill, S.; Meek, R. Husbandry notes on the Asian rat snake Gonyosoma oxycephala. Brit. Herp. Soc. Bull. 1988, 23, 23–24. [Google Scholar]
- Whitaker, R.; Captain, A. Snakes of India: the field guide. Draco Books, Chennai. India, 2004; pp. 1–479.
- Grismer, L.L. Field guide to the amphibians and reptiles of the Seribuat Archipelago (Peninsular Malaysia): a field guide. Edition Chimaira, Frankfurt am Main, Germany, 2011; pp. 1–239 pp.
- Cox, M.J.; Hoover M.F.; Chanhome, L.; Thirakhupt, K. The snakes of Thailand. Chulalongkorn University Museum of Natural History, Bangkok, Thailand, 2012; pp. 1–845.
- Wogan, G.; Vogel, G.; Neang, T.; Nguyen, T.Q.; Demegillo, A.; Diesmos, A.C.; Gonzalez, J.C. Gonyosoma oxycephalum. IUCN Red List of Threatened Species 2012b; Available online: e.T183196A1732988.
- Chakravarty, R.; Saw, I. Beobachtungen an Gonyosoma oxycephalum (Boie, 1827) beim erbeuten von Höhlenfledertieren auf den Andamanen, Indien. Sauria (Berlin) 2014, 36, 55–58. [Google Scholar]
- Dieckmann, S.; Norval, G.; Mao, J.J. A gravid Indonesian Red-tailed Green Ratsnake (Gonyosoma oxycephalum [Boie 1827]) in the pet trade. IRCF Rept. Amph. 2015, 22, 32–33. [Google Scholar] [CrossRef]
- Lang, R. de. The snakes of Java, Bali and surrounding islands. Edition Chimaira, Frankfurt am Main, Germany, 2017; pp. 1–435.
- Zhao, E.-M.; Huang, M.-H.; Zong, Y. Fauna Sinica. Reptilia Vol. 3, Squamata: Serpentes. Science Press, Beijing, 1998; pp. 1–522.
- Schmidt, K.P. New reptiles and a new salamander from China. Amer. Mus. Novit. 1925, 157, 1–5. [Google Scholar]
- Hu, B.-C.; Huang, M.-H.; Ho, S.-S.; Wei, K.-C. New records of snakes from Chekiang. Acta Zool. Sin., Beijing 1958, 10, 113–122. [Google Scholar]
- Sharma, R.C. Fauna of India and adjacent countries. Reptilia. Volume-III (Serpentes). Zoological Survey of India, Kolkata, India, 2007; pp. 1–410.
- You, C.-W.; Li S.-W.; Lau, A. 2013. Ratsnakes of Taiwan: an annotated photographic review. In Old World ratsnakes, Schulz, K.-D., Ed.; Bushmaster Publications, Berg, Germany, 2013; pp. 369–384.
- Wallach, V.; Williams, K.L.; Boundy, J. Snakes of the world: a catalogue of living and extinct snakes. CRC Press, Boca Raton, USA, 2014; pp. 1–1209.
- Wangyal, J.T.; Das, I. A Guide to the Reptiles of Bhutan. Bhutan Ecological Society, Thimphu, Bhutan, 2021; pp. 1–109.
- Li, P.; Lau, M. Gonyosoma frenatum. IUCN Red List of Threatened Species 2021; Available online: e.T191929A2016603.
- Messenger, K.R. 2021. The Asian ratsnakes and kin of Greater China. KDP Publishing, Nanjing, China, 2021; pp. 1–175.
- Bourret, R. Notes herpétologiques sur l'Indochine française. Bull. Gén. Instr. Publ., Hanoi 1934, 14, 73–83.
- Obst, F.J.; Richter, K.; Jacob, U. The completely illustrated atlas of reptiles and amphibians for the terrarium. T.F.H. Publications, Neptune City, USA. 1988; pp. 1–831.
- Fan, T.H. 1931. Preliminary report of reptiles from Yaoshan, Kwangsi, China. Bull. Depart. Biol., Sun Yatsen Univ. 1931, 11, 1–154. [Google Scholar]
- Brachtel, N. Das Portrait: Rhynchophis boulengeri Mocquard. Sauria (Berlin) 1998, 20, 2. [Google Scholar]
- Schulz, K.-D.; Ryabov S.; Wang, X. 2013. Contribution to the knowledge of the Oriental rhino ratsnake, Rhynchophis boulengeri Mocquard. In Old World ratsnakes. Schulz, K.-D., Ed.; Bushmaster Publications, Berg, Germany, 2013; pp. 385–394.
- Nguyen, L.T.; Kane, D.; Le, M.V.; Nguyen, T.T.; Hoang, H.V.; McCormack, T.E.M.; Tapley, B.; Nguyen, S.N. The southernmost distribution of the rhinocercos snake, Gonyosoma boulengeri (Mocquard, 1897) (Reptilia, Squamata, Colubridae), in Vietnam. Check List 2020, 16, 337–342. [Google Scholar] [CrossRef]
- Kane, D.; Gill, I.; Harding, L.; Capon, J.; Franklin, M.; Servini, F.; Tapley, B.; Michaels, C.J. Captive husbandry and breeding of Gonyosoma boulengeri. Herp. Bull. 2017, 139, 7–11. [Google Scholar]
- Orlov, N.L. Rare snakes of the mountainous forests on northern Indochina. Russ. J. Herp. 1995, 2, 179–183. [Google Scholar]
- Orlov, N.L.; Ryabov, S.A.; Schulz, K.D. Eine seltene Natter aus Nordvietnam, Rhynchophis boulengeri Mocquard, 1897 (Squamata: Serpentes: Colubridae). Sauria (Berlin) 1999, 21, 3–8. [Google Scholar]
- Devisch, F. Rhynchophis boulengeri. Vietnamese long-nosed snake, rhino rat snake. Litt. Serpent. 2010, 30, 78–85. [Google Scholar]
- Nguyen, Q.T.; Stenke, R.; Nguyen, H.X.; Ziegler, T. The terrestrial reptile fauna of the Biosphere Reserve Cat Ba Archipelago, Hai Phong, Vietnam. Bonn. Zool. Monogr. 2011 57, 99–115.
- Rao, D.Q.; Lau, M. Rhynchophis boulengeri. IUCN Red List of Threatened Species 2012; Available online: e.T190628A1955324.
- Ackermann, G. Ein Fall von Kannibalismus bei Gonyosoma boulengeri (Mocquard, 1897), mit Anmerkungen zur Inkubation im Terrarium. Sauria (Berlin) 2015, 37, 59–62. [Google Scholar]
- David, P.; Campbell, P.D.; Deuti, K.; Hauser, S.; Luu, V.Q.; Nguyen, T.Q.; Orlov, N.; Pauwels, O.S.G.; Scheinberg, L.; Sethy, P.G.S.; Smits, T.; Teynié, A.; Vogel, G. On the distribution of Gonyosoma prasinum (Blyth, 1854) and Gonyosoma coeruleum Liu, Hou, Ye Htet Lwin, Wang & Rao, 2021, with a note on the status of Gonyosoma gramineum Günther, 1864 (Squamata: Serpentes: Colubridae). Zootaxa 2022, 5154, 175–197. [Google Scholar]
- Schleich, H.H.; Kästle, W. Amphibians and reptiles of Nepal: biology, systematics, field guide, A.R.G. Gantner Verlag, Ruggell, Liechtenstein, 2002; pp. 1–1201.
- Grossmann, W. Elaphe prasina (Blyth). Sauria (Berlin) 2002 24, 573–576, (Suppl).
- Wogan, G.; Grismer, L.L.; Chan-Ard, T. Rhadinophis prasina. IUCN Red List of Threatened Species 2012a; Available online: e.T192082A2037579.
- Vassilieva, A.B.; Galoyan, E.A.; Poyarkov, N.A.; Geisssler, P. A photographic field guide to the amphibians and reptiles of the lowland monsoon forests of southern Vietnam. Edition Chimaira, Frankfurt am Main, Germany, 2016; pp. 1–324.
- Lalrinsanga; Lalremsanga, H.T.; Decemson, L.H.; Mathipi, V.; Tanpuii, L.; Muansanga, L.; Biakzuala, L. Contributions to the morphology and molecular phylogenetics of Gonyosoma prasinum (Blyth, 1854) (Reptilia: Squamata: Colubridae) from Mizoram, India. Hamadryad 2022, 39, 96–103.
- Holman, J.A. Pleistocene amphibians and reptiles in Britain and Europe. Oxford University Press, New York, USA, 1998; pp. 1–284.
- Ivanov, M. Miocene snakes of Eurasia: a review of the evolution of snake communities. In The origin and early evolutionary history of snakes. Gower, D.J.; Zaher, H., Eds.; Cambridge University Press, Cambridge, U.K., 2022; pp. 85–110.
- Figueroa, A.; McKelvy, A.D.; Grismer, L.L.; Bell, C.D.; Lailvaux, S.P. A species-level phylogeny of extant snakes with description of a new colubrid subfamily and genus. PLoS ONE 2016, 11, e0161070. [Google Scholar] [CrossRef]
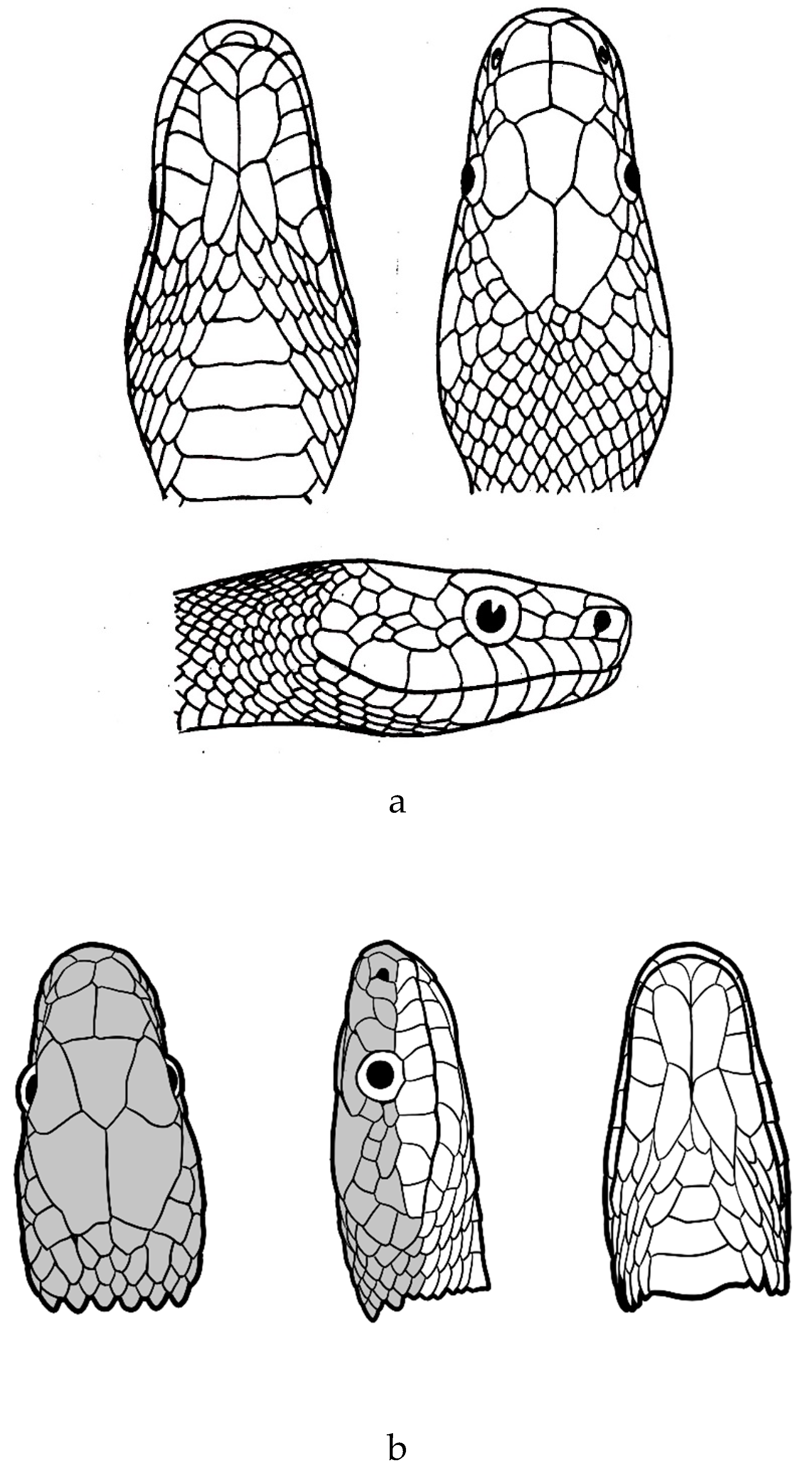
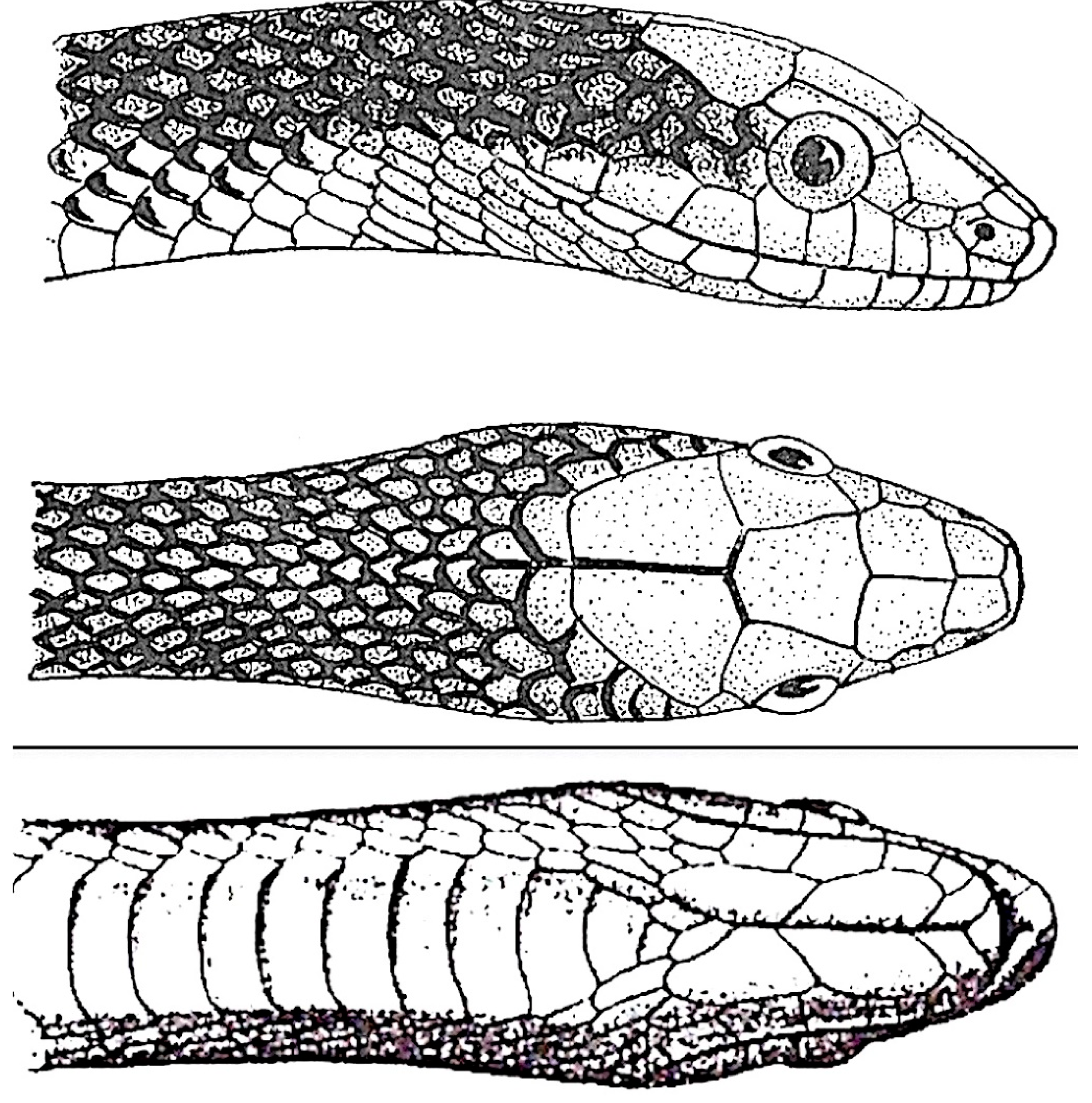
Figure 1.
Gonyophis margaritatus (after Peters [
33] & Rooij [
34]).
Figure 1.
Gonyophis margaritatus (after Peters [
33] & Rooij [
34]).
Figure 2.
Gonyosoma jansenii (after Schulz [
4]).
Figure 2.
Gonyosoma jansenii (after Schulz [
4]).
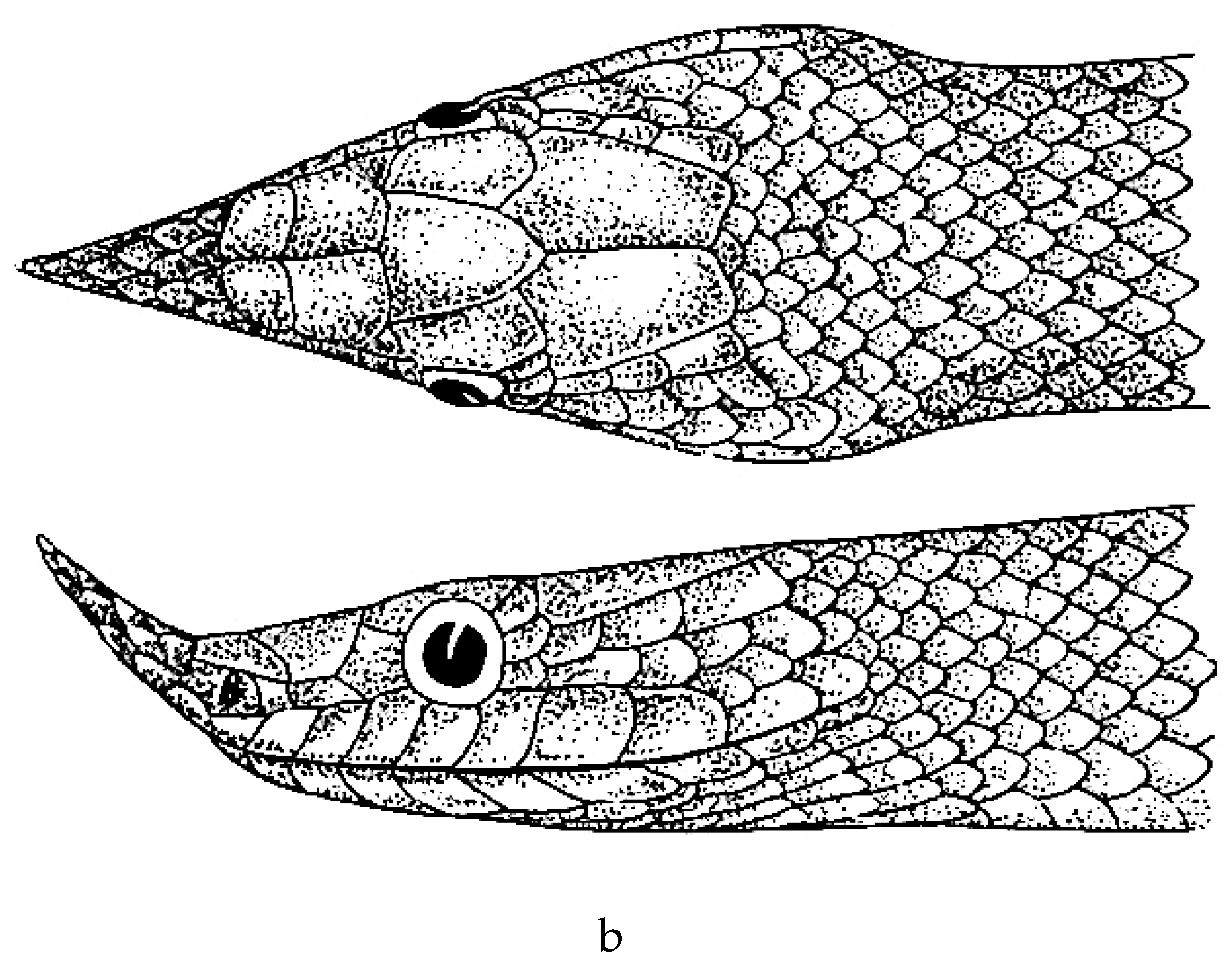
Figure 3.
a.
Gonyosoma oxycephalum (after Nutaphand [
46]). b.
Gonyosoma oxycephalum (after Schulz [
4]).
Figure 3.
a.
Gonyosoma oxycephalum (after Nutaphand [
46]). b.
Gonyosoma oxycephalum (after Schulz [
4]).
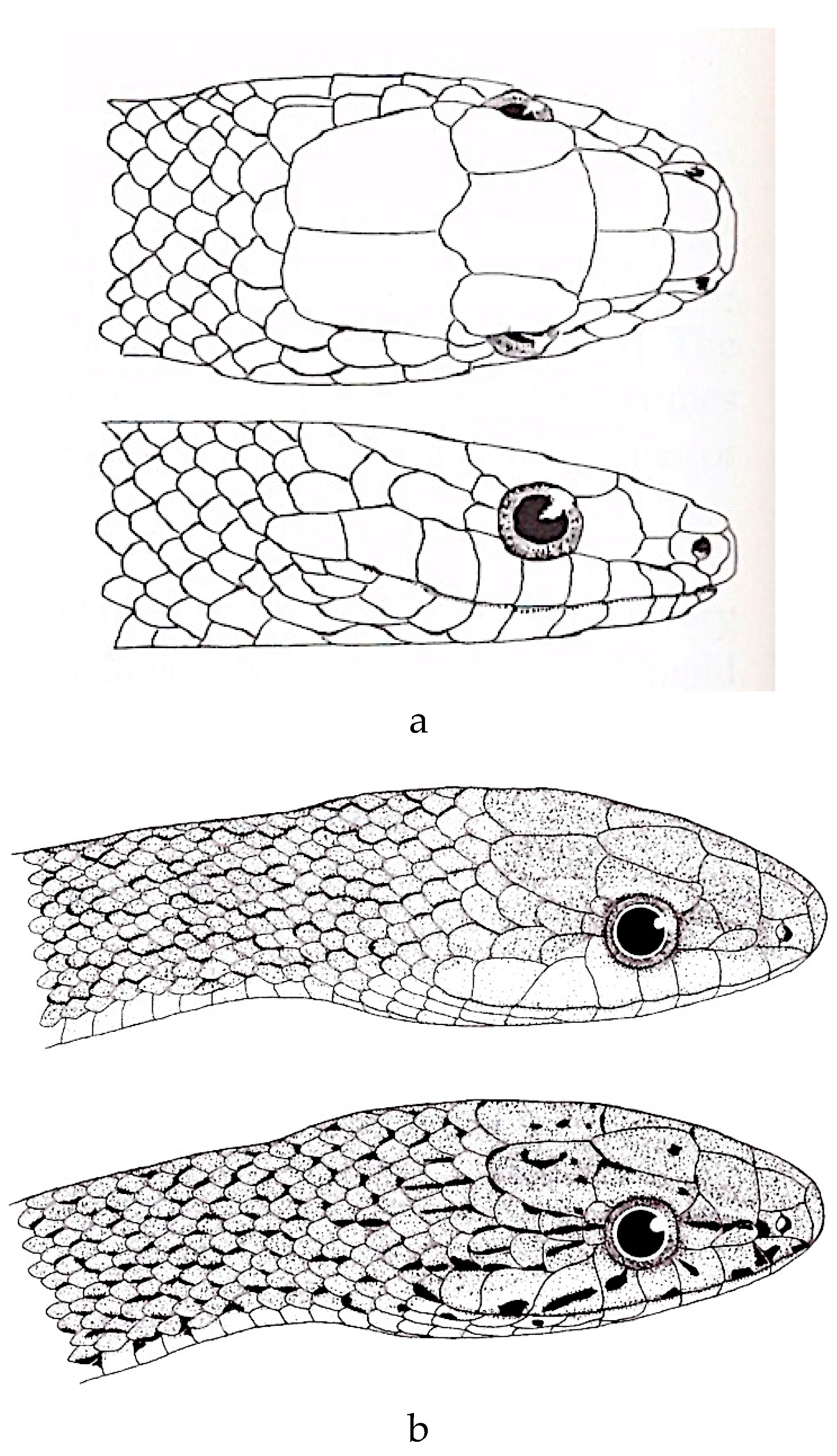
Figure 4.
a.
Rhadinophis frenatus (after Zhao et al. [
68]). b.
Rhadinophis frenatus (after Schulz [
4]).
Figure 4.
a.
Rhadinophis frenatus (after Zhao et al. [
68]). b.
Rhadinophis frenatus (after Schulz [
4]).
Figure 5.
a.
Rhynchophis boulengeri (after Bourret [
77]). b.
Rhynchophis boulengeri (after Obst et al. [
78]).
Figure 5.
a.
Rhynchophis boulengeri (after Bourret [
77]). b.
Rhynchophis boulengeri (after Obst et al. [
78]).
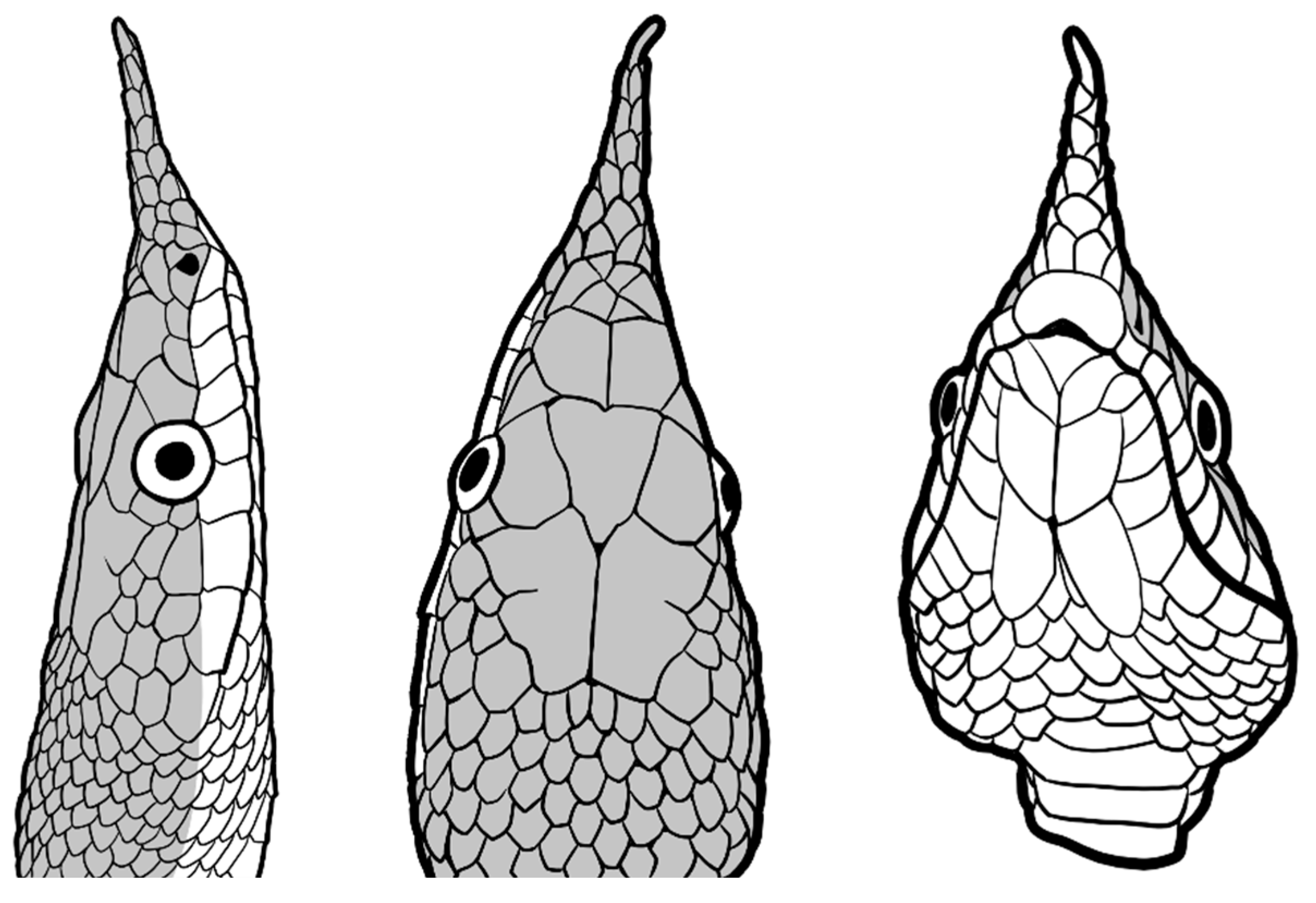
Figure 6.
Rhynchophis hainanensis (E. Hsiao from Peng et. al. [
28]).
Figure 6.
Rhynchophis hainanensis (E. Hsiao from Peng et. al. [
28]).
Figure 7.
a.
Verdigrophis coeruleus (after Zhao et al. [
68]). b.
Verdigrophis coeruleus (E. Hsiao from Liu et. al. [
28]).
Figure 7.
a.
Verdigrophis coeruleus (after Zhao et al. [
68]). b.
Verdigrophis coeruleus (E. Hsiao from Liu et. al. [
28]).
Figure 8.
a.
Verdigrophis prasinus (after Schleich & Kästle [
91]). b.
Verdigrophis prasinus (after Schulz [
4]).
Figure 8.
a.
Verdigrophis prasinus (after Schleich & Kästle [
91]). b.
Verdigrophis prasinus (after Schulz [
4]).
Table 1.
Specific characters of the Rhynchophiini. Elev. = elevation in m, ASR, MSR & PSR = anterior, midbody & posterior scale rows, C = carination type (S = smooth, k+s = partly keeled and partly smooth), SR = smooth scale rows, KR = keeled scale rows, V = ventrals, C.S. = cloacal shield (D = divided, E = entire), SC = subcaudals, L = loreal (present = 1, absent = 0, S = squarish, E = elongated), SL = supralabials, SLO = SL entering orbit, Pst = postoculars, AT, MT & PT = anterior, medial & posterior temporals, IL = infralabials, AG = IL contacting anterior genials, LOA = total length in mm, RTL = relative tail length (TL/LOA), UVI = umbilicus-vent interval (as % total ventrals), R.A. = rostral appendage, P-F = preocular-frontal contact, Fr = frontal shape (B = broad, M = moderate, N = narrow, T = tapered posteriorly, P = parallel, S = subtriangular), IN = internasal proportions (L = length, W = width), Max. = maxillary teeth, VLTS = ventrolateral tail stripe, J.C. = juvenile coloration (.Gr = green, Gy = gray, O = olive, B = brown), Eggs = eggs in clutch.
Table 1.
Specific characters of the Rhynchophiini. Elev. = elevation in m, ASR, MSR & PSR = anterior, midbody & posterior scale rows, C = carination type (S = smooth, k+s = partly keeled and partly smooth), SR = smooth scale rows, KR = keeled scale rows, V = ventrals, C.S. = cloacal shield (D = divided, E = entire), SC = subcaudals, L = loreal (present = 1, absent = 0, S = squarish, E = elongated), SL = supralabials, SLO = SL entering orbit, Pst = postoculars, AT, MT & PT = anterior, medial & posterior temporals, IL = infralabials, AG = IL contacting anterior genials, LOA = total length in mm, RTL = relative tail length (TL/LOA), UVI = umbilicus-vent interval (as % total ventrals), R.A. = rostral appendage, P-F = preocular-frontal contact, Fr = frontal shape (B = broad, M = moderate, N = narrow, T = tapered posteriorly, P = parallel, S = subtriangular), IN = internasal proportions (L = length, W = width), Max. = maxillary teeth, VLTS = ventrolateral tail stripe, J.C. = juvenile coloration (.Gr = green, Gy = gray, O = olive, B = brown), Eggs = eggs in clutch.
| Species |
Elev |
ASR |
MSR |
PSR |
C |
SR |
KR |
V |
C.S. |
SC |
L |
SL |
SLO |
Pst |
| G. margaritatus |
0–2000 |
19–21 |
19 |
15–17 |
k+s |
I–III/VI |
IV/VII-Vert |
230–249 |
D |
108–130 |
1S |
9 (8,10) |
3–5/4–6/5–7 (5–6) |
2 |
| Gon. jansenii |
100–1000 |
21–23 |
23–25 |
15–17(13) |
S |
All |
None |
245–257 |
D |
130–140 |
1E |
9–10 |
5–7 (6–7) |
2 |
| Gon. oxycephalum |
20–1400 |
23–27 |
23–25(27) |
15–17 |
S |
All |
None |
229–263 |
D |
120–157 |
1E(0) |
9–11 (7,8) |
4–5/5–6/6–7 /4–6$$$5–7/6–8 |
2 |
| Rha. frenatus |
170–2800 |
19(21) |
19(17) |
17(13–15) |
k+s |
I–VIII |
PV+V |
198–235 |
D |
108–149 |
0 |
8(9) |
3–5/4–6 |
2 |
| Rhy. boulengeri |
0–2000 |
19 |
19(17,18) |
15(13) |
k+s |
I–VI |
VII-V |
207–227 |
D |
101–133 |
1E |
9 (10) |
4–6/5–7 |
2 (3) |
| Rhy. hainanenesis |
80–900 |
19 |
19 |
15(13) |
k+s |
VL |
MD |
216–221 |
D |
122–133 |
2S |
9 |
4–6 |
2 |
| V. coeruleus |
250–1650 |
19(20) |
19(15,17) |
15(13,17) |
k+s |
I–IV/VI |
V/VII–Vert |
181–224 |
D |
88–128 |
1S |
9 (8,10) |
4–6 (3–5) |
2 |
| V. prasinus |
75–2560 |
19(17,21) |
19(17) |
15(13,14,17) |
k+s |
I–V/VI |
VI/VII–Vert |
186–209 |
E |
91–116 |
1S(0) |
9(8) |
4–6 (4–5) |
2 |
| Species |
AT |
MT |
PT |
IL |
AG |
LOA |
RTL |
UVI |
R.A. |
P-F |
Fr |
IN |
Max. |
VLTS |
J.C. |
Eggs |
| G. margaritatus |
2 |
2–3 |
2–3 |
10–12 |
5 |
424–1943 |
22.6–33.3 |
11.8–13.3 |
No |
No |
B/T |
W = 1.5L |
20–23 |
No |
MC |
? |
| Gon. jansenii |
1(2) |
2–3 |
3 |
11–13 |
1–5 |
420–2374 |
22.8–24.8 |
16.7 |
No |
Yes |
M/T |
L = W |
? |
No |
Gr |
2–9 |
| Gon. oxycephalum |
2(1,3) |
2(4,5) |
3 |
12–15 |
1–5(6,7) |
240–2400 |
20.9–27.7 |
14.2–18.7 |
No |
Yes |
M/T |
L = 2W |
20-25 |
No |
Gr |
2–12 |
| Rha. frenatus |
2(1,3) |
2–3 |
2–3(4) |
9–11 |
1–5/6 |
120–1475 |
23.7–31.0 |
12.5–14.1 |
No |
No |
N/P |
L > W |
19–25 |
Yes |
Gy/O/B |
6–12 |
| Rhy. boulengeri |
2(1) |
3(2) |
3 |
10–11 |
1–5(6) |
170–1630 |
20.6–30.2 |
13.1–13.7 |
Yes |
Yes |
S |
L = W |
16–22 |
Yes |
Gy/B |
5–17 |
| Rhy. hainanenesis |
2 |
2 |
3 |
10–12 |
1–5 |
718–1229 |
22.0–32.5 |
? |
Yes |
Yes |
S |
L = W |
? |
No |
Gy |
6 |
| V. coeruleus |
1–2 |
2(1,3) |
2–3 |
9–11 |
1–5 |
200–1192 |
22.4–27.5 |
13.7 |
No |
No |
B/T |
L = W |
20–21 |
No |
Gr |
3–11 |
| V. prasinus |
1–2 |
1–2(3) |
2(3) |
9–10 (12) |
1–5(6) |
150–1355 |
19.4–26.7 |
14.4 |
No |
No |
B/T |
L = W |
19–23 |
No |
Gr |
3–14 |
Table 2.
Generic characters of the Rhynchophiini. MSR = midbody scale rows, C = carination (S = smooth, k+s = feebly keeled and smooth), KR = number of keeled scale rows, Lor = loreal number, SL = supralabial number, P/F = preocular-frontal contact (0 = no, + = yes), Fr = frontal width and shape (B = broad, M = moderate, N = narrow, P = parallel, T = tapered), IN = internasals (L = length, W = width), Gen = genials (A = anterior pair, P = posterior pair), RA = rostral appendage, Head = dorsal head color (G = green, O = orange, R = red, Gr = gray, Bl = blue), GD = green dorsum (+ = yes, 0 = no), DP = dorsal pattern (U = uniform, R = posterior rings), Tail = dorsal tail color (G = green, B = black, O = orange, R = red, Br = brown), Iris = iris color (B = black, R = red, O = orange, Y = yellow, Bl = blue, Br = brown), T.L. = tracheal lung (0 = absent, + = present), MI = interior mouth color (P = pink, G = gray, Pu = purple), Ont = ontogenetic change of juvenile color and/or pattern to adult (0 = no, + = yes), L.L. = left lung length (% SVL).
Table 2.
Generic characters of the Rhynchophiini. MSR = midbody scale rows, C = carination (S = smooth, k+s = feebly keeled and smooth), KR = number of keeled scale rows, Lor = loreal number, SL = supralabial number, P/F = preocular-frontal contact (0 = no, + = yes), Fr = frontal width and shape (B = broad, M = moderate, N = narrow, P = parallel, T = tapered), IN = internasals (L = length, W = width), Gen = genials (A = anterior pair, P = posterior pair), RA = rostral appendage, Head = dorsal head color (G = green, O = orange, R = red, Gr = gray, Bl = blue), GD = green dorsum (+ = yes, 0 = no), DP = dorsal pattern (U = uniform, R = posterior rings), Tail = dorsal tail color (G = green, B = black, O = orange, R = red, Br = brown), Iris = iris color (B = black, R = red, O = orange, Y = yellow, Bl = blue, Br = brown), T.L. = tracheal lung (0 = absent, + = present), MI = interior mouth color (P = pink, G = gray, Pu = purple), Ont = ontogenetic change of juvenile color and/or pattern to adult (0 = no, + = yes), L.L. = left lung length (% SVL).
| Genus |
MSR |
C |
KR |
Lor |
SL |
P/F |
Fr |
IN |
Gen |
RA |
Head |
GD |
DP |
Tail |
Iris |
T.L. |
MI |
O |
L.L. |
| Gonyophis |
19 |
k+s |
7–13 |
1 |
9 |
0 |
B/T |
W > L |
A > P |
0 |
O |
0 |
R |
B/O |
B/R/Br |
0 |
P |
0 |
1.9 |
| Gonyosoma |
23–25 |
S |
0 |
1 |
9–11 |
+ |
M/T |
L > W |
P > A |
0 |
R/G/Gr/B |
+ |
U |
B/R/Br |
Y/O/G/Bl |
+ |
P |
0 |
5.6–7.5 |
| Rhadinophis |
19 |
k+s |
3 |
0 |
8 |
0 |
N/P |
L > W |
P > A |
0 |
G |
+ |
U |
G |
Y |
0 |
P/Pu |
+ |
1.3 |
| Rhynchophis |
19 |
k+s |
3 |
1–2 |
9 |
+ |
S |
L = W |
P > A |
+ |
G |
+ |
U |
G |
Br |
0 |
P |
+ |
0.8 |
| Verdigrophis |
19 |
k+s |
5–11 |
1 |
9 |
0 |
B/T |
W > L |
P = A |
0 |
Bl/G |
+ |
U |
G |
Bl/G |
0 |
G/P |
0 |
1.4 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).